Abstract
Spinal cord injury (SCI) is a widely disabling condition, constraining those affected by it to wheelchairs and requiring intense daily care and assistance. Cell replacement therapies, targeting regeneration of cells in the injured cord, are currently gaining momentum in the field of SCI research. Previous studies indicate that mesenchymal stem cells (MSCs) can reduce functional deficits through immunomodulation and production of trophic factors in a variety of neurological disorders. The present study assessed the efficacy of transplanted bone marrow–derived MSCs at different concentrations and locations for promoting functional recovery following SCI. Although effects were modest, MSCs facilitated an increase in the base of support, as measured by increased distance between the plantar surface of the hind paws, following incomplete contusive SCI, and reduced the density of astroglial scarring. Varying the concentrations or locations of transplanted cells did not provide additional benefits on these measures. These findings indicate that MSC transplants are safe at relatively high concentrations and confer therapeutic benefits that, when used as an adjunctive treatment, could significantly enhance functional recovery following SCI.
Keywords: spinal cord injury (SCI), mesenchymal stem cell (MSC), cell therapy, functional recovery
Introduction
Spinal cord injury (SCI) in humans is the second leading cause of paralysis in the United States, with as many as 12,000 new cases of SCI occurring each year in the United States alone,1 and an estimated 1,275,000 people currently living with SCI.2 Individual cases and their symptoms are highly variable based on the level and severity of the injury, though nearly all patients suffering from SCI demonstrate full or partial loss of mobility. Spontaneous recovery is possible to some degree in humans as well as animals.3 However, it is greatly limited and depends on the magnitude of the injury and its effects on the motor, sensory, and autonomic systems. Cell therapy is a category of treatment currently gaining momentum in the field of SCI research, showing promise in animal studies as well as human trials, though safety concerns still limit its use in the clinic. As such, safety and efficacy by this therapeutic approach must be assessed comprehensively in animal models before extensive testing begins in humans.
Human SCI is categorized according to its gross morphology, including solid cord injuries, contusive or cavity injuries, laceration or transection injuries, and compressive injuries.4 Most clinical cases of SCI are a result of multiple modes of injury that occur simultaneously, such as contusive forces at the moment of injury, compression on the cord from collapsed vertebrae, and lacerations that occur from bone fragments. However, when modeling SCI in rats, contusive injuries appear to best represent the pathology that is observed in humans. Following a contusive SCI, the surface of the cord appears undisturbed and the dura remains intact, with internal lesions developing first at the epicenter of injury and spreading outward in the rostral and caudal directions as a consequence of propagating necrosis. Following the initial insult, hemorrhaging of blood vessels results in hypoxia and a strong inflammatory response that leads to a secondary cascade of damage as the body attempts to limit further damage.5 This secondary cascade leads to the pathologies most common in cases of SCI: cell death/axonal dieback, demyelination, inflammation, glial scarring, and more.4,6–11 The multifaceted and highly variable aspect of SCI makes treatment difficult, as each individual case may have differing pathology based on the level, degree, and type of injury sustained, thus requiring therapeutic techniques that are specifically tailored to the individual.
After sustaining trauma, the spinal cord produces a physical barrier against the immune cascade, which is known as the glial scar. As its name suggests, the glial scar consists mainly of glial cells, including astrocytes, and extracellular matrix (ECM) proteins, such as proteoglycans and other glycoproteins.4 One of the major inhibitory components of the glial scar is the presence of chondroitin sulfate proteoglycans (CSPGs), which are known to block the growth of neurites in in vitro experiments12 as well as interfering with axonal sprouting and graft cell survival in animal models of SCI.3,10,13 Levels of CSPGs are highest in the lesion epicenter and may be broken down by the enzyme chondroitinase ABC (ChABC) before cell replacement therapies are attempted.3,13
Numerous types of stem cells from varying sources have been tested for efficacy in facilitating functional recovery from SCI. Among them, olfactory ensheathing cells, in addition to facilitating axonal sprouting, produced improved scores in motor-evoked potentials in the brain in a rat model of SCI, indicating reconnectivity across the lesion site.14,15 Similarly, neural precursor cells (NPCs) transplanted into the white matter surrounding the lesion have been shown to improve functional recovery in a rat model of SCI, as well as differentiate into oligodendrocyte lineages without producing tumorigenesis or neuropathic pain,7 and were able to survive and differentiate in the chronic phase as well as the subacute time point.13
Mesenchymal stem cells (MSCs) are obtained from tissues of mesodermal origins, can be found in fat, blood, and bone marrow, and have shown promising results in the treatment of many degenerative and traumatic conditions, including Huntington's disease,16 Alzheimer's disease,17 stroke,18 and SCI.19 These cells show properties such as low immunogenicity, secretion of trophic support factors, and the ability to differentiate into multiple lineages.16,19 MSCs transplanted into the human spinal cord have shown beneficial effects, including the return of bladder and bowel control in chronically injured individuals, and continued to improve function with no adverse findings for more than 2 y posttransplant using a small dose size.19 While some degree of neuropathic pain was reported in human trials, there was no confirmation that this pain was related to cellular transplants, but may instead be a part of the normal course of SCI recovery, as hyperalgesia is a common complication reported by post-SCI patients. Together, these studies support the idea that MSCs may be a highly beneficial treatment for SCI with few, if any, unfavorable outcomes.
Systematic assessment of concentrations and locations of transplanted MSCs, and their effects on functional recovery after SCI, has not been previously explored. Previous studies have transplanted cell concentrations ranging from 2.5 × 104 to 5 × 105 NPCs per 2 μL of vehicle13 or as low as 3 to 4 × 105 NPCs in 8 μL of vehicle.7 Although each concentration appears to provide significant benefits to the injured cord, direct comparisons are lacking, though higher concentrations on NPCs resulted in the same number of transplanted cells as lower concentrations.13 Similarly, comparisons of what location for transplanting MSCs will produce optimal benefits are also lacking.
There has been much debate as to whether the lesion epicenter in SCI is inhospitable to transplanted cells, thereby reducing cell survivability and rendering interlesion transplants ineffective.7 However, others have demonstrated significant behavioral improvement when cells were transplanted into the core of the lesion.11,13,20 Conceptually, cells transplanted within the lesion epicenter will release trophic factors that encourage growth from both the rostral and caudal ends of the lesion. Clinical trials in humans have distributed a small concentration of autologous cells, combining bone marrow-derived MSCs with Schwann cells, among the central injury site and the intact regions above and below the injury.19 Although transplant size was small, cell viability was high at 98%, and 5 of 8 patients reported some return of function.
Based on these findings, the current study sought to determine what specific distribution of cells, and what cell concentrations, may provide the greatest behavioral outcome in measures of functional recovery. Three different cell concentrations were tested, as well as 5 locations (Fig. 1), with the addition of a lesion-only control, which received an intraspinal injection of vehicle only. Measures of functional recovery, including the Basso, Beattie, and Bresnahan (BBB) scale for locomotor assessment,21 and gait analysis, as well as assessments of the effects of MSCs on glial scarring and demyelination were taken.
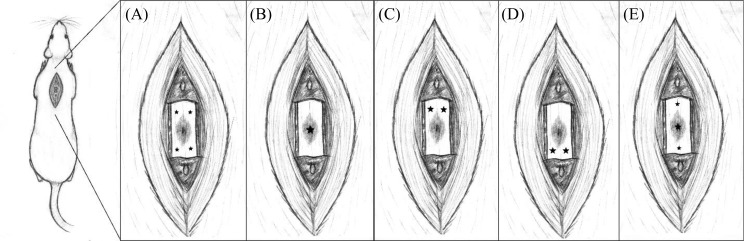
Fig. 1.
Transplant locations in regard to the lesion epicenter. Cell transplants were distributed among the starred points at the following volumes: (A) penumbra, 1 μL injected per starred site; (B) epicenter, 4 μL injected into the lesion core; (C) rostral bilateral, 2 μL injected per site; (D) caudal bilateral, 2 μL per site; (E) distributed, 1 μL rostral, 2 μL at epicenter, and 1 μL caudal.
Materials and Methods
Animal Subjects and Design
Sprague-Dawley (SD) rats at approximately 3 mo of age within a weight range of 250 to 300 g at the day of injury were utilized in this study. Animals were maintained on daylight cycle (lights on 08:00) in standard housing conditions and given ad libitum access to food and water for the duration of the study. All animals were housed in groups of 2 to 3 rats per cage to eliminate differences in social interaction and provided with Nylabone toys as enrichment (Bio-Serv, Flemington, NJ, USA). All procedures were in accordance with the university’s Institutional Animal Care and Use Committee (IACUC) approved protocols.
As individual variability is high in spinal cord injured rats, animals were counterbalanced according to scores on the BBB assessment 1 wk postinjury before being placed into experimental groups on the day of transplantation surgery. Experimental groups were arranged in a 2 × 2 factorial design, with the primary factors of the study being location of the transplant and concentration of cells transplanted (Table 1).
Table 1.
Multifactorial Design of Experimental Groups by Dose Size and Transplant Location.a
| Location Concentration | Penumbra: 1 μL Each Bilateral | Epicenter: 4 μL | Rostral: 2 μL Bilateral | Caudal: 2 μL Bilateral | Distributed: 1 μL Rostral, 2 μL Epicenter, 1 μL Caudal |
|---|---|---|---|---|---|
| Low: 25,000 cells/μL | Pen-Low | Epi-Low | Rostral-Low | Caudal-Low | Dist-Low |
| n = 6 | n = 5 | n = 5 | n = 5 | n = 6 | |
| 5.17 | 5.20 | 6.00 | 7.00 | 3.83 | |
| Med: 50,000 cells/μL | Pen-Med | Epi-Med | Rostral-Med | Caudal-Med | Dist-Med |
| n = 6 | n = 6 | n = 5 | n = 6 | n = 5 | |
| 5.50 | 5.17 | 4.60 | 5.67 | 5.40 | |
| High: 100,000 cells/μL | Pen-High | Epi-High | Rostral-High | Caudal-High | Dist-High |
| n = 5 | n = 4 | n = 5 | n = 5 | n = 5 | |
| 4.00 | 4.00 | 5.80 | 5.20 | 5.00 |
Abbreviation: BBB = Basso, Beattie, and Bresnahan scale for locomotor assessment.
aNumbers represent the sample size and group mean in BBB scores at 1 wk postinjury.
Stem Cell Acquisition and Culture
One healthy adult male rat was utilized as the source of cells for this study. The use of a single cell donor was implemented in order to more accurately reflect a method of using tissue bank allografts, rather than the costly method of individually-tailored autografts. The rat was humanely euthanized via CO2 asphyxiation, and the femurs cleanly excised. A small hole was bored in the distal end of each bone, and the marrow aspirated gently with a small amount of culture media (α-minimum essential medium (MEM) containing 10% fetal bovine serum (FBS), 10% horse serum, and 1% penicillin/streptomycin; (Life Technologies, Grand Island, NY, USA), followed by plating on a 25 cm2 cell culture flask with 5 mL of culture media. Cells were allowed to adhere to the plastic for at least 5 days, after which the media and debris were removed, adhering cells washed in sterile 0.1 M phosphate-buffered saline (PBS), and fresh media added. Cells were then allowed to expand in culture until confluent, defined here as coating approximately 80% of the plate surface, at which point they were either passaged or prepared for transplantation. Cells were passaged 3 to 8 times before being prepared for transplantation, in order to maximize the expression of beneficial cytokines22 and provide optimal biochemical support to the injured host tissue. To prepare cells for transplantation, cells were first incubated in a 1:500 dilution of Hoechst solution (Life Technologies) for 10 min to label the nuclei of transplanted cells, examined under fluorescent microscopy (Carl Zeiss Microimaging, LLC; Thornwood, NY, USA) for verification, then thoroughly washed in sterile PBS, detached, centrifuged, and counted as before. From this point, cells were centrifuged at 300g for 10 minutes again and the supernatant removed, then the pellet resuspended to the concentrations described above in Hank’s balanced salt solution (HBSS) containing calcium chloride and magnesium chloride (Life Technologies). This tube was then transported on ice to the surgical suite for transplantation.
Behavioral Assessments
BBB scale for locomotor assessment
Established by Basso, Beattie, and Bresnahan in 1995, the BBB is the most commonly used assessment for locomotor qualities in the injured rat spinal cord. Animals were first placed in a clean, empty wading pool and allowed to explore for 4 min while video systems recorded their behavior. Two or more raters, who remained blind to the experimental group of the rat, observed the range of motion in hind limb joints, weight support, stepping patterns, and other qualities of ambulation in order to assign each animal a number according to the standardized BBB scale.21 Testing was repeated weekly for the duration of the study.
Footprint analysis
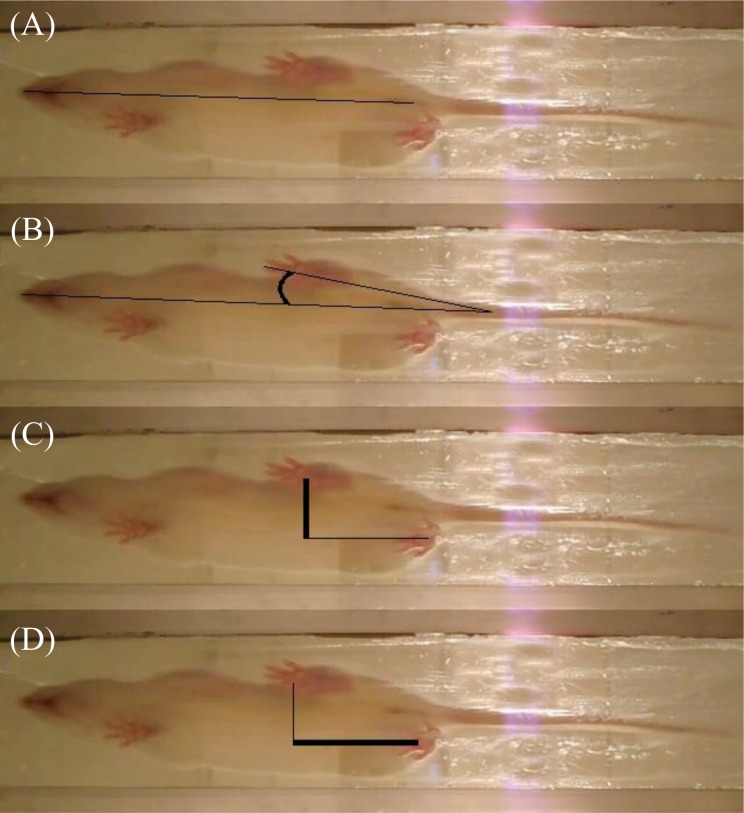
Following SCI, animals that attain advanced, near-baseline recovery scores on the BBB may still demonstrate altered footprint qualities. Adapted from the protocol of de Medinaceli et al.20 rats were enticed to attain a sweetened food treat by crossing a clear Plexiglas tunnel suspended between 2 raised platforms, while video surveillance recorded their footsteps from below. This allowed for frame-by-frame analysis, which included step length, as measured by the distance between the plantar surface of the hind paws parallel to the direction of gait, base of support, as measured by the distance between the plantar surface of the hind paws perpendicular to the direction of gait, and angle of rotation, as measured from the direction of gait to the projection of the third toe (Fig. 2).
Fig. 2.
Footprint analysis variables. Images were calibrated to the size of the rat (A), and tracked for (B) the angle of paw rotation, as measured by the deviation of the third toe from the direction of gait, (C) the base of support, as measured by the distance between the center of the plantar surfaces of the hind paws, and (D) the step length, as measured by the distance between the front of the plantar surfaces of the hind paws.
Contusive Injury and MSC Transplantation
Surgical procedures were adopted from the Multicenter Animal Spinal Cord Injury Study (MASCIS) Spinal Cord Contusion Model.23 Based on information from pilot animals, the present study utilized a contusive height of 18.75 mm, producing an incomplete SCI of moderate severity. Animals were anesthetized and maintained with a flow of 1% to 3% isoflurane (Vet One; Boise, ID, USA) in 0.5 to 1 L oxygen, administered through a nose cone. The backs of the animals were shaved and disinfected with chlorhexidine gluconate (Hibiclens; Norcross, GA, USA), followed by subcutaneous administration of buprenorphine (0.02 mg/kg; Med Vet International, Mettawa, IL, USA). The animals were then draped over a rolled, sterile surgical towel approximately 3 cm in diameter in order to create a gentle curvature of the spine. The rat’s prominent second thoracic (T2) spinous process was used as a reference mark to locate vertebral processes during surgical exposure. The location of the eighth thoracic vertebra (T8) was labeled on the skin, and the midline on the back cut from T6 to T9 to expose the ligaments over spinal processes in the midline and the underlying fascia. The muscle and other connective tissues were then sharply transected and any observed bleeding staunched with cauterization and/or sterile gauze. Once the vertebral process had been exposed, a laminectomy removed the dorsal processes from the T8 vertebra, leaving the dura over the spinal cord intact. Sterile bone clamps attached to the device held the spinous processes of the T7 and T9 vertebrae, to prevent the vertebrae from moving during spinal cord contusion.
The impactor was calibrated by lowering the plated tip onto the exposed cord until it just contacted the dura. It was then raised to the designated height of 18.75 mm and dropped onto the cord. Impact was confirmed by observation of a spasm in the lower limbs of the animal. The bone clamps were then released and moved out of the surgical field, the surgical site was rinsed with sterile saline, and paravertebral muscles sutured using an interrupted suture pattern with 4-0 polypropylene sutures, one above and one below the laminectomy site, to close bony deficiency over T8, followed by skin closure with 9-mm autoclips and application of topical lidocaine (Akorn Inc.; Lake Forest, IL, USA). The animal was then placed onto a heating pad maintained at 37°C, given meloxicam (1 mg/kg) (Putney Inc.; Portland, ME, USA) for prophylactic analgesia, and monitored until regaining consciousness and displaying normal eating behaviors. The rat was then returned to a clean cage, monitored closely, and provided with daily postoperative care.
Anesthesia and surgical preparation for MSC transplantation took place using the same protocols as described above. Animals were placed in a standard stereotaxic frame, and a midline incision was made in the same location as before. The muscles and connective tissues were carefully transected to expose the T8 spinal cord, using the previously placed sutures to identify the correct location and thereby limit additional tissue damage. Injections of either MSCs or vehicle (HBSS) took place using a 26-gauge Hamilton microsyringe (Hamilton Company, Reno, NV, USA), which was angled at approximately 30°, with the bevel facing upward to facilitate passage through the dura. The needle was placed to a depth at which the bevel was no longer visible, and cell suspensions were injected slowly at a rate of 0.33 µL/min, to a total volume of 4 µL. Vehicle-only injections were performed at the same locations as the distributed group in order to better translate to the methods of current clinical trials and introduced the same volume of vehicle as cell transplant groups. The cell concentrations tested for the present study included 25,000 cells/μL of vehicle as the lowest concentration, 50,000 cells/μL of vehicle as the medium concentration, and 100,000 cells/μL of vehicle as the highest concentration. Upon completion of the injection, the needle was left in place for 3 min and then removed slowly to prevent the backflow of fluid into the syringe. The animals were then sutured as before and received the same intensive postoperative care.
Postoperative monitoring continued for 7 to 10 days after all surgical procedures. Meloxicam (1.0 mg/kg subcutaneous) was administered once daily and buprenorphine (0.02 mg/kg subcutaneous) twice daily for 3 days as systemic analgesics. Animals were given 10 mL of lactated Ringer’s solution (Abbott Global, Abbott Park, IL, USA) on the day of surgery to prevent dehydration, with additional fluids and supplemental nutrition provided if dehydration was observed during postoperative assessment. Bladders were manually expressed through gentle palpation of the abdomen twice a day for the first 3 to 5 days postinjury, and then once a day until reflex urination occurred and continued. Any identified urinary tract infections were treated with a 3-day course of the antibiotic enrofloxacin (Baytril, 10 mg/kg subcutaneous; Bayer Healthcare, LLC, Shawnee Mission, KS, USA).
Euthanasia and Perfusion
At 4 wk posttransplantation, animals were euthanized with an overdose of 300 mg/kg Fatal-Plus Solution (Butler Schein, Dublin, OH, USA) and were given a transcardial perfusion of 0.1 M PBS, followed by 4% paraformaldehyde (PFA). A 1 cm segment of the spinal cord, containing the lesion at its center, was carefully extracted and a notch placed on the right rostral side to serve as a landmark for sectioning. Samples were stored in 4% PFA overnight, followed by a solution of 30% sucrose until no longer buoyant, then flash frozen in anhydrous 2-methylbutane (Sigma Aldrich; St. Louis, MO, USA) cooled by dry ice and stored at −80°C until ready for sectioning.
Histology
Specimens were sectioned longitudinally from dorsal to ventral in a cryostat at 30-μm thickness. For each sample, the tissue was placed into 12 wells of free-floating tissue in 2% PFA until ready for labeling. To ensure reliability in between-group comparisons, all tissues were labeled in large batches, with incubation and exposure times standardized using an injury-only control.
Immunohistochemistry
Standard immunohistological techniques were utilized for the identification of neurogenesis through beta III tubulin (Tuj 1), a neuron-specific tubulin marker for immature cells, and for two major characteristics of the glial scar, including glial fibrillary acidic protein (GFAP) for astrocyte content and CSPG for fibrous extracellular scar tissue. While MSCs were not expected to differentiate into a neuronal lineage, positive labeling for Tuj 1 served to indicate endogenous regeneration and provide an area for future exploration. Similarly, the use of GFAP and CSPG labeling provided an overview of the characteristics of the glial scar and indicated whether additional measures would focus more on cellular or fibrotic pathology. Briefly, well of free-floating tissue were washed in 0.1 M PBS 3 times, followed by blocking of nonspecific binding in a 10% solution of normal goat serum (NGS) in a 0.1% Triton X-100 (Sigma Aldrich) in PBS for 1 h. An additional blocking step of 20 min in 0.3% hydrogen peroxide (Sigma Aldrich) was utilized for GFAP and CSPG labeling prior to NGS blocking. Tissue was incubated overnight in primary antibody at a dilution of 1:1,000 for mouse anti-beta III tubulin (Ms × Tuj 1; Abcam, Cambridge, MA, USA), 1:1,000 for rabbit-anti GFAP (Rb × GFAP; Abcam), and 1:2,000 for mouse anti-CSPG (Ms × CSPG; EMD Millipore, Billerica, MA, USA) on a rotator at 4°C. The next day, the tissues were washed 3 times in PBS and then placed in secondary antibodies for 1 h at room temperature with the following dilutions: for Tuj 1, 1:500 mouse anti-488 (Alexa Fluor Rb × 488; Life Technologies) in 0.1% Triton in PBS; for GFAP, 1:3 goat antirabbit horseradish peroxidase (HRP) (Gt × Rb Poly-HRP; EMD Millipore); and for CSPG, 1:500 goat antimouse (Gt × Ms Biotin; Vector Labs, Burlingame, CA, USA). For CSPG, the signal was then amplified using avidin–biotin complex (Vector Labs) for 1 h and revealed using 3′3′-diaminobenzidine (DAB; Sigma-Aldrich, St. Louis, MO, USA). Tissues labeled with GFAP were revealed in DAB following secondary incubation without amplification. All tissues were washed in distilled water for a final time and immediately mounted onto gelatin-coated slides. Fluorescent-labeled slides were coverslipped using aqueous mounting solution (Sigma-Aldrich, St. Louis, MO, USA), while GFAP and CSPG slides underwent dehydration through graded ethanol and xylene before coverslipping.
Eriochrome cyanine RC for myelin content
The density of myelin present in the T8 spinal cord was assessed through the histological dye Eriochrome Cyanine RC (ECRC; Sigma-Aldrich, St. Louis, MO, USA), which oxidizes lipids to produce a rich blue stain that can be used to identify regions of myelin/demyelination. Tissues were first premounted on gelatin-coated slides and dried to promote adhesion and preservation of the specimens. Slides were then rinsed in 100% ethanol and cleared for 15 min in xylene, followed by an additional 10 min in 100% ethanol and rehydration in graded ethanol for 3 min each. Slides were placed in a 0.4% solution of ECRC in 0.5% H2SO4 for 10 min and washed in 2 rinses of tap water. To differentiate the gray matter, slides were dipped in a solution of 0.5% ammonium hydroxide and agitated for 30 s, followed by 2 rinses in tap water. The differentiation step was repeated as needed until the gray matter was clearly defined. Finally, slides were cleared and coverslipped in graded ethanol and xylene and allowed to dry overnight.
Microscopy
Fluorescent imaging
Fluorescently stained tissues were analyzed using a Zeiss Axiovert fluorescent microscope (Carl Zeiss Microimaging, LLC, Thornwood, NY, USA). Pictures were captured in the center of each graft, the location of which was determined by identifying the lesion epicenter, or section of least ECRC staining, as a reference point and moving to the appropriate region based on transplant location (Fig. 1). Positively stained cells of each type were manually counted for at least 3 lateral sections using ImageJ software (National Institute of Health, Bethesda, MD, USA) by a researcher who remained blind to the transplant concentration conditions, and the number of cells per square micrometer of tissue was recorded for each image as the dependent measures.
Densitometry
For ECRC, CSPG, and GFAP labeling, optical densitometry was used to determine the overall levels of myelin and glial scarring present in spinal cord tissues. This was done as a proof of concept to establish viability of MSC treatment, to be followed up with more detailed cell number quantification in future studies. Briefly, cleaned and coverslipped slides were scanned digitally using Nikon Scan 4.0 software on a Coolscan IV scanner (Nikon, Melville, NY, USA) and analyzed using ImageJ. Scanned images were first converted into 8-bit gray scale and inverted to facilitate bright-field measurements. A 7-mm long segment centered on the lesion epicenter, which was defined as the section with the lowest area of ECRC-positive staining, was traced for evaluation of mean density levels of each tissue sample. The mean densities of surviving tissue were collected without the inclusion of major cavitation, characterized as cavities which exceeded 50 μm2 in size. The main dependent variables included the mean density and area of the regions of interest. Overall measurements of density and tissue area for each spinal cord, with 3 to 4 samples evaluated per cord, were used to calculate group means for statistical comparisons.
Statistical Analysis
Statistics were performed using SPSS version 20 for Microsoft Windows 7 (International Business Machines Corp (IBM); Armonk, NY, USA). Behavioral and histological assessments were compared between groups using 1-way analysis of variance (ANOVA). Separate analyses were done for the factors of transplant location and cell concentration as well as for the interaction of transplant location and concentration. An α level of P < 0.05 was used to indicate significance, and protected least significant difference was used for post hoc comparisons when appropriate.
Results
MSC Transplantation Provides a Modest Therapeutic Effect on Base of Support
No statistical significance was observed in the BBB scores of animals receiving MSC transplants when compared to lesion-only controls based on location, F(5, 84) = 0.261, P > 0.05, concentration, F(3, 86) = 0.291, P > 0.05, or location–concentration interaction, F(15, 74) = 0.728, P > 0.05.
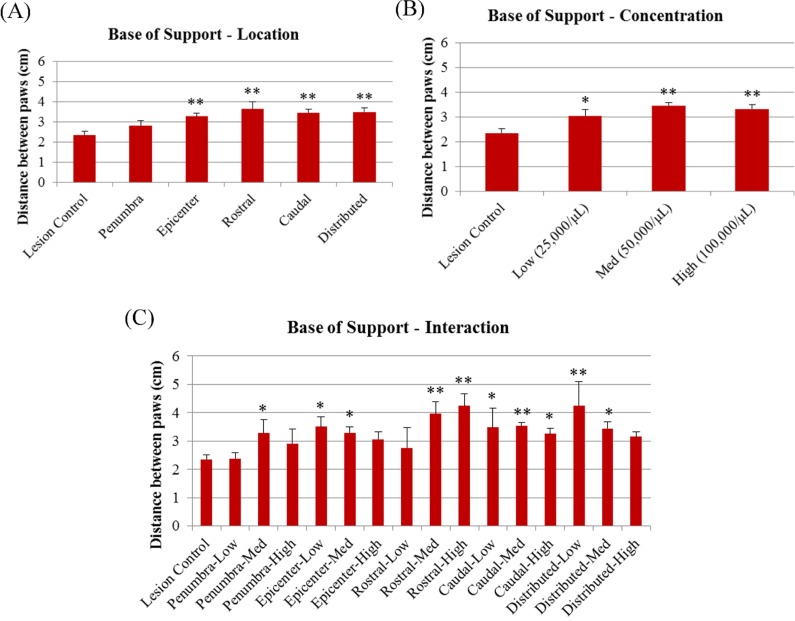
A significant difference was seen for the footprint analysis, with MSC-treated animals showing an increased base of support when compared to lesion-only controls in all experimental factors, location F(5, 65) = 4.350, P < 0.01, concentration F(3, 67) = 5.037, P < 0.01, interaction F(15, 55) = 2.777, P < 0.05, with the exception of those receiving cells in the penumbra location (Fig. 3).
Fig. 3.
Base of support in the footprint analysis at 4 wk posttransplantation for (A) transplant location, (B) cell concentration, and (C) interaction. *Significantly different from lesion-only controls, P < 0.05. **Significantly different from lesion-only controls, P < 0.01.
MSCs Are Able to Survive in the Lesion Environment for 4 wk Posttransplantation but Do Not Increase the Proliferation of New Neurons
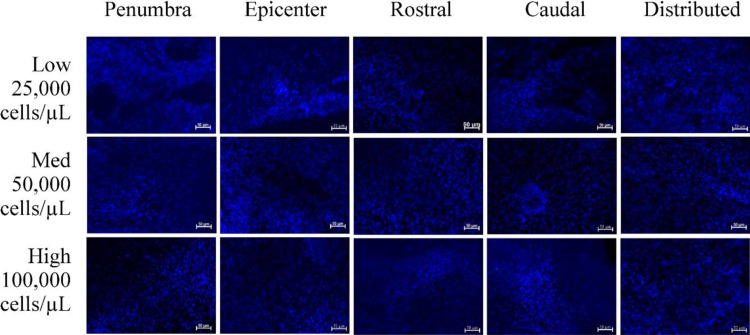
Cells prelabeled with Hoechst were readily observed in tissue 4 wk posttransplantation and were well integrated into host tissues. Images captured from the center of each graft produced comparable cell counts in all transplant locations, F(4, 41) = 0.312, P > 0.05, and for all cell concentrations, F(2, 12) = 0.796, P > 0.05, as well as interaction, F(14, 31) = 0.946, P > 0.05 (Fig. 4). No notable Tuj-1-positive labeling for immature neurons was observed in transplanted or lesion-only tissues. Though argued to contribute to cell death by affecting the DNA, previous studies by our group have observed no aberrant cell growth in response to Hoechst nuclear staining.16–18,22 This stain was also determined to be adequately specific to the transplanted cells, as no efflux of the Hoechst dye to endogenous structures was observed in any of the transplanted tissue samples. Likewise, as MSCs are nonimmunogenic, no abnormal growths beyond the expected trauma-induced histopathology were observed.
Fig. 4.
Cell survivability as indicated by positive labeling for Hoechst fluorescent nuclear stain. Scale bars represent 50 μm.
MSC Transplantation Facilitated Significant Sparing of Myelin Content
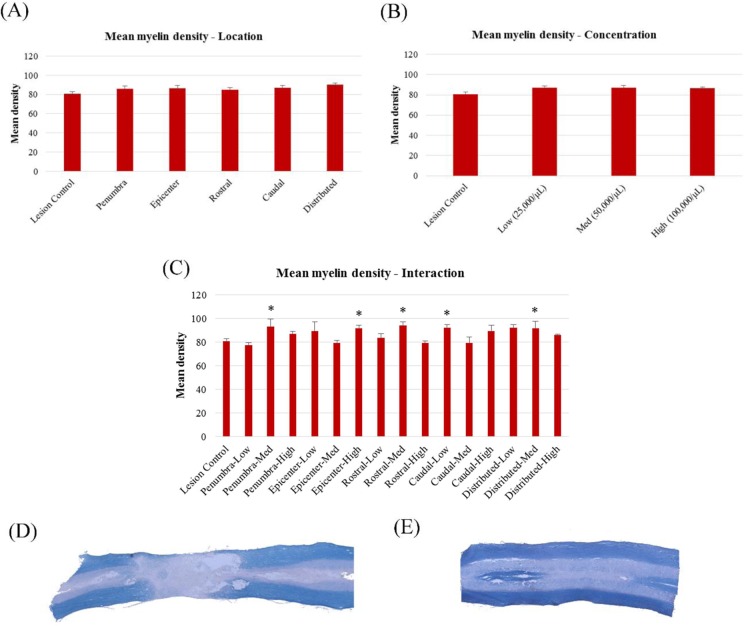
The density of myelin was found to be significantly higher in tissue that had received transplants of MSCs when compared to lesion-only controls in regard to the location–concentration interaction, F(15, 50) = 2.519, P < 0.01, but not for location alone, F(5, 61) = 0.848, P > 0.05, or concentration alone, F(3, 63) = 0.690, P > 0.05 (Fig. 5). There was a tendency, however, for transplant groups in all locations and concentrations to have increased myelin content compared to lesion-only controls.
Fig. 5.
Comparisons of mean myelin densities, as indicated by Eriochrome Cyanine RC (ECRC) labeling in mesenchymal stem cell (MSC)–treated animals. (A) Mean densities of transplant locations; (B) mean densities of transplant concentrations; (C) mean densities of location–concentration interaction; (D) sample images of ECRC staining in injured, sham-treated tissue; (E) sample images of ECRC staining in healthy control tissue. *Significantly different from lesion-only controls, P < 0.05.
Fibrotic Glial Scarring Was Not Affected by MSC Transplantation, though Astrocyte Density Was Significantly Reduced
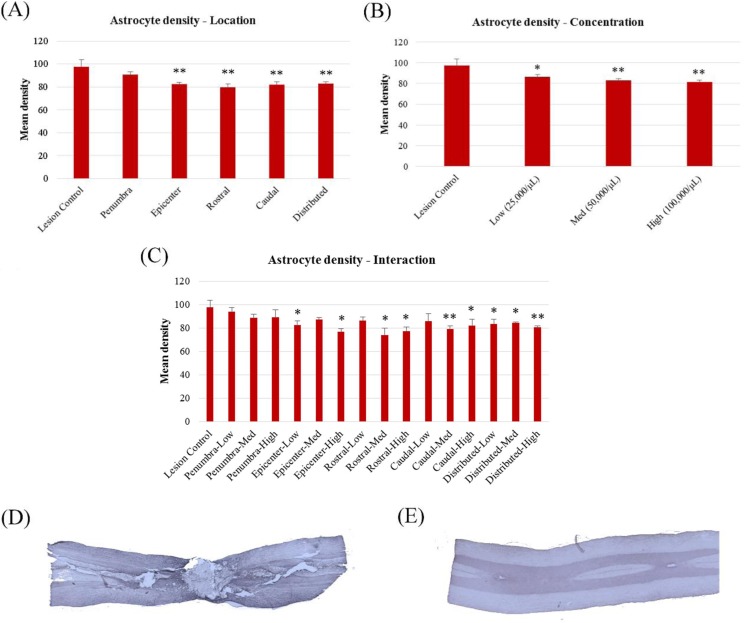
Fibrotic scarring within the lesion, as measured by the optical densities of CSPG labeling, was not significantly different between groups when comparing transplant locations, F(5, 60) = 1.883, P > 0.05, cell concentrations, F(3, 62) = 2.086, P > 0.05, or location–concentration interaction, F(15, 50) = 1.484, P > 0.05. In contrast, when examining the density of astrocytes within the scar tissue, GFAP labeling was found to be significantly lower in MSC transplant groups compared to lesion-only controls in regard to transplant location, F(5, 61) = 4.796, P < 0.01, cell concentration, F(3, 63) = 4.586, P < 0.01, and interaction, F(15, 51) = 2.345, P < 0.05. Reductions in GFAP density were most prominent in the rostral bilateral transplant group, followed by the distributed location (Fig. 6A). Animals receiving transplants to the penumbra, however, did not differ significantly from lesion-only controls, suggesting that this transplant location may not be efficacious in reducing astrocytic scarring. In terms of cell concentration, GFAP density tended to decrease as concentration increased, indicating a potential advantage of higher cell concentrations (Fig. 6B).
Fig. 6.
Comparisons of astrocytic scar densities, as measured by the intensity of glial fibrillary acidic protein (GFAP) immunolabeling in mesenchymal stem cell (MSC)–treated animals. (A) Mean densities of transplant locations; (B) mean densities of transplant concentrations; (C) mean densities of location–concentration interaction; (D) sample images of GFAP staining in injured, sham-treated tissue; (E) sample images of GFAP staining in healthy control tissue. *Significantly different from lesion-only controls, P < 0.05. **Significantly different from lesion-only controls, P < 0.01.
Higher Cell Concentrations May Provide Protection Against Overall Tissue Loss, but Transplant Location Does Not Affect Tissue Volume
The total calculated area of surviving tissue was collected excluding all major cavitation, or those which exceeded 50 μm2 in size, and was unaffected by cell transplantation when comparing transplant locations, F(5, 60) = 0.937, P > 0.05, or interaction, F(15, 50) = 1.496, P > 0.05. There was a trend toward significance in the comparison of cell concentrations, F(3, 62) = 2.086, P = 0.052, with a high cell concentration of 100,000 cells/µL appearing to have a more significant sparing effect on the total area of surviving tissue compared to untreated lesion controls. However, higher concentrations of cells introduced a potential complication during the transplant procedure, as cells were too dense to maintain a stable cell suspension within the transplant syringe and required resuspension between each injection. Increasing the cell number, therefore, would necessitate increased fluid volumes, which may place additional strain on host tissues.
Discussion
The primary findings of the present study were that mild improvements in behavioral recovery, as measured by footprint analysis, and reductions in astrocytic scarring were observed in animals receiving transplants of bone marrow–derived MSCs when compared to lesion-only controls, but the concentration of cells or location of the transplant did not appear to have a consistent effect on these outcomes. These findings suggest that MSCs remain a viable and low-risk treatment option that may serve as an adjunctive treatment but are unlikely to confer significant long-term benefits in terms of restoring function to the injured spinal cord. The lack of statistical significance in BBB ratings is unsurprising, as single-therapy approaches to treating SCI are far less likely to provide dramatic beneficial effects than multimodal approaches.19
The location of cell transplantation did have a significant effect on the density of astrocytes within the injured spinal cord, reducing the density of glial scarring in all groups except for those transplanted into the lesion penumbra but did not appear to affect functional recovery to a notable degree. Previous studies have suggested that the penumbral zone surrounding the lesion epicenter would provide the greatest therapeutic effect8 and that the epicenter itself would be ineffective due to the central cavitation of the injury.7 However, the results of the current study stand in stark contrast, demonstrating that in cases of moderately severe SCI, transplantation of cells within and above the lesion epicenter provided significantly greater reductions in astrocyte density and improvements in the base of support and thereby trunk stability, when compared to those transplanted into the lesion penumbra. It is possible that because lesion severity was moderate rather than severe for the present study, central cavitation was not significantly large enough to affect the efficacy of the transplant in the epicenter location. This could indicate a potential interaction between lesion severity and optimum transplant sites, and future research would benefit from the comparison of these transplant locations on varying degrees of injury.
Tissue from all cell concentrations showed significant reductions in astrocytic scar densities compared to lesion-only controls as well as increased base of support in the footprint analysis, and mild improvements in myelin sparing and functional recovery in the BBB, yet increasing the concentration of cell transplants beyond 50,000 cells/µL did not increase the overall efficacy of transplantation or survivability of grafts. Mean densities of surviving cells within the graft did not differ between groups, regardless of the total volume initially injected in to the site. The number of cells transplanted into traumatic injuries and degenerative conditions varies greatly from one study to another, with some testing high numbers of cells in hopes of improved integration into the host tissue and others using smaller amounts in order to avoid additional strain on the sensitized lesion environment. More concentrated transplants allow the compromise of using more cells without excessive fluid volumes that can increase pressure within the target environment but may be more viscous and therefore more difficult to deliver through minimally invasive methods. In the present study, higher concentrations of MSCs were found to survive equally well when compared to lower concentrations, though no additional benefits were gained from high-concentration transplants compared to the low and medium concentrations. Increasing the cell concentration from the current density of 100,000 cells/µL would therefore require diluting the cells with greater fluid volumes, and in absence of compression from the increased volume is likewise not expected to convey more benefits than the existed data portray. As such, we are able to conclude that MSCs are safe at reasonably high doses and may be further tested without risk of serious adverse reactions. Alternative cell types, however, may have dramatically different properties and should be examined separately for safety and efficacy before selecting an optimum dose size.
Myelin was found to have slightly higher densities in all transplant locations and cell concentrations when compared to lesion-only controls, though not to statistically significant levels, suggesting a potential, mild protective effect of MSCs on progressive degeneration of myelin postinjury. Myelin density did not differ significantly between cell concentrations, which again indicates that transplantation of higher cell numbers did not provide additional benefits for the protection of myelin content in the injured spinal cord.
The results of the present study overall support the principle that MSCs are a safe therapeutic option with little to no side effects, which appear to be most beneficial when transplanted at moderate concentrations and in regions within and rostral to the lesion epicenter. Although therapeutic effects of MSCs alone are mild, it is well known that the highly variable nature of SCI has a limited response to independent treatments and that multimodal approaches are likely to amplify the improvements provided by single-approach methods. While the present study explored a relatively short time span of 4 wk posttransplant, recovery from SCI in the rat reaches the chronic phase before this time point and becomes functionally static. Positive survivability outcomes at this time point serve as an encouraging proof of principle for the viability of MSCs as a potential treatment option. Future studies would benefit from extending this time point beyond the early chronic phase and into late chronic tissues.
The current findings primarily focus on the basic characteristics of post-SCI pathology, such as glial scarring and limitation of neurogenesis, yet also provide a stable proof of concept in favor of using MSCs as a potential therapy for SCI. However, based on the narrow range of benefits observed to be proffered by MSCs, it would likely be worthwhile to explore the addition of other cell types in combination with MSCs, such as Schwann cells or oligodendrocyte precursor cells, as well as more specific pathological measures, including reactivity of astrocytes and inflammatory markers, or ingrowth of axons into the lesion environment, which may change in response to MSC transplant therapy. Other groups have shown relative success in co-transplantation therapies, particularly those utilizing Schwann cells in tandem with MSCs, which was observed to contribute to an improved regain of function in rats compared to single-cell-type transplants24 and return of bowel and bladder function in humans.11 When combined with oligodendrocyte precursors, MSCs were also shown to improve myelin formation in the optic nerve of mice.25 With the new inclusion of data from the current study, which suggest that future studies select transplant locations specific to the injury type and severity of the individual, it is therefore our belief that MSC transplantation can improve the lives of those living with the debilitating effects of SCI.
Footnotes
Ethical Approval: This study was approved by the university's Institutional Animal Care and Use Committee.
Statement of Human and Animal Rights: All procedures were in accordance with the university's Institutional Animal Care and Use Committee (IACUC) approved protocols.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Central Michigan University College of Graduate Studies and College of Humanities and Social Behavioral Sciences, the Field Neurosciences Institute, and the John G. Kulhavi Professorship (to GLD).
References
- 1. National Spinal Cord Injury Statistical Center. Spinal cord injury facts and figures at a glance. J Spinal Cord Med. 2013. http://www.ncbi.nlm.nih.gov/pubmed/23820155. Accessed 36 4.
- 2. Christopher and Dana Reeve Foundation. One Degree of Separation: Paralysis and Spinal Cord Injury in the United States. SCI Stats 2014 2014.
- 3. Onifer SM, Smith GM, Fouad K. Plasticity after spinal cord injury: relevance to recovery and approaches to facilitate it. Neurotherapeutics. 2011;8(2):283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Norenberg MD, Smith J, Marcillo A. The pathology of human spinal cord injury: defining the problems. J Neurotrauma. 2004;21(4):12. [DOI] [PubMed] [Google Scholar]
- 5. Grill RJ. User-defined variables that affect outcome in spinal cord contusion/compression models. Exp Neurol. 2005;196(1):1–5. [DOI] [PubMed] [Google Scholar]
- 6. Houle JD, Cote MP. Axon regeneration and exercise-dependent plasticity after spinal cord injury. Ann N Y Acad Sci. 2013;1279(1):154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Morshead CM, Fehlings MG. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J Neurosci. 2006;26(13):3377–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mautes A, Weinzierl M, Donovan F, Noble L. Vascular events after sci: contribution to secondary pathogenesis. Phys Ther. 2000;80(7):17. [PubMed] [Google Scholar]
- 9. Rosenzweig ED, McDonald JW. Rodent models for treatment of spinal cord injury: research trends and progress toward useful repair. Curr Opin Neurol. 2004;17(2):11. [DOI] [PubMed] [Google Scholar]
- 10. Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5(2):146–156. [DOI] [PubMed] [Google Scholar]
- 11. Volarevic V, Erceg S, Bhattacharya SS, Stojkovic P, Horner P, Stojkovic M. Stem cell-based therapy for spinal cord injury. Cell Transplant. 2013;22(8):1309–1323. [DOI] [PubMed] [Google Scholar]
- 12. Li J, Lepski G. Cell transplantation for spinal cord injury: a systematic review. Biomed Res Int. 2013;2013(1):786475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nishimura S, Yasuda A, Iwai H, Takano M, Kobayashi Y, Nori S, Tsuji O, Fujiyoshi K, Ebise H, Toyama Y, et al. Time-dependent changes in the microenvironment of injured spinal cord affects the therapeutic potential of neural stem cell transplantation for spinal cord injury. Mol Brain. 2013;6(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feron F, Perry C, Cochrane J, Licina P, Nowitzke A, Urquhart S, Geraghty T, Mackay-Sim A. Autologous olfactory ensheathing cell transplantation in human spinal cord injury. Brain. 2005;128(12):10. [DOI] [PubMed] [Google Scholar]
- 15. Ziegler MD, Hsu D, Takeoka A, Zhong H, Ramon-Cueto A, Phelps PE, Roy RR, Edgerton VR. Further evidence of olfactory ensheathing glia facilitating axonal regeneration after a complete spinal cord transection. Exp Neurol. 2011;229(1):109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rossignol J, Boyer C, Thinard R, Remy S, Dugast AS, Dubayle D, Dey ND, Boeffard F, Delecrin J, Heymann D, et al. Mesenchymal stem cells induce a weak immune response in the rat striatum after allo or xenotransplantation. J Cell Mol Med. 2009;13(8b):2547–2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Matchynski-Franks JJ, Rossignol J, Pappas CA, Reinke TA, Fink K, Crane A, Lowarance SA, Twite A, Cheng S, Dunbar GL. Mesenchymal stem cells as treatment for behavioral deficits and neuropathology in the 5XFAD mouse model of Alzheimer's disease. Cell Transpl. 2016;25(4):687–703. [DOI] [PubMed] [Google Scholar]
- 18. Lowrance SA, Fink KD, Crane AT, Matyas JJ, Dey ND, Matchynski JJ, Dunbar GL. Bone marrow-derived mesenchymal stem cells attenuate cognitive deficits in an endothelin-1 rat model of stroke. Restor Neurol Neurosci. 2015;33(4):579–588. [DOI] [PubMed] [Google Scholar]
- 19. Yazdani SO, Hafizi M, Zali AR, Atashi A, Ashrafi F, Seddighi AS, Soleimani M. Safety and possible outcome assessment of autologous Schwann cell and bone marrow mesenchymal stromal cell co-transplantation for treatment of patients with chronic spinal cord injury. Cytotherapy. 2013;15(7):782–791. [DOI] [PubMed] [Google Scholar]
- 20. de Medinaceli L, Freed WJ, Wyatt RJ. An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp Neurol. 1982;77(3):634–643. [DOI] [PubMed] [Google Scholar]
- 21. Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12(1):21. [DOI] [PubMed] [Google Scholar]
- 22. Rossignol J, Boyer C, Leveque X, Fink KD, Thinard R, Blanchard F, Dunbar GL, Lescaudron L. Mesenchymal stem cell transplantation and DMEM administration in a 3NP rat model of Huntington’s disease: morphological and behavioral outcomes. Behav Brain Res. 2011;217(2):369–378. [DOI] [PubMed] [Google Scholar]
- 23. Young W. MASCIS spinal cord contusion model In: Chen J, editor. Animal models of acute neurological injuries. Totowa (NJ): Humana Press; 2009. P. 411–421. [Google Scholar]
- 24. Joghataei MT, Bakhtiari M, Pourheydar B, Mehdizadeh M, Faghihi A, Mehraein F, Behnam B, Pirhajati V. Co-transplantation of Schwann and bone marrow stromal cells promotes locomotor recovery in the rat contusion model of spinal cord injury. Yakhteh Med J. 2010;12(1):7–16. [Google Scholar]
- 25. Arriola A, Kiel ME, Shi Y, McKinnon RD. Adjunctive MSCs enhance myelin formation by xenogenic oligodendrocyte precursors transplanted in the retina. Cell Res. 2010;20(6):728–731. [DOI] [PubMed] [Google Scholar]