Abstract
Cell therapy for Parkinson’s disease (PD) began in 1979 with the transplantation of fetal rat dopamine-containing neurons that improved motor abnormalities in the PD rat model with good survival of grafts and axonal outgrowth. Thirty years have passed since the 2 clinical trials using cell transplantation for PD patients were first reported. Recently, cell therapy is expected to develop as a realistic treatment option for PD patients owing to the advancement of biotechnology represented by pluripotent stem cells. Medication using levodopa, surgery including deep brain stimulation, and rehabilitation have all been established as current therapeutic strategies. Strong therapeutic effects have been demonstrated by these treatment methods, but they have been unable to stop the progression of the disease. Fortunately, cell therapy might be a key for true neurorestoration. This review article describes the historical development of cell therapy for PD, the current status of cell therapy, and the future direction of this treatment method.
Keywords: cell therapy, dopaminergic neuron, ES cells, iPSCs, transplantation
Introduction
Since Cajal demonstrated the “neuron doctrine,”1 it was widely believed for many years that neural tissue in the central nervous system (CNS) never regenerates once it matures to differentiated neurons. However, Altman and Das first identified stem cells and neurogenesis in the adult mammalian brain in 1965 with autoradiography.2 Then, Reynolds and Weiss demonstrated in 1992 that neural stem cells (NSCs) exist in the adult mammalian brain,3 which gave rise to stem cell research and the many studies that followed.4–8 Biological technology involving various kinds of stem cells has been developed. Takahashi and Yamanaka established a method to induce pluripotent stem cells (iPSCs) from mouse embryonic and adult fibroblast cultures by 4 factors (i.e., octamer-binding transcription factor 3 or 4 (Oct3/4), sex determining region Y-box 2 [Sox2], c-Myc, and Kruppel-like factor 4 [Klf4]) in 20069 and from adult human fibroblasts in 2007.10 This discovery is accelerating the clinical trials of stem cell transplantation for patients with diseases in the CNS and widening the possibility of discovering new drugs or revealing the mechanisms of several diseases.
Parkinson’s disease (PD) is a neurodegenerative disease characterized by the loss of dopaminergic (DAergic) neurons in the nigrostriatal system. Resting tremors, rigidity, akinesia, and disturbance of postural reflex are the tetralogy of PD. Dopamine (DA) replacement therapy has been established and is now a gold standard for the treatment of PD.11 The advantage of medication is that it is less invasive, and oral administration leads to prompt effectiveness. On the other hand, the disadvantages include drug-induced dyskinesia and other side effects. Stereotactic surgery, such as deep brain stimulation and thermocoagulation, has also been established as a strong therapy option for PD.12,13 Surgery has dramatic and prompt effects, although it requires relatively invasive maneuvers and involves expensive medical care. Rehabilitation is also an established therapy for functional maintenance and recovery of PD patients.14,15 Basic research revealed that rehabilitation exerted neuroprotective effects on animal PD models with increased neurogenesis.16 Rehabilitation ameliorates the mental state of patients safely and at a low cost, although continuous efforts are needed to produce slow therapeutic effects. Although PD patients receive benefits from the existing therapies, these patients experience exacerbated symptoms over time.
PD pathology mainly lies in the degeneration of DAergic neurons in the nigrostriatal system brought on by both genetic and environmental triggers. Thus, PD is a good target for cell therapy. Fetal nigral cell transplantation17,18 was initially considered to have a “magical” effect on PD patients. In this review article, fetal nigral cell transplantation is described as part of the historical development of cell therapy for PD (see the following section). Subsequently, the current status of cell therapy and the future direction of cell therapy for PD will be discussed.
Historical Development of Cell Therapy for PD
Fetal Nigral Cells
Cell therapy for PD began when the transplantation of fetal rat DA-containing neurons improved motor abnormalities in the PD model of rats with good survival of grafts and axonal outgrowth in 1979.19 This study showed the potential of cell therapy to reverse the deficits after circumscribed destruction of brain tissue. In clinical practice, roughly 30 y have passed since the 2 clinical trials using cell transplantation for PD patients were reported in 1988.17,18 Fetal nigral cell transplantation was intended to achieve synapse formation between transplanted donor cells and preserved host neurons as well as to supply DA. This method was considered to be an effective and safe therapeutic option at that time, although there were immunological problems and ethical issues involved with using aborted fetuses. However, at the outset of the 21st century, randomized, double-blind studies revealed the insufficient functional recovery of older patients and delayed dyskinesia in some patients, although the increased DA uptake was recognized by positron emission tomography (PET) using 18F-fluorodopa.20–22 The results of these studies substantially reduced the momentum for fetal cell transplantation. On the other hand, the hopeful aspects of fetal nigral cell transplantation using a cell suspension were reported compared to the solid tissue transplantation used in the previous randomized, double-blind studies.23 Two patients showed improvement in motor function, reduction in levodopa (l-DOPA)-induced dyskinesia, and no dyskinesia in the off state. Four years after transplantation, postmortem analyses showed that many transplanted cells had survived with subtle immune reactions, despite the usage of immunosuppression for only 6 mo.23 Some patients receiving fetal nigral cell transplantation showed a continuous improvement of motor symptoms for over a decade.24 Thus, fetal nigral cell transplantation might exert strong therapeutic effects over an extended period if there is appropriate patient selection, transplantation protocol, and optimal trial design. The current status of the TRANSEURO trial (http://www.transeuro.org.uk), a European Union–funded multicenter clinical trial of fetal nigral cell transplantation, has been shown in detail.25 In order to increase the therapeutic effects and to minimize the risk of graft-induced dyskinesia, the overall protocol of TRANSEURO was strictly determined (Table 1). Most PD patients are idiopathic and age is considered as the major risk factor to exacerbate PD symptoms. Thus, a major flaw in the TRANSNEURO trial might be emphasis on younger subjects with early stage disease. In another article, it was confirmed that the patients enrolled in TRANSEURO were young, well-educated with higher cognitive scores, and possessed good motor function, compared to eligible PD patients not enrolled in TRANSEURO,26 although it is natural for the inclusion criteria to outline the characteristics of PD patients enrolled in such a trial. The results of TRANSEURO are highly anticipated and will strongly affect the future of cell therapy using DAergic neurons, although we need to consider the ethical issues of fetal nigral cell transplantation.
Table 1.
Information on TRANSEURO.a
| Issues | Conditions |
|---|---|
| Inclusion criteria | Aged less than 65 y |
| Disease duration less than 10 y | |
| Without cognitive impairment | |
| Without significant levodopa-induced dyskinesia | |
| Tissue preparation | Tissue collection in several centers |
| Transfer of tissue between centers | |
| Storage up to 4 d in hibernation medium | |
| Transplantation | Standardized procedure for grafting |
| Transplantation via 5 to 7 tracts into the posterior putamen | |
| Study design | Observation study with 150 patients |
| Randomized 40 of the 150 patients | |
| Assigned either to transplant or control group | |
| Immunosuppression for 1 y | |
| Primary end point | Three years after transplantation |
aThe condition and issues of criteria, such as patient selection, tissue composition, tissue placement, trial design, and primary end point, are described. The promising patients are selected with the intent of not causing graft-induced dyskinesia. Tissue is collected, stored, and transplanted in a stable fashion to ensure the quality of cell transplantation.
Autologous DA-secreting Cells
In the usage of autologous cells, there are no ethical issues and only a limited immune reaction. Autologous cell transplantation using various tissues to supply DA was also reported as a potential treatment for PD patients.27,28 A phase I/II study was performed using intrastriatal transplantation of the autologous carotid body in patients with advanced stage PD.28 Bilateral intrastriatal transplantation was performed in 13 PD patients, and no patients demonstrated graft-induced dyskinesia. Clinical amelioration was found in 10 patients. The carotid body is a paraneuron derived from the neural crest that is similar to the chromaffin cells of the adrenal medulla. Glial cell line–derived neurotrophic factor (GDNF) secreted from the carotid body might exert the neuroprotective effects of these transplanted cells.29 However, there have been no further clinical studies using this type of cells.
Current Status of Cell Therapy for PD
In the previous section, the clinical trials of cell transplantation with fetal nigral DAergic neurons and autologous DA-secreting cells were described. Next, we would like to examine the current status of cell therapy for PD. In the National Institutes of Health–registered clinical trials, 18 studies are identified when the terms “Parkinson’s disease” and “transplantation” are searched for (https://clinicaltrials.gov). Among these 18 studies, 5 involving cell transplantation are classified as open studies, which are currently recruiting participants or will be recruiting participants in the future as of February 2017. There are 2 studies using fetal DAergic cells, 1 study with allogeneic bone marrow–derived mesenchymal stem cells (BM-MSCs), 1 study with NSCs, and 1 study with encapsulated porcine choroid plexus cells. Thus far, various cell sources are still being studied in the United States.
In this section, we first briefly show our cell therapy strategy for PD. Subsequently, the current status of cell therapy using bone marrow–derived stem cells, embryonic stem cells (ESCs), and NSCs is described. Finally, the progress of the fast-evolving technology involving iPSCs is shown.
Our Strategy for PD
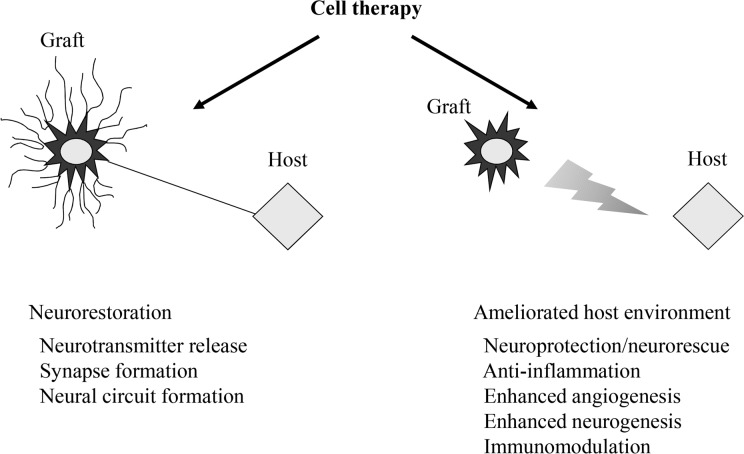
Twenty-seven and 21 y have passed since encapsulated cell transplantation for an animal PD model was initially reported in the world30 and in Japan,31 respectively. The merits of encapsulated cell transplantation include the ability to safely use various kinds of cells including genetically modified cells, to secrete a designed neurotransmitter, neurotrophic factor, or growth factor for at least 6 mo in vivo.32,33 The cells inside the capsule are protected from immunological rejection without the problem of tumor formation. Information on our method is described in the previous review article.34 GDNF-secreting NSCs were transplanted into the brains of PD model rats. In this study, behavioral amelioration and DAergic neuronal preservation were demonstrated with good engraftment of transplanted cells. In another study, MSCs were also demonstrated to be good candidates for cell therapy in the PD rat model.35 The intravenous administration of MSCs exerted therapeutic potentials through the neuroprotective effects of stromal cell-derived factor-1α. The benefits of cell therapy are (1) direct replacement of the host tissue and (2) trophic effect for the host tissue leading to neuroprotection, anti-inflammation, neurogenesis, and angiogenesis. The concept of MSC transplantation for PD is aimed at the latter benefit. Besides the development of the cell source, the timing of transplantation and the transplantation procedure should be thoroughly considered to ensure the appropriate evaluation of transplantation.36,37 In addition to cell therapy, the therapeutic mechanisms of carbamylated erythropoietin-Fc fusion protein,38 rehabilitation,16 spinal cord stimulation,39 and an antibody against high mobility group box 1 (HMGB1), which displays an inflammatory cytokine activity in the extracellular space,40 were explored for PD animal models. The therapeutic effects of anti-HMGB1 antibody administered intravenously showed the possibility of inflammatory control for PD. The aims of cell therapy for PD are not only the restoration of DAergic neural structure but also the amelioration of the host tissue environment (Fig. 1).
Figure 1.
Two keystones of cell therapy: neural restoration and amelioration of the host tissue environment. The idealistic form of regenerative medicine might be neural restoration, but the positive effects of host tissue should also be considered.
Cell Therapy Using MSCs, ESCs, and NSCs
In the stem cell era, various kinds of stem cells were explored in terms of their potential for PD treatment. MSCs are easily harvested and amplified via differentiation capacity. Dezawa et al. reported a method for inducing the differentiation of MSCs into the neuronal lineages by gene transfection with a Notch intracellular domain and subsequent administration of basic fibroblast growth factor (bFGF), forskolin, and ciliary neurotrophic factor (CNTF).5 Additional GDNF treatment increased the proportion of DAergic neurons. Next, they found multilineage-differentiating stress-enduring (Muse) cells41 with stage-specific embryonic antigen-3 (SSEA-3). The protocol for isolation and culture takes less time and labor than that of other stem cells. The use of Muse cells for the treatment of CNS disorders is another hope. Intranasal delivery of MSCs might also be attractive for clinical application.42,43 So far, however, there have been no successful clinical data involving MSCs for PD patients.
ESCs have also been studied vigorously. In 2000, 2 essential methods of neuronal differentiation from ESCs were reported. Kawasaki et al. found that the coculture of ESCs and stromal cells (stromal cell-derived inducing activity [SDIA] method) induce ESCs to become neuron-like cells with a high proportion of DAergic neuron-like cells.44 On the other hand, Lee et al. described a method that involves going through the embryoid body.45 Both methods were refined in various ways to realize a clinical application.46,47 With the SDIA method, Doi et al.48 and Takagi et al.49 conducted a preclinical trial using a monkey model. Recent studies revealed that markers of the caudal ventral mesencephalon are associated with higher DAergic neuronal differentiation from ESCs.50 From another institute, purification of human ESC- and iPSC-derived DAergic neurons with a cell surface marker of midbrain DAergic neurons, leucine-rich repeat and transmembrane domains 1, which belongs to the extracellular leucine-rich repeats superfamily, was reported as a tool for efficient cell therapy for PD patients.8 These results might improve the protocol for DAergic neurons from ESCs with subsequent outcomes of transplantation itself. The ethical issues and tumor formation are critical problems, but the continuous efforts to overcome tumorigenesis are impressive.
NSCs offer another source of hope.51 There are several research papers on the human-derived NSC line for an animal model of PD.5,52,53 The neuroprotective effects of NSCs were mediated by secreted trophic factor as well as by neuronal differentiation.6 Clonal human DAergic neuron precursors might exert stable therapeutic effects and be a good design for in vivo experiments.52 Recently, a phase I study of transplantation of NSCs for PD patients was reported from Turkey.54 Twenty-one PD patients were transplanted with NSCs derived from ESCs into the bilateral striatum at specific intervals, and the motor function of the patients improved significantly with no apparent side effects.
Current Status of iPSCs
As described briefly in the Introduction section, biotechnology using iPSCs opened new doors for cell therapy. After mouse- and human-derived iPSCs were established,9,10 the technology progressed rapidly. Tumorigenesis is a major concern in terms of the clinical application of iPSCs, and various modifications have been developed to reduce the risk of tumor formation. Methods have been identified to generate iPSCs without c-Myc,55 with only Oct3/4 and Klf4,56 with Oct4 from mouse NSCs,57 with recombinant proteins,58 without viral vectors,59 or without exogenous reprogramming factors.60 In 2011, Gli-similar 1, enriched in unfertilized oocytes, was shown to be another important factor to promote the direct reprogramming of somatic cells during iPSC generation.61 Thus, the efficient generation of iPSCs has been explored using safe methods. In Japan, the clinical application of iPSC-derived tissue may commence for age-related maculopathy. Very recently, it was reported that autologous iPSC-derived retinal pigment epithelial sheets survived for 1 y after transplantation with no adverse events.62 After the clinical study reveals the safety of this approach, PD might be an effective target for iPSC technology.63 There are several planned clinical trials of iPSC-based therapies around the world.64 In 2016, the first approved clinical trial using iPSCs to treat PD patients was started in Melbourne, Australia, by the International Stem Cell Corporation.65 iPSC technology is also expected to reveal pathological conditions using patient-derived iPSC research.66–69 DAergic neurons from PD patient-derived iPSCs produce double the amount of α-synuclein protein compared to neurons from unaffected donors.66 A recent study revealed significant differences in gene expression of DAergic neurons derived from iPSCs of PD patients, especially in genes related to neuronal maturity compared to primary midbrain DAergic neurons.69 Using PD patient-derived iPSCs and differentiated DAergic neurons, the genetic alteration, reaction to drugs, and fate of the cells might clarify what is beneficial and what is harmful for PD patients. Drug discoveries from iPSC technology are highly anticipated.64 Alternatively, the direct conversion or transdifferentiation of fibroblasts into neurons without going through the iPSC stage is another hopeful technique.70,71 Suppression of p53 combined with cell cycle arrest at G1 increased the efficiency in the direct conversion of human fibroblasts to DAergic neurons.71
Future Direction of Cell Therapy for PD
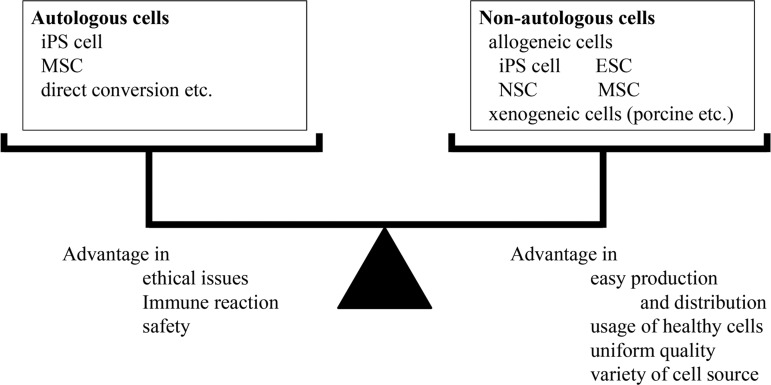
When considering the future direction of cell therapy, issues related to the cell source, conditions of cell therapy, and the mechanisms involved are all important concerns. Transplanted cells can be divided broadly into 2 groups: autologous cells and nonautologous cells (Fig. 2). We can choose either or both when analyzing the advantages and disadvantages of cell types and the target disease. Generally speaking, the advantages of autologous cells are (1) few ethical issues, (2) no need for immunosuppression, and (3) relative safety. The disadvantages of autologous cells are (1) pathologically affected cells in some degenerative or genetic diseases such as PD; (2) considerable time and effort required for isolation, amplification, and purification when cells are prepared just before transplantation; and (3) efforts and cost for preserving cells when cells are prepared in advance. The advantages of nonautologous cells are (1) easy production, distribution, and handy usage of the cells after thawing preserved cells; (2) cells originating from healthy volunteers can be used; and (3) a greater variety of cells are usable compared to autologous cells. The disadvantages of nonautologous cells are (1) ethical issues and (2) immune rejection, although it depends largely on which cells are used for transplantation (e.g., iPSCs, ESCs, NSCs, MSCs). Furthermore, the generation of iPSCs from several critical human leukocyte antigen–homozygous donors might overcome the immune rejection limitation for most Japanese patients.72
Figure 2.
Consideration of cell source. Transplanted cells can be divided into 2 groups. Autologous cells can be used with few ethical issues and need no immunosuppression, while nonautologous cells can be produced like a drug and offer uniform quality.
As a cell source, autologous DAergic neurons from patient iPSCs are ideal. Autologous iPSC-derived DAergic neurons transplanted into nonhuman primates survived for 2 y without immunosuppression and caused functional recovery.73 In terms of drug discovery, iPSCs from patients as well as the clarification of mechanisms are useful, although the variable characteristics of the clones of respective iPSCs should be considered.74 Preclinical tests using iPSCs from healthy volunteers to evaluate safety, pharmacokinetics, or drug efficacy are also meaningful. We should also consider the cost–benefit performance so as not to put undue strain on the medical economy.
In applying cell therapy for PD, one paradoxical issue should be considered. The most hopeful candidates for cell therapy might be younger PD patients in the relatively early stages of the disease. These patients are usually treated using current therapeutic options, but it is clear that healthier patients might enjoy more powerful benefits from newer and potentially more effective treatment methods. The study design and inclusion criteria of cell therapy should be carefully considered when demonstrating the true therapeutic efficacy of TRANSEURO,25,26 although emphasis on younger subjects with early stage disease might be an inevitable flaw. In contrast, the condition of candidates for cell therapy is not that favorable at present. In other words, cell therapy for PD is expected to impart therapeutic effects on PD patients in the advanced stages of the disease, those who will receive fewer benefits from cell therapy. To overcome this dilemma, the fundamental restoration of DAergic neurons is desired, although it is a difficult challenge. The differentiation, isolation, and purification of the DAergic neurons might affect the study results of cell therapy for PD patients. Some basic research has shown that serotonin neurons in the DAergic neuron graft are associated with graft-induced dyskinesia.75 Conversely, the involvement of serotonergic neurons among the graft in dyskinesia was demonstrated by the fact that l-DOPA–induced dyskinesia of PD patients was suppressed via administration of the serotonin receptor type 1A agonist buspirone prior to l-DOPA.76 Simple and effective purification is needed to obtain DAergic neuronal grafts without serotonergic neurons for fewer complications such as graft-induced dyskinesia.77
Based on the historical achievements in this field, technological developments have often introduced new eras. However, the outcomes of randomized, controlled trials often contrast with the favorable results of early open-label trials. The limitation of clinical trials for PD patients might lie in the placebo responses78 or cognitive/psychological impairment.79 We have repeatedly experienced difficulty in obtaining good results from randomized, controlled studies. In order to overcome the somewhat static current status, we should not only refine the protocol of clinical trials but should also search for more predictive animal models80 to recreate clinical trials, perform carefully retrospective analyses, and continue our efforts to realize cell therapy for PD. At the same time, we need to consider how we can reduce the running cost of cell therapy for PD. The isolation, amplification, purification, and storing of cells should also be improved from an economic perspective. To benefit the patient, hybrid surgery might be a realistic method for transplantation. The concept was first described 16 y ago,81 and a viral vector or stem cell implantation combined with deep brain stimulation was recently discussed as a new form of hybrid stereotactic surgery.82 Finally, a safety net after transplantation should be considered. The control of the tumor formation after iPSC transplantation by γ-ray irradiation was confirmed in rats as a fail-safe therapy.83 Some switching of cell death after transplantation might also be useful, although additional genetic modification might be needed.
In this article, we have reviewed cell therapy for PD. From the historical development of cell therapy for PD, we can see the difficulty associated with achieving a positive result in randomized, controlled trials. With the review of the current status of cell therapy, various cell sources are promising, although each has several hurdles that must first be overcome. From the perspective of cell therapy, we realize that a breakthrough in this field, as represented by iPSC technology, might cause a paradigm shift in PD treatment. We need to continue our daily efforts in our clinical and research work and take every opportunity to try and achieve a breakthrough.
Acknowledgment
The authors express their sincere gratitude for the dedicated work of the following doctors: Professor Cesario V. Borlongan from the Center of Excellence for Aging and Brain Repair, Department of Neurosurgery and Brain Repair, University of South Florida Morsani College of Medicine, and Drs. Tetsuro Shingo, Kazuki Kobayashi, Akira Takeuchi, Akimasa Yano, Takashi Agari, Kenichiro Muraoka, Kazuya Takahashi, Satoshi Kuramoto, Yuan WJ, Akihiko Kondo, Meng Jing, Takamasa Morimoto, Feifei Wang, Tomohito Kadota, Judith Thomas Tayra, Aiko Shinko, Takaaki Wakamori, Susumu Sasada, Atsuhiko Toyoshima, Hayato Takeuchi, Jun Morimoto, Mihoko Okazaki, Kyohei Kin, Michiari Umakoshi, Ittetsu Kin, Ken Kuwahara, and Yousuke Tomita from the Department of Neurological Surgery, Okayama University Graduate School of Medicine.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article. This work was supported in part by Grants-in-Aid for Scientific Research and the grant of the project for realization of regenerative medicine from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
- 1. Cajal SRY. The structure and connexions of neurons. Novel Lecture. 1906. [Google Scholar]
- 2. Altman J, Das GD. Post-natal origin of microneurones in the rat brain. Nature. 1965;207(5000):953–956. [DOI] [PubMed] [Google Scholar]
- 3. Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255(5052):1707–1710. [DOI] [PubMed] [Google Scholar]
- 4. Borlongan CV, Sanberg PR, Freeman TB. Neural transplantation for neurodegenerative disorders. Lancet. 1999;353(Suppl 1):SI29–SI30. [DOI] [PubMed] [Google Scholar]
- 5. Dezawa M, Kanno H, Hoshino M, Cho H, Matsumoto N, Itokazu Y, Tajima N, Yamada H, Sawada H, Ishikawa H, et al. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J Clin Invest. 2004;113(12):1701–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yasuhara T, Matsukawa N, Hara K, Yu G, Xu L, Maki M, Kim SU, Borlongan CV. Transplantation of human neural stem cells exerts neuroprotection in a rat model of Parkinson’s disease. J Neurosci. 2006;26(48):12497–12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gibson SA, Gao GD, McDonagh K, Shen S. Progress on stem cell research towards the treatment of Parkinson’s disease. Stem Cell Res Ther. 2012;3(2):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Samata B, Doi D, Nishimura K, Kikuchi T, Watanabe A, Sakamoto Y, Kakuta J, Ono Y, Takahashi J. Purification of functional human ES and iPSC-derived midbrain dopaminergic progenitors using LRTM1. Nat Commun. 2016;7:13097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. [DOI] [PubMed] [Google Scholar]
- 10. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. [DOI] [PubMed] [Google Scholar]
- 11. Sethi KD. The impact of levodopa on quality of life in patients with Parkinson disease. Neurologist. 2010;16(2):76–83. [DOI] [PubMed] [Google Scholar]
- 12. Benabid AL, Krack PP, Benazzouz A, Limousin P, Koudsie A, Pollak P. Deep brain stimulation of the subthalamic nucleus for Parkinson’s disease: methodologic aspects and clinical criteria. Neurology. 2000;55(12 Suppl 6):S40–S44. [PubMed] [Google Scholar]
- 13. Vitek JL, Bakay RA, Freeman A, Evatt M, Green J, McDonald W, Haber M, Barnhart H, Wahlay N, Triche S, et al. Randomized trial of pallidotomy versus medical therapy for Parkinson’s disease. Ann Neurol. 2003;53(5):558–569. [DOI] [PubMed] [Google Scholar]
- 14. Prodoehl J, Rafferty MR, David FJ, Poon C, Vaillancourt DE, Comella CL, Leurgans SE, Kohrt WM, Corcos DM, Robichaud JA, et al. Two-year exercise program improves physical function in Parkinson’s disease: the PRET-PD randomized clinical trial. Neurorehabil Neural Repair. 2015;29(2):112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sturkenboom IH, Graff MJ, Hendriks JC, Veenhuizen Y, Munneke M, Bloem BR, Nijhuis-van der Sanden MW. OTiP Study Group. Efficacy of occupational therapy for patients with Parkinson’s disease: a randomised controlled trial. Lancet Neurol. 2014;13(6):557–566. [DOI] [PubMed] [Google Scholar]
- 16. Tajiri N, Yasuhara T, Shingo T, Kondo A, Yuan W, Kadota T, Wang F, Baba T, Tayra JT, Morimoto T, et al. Exercise exerts neuroprotective effects on Parkinson’s disease model of rats. Brain Res. 2010;1310:200–207. [DOI] [PubMed] [Google Scholar]
- 17. Madrazo I, Leon V, Torres C, Aguilera MC, Varela G, Alvarez F, Fraga A, Drucker-Colin R, Ostrosky F, Skurovich M, et al. Transplantation of fetal substantia nigra and adrenal medulla to the caudate nucleus in two patients with Parkinson’s disease. N Engl J Med. 1988;318(1):51. [DOI] [PubMed] [Google Scholar]
- 18. Lindvall O, Rehncrona S, Gustavii B, Brundin P, Astedt B, Widner H, Lindholm T, Bjorklund A, Leenders KL, Rothwell JC, et al. Fetal dopamine-rich mesencephalic grafts in Parkinson’s disease. Lancet. 1988;2(8626–8627):1483–1484. [DOI] [PubMed] [Google Scholar]
- 19. Perlow MJ, Freed WJ, Hoffer BJ, Seiger A, Olson L, Wyatt RJ. Brain grafts reduce motor abnormalities produced by destruction of nigrostriatal dopamine system. Science. 1979;204(4393):643–647. [DOI] [PubMed] [Google Scholar]
- 20. Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, Dillon S, Winfield H, Culver S, Trojanowski JQ, et al. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med. 2001;344(10):710–719. [DOI] [PubMed] [Google Scholar]
- 21. Ma Y, Feigin A, Dhawan V, Fukuda M, Shi Q, Greene P, Breeze R, Fahn S, Freed C, Eidelberg D. Dyskinesia after fetal cell transplantation for Parkinsonism: a PET study. Ann Neurol. 2002;52(5):628–634. [DOI] [PubMed] [Google Scholar]
- 22. Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, Brin MF, Shannon KM, Nauert GM, Perl DP, Godbold J, et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann Neurol. 2003;54(3):403–414. [DOI] [PubMed] [Google Scholar]
- 23. Mendez I, Sanchez-Pernaute R, Cooper O, Vinuela A, Ferrari D, Bjorklund L, Dagher A, Isacson O. Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson’s disease. Brain. 2005;128(pt 7):1498–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ma Y, Peng S, Dhawan V, Eidelberg D. Dopamine cell transplantation in Parkinson’s disease: challenge and perspective. Br Med Bull. 2011;100:173–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Petit GH, Olsson TT, Brundin P. The future of cell therapies and brain repair: Parkinson’s disease leads the way. Neuropathol Appl Neurobiol. 2014;40(1):60–70. [DOI] [PubMed] [Google Scholar]
- 26. Moore SF, Guzman NV, Mason SL, Williams-Gray CH, Barker RA. Which patients with Parkinson’s disease participate in clinical trials? One centre’s experiences with a new cell based therapy trial (TRANSEURO). J Parkinsons Dis. 2014;4(4):671–676. [DOI] [PubMed] [Google Scholar]
- 27. Date I, Asari S, Ohmoto T. Two-year follow-up study of a patient with Parkinson’s disease and severe motor fluctuations treated by co-grafts of adrenal medulla and peripheral nerve into bilateral caudate nuclei: case report. Neurosurgery. 1995;37(3):515–518; discussion 518–9. [DOI] [PubMed] [Google Scholar]
- 28. Minguez-Castellanos A, Escamilla-Sevilla F, Hotton GR, Toledo-Aral JJ, Ortega-Moreno A, Mendez-Ferrer S, Martin-Linares JM, Katati MJ, Mir P, Villadiego J, et al. Carotid body autotransplantation in Parkinson disease: a clinical and positron emission tomography study. J Neurol Neurosurg Psychiatry. 2007;78(8):825–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Toledo-Aral JJ, Mendez-Ferrer S, Pardal R, Echevarria M, Lopez-Barneo J. Trophic restoration of the nigrostriatal dopaminergic pathway in long-term carotid body-grafted parkinsonian rats. J Neurosci. 2003;23(1):141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jaeger CB, Greene LA, Tresco PA, Winn SR, Aebischer P. Polymer encapsulated dopaminergic cell lines as “alternative neural grafts.” Prog Brain Res. 1990;82:41–46. [PubMed] [Google Scholar]
- 31. Date I, Ohmoto T. Neural transplantation and trophic factors in Parkinson’s disease: special reference to chromaffin cell grafting, NGF support from pretransected peripheral nerve, and encapsulated dopamine-secreting cell grafting. Exp Neurol. 1996;137(2):333–344. [DOI] [PubMed] [Google Scholar]
- 32. Yasuhara T, Shingo T, Kobayashi K, Takeuchi A, Yano A, Muraoka K, Matsui T, Miyoshi Y, Hamada H, Date I. Neuroprotective effects of vascular endothelial growth factor (VEGF) upon dopaminergic neurons in a rat model of Parkinson’s disease. Eur J Neurosci. 2004;19(6):1494–1504. [DOI] [PubMed] [Google Scholar]
- 33. Kobayashi K, Yasuhara T, Agari T, Muraoka K, Kameda M, Ji Yuan W, Hayase H, Matsui T, Miyoshi Y, Shingo T, et al. Control of dopamine-secretion by Tet-Off system in an in vivo model of parkinsonian rat. Brain Res. 2006;1102(1):1–11. [DOI] [PubMed] [Google Scholar]
- 34. Yasuhara T, Date I. Intracerebral transplantation of genetically engineered cells for Parkinson’s disease: toward clinical application. Cell Transplant. 2007;16(2):125–132. [PubMed] [Google Scholar]
- 35. Wang F, Kameda M, Yasuhara T, Tajiri N, Kikuchi Y, Liang HB, Tayra JT, Shinko A, Wakamori T, Agari T, et al. GDNF-pretreatment enhances the survival of neural stem cells following transplantation in a rat model of Parkinson’s disease. Neurosci Res. 2011;71(1):92–98. [DOI] [PubMed] [Google Scholar]
- 36. Yasuhara T, Kameda M, Agari T, Date I. Regenerative medicine for Parkinson’s disease. Neurol Med Chir (Tokyo). 2015;55(Suppl 1):113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Toyoshima A, Yasuhara T, Kameda M, Morimoto J, Takeuchi H, Wang F, Sasaki T, Sasada S, Shinko A, Wakamori T, et al. Intra-arterial transplantation of allogeneic mesenchymal stem cells mounts neuroprotective effects in a transient ischemic stroke model in rats: analyses of therapeutic time window and its mechanisms. PLoS One. 2015;10(6):e0127302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thomas Tayra J, Kameda M, Yasuhara T, Agari T, Kadota T, Wang F, Kikuchi Y, Liang H, Shinko A, Wakamori T, et al. The neuroprotective and neurorescue effects of carbamylated erythropoietin Fc fusion protein (CEPO-Fc) in a rat model of Parkinson’s disease. Brain Res. 2013;1502:55–70. [DOI] [PubMed] [Google Scholar]
- 39. Shinko A, Agari T, Kameda M, Yasuhara T, Kondo A, Tayra JT, Sato K, Sasaki T, Sasada S, Takeuchi H, et al. Spinal cord stimulation exerts neuroprotective effects against experimental Parkinson’s disease. PLoS One. 2014;9(7):e101468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sasaki T, Liu K, Agari T, Yasuhara T, Morimoto J, Okazaki M, Takeuchi H, Toyoshima A, Sasada S, Shinko A, et al. Anti-high mobility group box 1 antibody exerts neuroprotection in a rat model of Parkinson’s disease. Exp Neurol. 2016;275(Pt 1):220–231. [DOI] [PubMed] [Google Scholar]
- 41. Kuroda Y, Wakao S, Kitada M, Murakami T, Nojima M, Dezawa M. Isolation, culture and evaluation of multilineage-differentiating stress-enduring (Muse) cells. Nat Protoc. 2013;8(7):1391–1415. [DOI] [PubMed] [Google Scholar]
- 42. Danielyan L, Beer-Hammer S, Stolzing A, Schäfer R, Siegel G, Fabian C, Kahle P, Biedermann T, Lourhmati A, Buadze M, et al. Intranasal delivery of bone marrow-derived mesenchymal stem cells, macrophages, and microglia to the brain in mouse models of Alzheimer’s and Parkinson’s disease. Cell Transplant. 2014;23(Suppl 1): S123–S139. [DOI] [PubMed] [Google Scholar]
- 43. Salama M, Sobh M, Emam M, Abdalla A, Sabry D, El-Gamal M, Lotfy A, El-Husseiny M, Sobh M, Shalash A, et al. Effect of intranasal stem cell administration on the nigrostriatal system in a mouse model of Parkinson’s disease. Exp Ther Med. 2017;13(3):976–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kawasaki H, Mizuseki K, Nishikawa S, Kaneko S, Kuwana Y, Nakanishi S, Nishikawa SI, Sasai Y. Induction of midbrain dopaminergic neurons from ES cells by stromal cell-derived inducing activity. Neuron. 2000;28(1):31–40. [DOI] [PubMed] [Google Scholar]
- 45. Lee SH, Lumelsky N, Studer L, Auerbach JM, McKay RD. Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat Biotechnol. 2000;18(6):675–679. [DOI] [PubMed] [Google Scholar]
- 46. Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27(3):275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Morizane A, Doi D, Kikuchi T, Nishimura K, Takahashi J. Small-molecule inhibitors of bone morphogenic protein and activin/nodal signals promote highly efficient neural induction from human pluripotent stem cells. J Neurosci Res. 2011;89(2):117–126. [DOI] [PubMed] [Google Scholar]
- 48. Doi D, Morizane A, Kikuchi T, Onoe H, Hayashi T, Kawasaki T, Motono M, Sasai Y, Saiki H, Gomi M, et al. Prolonged maturation culture favors a reduction in the tumorigenicity and the dopaminergic function of human ESC-derived neural cells in a primate model of Parkinson’s disease. Stem Cells. 2012;30(5):935–945. [DOI] [PubMed] [Google Scholar]
- 49. Takagi Y, Takahashi J, Saiki H, Morizane A, Hayashi T, Kishi Y, Fukuda H, Okamoto Y, Koyanagi M, Ideguchi M, et al. Dopaminergic neurons generated from monkey embryonic stem cells function in a Parkinson primate model. J Clin Invest. 2005;115(1):102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kirkeby A, Nolbrant S, Tiklova K, Heuer A, Kee N, Cardoso T, Ottosson DR, Lelos MJ, Rifes P, Dunnett SB, et al. Predictive markers guide differentiation to improve graft outcome in clinical translation of hESC-based therapy for Parkinson’s disease. Cell Stem Cell. 2017;20(1):135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yao Y, Huang C, Gu P, Wen T. Combined MSC-secreted factors and neural stem cell transplantation promote functional recovery of PD rats. Cell Transplant. 2016;25(6):1101–1113. [DOI] [PubMed] [Google Scholar]
- 52. Ramos-Moreno T, Lendinez JG, Pino-Barrio MJ, Del Arco A, Martinez-Serrano A. Clonal human fetal ventral mesencephalic dopaminergic neuron precursors for cell therapy research. PLoS One. 2012;7(12):e52714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zuo F, Xiong F, Wang X, Li X, Wang R, Ge W, Bao X. Intrastriatal transplantation of human neural stem cells restores the impaired subventricular zone in Parkinsonian mice. Stem Cells. 2017;35(6):1519–1531. [DOI] [PubMed] [Google Scholar]
- 54. Lige L, Zengmin T. Transplantation of neural precursor cells in the treatment of Parkinson disease: an efficacy and safety analysis. Turk Neurosurg. 2016;26(3):378–383. [DOI] [PubMed] [Google Scholar]
- 55. Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26(1):101–106. [DOI] [PubMed] [Google Scholar]
- 56. Kim JB, Zaehres H, Wu G, Gentile L, Ko K, Sebastiano V, Arauzo-Bravo MJ, Ruau D, Han DW, Zenke M, et al. Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature. 2008;454(7204):646–650. [DOI] [PubMed] [Google Scholar]
- 57. Kim JB, Sebastiano V, Wu G, Arauzo-Bravo MJ, Sasse P, Gentile L, Ko K, Ruau D, Ehrich M, van den Boom D, et al. Oct4-induced pluripotency in adult neural stem cells. Cell. 2009;136(3):411–419. [DOI] [PubMed] [Google Scholar]
- 58. Zhou H, Wu S, Joo JY, Zhu S, Han DW, Lin T, Trauger S, Bien G, Yao S, Zhu Y, et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4(5):381–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322(5903):949–953. [DOI] [PubMed] [Google Scholar]
- 60. Kaji K, Norrby K, Paca A, Mileikovsky M, Mohseni P, Woltjen K. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009;458(7239):771–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Maekawa M, Yamaguchi K, Nakamura T, Shibukawa R, Kodanaka I, Ichisaka T, Kawamura Y, Mochizuki H, Goshima N, Yamanaka S. Direct reprogramming of somatic cells is promoted by maternal transcription factor Glis1. Nature. 2011;474(7350):225–229. [DOI] [PubMed] [Google Scholar]
- 62. Mandai M, Watanabe A, Kurimoto Y, Hirami Y, Morinaga C, Daimon T, Fujihara M, Akimaru H, Sakai N, Shibata Y, et al. Autologous induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med. 2017;376(11):1038–1046. [DOI] [PubMed] [Google Scholar]
- 63. Morizane A, Takahashi J. A challenge towards the clinical application of induced pluripotent stem cell technology for the treatment of Parkinson’s disease [in Japanese]. Brain Nerve. 2012;64(1):29–37. [PubMed] [Google Scholar]
- 64. Okano H, Yamanaka S., iPS cell technologies: significance and applications to CNS regeneration and disease. Mol Brain. 2014;7:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Barker RA, Parmar M, Kirkeby A, Bjorklund A, Thompson L, Brundin P. Are stem cell-based therapies for Parkinson’s disease ready for the clinic in 2016? J Parkinsons Dis. 2016;6(1):57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Devine MJ, Ryten M, Vodicka P, Thomson AJ, Burdon T, Houlden H, Cavaleri F, Nagano M, Drummond NJ, Taanman JW, et al. Parkinson’s disease induced pluripotent stem cells with triplication of the α-synuclein locus. Nat Commun. 2011;2:440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kiskinis E, Eggan K. Progress toward the clinical application of patient-specific pluripotent stem cells. J Clin Invest. 2010;120(1):51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Qiu Z, Farnsworth SL, Mishra A, Hornsby PJ. Patient-specific induced pluripotent stem cells in neurological disease modeling: the importance of nonhuman primate models. Stem Cells Cloning. 2013;6:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Xia N, Zhang P, Fang F, Wang Z, Rothstein M, Angulo B, Chiang R, Taylor J, Reijo Pera RA. Transcriptional comparison of human induced and primary midbrain dopaminergic neurons. Sci Rep. 2016;6:20270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463(7284):1035–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jiang H, Xu Z, Zhong P, Ren Y, Liang G, Schilling HA, Hu Z, Zhang Y, Wang X, Chen S, Yan Z, Feng J. Cell cycle and p53 gate the direct conversion of human fibroblasts to dopaminergic neurons. Nat Commun. 2015;6:10100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Okita K, Matsumura Y, Sato Y, Okada A, Morizane A, Okamoto S, Hong H, Nakagawa M, Tanabe K, Tezuka K, et al. A more efficient method to generate integration-free human iPS cells. Nat Methods. 2011;8(5):409–412. [DOI] [PubMed] [Google Scholar]
- 73. Hallett PJ, Deleidi M, Astradsson A, Smith GA, Cooper O, Osborn TM, Sundberg M, Moore MA, Perez-Torres E, Brownell AL, et al. Successful function of autologous iPSC-derived dopamine neurons following transplantation in a non-human primate model of Parkinson’s disease. Cell Stem Cell. 2015;16(3):269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hayakawa T, Mizuguchi H. Perspectives regarding the potential use of human induced pluripotent stem cells for the development of and research on medicinal products [in Japanese]. Brain Nerve. 2012;64(1):47–57. [PubMed] [Google Scholar]
- 75. Carlsson T, Carta M, Munoz A, Mattsson B, Winkler C, Kirik D, Bjorklund A. Impact of grafted serotonin and dopamine neurons on development of L-DOPA-induced dyskinesias in parkinsonian rats is determined by the extent of dopamine neuron degeneration. Brain. 2009;132(Pt 2):319–335. [DOI] [PubMed] [Google Scholar]
- 76. Politis M, Wu K, Loane C, Brooks DJ, Kiferle L, Turkheimer FE, Bain P, Molloy S, Piccini P. Serotonergic mechanisms responsible for levodopa-induced dyskinesias in Parkinson’s disease patients. J Clin Invest. 2014;124(3):1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tronci E, Fidalgo C, Carta M. Foetal cell transplantation for Parkinson’s disease: focus on graft-induced dyskinesia. Parkinsons Dis. 2015;2015:563820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Goetz CG, Wuu J, McDermott MP, Adler CH, Fahn S, Freed CR, Hauser RA, Olanow WC, Shoulson I, Tandon PK, et al. Placebo response in Parkinson’s disease: comparisons among 11 trials covering medical and surgical interventions. Mov Disord. 2008;23(5):690–699. [DOI] [PubMed] [Google Scholar]
- 79. Wakamori T, Agari T, Yasuhara T, Kameda M, Kondo A, Shinko A, Sasada S, Sasaki T, Furuta T, Date I. Cognitive functions in Parkinson’s disease: relation to disease severity and hallucination. Parkinsonism Relat Disord. 2014;20(4):415–420. [DOI] [PubMed] [Google Scholar]
- 80. Gonzalez R, Garitaonandia I, Poustovoitov M, Abramihina T, McEntire C, Culp B, Attwood J, Noskov A, Christiansen-Weber T, Khater M, et al. Neural stem cells derived from human parthenogenetic stem cells engraft and promote recovery in a nonhuman primate model of Parkinson’s disease. Cell Transplant. 2016;25(11):1945–1966. [DOI] [PubMed] [Google Scholar]
- 81. During MJ, Kaplitt MG, Stern MB, Eidelberg D. Subthalamic GAD gene transfer in Parkinson disease patients who are candidates for deep brain stimulation. Hum Gene Ther. 2001;12(12):1589–1591. [PubMed] [Google Scholar]
- 82. Rowland NC, Kalia SK, Kalia LV, Larson PS, Lim DA, Bankiewicz KS. Merging DBS with viral vector or stem cell implantation: “hybrid” stereotactic surgery as an evolution in the surgical treatment of Parkinson’s disease. Mol Ther Methods Clin Dev. 2016;3:15051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Katsukawa M, Nakajima Y, Fukumoto A, Doi D, Takahashi J. Fail-safe therapy by gamma-ray irradiation against tumor formation by human-induced pluripotent stem cell-derived neural progenitors. Stem Cells Dev. 2016;25(11):815–825. [DOI] [PubMed] [Google Scholar]