Abstract
Background
Cognitive deficits are prevalent in people with schizophrenia and associated with functional impairments. In addition to antipsychotics, pharmacotherapy in schizophrenia often includes other psychotropics, and some of these agents possess anticholinergic properties, which may impair cognition. The objective of this study was to explore the association between medication anticholinergic burden and cognition in schizophrenia.
Methods
Seven hundred five individuals with schizophrenia completed a neuropsychological battery comprising Judgment of Line Orientation Test, Wechsler Abbreviated Scale of Intelligence Matrix Reasoning, Continuous Performance Test–Identical Pairs Version, and the Brief Assessment of Cognition in Schizophrenia. Cognitive g and 3 cognitive factor scores that include executive function, memory/fluency, and speed of processing/vigilance, which were derived from a previously published analysis, were entered as cognitive variables. Anticholinergic burden was computed using 2 anticholinergic scales: Anticholinergic Burden Scale and Anticholinergic Drug Scale. Duration and severity of illness, antipsychotic dose, smoking status, age, and sex were included as covariates.
Results
Anticholinergic burden was associated with poorer cognitive performance in cognitive g, all 3 cognitive domains and most cognitive tasks in multivariate analyses. The associations were statistically significant, but the effect sizes were small (for Anticholinergic Burden Scale, Cohen f2 = 0.008; for Anticholinergic Drug Scale, Cohen f2 = 0.017).
Conclusions
Although our results showed a statistically significant association between medications with anticholinergic properties and cognition in people with schizophrenia, the impact is of doubtful or minimal clinical significance.
Key Words: anticholinergic, cognition, schizophrenia
Cognitive deficits are prevalent and stable in people with schizophrenia1–3 and are associated with functional outcomes.4 Both the dopaminergic and cholinergic systems and the balance of the dopaminergic-cholinergic system have been thought to be essential in cognition.5–8 Evidence from animal, neuropharmacological, and magnetic resonance imaging studies shows that the cholinergic system is involved in the modulation of attention and memory encoding.9,10 Sellin et al11 suggested that muscarinic acetylcholine receptors, especially M1 receptors, may be altered in schizophrenia, and this may contribute to deficits in memory and learning in schizophrenia. Goff et al5 reviewed the treatment of cognitive impairment in schizophrenia and suggested strong evidence in the role of muscarinic and nicotinic acetylcholine receptors in cognitive impairment in schizophrenia. A review by Spohn and Strauss12 suggested that anticholinergics affect memory in schizophrenia patients, whereas researchers suggested that muscarinic and nicotinic agonists, cholinesterase inhibitors, and allosteric activators5,11,13–15 may be efficacious in treating cognitive impairment in schizophrenia. Taken together, these findings suggest that cholinergic neurotransmission plays a vital role in cognition and that abnormal cholinergic regulation is associated with cognitive impairment.9
Antipsychotic medications form the mainstay of pharmacological interventions in schizophrenia, whereas concomitant medications such as antidepressants, mood stabilizers, and anxiolytics are not uncommon.16 Some of these medications possess anticholinergic properties, which have been suggested to impair cognitive functions. Furthermore, anticholinergic medications are often coprescribed to ameliorate the extrapyramidal adverse effects brought on by antipsychotics, specifically the typical agents.
Most of the studies on the association between anticholinergic burden and cognition were conducted in the elderly. A review of studies on anticholinergic burden and cognition in the elderly found that all but 2 of 27 studies showed an association between the use of anticholinergic medications and poorer cognition, with specific deficits in processing speed, attention, language, problem solving, and psychomotor performance.17 Ancelin et al18 found anticholinergic drug use to be a strong predictor of mild cognitive impairment in the elderly. Mulsant et al19 found that even low serum anticholinergic activity (SAA) was significantly associated with cognitive impairment in community geriatric sample. Dose-response relationship between Anticholinergic Burden Scale (ACB) total score and cognition as measured by a 6-item orientation memory concentration test was observed, and drug use as identified by ACB scale has been associated with more severe cognitive impairment in elderly people.20 Similarly, an 8-year longitudinal study on patients with Parkinson disease reported that anticholinergic load and duration of anticholinergic drug use were associated with decline in Mini–Mental State Examination.21
Studies on the association between medication anticholinergic burden and cognition in schizophrenia are limited. Serum anticholinergic activity, a suggested biomarker for anticholinergic burden, was found to be higher in schizophrenia patients with poorer verbal recall22–24 and poorer verbal working memory, verbal learning, and memory.25 In addition, SAA level was inversely associated with improvements from a computerized cognitive training in individuals with schizophrenia.25 In another study, exposure to medications with anticholinergic properties was shown to impair attention and declarative memory in schizophrenia, but had no effects on other aspects of cognition, including intelligence, working memory, executive functioning, and motor speed.26
The studies cited above were conducted on relatively small samples, ranging from 10 to 106 participants, which might have affected the consistency in findings between the elderly and people with schizophrenia and even within schizophrenia studies. In addition, the different anticholinergic scales and different cognition measures adopted might have contributed to the variations. The objective of the present study was to examine the association between anticholinergic burden and cognition in people with schizophrenia. For increased reliability, we adopted 2 independently established anticholinergic rating scales to examine the concordance in findings. We hypothesized that a higher anticholinergic burden is associated with poorer cognitive performance in schizophrenia.
MATERIALS AND METHODS
Study Participants
Seven hundred five Chinese individuals with schizophrenia, aged 21 to 55 years, were included in this study. Patients were recruited from both outpatient and inpatient settings in the Institute of Mental Health, Singapore, as well as from community care centers and rehabilitation centers in Singapore. The diagnosis of schizophrenia was ascertained on the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Exclusion criteria were a history of mental retardation, current substance use, clinically significant neurological disorder or brain injuries, and color blindness. Ethics approval for this study was granted by the National Healthcare Group Domain Specific Review Board. All participants provided informed consent to participate in the study.
Cognitive Assessments
All participants were assessed on a comprehensive cognitive battery composed of Benton's Judgment of Line Orientation Test, Wechsler Abbreviated scale of Intelligence Matrix Reasoning, Continuous Performance Test (CPT)–Identical Pairs Version, and Brief Assessment of Cognition in Schizophrenia (BACS).27 The BACS includes Verbal Memory (VM), Digit Sequencing, Token Motor Task (TMT), Semantic Fluency (SF), Symbol Coding (SC), and Tower of London. The SF test includes 3 measures of semantic fluency—animal, fruits, and vegetable. A cognitive model comprising 3 domains, namely, executive function, fluency/memory, and speed/vigilance, was previously published using the same data set.28 The executive function domain consisted of Judgment of Line Orientation Test and Wechsler Abbreviated scale of Intelligence Matrix Reasoning items. The fluency/memory domain consisted of BACS SF and VM items. The speed/vigilance domain consisted of CPT–Identical Pairs Version and BACS TMT and SC items. Cognitive scores for these 3 domains were generated from the model using regression.
Assessment of Anticholinergic Burden
Medication data were collected from the participants' medical record. Total anticholinergic burden was computed using the ACB and Anticholinergic Drug Scale (ADS). Anticholinergic Burden Scale is an expert-rated scale based on systematic review of the literature. Information from the MEDLINE database from 1966 to 2007 on drug anticholinergic properties and cognitive function in older adults was provided to a multidisciplinary team composed of geriatricians, pharmacists, psychiatrists, general physicians, nurses, and aging-brain researchers who categorized the medications into 3 classes of mild, moderate, and severe cognitive anticholinergic negative effects. The ADS was previously referred to as the Clinician-Rated Anticholinergic Scale–modified version. Clinician-Rated Anticholinergic Scale has 340 medications that were rated by 3 geriatric clinicians based on their experience and knowledge. The median of the ratings has strong agreement with Summers' Drug Risk Number and laboratory data.29 Clinician-Rated Anticholinergic Scale was then renamed as ADS and validated by Carnahan et al.30 The ADS was also reported to be associated with serum anticholinergic activities.30 Effect sizes of cognitive g were estimated using Cohen f2, where 0.02, 0.15, and 0.35 represent small, medium, and large effect sizes, respectively.31,32
Statistical Analyses
To explore the association between total anticholinergic loading and cognition, bivariate correlation analyses were performed. Univariate analyses were conducted with total anticholinergic loading of each scale as an independent variable and each normalized cognitive score as a dependent variable. Covariates such as sex, age, smoking status, duration of illness, severity of illness (measured by Positive and Negative Syndrome Scale [PANSS] total score), and impact of antipsychotics were included in subsequent multivariate regression analyses with anticholinergic loading as an independent variable and cognitive score as a dependent variable. The analyses were repeated for each cognitive variable.
All statistical analyses were performed using IBM SPSS statistics 23 (IBM Corp, Armonk, NY). Statistical significance was determined at P < 0.05.
RESULTS
The demographic information of the study sample is presented in Table 1. Correlational analyses revealed statistically significant but weak associations between anticholinergic burden (both ACB and ADS total scores) and all cognitive variables (for ACB, r = −0.080 to −0.238, all P < 0.05; for ADS, r = −0.094 to −0.272, all P < 0.05). The results showed a trend suggesting patients with higher ACB and ADS total scores performed poorer in all cognitive tasks, but the impact of medication on cognition may be minimal. The association between cognitive g and ACB and association between cognitive g and ADS are presented in Figures 1 and 2. Frequency and rating of medications with anticholinergic activity are presented in Table 2.
TABLE 1.
Characteristics of Participants (n = 705)

FIGURE 1.
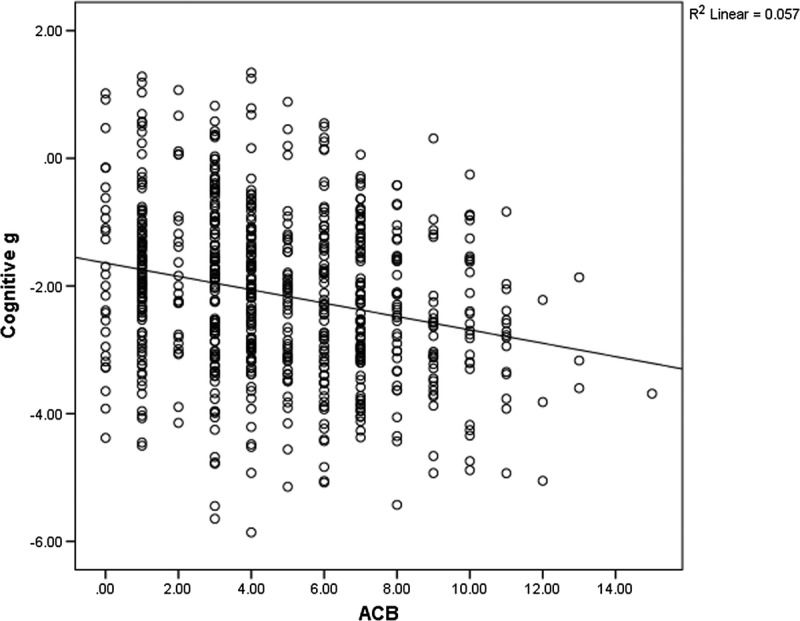
Scatterplot of ACB and cognition g.
FIGURE 2.
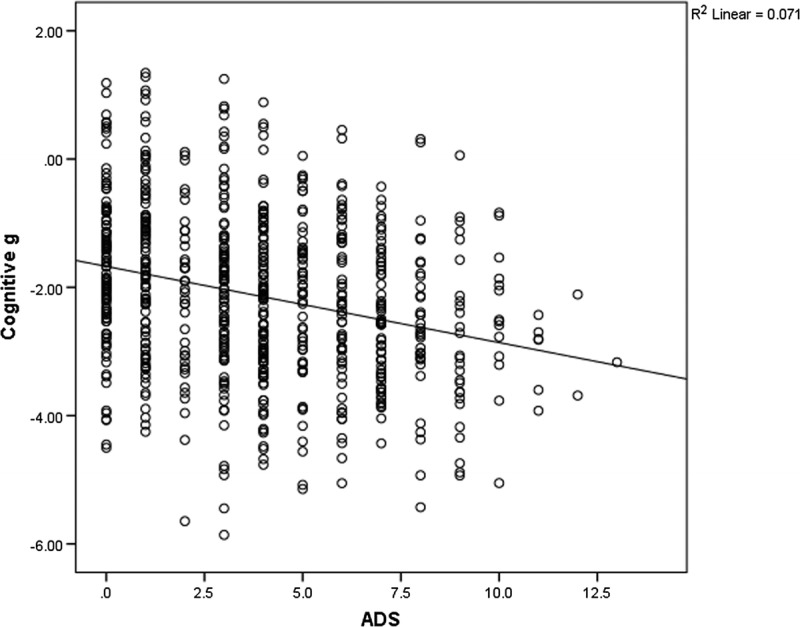
Scatterplot of ADS and cognition g.
TABLE 2.
Frequency and Rating of Medications With Anticholinergic Activity
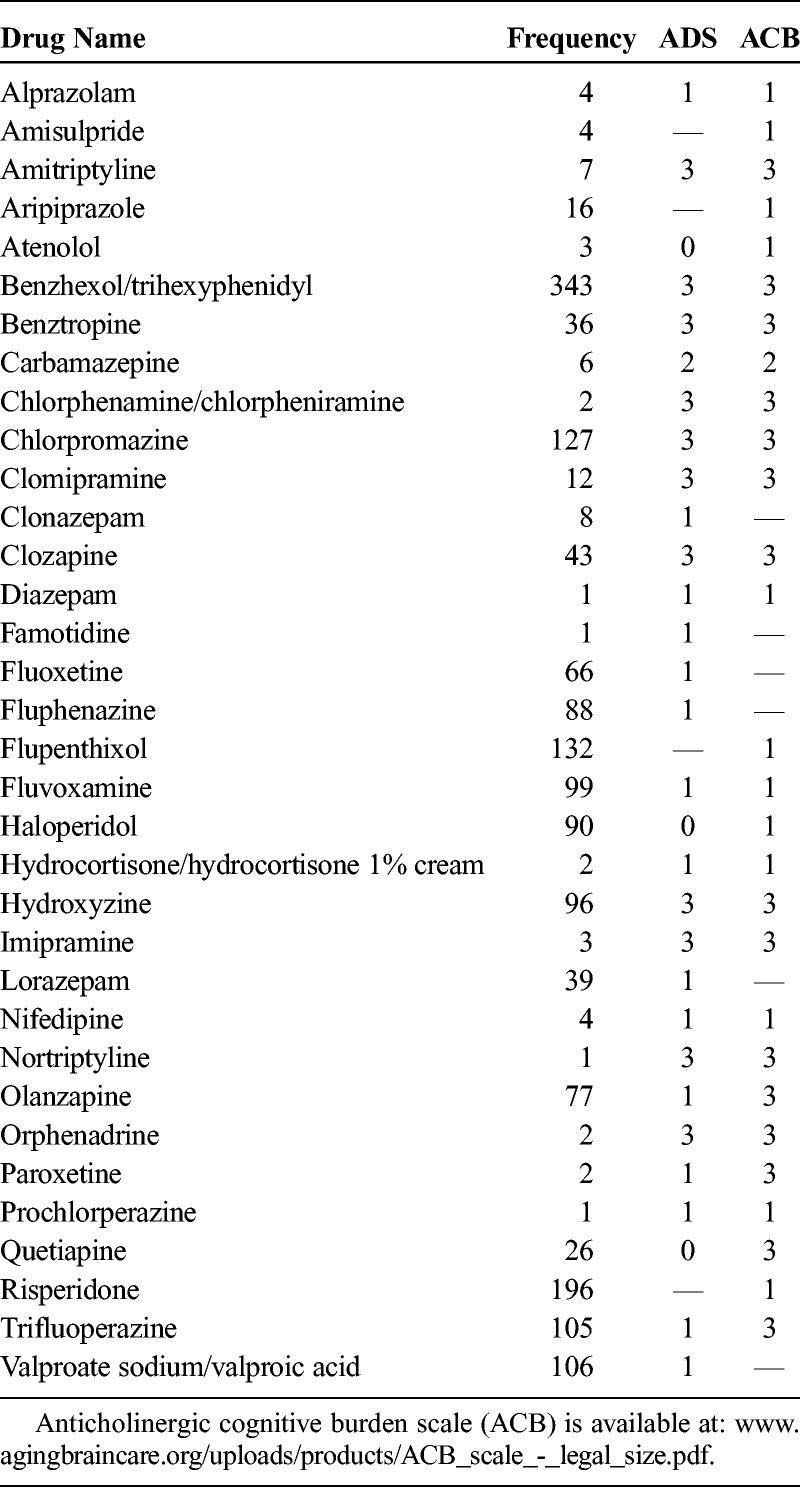
Anticholinergic Burden Scale
Linear regression analyses revealed that higher ACB total scores significantly predicted poorer performance in all cognitive variables (P < 0.001 to 0.040). However, the variances explained by the models were small (R2 = 0.006 to 0.057), and the regression coefficients of ACB total scores were small (B = −0.028 to −0.105). After adjusting for covariates, ACB total scores were significantly and negatively associated with poorer cognitive scores for most cognitive variables. The coefficients for ACB total scores were small (B = 0.004 to −0.047). Effect size of cognitive g was also small (Cohen f2 = 0.008). Results of the analyses are presented in Table 3.
TABLE 3.
Linear Regression Analyses on ACB Total Scores
Anticholinergic Drug Scale
All cognitive test scores, 3-factor scores and global cognition g, were significantly associated with ADS total scores, with P values ranging from P < 0.001 to 0.013. The results indicated that a higher ADS total score was associated with poorer cognitive performance (Table 4). The variances accounted by ADS total scores were small (R2 = 0.009 to 0.074), and the coefficients of ADS total scores were small (B = −0.034 to −0.119). Similar to the results in ACB, after adjusting for covariates, ADS total scores had significant inverse association with most cognitive variables. The unique effects of ADS total scores on cognition were small (B = −0.006 to −0.064). Effect size of cognitive g was small (Cohen f2 = 0.017). The results are shown in Table 4.
TABLE 4.
Linear Regression Analyses on ADS Total Scores

DISCUSSION
The present study sought to examine the relationship between medication anticholinergic burden and cognition. The results suggest an inverse relationship between cumulative anticholinergic activity and cognition; that is, individuals with higher medication anticholinergic burden performed poorer in cognitive tasks. Although some specific cognitive tasks were not significantly associated with both ACB and ADS total scores, the cognitive domains represented by the tasks were significantly associated with both anticholinergic burden scales. The results indicated that higher cumulative anticholinergic burden was associated with poorer executive functioning, memory/fluency, processing speed, and global cognition. However, the magnitude of association between both ACB total scores and ADS total scores with cognitive variables seemed to be small. In addition, effect size (Cohen f2) that is independent of sample size was shown to be small in both ACB and ADS accumulative anticholinergic measures.
Our results were generally consistent with findings collected on samples with schizophrenia22,25,26 and geriatric populations. In geriatric studies, use of drugs with anticholinergic properties was associated with poorer visual memory and verbal fluency, as well as poorer performance in the Mini–Mental State Examination.19,33 In addition, higher dose and longer duration of central nervous system medication use were associated with cognitive decline.21,34 The zero-order correlations and regression coefficients in our data seem to be similar to those reported in Lanctôt et al.35 In the study conducted by Vinogradov et al25 in outpatients with chronic schizophrenia, SAA accounted for 7% of variance in verbal working memory as well as verbal learning and memory as indicated by squared semipartial correlation, after controlling for the impact of age, IQ, and symptom severity. However, our study found that ACB and ADS uniquely accounted for approximately 1% to 2% variance in memory/fluency factor. The difference in magnitude of impact may be due to the measure adopted (SAA vs ACB). In addition, the findings may be partly due to the younger age in this sample, as the cholinergic system of younger individuals may be more resilient than older individuals.36–38
The inconsistencies in findings may be due to the different measures of anticholinergic burden. Lertxundi et al39 found poor agreement between 3 anticholinergic burden scales that they reviewed. In addition, Durán et al40 and Salahudeen et al41 reviewed 7 anticholinergic burden scales and found inconsistent rating of medication anticholinergic burden in the scales. In this study, we relied on the ADS and ACB, 2 of the scales that are widely used in assessing medication anticholinergic burden. Available measures of anticholinergic burden have their limitations. Serum anticholinergic activity as the criterion standard measure of cumulative anticholinergic activity may only reflect a transitional cholinergic state outside the brain, whereas the expert-based scales may still be biased by the professionals' boundary of expertise. The scales also assume that medications have additive anticholinergic properties. Also, medication dose and frequency were not considered in estimating anticholinergic burden in these scales. Although dose-adjusted ADS score did not improve the variance explained in SAA than the traditionally calculated ADS score,30 this adjustment may strengthen the association between medication burden and physical functional capacity and cognitive measures.42 Therefore, these anticholinergic measures would serve only as an estimate that approximates medication anticholinergic burden. Despite the limitation, they may still serve as a simple and practical guide for practitioner in prescribing medication.
Although statistically significant, the magnitude of the associations between anticholinergic burden and cognition in our data was small. The significant results may also be due to the large sample size in this study.43 Therefore, it seems that the impact of anticholinergic medication on cognition in patients with schizophrenia may be minimal. Nevertheless, it is an important clinical consideration especially in a population where cognitive deficits are prevalent and have been shown to have significant impact on functioning.
Some limitations of the present study need to be discussed. The total anticholinergic burden of each scale may not be fully indicative of the true anticholinergic burden of patients. This might be due to other undocumented concomitant medications that the participants were taking at the time of the study. In estimating anticholinergic burden, medication dose and frequency were not adjusted for, and this might influence our findings. However, a previous study showed that dose-adjusted ADS total scores did not account for more variance in SAA than the traditionally computed ADS scores.30 Medication adherence was also not assessed in this study. Interaction between ACB or ADS and whether typical antipsychotics were taken was not controlled for in the analyses. This may be a potential confounder because the use of anticholinergics is associated with the use of typical antipsychotics, resulting in higher ACB and ADS scores. Concomitant disorders were not recorded in this study; therefore, the impact of other disorders on cognition is not controlled. Also, it should be noted that the significant association between anticholinergic burden and cognition does not indicate causal relationship between the variables.
To conclude, the findings in this study shed light on the impact of medication anticholinergic burden on different cognitive domains in patients with schizophrenia, indicating that all cognitive aspects were inversely associated with medication anticholinergic burden. However, this impact seems to be of little clinical significance.
AUTHOR DISCLOSURE INFORMATION
R.S.E.K. currently or in the past 3 years has received investigator-initiated research funding support from the Department of Veterans Affairs, Feinstein Institute for Medical Research, National Institute of Mental Health, Research Foundation for Mental Hygiene, Inc, and the Singapore National Medical Research Council. He currently or in the past 3 years has received honoraria and served as a consultant, speaker, or advisory board member for Abbvie, Akebia, Asubio, Avanir, AviNeuro/ChemRar, Biogen, BiolineRx, Biomarin, Boehringer-Ingelheim, Cerecor, CoMentis, FORUM, Global Medical Education (GME), GW Pharmaceuticals, Janssen, Lundbeck, MedScape, Merck, Minerva Neurosciences Inc., Mitsubishi, Moscow Research Institute of Psychiatry, Neuralstem, Neuronix, Novartis, NY State Office of Mental Health, Otsuka, Pfizer, Reviva, Roche, Sanofi, Shire, Sunovion, Takeda, Targacept, University of Moscow, and University of Texas Southwest Medical Center. R.S.E.K. also receives royalties from the BACS testing battery and the MATRICS Battery (BACS SC). He is a shareholder in NeuroCog Trials, Inc, and Sengenix. J.L. has received honoraria from Roche and Janssen-Cilag in the past 5 years. The other authors declare no conflicts of interest.
Footnotes
This study was supported by the National Research Foundation Singapore under the National Medical Research Council Translational and Clinical Research Flagship Programme (grant NMRC/TCR/003/2008). M.S.A., N.A.A.R., M.L., and A.R. are supported by the Singapore Ministry of Health's National Medical Research Council under the Centre Grant Programme (grant NMRC/CG/004/2013).
Presented at the Singapore Health & Biomedical Congress; Singapore; September 24, 2016.
REFERENCES
- 1.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. [DOI] [PubMed] [Google Scholar]
- 2.Keefe RS, Bilder RM, Harvey PD, et al. Baseline neurocognitive deficits in the CATIE schizophrenia trial. Neuropsychopharmacology. 2006;31:2033–2046. [DOI] [PubMed] [Google Scholar]
- 3.Talreja BT, Shah S, Kataria L. Cognitive function in schizophrenia and its association with socio-demographics factors. Ind Psychiatry J. 2013;22:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green MF, Kern RS, Braff DL, et al. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26:119–136. [DOI] [PubMed] [Google Scholar]
- 5.Goff DC, Hill M, Barch D. The treatment of cognitive impairment in schizophrenia. Pharmacol Biochem Behav. 2011;99:245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peuskens J, Demily C, Thibaut F. Treatment of cognitive dysfunction in schizophrenia. Clin Ther. 2005;27:S25–S37. [DOI] [PubMed] [Google Scholar]
- 7.Raedler TJ, Bymaster FP, Tandon R, et al. Towards a muscarinic hypothesis of schizophrenia. Mol Psychiatry. 2007;12:232–246. [DOI] [PubMed] [Google Scholar]
- 8.Rudd KM, Raehl CL, Bond CA, et al. Methods for assessing drug-related anticholinergic activity. Pharmacotherapy. 2005;25:1592–1601. [DOI] [PubMed] [Google Scholar]
- 9.Hasselmo ME, Sarter M. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology. 2011;36:52–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarter M, Lustig C, Taylor SF. Cholinergic contributions to the cognitive symptoms of schizophrenia and the viability of cholinergic treatments. Neuropharmacology. 2012;62:1544–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sellin AK, Shad M, Tamminga C. Muscarinic agonists for the treatment of cognition in schizophrenia. CNS Spectr. 2008;13:985–996. [DOI] [PubMed] [Google Scholar]
- 12.Spohn HE, Strauss ME. Relation of neuroleptic and anticholinergic medication to cognitive functions in schizophrenia. J Abnorm Psychol. 1989;98:367–380. [DOI] [PubMed] [Google Scholar]
- 13.Jones CK, Byun N, Bubser M. Muscarinic and nicotinic acetylcholine receptor agonists and allosteric modulators for the treatment of schizophrenia. Neuropsychopharmacology. 2012;37:16–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langmead CJ, Watson J, Reavill C. Muscarinic acetylcholine receptors as CNS drug targets. Pharmacol Ther. 2008;117:232–243. [DOI] [PubMed] [Google Scholar]
- 15.Matera C, Tata AM. Pharmacological approaches to targeting muscarinic acetylcholine receptors. Recent Pat CNS Drug Discov. 2014;9:85–100. [DOI] [PubMed] [Google Scholar]
- 16.Tan C, Shinfuku N, Sim K. Psychotropic prescription practices in East Asia: looking back and peering ahead. Curr Opin Psychiatry. 2008;21:645–650. [DOI] [PubMed] [Google Scholar]
- 17.Campbell N, Boustani M, Limbil T, et al. The cognitive impact of anticholinergics: a clinical review. Clin Interv Aging. 2009;4:225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ancelin ML, Artero S, Portet F, et al. Non-degenerative mild cognitive impairment in elderly people and use of anticholinergic drugs: longitudinal cohort study. BMJ. 2006;332:455–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulsant BH, Pollock BG, Kirshner M, et al. Serum anticholinergic activity in a community-based sample of older adults: relationship with cognitive performance. Arch Gen Psychiatry. 2003;60:198–203. [DOI] [PubMed] [Google Scholar]
- 20.Pasina L, Djade CD, Lucca U, et al. Association of anticholinergic burden with cognitive and functional status in a cohort of hospitalized elderly: comparison of the anticholinergic cognitive burden scale and anticholinergic risk scale: results from the REPOSI study. Drugs Aging. 2013;30:103–112. [DOI] [PubMed] [Google Scholar]
- 21.Ehrt U, Broich K, Larsen JP, et al. Use of drugs with anticholinergic effect and impact on cognition in Parkinson's disease: a cohort study. J Neurol Neurosurg Psychiatry. 2010;81:160–165. [DOI] [PubMed] [Google Scholar]
- 22.Perlick D, Stastny P, Katz I, et al. Memory deficits and anticholinergic levels in chronic schizophrenia. Am J Psychiatry. 1986;143:230–232. [DOI] [PubMed] [Google Scholar]
- 23.Strauss ME, Reynolds KS, Jayaram G, et al. Effects of anticholinergic medication on memory in schizophrenia. Schizophr Res. 1990;3:127–129. [DOI] [PubMed] [Google Scholar]
- 24.Tune LE, Strauss ME, Lew MF, et al. Serum levels of anticholinergic drugs and impaired recent memory in chronic schizophrenic patients. Am J Psychiatry. 1982;139:1460–1462. [DOI] [PubMed] [Google Scholar]
- 25.Vinogradov S, Fisher M, Warm H, et al. The cognitive cost of anticholinergic burden: decreased response to cognitive training in schizophrenia. Am J Psychiatry. 2009;166:1055–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minzenberg MJ, Poole JH, Benton C, et al. Association of anticholinergic load with impairment of complex attention and memory in schizophrenia. Am J Psychiatry. 2004;161:116–124. [DOI] [PubMed] [Google Scholar]
- 27.Keefe RS, Goldberg TE, Harvey PD, et al. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68:283–297. [DOI] [PubMed] [Google Scholar]
- 28.Lam M, Collinson SL, Eng GK, et al. Refining the latent structure of neuropsychological performance in schizophrenia. Psychol Med. 2014;44:3557–3570. [DOI] [PubMed] [Google Scholar]
- 29.Han L, McCusker J, Cole M, et al. Use of medications with anticholinergic effect predicts clinical severity of delirium symptoms in older medical inpatients. Arch Intern Med. 2001;161:1099–1105. [DOI] [PubMed] [Google Scholar]
- 30.Carnahan RM, Lund BC, Perry PJ, et al. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46:1481–1486. [DOI] [PubMed] [Google Scholar]
- 31.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 32.Selya AS, Rose JS, Dierker LC, et al. A practical guide to calculating Cohen's f(2), a measure of local effect size, from PROC MIXED. Front Psychol. 2012;3:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lechevallier-Michel N, Molimard M, Dartigues JF, et al. Drugs with anticholinergic properties and cognitive performance in the elderly: results from the PAQUID Study. Br J Clin Pharmacol. 2005;59:143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wright RM, Roumani YF, Boudreau R, et al. Effect of central nervous system medication use on decline in cognition in community-dwelling older adults: findings from the health, aging and body composition study. J Am Geriatr Soc. 2009;57:243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lanctôt KL, O'Regan J, Schwartz Y, et al. Assessing cognitive effects of anticholinergic medications in patients with coronary artery disease. Psychosomatics. 2014;55:61–68. [DOI] [PubMed] [Google Scholar]
- 36.Perry EK. The cholinergic system in old age and Alzheimer's disease. Age Ageing. 1980;9:1–8. [DOI] [PubMed] [Google Scholar]
- 37.Strong R, Hicks P, Hsu L, et al. Age-related alterations in the rodent brain cholinergic system and behavior. Neurobiol Aging. 1980;1:59–63. [DOI] [PubMed] [Google Scholar]
- 38.White P, Hiley CR, Goodhardt MJ, et al. Neocortical cholinergic neurons in elderly people. Lancet. 1977;1:668–671. [DOI] [PubMed] [Google Scholar]
- 39.Lertxundi U, Domingo-Echaburu S, Hernandez R, et al. Expert-based drug lists to measure anticholinergic burden: similar names, different results. Psychogeriatrics. 2013;13:17–24. [DOI] [PubMed] [Google Scholar]
- 40.Durán CE, Azermai M, Vander Stichele RH. Systematic review of anticholinergic risk scales in older adults. Eur J Clin Pharmacol. 2013;69:1485–1496. [DOI] [PubMed] [Google Scholar]
- 41.Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr. 2015;15:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hilmer SN, Mager DE, Simonsick EM, et al. A drug burden index to define the functional burden of medications in older people. Arch Intern Med. 2007;167:781–787. [DOI] [PubMed] [Google Scholar]
- 43.Harmatz JS, Greenblatt DJ. Regression and correlation. Clin Pharmacol Drug Dev. 2015;4:161–162. [DOI] [PubMed] [Google Scholar]


