Abstract
Intraperitoneal transplantation of hepatocyte microbeads is an attractive option for the management of acute liver failure. Encapsulation of hepatocytes in alginate microbeads supports their function and prevents immune attack of the cells. Establishment of banked cryopreserved hepatocyte microbeads is important for emergency use. The aim of this study was to develop an optimized protocol for cryopreservation of hepatocyte microbeads for clinical transplantation using modified freezing solutions. Four freezing solutions with potential for clinical application were investigated. Human and rat hepatocytes cryopreserved with University of Wisconsin (UW)/10% dimethyl sulfoxide (DMSO)/5% (300 mM) glucose and CryoStor CS10 showed better postthawing cell viability, attachment, and hepatocyte functions than with histidine–tryptophan–ketoglutarate/10% DMSO/5% glucose and Bambanker. The 2 freezing solutions that gave better results were studied with human and rat hepatocytes microbeads. Similar effects on cryopreserved microbead morphology (external and ultrastructural), viability, and hepatocyte-functions post thawing were observed over 7 d in culture. UW/DMSO/glucose, as a basal freezing medium, was used to investigate the additional effects of cytoprotectants: a pan-caspase inhibitor (benzyloxycarbonyl-Val-Ala-dl-Asp-fluoromethylketone [ZVAD]), an antioxidant (desferoxamine [DFO]), and a buffering and mechanical protectant (human serum albumin [HSA]) on RMBs. ZVAD (60 µM) had a beneficial effect on cell viability that was greater than with DFO (1 mM), HSA (2%), and basal freezing medium alone. Improvements in the ultrastructure of encapsulated hepatocytes and a lower degree of cell apoptosis were observed with all 3 cytoprotectants, with ZVAD tending to provide the greatest effect. Cytochrome P450 activity was significantly higher in the 3 cytoprotectant groups than with fresh microbeads. In conclusion, developing an optimized cryopreservation protocol by adding cytoprotectants such as ZVAD could improve the outcome of cryopreserved hepatocyte microbeads for future clinical use.
Keywords: hepatocyte microbeads, alginate encapsulation, acute liver failure, cryopreservation, cytoprotectants, apoptosis, clinical grade
Introduction
Acute liver failure (ALF) is a rare but life-threatening condition without liver transplantation. However, the tremendous regenerative capacity of the liver enables spontaneous recovery, avoiding liver transplantation and lifelong immunosuppression. Current liver support systems like Molecular Adsorbent Recirculation System® and the fractionated plasma separation and absorption system (Prometheus®) do not provide any synthetic function while nonselective exchange of biological fluid could lead to low liver trophic factors negatively affecting regeneration.1–3 Transplantation of alginate microencapsulated hepatocytes (microbeads) has been studied in animal models of ALF with promising outcomes.4–7 Hepatocyte microbeads can replace the missing detoxification and synthetic functions of damaged hepatocytes either for a short period, bridging the patient to liver transplantation, or allowing time for the liver to regenerate and recover, avoiding liver transplantation altogether.
Cryopreservation of hepatocytes is a key to cell therapy for emergency transplantation in patients with ALF. Establishment of banked cryopreserved hepatocyte microbeads for long-term storage would be of great benefit, allowing on-demand usage for emergency cases. However, the difficulty with cryopreservation is due to cells being subjected to damaging conditions during both freezing and thawing steps.
Previously published studies have shown protective effects of alginate microencapsulation on hepatocytes during the cryopreservation process.8–11 The challenges are the high water content (>90%) together with the relatively large size of the microbeads and the number of cells compared to single cells. This makes microbeads susceptible to cryodamage by ice-crystal formation, leading to cell death.12 Studies on cryopreservation of microencapsulated cells have been reported with variable effectiveness related to differences in cryopreservation methods. Most of the previous studies of cryopreservation of microencapsulated hepatocytes used a cryopreservation medium consisting of culture medium, 10% fetal calf serum (FCS), and 10% dimethyl sulfoxide (DMSO) placed into cryovials and stored in liquid nitrogen.8–10,13 Although some of these protocols provided relatively good viability and function after thawing, they are not acceptable for clinical transplantation.
Recent studies demonstrated that apoptosis plays an important role in cryopreservation-induced cell injury.14,15 The understanding of pathways involved with cryopreservation-induced molecular cell death could lead to improvement in the cryopreservation outcome, as cytoprotectants can be added to specifically target or control the apoptosis pathway. Apoptosis signal transduction can be initiated at different levels (cell membrane or mitochondria) but is then followed by common proteolytic caspase cascade pathways (caspase-8, -9, and -3).16 The pan-caspase inhibitor benzyloxycarbonyl-Val-Ala- dl-Asp-fluoromethylketone (ZVAD) has been shown to have beneficial effects on cell viability and function under stressful conditions prone to apoptosis such as isolation and cryopreservation of encapsulated hepatocytes.11,17
The hypothesis of this study was that cryopreservation of hepatocyte microbeads could be improved by using an appropriate freezing solution with added cytoprotective agents such as the anti-apoptosis compound ZVAD, an iron chelator such as desferoxamine (DFO), and human serum albumin (HSA) to protect hepatocytes from oxidative stress and apoptosis15,18,19 and maintain microbead integrity.20 The aim of this study was to develop an optimized protocol for cryopreservation of human hepatocyte microbeads (HMBs) for clinical transplantation. Stepwise studies of different types of cryopreservation solutions and cytoprotectants that can potentially be used for clinical application were investigated.
Materials and Methods
Experimental Design
Firstly, the effects of 4 different freezing solutions were evaluated on isolated human and rat hepatocytes. The 2 freezing solutions that resulted in the greatest cell viability and function after thawing were then selected for study with HMBs and rat hepatocyte microbeads (RMBs), respectively, cryopreservation. Following this, cytoprotectants were added in order to improve the microbead cryopreservation protocol.
Hepatocytes were isolated from the same donor tissues for each set of experiments. Fresh isolated cells and microbeads were prepared identically and maintained in cultures as cryopreserved samples. All assays on cryopreserved samples were performed and compared to fresh samples at similar time points.
Hepatocyte Isolation
Human hepatocyte isolation
Human hepatocytes were isolated from donor liver tissues (rejected or unused for transplantation) or from the nontumoral margin of liver resections from metastatic cancer cases using a collagenase perfusion technique according to Mitry.21 Total hepatocyte number and their viability were estimated using the standard trypan blue (Sigma-Aldrich, Dorset, UK) exclusion test; cell viability was ≥60%. All human tissues were approved for research use in accordance with the Research Ethics Committee of King’s College Hospital. Written informed consent was obtained from donor relatives or patients.
Rat hepatocyte isolation
Male Sprague Dawley (Harlan Olec, Bicester, UK) rats (8–10 wks old and weighed between 200 g and 300 g) were used as hepatocyte donors. Animals were maintained in conventional housing facilities and received standard care. They were housed in a room kept at 21 ± 2 °C, humidity of 55% ± 10% and 12-h light–dark cycle with ad libitum food and water for 1 wk prior to the procedure. All animal donors were handled following protocols approved by the ethical review process of King’s College London in accordance with the UK Animals (Scientific) Procedures Act of 1986. Rat hepatocyte isolation was performed using in situ collagenase perfusion of the liver as previously described.22,23 The total number of hepatocytes and viability were determined using the standard trypan blue exclusion test.
Encapsulation of Hepatocytes
Human and rat hepatocytes were encapsulated using the optimized technique described by Jitraruch et al.,4 where the microbeads were produced at a cell density of 3.5 × 106 cell/mL alginate and polymerized for 15 min. Briefly, hepatocyte microbeads were produced using the IE-50R encapsulator (Inotech Encapsulation AG, Dottikon, Switzerland) and sterile clinical grade reagents. Ultrapure sodium alginate, with low-viscosity and high-glucoronic acid (PRONOVA™ SLG20; NovaMatrix, Sandvika, Norway) was dissolved in 0.9% NaCl to give a final concentration of 1.5% alginate solution (w/v). Microbeads were produced using a 250-µm nozzle and polymerized in 1.2% CaCl2 solution. The microbeads were washed twice with 0.9% NaCl to remove excess Ca2+ ions. The microbeads size was 500 ± 100 µm, containing approximately 450 cells/beads.
Cryopreservation of Hepatocytes in Different Freezing Solutions
Human and rat hepatocytes were used to evaluate 4 different cryopreservation media consisting of (a) 2 hypothermic organ preservation solutions namely University of Wisconsin (UW, Bristol-Myers Squibb AB, Hounslow, UK) and histidine–tryptophan–ketoglutarate (HTK: Custodiol™; Dr. Franz Kohler Chemie GMB, Alsbach-Hähnlein, Germany) and (b) 2 commercial cryopreservation solutions which are Bambanker (Nippon Genetics, Tokyo, Japan) and CryoStor CS10™ (Sigma-Aldrich). All cryopreservation solutions contained 10% DMSO (WAK-Chemie Medical GmbH, Steinbach, Germany). Based on published and standard protocols used for cryopreservation of human hepatocytes at King’s College Hospital,24 5% glucose (300mM) was added to UW and HTK (Table 1). Briefly, isolated hepatocytes were split into 4 groups. In each group, hepatocytes were resuspended in ice-cold freezing medium at a density of 1 × 107 cells/mL. Hepatocyte suspension was then transferred into 2-mL cryovials. Cryopreservation was performed using the standard freezing protocol at King’s College Hospital in a controlled rate freezer (CRF, Planer, Model: Kryo10 CRF; Planer PLC, Middlesex, UK).
Table 1.
Cryopreservation Solution Mixtures.
| Main Solution | UW (ViaSpan®) | HTK (Custodiol™) | Bambankera | CryoStor CS10a |
|---|---|---|---|---|
| Application | Organ preservation solution | Organ preservation solution | Cell cryopreservation medium | Cell cryopreservation medium |
| Ingredients | K+-lactobionate (100 mM) KH2PO4 (25 mM) Na+ (30 mM) Raffinose (30 mM) Adenosine (5 mM) Glutathione (3 mM) Allopurinol (1 mM) Hydroxyethyl starch (50 g/L) Dexamethasone (8 mg/L) Penicillin (200,000 U/L) Insulin (40 U/L) Osmolarity 320 mOsM pH 7.4 | Na+ (15 mM) K+ (10 mM) Mg2+ (4 mM) Ca2+ (0.015) Cl− (50 mM) l-Histidine (198 mM) Ketoglutarate (1 mM) Tryptophan (2 mM) Mannitol (30 mM) Osmolarity 310 mOsM pH 7.2 | Ready to use Serum free DMSO (10%) | Ready to use Serum free DMSO (10%) [Modified from HTS] Composition of HTS: Na+ (100 mM) K+ (42.5 mM) Mg2+ (5 mM) Ca2+ (0.05 mM) Cl− (17.1 mM) H2PO4 − (10 mM) HCO3 − (5 mM) HEPES (25 mM) Lactobionate (100 mM) Sucrose (20mM) Mannitol (20mM) Glucose (5 mM) Dextran-40 (6%) Adenosine (2 mM) Glutathione (3 mM) Osmolarity 360 mOsM pH 7.6 |
| Additives | ||||
| DMSO | 10% | 10% | — | — |
| Glucose | 5% | 5% | — | — |
Note. UW, University of Wisconsin solution; HTK, histidine, tryptophan, ketoglutarate solution; HTS, hypothermosol; DMSO, dimethyl sulfoxide.
aThe actual composition of Bambanker and CryoStor CS10 are undisclosed.
Cryopreservation of Hepatocyte Microbeads Using Different Freezing Solutions
The 2 best freezing solutions were further evaluated for cryopreservation of hepatocyte microbeads (HMBs and RMBs). Each ice-cold freezing medium was slowly added to the microbeads (1-mL microbeads + 4-mL freezing solution in a 5-mL cryovial). The microbead suspensions were then maintained on ice for 15–30 min before transfer to the CRF. The freezing protocol was chosen based on results of a preliminary study. The published protocols by Terry et al.24 for cryopreservation of human hepatocytes and the protocol for freezing alginate-encapsulated liver cell spheroids by Massie et al.25 were compared. These 2 protocols are multistep with slow cooling and contain an important holding step to allow samples to reach equilibrium followed by a shock cooling step (from −8 °C to−28 °C) to control nucleation of ice. They are different in terms of starting temperature (8 °C vs. 0 °C) and total duration of cryopreservation (62 min vs. 39 min; Table 2). The findings from a pilot study showed that HMBs cryopreserved according to the Massie et al.’s protocol provided intact microbeads and higher 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) activity and albumin production at day 1 and 3 post thawing than those cryopreserved with the standard protocol for hepatocytes by Terry et al.24 As a result, the freezing protocol by Massie et al. was applied in all of the following experiments of microbead cryopreservation.
Table 2.
Multistep Slow-cooling Cryopreservation Protocols Using Controlled Rate Freezer.
| Step | Start Temperature | Rate | Time | End Temperature |
|---|---|---|---|---|
| Protocol for human hepatocytes24 | ||||
| 1 | 8 °C | −1 °C/min | 8 min | 0 °C |
| 2 | 0 °C | Hold | 8 min | 0 °C |
| 3 | 0 °C | −2 °C/min | 4 min | −8 °C |
| 4 | −8 °C | −35 °C/min | 33 s | −28 °C |
| 5 | −28 °C | −2.5 °C/min | 2 min | −33 °C |
| 6 | −33 °C | +2.5 °C /min | 2 min | −28 °C |
| 7 | −28 °C | −1 °C/min | 32 min | −60 °C |
| 8 | −60 °C | −10 °C/min | 4 min | −100 °C |
| 9 | −100 °C | −20 °C/min | 2 min | −140 °C |
| Protocol for encapsulated liver spheroids25 | ||||
| 1 | 0 °C | Hold | 8 min | 0 °C |
| 2 | 0 °C | −2 °C/min | 4 min | −8 °C |
| 3 | −8 °C | −35 °C/min | 6 s | −28 °C |
| 4 | −28 °C | −2.5 °C/min | 2 min | −33 °C |
| 5 | −33 °C | +2.5 °C/min | 2 min | −28 °C |
| 6 | −28 °C | −2 °C/min | 16 min | −60 °C |
| 7 | −60 °C | −10 °C/min | 4 min | −100 °C |
| 8 | −100 °C | −20 °C/min | 3 min | −160 °C |
Effect of Cytoprotectants on Hepatocyte Microbeads
The cytoprotectant compounds were chosen based on published data which demonstrated beneficial effects during culture, cold storage, or cryopreservation of cells or microencapsulated cells. Compounds that have different mechanisms to protect cells, including a caspase inhibitor (ZVAD), an iron chelator (DFO), and HSA, were evaluated. The concentrations used in current study were based on those used previously (ZVAD; R&D Systems, Abington, UK) at 60 µM,11,17 DFO (Sigma-Aldrich) at 1 mM,19,26 and HSA (20%; Baxter Healthcare UK, Berkshire, UK) at 2%.20,27 The effects of the compounds were tested on RMBs by adding them to the most effective freezing medium from the above experiments. There were 4 experimental groups: (1) basal freezing media alone, (2) basal freezing media with HSA, (3) basal freezing media with DFO, and (4) basal freezing media with ZVAD. Microbead suspensions were then processed and cryopreserved as described above.
Thawing and Culturing Cryopreserved Cells and Microbeads
Following storage at −140 °C for 2 wk, isolated cells and microbeads were rapidly thawed in a 37 °C water bath24,28 using ice-cold Eagle’s minimum essential medium (EMEM) (EMEM; Lonza, Verviers, Belgium) as thawing media. Similarly, cryopreserved HMBs were thawed with 2% (v/v) HSA added in EMEM. Thawed hepatocytes were then plated and cultured in William’s E medium (WEM; Sigma-Aldrich) supplemented with 10% FCS (Invitrogen, Paisley, UK), 10-mM 4-(2-hydroxyethyl)-1-piperazine-ethanesulfonic acid (HEPES; Lonza), 2-mM l-glutamine (Sigma-Aldrich), 0.1-μM dexamethasone (Sigma-Aldrich), 0.1-μM insulin (Sigma-Aldrich), penicillin (50 U/mL; Sigma-Aldrich), and streptomycin (50 μg/mL; Sigma-Aldrich). Thawed microbeads were cultured in the same medium as the same as hepatocytes except Connaught Medical Research Laboratories medium (Mediatech was used, Inc., Vancouver, Canada) instead of WEM without HEPES. The cells and microbeads were maintained in a humidified incubator at 37 °C, 5% CO2 for 7 d.
Plated Hepatocyte Viability and Function
Cell viability was determined immediately after thawing using the trypan blue exclusion test. Mitochondrial dehydrogenase activity, cell attachment, and hepatocyte specific function were evaluated after maintenance in culture for 24 h. For mitochondrial dehydrogenase activity and cell attachment assay, cells were cultured in collagen-coated 96-well plates (5.0 × 104 cells/well). The mitochondrial dehydrogenase activity of hepatocytes was assessed using an MTT assay.29 Hepatocyte attachment was assessed using the sulforhodamine B (SRB) assay.30 Albumin and urea synthesis were assessed for cells cultured in collagen-coated 24-well plates (3.0 × 105 cells/well). Albumin production was quantified in supernatant using human or rat albumin enzyme-linked immunosorbent assay (ELISA) quantitation kits (Bethyl Laboratories, Montgomery, TX, USA). Urea synthesis was assessed after challenging hepatocytes with ammonium chloride (5 mM) for 6 h at 37 °C. Supernatant was collected and analyzed using the QuantiChrom™ Urea Assay Kit (BioAssay Systems, Hayward, CA, USA).
Hepatocyte Microbead Viability and Function
Viability and function of microbeads (HMBs and RMBs) were assessed according to Jitraruch et al.4 immediately postthaw (day 0), day 1, day 3, and day 7. Briefly, viability of hepatocyte microbeads was evaluated by a cell membrane integrity assay using 10-µg fluorescein diacetate (FDA; Sigma-Aldrich, Gilingham, UK) and 20-µg propidium iodide (PI; Sigma-Aldrich) incubated in the dark for 90 s then visualized under a fluorescent microscope (Leica Microsystems Ltd, Milton Keynes, UK). In addition, an MTT assay was performed to assess overall metabolic activity of encapsulated hepatocytes. Hepatocyte specific functions of microbeads including of albumin production, urea synthesis, and cytochrome P450 (CYP1A1/2) were studied in supernatants on day 1, day 3, and day 7. The amount of albumin and urea secreted by HMBs and RMBs were evaluated by the same method as for isolated cells. CYP1A1/2 activity was measured using the ethoxyresorufin-O-deethylase method.31
Morphological Examination Using Light Microscopy
The morphology of hepatocytes from each group was examined after thawing and plating on collagen-coated plates for 24 h. Microbead morphology was evaluated immediately post thawing.
Hepatocyte Microbead Ultrastructure Examination Using Electron Microscopy
The exterior and interior ultrastructural morphology of hepatocyte microbeads were studied using scanning electron microscopy (SEM) and transmission electron microscopy (TEM). Microbeads were processed at the Centre for Ultrastructural Imaging, King’s College London. Briefly, samples were fixed overnight with 2.5% glutaraldehyde in 0.15 M cacodylate buffer (pH 7.3) at 4 °C. For SEM, after the initial fixation, samples were rinsed several times in cacodylate buffer and postfixed with 1% osmium tetroxide in 0.15 M cacodylate buffer (pH 7.3) for 1 h. Samples were then washed and dehydrated in a graded series of ethanol and dried by hexamethyldislazane. Dried samples were mounted on stubs (Hitachi; NanoAndMore GmbH, Wetzlar, Germany) with adhesive carbon tab (TAAB) and sputter coated with gold (Emitech K550X; Quorum Technologies Ltd, East Sussex, UK) before examination under an SEM. Images recorded using an FEI Quanta 200F field emission SEM (FEI Company, Hillsboro, OR, USA) operated at 5 kV in high vacuum mode.
For TEM, after the initial fixation, samples were rinsed several times in cacodylate buffer and postfixed with 1% osmium tetroxide in 0.15 M cacodylate buffer (pH 7.3) for 1.5 h. Samples were then washed, dehydrated in a graded series of ethanol solutions, and equilibrated with propylene oxide before infiltration with SPURR resin (TAAB). Samples were embedded and polymerized at 70 °C for 24 h. Ultrathin sections (70–90 nm) were prepared using a Reichert-Jung Ultracut E Ultramicrotome (Reichert-Jung, Vienna, Austria), mounted on 150 mesh copper grids, contrasted using uranyl acetate and lead citrate, and examined on an FEI Tecnai 12 transmission microscope (FEI Company, USA) operated at 120 kV. Images were acquired with an AMT 16000M digital camera (Advanced Microscopy Techniques, Corp., Woburn, MA, USA).
Detection of Apoptotic Cells Using Flow Cytometry
Cryopreserved hepatocyte microbeads from the 4 groups of the cytoprotectant study (1 mL/group) were thawed and cultured in 6-well plates. Additional samples of hepatocyte microbeads cryopreserved in freezing solution without any cryoprotectant were thawed and then incubated with ZVAD (60 µM) for 30 min prior to culture. After 24 h in culture, hepatocytes were released from the microbeads.32 Cells were then washed twice with phosphate-buffered saline (PBS)/1% FCS followed by PI staining. Stained cells were incubated at 4 °C for 30 min in the dark, then washed with PBS/1% FCS, and resuspended in 400-μL PBS/1% FCS. An equivalent number of cells from each sample were acquired for fluorescence-activated cell sorting (FACS) analysis (FACSCanto II, BD Biosciences, San Jose, CA). FACS was set to detect PI fluorescence at 620 ± 10 nm.
Statistical Analysis
Data were analyzed using GraphPad Prism® 6 software (GraphPad, San Diego, CA, USA). All data are presented as the mean ± standard deviation (SD) from at least 4 independent experiments. Student t-tests were applied to compare 2 independent groups. Comparisons between 3 or more groups with 1 independent variable were performed using one-way analysis of variance (ANOVA) and multiple comparisons were adjusted by using the Tukey’s post hoc test. A two-way repeated measurement ANOVA and Tukey’s post hoc multiple comparisons tests were used to compare groups exposed to different conditions. A P value of ≤0.05 was considered statistically significant.
Results
Effect of Freezing Solutions on Hepatocytes
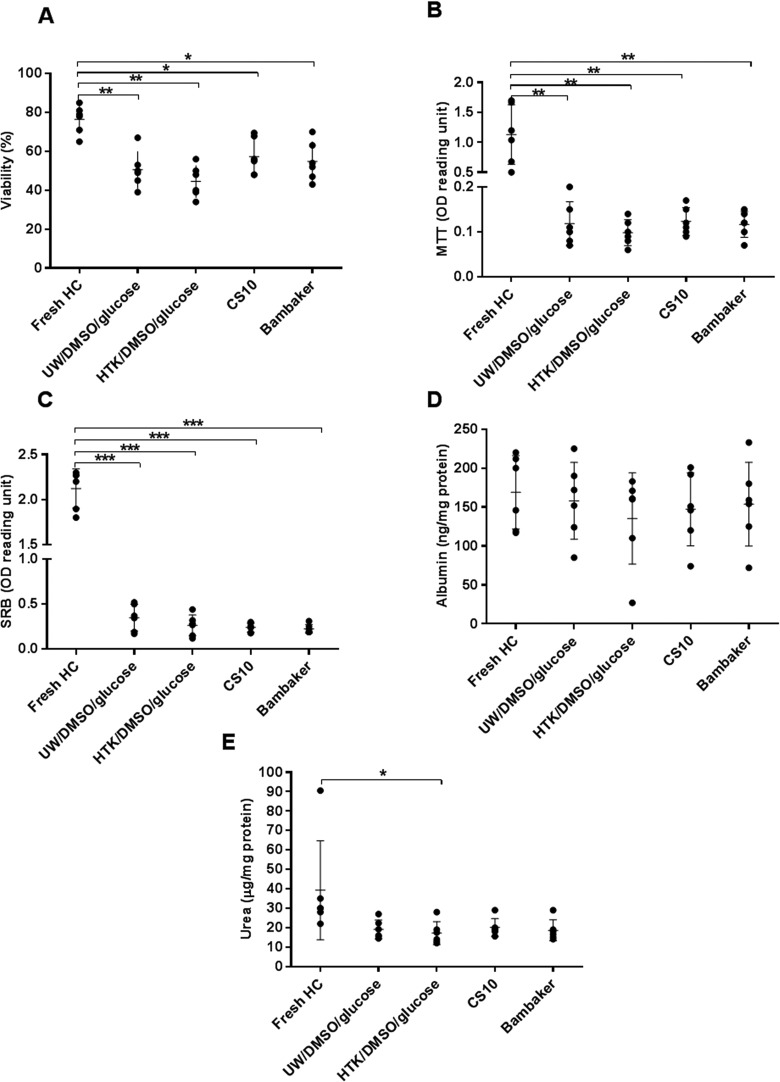
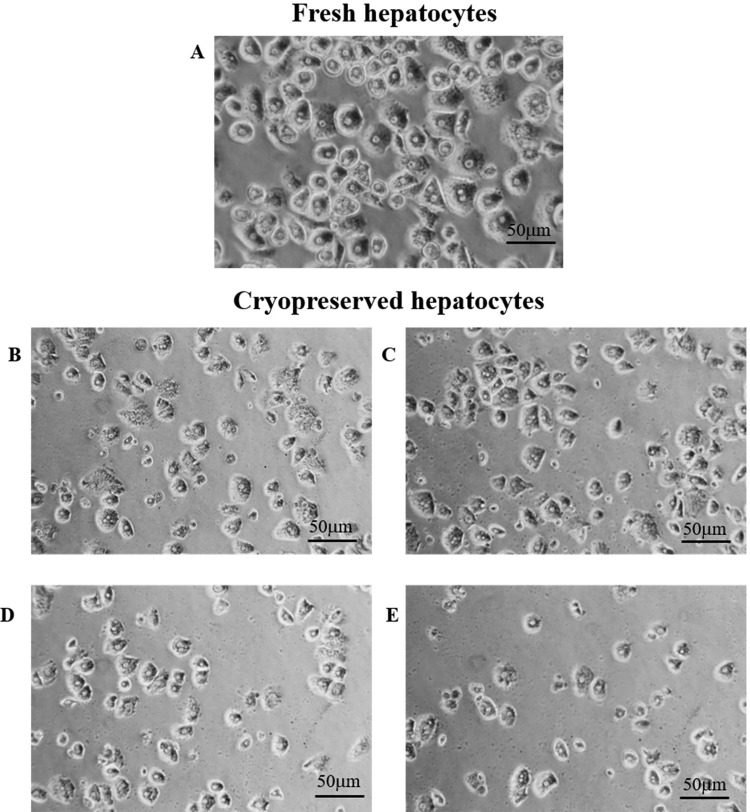
Cell viability of fresh human hepatocytes was assessed by trypan blue immediately after isolation, and overall mitochondrial activity was assessed immediately after plating by MTT assay (77.0% ± 7.3% and 1.13 ± 0.69 optical density (OD) reading units, respectively). There was a significant decrease in cell viability evaluated immediately after thawing and after plating with all freezing media compared to fresh cells (trypan blue: P < 0.05 and MTT assay: P < 0.001). There was no significant difference in postthawing viability between cells cryopreserved with the 4 different solutions (CryoStor CS10: 57.3% ± 13.9%, Bambanker: 54.7% ± 10.3%, UW/DMSO/glucose: 50.4% ± 10.8%, and HTK/DMSO/glucose: 44.5% ± 12.7%). Cell attachment (SRB assay) of cryopreserved cells was significantly lower than that of fresh cells and showed a similar trend to that observed with cell viability. However, the UW/DMSO/glucose group had the highest attachment efficacy (0.35 ± 0.21 OD reading units), but this did not reach statistical significance (Fig. 1A–C). The typical hexagonal shape of hepatocytes was observed in cryopreserved cells under light microscopy. There was no obvious difference in morphological appearance between cryopreserved groups, except in the Bambanker group which showed some “damaged” hepatocytes with irregular cell shape (Fig. 2). Comparison of albumin synthesis between fresh and cryopreserved groups showed a minimal decrease with no statistically significant difference. Nevertheless, urea synthesis was significantly reduced in HTK/DMSO/glucose cryopreserved cells compared to fresh cells (P = 0.04; Fig. 1D, E).
Fig. 1.
Effects of cryopreservation using the 4 different freezing solutions on human hepatocytes viability and function (N = 6). (A) Cell viability immediately after thawing assessed by trypan blue exclusion, (B) cell viability assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay, (C) cell attachment assessed by sulforhodamine B assay, (D) albumin production, and (E) urea synthesis after thawed and plated for 24 h. Statistical significance as compared to fresh hepatocytes (controls): *P < 0.05, **P < 0.01, and ***P < 0.001.
Fig. 2.
Representative images of human hepatocyte morphology after plating and maintenance in culture for 24 h (under light microscopy; 400×), (A) fresh hepatocytes (control). Cryopreserved hepatocytes using (B) University of Wisconsin solution/dimethyl sulfoxide (DMSO)/glucose, (C) CryoStorCS10, (D) histidine–tryptophan–ketoglutarate/DMSO/glucose, and (E) Bambanker.
The results obtained from experiments using rat hepatocytes showed a similar outcome to human hepatocytes. Comparing cryopreserved cells, the CryoStor CS10 group (54.0% ± 5.70%) demonstrated significantly higher cell viability than Bambanker (P = 0.003), HTK/DMSO/glucose (P = 0.002), or UW/DMSO/glucose (P = 0.04). Cells cryopreserved in UW/DMSO/glucose were able to maintain their overall mitochondrial activity and attachment after plating and culturing for 24 h better than the other 3 groups. CryoStor CS10 and UW/DMSO/glucose showed a promising outcome in both human and rat hepatocytes. Hence, these 2 freezing solutions were chosen for the study of cryopreservation of microbeads.
Effect of Freezing Solutions on Hepatocyte Microbeads
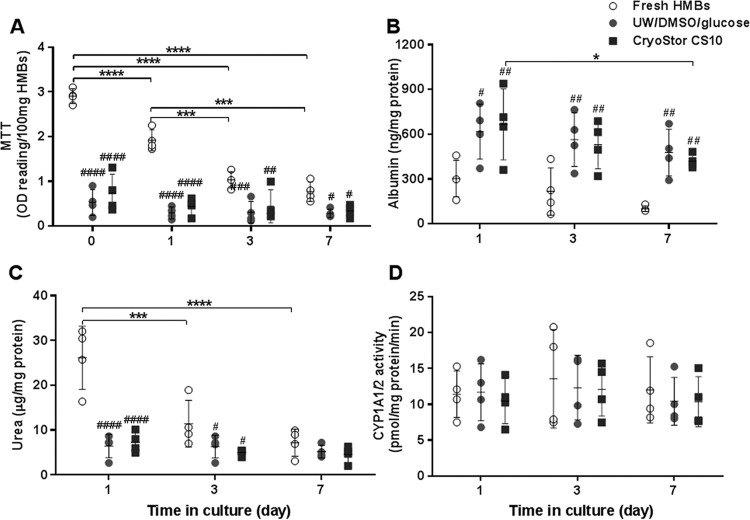
There was no significant difference between cell viability of cryopreserved HMBs immediately postthawing and after maintenance in culture for 7 d, as assessed by FDA/PI (data not shown) and MTT assay (Fig. 3A). Hepatocyte function of cryopreserved HMBs was assessed by albumin production, urea synthesis, and CYP1A1/2; comparable results between the 2 freezing solutions were observed (Fig. 3B–D). Interestingly, there was a small difference in cell viability and functionality of cryopreserved HMBs after thawing and then after maintenance in culture throughout the 7 d, while fresh HMBs showed a significant decrease in cell viability and urea production. When comparing fresh and cryopreserved HMBs, there was a significant decrease in both viability and urea synthesis in cryopreserved HMBs. These reductions were more apparent immediately after thawing and on day 1 in culture (P < 0.0001) than on days 3 and 7. However, albumin production was unexpectedly significantly higher in cryopreserved HMBs in both groups than those of fresh cells at each time point (P ≤ 0.01). In contrast, there was no significant difference in CYP1A1/2 activity between fresh and cryopreserved HMBs.
Fig. 3.
Effects of 2 freezing solutions on cell viability and function of human hepatocyte microbeads (HMBs) after thawing and maintenance in culture for 7 d (N = 4); (A) cell viability, (B) albumin production, (C) urea synthesis, and (D) cytochrome P450 activity. Statistical significance: *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. Statistical significance compared to fresh HMBs: #P < 0.05, ##P < 0.01, ###P < 0.001, and ####P < 0.0001.
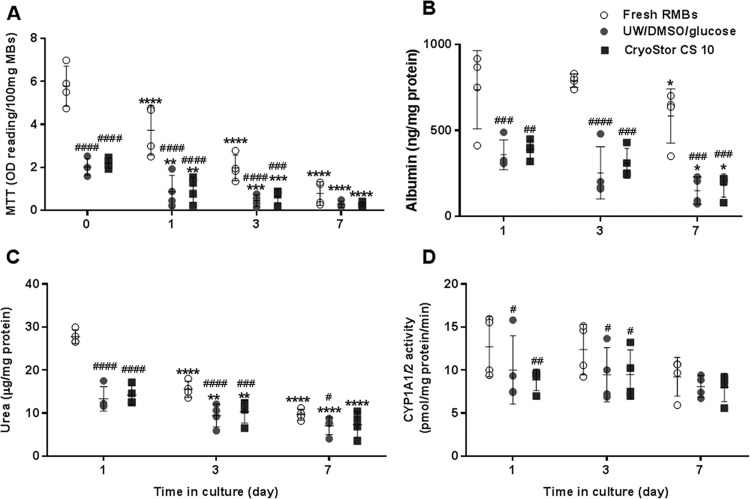
Similar effects of freezing solutions on HMBs were also observed in RMBs; there were no significant differences found between UW/DMSO/glucose and CryoStor CS10 (Fig. 4). However, the difference between fresh and cryopreserved RMBs was more prominent than in the HMB experimental groups.
Fig. 4.
Effects of 2 freezing solutions on cell viability and function of rat hepatocyte microbeads after thawing and 7 d maintenance in culture (N = 4). (A) Cell viability, (B) albumin production, (C) urea synthesis, and (D) cytochrome P450 family activity. Statistical significance compared to either immediately post thawing or compared to day 1: *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 and compared to fresh human hepatocyte microbeads: #P < 0.05 and ##P < 0.01, ###P < 0.001 and ####P < 0.0001.
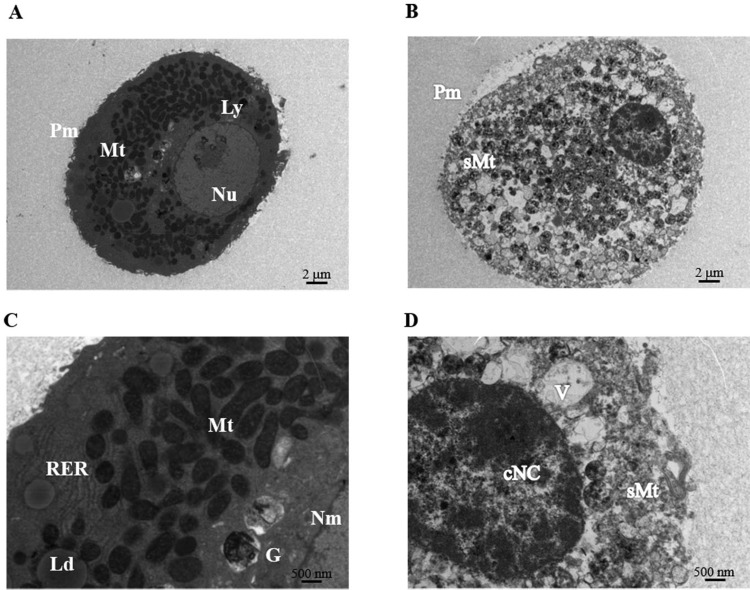
The morphology of cryopreserved microbeads (HMBs and RMBs) was examined under light microscopy and SEM. The morphologies were intact and well-preserved when compared to fresh samples. However, further investigation of the ultrastructure of hepatocytes within microbeads using TEM showed that microbeads cryopreserved in CryoStor CS10 provided slightly more cells with preserved ultrastructure than those in UW/DMSO/glucose. Figure 5 shows TEM images of examples of healthy hepatocytes versus necrotic and apoptotic hepatocytes within microbeads. Healthy hepatocytes exhibited well-defined nuclear membranes and contained abundant smooth membranes with intact cristae in mitochondria. In contrast, apoptotic hepatocytes showed condensation of nuclear chromatin, irregular plasma membranes, and swelling mitochondria. Overall, UW/DMSO/glucose and CryoStor CS10 had similar effects on cryopreservation of hepatocyte microbeads. Therefore, UW/DMSO/glucose which is the freezing media used for cryopreservation of clinical grade human hepatocytes was chosen as the freezing solution in further cryopreservation experiments.
Fig. 5.
Transmission electron microscopy (TEM) of hepatocytes within microbeads after 24-h culture. Representative TEM images of normal healthy hepatocytes (left panel) and necrotic/apoptotic hepatocytes (right panel). (A, B) at magnification of 1,400×, and (C, D) at high magnification of 4,800×. cNC, condensed nuclear chromatin; G, Golgi apparatus; Ld, lipid droplet; Ly, lysosomes, Mt, mitochondria; N, nuclei; Nu, nucleoli; Nm, nuclear membrane; Pm, plasma membrane; RER, rough-surfaced endoplasmic reticulum; sMt, swelling mitochondria; V, vacuole.
Effects of Cytoprotectants on Hepatocyte Microbeads
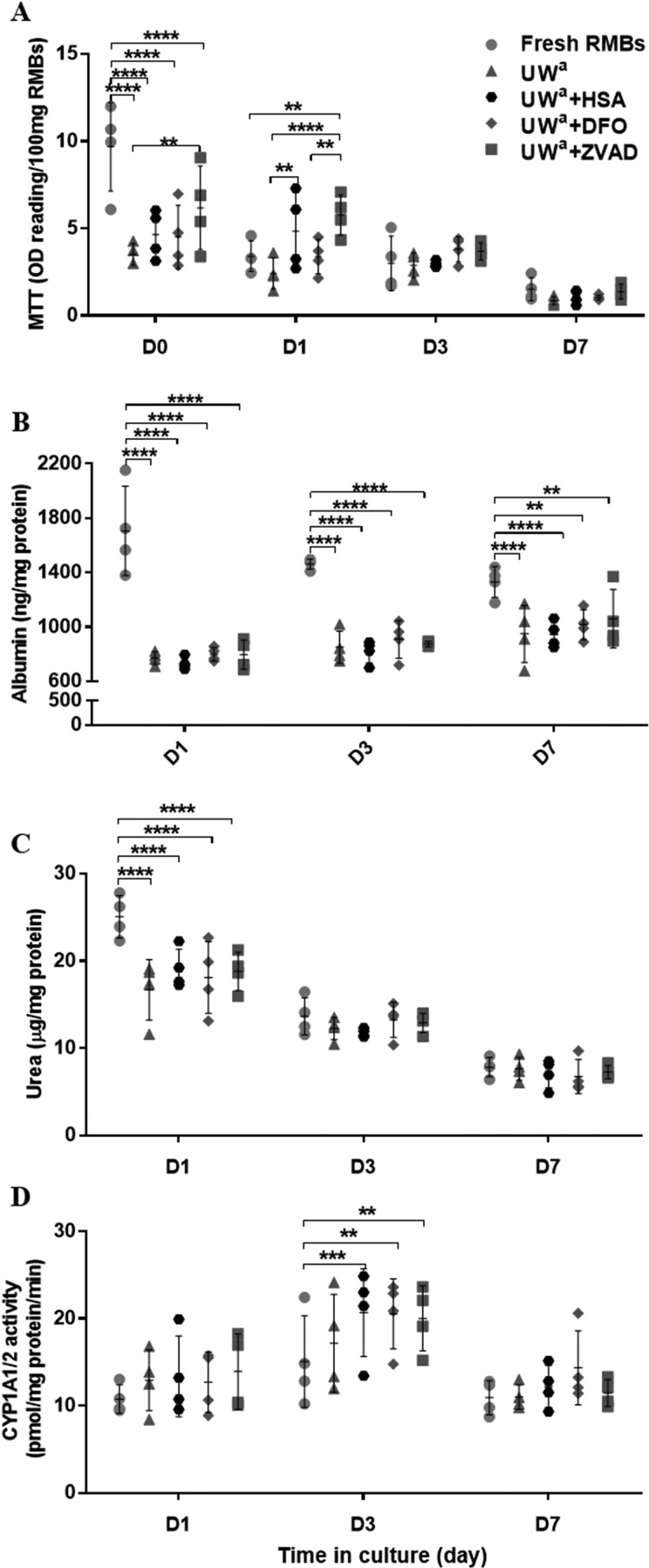
RMBs cryopreserved in UW/DMSO/glucose (basal freezing medium) containing 60-µM ZVAD resulted in a significantly higher cell viability immediately post thawing (MTT = 6.12 ± 2.24 OD reading/100-mg RMBs) than those cryopreserved with standard basal freezing medium alone (3.51 ± 0.62, P = 0.002). After 1 d in culture, the cell viability of the ZVAD group (5.79 ± 1.16) remained significantly higher than that of basal medium alone (2.42 ± 0.90, P < 0.0001), the DFO group (3.39 ± 0.98, P = 0.006), or the fresh RMBs (3.41 ± 0.88, P = 0.007). Moreover, RMBs in the HSA group gave significantly higher viability (4.48 ± 2.22, P = 0.006) than those in the basal medium group. Interestingly, fresh RMBs gave better viability compared to the other groups (P < 0.0001) on the day of production (day 0) but had similar viability at days 3 and 7 (Fig. 6A). The results of cell viability assessed by fluorescence staining correlated well with the MTT assay data.
Fig. 6.
Effect of cryopreservation with different cytoprotectants on viability and hepatocyte-specific functions of rat hepatocyte microbeads after thawing and maintenance in culture for 7 d (N = 4). (A) Cell viability, (B) albumin production, (C) urea synthesis, and (D) cytochrome P450 family activity. Statistical significance: *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
There were no significant differences between hepatocyte-specific functions in the 4 RMB cryopreserved groups. The fresh RMBs had significantly higher albumin production at day 1 (P < 0.0001) and day 3 (P < 0.0001) compared to the other 4 groups. At day 7, albumin synthesis of the fresh RMB group (1,331 ± 111.6 ng/mg protein) remained higher than those of the basal medium (950.3 ± 209.6, P = 0.03) or HSA (943.9 ± 97.0, P = 0.02) groups but was not significantly different compared to both the DFO (1,017 ± 101.2) and ZVAD (1,061 ± 215.2) groups. Similar findings were observed in urea production, where the fresh RMB group provided significantly higher production at day 1 than all of the other groups (P < 0.0001), but not on days 3 and 7. Interestingly, on day 3, there was significantly higher CYP1A1/2 activity in RMBs cryopreserved using HSA (20.7 ± 5.0 pmol/mg protein/min, P = 0.001), DFO (20.6 ± 4.0, P = 0.001), and ZVAD (20.0 ± 3.8, P = 0.003) compared to fresh RMBs (15.1 ± 5.2; Fig. 6B–D).
Among the cryopreserved RMB groups, the ZVAD group had the highest percentage of well-preserved hepatocyte ultrastructure within microbeads (76.9% of the fresh RMBs group), followed by the HSA, the DFO, and the basal medium groups, at 67.4%, 44.7%, and 34.8% of the fresh RMB group, respectively.
Detection of Apoptotic Cells in Cryopreserved RMBs
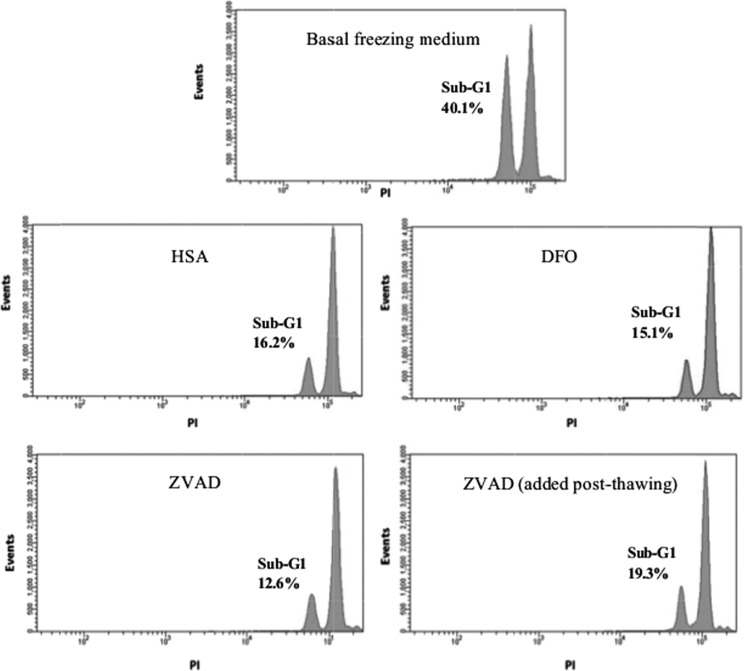
The anti-apoptotic effects of cytoprotectants on RMBs were assessed by detection of the sub-G1 phase of the cell cycle using PI staining and FACS analysis. Apoptotic cells (fragmented nuclei) were arrested in the sub-G1 cycle. RMBs in the basal medium alone had the highest percentage of cell in sub-G1 stage (40.1%), whereas the lowest was detected in the ZVAD group (12.6%). Noticeably, the sub-G1 arrest of RMBs in the basal medium alone was decreased to 19.3% when incubated with ZVAD (60 µM) for 30 min immediately after thawing before maintenance in culture. Figure 7 shows representative FACS histograms of cell populations after thawing and maintenance in culture for 24 h.
Fig. 7.
Representative histograms of cell cycle analysis using fluorescence activated cell sorting (FACS). Cryopreserved rat hepatocyte microbeads were thawed and then cultured for 24 h. Hepatocytes were released from microbeads and analyzed using propidium iodide staining and FACs.
Discussion
This study focused on optimizing cryopreservation solutions that can potentially be used for cryopreservation of clinical grade microbeads. The study investigated the effect of cryopreservation solutions on cell viability and function of cryopreserved hepatocyte microbeads. The important issue was the scarcity of good quality donor livers for hepatocyte isolation; our study was carried out in a stepwise strategy to address this problem. Firstly, the efficacy of freezing solutions was compared on both isolated human and rat hepatocytes, followed by testing of human and rat microbeads. The results obtained from these initial experiments demonstrated comparable outcomes for both human and rat hepatocytes. Therefore, RMBs were considered suitable for further experiments to assess cryoprotectants.
In the initial experiments, we attempted to determine the efficacy of basal freezing solutions for cryopreservation of isolated hepatocytes. The results showed that these freezing media alone did not have significantly different effects on the outcome of cryopreservation. Nevertheless, UW/DMSO/glucose and CryoStor CS10 were superior to both HTK/DMSO/glucose and Bambanker for cryopreservation of hepatocyte suspensions. Hepatocyte cryopreservation in UW/DMSO/glucose tended to result in higher cell attachment and overall mitochondrial activity after 24 h in culture, while in CryoStor CS10, cells had a higher viability immediately postthawing. This could be explained by solutions containing similar compounds that are beneficial for protection of the cells during cryopreservation (Table 1). UW has shown beneficial effects for cryopreservation of hepatocytes,33–36 as it includes important components to prevent cells from swelling, stabilizes cell membranes, and promotes adenosine triphosphate (ATP) production. Moreover, addition of a high concentration of glucose (300 mM) enhances the efficacy of UW, so that it leads to an increase in cell ATP content and protects cell attachment molecules on the plasma membrane, resulting in better hepatocyte mitochondrial function and improvement of cell attachment, respectively.24,37 CryoStor CS10 is a recently developed freezing solution that has been used clinically for stem cell banking.38 It was developed from hypothermosol solution consisting of multiple components to maintain ionic and osmotic balance under hypothermic conditions. It reduces cryopreservation-induced delayed-onset cell death, which develops over time postthawing.14 However, it contains a low level of glucose (5 mM) to provide energy for the cells. Extra glucose was not added either to CryoStor CS10 or to Bambanker, as they are ready-made cryopreservation solutions and their actual ingredients are not disclosed by the suppliers, and addition of glucose may have led to unexpected interaction(s).
The HTK-based freezing solution used in this study was the least effective for cryopreservation of both human and rat hepatocytes. This finding supports previously published studies demonstrating the superiority of UW solution over HTK solutions for cold storage of human hepatocytes and rat livers by limiting cell necrosis and mitochondrial dysfunction.39,40 Of note, the histidine and phosphate (buffering system) in HTK have been suggested to be the cause of toxicity to cells during cold storage.41
Bambanker provided good results for freezing a wide variety of cells including human gastric epithelial cells, human T cells, and stem cells.42–44 However, there was no study using Bambanker for cryopreservation of hepatocytes. The current results showed that Bambanker is not suitable for hepatocyte cryopreservation, as it causes some damage to hepatocytes and abnormal cell morphology after thawing. The possible explanation for this is not known, as the actual composition of this solution is not disclosed.
UW/DMSO/glucose and CryoStor CS10 were further studied in order to compare the efficacy on cryopreservation of hepatocyte microbeads. The results showed that there were no significant differences between CryoStor CS10 and UW/DMSO/glucose with either cryopreserved HMBs or RMBs. Both freezing solutions were able to maintain microbead integrity after thawing. Morphology under light microscopy and SEM indicated that the appearance of cryopreserved microbeads was comparable to fresh microbeads. This suggested that alginate microbeads could be cryopreserved with 10% DMSO using a multistep, slow-freezing step protocol without damaging microbead integrity. Cryopreserved microbeads with a DMSO concentration lower than 10% or higher at 25% using a rapid cooling protocol resulted in low postthawing viability and damage to microbeads.12,45 Noticeably, viability and functionality of microbeads after thawing were dramatically decreased compared to fresh microbeads in this stage of study. This indicated that these 2 freezing media for cryopreservation of hepatocyte microbeads were not sufficient to protect cells against cell death related to cryopreservation. However, CYP1A1/2 activities were well-preserved and showed minimal reduction compared to fresh microbeads. It has been demonstrated that cryopreserved microencapsulated hepatocytes retain gene expression of organic ion transporter 22 and CYP3A2/9.8 The results in the present study also showed that cryopreserved HMBs had higher albumin production than fresh HMBs at all times. The viability and functionality of cryopreserved HMBs were maintained over 7 d while those of fresh HMBs continued to decrease. The reason for this is unknown; however, it has been postulated that cryopreservation “selects” only good quality hepatocytes which are viable on thawing.13 UW-based freezing solution was chosen as a basal freezing medium for further experiments, as it has been widely used in clinical transplantation and is less expensive than CryoStor CS10. In this study, 3 cytoprotectants targeting different sites of cryopreservation-induced cell death in cryopreserved hepatocyte microbeads were studied. The results demonstrated that ZVAD had positive effects on cell viability and functionality of RMBs when this cytoprotectant was added to the basal freezing media. The beneficial effects on cell viability were greater than with either DFO or HSA. This was shown by both overall mitochondrial activity and fluorescent staining for viable cells. RMBs cryopreserved with ZVAD had a reduction in cell apoptosis observed on TEM following cryopreservation when compared to basal freezing media alone or other cytoprotectant groups. This was also supported by the PI staining (DNA defragmentation)/FACS, which showed that the sub-G1 cell population was lowest in the ZVAD group. After thawing, ZVAD also reduced the degree of apoptosis, but this was not as effective as including it in the freezing medium. A possible explanation is that the process of apoptosis has already started at the time of hepatocyte isolation and continues throughout cryopreservation and after thawing. Therefore, the earlier the addition of the anti-apoptosis agent, the more beneficial effects will be observed. The current data are consistent with previously published studies showing the beneficial effects of ZVAD when used in cryopreservation and cold storage.11,15 It is worth mentioning that the cytoprotective effects of ZVAD are dose-dependent and related to the cell type to be preserved and 60 µM was the optimal dose for hepatocytes.17
The addition of HSA showed some beneficial effects on cell viability and function. In fact, HSA seemed to be a good additive, as it has a buffering capacity and reduced DMSO toxicity which may have protected cells from apoptosis. Addition of HSA to the freezing medium is believed to be beneficial not only for the cells but also for the physical integrity of microbeads as well.20 Importantly, it is widely used and clinically approved. However, the results obtained showed that HSA was not as effective as ZVAD. This could be due to the concentration used being too low (2% v/v) to overcome the stress during cryopreservation and postthaw. There have been no previously reported studies using HSA for cryopreservation of microbeads. Similar to HSA, DFO also had positive effects on cryopreserved RMBs but less than ZVAD. The reason could be that a suboptimal concentration of DFO was used in this study. DFO has been reported to be effective in the reduction of necrosis and apoptosis of hepatocytes during cold storage by reducing reactive oxygen species associated with an increased intracellular chelatable iron.19,46 DFO was one of the choices as a cytoprotectant compound due to its properties and being approved for clinical use. The testing of various concentrations of HSA and DFO and the possible synergistic effects when added together with ZVAD (HSA-ZVAD, DFO-ZVAD, or HSA-DFO-ZVAD) to the cryopreservation media at the same time would be important for future studies. It must be noted that this set of experiments was done using only RMBs due to the lack of good quality fresh human hepatocytes at that time. Nevertheless, the initial results showed similar outcomes using both human and rat hepatocytes. The results obtained from this study could be considered as an important step in the development of an optimized protocol for cryopreservation of HMBs for clinical use.
In conclusion, cryopreservation of hepatocyte microbeads using UW solution containing 10% DMSO, 5% glucose, and a cytoprotectant such as ZVAD were shown to have beneficial effects on cellular and physical damage leading to maintenance of cell viability and function post thawing. This initial optimization protocol for cryopreservation of hepatocyte microbeads supports the hypothesis that the development of freezing solution containing cryoprotective agents and compounds targeting apoptosis during the cryopreservation process could improve the outcome of cryopreservation. We believe that clinically a large amount of hepatocyte microbeads may be required immediately for emergency treatment in conditions such as ALF. Establishment of a bank of cryopreserved clinical grade hepatocyte microbeads is an important goal.
Acknowledgments
We acknowledge the support of The National Institute for Health Research (NIHR), King’s College Hospital Chari Trust, Department of Health, WellChild, and Medical Research Council (MRC) Centre for Transplantation, King’s College London, UK. We would like to thank the Liver Transplant and Hepatobiliary Surgery teams and The Liver Pathology Service at King’s College Hospital.
Authors’ Note: Anil Dhawan and Ragai R. Mitry are joint senior authors.
Ethical Approval: This study was approved by our institutional review board.
Statement of Human and Animal Rights: All human tissues were approved for research use in accordance with the Research Ethics Committee of King's College Hospital. Written informed consent was obtained from donor relatives or patients.
All animal donors were handled following protocols approved by the ethical review process of King's College London in accordance with the UK Animals (Scientific) Procedures Act of 1986.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Khuroo M, Khuroo M, Farahat K. Molecular adsorbent recirculating system for acute and acute-on-chronic liver failure: a meta-analysis. Liver Transpl. 2004;10(9):1099–1106. [DOI] [PubMed] [Google Scholar]
- 2. Oppert M, Rademacher S, Petrasch K, Jörres A. Extracorporeal liver support therapy with Prometheus in patients with liver failure in the intensive care unit. Ther Apher Dial. 2009;13(5):426–430. [DOI] [PubMed] [Google Scholar]
- 3. Struecker B, Raschzok N, Sauer I. Liver support strategies: cutting-edge technologies. Nat Rev Gastroenterol Hepatol. 2014;11(3):166–176. [DOI] [PubMed] [Google Scholar]
- 4. Jitraruch S, Dhawan A, Hughes R, Filippi C, Soong D, Philippeos C, Lehec S, Heaton N, Longhi M, Mitry R. Alginate microencapsulated hepatocytes optimised for transplantation in acute liver failure. PLoS One. 2014;9(12):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mai G, Nguyen T, Morel P, Mei J, Andres A, Bosco D, Baertschiger R, Toso C, Berney T, Majno P, et al. Treatment of fulminant liver failure by transplantation of microencapsulated primary or immortalized xenogeneic hepatocytes. Xenotransplantation. 2005;12(6):457–464. [DOI] [PubMed] [Google Scholar]
- 6. Sgroi A, Mai G, Morel P, Baertschiger R, Gonelle-Gispert C, Serre-Beinier V, Buhler L. Transplantation of encapsulated hepatocytes during acute liver failure improves survival without stimulating native liver regeneration. Cell Transplant. 2011;20(11–12):1791–1803. [DOI] [PubMed] [Google Scholar]
- 7. Umehara Y, Hakamada K, Seino K, Aoki K, Toyoki Y, Sasaki M. Improved survival and ammonia metabolism by intraperitoneal transplantation of microencapsulated hepatocytes in totally hepatectomized rats. Surgery. 2001;130(3):513–520. [DOI] [PubMed] [Google Scholar]
- 8. Aoki T, Koizumi T, Kobayashi Y, Yasuda D, Izumida Y, Jin Z, Nishino N, Shimizu Y, Kato H, Murai N, et al. A novel method of cryopreservation of rat and human hepatocytes by using encapsulation technique and possible use for cell transplantation. Cell Transplant. 2005;14(9):609–620. [DOI] [PubMed] [Google Scholar]
- 9. Hang H, Shi X, Gu G, Wu Y, Gu J, Ding Y. In vitro analysis of cryopreserved alginate-poly-L-lysine-alginate-microencapsulated human hepatocytes. Liver Int. 2010;30(4):611–622. [DOI] [PubMed] [Google Scholar]
- 10. Kusano T, Aoki T, Yasuda D, Matsumoto S, Jin Z, Nishino N, Hayashi K, Odaira M, Yamada K, Koizumi T, et al. Microencapsule technique protects hepatocytes from cryoinjury. Hepatol Res. 2008;38(6):593–600. [DOI] [PubMed] [Google Scholar]
- 11. Mahler S, Desille M, Frémond B, Chesné C, Guillouzo A, Campion J, Clément B. Hypothermic storage and cryopreservation of hepatocytes: the protective effect of alginate gel against cell damages. Cell Transplant. 2003;12(6):579–592. [DOI] [PubMed] [Google Scholar]
- 12. Chin H, Yu H, Chye N. Strategies for the cryopreservation of microencapsulated cells. Biotechnol Bioeng. 2004;85(2):202–213. [DOI] [PubMed] [Google Scholar]
- 13. Dixit V, Arthur M, Gitnick G. A morphological and functional evaluation of transplanted isolated encapsulated hepatocytes following long-term transplantation in Gunn rats. Biomater Artif Cells Immobilization Biotechnol. 1993;21(2):119–133. [DOI] [PubMed] [Google Scholar]
- 14. Baust J, Vogel M, Van B, Baust J. A molecular basis of cryopreservation failure and its modulation to improve cell survival. Cell Transplant. 2001;10(7):561–571. [PubMed] [Google Scholar]
- 15. Yagi T, Hardin J, Valenzuela Y, Miyoshi H, Gores G, Nyberg S. Caspase inhibition reduces apoptotic death of cryopreserved porcine hepatocytes. Hepatology. 2001;33(6):1432–1440. [DOI] [PubMed] [Google Scholar]
- 16. Baust J, Van B, Baust J. Modulation of the cryopreservation cap: elevated survival with reduced dimethyl sulfoxide concentration. Cryobiology. 2002;45(2):97–108. [DOI] [PubMed] [Google Scholar]
- 17. Nyberg S, Hardin J, Matos L, Rivera D, Misra S, Gores G. Cytoprotective influence of ZVAD-fmk and glycine on gel-entrapped rat hepatocytes in a bioartificial liver. Surgery. 2000;127(4):447–455. [DOI] [PubMed] [Google Scholar]
- 18. Fujita R, Hui T, Chelly M, Demetriou A. The effect of antioxidants and a caspase inhibitor on cryopreserved rat hepatocytes. Cell Transplant. 2005;14(6):391–396. [DOI] [PubMed] [Google Scholar]
- 19. Kerkweg U, Li T, de G, Rauen U. Cold-induced apoptosis of rat liver cells in University of Wisconsin solution: the central role of chelatable iron. Hepatology. 2002;35(5):560–567. [DOI] [PubMed] [Google Scholar]
- 20. Schneider S, Feilen P, Cramer H, Hillgärtner M, Brunnenmeier F, Zimmermann H, Weber M, Zimmermann U. Beneficial effects of human serum albumin on stability and functionality of alginate microcapsules fabricated in different ways. J Microencapsul. 2003;20(5):627–636. [DOI] [PubMed] [Google Scholar]
- 21. Mitry R. Isolation of human hepatocytes. Methods Mol Biol. 2009;481:17–23. [DOI] [PubMed] [Google Scholar]
- 22. Neufeld D. Isolation of rat liver hepatocytes. Methods Mol Biol. 1997;75:145–151. [DOI] [PubMed] [Google Scholar]
- 23. Wu Y, Gupta S. Hepatic preconditioning for transplanted cell engraftment and proliferation. Methods Mol Biol. 2009;481:107–116. [DOI] [PubMed] [Google Scholar]
- 24. Terry C, Dhawan A, Mitry R, Lehec S, Hughes R. Optimization of the cryopreservation and thawing protocol for human hepatocytes for use in cell transplantation. Liver Transpl. 2010;16(2):229–237. [DOI] [PubMed] [Google Scholar]
- 25. Massie I, Selden C, Hodgson H, Fuller B. Cryopreservation of encapsulated liver spheroids for a bioartificial liver: reducing latent cryoinjury using an ice nucleating agent. Tissue Eng Part C Methods. 2011;17(7):765–774. [DOI] [PubMed] [Google Scholar]
- 26. Niu X, Arthur P, Jeffrey G. Iron and oxidative stress in cold-initiated necrotic death of rat hepatocyte. Transplant Proc. 2010;42(5):1563–1568. [DOI] [PubMed] [Google Scholar]
- 27. De C, Orive G, Gascón A, Hernandez R, Pedraz J. Evaluation of human serum albumin as a substitute of foetal bovine serum for cell culture. Int J Pharm. 2006;310(1–2):8–14. [DOI] [PubMed] [Google Scholar]
- 28. Steinberg P, Fischer T, Kiulies S, Biefang K, Platt K, Oesch F, Böttger T, Bulitta C, Kempf P, Hengstler J. Drug metabolizing capacity of cryopreserved human, rat, and mouse liver parenchymal cells in suspension. Drug Metab Dispos. 1999;27(12):1415–1422. [PubMed] [Google Scholar]
- 29. Mitry R, Hughes R, Bansal S, Lehec S, Wendon J, Dhawan A. Effects of serum from patients with acute liver failure due to paracetamol overdose on human hepatocytes in vitro. Transplant Proc. 2005;37(5):2391–2394. [DOI] [PubMed] [Google Scholar]
- 30. Mitry R, Sarraf C, Havlík R, Habib N. Detection of adenovirus and initiation of apoptosis in hepatocellular carcinoma cells after Ad-p53 treatment. Hepatology. 2000;31(4):885–889. [DOI] [PubMed] [Google Scholar]
- 31. Donato M, Gómez-Lechón M, Castell J. A microassay for measuring cytochrome P450IA1 and P450IIB1 activities in intact human and rat hepatocytes cultured on 96-well plates. Anal Biochem. 1993;213(1):29–33. [DOI] [PubMed] [Google Scholar]
- 32. Chayosumrit M, Tuch B, Sidhu K. Alginate microcapsule for propagation and directed differentiation of hESCs to definitive endoderm. Biomaterials. 2010;31(3):505–514. [DOI] [PubMed] [Google Scholar]
- 33. Arikura J, Kobayashi N, Okitsu T, Noguchi H, Totsugawa T, Watanabe T, Matsumura T, Maruyama M, Kosaka Y, Tanaka N, et al. UW solution: a promising tool for cryopreservation of primarily isolated rat hepatocytes. J Hepatobiliary Pancreat Surg. 2002;9(6):742–749. [DOI] [PubMed] [Google Scholar]
- 34. Dandri M, Burda M, Gocht A, Török E, Pollok J, Rogler C, Will H, Petersen J. Woodchuck hepatocytes remain permissive for hepadnavirus infection and mouse liver repopulation after cryopreservation. Hepatology. 2001;34(4 pt 1):824–833. [DOI] [PubMed] [Google Scholar]
- 35. Kunieda T, Maruyama M, Okitsu T, Shibata N, Takesue M, Totsugawa T, Kosaka Y, Arata T, Kobayashi K, Ikeda H, et al. Cryopreservation of primarily isolated porcine hepatocytes with UW solution. Cell Transplant. 2003;12(6):607–616. [DOI] [PubMed] [Google Scholar]
- 36. Terry C, Mitry R, Lehec S, Muiesan P, Rela M, Heaton N, Hughes R, Dhawan A. The effects of cryopreservation on human hepatocytes obtained from different sources of liver tissue. Cell Transplant. 2005;14(8):585–594. [DOI] [PubMed] [Google Scholar]
- 37. Stéphenne X, Najimi M, Ngoc D, Smets F, Hue L, Guigas B, Sokal E. Cryopreservation of human hepatocytes alters the mitochondrial respiratory chain complex 1. Cell Transplant. 2007;16(4):409–419. [DOI] [PubMed] [Google Scholar]
- 38. Woods E, Perry B, Hockema J, Larson L, Zhou D, Goebel W. Optimized cryopreservation method for human dental pulp-derived stem cells and their tissues of origin for banking and clinical use. Cryobiology. 2009;59(2):150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Janssen H, Janssen P, Broelsch C. Celsior solution compared with University of Wisconsin solution (UW) and histidine-tryptophan-ketoglutarate solution (HTK) in the protection of human hepatocytes against ischemia-reperfusion injury. Transpl Int. 2003;16(7):515–522. [DOI] [PubMed] [Google Scholar]
- 40. Straatsburg I, Abrahamse S, Song S, Hartman R, Van G. Evaluation of rat liver apoptotic and necrotic cell death after cold storage using UW, HTK, and Celsior. Transplantation. 2002;74(4):458–464. [DOI] [PubMed] [Google Scholar]
- 41. Rauen U, de G. Inherent toxicity of organ preservation solutions to cultured hepatocytes. Cryobiology. 2008;56(1):88–92. [DOI] [PubMed] [Google Scholar]
- 42. Hikichi T, Wakayama S, Mizutani E, Takashima Y, Kishigami S, Van T, Ohta H, Bui H, Nishikawa S, Wakayama T. Differentiation potential of parthenogenetic embryonic stem cells is improved by nuclear transfer. Stem Cells. 2007;25(1):46–53. [DOI] [PubMed] [Google Scholar]
- 43. Naito H, Yoshimura M, Mizuno T, Takasawa S, Tojo T, Taniguchi S. The advantages of three-dimensional culture in a collagen hydrogel for stem cell differentiation. J Biomed Mater Res A. 2013;101(10):2838–2845. [DOI] [PubMed] [Google Scholar]
- 44. Tamai Y, Hasegawa A, Takamori A, Sasada A, Tanosaki R, Choi I, Utsunomiya A, Maeda Y, Yamano Y, Eto T, et al. Potential contribution of a novel Tax epitope-specific CD4+ T cells to graft-versus-Tax effect in adult T cell leukemia patients after allogeneic hematopoietic stem cell transplantation. J Immunol. 2013;190(8):4382–4439. [DOI] [PubMed] [Google Scholar]
- 45. Pravdyuk A, Petrenko Y, Fuller B, Petrenko A. Cryopreservation of alginate encapsulated mesenchymal stromal cells. Cryobiology. 2013;66(3):215–222. [DOI] [PubMed] [Google Scholar]
- 46. Vairetti M, Griffini P, Pietrocola G, Richelmi P, Freitas I. Cold-induced apoptosis in isolated rat hepatocytes: protective role of glutathione. Free Radic Biol Med. 2001;31(8):954–961. [DOI] [PubMed] [Google Scholar]