Abstract
Strategies to reduce the immunogenicity of pancreatic islets and to prevent the activation of proinflammatory events are essential for successful islet engraftment. Pretransplant islet culture presents an opportunity for preconditioning to improve outcomes of islet transplantation. We previously demonstrated that ex vivo mitomycin C (MMC) pretreatment and subsequent culture significantly prolonged graft survival. Fully understanding the biological process of pretreatment could result in the development of a protocol to improve the survival of islet grafts. Microarrays were employed to conduct a comprehensive analysis of genes expressed in untreated or MMC-treated rat islets that were subsequently cultured for 3 d. A bioinformatics software was used to identify biological processes that were most affected by MMC pretreatment, and validation studies, including in vivo and in vitro assay, were performed. The gene expression analysis identified significant downregulation of annotated functions associated with cellular movement and revealed significant downregulation of multiple genes encoding proinflammatory mediators with chemotactic activity. Validation studies revealed significantly decreased levels of interleukin 6 (IL-6), monocyte chemoattractant protein 3 (MCP-3), and matrix metallopeptidase 2 (MMP2) in culture supernatants of MMC-treated islets compared with controls. Moreover, we showed the suppression of leukocyte chemotactic activity of MMC-treated islets in vitro. We also showed that MMC-treated islets secreted lower levels of chemoattractants that synergistically reduced the immunogenic potential of islets. Histological and immunohistochemical analyses of the implant site revealed that infiltration of monocytes, CD3-positive T cells, and B cells was decreased in MMC-treated islets. In conclusion, the ex vivo pretreatment of islets with MMC and subsequent culture can reduce the immunogenic potential and prolong the survival of islet grafts by inducing the suppression of multiple leukocyte chemotactic factors.
Keywords: islets transplantation, preconditioning, inflammation, tolerance
Introduction
The transplantation of pancreatic islets is a minimally invasive therapy for patients with type 1 diabetes mellitus with severe hypoglycemia unawareness, despite adequate insulin therapy. The short-term results of recent clinical trials of islet transplantation are promising, although multiple donors were required to achieve sustained insulin independence of 1 recipient, and the function of implanted islets decreased with time after transplantation.1,2 Early impairment of islet engraftment might be attributed to numerous factors involved in intrahepatic islet infusion such as instant blood-mediated inflammatory response (IBMIR), thrombosis, and hepatic tissue ischemia.3 Furthermore, cell stress probably initiated by both isolation and transplant-induced adaptive immune responses might be involved and play a significant role in islet loss.4,5 Islet grafts are stressed and inflamed by the isolation procedure6,7 and secrete or express multiple proinflammatory mediators immediately after isolation or even after a couple of days in culture.8,9 Numerous studies over the past decade have described the presence of proinflammatory cytokines including C-X-C chemokine ligand 8 (CXCL8)/interleukin 8 (IL-8), CXCL6/granulocyte chemotactic protein (GCP) 2, chemokine (C-X-C motif) ligand (CXCL) 2, CXCL1, CXCL5, C-C chemokine ligand (CCL) 2, CXCL12, and CCL28 and danger signals induced by islet isolation.10 Targeting these cytokines was found to be effective in restoring glucose tolerance or prolonging graft survival,11 but the effect was insufficient when tested against a single molecule.12 We hypothesized that the regulation of multiple inflammatory cytokines together will be more effective and could induce immunological unresponsiveness to islet grafts. This hypothesis was clarified in our model using mitomycin C (MMC) pretreatment of islets and culture, in which an indefinite survival of islet allografts and xenografts was obtained without any immunosuppressive treatment.13,14 MMC is categorized as a pharmacological alkylating agent that has genotoxic activity by inhibition of DNA replication to influence intracellular metabolism via various pathways.15,16 Ex vivo islet pretreatment exposure of islets to sublethal genotoxic stressors such as ultraviolet (UV) irradiation,17 γ irradiation,18 or mitomycin-C19 reduced the postgrafting immune response and enhanced islets engraftment. In our previous studies, the finding that the enhancement of MMC-induced engraftment was the greatest when islets were cultured for 3 d after MMC treatment is particularly interesting,13 indicating that biological processes relevant to engraftment were induced in MMC-treated islets over the periods. Here, we showed that comprehensive analyses of gene expression by MMC treatment provided evidence of its potential as a regulator of multiple proinflammatory cytokines, as validated in in vitro and in vivo studies.
Materials and Methods
Animals
Islet donors were male Wistar (Jcl) rats (CLEA Japan, Inc., Tokyo, Japan) aged 8 to 10 wk, weighing 250 to 320 g. We induced diabetes in male C57BL/6 (H-2b) mice (B6 mice; Nihon Clea, Inc. Shizuoka, Japan) aged 8 to 9 wk using a single intraperitoneal (i.p.) injection of streptozotocin (STZ; 250 mg/kg body weight; Sigma-Aldrich, St Louis, MO, USA). Rats and mice were housed in cages at a controlled temperature and under a suitable light–dark cycle. Mice with 2 consecutive nonfasting blood glucose levels of >400 mg/dL served as recipients. Monocytes were isolated from bone marrow of male B6 mice aged 8 to 10 wk. All animals were anesthetized using isoflurane inhalation. The Ethics Review Committee for Animal Experimentation of Fukushima Medical University approved this study.
Isolation of Pancreatic Islets, MMC Treatment Culture, and Transplantation
Islets were isolated using a method similar to that reported previously.20 In brief, pancreatic islets were isolated from Wistar rats using stationary collagenase digestion (Liberase; 0.23 mg/mL; Roche, Basal, Switzerland), followed by centrifugation through a discontinuous gradient of Ficoll (Ficoll 400; Sigma-Aldrich). The crude islets obtained after the second Ficoll separation were divided into 2 groups as follows: (1) MMC-treated islets were incubated for 30 min in Roswell Park Memorial Institute (RPMI)-1640 medium with 10 µg/mL MMC (Kyowa Hakko Kogyo, Tokyo, Japan) (13) and (2) nontreated islets were incubated for the same time in RPMI-1640. After washing thrice in RPMI-1640 containing 10% fetal bovine serum (FBS) (Nichirei Bioscience Inc. Tokyo, Japan), the islets were cultured in complete medium (RPMI-1640 with 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 2 mM l-glutamine (MP Biomedicals, Santa Ana, CA, USA), 100 U/mL penicillin (Life Technologies, Carlsbad, CA, USA), 100 µg/mL streptomycin sulfate (Life Technologies), 0.5 µg/mL amphotericin B, and 10% FBS) at 37 °C in a humidified atmosphere containing 5% CO2 and 95% air. Our previous study demonstrated that 10 µg/mL MMC concentration was optimal pretreatment dose, in which glucose stimulated insulin secretion and the percentage of viable islets was not decreased comparing nontreated islets13 which were cultured for 3 d, were implanted into the left renal subcapsular space of STZ-induced diabetic B6 mice. Graft rejection was defined as a blood glucose level of >300 mg/dL in 2 consecutive measurements.
RNA Purification, Amplification, and Microarray Analysis
RNA was purified from MMC-treated or nontreated islets immediately after 30 min of treatment (d0-islets) and from 1-d (d1-islets) and 3-d cultures (d3-islets; n = 3). We used a microscope to assist manual harvesting of 200 islets that were immediately stored at −80 °C. Total islet RNA was purified using the RNeasy Mini Kit (Qiagen N.V., Venlo, The Netherlands) according to the manufacturer’s protocol. RNA quantity and purity were measured using a NanoDrop 1000 spectrophotometer and the Agilent RNA 6000 Nano Kit (Agilent Technologies, Santa Clara, CA, USA). Samples with an RNA integrity number of >7.0 and concentrations of >33 ng/μL were used for amplifications. Each total RNA sample was reverse-transcribed into double-stranded complementary DNA (cDNA) that was used in in vitro transcription reactions to amplify antisense mRNA (complementary RNA [cRNA]) using the Ambion WT Expression Kit (Thermo Fisher Scientific Inc). The cRNA was labeled and fragmented using the Affynmetrix GeneChip WT Terminal Labeling Kit (Affymetrix, Santa Clara, CA, USA). The labeled cDNA from each islet sample was hybridized to a single GeneChip Rat Gene 1.0 ST Array (Affymetrix, Santa Clara, CA, USA). This array can be used to detect >30,000 transcripts of known rat genes and potentially expressed sequences. The hybridized arrays were scanned using Gene Chip Scanner 3000 7G (Thermo Fisher Scientific, Inc.) to generate images of fluorescence intensity for data analysis. Image data were quantified using GeneChip Command Console Software (Affymetrix).
Identification of the Functions of Differentially Expressed Genes
Normalization and filtering of microarray data were performed using Gene Spring GX software Version 12.5 (Agilent Technologies, Santa Clara, CA, USA). Differentially expressed genes were defined as those levels that changed > 2-fold or ≤ 2-fold (log2) compared with the respective control. Ingenuity Pathway Analysis (Qiagen, Redwood City, CA, USA; qiagen.com/ingenuity) was used to identify the biological processes that were differentially affected in MMC- or nontreated cultured islets. This tool provides information about a disease, molecular and cellular functions, and physiological and development function categories related to genes obtained from the microarray analysis. To determine the biological functions most closely associated with MMC-induced engraftment, we used an activation z-score of >2 or ≤2 and P value of <0.05. The activation z-score assesses the match between observed and predicted upregulation or downregulation patterns that indicate significant differential biological activity of MMC-treated islets compared with that of control islets (nontreated d0-islets). The P value, which was calculated using the Fisher exact test, indicates the statistical significance of the association of a biological function with a set of differentially expressed genes.
Immunoassay of Cytokines and Peptidase Activity in Islet Culture Supernatants
Aliquots of pancreatic islet preparations (240 islets/mL) were cultured in 3 mL of RPMI-1640 medium in a 3.5-cm dish (Sumilon, Sumitomo Bakelite Co., Utsunomiya, Japan) in a CO2 incubator. Islet culture supernatants were collected after 1 and 3 d of culture and immediately stored at −80 °C. The levels of interleukin 6 (IL-6), monocyte chemoattractant protein 3 (MCP-3), and matrix metallopeptidase 2 (MMP-2) were determined using the Quantikine enzyme-linked immunosorbent assay (ELISA) Kit (R&D Systems, Minneapolis, MN, USA), MCP-3 ELISA Kit (Cloud-Clone Corp. Houston, TX, USA), and MMP-2 ELISA kit (Abnova, Taipei, Taiwan). Each reaction was performed in duplicate. The lower limits of detection density was measured at 450 nm using a microplate reader (Multiskan GO: Thermo Scientific, Yokohama, Japan).
Isolation of Murine Bone Marrow Monocytes
B6 mice were sacrificed by inhalation of an analgesic, and the femur and tibia were removed to collect bone marrow cells, as described previously.21 Monocytes were isolated from bone marrow cells using auto magnetic cell sorting (MACS). T cells, granulocytes, natural killer cells, B cells, dendritic cells (DCs), and basophils were depleted from the bone marrow cell population using a mixture of antibodies against CD3, CD7, CD19, CD45RA, CD56, and anti-immunoglobulin E (IgE; Monocyte Isolation Kit; Miltenyi Biotec, Bergisch-Gladbach, Germany) and an autoMACS Pro Separator (Miltenyi Biotec).
Chemotaxis Assay
The chemotaxis of mouse monocytes toward rat islet supernatants was evaluated using a 5-µm pore Transwell membrane (Corning Inc., Corning, NY, USA), as described previously.22 The rat islet culture supernatants used in this assay were the same as those used to determine cytokine concentrations. MACS-enriched monocytes (1.0 × 105) were seeded in the upper chamber in 100 µL of serum-free medium, and 600 µL of islet supernatant or RPMI was placed in the lower chamber. Chemotactic activity is expressed as the mean number of migrated monocytes in 10 high-power microscope fields. Monocyte migration toward RPMI served as the negative control.
To validate the effects of MCP-3 on chemotaxis, anti-MCP-3 monoclonal antibody (mAb; 2 µg/mL; Abcam, Cambridge, UK) was added to the islet supernatant to neutralize MCP-3. Isotype-matched mouse immunoglobulin G (2 µg/mL; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) served as the control. To inhibit chemotaxis induced by MMPs, the MMP inhibitor GM6001 (10 μmol/L; Santa Cruz Biotechnology, Inc.) was used, as described previously.23 GM6001 inhibits MMP-1, MMP-2, MMP-3, MMP-7, MMP-8, MMP-9, MMP-12, MT-MMP1, and MMP-26.
Histological and Immunohistochemical Analyses of the Implant Site
STZ-induced diabetic B6 mice were implanted in the left renal subcapsular space with 300 MMC-treated or nontreated islets cultured for 3 d. Mice with normalized blood glucose levels were killed on days 4 or 8 after grafting. Specimens of the transplanted kidney containing the islet graft were fixed in 10% formalin (GraphPad, La Jolla, CA, USA), embedded in paraffin (Sakura Finetek Japan Co, Tokyo, Japan), and serially cut into 4-µm thick sections. The sections were stained with hematoxylin and eosin (H&E) to estimate the host’s cell-mediated immune response. Subsequently, monocytes, T cells, and B cells that migrated to the transplantation site were subjected to immunohistochemical analyses.
To detect monocytes and T cells, we used anti-F4/80 mAb (1:200 dilution) and anti-CD3 mAb (1:100 dilution), each purchased from Abcam, and a rabbit anti-rat IgG (DAKO, Glostrup, Denmark) secondary antibody. For the detection of B cells, we used biotin-conjugated rat anti-mouse CD45 R mAb (1:50 dilution; Thermo Fisher Scientific Inc, Waltham, MA, USA) and rabbit anti-rat IgG (DAKO).
Statistical Analysis
Data are expressed as the mean ± standard error of mean (SEM). The IL-6, MCP-3, and MMP-2 concentrations in islet culture supernatants were compared using the Student t test, and chemotactic activities were compared, as appropriate, using the Student t test or 1-way analysis of variance (ANOVA) with Bonferroni adjustment. Statistical significance was defined as P < 0.05. All statistical analyses were performed using Graph-Pad PRISM 5.0 Software (GraphPad, La Jolla, CA, USA).
Results
Comprehensive Analysis of Gene Expression by Rat Islets in Response to MMC Treatment and Subsequent Culture
Compared with control islets (nontreated d0-islets), the gene expression analysis revealed 735 differentially expressed transcripts in nontreated d3-islets, including 526 upregulated and 209 downregulated transcripts. In MMC-treated islets, 115 differentially upregulated transcripts in d0-islets and 812 differentially expressed transcripts in d3-islets, including 335 upregulated and 477 downregulated transcripts (Table 1). These analyses indicate that upregulated transcripts increased during the culture period in nontreated islets, whereas the repression of gene expression by pretreatment with MMC is enhanced during the 3 d of culture.
Table 1.
Comprehensive Analysis of Gene Expression by Non-Treated and MMC-Treated Rat Islets.a
| Islet Sample | Number of Differentially Expressed Genes | ||
|---|---|---|---|
| Non-treated islets | d0-Islets (Control) | d1-Islets | d3-Islets |
| Total genes | 723 | 735 | |
| Upregulated genes | 398 | 526 | |
| Downregulated genes | 325 | 209 | |
| MMC-treated islets | d0-Islets | d1-islets | 3d-islets |
| Total genes | 115 | 797 | 812 |
| Upregulated genes | 115 | 376 | 335 |
| Downregulated genes | 0 | 421 | 477 |
aGene expression analysis: differentially expressed genes were characterized as those with a fold-change >2, or ≤2 (log2) compared with the control (untreated d0-islets).
Identification of Differences in Biological Functions between MMC-Treated and Untreated Islets
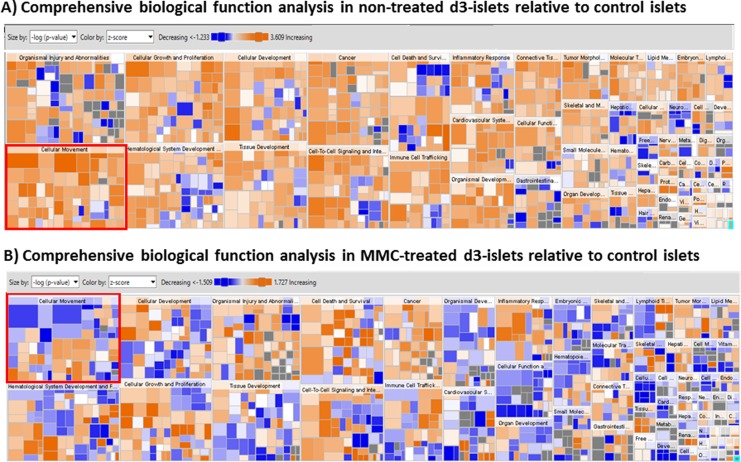
The hierarchical gene cluster map based on the activation z-score and P value indicated that upregulated biological functions were dominant in nontreated islets in response to 3-d culture, whereas biological functions, including “cellular movement,” were downregulated in MMC-treated islets (Fig. 1). Among molecular and cellular functions, expression levels of numerous genes involved function such as “cellular movement” were upregulated in nontreated d3-islets (Fig. 2). On the other hand, more than half of the functions, including “cellular movement,” were downregulated in MMC-treated d3-islets (Fig. 3A and B). These findings indicate that the functional category associated with “cellular movement” was more significantly changed between nontreated d3-islets and MMC-treated d3-islets.
Fig. 1.
Comprehensive interpretation of biological functions in nontreated and mitomycin C (MMC)-treated islets in response to 3-d culture. We used the ingenuity pathway analysis (IPA) algorithm to determine the z-score and the P value to identify significant biological functions associated with MMC-induced engraftment (MMC- and nontreated d3-islets compared with control islets [nontreated d0-islets]). Each category was sized and arranged in order of –log (P value) and colored by the activation z-score (red; upregulation, blue; downregulation). Compared to nontreated islets, many biological functions were downregulated in MMC-treated d3-islets; most significantly downregulated function was cellular movement.
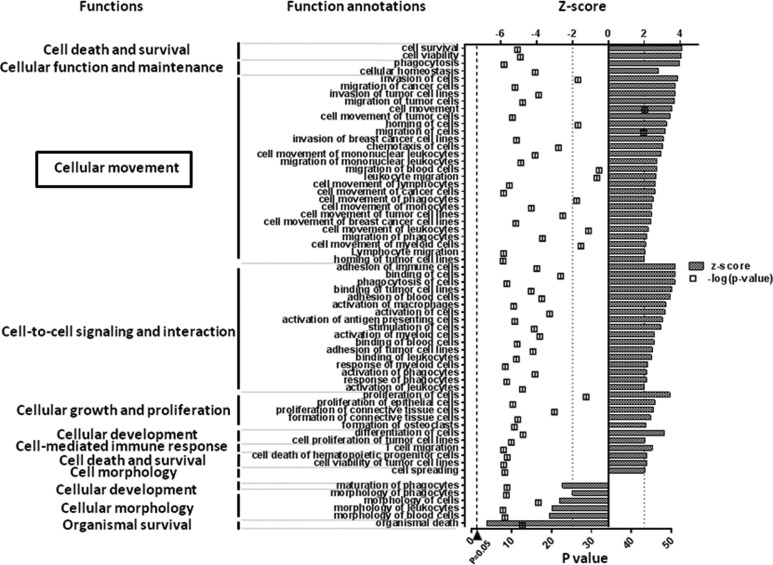
Fig. 2.
Significant alteration of annotated molecular and cellular functions in nontreated islets. We sorted molecular and cellular function annotations according to the z-score as follows: >2 (upregulation) or ≤2 (downregulation). The P value (square dot), which was calculated using the Fisher exact test, indicates the statistical significance of the association between the expression of a set of genes and a biological function (P value ≤ 0.05, −log10 ≥ 1.3). Expression levels of numerous genes involved function such as “cellular movement” were upregulated significantly in nontreated d3-islets during culture period prior to transplantation.
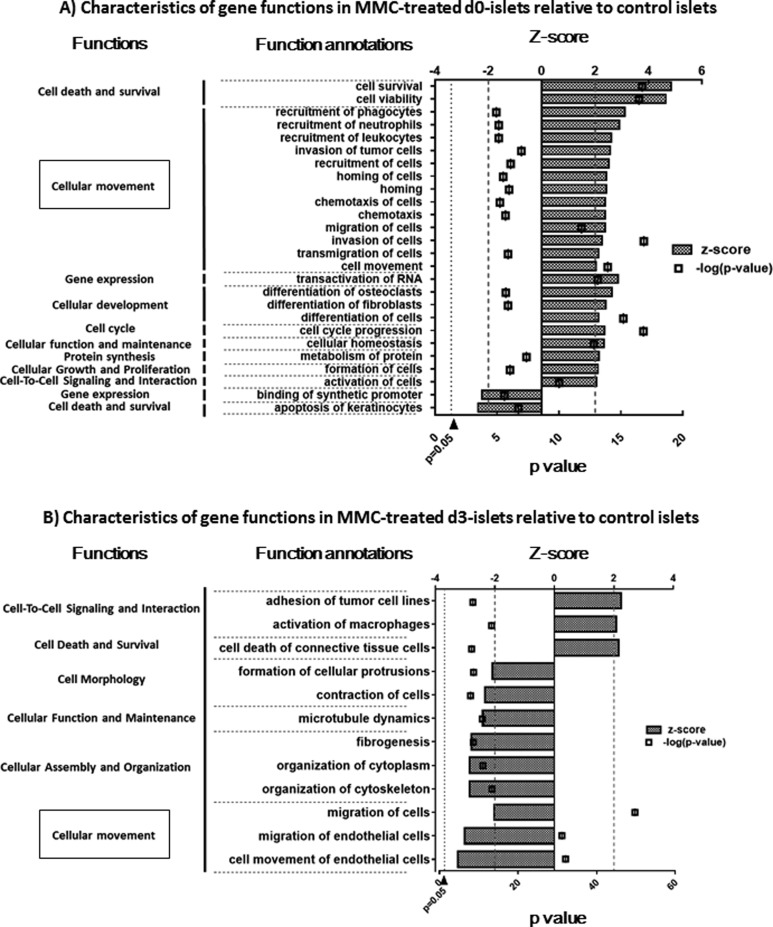
Fig. 3.
Significant alteration of annotated molecular and cellular functions in mitomycin C (MMC)-treated islets. Significant alteration of annotated molecular and cellular functions in MMC-treated islet was evaluated, as described in Fig. 2. A number of downregulated functions were increased over subsequent 3-d culture period in MMC-treated islets, and more than half of the functions, including “cellular movement,” were downregulated in MMC-treated d3-islets.
Among the genes that constituted the “cellular movement” category, the number of downregulated genes that encode chemoattractants, such as cytokines and peptidases, was increased in MMC-treated islets as a function of time in culture (Table 2). Many more genes associated with cytokine and peptidase were upregulated in nontreated d3-islets than in control islets (data were not shown). These findings indicate that multiple chemoattractant genes were upregulated in isolated islets as a result of the stress of isolation procedures, whereas ex vivo pretreatment with MMC reduced the immunogenicity of isolated islets by repressing the gene expression of multiple proinflammatory molecules.
Table 2.
Time-Dependent Alterations of Expressed Genes Involved in Cytokine and Peptidase Functions of MMC-Treated Islets Relative to Non-Treated Islets.a
| Gene Category | d0-Islets | d1-Islets | d3-Islets | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Symbol | Gene Name | FC | Symbol | Gene Name | FC | Symbol | Gene Name | FC | |
| Cytokines | IL6 | Interleukin 6 | 9.041 | CXCL10 | Chemokine ligand 10 | 4.530 | CXCL10 | Chemokine ligand 10 | 8.839 |
| CCL13 | Chemokine ligand 13 | 7.102 | WNT5A | Wingless-type MMTV integration site family, member 5A | −2.085 | IL15 | Interleukin 15 | 2.374 | |
| CXCL10 | Chemokine ligand 10 | 5.657 | IL11 | Interleukin 11 | −3.936 | CXCL13 | Chemokine ligand 13 | −2.258 | |
| CXCL2 | Chemokine ligand 2 | 4.629 | CXCL14 | Chemokine ligand 14 | −2.311 | ||||
| CXCL3 | Chemokine ligand 3 | 4.350 | IL10 | Interleukin 10 | −2.969 | ||||
| WNT5A | Wingless-type MMTV integration site family, member 5A | −3.050 | |||||||
| CCL7 (MCP3) | chemokine ligand 7 | −3.819 | |||||||
| IL33 | Interleukin 33 | −5.372 | |||||||
| IL6 | Interleukin 6 | −7.659 | |||||||
| Peptidase | ND | ND | ND | ADAM8 | ADAM metallopeptidase domain 8 | 3.812 | ADAM*8 | ADAM* metallopeptidase domain 8 | 11.024 |
| C3 | Complement component 3 | −3.454 | LOC286960 | LOC286960 | 3.782 | ||||
| CASP1 | Caspase 1, apoptosis-related cysteine peptidase | −2.360 | |||||||
| MMP9 | Matrix metallopeptidase 9 | −2.406 | |||||||
| PLAU | Plasminogen activator, urokinase | −2.507 | |||||||
| MMP7 | Matrix metallopeptidase 7 | −3.203 | |||||||
| MMP14 | Matrix metallopeptidase 14 | −3.872 | |||||||
| C3 | Complement component 3 | −4.536 | |||||||
| MMP2 | matrix metallopeptidase 2 | −4.721 | |||||||
| CTSC | Cathepsin C | −4.843 | |||||||
| GZMB | Granzyme B | −4.997 | |||||||
Abbreviations: ADAM, A disintegrin and A metalloprotease; FC, Fold change; MMP, metallopeptidase 2; ND, not determined.
aGene lists were created by comparing expressed genes involved in cellular movement between MMC-treated islets and untreated islets.
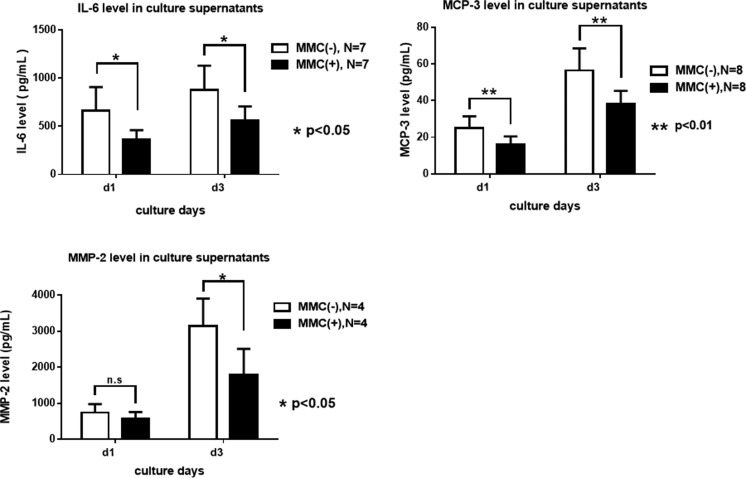
Analysis of IL-6, MCP-3, and MMP-2 Levels in Islet Supernatants
To validate microarray data, we selected cytokines and peptidase genes with the highest differences in mRNA levels (IL-6, fold-increase [FC] −7.659; MCP-3, FC −3.819, and matrix metallopeptidase-2 (MMP-2), FC −4.721; Table 2). The levels of IL-6, MCP-3, and MMP-2 in islet culture supernatants were more significantly decreased in MMC-treated islets than in untreated islets (Fig. 4). These data are consistent with the gene expression data.
Fig. 4.
Rat pancreatic islets produce and secrete interleukin 6 (IL-6), monocyte chemoattractant protein 3 (MCP-3), and metallopeptidase 2 (MMP-2). Supernatants from primary cultures of rat islets were analyzed using the enzyme-linked immunosorbent assay (ELISA) Kit. The IL-6, MCP-3, and MMP2 levels in islet culture supernatants were significantly higher in untreated islets than in MMC-treated islets.
Chemotaxis of Mouse Monocytes in Response to Culture Supernatants from MMC-Treated or Untreated Islet Cultures
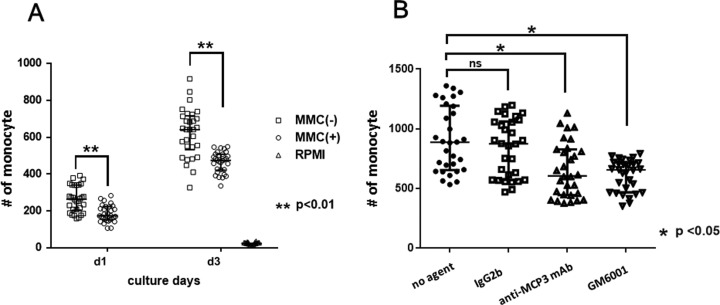
We next attempted to answer the crucial question of whether ex vivo MMC treatment reduces the chemotactic activity of leukocytes in response to islets. We found that mean number of monocytes that migrated toward the islet culture supernatant in 1 high-power field was significantly lower for MMC-treated islets than for untreated islets (Fig. 5).
Fig. 5.
Mouse monocyte chemotactic activity of supernatants harvested from cultures of MMC-treated or untreated islets. Undiluted supernatants of cultured islets (day 1 or 3) were used in chemotaxis assays of mouse monocytes. The results are expressed as the mean number of migrated monocytes toward the islet culture supernatant per high-power field. (A) The number of monocytes was significantly lower in MMC-treated islets than in untreated islets. (B) Compared with controls, the mean number of migrated monocyte was significantly decreased in islet culture supernatants treated with anti- monocyte chemoattractant protein 3(MCP-3) monoclonal antibody (mAb) or the metallopeptidase 2 (MMP) inhibitor GM6001.
To determine whether the validated molecules encoded by highly downregulated genes function as key components of chemoattractants secreted from islets, we assessed monocyte chemotaxis in response to supernatants treated with MCP-3-neutralizing antibody (anti-MCP-3 mAb) or an inhibitor of MMP activity (GM6001). The mean number of migrated monocytes significantly decreased under both conditions (Fig. 5).
Histology of Representative Transplanted Islets Harvested from C57BL/6 Recipients on Day 4 or 8
To assess the localized immunological response to an implanted graft in a concordant xeno-transplantation experiment (B6 mice engrafted with Wistar rat islets), we used immunohistochemistry to detect leukocytes that infiltrated the transplantation site. In this transplantation experiment, prolonged graft survival induced by ex vivo MMC treatment was determined. The median survival time (MST) of engrafted MMC-treated d3-islets was significantly prolonged compared with that of untreated d3-islets (median survival time [MST]; 98 days vs. 17 days, P < 0.01), which is consistent with the results of our previous study.13
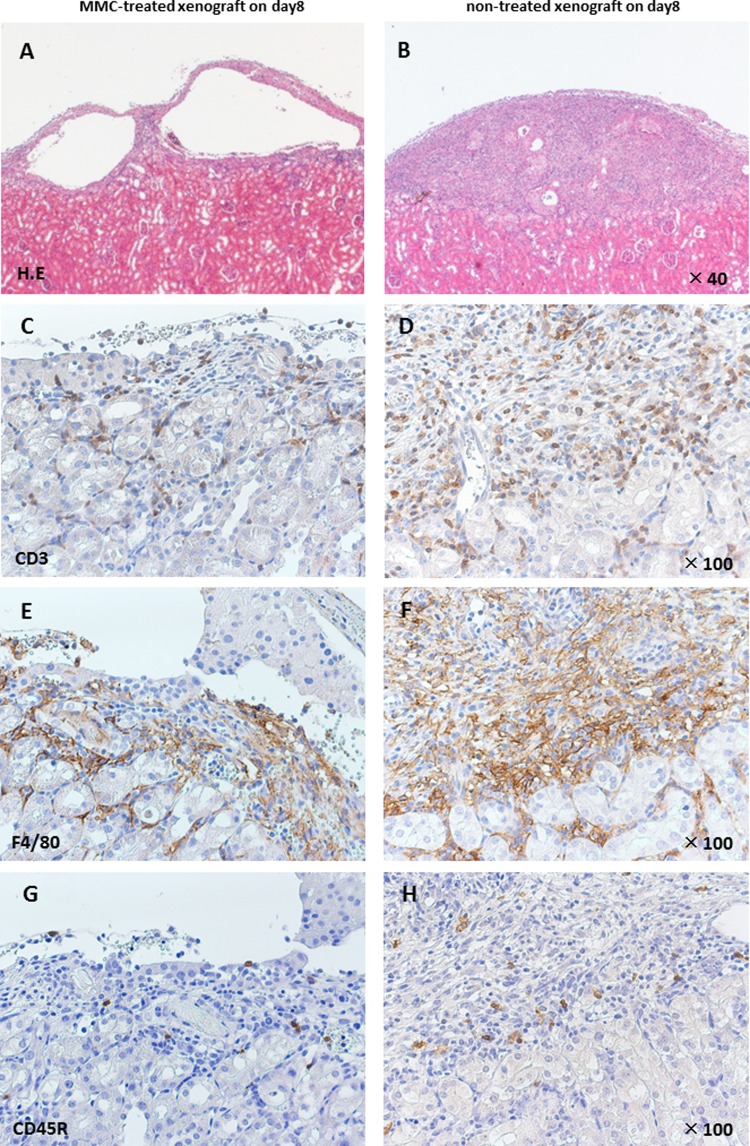
Histological analysis revealed marked infiltration of inflammatory cells in untreated xenografts, whereas significantly fewer leukocytes were observed in MMC-treated xenografts 8 d after transplantation (Fig. 6).
Fig. 6.
Histology of representative Wistar rat islets harvested from C57BL/6 recipient on day 4 or 8. Representative sections of xenografts treated with 10 µg/mL mitomycin C (MMC; A, C, E, and G) or untreated xenografts (B, D, F, and H). Paraffin-embedded sections were reacted with antibodies specific to T cells (CD3), macrophages (F4/80), and B cells (CD45 R).
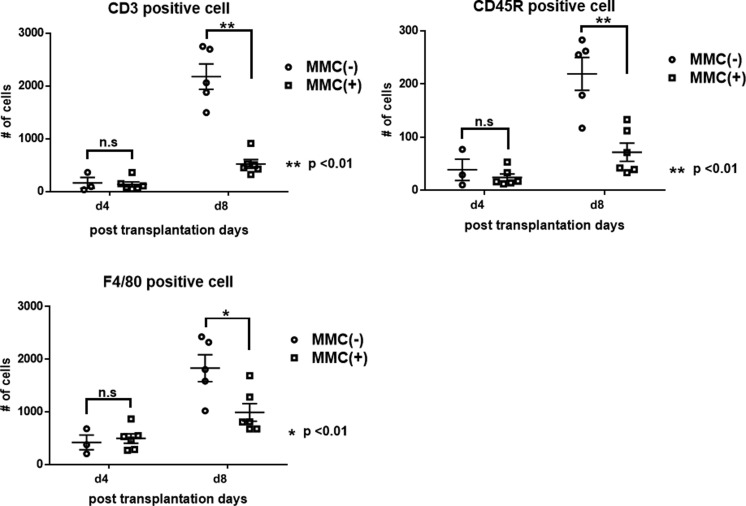
Immunohistochemical analysis of the leukocytes that infiltrated the site of engraftment in the kidneys revealed that ex vivo pretreatment with MMC significantly suppressed the infiltration of monocytes, T cells, and B cells 8 d after engraftment, although there was no significant difference in leukocyte infiltration between the 2 groups after 4 d (Fig. 7). These findings demonstrates that pretreating islets with MMC inhibits the chemotaxis of host leukocytes to xenografts in vivo and prolongs the survival of the grafts.
Fig. 7.
Analysis of metallopeptidase 2 (MMC)-induced immunosuppression of xenografts. Immune cells that infiltrated the xenografts were stained and counted. MMC-treated (circles) and untreated (squares) islet xenografts. The dominant components of the leukocyte population that migrated to the xenograft were CD3-positive T cells and macrophages. The numbers of T cells, macrophages, and B cells significantly decreased in MMC-treated islet xenografts (P < 0.05).
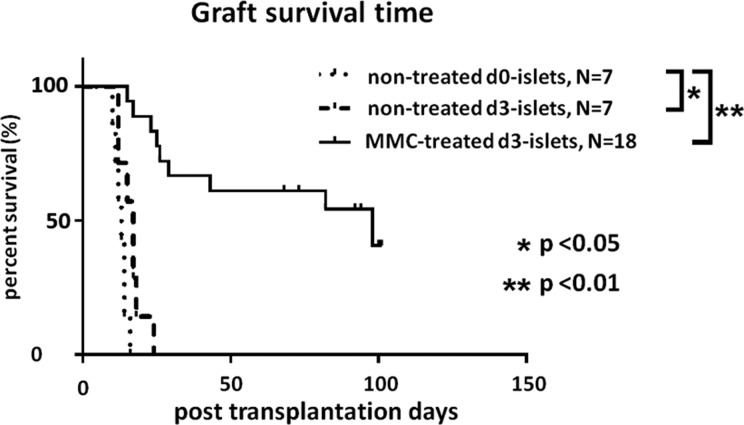
Survival of Untreated and MMC-Treated Engrafted Islets
Prolonged graft survival induced by ex vivo MMC treatment was determined in a concordant model of islet xeno-transplantation. The MST of engrafted MMC-treated d3-islets was significantly prolonged compared with that of untreated d3-islets (MST; 98 days vs. 17 days, P < 0.01; Fig. 8). Notably, 40% of the MMC-treated grafts survived for >100 days without adding immunosuppressive agents, which is consistent with the results of a previous study.13
Fig. 8.
Survival of metallopeptidase 2 (MMC)- and untreated islet xenografts (Wistar rat islets to B6 mice). Prolonged survival of MMC-treated d3-islets (solid line) compared with that of untreated d0- and d3-islets (dotted lines; P < 0.01).
Discussion
The present study provides a comprehensive interpretation of the biological characteristics of islets following their exposure to MMC ex vivo and subsequent short-term (3 d) culture. Specifically, our islet-preconditioning protocol induced simultaneous downregulation of 3 important chemoattractants (IL-6, MCP-3, and MMP-2) as compared to nontreated islets, and the secretion and chemoattractant activities of islets were validated to be suppressed in in vitro studies. The in vivo study investigating the host immune response to implanted grafts demonstrated that infiltration of monocytes and CD3-positive T cells were significantly decreased in MMC-treated islets. To the best of our knowledge, this is the first report using a single protocol to induce the downregulation of multiple cytokines, suggesting a clinical application of this pretreatment protocol.
The comprehensive analysis of gene expression demonstrated a contrasting change between MMC-treated and nontreated islets. The upregulated transcripts increased during the culture period in nontreated islets, whereas the number of expressed transcripts in MMC-treated islets further decreased the following culture (Table 1). Among molecular and cellular function categories, the expression levels of numerous genes involved in functions such as “cellular movement” were upregulated in nontreated d3-islets, but downregulated in MMC-treated d3-islets (Figs. 2 and 3).
The levels of upregulated multiple proinflammatory genes in isolated islets during subsequent culture were comparable with those of a previous finding by Cowley et al, who showed that isolated human islets express high levels of a set of chemokines in association with a marked nuclear factor (NF-κB)-dependent proinflammatory response based on a comprehensive analysis of gene expression.8 In addition, they showed that the inflammatory profile of isolated human islets was traced even after transplantation into severe combined immunodeficient (SCID) mouse recipients. They also found that the forced expression of NF-κB in rodent islets induced the same proinflammatory chemokine profile and glucose intolerance in syngeneic recipients and accelerated rejection in allogeneic recipients.
MMC treatment prolonged islet xenograft survival with a half of the islet grafts surviving indefinitely in nonimmunosuppressed STZ-induced diabetic mice (Fig. 8). A significant inhibition of inflammatory response against islet grafts has consistently been observed in histological studies.13,24 In line with these observations, we reasonably assumed that a multiple cytokine regulatory regimen in the MMC pretreatment of islets protects islets against an acquired immune response to grafts.
Thus, questions naturally arise as to candidate chemoattractants to injure islets and to initiate rejection responses to islet grafts. Proinflammatory cytokines including tumor necrosis factor α ,25 MCP-1,22 and the alarmin high mobility group box 1 (HMGB1)26 act as strong stimulators of the host innate cell-mediated immune response. These molecules may serve as therapeutic targets in efforts to enhance islet transplantation11,12; however, the effect was limited. Citro et al.12 insisted that anti-inflammatory treatment that targets a single proinflammatory axis is insufficient because of the redundancy and promiscuity of chemokine signaling mechanisms.
Our bioinformatics analysis revealed that ex vivo pretreatment of islets with MMC downregulated various cytokine genes and suppressed the secretion of IL-6, MCP-3, and MMP-2 by isolated islets as well as the chemotaxis of monocytes toward islets. These findings suggest that such treatment reduces early-phase proinflammatory events provoked by damaged and inflamed islets. Further, the suppression of monocyte chemotaxis toward the islet culture medium was reproduced using anti-MCP-3 mAb and a pharmacological inhibitor of MMPs, suggesting that MCP-3 and MMPs play an important role in monocyte recruitment to grafted islets during the early stage of the innate immune response and therefore serve as potential therapeutic targets.
Few studies have shown that MCP-3 and MMPs contribute to the outcome of islet transplantation. The chemokine MCP-3, which is a member of the CC-chemokine subfamily, is one of the most pleiotropic chemokines because it activates all major leukocytes by stimulating the specific receptors CC-chemokine receptor (CCR) 1, CCR2, CCR3, and CCR5.27 Accordingly, Ali et al.28 employed a mouse model of skin transplantation to show that an MCP-3 antagonist reduces the infiltration of grafts by CD3+ lymphocytes and consequently increases the survival of allografts.
MMPs are members of a family of zinc-dependent endopeptidases that mediate the turnover of the extracellular matrix as well as the invasion of grafts by inflammatory cells.29 Consistent with the findings of the present study, Barro et al.30 found that mRNAs encoding MMP-2, MMP-3, MMP-9, MMP-14, tissue inhibitor of metalloproteinase 1 (TIMP-1), and TIMP-2 are expressed by isolated rat pancreatic islets. Further, Lingwal et al.23 found that MMP-9 expression and activity after islet transplantation are relevant to leukocyte chemotaxis and early islet graft function.
The IL-6 gene was downregulated by MMC treatment. IL-6 plays an important role in inflammation by stimulating the section of IL-8 and MCP-1 chemokines and upregulating the expression of adhesion molecules such as intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1).31 The downregulation of IL-6 gene expression might be related to the reduced immunogenicity of islets. Our interpretation regarding the role of IL-6 in islet transplantation is supported by the findings of Itoh et al.,32 who revealed that the anti-IL-6 R antibody prevents the early loss of intrahepatic islet grafts in a syngeneic and an allogeneic combination.
Our findings lead us to the conclusion that preconditioning islets to suppress chemoattractant secretion is a reasonable approach because reduction in the immunogenic potential of islets before implantation may contribute to the suppression of the early innate immune response mediated by the infiltration of immunocytes such as neutrophils or macrophages. We showed that the infiltration of the graft by monocytes and CD3-positive T cells in vivo was significantly suppressed by ex vivo MMC pretreatment (Figs. 6 and 7), which is consistent with the results of a previous study.24
There are certain limitations associated with the present study. First, biological functions other than “cellular movement” may be involved in MMC-induced engraftment because suppression of multiple chemotactic factors from islets is insufficient evidence for revealing the underlying mechanism of the MMC-induced inhibition of inflammatory cellular infiltration (Figs. 6 and 7) and engraftment with no immunosuppressive treatment. Further research that focuses on induction of donor-specific tolerance by MMC pretreatment as well as cellular movement is required. Second, the cytokine profile of post-grafting islets in vivo is recommended in future studies, in which we will know how the in vitro difference contributes to the diversity of cytokine profiles in in vivo situations. Finally, in the present study, the effects of ex vivo MMC pretreatment were demonstrated in rodent islets. Validation of the present results in human islets would significantly increase the relevance of our study.
In conclusion, the results of our comprehensive analysis of genes differentially expressed by isolated pancreatic islets reveal that ex vivo pretreatment of islets with MMC simultaneously suppresses the secretion of leukocyte chemotactic factors inducing the synergistic suppression of leukocyte chemotaxis, leading to enhanced islet engraftment without immunosuppressive agents. These findings suggest that this ex vivo pretreatment of islets reduces the immunogenic potential of islets and prolongs the survival of islet grafts. A better understanding of the mechanism will likely contribute to the clinical application of this pretreatment protocol.
Acknowledgments
We thank Professor Teru Okitsu for inspired guidance and invaluable suggestions and Yukiko Kikuta, Eiko Otomo, Yuiko Watanabe, Yukari Yuda, and Hidemi Sasaki for excellent technical support.
Footnotes
Ethical Approval: The Ethics Review Committee for Animal Experimentation of Fukushima Medical University approved this study.
Statement of Human and Animal Rights: Rats and mice were housed in cages at a controlled temperature and under a suitable light–dark cycle. All animals were anesthetized using isoflurane inhalation. The Ethics Review Committee for Animal Experimentation of Fukushima Medical University approved this study.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: This work was supported by a Grant-in-Aid for Scientific Research 15K10033 and 15K199902 from the Japan Society for the Promotion of Science.
References
- 1. Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355(13):1318–1330. [DOI] [PubMed] [Google Scholar]
- 2. Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JR, Shapiro AM. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54(7):2060–2069. [DOI] [PubMed] [Google Scholar]
- 3. Bennet W, Groth CG, Larsson R, Nilsson B, Korsgren O. Isolated human islets trigger an instant blood mediated inflammatory reaction: implications for intraportal islet transplantation as a treatment for patients with type 1 diabetes. Upsala J Med Sci. 2000;105(2):125–133. [DOI] [PubMed] [Google Scholar]
- 4. Monti P, Vignali D, Piemonti L. Monitoring inflammation, humoral and cell-mediated immunity in pancreas and islet transplants. Curr Diabetes Rev. 2015;11(3):135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sleater M, Diamond AS, Gill RG. Islet allograft rejection by contact-dependent CD8+ T cells: perforin and FasL play alternate but obligatory roles. Am J Transplantat. 2007;7(8):1927–1933. [DOI] [PubMed] [Google Scholar]
- 6. Bottino R, Balamurugan AN, Tse H, Thirunavukkarasu C, Ge X, Profozich J, Milton M, Ziegenfuss A, Trucco M, Piganelli JD. Response of human islets to isolation stress and the effect of antioxidant treatment. Diabetes. 2004;53(10):2559–2568. [DOI] [PubMed] [Google Scholar]
- 7. Noguchi H, Naziruddin B, Jackson A, Shimoda M, Ikemoto T, Fujita Y, Chujo D., Takita M, Peng H, Sugimoto K, et al. Fresh islets are more effective for islet transplantation than cultured islets. Cell Transplant. 2012;21(2-3):517–523. [DOI] [PubMed] [Google Scholar]
- 8. Cowley MJ, Weinberg A, Zammit NW, Walters SN, Hawthorne WJ, Loudovaris T, Thomas H, Kay T, Gunton JE, Alexander SI, et al. Human islets express a marked proinflammatory molecular signature prior to transplantation. Cell Transplant. 2012;21(9):2063–2078. [DOI] [PubMed] [Google Scholar]
- 9. Negi S, Jetha A, Aikin R, Hasilo C, Sladek R, Paraskevas S. Analysis of beta-cell gene expression reveals inflammatory signaling and evidence of dedifferentiation following human islet isolation and culture. PloS One. 2012;7(1): e30415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nano R, Racanicchi L, Melzi R, Mercalli A, Maffi P, Sordi V, Ling Z, Scavini M, Korsgren O, Celona B, et al. Human pancreatic islet preparations release HMGB1: (ir)relevance for graft engraftment. Cell Transplant. 2013;22(11):2175–2186. [DOI] [PubMed] [Google Scholar]
- 11. Merani S, Truong WW, Hancock W, Anderson CC, Shapiro AM. Chemokines and their receptors in islet allograft rejection and as targets for tolerance induction. Cell Transplant. 2006;15(4):295–309. [PubMed] [Google Scholar]
- 12. Citro A, Cantarelli E, Piemonti L. Anti-inflammatory strategies to enhance islet engraftment and survival. Curr Diabetes Rep. 2013;13(5):733–744. [DOI] [PubMed] [Google Scholar]
- 13. Gunji T, Saito T, Sato Y, Matsuyama S, Ise K, Kimura T, Anazawa T, Gotoh M. Mitomycin-C treatment followed by culture produces long-term survival of islet xenografts in a rat-to mouse model. Cell Transplant. 2008;17(6):619–629. [DOI] [PubMed] [Google Scholar]
- 14. Matsuyama S, Gunji T, Ise K, Sato Y, Saito T, Gotoh M. Permanent acceptance of mitomycin C-treated islet allograft. Transplantation. 2003;76(1):65–71. [DOI] [PubMed] [Google Scholar]
- 15. Cummings J, Spanswick VJ, Tomasz M, Smyth JF. Enzymology of mitomycin C metabolic activation in tumour tissue: implications for enzyme-directed bioreductive drug development. Biochem Pharmacol. 1998;56(4):405–414. [DOI] [PubMed] [Google Scholar]
- 16. Tomasz M, Palom Y. The mitomycin bioreductive antitumor agents: cross-linking and alkylation of DNA as the molecular basis of their activity. Pharmacol Ther. 1997;76(1-3):73–87. [DOI] [PubMed] [Google Scholar]
- 17. Hardy MA, Lau H, Weber C, Reemtsma K. Pancreatic islet transplantation. Induction of graft acceptance by ultraviolet irradiation of donor tissue. Ann Surg. 1984;200(4):441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kanai T, Porter J, Gotoh M, Monaco AP, Maki T. Effect of gamma-irradiation on mouse pancreatic islet-allograft survival. Diabetes. 1989;38(suppl 1):154–156. [DOI] [PubMed] [Google Scholar]
- 19. Grtochowiecki T, Gotoh M, Dono K, Takeda Y, Nishihara M, Ohta Y, Ota H, Ohzato H, Okuyama M, Shimizu J, et al. Pretreatment of crude pancreatic islets with mitomycin C prolongs graft survival time in xenogeneic rat-to-mouse model. Transplantation. 1999;67(11):1474–1477. [DOI] [PubMed] [Google Scholar]
- 20. Saito T, Ohashi K, Utoh R, Shimizu H, Ise K, Suzuki H, Yamato M, Okano T, Gotoh M. Reversal of diabetes by the creation of neo-islet tissues into a subcutaneous site using islet cell sheets. Transplantation. 2011;92(11):1231–1236. [DOI] [PubMed] [Google Scholar]
- 21. Yona S, Hayhoe R, Avraham-Davidi I. Monocyte and neutrophil isolation and migration assays. Current Protoc Immunol. 2010;Chapter 14: Unit 14.15. [DOI] [PubMed] [Google Scholar]
- 22. Piemonti L, Leone BE, Nano R, Saccani A, Monti P, Maffi P, Bianchi G, Sica A, Peri G, Melzi R, et al. Human pancreatic islets produce and secrete MCP-1/CCL2: relevance in human islet transplantation. Diabetes. 2002;51(1):55–65. [DOI] [PubMed] [Google Scholar]
- 23. Lingwal N, Padmasekar M, Samikannu B, Bretzel RG, Preissner KT, Linn T. Inhibition of gelatinase B (matrix metalloprotease-9) activity reduces cellular inflammation and restores function of transplanted pancreatic islets. Diabetes. 2012;61(8):2045–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ise K, Kanazawa Y, Sato Y, Matsuyama S, Gunji T, Endo Y, Hojo H, Abe M, Gotoh M. Survival of mitomycin C-treated pancreatic islet xenografts is mediated by increased expression of transforming growth factor-beta. Transplantation. 2004;77(6):907–914. [DOI] [PubMed] [Google Scholar]
- 25. Angaswamy N, Fukami N, Tiriveedhi V, Cianciolo GJ, Mohanakumar T. LMP-420, a small molecular inhibitor of TNF-alpha, prolongs islet allograft survival by induction of suppressor of cytokine signaling-1: synergistic effect with cyclosporin-A. Cell Transplant. 2012;21(6):1285–1296. [DOI] [PubMed] [Google Scholar]
- 26. Matsuoka N, Itoh T, Watarai H, Sekine-Kondo E, Nagata N, Okamoto K, Mera T, Yamamoto H, Yamada S, Maruyama I, et al. High-mobility group box 1 is involved in the initial events of early loss of transplanted islets in mice. J Clin Invest. 2010;120(3):735–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu LL, McVicar DW, Ben-Baruch A, Kuhns DB, Johnston J, Oppenheim JJ, Wang JM. Monocyte chemotactic protein-3 (MCP3) interacts with multiple leukocyte receptors: binding and signaling of MCP3 through shared as well as unique receptors on monocytes and neutrophils. Eur J Immunol. 1995;25(9):2612–2617. [DOI] [PubMed] [Google Scholar]
- 28. Ali S, O’Boyle G, Hepplewhite P, Tyler JR, Robertson H, Kirby JA. Therapy with nonglycosaminoglycan-binding mutant CCL7: a novel strategy to limit allograft inflammation. Am J Transplant. 2010;10(1):47–58. [DOI] [PubMed] [Google Scholar]
- 29. Song J, Wu C, Zhang X, Sorokin LM. In vivo processing of CXCL5 (LIX) by matrix metalloproteinase (MMP)-2 and MMP-9 promotes early neutrophil recruitment in IL-1beta-induced peritonitis. J Immunol. 2013;190(1):401–410. [DOI] [PubMed] [Google Scholar]
- 30. Barro C, Zaoui P, Morel F, Benhamou PY. Matrix metalloproteinase expression in rat pancreatic islets. Pancreas. 1998;17(4):378–382. [DOI] [PubMed] [Google Scholar]
- 31. Ro H, Lee EW, Hong JH, Han KH, Yeom HJ, Kim HJ, Kim MG, Jung HS, Oh KH, Park KS, et al. Roles of islet Toll-like receptors in pig to mouse islet xenotransplantation. Cell Transplant. 2013;22(9):1709–1722. [DOI] [PubMed] [Google Scholar]
- 32. Itoh T, Nitta T, Nishinakamura H, Kojima D, Mera T, Ono J, Kodama S, Yasunami Y. HMGB1-mediated early loss of transplanted islets is prevented by anti-IL-6 R antibody in mice. Pancreas. 2015;44(1):166–171. [DOI] [PubMed] [Google Scholar]