Abstract
Olfactory mucosa mesenchymal stem cells (OM-MSCs) display significant clonogenic activity and may be easily propagated for Parkinson’s disease therapies. Methods of inducing OM-MSCs to differentiate into dopaminergic (DAergic) neurons using olfactory ensheathing cells (OECs) are thus an attractive topic of research. We designed a hypoxic induction protocol to generate DAergic neurons from OM-MSCs using a physiological oxygen (O2) level of 3% and OEC-conditioned medium (OCM; HI group). The normal induction (NI) group was cultured in O2 at ambient air level (21%). The role of hypoxia-inducible factor-1α (HIF-1α) in the differentiation of OM-MSCs under hypoxia was investigated by treating cells with an HIF-1α inhibitor before induction (HIR group). The proportions of β-tubulin- and tyrosine hydroxylase (TH)-positive cells were significantly increased in the HI group compared with the NI and HIR groups, as shown by immunocytochemistry and Western blotting. Furthermore, the level of dopamine was significantly increased in the HI group. A slow outward potassium current was recorded in differentiated cells after 21 d of induction using whole-cell voltage-clamp tests. A hypoxic environment thus promotes OM-MSCs to differentiate into DAergic neurons by increasing the expression of HIF-1α and by activating downstream target gene TH. This study indicated that OCM under hypoxic conditions could significantly upregulate key transcriptional factors involved in the development of DAergic neurons from OM-MSCs, mediated by HIF-1α. Hypoxia promotes DAergic neuronal differentiation of OM-MSCs, and HIF-1α may play an important role in hypoxia-inducible pathways during DAergic lineage specification and differentiation in vitro.
Keywords: olfactory mucosa mesenchymal stem cells, differentiation, DAergic neuron, hypoxia-inducible factor-1α
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disorder of the central nervous system (CNS) that mainly affects the motor system.1 Functional recovery is possible after CNS injury and neurodegeneration2 by neurorestorative strategies including cell therapy,3 neurostimulation or neuromodulation, neuroprosthesis or related advanced assistive devices, bioengineering or tissue engineering, neurotization or nerve bridging, neurorehabilitation, drug or growth factors, and other novel treatment procedures.4,5 PD symptoms such as muscle tremors, slowness of movement, and rigidity are caused by the destruction of dopaminergic (DAergic) neurons that produce dopamine (DA).6,7 Nasal olfactory mucosa mesenchymal stem cells (OM-MSCs) possess the capacity of multidirectional differentiation and the ability to promote nervous system regeneration in vivo.8 Notably, OM-MSCs could be obtained from the patients themselves and used for autologous transplantation9 and have thus recently been considered a promising approach for PD therapeutic applications.10–12 This approach has several advantages including ease of acquisition, convenient location, and high versatility.13 OM-MSCs have previously been used successfully in mammalian models of neurodegenerative diseases and nerve damage.9,14,15
To optimize their therapeutic effect for PD, OM-MSCs should be more committed to differentiate into a neural lineage, with a high potential to become functional DAergic neurons. Inducing OM-MSCs to differentiate into appropriate neural cells is thus a key technological goal of cell replacement therapy. Oxygen (O2) is a component of the stem cell niche and acts as an important regulator of stem cell fate specification. The level of O2 plays a fundamental role in maintaining the stem cell niche, and recent studies have investigated the effect of hypoxemia on stem cell differentiation. Reduced O2 levels can promote the survival, proliferation, and catecholaminergic differentiation of neural stem cells (NSCs) and bone marrow–derived mesenchymal stem cells.16–18 Accumulated evidence suggests that these beneficial effects might be associated with the increased expression of hypoxia-inducible factor-1α (HIF-1α).
In this study, we assessed the effects of hypoxemia on the differentiation of OM-MSCs and their ability to produce functional neurons. Based on indications that these beneficial effects might be associated with the increased expression of HIF-1α induced by hypoxia/ischemia/hyperthermia preconditioning,19,20 we also hypothesized that HIF-1α might play a key role in cell fate specification and differentiation and that upregulation of HIF-1α by hypoxemic conditioning might stimulate OM-MSCs to differentiate into a DAergic lineage.
Materials and Methods
Ethics Statement
All surgical operations were executed according to the Chinese legislations involving animal protection and were approved by the ethics committee of Hunan Normal University. The use of human nasal mucosa biopsy tissues was permitted by the ethical committee of Hunan Normal University and the patients gave written informed consent.
Isolation and Culture of Human OM-MSCs (hOM-MSCs)
The culture of hOM-MSCs was carried out using a protocol from a previous study.8 Then substantial OM (1–2 mm3) was collected from the surface interior of the concha nasalis media, washed 3 to 5 times with penicillin–streptomycin (Invitrogen, Carlsbad, CA, USA) under room temperature, and then cut into approximately 0.5 mm3 pieces of tissue block. The tissues were cultured in Dulbecco’s modified Eagle’s medium:nutrient mixture F12 (DMEM/F12; Invitrogen) containing 10% fetal bovine serum (FBS; Invitrogen) and incubated at 37 °C in 5% CO2. The medium (DMEM/F12 + 10% FBS) was changed every 3 d. After 5 to 7 d, the cells began to climb the culture bottle. We then used flow cytometry to test cell surface correlation antigens after 2 to 3 weeks. Four to 5 passages of cells were used in our experiment.
Primary Culture and Purification of Olfactory Ensheathing Cells (OECs)
The 3-d old Kunming mice (male and female unlimited) were obtained from the Experimental Animal Centre of Central South University. Primary culture and purification of OECs were performed according to the isolation protocol described in our previous study.21 Briefly, the meningeal membranes were separated with dissecting forceps and the olfactory bulb’s outer layers were removed. The collected tissues were digested at 37 °C in 0.25% trypsin (Sigma-Aldrich, St Louis, MO, USA) for 10 min. This step was stopped by adding DMEM/F12 supplemented with 10% FBS. Then the tissues were cut into pieces and centrifuged at 1,200g for 5 min. The tissues were resuspended and placed into a culture bottle with the medium DMEM/F12 supplemented with 10% FBS and penicillin–streptomycin (50 mg/mL). Then the tissues were incubated at 37 °C in 5% CO2. Serum-free OEC culture medium was used for cell purification. We began to collect OEC-conditioned medium (OCM) while the OECs’ purity was up to 90%.
Preparation of OCM
When the cell confluence reached 80%, the cells were washed twice with phosphate-buffered saline (PBS). Then the medium containing FBS was replaced by fresh DMEM without FBS. OEC-conditioned medium was collected (48-h incubation) by a series of centrifugation steps (200g for 5 min; 1,000g for 10 min) and filtered through a 0.45-µm syringe (Invitrogen) to remove detached cells and cellular debris. OCM was stored in a low-temperature refrigerator (−80 °C) as an inductive agent of OM-MSCs.
Neuronal Differentiation of OM-MSCs
When OM-MSCs reached passage 4, the medium containing FBS was replaced by OCM after washing the cells twice with PBS; half of the medium was replaced every 2 d. Immunofluorescence was performed using standard protocols8 after being induced by OCM for 21 to 24 d. Briefly, after fixation and washing, the cultures were blocked with 10% normal goat or donkey serum in 0.3% Triton X-100 (Sigma-Aldrich) for 1 h at room temperature and then incubated with the primary antibody at 4 °C overnight. The following primary antibodies were used: monoclonal rabbit anti-III β-tubulin (anti-Tuj-1, 1:1,000; Abcam, Cambridge, United Kingdom) and monoclonal anti-tyrosine hydroxylase (anti-TH, 1:500; Abcam) for neurons. The cultures were then incubated with fluorescence-conjugated secondary antibodies for 1 h at room temperature and mounted with a coverslip and media containing 4′,6-diamidino-2-phenylindole (DAPI) (Beyotime, Hangzhou, China) to counterstain the nuclei. Images were taken with a fluorescence microscope (Carl Zeiss Axioskop2+, Jena, Germany).
Western Blot
Cells were dissolved with sodium dodecyl sulfate (SDS; Amresco, Solon, OH, USA) buffer (62.5 mM Tris–HCl, 10% glycerol, 2% SDS, and 50 mM dithiothreitol). The proteins were then transferred to polyvinylidene difluoride (PVDF) (Amresco) membranes. The blots were blocked in 4% bovine serum albumin (Amresco) in Tris-buffered saline/Tween-20 (Amresco) solution for 30 min at room temperature and then incubated at 4 °C overnight with the following primary antibodies: mouse monoclonal anti-P75 (Sigma-Aldrich), mouse monoclonal anti–glial fibrillary acidic protein (GFAP; Sigma-Aldrich), human monoclonal anti-HIF-1α (Sigma-Aldrich), human monoclonal anti-III beta-tubulin (Sigma-Aldrich), human monoclonal anti-TH (Sigma-Aldrich), human monoclonal anti-GFAP (Sigma-Aldrich), human monoclonal anti-nuclear receptor related 1 protein (Nurr1; Sigma-Aldrich), human monoclonal anti-pituitary homeobox 3 (Pitx3; Sigma-Aldrich), human monoclonal anti-Lmx1b (Sigma-Aldrich), and human monoclonal anti-actin (Sigma-Aldrich). After incubation with secondary antibodies at room temperature for 1 h, the blot was visualized using ChemiDoc XRS imaging system (Bio-Rad Laboratories, Hercules, CA, USA).
RNA Extraction and Quantitative Polymerase Chain Reaction
The total RNA was extracted from cells using the acid guanidinium isothiocyanate–phenol–chloroform method with TRIzol reagent (Sigma-Aldrich) and reverse-transcribed for complementary DNA (cDNA) synthesis with SuperScript III cDNA synthesis kit (Sigma-Aldrich). Each cDNA subpopulation was subjected to polymerase chain reaction (PCR) amplification using the specific primers. The sense and antisense primers for each gene were as follows: HIF-1α, human HIF-1α-F: 5′-AAGTGTACCCTAACTAGCCG-3′ and human HIF-1α-R: 5′-CACAAATCAGCACCAAGC-3′, product length: 160 bp; TH (tyrosine hydroxylase), human TH-F: 5′-AGGAGGTCTACACCACGCTGAAGGG-3′ and human TH-R: 5′-TGCACTGGAACACGCGGAAGG-3′, product length: 234 bp; actin, actin-F: 5′-CATCCTGCGTCTGGACCTGG-3′ and actin-R: 5′-TAATGTCACGCACGATTTCC-3′, product length: 107 bp; engrailed-1 (En1), human En1-F: 5′-CTGACTCGCAGCAGCCTCTCGT-3′ and human En1-R: 5′-GCCGCTTGTCCTCCTTCTCGTT-3′, product length: 126 bp; En2, human En2-F: 5′-GCTGAGCCTCAACGAGTCAC-3′ and human En2-R: 5′-TACTCGCTGTCCGACTTGCC-3′, product length: 162 bp; Nurr1, human Nurr1-F: 5′-GCCACTACGCACATGATCGAG-3′ and human Nurr1-R: 5′-AGCGCATCTGGCAACTAGACA-3′, product length: 109 bp; Pitx3, human Pitx3-F: 5′-GACTAGGCCCTACACACAGACCG-3′ and human Pitx3-R: 5′-TTTTGACAGTCCGCGCACGTT-3′, product length: 159 bp; LIM homeobox transcription factor 1-beta (Lmx1b), human Lmx1b-F: 5′-ACCAGCTGCTACTTCCGGGAT-3′ and human Lmx1b-R: 5′-CCCTTGCGTAGCTGCCGTTC-3′, product length: 185 bp; actin, actin-F: 5′-CATCCTGCGTCTGGACCTGG-3′ and actin-R: 5′-TAATGTCACGCACGATTTCC-3′, product length: 107 bp. The PCR products were mixed with a loading buffer (0.25% bromophenol blue, 0.25% xylene cyanol, and 40% sucrose; Sigma-Aldrich) and separated on 2% agarose gels. The data were analyzed using MxPro QPCR software (Thermo Fisher Scientific, Waltham, MA, USA).
Patch-Clamp Recording
Potassium currents of OM-MSCs after neural differentiation were recorded using a patch clamp under the room temperature environment described in our previous study.22 Briefly, pipettes with a tip resistance of 2 to 4 MΩ were made from borosilicate capillaries with a Brown–Flaming micropipette puller. The cells were accessed by the patch electrode using an infrapatch system. The cells and the recording electrode were recorded with a digital camera. The current signals were amplified with an EPC-9 amplifier with a low-pass filter at 10 kHz. Data collection and analysis were all performed with PulseFit + Pulse 810 (HEKA Elektronik, Lambrecht/Pfalz, Germany).
Enzyme-Linked Immunosorbent Assay (ELISA)
The supernatant liquors of the normal induction group (NI group), the hypoxia induction group (HI group), and the group with the addition of an HIF-1α inhibitor to the OM-MSCs under hypoxic conditions (HIR group) were collected, and the contents of DA, which were released, were detected in triplicate using a commercially available ELISA kit (BD Biosciences, New York, NJ, USA) according to the recommended protocol.
Statistical Analysis
All data were presented as mean ± standard error of the mean (SEM). Statistical significance was assessed with t tests or one-way analysis of variance (ANOVA) using Prism 4.0 software (GraphPad Software; Autodesk, Inc, San Rafael, CA, USA). P values less than 0.05 were considered as statistically significant differences and values less than 0.01 were considered as statistically significant differences.
Results
Characteristics of hOM-MSCs and mouse olfactory ensheathing cells
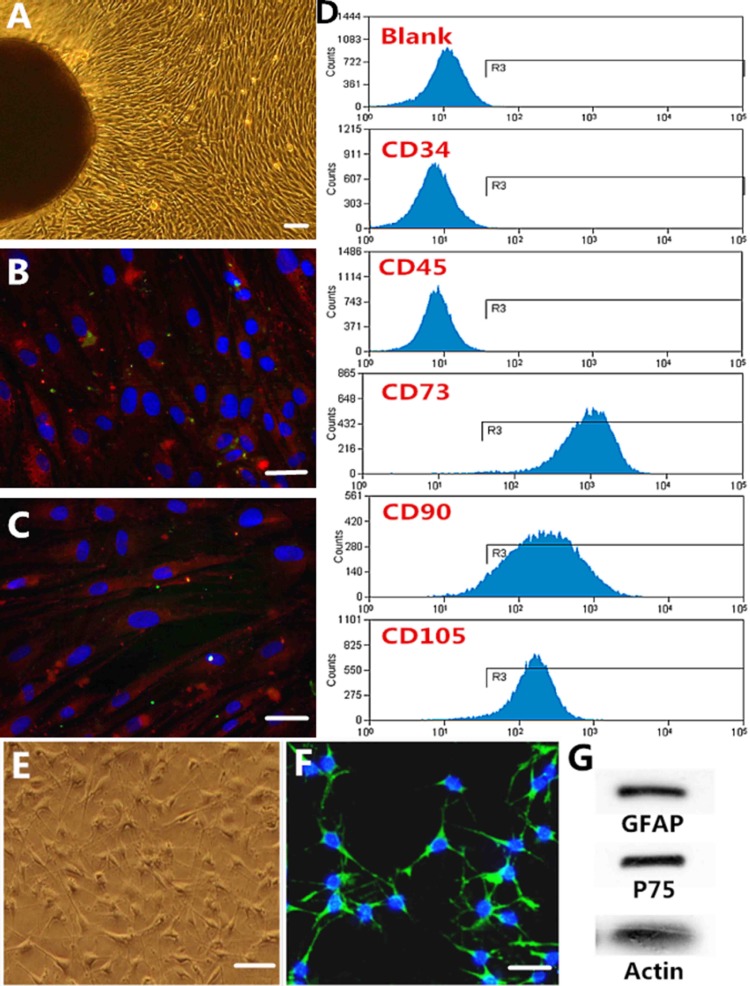
Tissue samples were obtained from the root of the medial aspect of the middle turbinate in patients undergoing endoscopic nasal surgery. The OM usually healed within 1 month after injury. Olfactory procedures had no effect on the patients’ sense of smell. Following the protocols, we successfully cultured MSC-like cells from the OM samples. Adherent cells migrated from the explants and most cells became spindle shaped after 6 to 8 d in culture (Fig. 1). After passaging, the cells grew rapidly and nuclear disintegration and cell division could be observed, indicating active proliferation. Moreover, purified cells were immunopositive for the characteristic OM-MSC markers stromal cell antigen-1 (STRO-1) and nestin (Fig. 1B, C). We determined the immunophenotyping profile of OM-MSCs by assessing cell surface antigens by flow cytometry. The cultured cells expressed the MSC markers CD73, CD90, and CD105, but not the hematopoietic cell markers CD34 and CD45 (Fig. 1D). The results suggested that these cells were mainly composed of MSCs.
Figure. 1.
Characterization of olfactory mucosa mesenchymal stem cells (OM-MSCs). (A) Adherent cells migrated from the explants and most cells became spindle shaped. (B and C) Immunocytochemistry of the characteristic markers of human OM-MSCs: nestin (B) and stromal cell antigen-1 (STRO-1; C). (D) Surface marker expression. Flow cytometric analysis of these cells showed that they express the MSC markers CD73, CD90, and CD105, but not CD34 and CD45, which are characteristic of hematopoietic cells. (E and F) Characterization of olfactory ensheathing cells (OECs; E) and immunocytochemistry of the characteristic markers of OECs: p75 (F). (G) Western blot of the OEC characteristic markers of glial fibrillary acidic protein (GFAP) and p75. A to F: Scale bar = 100 µm.
After 7 d of culture, the OECs grew vigorously and most of the cells showed bipolar or multipolar shapes with spindle-like morphology. Immunocytochemistry for the characteristic OECs markers showed positive expression of p75 (Fig. 1F). Western blot analysis of OECs showed positive immunoreactivity for nerve growth factor (NGF) receptor, p75, and GFAP (Fig. 1G).
Culture in Reduced O2 Promotes DAergic Neuronal Differentiation of OM-MSCs
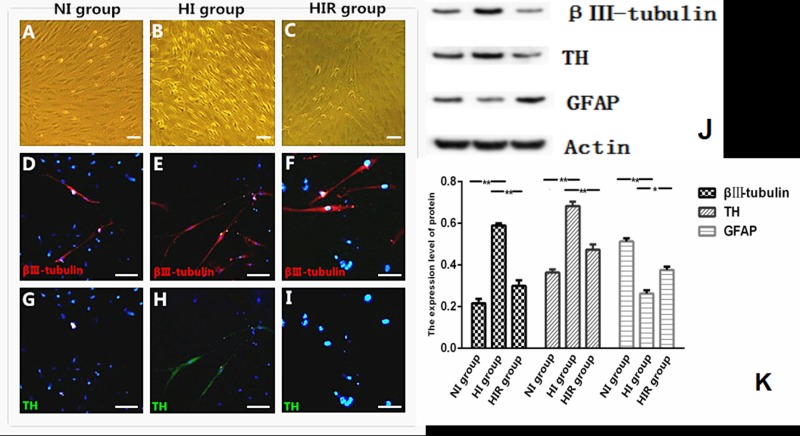
OM-MSCs cultured in DMEM supplemented with 10% FBS retained their proliferative and undifferentiated state for a prolonged period. However, after cultivation in OCM, OM-MSCs began to differentiate after 48 h, including demonstrating soma growth and synapse formation. Over the first week, cells progressively assumed the morphology of multipolar neurons with further morphological changes over the subsequent 7 d to yield network-like structures. By day 21 of induction, the cells in all 3 groups displayed neuron-like morphologies with long processes (Fig. 2).
Figure 2.
Hypoxia promoted neuronal differentiation more efficiently in vitro compared with routine culture group. A-C: Morphological evaluation of differentiated cells. D-I: representative images of Tuj-1 and TH immunostaining of N-I, H-I and H-I-R group. J: Western blot analysis of Tuj-1, TH and GFAP of three groups. K: quantitation of protein bands. *P < 0.05, **P < 0.01. A-I: Scale bar = 100 μm.
The differentiated cells expressed the neuron-specific class marker TUJ-1, the DAergic neuron-specific marker TH, and the astrocyte-specific marker GFAP (Fig. 2). TH is the rate-limiting enzyme in the production of DA and considered to be a basic marker for DAergic neurons. Compared with the NI and HIR groups, cells in the HI group had significantly higher proportions of TUJ-1-positive and TH-positive cells. Expression levels of TUJ-1 and TH were much higher in the HI group compared with the NI and HIR groups after 21 d of induction according to Western blotting, while GFAP expression was lower in HI than in the other 2 groups (Fig. 2).
Function of Terminally Differentiated DAergic Neurons
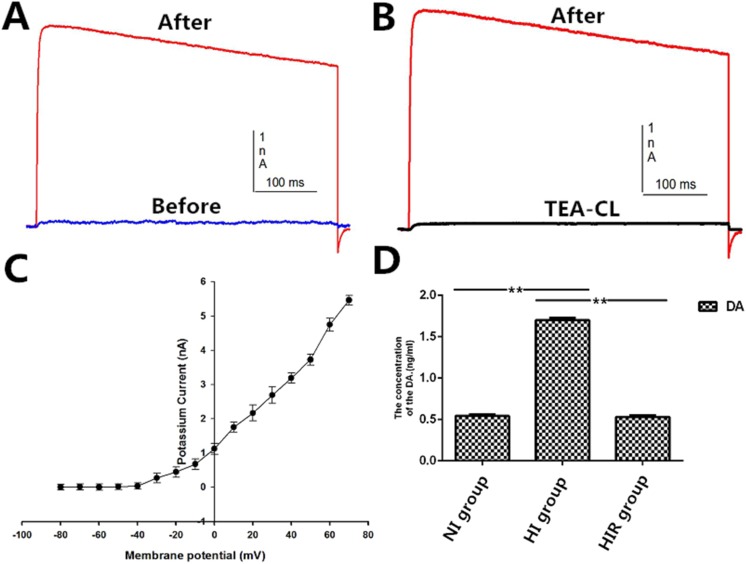
We characterized the function of the differentiated DAergic neurons induced from OM-MSCs in vitro and assessed DA release by ELISA. The DA levels were significantly increased in the HI group compared with the NI and HIR groups (1.70 ± 0.03 ng/mL vs. 0.54 ± 0.02 ng/mL vs. 0.53 ± 0.02 ng/mL, respectively; Fig. 3).
Figure 3.
Potassium currents and dopamine (DA) checked in differentiated neurons. (A) Depolarizing pulses evoked a slow outward current. (B) Potassium currents were blocked when tetraethylammonium chloride (TEA) was added into the extracellular solution. (C) Current–voltage curves (I-V curves). (D) Concentrations of DA that is released by differentiated cells. *P < 0.05. **P < 0.01.
We investigated the electrophysiological characteristics of OM-MSCs after neuronal differentiation induced by OCM. We measured voltage-gated potassium currents by whole-cell voltage clamping. Depolarizing pulses from −80 to 30 mV evoked a slow outward potassium current, which was blocked by the addition of tetraethylammonium chloride (TEA) to the extracellular solution. The I-V curves showed that the potassium currents were activated at −20 mV, with slow activation and deactivation (Fig. 3).
Involvement of HIF-1α in DAergic Neuronal Differentiation
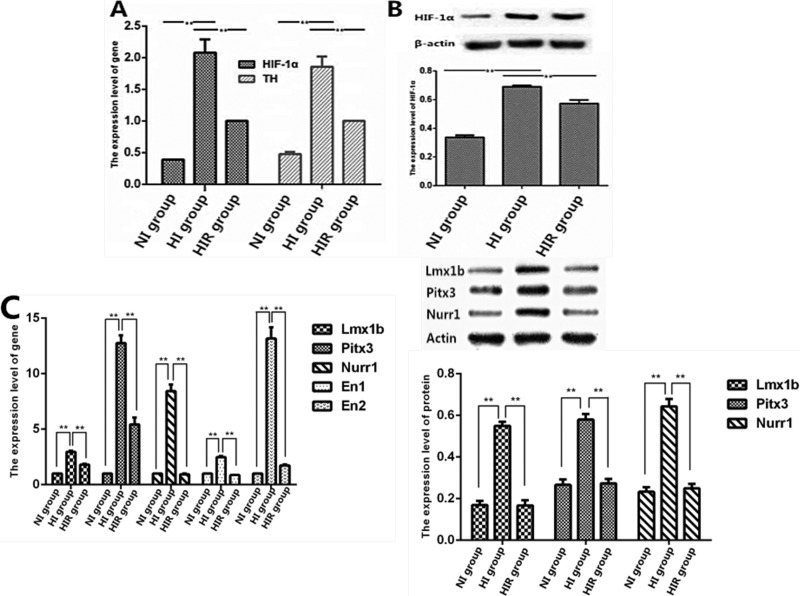
To clarify the mechanisms involved in neuronal differentiation under low-O2 conditions, we investigated the effects of hypoxia on OM-MSC differentiation. Expression levels of HIF-1α and TH were upregulated in the HI group compared with the NI and HIR groups (Fig. 4). These results indicated that upregulation of HIF-1α expression increased TH expression in OM-MSCs, while addition of a novel HIF-1α inhibitor decreased TH expression in differentiated cells. This suggested that upregulation of TH was closely related to enhanced expression of HIF-1α during neural differentiation of OM-MSCs under hypoxia (Fig. 4). In order to confirm their potential for DAergic neuronal differentiation, we examined some important key transcriptional factors involved in the development of DAergic neurons, including Lmx1b, Pitx3, Nurr1, En1, and En2. These genes were significantly upregulated in the HI group compared to the NI and HIR groups (Fig. 4C). Western blot further demonstrated overexpression of protein in the HI group, including Lmx1b, Pitx3, and Nurr1 (Fig. 4D). These results indicated that OCM under hypoxic conditions could promote OM-MSCs to differentiate into neurons, typically DAergic neurons.
Figure 4.
Quantitative polymerase chain reaction (Q-PCR) and Western blot analysis of hypoxia on expression of hypoxia-inducible factor-1α (HIF-1α) and tyrosine hydroxylase (TH). (A) Quantitative analysis of the HIF-1α and TH. (B) Representative experiment of Western blot analysis of HIF-1α and quantifications of HIF-1α expression. (C) The transcription levels of key transcriptional factors involved in the development of DAergic neurons (LIM homeobox transcription factor 1-beta [Lmx1b], pituitary homeobox 3 [Pitx3], nuclear receptor related 1 protein [Nurr1], engrailed 1 (En1), and En2) were detected by Q-PCR. (D) Western blot analysis of Lmx1b, Pitx3, and Nurr1. *P < 0.05. **P < 0.01.
The above results were in accord with the results for DA detection. The DA levels were higher in the HI group compared with the NI group (Fig. 3). Addition of an HIF-1α inhibitor to OM-MSCs under hypoxic conditions (HIR group) reduced DA expression in differentiated cells. These results indicated that neural differentiation of OM-MSCs in a low-O2 environment was associated with upregulation of HIF-1α expression and that HIF-1α may be involved in regulating the fate of DAergic neurons, given that the DAergic neural differentiation–promoting effect could be blocked by an HIF-1α inhibitor.
Discussion
OM-MSCs possess significant clonogenic activity and could be easily propagated for the purpose of PD therapies. Standard in vitro cell culture procedures include culture at 37 °C in an atmosphere of 5% CO2 and 95% air. However, O2 levels in cell culture in vitro are not adjusted to the normal physiological levels found in vivo. The mean O2 concentration under physiological conditions is 3%, which is much lower than that in ambient air.16 The O2 level in mammalian brain tissue ranges from 1% to 5% and is even lower in some brain regions, for example, O2 levels in mouse brain ranged from 0.55% in the mesencephalon to 8% at the surface.23 Furthermore, there is evidence to suggest that low-O2 levels may be important for the differentiation of stem cells in vitro. Morrison et al. suggested that O2 levels influenced the fate of neural crest stem cells,16 while Studer et al. indicated that reduced O2 levels also promoted the survival, proliferation, and catecholaminergic differentiation of CNS stem cells.24 In accord with these previous studies, our results demonstrated that decreased O2 levels promoted the commitment of OM-MSCs to the DAergic lineage in vitro. We characterized the function of DAergic neurons differentiated from OM-MSCs by measuring released DA by ELISA. Consistent with the results of differentiation, DA release was significantly elevated in the HI group compared with the NI and HIR groups. Overall, these results suggest that decreased O2 levels promote functional DAergic neuronal differentiation of OM-MSCs.
Although the mechanism whereby hypoxia promotes DAergic neuronal differentiation is uncertain and complex, our data suggested that the enhancement of TH expression in differentiated OM-MSCs by reduced O2 levels may be regulated by HIF-1. HIF-1 is a low-O2 sensor that plays a central role in response to hypoxia. It is a heterodimeric transcription factor composed of α and β subunits, with regulation of HIF-1 activity being largely dependent on the α subunit.25,26
HIF targets and regulates >1,000 genes, either directly or indirectly. HIF-1 markedly influences the expression levels of several genes encoding transcription factors, enzymes, receptors, receptor-associated kinases, and differentiation factors in various cell types, thus supporting a potential role for HIF in the hypoxia-induced promotion of OM-MSC differentiation into DAergic lineage cells. Previous studies showed that HIF-1α was the main regulator controlling the metabolic fate and multipotency of MSCs.27,28 Stable expression of HIF-1α in MSCs led to induction of octamer-4 and Kruppel-like factor 4 (KLF4) and influenced terminal differentiation.29,30 In terms of CNS-derived stem cells, hypoxia has been indicated to promote DAergic neuronal differentiation through the activation of HIF-1α,31,32 though the mechanism whereby hypoxia affected DAergic commitment was complex. Consistent with previous studies, our results suggested that hypoxia enhanced the number of TH-positive cells and increased DA release by OM-MSCs in vitro, mediated by HIF-1α. This was supported by the blocking of this positive effect by the HIF-1α inhibitor YC-1. Furthermore, our study examined some important key genes involved in the development of DAergic neurons, including Lmx1b, Pitx3, Nurr1, En1, and En2. These genes were significantly upregulated in the HI group compared to the NI and HIR groups. Western blot further demonstrated overexpression of proteins in the HI group, including Lmx1b, Pitx3, and Nurr1. These results indicated that OCM under hypoxic conditions could significantly upregulate key transcriptional factors involved in the development of DAergic neurons of OM-MSCs, mediated by HIF-1α.
In the current study, we cultured OECs and collected OCM for DAergic induction of OM-MSCs. OECs have previously been reported to secrete a variety of neurotrophins and matrix molecules related to nerve regeneration and growth, such as nerve growth factor, brain-derived neurotrophic factor (BDNF), glial cell line–derived neurotrophic factor (GDNF), and neurotrophin,33,34 which have been found to induce and promote the differentiation of NSCs into neurons.35–37 Moreover, some reports showed that OECs could promote NSCs to differentiate into DAergic neurons and cholinergic neurons.38,39 The expression of neuron-like sodium and potassium currents in in vitro–differentiated OM-MSCs can be considered as strong evidence that OM-MSCs could turn into electrophysiologically active neurons when exposed to appropriate neuronal differentiating conditions. We previously showed that neurons derived from NSCs had active electrophysiological properties. Not only did the neurons exhibit typical voltage-dependent transient inward sodium currents that were blocked by tetrodotoxin, but they also displayed voltage-dependent slowly rectifying outward currents that were blocked by TEA.40 In the current study, we measured voltage-gated sodium and potassium currents in differentiated OM-MSCs by whole-cell voltage clamping but only detected slow outward potassium currents, and no sodium currents were detected in differentiated OM-MSCs.
Overall, the results of this study suggest that the induction of OM-MSCs by OCM under hypoxic culture conditions improves the production of DAergic neurons. Moreover, HIF-1 appears to play an important role in hypoxia-inducible pathways in DAergic-lineage specification and differentiation in vitro. This novel induction protocol is simple, effective, and safe and could significantly increase the DAergic neuronal differentiation potential of OM-MSCs, thus representing an important development in the treatment of PD.
Footnotes
Author Contribution: Yi Zhuo, Lei Wang, and Lite Ge contributed equally to this work.
Ethical Approval: This study was approved by ethics committee of Hunan Normal University.
Statement of Human and Animal Rights: All surgical operations were executed according to the Chinese legislations involving animal protection and approved by the Ethics Committee of Hunan Normal University.
Statement of Informed Consent: The use of human nasal mucosa biopsy tissues was permitted by the Ethical Committee of Hunan Normal University and the patients gave written informed consent.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: This project was supported by grants from the National Natural Science Foundation of China (grant no. 81360190 to Y.X. and grant no. 81371358 to M.L.), Hunan Provincial Natural Science Foundation of China (grant/award no. 14JJ2060), and Hunan Provincial Innovation Foundation for Postgraduate (no. CX2015B188).
References
- 1. Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson’s disease. Annu Rev Neurosci. 1999;22:123–144. [DOI] [PubMed] [Google Scholar]
- 2. Young W, AlZoubi ZM, Saberi H, Sharma A, Muresanu D, Feng S, Chen L, Huang H. Beijing declaration of international association of neurorestoratology. J Neurorestoratol. 2015;3:121–122. [Google Scholar]
- 3. Huang H, Mao G, Chen L, Liu A. Progress and challenges with clinical cell therapy in neurorestoratology. J Neurorestoratol. 2015;3:91–95. [Google Scholar]
- 4. Huang H, Sharma HS. Neurorestoratology: one of the most promising new disciplines at the forefront of neuroscience and medicine. J Neurorestoratol. 2013;1:37–41. [Google Scholar]
- 5. Huang H, Chen L, Sanberg PR. Clinical achievements, obstacles, falsehoods, and future directions of cell-based neurorestoratology. Cell Transplant. 2012;21(Suppl 1):S3–S11. [DOI] [PubMed] [Google Scholar]
- 6. Chen C, Duan J, Shen A, Wang W, Song H, Liu Y, Lu X, Wang X, You Z, Han Z, et al. Transplantation of human umbilical cord blood-derived mononuclear cells induces recovery of motor dysfunction in a rat model of Parkinson’s disease. J Neurorestoratol. 2016;4:23–33. [Google Scholar]
- 7. Chen L, Huang HY, Duan WM, Mao GS. Clinical neurorestorative progress in Parkinson’s disease. J Neurorestoratol. 2015;3:101–107. [Google Scholar]
- 8. Ge L, Jiang M, Duan D, Wang Z, Qi L, Teng X, Zhao Z, Wang L, Zhuo Y, Chen P, et al. Secretome of olfactory mucosa mesenchymal stem cell, a multiple potential stem cell. Stem Cells Int. 2016:1243659 doi:10.1155/2016/1243659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Murrell W, Wetzig A, Donnellan M, Féron F, Burne T, Meedeniya A, Kesby J, Bianco J, Perry C, Silburn P, et al. Olfactory mucosa is a potential source for autologous stem cell therapy for Parkinson’s disease. Stem Cells. 2008;26(8):2183–2192. [DOI] [PubMed] [Google Scholar]
- 10. Murrell W, Féron F, Wetzig A, Cameron N, Splatt K, Bellette B, Bianco J, Perry C, Lee G, Mackay-Sim A. Multipotent stem cells from adult olfactory mucosa. Dev Dyn. 2005;233(2):496–515. [DOI] [PubMed] [Google Scholar]
- 11. Delorme B, Nivet E, Gaillard J, Häupl T, Ringe J, Devèze A, Magnan J, Sohier J, Khrestchatisky M, Roman FS, et al. The human nose harbors a niche of olfactory ectomesenchymal stem cells displaying neurogenic and osteogenic properties. Stem Cells Dev. 2010;19(6):853–866. [DOI] [PubMed] [Google Scholar]
- 12. Boone N, Bergon A, Loriod B, Devèze A, Nguyen C, Axelrod FB, Ibrahim EC. Genome-wide analysis of familial dysautonomia and kinetin target genes with patient olfactory ecto-mesenchymal stem cells. Hum Mutat. 2012;33(3):530–540. [DOI] [PubMed] [Google Scholar]
- 13. Guo Z, Draheim K, Lyle S. Isolation and culture of adult epithelial stem cells from human skin. J Vis Exp. 2011;(49):2561 doi:10.3791/2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Toft A, Tomé M, Lindsay SL, Barnett SC, Riddell JS. Transplant-mediated repair properties of rat olfactory mucosal OM-I and OM-II sphere-forming cells. J Neurosci Res. 2012;90(3):619–631. [DOI] [PubMed] [Google Scholar]
- 15. Nivet E, Vignes M, Girard SD, Pierrisnard C, Baril N, Devèze A, Magnan J, Lanté F, Khrestchatisky M, Féron F, Roman FS, et al. Engraftment of human nasal olfactory stem cells restores neuroplasticity in mice with hippocampal lesions. J Clin Invest. 2011;121(7):2808–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morrison SJ, Csete M, Groves AK, Melega W, Wold B, Anderson DJ. Culture in reduced levels of oxygen promotes clonogenic sympathoadrenal differentiation by isolated neural crest stem cells. J Neurosci. 2000;20(19):7370–7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Storch A, Paul G, Csete M, Boehm BO, Carvey PM, Kupsch A, Schwarz J. Long-term proliferation and dopaminergic differentiation of human mesencephalic neural precursor cells. Exp Neurol. 2001;170(2):317–325. [DOI] [PubMed] [Google Scholar]
- 18. Wang Y, Yang J, Li H, Wang X, Zhu L, Fan M, Wang X. Hypoxia promotes dopaminergic differentiation of mesenchymal stem cells and shows benefits for transplantation in a rat model of Parkinson’s disease. PLoS One. 2013;8(1):e54296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Du F, Wu XM, Gong Q, He X, Ke Y. Hyperthermia conditioned astrocyte-cultured medium protects neurons from ischemic injury by the up-regulation of HIF-1 alpha and the increased anti-apoptotic ability. Eur J Pharmacol. 2011;666(1–3):19–25. [DOI] [PubMed] [Google Scholar]
- 20. Zhu L, Wu XM, Yang L, Du F, Qian ZM. Up-regulation of HIF-1alpha expression induced by ginkgolides in hypoxic neurons. Brain Res. 2007;1166:1–8. [DOI] [PubMed] [Google Scholar]
- 21. Wang L, Jiang M, Duan D, Zhao Z, Ge L, Teng X, Liu B, Liu B, Chen P, Lu M. Hyperthermia-conditioned OECs serum-free-conditioned medium induce NSC differentiation into neuron more efficiently by the upregulation of HIF-1 alpha and binding activity. Transplantation. 2014;97(12):1225–1232. [DOI] [PubMed] [Google Scholar]
- 22. Zeng Y, Rong M, Liu Y, Liu J, Lu M, Tao X, Li Z, Chen X, Yang K, Li C, et al. Electrophysiological characterisation of human umbilical cord blood-derived mesenchymal stem cells induced by olfactory ensheathing cell-conditioned medium. Neurochem Res. 2013;38(12):2483–2489. [DOI] [PubMed] [Google Scholar]
- 23. Erecińska M, Silver IA. Tissue oxygen tension and brain sensitivity to hypoxia. Respir Physiol. 2001;128(3):263–276. [DOI] [PubMed] [Google Scholar]
- 24. Studer L, Csete M, Lee SH, Kabbani N, Walikonis J, Wold B, McKay R. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J Neurosci. 2000;20(19):7377–7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Perez-Pinzon MA. Mechanisms of neuroprotection during ischemic preconditioning: lessons from anoxic tolerance. Comp Biochem Physiol A Mol Integr Physiol. 2007;147(2):291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ma J, Li J, Wang KS, Mi C, Piao LX, Xu GH, Li X, Lee JJ, Jin X. Perillyl alcohol efficiently scavenges activity of cellular ROS and inhibits the translational expression of hypoxia-inducible factor-1α via mTOR/4E-BP1 signaling pathways. Int Immunopharmacol. 2016;39:1–9. [DOI] [PubMed] [Google Scholar]
- 27. Buravkova LB, Andreeva ER, Gogvadze V, Zhivotovsky B. Mesenchymal stem cells and hypoxia: where are we? Mitochondrion. 2014;19 Pt A:105–112. [DOI] [PubMed] [Google Scholar]
- 28. Lampert FM, Kütscher C, Stark GB, Finkenzeller G. Overexpression of Hif-1α in mesenchymal stem cells affects cell-autonomous angiogenic and osteogenic parameters. J Cell Biochem. 2016;117(3):760–768. [DOI] [PubMed] [Google Scholar]
- 29. Palomäki S, Pietilä M, Laitinen S, Pesälä J, Sormunen R, Lehenkari P, Koivunen P. HIF-1α is upregulated in human mesenchymal stem cells. Stem Cells. 2013;31(9):1902–1909. [DOI] [PubMed] [Google Scholar]
- 30. Park IH, Kim KH, Choi HK, Shim JS, Whang SY, Hahn SJ, Kwon OJ, Oh IH. Constitutive stabilization of hypoxia-inducible factor alpha selectively promotes the self-renewal of mesenchymal progenitors and maintains mesenchymal stromal cells in an undifferentiated state. Exp Mol Med. 2013;45:e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stacpoole SR, Bilican B, Webber DJ, Luzhynskaya A, He XL, Compston A, Karadottir R, Franklin RJ, Chandran S. Efficient derivation of NPCs, spinal motor neurons and midbrain dopaminergic neurons from hESCs at 3% oxygen. Nat Protoc. 2011;6(8):1229–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Milosevic J, Maisel M, Wegner F, Leuchtenberger J, Wenger RH, Gerlach M, Storch A, Schwarz J. Lack of hypoxia-inducible factor-1 alpha impairs midbrain neural precursor cells involving vascular endothelial growth factor signaling. J Neurosci. 2007;27(2):412–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Woodhall E, West AK, Chuah MI. Cultured olfactory ensheathing cells express nerve growth factor, brain-derived neurotrophic factor, glia cell line-derived neurotrophic factor and their receptors. Brain Res Mol Brain Res. 2001;88(1–2):203–213. [DOI] [PubMed] [Google Scholar]
- 34. Lipson AC, Widenfalk J, Lindqvist E, Ebendal T, Olson L. Neurotrophic properties of olfactory ensheathing glia. Exp Neurol. 2003;180(2):167–171. [DOI] [PubMed] [Google Scholar]
- 35. Choi KC, Yoo DS, Cho KS, Huh PW, Kim DS, Park CK. Effect of single growth factor and growth factor combinations on differentiation of neural stem cells. J Korean Neurosurg Soc. 2008;44(6):375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pellitteri R, Spatuzza M, Russo A, Zaccheo D, Stanzani S. Olfactory ensheathing cells represent an optimal substrate for hippocampal neurons: an in vitro study. Int J Dev Neurosci. 2009;27(5):453–458. [DOI] [PubMed] [Google Scholar]
- 37. Al-Zoubi A, Altwal F, Khalifeh F, Hermas J, Al-Zoubi Z, Jafar E, El-Khateeb M. Ex vivo differentiation of human bone marrow-derived stem cells into neuronal cell-like lineages. J Neurorestoratol. 2016;4:35–44. [Google Scholar]
- 38. Wang L, Duan D, Zhao Z, Teng X, Liu B, Ge L, Lu M. [Differentiation of C17.2 neural stem cells into neural cells induced by serum-free conditioned medium of olfactory ensheathing cells and cell viability detection of differentiated cells]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2014;28(5):633–638. [PubMed] [Google Scholar]
- 39. Srivastava N, Seth K, Khanna VK, Ansari RW, Agrawal AK. Long-term functional restoration by neural progenitor cell transplantation in rat model of cognitive dysfunction: co-transplantation with olfactory ensheathing cells for neurotrophic factor support. Int J Dev Neurosci. 2009;27(1):103–110. [DOI] [PubMed] [Google Scholar]
- 40. Duan D, Rong M, Zeng Y, Teng X, Zhao Z, Liu B, Tao X, Zhou R, Fan M, Peng C, et al. Electrophysiological characterization of NSCs after differentiation induced by OEC conditioned medium. Acta Neurochir (Wien). 2011;153(10):2085–2090. [DOI] [PubMed] [Google Scholar]