Abstract
Clofarabine is a second-generation purine nucleoside analog that has been synthesized to overcome the limitations and incorporate the best qualities of fludarabine and cladribine. Clofarabine acts by inhibiting ribonucleotide reductase and DNA polymerase, thereby depleting the amount of intracellular deoxynucleoside triphosphates available for DNA replication. Compared to its precursors, clofarabine has an increased resistance to deamination and phosphorolysis, and hence better stability as well as higher affinity to deoxycytidine kinase (dCyd), the rate-limiting step in nucleoside phosphorylation. Since the initiation of the first phase I study of clofarabine in 1993 in patients with hematologic and solid malignancies, clofarabine has demonstrated single-agent antitumor activity in adult acute leukemia, including acute myeloid leukemia (AML). Due to its unique properties of biochemical modulation when used in combination with other chemotherapy drugs, mainly cytarabine, combination regimens containing clofarabine have been evaluated. A review of the English literature was performed that included original articles and related reviews from the MEDLINE (PubMed) database and from abstracts based on the publication of meeting materials. This review describes the development, pharmacology and clinical activity of clofarabine, as well as its emerging role in the treatment of adult patients with AML and myelodysplastic syndrome.
Keywords: Nucleoside analog, clofarabine, hematologic malignancies, solid tumors
Introduction
Clofarabine(2-chloro-9-[2′-deoxy-2′-fluoro-β-D-arabinofuranosyl]-9H-purine-6-amine; Cl-F-ara-A; CAFdA) is a rationally designed, second-generation purine nucleoside analog. Clofarabine was synthesized based on the experience with the earlier deoxyadenosine analogs fludarabine and cladribine. It was designed as a hybrid molecule to overcome the limitations and incorporate the best qualities of fludarabine and cladribine, both of which are used for the treatment of hematologic malignancies. Clofarabine has a chloro-group at the 2-position of adenine; its chemical structure is more closely related to cladribine than to fludarabine. Halogenation at the 2-position of adenine renders this class of compounds resistant to intracellular degradation by the enzyme adenosine deaminase. Substitution of a fluorine at the C-2′-position of the arabinofuranosyl moiety of clofarabine increases its stability in gastric acid and decreases its susceptibility to phosphorolytic cleavage by the bacterial enzyme Escherichia coli purine nucleoside phosphorylase in the gastrointestinal tract, both of which may lead to enhanced oral bioavailability [1–3].
These pharmacologic features confer several advantages to clofarabine compared with fludarabine and cladribine: (1) increased resistance to deamination and phosphorolysis, hence better stability; (2) higher affinity to deoxycytidine kinase (dCyd); (3) prolonged retention of the triphosphate compound in leukemic blasts; and (4) potent inhibition of DNA synthesis and of ribonucleotide reductase (RNR) [1–4]. Clofarabine has been approved by the Food and Drug Administration (FDA) for the treatment of pediatric patients with relapsed or refractory acute lymphoblastic leukemia (ALL) after at least two prior regimens, based on the induction of complete responses [5]. Since clofarabine also has significant activity in patients with acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS), its use in treating these patients is an area of active research and is the focus of this review.
Mechanism of action
Clofarabine is S-phase specific and cell cycle phase non-specific. Its precise mechanism of action on dividing and non-dividing cells is unknown. Like other nucleoside analogs (e.g. cytarabine [ara-C], vidarabine [ara-A], cladribine, fludarabine), clofarabine must be serially phosphorylated, first by deoxycytidine kinase (dCK) and then by other kinases, to be active within cells. Clofarabine is more efficient as a substrate for purified recombinant dCK, exceeding cladribine and the natural substrate, deoxycytidine [3]. There is evidence that the primary cytotoxic effect of clofarabine is due to its inhibition of deoxyribonucleic acid (DNA) synthesis. The triphosphate form of clofarabine is an inhibitor of both DNA polymerase α and ribonucleotide reductase [6]. These effects lead to depletion of intracellular deoxynucleotide triphosphate pools, and inhibition of elongation of DNA strands during synthesis [7]. For inhibition of ribonucleotide reductase, clofarabine and cladribine are superior to fludarabine. For inhibition of DNA polymerase α, clofarabine and fludarabine are similar and both are superior to cladribine [6].
Thus, in comparison to cladribine and fludarabine, clofarabine inhibits better both ribonucleotide reductase and DNA polymerase α. Unlike fludarabine, clofarabine is active in non-dividing cells and in cells with a low proliferation rate. Clofarabine has been shown to disrupt the integrity of mitochondria in primary chronic lymphocytic leukemia (CLL) cells. The damage leads to release of pro-apoptotic mitochondrial factors [8]. In addition to its anti-leukemic activity as a single agent, in vitro studies supported a possible role for clofarabine in biochemical modulation strategies to enhance the efficacy of other nucleoside analogs such as cytarabine [4,6,7,9,10].
Preclinical experience
Although clofarabine had been synthesized in the 1980s, there was little interest in its development by pharmaceutical companies because of the availability of other nucleoside analogs, and the lack of compelling evidence for activity outside the lymphoproliferative disorders. In 1993, the development of clofarabine was initiated at M. D. Anderson Cancer Center (H. Kantarjian, unpublished data). A dose of 15 mg/m2 daily for 5 days was chosen for human phase I clinical trials. Oral clofarabine has since demonstrated excellent anti-tumor activity in both solid and hematological tumor xenograft mouse models [2,11–15]. Clofarabine was cytotoxic at lower IC50 (50% inhibitory concentration) levels than fludarabine in vitro against numerous human cell lines, including L1210 and K562 leukemic cells [3,11].
Pharmacokinetics and pharmacology of clofarabine in adult patients
A phase I clinical trial was conducted in 51 patients with indolent and acute hematologic malignancies and in solid tumors to identify the dose limiting toxicities (DLTs) of clofarabine and to define the maximum tolerated dose (MTD) on a daily-times-5-days schedule [16]. Clofarabine, at escalating doses, was administered daily for 5 days as a 1 h intravenous (IV) infusion. The DLT was reached at 2 and 4 mg/m2/day for 5 days in patients with solid tumors and chronic lymphoid malignancies, respectively. At these levels, myelosuppression was observed. A maximum tolerated level of 40 mg/m2 was identified. At this level, severe but reversible hepatotoxicity was observed, thus defining the DLT. The clofarabine concentration projected at the end of the infusion of the MTD (40 mg/m2/day) was well above the toxic level for leukemia cell lines growing in culture [4,6]. Subsequently, the dose level of 40 mg/m2 IV daily for 5 days was explored in additional patients. The clofarabine dose schedule of 40 mg/m2 IV daily for 5 days was judged to be the recommended phase II dose schedule for adult acute leukemia. Plasma and cellular pharmacology studies established many of the characteristics that would assist the design of subsequent studies [17]. Following the completion of the phase I study in adult leukemia, several phase II single-agent clofarabine and clofarabine combination studies were conducted.
At the given 52 mg/m2 dose, similar concentrations were obtained over a wide range of body surface areas. Clofarabine was 47% bound to plasma proteins, predominantly to albumin. Elimination was primarily renal, with 49–60% of the dose excreted unchanged in the urine. Non-renal excretion pathways are yet to be determined. No apparent differences in pharmacokinetics were observed between patients with AML and ALL [18]. The pharmacokinetics of clofarabine has not been evaluated in patients with renal or hepatic dysfunction. The pharmacokinetics of oral clofarabine has also been studied in 23 adult patients with refractory advanced solid tumors (CLO-152 study) [15]. Oral availability was found to be around 50% with a mean absorption time of 2 h in this patient population. The results obtained are being used in ongoing clinical trials with oral clofarabine.
Rationale for combination studies
Phase I and II studies of single-agent clofarabine established its dose schedule and confirmed its activity in leukemia. Emphasis in AML shifted to combinations with cytarabine and anthracyclines. Initial clofarabine combinations were with cytarabine for two reasons: (1) cytarabine is the most active agent against AML and is the backbone of many combination regimens in AML, and (2) favorable biochemical modulation of cytarabine triphosphate (ara-CTP) by fludarabine and clofarabine was noted in AML blasts [19–21]. Biochemical modulation strategies aimed to increase intracellular nucleoside concentrations, such as ara-CTP, and synergy between cytarabine and clofarabine has been demonstrated in vitro [19–23].
Clofarabine experience in adults
Single-agent clofarabine in relapsed/refractory adult leukemia and myelodysplastic syndrome (Table I)
Table I.
Summary of important studies using single-agent clofarabine in the treatment of adult patients with AML and MDS.
| Reference | Phase of study | Number of patients | Patient characteristics | Median age (years) | Treatment schema | ORR (%) | CR (%) | Remarks |
|---|---|---|---|---|---|---|---|---|
| Kantarjian et al. (2003) [18] | II | 39 | Refractory AML and MDS | 54 | Clofarabine induction | 56 | 38 | ORR=87% for patients with AML with prolonged first CR |
| Krawczyk et al. (2010) [26] | II | 8 | Frontline therapy AML | 67.5 | Clofarabine induction ± consolidation | 61 | 50 | Induction mortality = 17% |
| Faderl et al. (2010) [28] | II | 32 | High risk MDS | 70 | Three doses of oral clofarabine | 43 | 25 | Low dose as efficacious, less toxic |
| Faderl et al. (2012) [27] | II | 58 | High risk MDS | 68 | Low vs. high dose clofarabine | 36 | 26 | Median OS = 7.4 months; low dose as efficacious, less toxic |
| Burnett et al. (2010) [44] | Two consecutive phase II | 106 | Frontline elderly AML | 71 | Reduced dose clofarabine induction and consolidation | 48 | 32 | |
| Kantarjian et al. (2010) [45] | II | 112 | Frontline elderly AML | 71 | Reduced dose clofarabine induction and consolidation | 46 | 38 | Median OS = 42 weeks |
| Foran et al. (2003) [24] | II | 40 | Relapsed/refractory AML | 55.6 | Clofarabine induction and consolidation | 5 | 0 |
AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; ORR, overall response rate; CR, complete remission; OS, overall survival.
Following the lead of the phase I studies, a single-institution phase II study of clofarabine (single agent) was conducted in 62 patients including 31 patients with refractory AML, eight patients with MDS, 11 patients with chronic myeloid leukemia in blast phase and 12 patients with acute lymphoblastic leukemia [18]. All patients received clofarabine at an IV dose of 40 mg/m2 daily for 5 days every 3–6 weeks. Of the 31 patients with AML, 13 (42%) achieved a complete remission (CR) and four (13%) a CR with incomplete platelet recovery (CRp), for an overall response rate (ORR) of 55%. Response rates were higher in patients who had preceding CR durations for more than 12 months and in patients who received clofarabine in first salvage. Among the six patients with MDS, two achieved CR and two CRp, for an ORR of 50%. The duration of responses ranged from 1 to 10 months for patients with AML and ALL. The ORR was 52% for those with diploid karyotype and 56% for those with unfavorable karyotype. Frequently observed adverse events were transient liver dysfunction, skin rashes, palmoplantar ertythrodysesthesia and mucositis. Foran and colleagues conducted a similar phase II study in 40 patients with relapsed or refractory AML [24]. The response rate was considerably lower with no CR, one CRp and one PR, for an ORR of 5%.
Racil et al. examined the role of clofarabine in the treatment of AML with molecular relapse [25]. In that study, eight patients with AML exhibited a molecular relapse and were treated with clofarabine monotherapy. Three patients had CBFB/MYH11, three patients had NPM1 mutation, one patient had the MLL/ELL fusion gene and one patient had RUNX1/RUNX1T1. Molecular relapse was defined as the reappearance of a molecular marker in peripheral blood or bone marrow (BM) samples, or a 10-fold increase if detected repeatedly, when the simultaneously assessed BM morphology, immunophenotype and cytogenetics remained normal. Quantitative reverse-transcription polymerase chain reaction (PCR) and real-time PCR were used to monitor for molecular relapse. The median age of patients at the time of molecular relapse was 51 years. Primary therapy of AML consisted of standard induction cytarabine and daunorubicin in all patients, followed by post-remission therapy (conventional chemotherapy or transplant). After one cycle of clofarabine reinduction, all patients had a sustainable complete hematological remission (CHR). A molecular response (MoR) was achieved in seven of eight patients (87.5%), six patients (75%) achieved complete MoR (CMoR) and one patient (12.5%) achieved a partial MoR (PMoR). The 6-month overall survival (OS) rate for the evaluated group of patients with AML was 100%, and the 6-month event-free survival (EFS) as well as disease-free survival (DFS) was 75% (95% confidence interval [CI]: 50.3–100%), each. Incorporating minimal residual disease data in therapeutic regimens for patients with AML and the optimal use of clofarabine for patients with molecular relapse are yet to be defined.
The role of IV single-agent clofarabine was also studied in a smaller cohort of patients with AML with poor risk features, deemed unsuitable for standard therapy [26]. Twenty-two patients were treated with clofarabine, alone (eight patients) or in combination (14 patients) for up to three cycles of treatment. The median age was 67.5 years (range, 24–76), with 16 patients older than 60 years. Four patients intolerant to standard induction received clofarabine as consolidation. The ORR for 18 patients with active AML was 61%, nine patients (50%) achieving a CR. Induction and consolidation were well tolerated with no unexpected toxicities. Predictably, all patients developed grade 4 neutropenia but the median duration was only 20 days (range, 17–120). Induction mortality was 17%.
Faderl and colleagues evaluated the activity and safety of two different doses (15 mg/m2 vs. 30 mg/m2 daily for 5 days) of IV clofarabine in patients with higher-risk MDS. Fifty-eight patients with a median age of 68 years (range, 25–89) including 15 patients (28%) with secondary MDS and 35 patients (60%) who received prior DNA methyltransferase (DNMT) inhibitors were randomized between the two dose cohorts. The ORR was 36% including 26% with CR. Responses were lower in the higher dose cohort and in patients who failed DNMT inhibitors (ORR, 17%; CR rate, 14%). The 8-week mortality rate was 19%. Median OS was 7.4 months for all patients, 13.4 months for responders and 21.7 months for complete responders. Hepatic and renal toxicities were more severe in patients randomized to 30 mg/m2 of clofarabine. Thus, the lower dose of IV clofarabine appeared to be efficacious and less toxic as compared with the higher dose [27].
Faderl et al. also evaluated the safety and efficacy of oral clofarabine used as a single agent for the treatment of patients with high-risk MDS [28,29]. Three doses of clofarabine were evaluated: 40 mg/m2, 30 mg/m2 and 20 mg/m2 daily for 5 days. Courses were repeated every 4–8 weeks. Thirty-two patients were treated, of whom 22 had intermediate-2 or high-risk disease using the International Prognostic Scoring System (IPSS). Median age was 70 years (range, 53–86), nine patients had secondary MDS, and 20 patients experienced prior therapy failure with hypomethylating agents. Eight patients (25%) achieved CR and the ORR was 43%. No patients died within 6 weeks of induction. Renal failure occurred in four patients in the context of myelosuppression-associated infectious complications. Myelosuppression was common, but prolonged myelosuppression (> 42 days) was rare. The toxicity profile was better with lower doses of clofarabine, whereas response rates did not differ significantly. However, the optimal dose and schedule and the appropriate patient population for oral clofarabine therapy remain to be defined.
Clofarabine based combination therapies (Table II)
Table II.
Summary of important studies using clofarabine-containing regimen in the treatment of adult patients with AML and MDS.
| Reference | Phase | Number of patients | Patient characteristics | Median age (years) | Treatment schema | ORR (%) | CR (%) | Remarks |
|---|---|---|---|---|---|---|---|---|
| Faderl et al. (2005) [29] | I/II | 29 | Relapsed AML and high risk MDS | 63 | Ara-C and clofarabine combination | 40 | 24 | Low induction mortality (3%), median OS: 5.5 months |
| Agura et al. (2011) [32] | II | 30 | Relapsed/refractory AML | 67 | Ara-C and clofarabine combination | 53 | 47 | Median DFS: 9.5 months |
| Faderl et al. (2012) [46] | III | 320 | Relapsed/refractory AML >55 years | 67 | Induction with clofarabine + ara-C vs. ara-C | 46.9 vs. 22.9 | 35 vs. 18 | Better CR, EFS and ORR in combination arm, no difference in OS |
| Becker et al. (2011) [34] | I/II | 50 | Relapsed/refractory AML | 53 | Clofarabine and high dose ara-C with G-CSF priming (GCLAC) (induction and consolidation) | 61 | 46 | Response independent of age and cytogenetic risk category |
| Faderl et al. (2008) [31] | III | 70 | Elderly frontline AML or high risk MDS | 71 | Induction and consolidation clofarabine vs. clofarabine + ara-C | 59 (67 vs. 31 favoring combination arm) | 63 vs. 31 favoring combination arm | Median OS: 11.4 vs. 5.8 months favoring combination arm (non-significant) |
| Faderl et al. (2012) [33] | II | 60 | Frontline elderly AML | 70 | Ara-C and clofarabine combination, consolidation alternating with decitabine | 66 | 58 | Median OS: 12.7 months |
| Nazha et al. (2011) [38] | II | 51 | Frontline AML | 49 | Combination of ara-C, clofarabine and idarubicin (induction and consolidation) | 71 | 69 | Low 1-month induction mortality (4%), median OS not reached |
| Amadori et al. (2011) [42] | II | 53 | Relapsed/refractory AML | 69 | Induction with clofarabine and temsirolimus, maintenance temsirolimus | 21 | 8 | >50% in vivo inhibition of S6 ribosomal protein phosphorylation highly correlated with response rate |
| Mathisen et al. (2012) [39] | I/II | 31 | Frontline or relapsed/refractory AML | 56 | Combination of clofarabine, idarubicin and cytarabine (CIA) | 44% for phase I relapsed/refractory patients; phase II CIA: 100% for front-line cohort; 43% for relapsed/refractory | 100% for front-line cohort; 36% for relapsed/refractory CIA cohort | Low early mortality rate |
AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; ORR, overall response rate; CR, complete remission; OS, overall survival; DFS, disease-free survival; EFS, event-free survival.
Trials of clofarabine in combination with cytarabine
Cooper and colleagues demonstrated that clofarabine modulates ara-CTP accumulation and increases the antileukemic activity of ara-C [22]. A phase I/II study of clofarabine plus ara-C was conducted in 32 patients with relapsed acute leukemia (25 AML, two ALL, four MDS and one chronic myeloid luekemia [CML] in blast phase) [29]. Clofarabine was given at escalating doses of 15–40 mg/m2 IV daily for 5 days (days 2–6) followed 4 h later by ara-C at 1 g/m2 IV over 2–3 h daily for 5 days (days 1–5). Preclinical studies had demonstrated that optimal up-regulation of ara-CTP levels occurred when clofarabine was dosed 4 h prior to ara-C exposure and given as a 2 h infusion [22]. The phase II dose of clofarabine was 40 mg/m2 IV daily for 5 days. The ORR in AML was 40% (28% CR, 12% CRp) and 2/4 patients with MDS responded. The median duration of remission was 3.2 months and the median OS was 5.5 months. Patients achieving CR or CRp had a median OS of 7.9 months. Of note, the CR rate of 24% in patients with AML/MDS in this study was lower than the rate observed in the phase II trial evaluating clofarabine as a single agent, where the complete response rate in AML patients was 42%.
Based on these data, Faderl and colleagues [30] pursued a phase I–II trial evaluating the efficacy of clofarabine in combination with cytarabine as front-line therapy in adults 50 years of age and older, with AML or high-risk MDS. Patients with good prognosis karyotype were excluded. Both clofarabine and cytarabine were given as in the phase II study described above. In total, 60 patients with a median age of 61 years (range, 50–74 years) were enrolled. Of these patients, 53 had AML and eight had high-risk MDS. Thirty percent of the patients had adverse cytogenetics or AML transformed from MDS. A total of 31 patients (52%) achieved a CR and five (8%) a CRp, for an ORR of 60%. Among the 30 patients with adverse cytogenetics, a CR was achieved in 43% of the patients for an ORR of 53% in this subgroup. Among 18 patients with secondary AML and antecedent MDS, 33% achieved a CR and the ORR was 50% in this subgroup. Nine patients (15%) died while on study, and most adverse effects were gastrointestinal and were of grades 1 and 2. The median OS for patients who achieved a CR was 23.5 months. The median OS for the intent-to-treat population was 10.3 months.
A randomized phase III study by the same authors compared clofarabine monotherapy to clofarabine combined with low-dose cytarabine in patients aged 60 years or older with untreated AML or high-risk MDS [31]. Inclusion criteria were Eastern Cooperative Oncology Group (ECOG) perfomance status (PS) of 2 or less and adequate end organ function. Of the 70 patients enrolled, monotherapy was administered to 16 and combination therapy to 54. The randomization for the first 20 patients was balanced with a 50% probability of being randomized to either arm. According to the method of Bayesian randomization, assignment probabilities were based on results of preceding patients. As efficacy data became available, assignment probabilities shifted, favoring the arm with a higher CR rate. The median age was 71 years and 10% were 80 years or older. Both treatment arms received induction with clofarabine 30 mg/m2 daily on days 1 through 5. The combination arm received subcutaneous cytarabine 20 mg/m2 daily on days 1 through 14 (4 h after clofarabine on days 1–5). For consolidation, clofarabine 30 mg/m2 daily was administered for 3 days (days 1–3) in the monotherapy arm and in the combination arm was combined with daily subcutaneous cytarabine for 7 days (days 1–7). Up to 12 consolidation courses were allowed for those achieving at least a CR with incomplete blood count recovery. All patients (16/16) in the monotherapy group and 50/54 patients (92%) in the combination group had AML. Overall, 50% of patients had secondary AML/MDS. Unfavorable cytogenetics was present in 31% of patients receiving monotherapy and 37% receiving combination therapy. The ORR (including CR and CRp) for the study group was 59%. A higher ORR was observed in patients receiving the combination regimen versus the single-agent therapy: 67% vs. 31% (p = 0.012). Similarly, the CR rate was significantly higher with the combination (63% vs. 31%; p = 0.025). The median OS was 11.4 months for the combination arm and 5.8 months for the monotherapy arm (p = 0.1). Of the patients with unfavorable cytogenetics, a CR was achieved in 19% in the monotherapy arm and 24% in the combination group. During the first induction, the mortality rate was 31% in the monotherapy arm and 19% in the combination group (p = 0.276). The most frequent non-hematologic adverse events were gastrointestinal and hepatic and did not differ significantly between treatment arms. The most frequent grade 3/4 toxicities were diarrhea and increased liver enzymes. Grade 3/4 renal failure, requiring dialysis, occurred in 19% in the monotherapy arm and 15% in the combination arm. Prolonged myelosuppression was rare, occurring in only six patients (11%) in the combination arm during induction. Based on the results of this first comparative study, clofarabine plus low-dose cytarabine appeared to have a higher response rate than clofarabine alone with comparable toxicity.
Agura and colleagues also studied the activity and safety of the combination of clofarabine and ara-C in 30 relapsed/refractory and untreated elderly patients with AML at high risk of anthracycline toxicity [32]. Patients received clofarabine 40 mg/m2/day, followed 4 h later by ara-C 1 g/m2/day 2 h IV infusion daily for 5 days (days 1 through 5). The median age in this study was 67 years (range, 38–82); 60% had received at least one prior cytotoxic regimen. High-risk cytogenetic abnormalities were present in 14 patients (47%). The ORR (CR and PR) was 53%, including CR in 14 patients (47%). Responses were observed in all cytogenetic groups. Half of the patients who achieved CR were able to proceed to stem cell transplant with curative intent. The median DFS interval was 9.5 months. The results of this study further confirm the clinical activity and tolerability of clofarabine in combination with cytarabine, and the therapeutic activity was again seen across all cytogenetic subgroups including patients with adverse prognosis cytogenetics.
Clofarabine plus low-dose cytarabine followed by prolonged consolidation alternating with decitabine have shown significant activity in a phase II study in elderly patients with AML [30,33]. Sixty patients (median age 70 years) with newly diagnosed AML were treated with clofarabine 20 mg/m2 IV daily for 5 days plus cytarabine 20 mg subcutaneously twice daily for 10 days. Responders continued for up to 17 consolidation courses. Courses 1, 2, 6–8 and 12–14 were composed of clofarabine 20 mg/m2 IV daily for days 1–3 plus cytarabine 20 mg subcutaneously twice daily for days 1–7. Consolidation courses 3–5, 9–11 and 15–17 were composed of decitabine 20 mg/m2 IV daily for 5 days. Among 59 evaluable patients, 40 (66%) responded. The CR rate was 58%. The median relapse-free survival (RFS) and OS were 14.1 and 12.7 months, respectively. The median OS was 24.2 months for patients with CR and CRp. Most toxicities were grade 2 or less. When these results were compared with a historical group of patients who received clofarabine plus low-dose cytarabine with a shorter consolidation, RFS was not statistically different. Thus, although a clofarabine based combination appeared to be effective in inducing remissions, the benefits of a prolonged consolidation remain unproven in this population.
More recently, another clofarabine and cytarabine combination was tested using clofarabine with high-dose cytarabine and granulocyte colony-stimulating factor (G-CSF) in patients with relapsed and refractory AML (GCLAC) [34]. This phase I/II trial included 50 patients of median age 53 years (range, 19–69). Baseline cytogenetics for the majority of patients were intermediate (54%) and unfavorable (40%), with a minority (6%) having favorable cytogenetic features. One day before initiating chemotherapy, 5 μg/kg of G-CSF was administered daily until the absolute neutrophil count reached 2 × 109/L for 2 consecutive days. Daily clofarabine was then administered IV for 5 days at 15, 20 and 25 mg/m2. Cytarabine 2 g/m2 over 2 h was administered IV for 5 days. Consolidation therapy, consisting of clofarabine dosed at 5 mg/m2 less than the induction dose for 5 days, was administered to those in CR, followed 4 h later by cytarabine 1 g/m2 for 5 days. The same G-CSF dosing schema was used for induction and consolidation. Among 46 evaluable patients, the CR rate was 46%. The rate of CR plus CRp was 61%. The most frequent treatment-related grade 3/4 adverse event was infection (bacterial or fungal), occurring in 40%. Grade 3/4 pulmonary toxicity was also observed in 46%. Importantly, multivariate analysis showed that responses to GCLAC were independent of age, cytogenetic risk category and number of prior salvage regimens. Thus, this study showed that GCLAC is highly active in relapsed and refractory AML and warrants prospective comparison to other regimens in this setting.
Other clofarabine-based combinations (Table II)
The increase in cytotoxicity when combining clofarabine with alkylating agents has been shown to be proportional to the initial magnitude of the DNA incision and to the extent of repair, suggesting a close correlation between repair inhibition and induction of cell death [35]. Anthracyclines are the most common combination partner of cytarabine. Interactions of 2-chlorodeoxyadenosine (2-CdA, cladribine) with three anthracyclines (doxorubicin, idarubicin and mitoxantrone) have been evaluated on murine leukemias P388 and L1210 [36]. Combinations of all three anthracyclines potentiated the antileukemic activity of cladribine. In a phase I study of patients with primary refractory or first-relapse AML, idarubicin was combined with either clofarabine alone (CI), or with clofarabine plus cytarabine (CIA) [37]. ORRs were 22% (three CR and two CRp) and 48% (10 CR) for patients treated with CI and CIA, respectively. These preliminary results indicated the feasibility of the combination of clofarabine with idarubicin and ara-C, and suggested a potential for a high response rate with the CIA triple combination therapy.
The CIA regimen is being studied in the front-line setting for newly diagnosed patients with AML younger than 61 years. Preliminary results reported by Nazha et al. showed that among 51 patients, 35 (69%) achieved a CR and one (2%) a CRp; the ORR was 71% [38]. Sixteen patients (31%) proceeded with an allogeneic hematopoietic stem cell transplant (AHSCT) in first CR. The induction/consolidation therapy consisted of clofarabine 20–22.5 mg/m2 daily (days 1–5), idarubicin 6–8 mg/m2 daily (days 1–3) and cytarabine 0.75 g/m2 daily (days 1–5). The majority of toxicities were manageable (grade 2 or less) and included: rash (41%), nausea (29%), diarrhea (23%), elevated transaminases (21%) and elevated bilirubin (17%).
A phase I/II study by Mathisen et al. similarly examined the efficacy of CIA as well as fludarabine, idarubicin and cytarabine (FIA) in newly diagnosed or relapsed/refractory patients with AML [39]. In over 50% of the relapsed/refractory patients, the study treatment served as salvage 2 or higher. Patients with relapsed/refractory disease were treated in the phase I portion of the study to define the MTD of clofarabine. Idarubicin was given at the dose of 10 mg/m2 on days 1–3 and cytarabine was given at the dose of 1 g/m2 on days 1–5. Clofarabine starting dose was 15 mg/m2. Doses were escalated to 25 mg/m2 on days 1–5 in subsequent study cohorts. The ORR for the nine patients enrolled in the phase I portion of the study was 44%.
During the phase II portion of the study, patients were randomized to CIA (clofarabine at the MTD) or FIA (fludarabine at 30 mg/m2 on days 1–5). Fifty patients were enrolled so far, including 16 newly diagnosed (nine CIA, seven FIA) and 34 relapsed/refractory (14 CIA, 20 FIA) patients. In the front-line cohort, the CR rate was 100% in both treatment groups. The ORR of both CIA and FIA combined was 100% in the front-line cohort and 32% for relapsed/refractory patients. The ORR for CIA was 100% in the front-line cohort and 43% for the relapsed/refractory cohort. The CR rate for relapsed/refractory CIA-treated patients was 36% and the CRp was 7%. Toxicities for both regimens included elevated liver function tests. Hand–foot syndrome was noted in the clofarabine group [39].
A pilot trial (AML16) examined the feasibility of combining daunorubicin and gemtuzumab ozogamycin (GO) with clofarabine [40]. Patients with untreated and refractory AML were enrolled into five treatment cohorts containing six patients each. All patients received daunorubicin 50 mg/m2 daily × 3 (days 1, 3 and 5). Cohorts 1, 2, 3 and 4 received clofarabine at the doses of 15 mg/m2/day, 20 mg/m2, 25 mg/m2 and 30 mg/m2, respectively on days 1–5 of the dosing regimen. Cohort 5 received the feasible dose of clofarabine derived from cohorts 1–4 in addition to GO 3 mg/m2 on day 1 of the dosing regimen. Clofarabine at the dose of 20 mg/m2/day was chosen for the combination with daunorubicin and GO in cohort 5. Aspartate transaminase (AST) and alanine transaminase (ALT) toxicities greater than grade 2 were not observed in any patients. Grade 3 or 4 cardiac toxicities were observed at the doses of 30 mg/m2, 25 mg/m2 and 20 mg/m2 of clofarabine with daunorubicin and GO. Grade 4 renal toxicity was observed in one patient in the clofarabine 30 mg/m2 cohort. Efficacy data were available in 34 patients: 24 (65%) achieved a CR. Thus, this combination was effective and toxicities were equivalent to other intensive chemotherapy regimens used for treatment of patients with AML.
Recently, another phase I study was conducted to determine the MTD and DLT of clofarabine when combined with GO in adult patients with relapsed or refractory AML [41]. Twenty patients received clofarabine (10, 20 or 30 mg/m2) on days 1–5, with GO 3 mg/m2/day on days 1, 4 and 7. Common DLTs were prolonged myelosuppression and hepatotoxicity. Clofarabine 20 mg/m2 was the MTD, but the DLT rate of 0.38 was too high for this dose to be used in phase II studies. The ORR (CR and CRp) was 42% among all patients. Thus, this combination again demonstrated activity in relapsed and refractory patients, but further testing of the combination using lower doses of GO may identify more favorable rates of toxicity while maintaining efficacy.
Amadori et al. explored the combination of temsirolimus, a mammalian target of rapamycin (mTOR) inhibitor, and lower-dose clofarabine as salvage therapy in older patients with AML [42]. Induction consisted of clofarabine 20 mg/m2 on days 1–5 and temsirolimus 25 mg on days 1, 8 and 15. Patients achieving CR or CRp could receive monthly temsirolimus maintenance. In 53 evaluable patients, the ORR was 21% (8% CR, 13% CRp). Median DFS was 3.5 months, and median OS was 4 months (9.1 months for responders). The most common non-hematological severe adverse events included infection (48%), febrile neutropenia (34%) and transaminitis (11%). The 30-day all-cause induction mortality was 13%. Laboratory data from 25 patients demonstrated that a > 50% in vivo inhibition of S6 ribosomal protein phosphorylation highly correlated with response rate, suggesting that targeting the mTOR pathway is clinically relevant. The acceptable safety profile and the predictive value of target inhibition could encourage further investigation of this novel regimen.
Clofarabine therapy in elderly patients
Another step in the development of clofarabine has been a focus on older patients with AML, a group with poor survival with standard chemotherapy. With high 4-week mortality rates and low OS rates (under 10% at 3–5 years), intensive chemotherapy may not benefit most patients with AML 70 years or older and could be harmful to some [43]. With the exception of elderly patients with AML with core binding factor leukemias, all other such patients are thought to be better candidates for investigational low intensity treatments or clinical trials, comparing intensive versus low intensity chemotherapy. For this reason, there has been a significant interest in clofarabine as a treatment for elderly patients unsuitable for intensive chemotherapy.
Burnett et al. analyzed previously untreated elderly patients with AML and combined the results of two consecutive phase II studies of 106 elderly patients (median age 71) who were administered clofarabine at reduced doses of 30 mg/m2 for 5 days for induction and 20–30 mg/m2 for consolidation [44]. The patients were older and considered unfit for conventional chemotherapy. The ORR was reported as 48% (CR in 32% and 16% with CRp). In the first phase II cohort of 66 patients, 14 (21%) had serious neutropenic sepsis during course 1 and six (24%) in course 2. Overall, for both cohorts, hepatic grade 3/4 toxicity occurred in 8% in course 1 and 12% in course 2. Additionally, grade 3/4 renal toxicity occurred in 14% in course 1 and 16% in course 2.
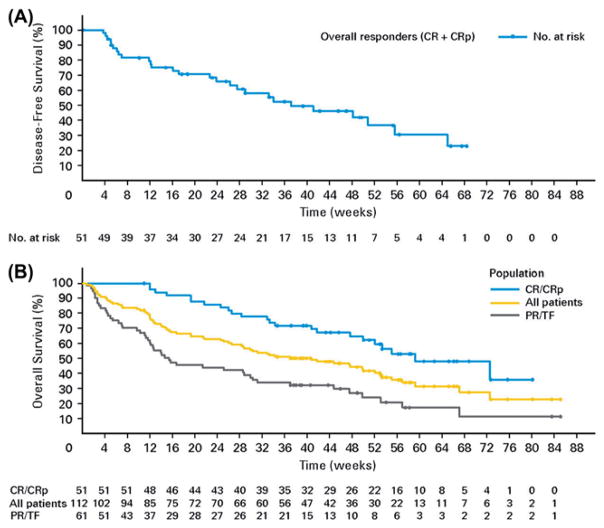
Kantarjian and colleagues studied single-agent clofarabine in previously untreated older patients with AML who were unlikely to benefit from conventional induction chemotherapy [45]. The study included 112 patients 60 years or older (median age 71) with one or more adverse prognostic factors. Treatment consisted of clofarabine induction with 30 mg/m2/day IV for 5 days, followed by consolidation with 20 mg/m2/day for 5 days if they achieved a CR. Reinduction on day 28 with a second cycle of 30 mg/m2 daily for 5 days was administered if patients had residual leukemia but did not meet criteria for progression. An ORR of 46% was obtained, with a CR rate of 38%. The ORRs were 39%, 32%, 51% and 42%, for patients 70 years or older, with an ECOG PS of 2, with an antecedent hematologic disorder and with unfavorable cytogenetics, respectively. The majority of responses occurred after cycle 1. As shown in the Kaplan–Meier curve in Figure 1(A), the estimated median DFS was 37 weeks. Figure 1(B) shows Kaplan–Meier estimates for the OS of all patients, those who achieved CR or CRp, as well as those with partial responses or treatment failure. For all patients, the median OS was 41 weeks. For patients who achieved a CR or CRp, the median OS was 59 weeks. Among patients with partial responses or treatment failure, the median OS was 15 weeks.
Figure 1.
(A) The Kaplan–Meier curve shows that the estimated median disease-free survival (DFS) was 37 weeks. (B) The Kaplan–Meier curve shows the estimated overall survival (OS) for all patients (yellow line), patients with a complete remission (CR) or complete remission with incomplete platelet recovery (CRp) (blue line) and patients with partial response (PR) or tr eatment failure (TF) (black line). For all patients, the median OS was 41 weeks. For patients who achieved a CR or CRp, the median OS was 59 weeks. Among those patients with PR or TF, the median OS was 15 weeks.
Like previous studies of clofarabine monotherapy, transient grade 3/4 AST or ALT elevations occurred frequently. Grade 3 and 4 AST elevations occurred in 21% and 3% of patients, respectively. Myelosuppression was the most common grade 3/4 toxicity. Grade 3/4 neutropenia occurred in 5% and 46% of patients, respectively. The all-cause 30-day and 60-day mortality rates were 9.8% and 16% respectively, which compared favorably to similar patient populations treated with 7 + 3 induction therapy. Based on this study, single-agent clofarabine could be safe and active in this patient population.
A phase III randomized trial evaluated the combination of clofarabine and high-dose cytarabine (HDAC) compared to HDAC monotherapy in older adult patients (> 55 years) with relapsed/refractory AML [46]. Patients received clofarabine (40 mg/m2 IV) or placebo followed by HDAC 1 g/m2 IV daily for 5 days; 320 patients with confirmed AML (median age: 67 years) were enrolled. Median OS was 6.6 and 6.3 months in the combination and HDAC alone arms, respectively (p = 1.00). Although the study did not show a significant difference in OS, the combination demonstrated statistically significant improvement in ORR (46.9% vs. 22.9%, respectively, p < 0.01) and 4-month EFS (37.7% vs. 16.6%, respectively, p < 0.01), favoring the combination arm in the overall patient population as well as the relapsed patients stratum. Overall 30-day mortality was 16% and 5% in the combination and HDAC alone arms, respectively. Serious adverse effects occurred in 60% and 49%, and infections (≥grade 3) occurred in 65% vs. 48% of patients, respectively. More patients in the combination arm (16% vs. 9%) underwent transplant in remission.
Clofarabine and transplant in patients with AML and MDS (Table III)
Table III.
Summary of important studies using clofarabine-containing regimen prior to allogeneic stem cell transplant in patients with AML and high risk MDS.
| Study design | Number of patients | Regimen | Baseline disease status | Median age | Outcome |
|---|---|---|---|---|---|
| Phase II | 7 | As a pre-AHSCT conditioning regimen: clofarabine 40 mg/m 2 IV on days −6 to −2; cytarabine 1 g/m 2 IV on days −6 to −2; 1 mg/kg of antithymocyte globulin (ATG) on day −4 and 2.5 mg/kg of ATG on days −3 and −2 | Three patients with MDS, four with AML | 54 | 3/7 expired on days 15, 26 and 32; ¾ relapsed with a median time to progression of 152 days |
| Phase I | 15 | Busulfan 0.8 mg/kg every 6 h IV on days −6 to −3 and clofarabine 30–60 mg/m 2 per day on days −6 to −2 | Relapsed/refractory AML or ALL | 48 | 1-year EFS and OS rates were 53% and 60%, respectively |
| Retrospective | 17 | As a cytoreductive regimen prior to AHSCT: 30–40 mg/m2 daily for 5 days with plans to initiate conditioning during the nadir | Relapsed/refractory AML | 58 | One-year PFS and OS rates were 25 and 38%, respectively. Two patients were alive in remission at 18 and 52 months |
| Retrospective | 27 | AHSCT after treatment with clofarabine and ara-C for 5 days and reduced intensity conditioning (4 Gy total body irradiation/cyclophosphamide/ATG) | High-risk, relapsed or refractory AML or MDS | 58 | The 2-year OS and RFS rates were 56% and 52%, respectively; seven patients relapsed within a median of 5.7 months |
AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; AHSCT, allogeneic hematopoietic stem cell transplant; ALL, acute lymphoblastic leukemia; EFS, event-free survival; OS, overall survival; PFS, progression-free survival; RFS, relapse-free survival.
The use of clofarabine has also been tested in conditioning regimens for patients with AML and MDS receiving AHSCT. Martin et al. published a small study which enrolled patients with MDS and AML who received clofarabine 40 mg/m2 IV on days −6 to −2, cytarabine 1 g/m2 IV on days −6 to −2 and 1 mg/kg of antithymocyte globulin (ATG) on day −4 and 2.5 mg/kg of ATG on days −3 and −2 as a pre-AHSCT conditioning regimen [47]. Seven patients were enrolled (three with MDS, four with AML). Their median age was 54 years. The median duration of neutropenia was 14 days and that of thrombocytopenia was 22 days. No acute graftversus-host disease (GVHD) was observed. Enrollment to the trial was halted after three of the first seven patients expired on days 15, 26 and 32. Three of the four surviving patients have relapsed, with a median time to progression of 152 days. This regimen was not sufficiently immunosuppressive to ensure engraftment, and was associated with substantial morbidity and mortality.
More encouraging results were obtained with the combination of clofarabine and busulfan in a phase I trial done to determine the MTD of clofarabine with high-dose busulfan followed by AHSCT in patients with relapsed/refractory acute leukemia [48]. Patients received busulfan 0.8 mg/kg every 6 h IV on days −6 to −3 and clofarabine 30–60 mg/m2 per day on days −6 to −2. A total of 15 patients, median age 48 years (range, 30–58), were treated at four clofarabine dose levels: 30, 40, 50 and 60 mg/m2 per day with busulfan. All patients engrafted, and the MTD was not reached. Most common grades 3–4 non-hematological toxicities included mucositis and reversible elevation of AST/ALT. The 1-year EFS and OS rates were 53% and 60%, respectively. Given the good tolerability and promising results, the authors recommended clofarabine 60 mg/m2 per day for 5 days as a phase II dose in combination with busulfan (12.8 mg per kg total dose) for further study as a myeloablative regimen for AHSCT for high-risk acute leukemia.
Clofarabine has also been recently studied as part of cytoreductive regimens prior to AHSCT for patients with relapsed/refractory AML. Locke et al. reported on 17 patients who received clofarabine 30–40 mg/m2 daily for 5 days with plans to initiate conditioning during the nadir, 14 days later [49]. Bone marrow biopsy 12 days after clofarabine showed effective cytoreduction (less than 20% cellularity with less than 10% blasts) in 10 of 17 patients (59%). Ineffective cytoreduction correlated with significantly lower PFS (3.8 vs. 6.4 months) and OS (5.1 vs. 16.6 months). Significant toxicities before AHSCT, attributable to clofarabine, were mostly grade 1–2, except for 18% of the patients who had grade 3–4 transaminitis. Sixteen patients proceeded to stem cell infusion at a median of 22 days after initiation of clofarabine. Day-100 and 2-year transplant-related mortality (TRM) was 6 and 36%, respectively. Nine patients relapsed. One-year PFS and OS rates were 25 and 38%, respectively. Two patients remained alive in remission at 18 and 52 months, respectively. Clofarabine cytoreduction followed by immediate AHSCT appeared to be feasible, with acceptable toxicity and TRM.
In a retrospective analysis, Buchholz et al. evaluated a clofarabine-based combination cytoreductive chemotherapy followed by reduced intensity conditioning (RIC) and AHSCT for high-risk, relapsed or refractory AML or MDS [50]. A total of 27 patients underwent AHSCT after treatment with clofarabine and ara-C for 5 days and RIC (4 Gy total body irradiation [TBI]/cyclophosphamide/anti-thymocyte globulin). Non-hematological toxicities of this regimen mainly affected liver and skin and were all reversible. Seven patients relapsed within a median time of 5.7 months. The 2-year OS and RFS rates were 56% and 52%, respectively. In this cohort of patients, cytoreduction with clofarabine/ara-C followed by RIC AHSCT was well tolerated and showed good antileukemic efficacy even in patients with high-risk AML or MDS, with engraftment and GVHD incidence comparable to other RIC regimens.
Conclusion
Although clofarabine has demonstrated efficacy as front-line and salvage therapy of adult myeloid leukemia, its only FDA-approved use remains for pediatric patients with relapsed/refractory ALL after the failure of at least two prior regimens. Clofarabine is a nucleoside analog that provides a striking example of how seemingly minor differences in a molecular structure are associated with significant differences in metabolic and pharmacokinetic behavior as well as in the spectrum of activity. These interesting properties of clofarabine, as well as the striving need to develop new therapies for adult patients with leukemia, explain the increased interest in this agent over the past decade. Its toxicity profile makes the drug potentially useful for patients excluded from intensive chemotherapy at diagnosis. Compared to intensive chemotherapy regimens, clofarabine seems to be associated with similar efficacy and lower induction mortality. Clofarabine may thus be an appropriate alternative treatment option for “unfit” patients or those who are unable to tolerate anthracyclines, which are included in most intensive AML induction regimens. The addition of cytarabine to clofarabine seems also to improve clinical responses especially in patients with unfavorable cytogenetics. Exciting results have been achieved with single line or clofarabine based combinations in that latter group, and the triple combination of clofarabine, cytarabine and fludarabine or anthracycline appears to achieve promising results. Further studies are needed to validate the role of clofarabine in the front-line setting as well as prior to transplant. Additional randomized controlled trials are also necessary to directly compare the efficacy of clofarabine to intensive chemotherapy regimens across all risk groups, as well as to confirm the potential benefit of combining clofarabine with cytarabine in adults and older patients.
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
References
- 1.Parker WB, Allan PW, Hassan AE, et al. Antitumor activity of 2-fluoro-2′-deoxyadenosine against tumors that express Escherichia coli purine nucleoside phosphorylase. Cancer Gene Ther. 2003;10:23–29. doi: 10.1038/sj.cgt.7700520. [DOI] [PubMed] [Google Scholar]
- 2.Carson DA, Wasson DB, Esparza LM, et al. Oral antilymphocyte activity and induction of apoptosis by 2-chloro-2′-arabino-fluoro-2′-deoxyadenosine. Proc Natl Acad Sci USA. 1992;89:2970–2974. doi: 10.1073/pnas.89.7.2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lotfi K, Mansson E, Spasokoukotskaja T, et al. Biochemical pharmacology and resistance to 2-chloro-2′-arabino-fluoro-2′-deoxyadenosine, a novel analogue of cladribine in human leukemic cells. Clin Cancer Res. 1999;5:2438–2444. [PubMed] [Google Scholar]
- 4.Xie C, Plunkett W. Metabolism and actions of 2-chloro-9-(2-deoxy-2-fluoro-beta-D-arabinofuranosyl)-adenine in human lymphoblastoid cells. Cancer Res. 1995;55:2847–2852. [PubMed] [Google Scholar]
- 5.Pui CH, Jeha S. Clofarabine. Nat Rev Drug Discov. 2005;(Suppl):S12–S13. doi: 10.1038/nrd1724. [DOI] [PubMed] [Google Scholar]
- 6.Parker WB, Shaddix SC, Chang CH, et al. Effects of 2-chloro-9-(2-deoxy-2-fluoro-beta-D-arabinofuranosyl)adenine on K562 cellular metabolism and the inhibition of human ribonucleotide reductase and DNA polymerases by its 5′-triphosphate. Cancer Res. 1991;51:2386–2394. [PubMed] [Google Scholar]
- 7.Xie KC, Plunkett W. Deoxynucleotide pool depletion and sustained inhibition of ribonucleotide reductase and DNA synthesis after treatment of human lymphoblastoid cells with 2-chloro-9-(2-deoxy-2-fluoro-beta-D-arabinofuranosyl) adenine. Cancer Res. 1996;56:3030–3037. [PubMed] [Google Scholar]
- 8.Genini D, Adachi S, Chao Q, et al. Deoxyadenosine analogs induce programmed cell death in chronic lymphocytic leukemia cells by damaging the DNA and by directly affecting the mitochondria. Blood. 2000;96:3537–3543. [PubMed] [Google Scholar]
- 9.Parker WB, Shaddix SC, Rose LM, et al. Comparison of the mechanism of cytotoxicity of 2-chloro-9-(2-deoxy-2-fluoro-beta-D-arabinofuranosyl) adenine, 2-chloro-9-(2-deoxy-2-fluoro-beta-D-ribofuranosyl)adenine, and 2-chloro-9-(2-deoxy-2,2-difluoro-beta-D-ribofuranosyl)adenine in CEM cells. Mol Pharmacol. 1999;55:515–520. [PubMed] [Google Scholar]
- 10.Parker WB, Bapat AR, Shen JX, et al. Interaction of 2-halogenated dATP analogs (F, Cl, and Br) with human DNA polymerases, DNA primase, and ribonucleotide reductase. Mol Pharmacol. 1988;34:485–491. [PubMed] [Google Scholar]
- 11.Takahashi T, Kanazawa J, Akinaga S, et al. Antitumor activity of 2-chloro-9-(2-deoxy-2-fluoro-beta-D-arabinofuranosyl) adenine, a novel deoxyadenosine analog, against human colon tumor xenografts by oral administration. Cancer Chemother Pharmacol. 1999;43:233–240. doi: 10.1007/s002800050889. [DOI] [PubMed] [Google Scholar]
- 12.Waud WR, Schmid SM, Montgomery JA, et al. Preclinical antitumor activity of 2-chloro-9-(2-deoxy-2-fluoro-beta-D-arabinofuranosyl) adenine (Cl-F-ara-A) Nucleosides Nucleotides Nucleic Acids. 2000;19:447–460. doi: 10.1080/15257770008033020. [DOI] [PubMed] [Google Scholar]
- 13.Avramis VI, Plunkett W. Metabolism of 9-beta-D-arabinosyl-2-fluoroadenine-5′-phosphate by mice bearing P388 leukemia. Cancer Drug Deliv. 1983;1:1–10. doi: 10.1089/cdd.1983.1.1. [DOI] [PubMed] [Google Scholar]
- 14.Response of subcutaneous HT-29 tumor to clofarabine or CPT-11 (Final report ILEX 27) Birmingham, AL: Southern Research Institute; 2003. [Google Scholar]
- 15.Final report. 6. San Antonio, TX: Genzyme Corp; 2007. Clofarabine investigator’s brochure. [Google Scholar]
- 16.Kantarjian HM, Gandhi V, Kozuch P, et al. Phase I clinical and pharmacology study of clofarabine in patients with solid and hematologic cancers. J Clin Oncol. 2003;21:1167–1173. doi: 10.1200/JCO.2003.04.031. [DOI] [PubMed] [Google Scholar]
- 17.Gandhi V, Kantarjian H, Faderl S, et al. Pharmacokinetics and pharmacodynamics of plasma clofarabine and cellular clofarabine triphosphate in patients with acute leukemias. Clin Cancer Res. 2003;9:6335–6342. [PubMed] [Google Scholar]
- 18.Kantarjian H, Gandhi V, Cortes J, et al. Phase 2 clinical and pharmacologic study of clofarabine in patients with refractory or relapsed acute leukemia. Blood. 2003;102:2379–2386. doi: 10.1182/blood-2003-03-0925. [DOI] [PubMed] [Google Scholar]
- 19.Gandhi V, Plunkett W. Modulation of arabinosylnucleoside metabolism by arabinosylnucleotides in human leukemia cells. Cancer Res. 1988;48:329–334. [PubMed] [Google Scholar]
- 20.Plunkett W, Iacoboni S, Estey E, et al. Pharmacologically directed ara-C therapy for refractory leukemia. Semin Oncol. 1985;12:20–30. [PubMed] [Google Scholar]
- 21.Estey E, Plunkett W, Dixon D, et al. Variables predicting response to high dose cytosine arabinoside therapy in patients with refractory acute leukemia. Leukemia. 1987;1:580–583. [PubMed] [Google Scholar]
- 22.Cooper T, Ayres M, Nowak B, et al. Biochemical modulation of cytarabine triphosphate by clofarabine. Cancer Chemother Pharmacol. 2005;55:361–368. doi: 10.1007/s00280-004-0906-y. [DOI] [PubMed] [Google Scholar]
- 23.Moufarij MA, Sampath D, Keating MJ, et al. Fludarabine increases oxaliplatin cytotoxicity in normal and chronic lymphocytic leukemia lymphocytes by suppressing interstrand DNA crosslink removal. Blood. 2006;108:4187–4193. doi: 10.1182/blood-2006-05-023259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foran JM, Faderl S, Wetzler M, et al. A phase II, open-label study of clofarabine in adult patients with refractory or relapsed acute myelogenous leukemia. Proc Am Soc Clin Oncol. 2003;22 Abstract 2360. [Google Scholar]
- 25.Racil Z, Toskova M, Dvorakova D, et al. Treatment of molecular relapse in patients with acute myeloid leukemia using clofarabine monotherapy. Am J Hematol. 2011 Oct 12; doi: 10.1002/ajh.22213. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 26.Krawczyk J, Ansar N, Swords R, et al. Clofarabine in the treatment of poor risk acute myeloid leukaemia. Hematol Oncol. 2010;28:118–123. doi: 10.1002/hon.922. [DOI] [PubMed] [Google Scholar]
- 27.Faderl S, Garcia-Manero G, Jabbour E, et al. A randomized study of 2 dose levels of intravenous clofarabine in the treatment of patients with higher-risk myelodysplastic syndrome. Cancer. 2012;118:722–728. doi: 10.1002/cncr.26327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faderl S, Garcia-Manero G, Estrov Z, et al. Oral clofarabine in the treatment of patients with higher-risk myelodysplastic syndrome. J Clin Oncol. 2010;28:2755–2760. doi: 10.1200/JCO.2009.26.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faderl S, Gandhi V, O’Brien S, et al. Results of a phase 1–2 study of clofarabine in combination with cytarabine (ara-C) in relapsed and refractory acute leukemias. Blood. 2005;105:940–947. doi: 10.1182/blood-2004-05-1933. [DOI] [PubMed] [Google Scholar]
- 30.Faderl S, Gandhi V, Verstovsek S, et al. Clofarabine plus cytarabine (ara-C) combination is active in newly diagnosed patients (pts) > = age 50 with acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) Blood. 2004;104(Suppl 1) Abstract 875. [Google Scholar]
- 31.Faderl S, Ravandi F, Huang X, et al. A randomized study of clofarabine versus clofarabine plus low-dose cytarabine as front-line therapy for patients aged 60 years and older with acute myeloidleukemia and high-risk myelodysplastic syndrome. Blood. 2008;112:1638–1645. doi: 10.1182/blood-2007-11-124602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agura E, Cooper B, Holmes H, et al. Report of a phase II study of clofarabine and cytarabine in de novo and relapsed and refractory AML patients and in selected elderly patients at high risk for anthracycline toxicity. Oncologist. 2011;16:197–206. doi: 10.1634/theoncologist.2010-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faderl S, Ravandi F, Huang X, et al. Clofarabine plus low-dose cytarabine followed by clofarabine plus low-dose cytarabine alternating with decitabine in acute myeloid leukemia frontline therapy for older patients. Cancer. 2012;118:4471–4477. doi: 10.1002/cncr.27429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Becker PS, Kantarjian HM, Appelbaum FR, et al. Clofarabine with high dose cytarabine and granulocyte colony-stimulating factor (G-CSF) priming for relapsed and refractory acute myeloid leukaemia. Br J Haematol. 2011;155:182–189. doi: 10.1111/j.1365-2141.2011.08831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamauchi T, Nowak BJ, Keating MJ, et al. DNA repair initiated in chronic lymphocytic leukemia lymphocytes by 4-hydroper-oxycyclophosphamide is inhibited by fludarabine and clofarabine. Clin Cancer Res. 2001;7:3580–3589. [PubMed] [Google Scholar]
- 36.Szmigielska-Kaplon A, Ciesielska E, Szmigiero L, et al. Anthracyclines potentiate activity against murine leukemias L1210 and P388 in vivo and in vitro. Eur J Haematol. 2002;68:370–375. doi: 10.1034/j.1600-0609.2002.01598.x. [DOI] [PubMed] [Google Scholar]
- 37.Faderl S, Ferrajoli A, Wierda W, et al. Clofarabine combinations as acute myeloid leukemia salvage therapy. Cancer. 2008;113:2090–2096. doi: 10.1002/cncr.23816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nazha A, Ravandi F, Kantarjian HM, et al. Clofarabine, idarubicin, and cytarabine (CIA) as frontline therapy for patients younger than 61 years with newly diagnosed acute myeloid leukemia (AML) Blood. 2011;118(Suppl 1) doi: 10.1002/ajh.23544. Abstract 1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mathisen M, Kantarjian H, Faderl S, et al. Interim results of a phase I/II randomized study of clofarabine, idarubicin, and cytarabine (CIA) versus fludarabine, idarubicin, and cytarabine (FIA) for newly diagnosed or relapsed patients (pts) with acute myeloid leukemia (AML) J Clin Oncol. 2012;30(Suppl) Abstract 6607. [Google Scholar]
- 40.Burnett AK, Kell WJ, Hills RK, et al. The feasibility of combining daunorubicin, clofarabine and gemtuzumab ozogamicin is feasible and effective. A pilot study. Blood. 2006;108(Suppl) Abstract 1950. [Google Scholar]
- 41.Foster MC, Amin C, Voorhees PM, et al. A phase I dose-escalation study of clofarabine in combination with fractionated gemtuzumab ozogamicin in patients with refractory or relapsed acute myeloid leukemia. Leuk Lymphoma. 2012;53:1331–1337. doi: 10.3109/10428194.2011.647313. [DOI] [PubMed] [Google Scholar]
- 42.Amadori S, Stasi R, Martelli AM, et al. Temsirolimus, an mTOR inhibitor, in combination with lower-dose clofarabine as salvage therapy for older patients with acute myeloid leukaemia: results of a phase II GIMEMA study (AML-1107) Br J Haematol. 2012;156:205–212. doi: 10.1111/j.1365-2141.2011.08940.x. [DOI] [PubMed] [Google Scholar]
- 43.Kantarjian H, Ravandi F, O’Brien S, et al. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood. 2010;116:4422–4429. doi: 10.1182/blood-2010-03-276485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burnett AK, Russell NH, Kell J, et al. European development of clofarabine as treatment for older patients with acute myeloid leukemia considered unsuitable for intensive chemotherapy. J Clin Oncol. 2010;28:2389–2395. doi: 10.1200/JCO.2009.26.4242. [DOI] [PubMed] [Google Scholar]
- 45.Kantarjian HM, Erba HP, Claxton D, et al. Phase II study of clofarabine monotherapy in previously untreated older adults with acute myeloid leukemia and unfavorable prognostic factors. J Clin Oncol. 2010;28:549–555. doi: 10.1200/JCO.2009.23.3130. [DOI] [PubMed] [Google Scholar]
- 46.Faderl S, Wetzler M, Rizzieri D, et al. Clofarabine plus cytarabine compared with cytarabine alone in older patients with relapsed or refractory acute myelogenous leukemia: results from the CLASSIC I trial. J Clin Oncol. 2012;30:2492–2499. doi: 10.1200/JCO.2011.37.9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin MG, Uy GL, Procknow E, et al. Allo-SCT conditioning for myelodysplastic syndrome and acute myeloid leukemia with clofarabine, cytarabine and ATG. Bone Marrow Transplant. 2009;44:13–17. doi: 10.1038/bmt.2008.423. [DOI] [PubMed] [Google Scholar]
- 48.Farag SS, Wood LL, Schwartz JE, et al. Phase I trial and pharmacokinetic study of high-dose clofarabine and busulfan and allogeneic stem cell transplantation in adults with high-risk and refractory acute leukemia. Leukemia. 2011;25:599–605. doi: 10.1038/leu.2010.319. [DOI] [PubMed] [Google Scholar]
- 49.Locke FL, Artz A, Rich E, et al. Feasibility of clofarabine cytoreduction before allogeneic transplant conditioning for refractory AML. Bone Marrow Transplant. 2010;45:1692–1698. doi: 10.1038/bmt.2010.32. [DOI] [PubMed] [Google Scholar]
- 50.Buchholz S, Dammann E, Stadler M, et al. Cytoreductive treatment with clofarabine/ara-C combined with reduced-intensity conditioning and allogeneic stem cell transplantation in patients with high-risk, relapsed, or refractory acute myeloid leukemia and advanced myelodysplastic syndrome. Eur J Haematol. 2012;88:52–60. doi: 10.1111/j.1600-0609.2011.01703.x. [DOI] [PubMed] [Google Scholar]