Abstract
Objective
Non-coding RNAs constitute a major fraction of the β-cell transcriptome. While the involvement of microRNAs is well established, the contribution of long non-coding RNAs (lncRNAs) in the regulation of β-cell functions and in diabetes development remains poorly understood. The aim of this study was to identify novel islet lncRNAs differently expressed in type 2 diabetes models and to investigate their role in β-cell failure and in the development of the disease.
Methods
Novel transcripts dysregulated in the islets of diet-induced obese mice were identified by high throughput RNA-sequencing coupled with de novo annotation. Changes in the level of the lncRNAs were assessed by real-time PCR. The functional role of the selected lncRNAs was determined by modifying their expression in MIN6 cells and primary islet cells.
Results
We identified about 1500 novel lncRNAs, a number of which were differentially expressed in obese mice. The expression of two lncRNAs highly enriched in β-cells, βlinc2, and βlinc3, correlated to body weight gain and glycemia levels in obese mice and was also modified in diabetic db/db mice. The expression of both lncRNAs was also modulated in vitro in isolated islet cells by glucolipotoxic conditions. Moreover, the expression of the human orthologue of βlinc3 was altered in the islets of type 2 diabetic patients and was associated to the BMI of the donors. Modulation of the level of βlinc2 and βlinc3 by overexpression or downregulation in MIN6 and mouse islet cells did not affect insulin secretion but increased β-cell apoptosis.
Conclusions
Taken together, the data show that lncRNAs are modulated in a model of obesity-associated type 2 diabetes and that variations in the expression of some of them may contribute to β-cell failure during the development of the disease.
Keywords: Diabetes, Insulin, Pancreatic islet, Obesity, Gene expression
Highlights
-
•
Mouse pancreatic islets express a large number of novel long non-coding RNAs.
-
•
Many long non-coding RNAs are differentially expressed in the islets of obese mice.
-
•
The level of two islet long non-coding RNAs correlates to body weight and glycemia.
-
•
The expression of these islet long non-coding RNAs is altered in Type 2 diabetes.
-
•
Altered expression of these long non-coding RNAs sensitise β-cells to apoptosis.
1. Introduction
Type 2 diabetes (T2D) is characterized by reduced insulin action and/or insulin deficiency [1], [2]. Insulin is responsible for the control of blood glucose levels and its release is finely tuned by nutrients, hormones, and neurotransmitters. Under obese conditions, a major risk factor for T2D, the peripheral tissues become less sensitive to insulin [3]. To maintain euglycemia and overcome peripheral insulin resistance, β-cells expand and increase their secretory activity [3]. However, in genetically predisposed individuals, defects in this process can lead to progressive deterioration of β-cell function and loss of β-cell by apoptosis, promoting the development of diabetes [3], [4], [5].
The mechanisms underlying compensatory β-cell mass expansion, β-cell failure and progression of diabetes are still largely unknown. The proposed causes of β-cell failure include, mitochondrial dysfunction, oxidative and endoplasmic reticulum stress, dysfunctional triglyceride/free fatty acid cycling, and glucolipotoxicity [3], [5]. These phenomena trigger alterations in the level of key protein-coding genes and microRNAs [3], [6], [7], a class of small non-coding RNAs playing central roles in numerous physiological and pathological processes [8]. Beside protein-coding genes and microRNAs, transcriptome analysis identified another large class of non-coding RNAs, the long non-coding RNAs (lncRNAs) [9], [10], [11], [12]. These non-coding transcripts participate in diverse gene-regulatory mechanisms [13], [14], [15] and their dysregulation has been implicated in many human diseases [16]. Recently, lncRNAs were found to contribute to β-cell development and glucose homeostasis [17], [18] and to be involved in β-cell demise during the initial phases of type 1 diabetes [19], [20]. However, so far only very few lncRNAs have been functionally characterized and very little is known about their possible contribution to T2D development.
The aim of this study was to identify novel islet lncRNAs and to investigate their role in the regulation of β-cell functions. The expression of lncRNAs is highly cell-type and context specific. In view of this, we used RNA-sequencing to explore unbiasedly the islet transcriptome for novel lncRNAs expressed in mice fed a high-fat diet, a model of mild diabetes associated with obesity that corresponds to early diabetes in human [21]. We identified several not yet annotated lncRNAs, a number of which displayed expression changes between islets of lean and obese mice. Analogous changes of selected lncRNAs were also observed in islets of obese diabetic db/db mice and in the islets of T2D donors. In addition, the modulation of some of these lncRNAs in dissociated mouse islet cells sensitised the β-cells to apoptosis. Overall, the results show that lncRNAs are modulated in islets from obese diabetic mice and T2D individuals and may contribute to β-cell failure during T2D development.
2. Material and methods
2.1. Chemicals
IL-1β, leptomycin B, collagenase, and Histopaque were purchased from Sigma–Aldrich (St Louis, MO, USA), TNF-α from Enzo Life sciences (Farmingdale, NY, USA) and IFN-γ from R&D systems (Minneapolis, MN, USA).
2.2. Animals
Five-week old male C57BL/6 mice (Charles River Laboratories, Raleigh, NC, USA) were fed a normal (ND) or a high-fat diet (HFD) for 8 weeks (Bioserv F-3282, 60% energy from fat, Frenchtown, NJ, USA) [21]. The animals on high fed diet were subdivided in low (LDR) and high responders (HDR) according to the criteria defined in Peyot et al., 2010 [21]. The mice in the LDR group weighted between 33 and 39 g after 7.5 weeks on HFD while the animals in the HDR group between 39 and 45 g. C57BL/KsJ db/db mice (13–16 weeks) and age-matched lean db/+ littermates were obtained from the Garvan Institute breeding colonies (Sydney, NSW, Australia) [22]. Mice expressing the enhanced yellow florescence protein in β-cells (RIPYY) were obtained by crossing rosa-26EYFP with RIP-Cre mice [23]. Animal procedures were performed in accordance with National Institutes of Health guidelines and were approved by research councils and veterinary offices.
2.3. Islet and insulin-secreting cells
Mice islets were isolated by collagenase digestion followed by Histopaque density gradient [24] and cultured in RPMI medium [19]. Islets from RIPYY mice were either directly used to isolate the RNA or dissociated to separate β- from non-β cells by FAC-sorting [23]. Human islets were provided by the Cell Isolation and Transplantation Center (University of Geneva) or by the Human Tissue Lab of EXODIAB/Lund University Diabetes Centre through the Nordic Network for Islet Transplantation, Uppsala University. After isolation, the human islets were utilized for RNA isolation or cultured in CMRL medium (Invitrogen) supplemented with 10% FCS, 100 μg/mL streptomycin and 100 IU/mL penicillin (Invitrogen), 2 mmol/L l-glutamine and 250 μmol/L HEPES. Dissociation of mice and human islets was achieved by incubation at 37 °C in PBS containing 3 mM EGTA and 0.002% trypsin. MIN6B1 cells were cultured in DMEM-Glutamax medium (Invitrogen) [25].
2.4. RNA-sequencing and analysis
RNA was isolated using the RNeasy kit (Qiagen), followed by DNase treatment (Promega). Ribosomal RNA was removed using the Ribo-Zero Magnetic Gold kit (MRZG126, Illumina), and sequence libraries were prepared using the Illumina TruSeq stranded mRNA LT kit without poly(A) selection in order to include also all the lncRNA transcripts that are not polyadenylated. Libraries were sequenced with the Illumina HiSeq2000. 100nt paired-end reads from 6 samples were mapped to mm9 reference genome using Tophat software version 2.0.8 [26] with the option ”Gene model” –G, using mm9 UCSC reference genes GTF [27]. Ab initio transcript reconstruction was performed using Cufflinks, version 2.1.1 [26], with option –G and the reference UCSC genome. The resulting GTFs were merged using Cuffmerge v2.1.1 [28] to distinguish known and novel transcripts. Using the output of Cuffmerge, the transcripts were divided into 3 categories: known mRNAs, known lncRNAs (UCSC as reference), and novel lncRNAs. Novel transcripts were filtered for having at least 2 exons. Read counts were then calculated per gene from the alignment bam files using HTSeq (v0.5.4p3) with options –m union –stranded no. Genes were then filtered for minimal expression (mean counts ≥5 across all conditions). The protein-coding potential of transcripts was evaluated using the program GeneID [29], v1.4.4, applied to transcript sequences in FASTA format, with parameters adapted for vertebrates as provided by the authors in file GeneID.human.070123.param and with options –s and –G. Transcripts with a coding potential >4 were removed from the analysis. Differentially expressed genes were detected using the limma package in R by first transforming the raw count data to log2 counts per million reads using the voom function. Empirical Bayes moderated t statistics and corresponding p-values were computed for the comparison and p-values adjusted for multiple comparisons using the Benjamini-Hochberg procedure [30]. Genes with an adjusted p-value of ≤0.05 were considered differentially expressed. Differential analysis by transcripts was done using Cuffdiff, v2.1.1 [28], on a gtf file containing the coordinates of the novel transcripts. Gene ontology analysis was performed by submitting the genes lists to the DAVID Functional annotation clustering tool using default parameters (https://david.ncifcrf.gov/tools.jsp).
2.5. Measurement of lncRNAs expression
RNA was reverse transcribed using M-MLV reverse transcriptase, RNAse H minus (Promega). Quantitative PCR was performed using iQ SYBR Green mix and samples were amplified using the CFX Connect Real-time system (Bio-Rad). Islets of human control and T2D patients were homogenized by vortexing in 700 uL Qiazol lysis buffer and the RNA extracted using the miRNeasy kit (Qiagen) with DNase treatment. 100ng total RNA was used for reverse transcription using the High Capacity cDNA kit with RNAse inhibitor (ThermoFisher). For qPCR, PowerUP SYBR Green Master Mix (Applied Biosystems) was used with assay-specific primers (Supplemental Table 1).
2.6. Subcellular fraction
MIN6B1 cells were incubated for 15 min in ice-cold lysis buffer (10 mM Tris–HCl, pH 7.5, 0.05% NP40, 3 mM MgCl2, 10 mM NaCl and 5 mM EGTA) and then centrifuged 10 min at 2,000× g. The supernatant (cytoplasmic fraction) was recovered while the pellet was resuspended in 10 mM HEPES, pH 6.8, 300 mM sucrose, 3 mM MgCl2, 25 mM NaCl, 1 mM EGTA, 0.5% Triton-X-100 and treated with 700 U/ml DNAse I for 30 min at 4 °C. The samples were then centrifuged at 17,000× g for 20 min and the pellet collected as nucleoplasmic fraction.
2.7. Transfection
Overexpression of lncRNAs was achieved by transfecting pcDNA3-based plasmids with Lipofectamine 2000 (MIN6B1 cells) or 3000 (primary cells) (Invitrogen). Down-regulation was carried out by transfecting Gapmers (Exiqon) with RNAiMax (Invitrogen) (gapmers sequences in Supplemental Table 1).
2.8. Insulin secretion
Insulin secretion of MIN6B1 cells was carried out as described [25].
2.9. Assessment of cell death
Apoptotic cells displaying pyknotic nuclei were scored under blind conditions by fluorescence microscopy (AxioCam MRc5, Zeiss, Feldbach, Switzerland) after incubation with 1 μg/ml Hoechst [25] or TUNEL assay (Roche).
2.10. NF-κB nuclear translocation
MIN6B1 cells were transfected with a plasmid expressing a GFP-tagged form of NF-κB subunit p65 (Rela) and/or the plasmid expressing the lncRNA. 24 h later, the cells were treated with the indicated compounds for 3 h, fixed, and mounted on a coverslip for microscopic examination.
2.11. Statistical analysis
Data are presented as mean ± sem. Statistical differences were assessed by two-tailed paired Student's t-test when only two sets of data were present or by one-way ANOVA with more than 2 groups with a discriminating p-value of 0.05 (GraphPad Prism). Correlations between lncRNA expression and different characteristics of the individuals were performed by linear regression, where F-test was used to determine significance at p < 0.05.
ACCESION NUMBERS: RNA-sequencing data have been deposited in the Gene Expression Omnibus Database, accession number GSE92602.
3. Results
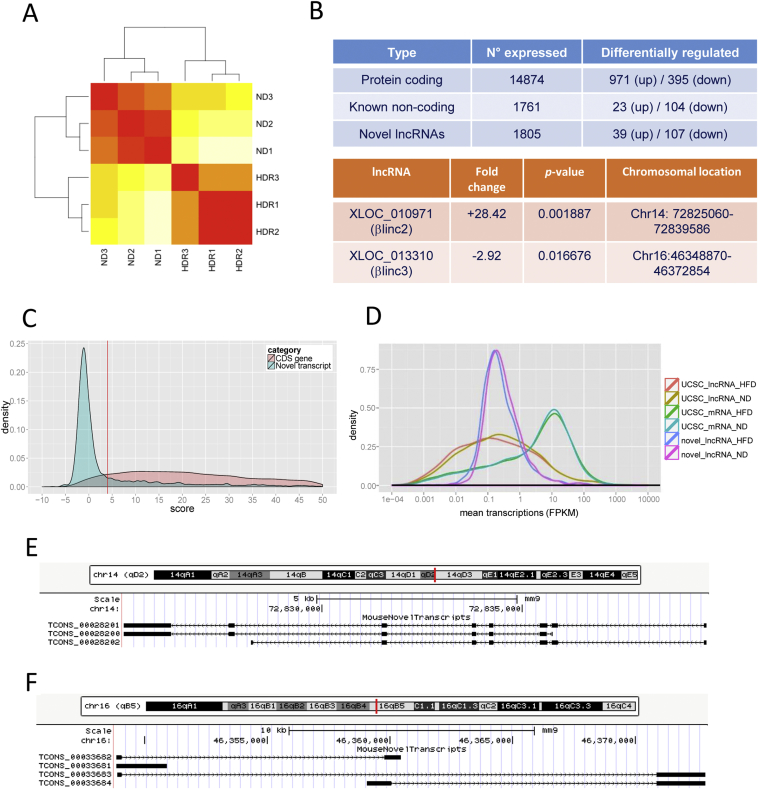
To investigate the contribution of lncRNAs to β-cell dysfunction and T2D development, we compared the transcriptome of islets from mice fed a regular and a high-fat diet. The metabolic characteristics of these animals are summarized in Supplemental Table 2. RNA-sequencing yielded ∼500.000.000 reads per sample (accession number GSE92602), of which, ∼75% were mapped to the mouse genome. Ab initio transcript assembly was performed using Cufflinks [26], and novel transcripts were classified from known lncRNAs and protein-coding mRNAs. Hierarchical clustering showed a distinguishable expression profile between the two groups (Figure 1A). The analysis detected 14874 protein-coding genes, of which, 971 were upregulated and 395 downregulated in mice fed a high-fat diet (Figure 1B and Supplemental Table 3). Functional annotation of the differentially expressed protein-coding genes showed enrichment for biological pathways related to protein localization and transport, redox processes, intracellular transport (Supplemental Fig. 1). Of these differentially expressed genes, 21.2% overlapped with those previously identified by microarray [31]. We also detected 1761 UCSC annotated lncRNAs (23 upregulated and 104 downregulated) and 1996 non-annotated UCSC lncRNA genes (4303 transcripts), of which, 39 were upregulated and 107 downregulated (Figure 1B, Supplemental Tables 4 and 5). Amongst the non-annotated lncRNAs, 438 overlapped with recently published transcripts [32], [33] while 1558 were novel. We compared our mouse data with those of Moran et al. [34] obtained in human using TransMap, a cross species mRNA alignment tool. A list of mouse lncRNA genes for which we were able to identify the corresponding human orthologues is provided in the Supplemental Table 6. The GeneID-coding potential score revealed that our novel lncRNA candidates have minimal protein-coding potential (Figure 1C). The expression level of the novel lncRNAs overlapped that of UCSC-annotated lncRNAs and was usually lower than that of protein-coding genes (Figure 1D). The coordinates of all newly annotated transcripts is available on GEO (accession number GSE92602).
Figure 1.
Overview of the RNA-sequencing results. A. Hierarchical clustering of samples using the 500 genes displaying the highest mean expression. Colors display Euclidian distance, red represents no distance, yellow means there is a longer distance. ND, normal diet; HDR, high-(high fat diet) responders. B. Summary of differentially expressed genes (up right) and studied lncRNAs with fold changes and p-values (bottom right). C. Coding potential for novel transcripts compared to known coding genes. The red line represents the cutoff used to filter and classify the novel transcripts (<4, GeneID). D. Size distribution of protein-coding genes, known and novel lncRNAs. E. Locus architecture and isoforms of the βlinc2 gene. F. Locus architecture and isoforms of the βlinc3 gene.
We then used different criteria to select candidate lncRNAs for further analysis, including clearly detectable expression changes in response to high-fat diet, the presence of a small number of isoforms to avoid dealing with several overlapping transcripts with potentially different functions, and the putative presence of human orthologues. Multidimensional analysis revealed that the transcriptome of one of the animals (HDR3) was slightly different from the other mice on high-fat diet (Supplemental Fig.2). To avoid missing potentially interesting candidates, we included in our initial screening also the lncRNAs showing significant differences between control mice and the other two mice on HFD (Supplemental Table 7). We selected two intergenic lncRNAs (XLOC_010971 and XLOC_013310) for further analysis. The chromosomal location and the fold changes of these two novel lncRNAs are shown in Figure 1B. Since there is not yet a consensus for the nomenclature of mouse lncRNAs, they are hereafter referred to as βlinc2 and βlinc3 (β long intergenic noncoding RNA 2 and 3) to follow Arnes et al. nomenclature [18]. The locus architecture, the number of isoforms, and the coding potential of βlinc2 and βlinc3 are provided in Figure 1E–F and in the Supplemental Table 8. Subcellular fractionation of MIN6B1 cells revealed that βlinc2 is present both in the cytosolic and in the nuclear fractions while βlinc3 is essentially nuclear (Supplemental Fig. 3).
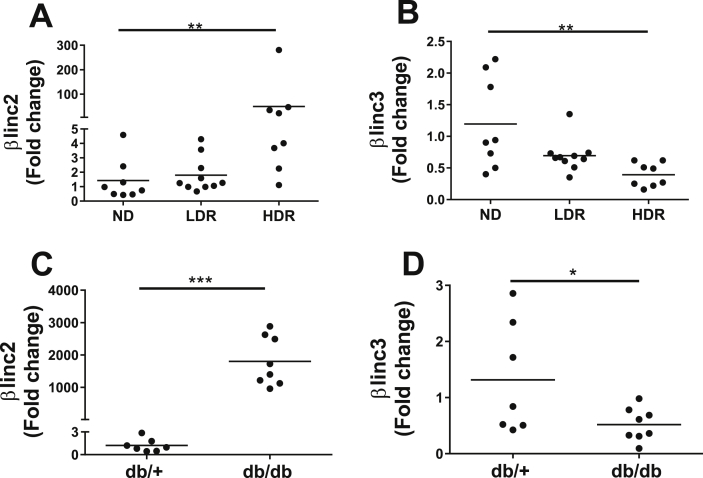
The changes observed by RNA-sequencing were confirmed by qRT-PCR in additional samples of high responders to the high-fat diet (HDR), as well as in low responders (LDR), a group of animals displaying the phenotypic characteristic of pre-diabetes when compared to obese humans [21]. The level of βlinc2 was not increased in the LDR group with no glycemic alterations but was upregulated 49 times in the HDR group that shows mild hyperglycemia [31]. The expression of βlinc3 tended to decrease already in the LDR group but reached significance only in the HDR group that displayed 60% lower levels compared to controls (Figure 2A–B). The expression of these transcripts was also analyzed in the islets of db/db mice, which lack the leptin receptor and develop severe obesity associated with T2D [22], [35]. The increase of βlinc2 was more pronounced than in HDR mice, with an up-regulation of 1802-fold, whereas the decrease in βlinc3 expression was similar to that observed in response to high-fat diet HDR group (Figure 2C–D).
Figure 2.
The expression levels of two lncRNAs are modified in islets from mice fed a high fat diet and in db/db mice. Expression of βlinc2 and βlinc3 in C57BL/6 mice fed a normal diet (ND) and in low-(high fat diet) responders (LDR) and in high-(high fat diet) responders (HDR) (A,B) and from db/+, db/db mice (C,D). Islets were isolated from mice of 14 weeks of age after being fed a standard or a high fat diet for 8 weeks and from db/db mice of 13–16 weeks of age. The expression levels of the lncRNAs were measured by real-time PCR and normalized to Gapdh. t-test or ANOVA, Kruskal–Wallis post hoc test, *P < 0.05 **P < 0.01, ***p < 0.001 vs ND or db/+.
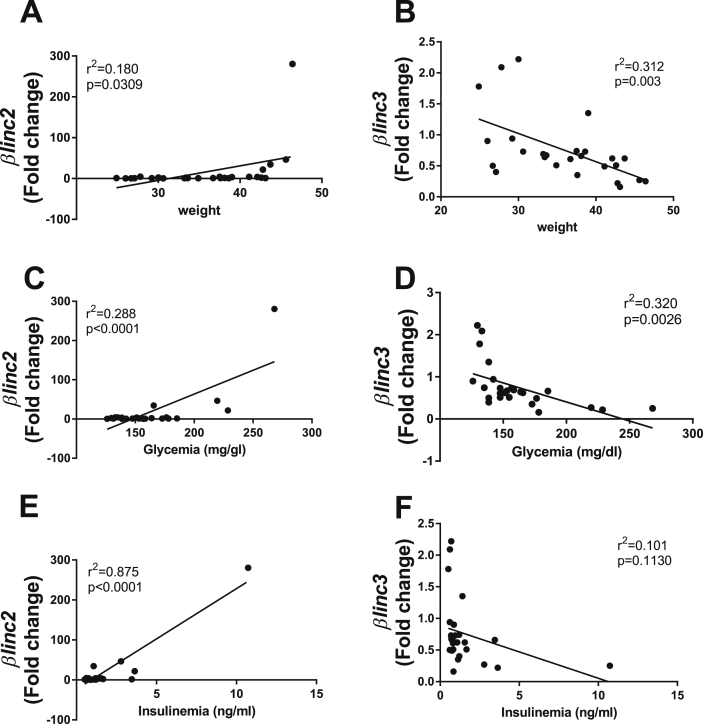
We then tested whether the expression of these two lncRNAs correlated with body weight, glycemia, and insulinemia of animals fed normal and high-fat diets (metabolic characteristics are provided in Supplemental Table 9). As shown in Figure 3 A, C, E we found a positive correlation between the level of βlinc2 with body weight, glycemia, and insulinemia. This was true even when performing the analysis after the exclusion of the highest point (Supplemental Fig. 4). The raise of βlinc2 was mainly observed in animals weighing >40 g, suggesting the existence of a threshold. In contrast, the expression of βlinc3 was negatively correlated with these parameters except for insulinemia (Figure 3B,D,F).
Figure 3.
Correlations of the expression of βlinc2 and βlinc3 with body weight, insulinemia, and glycemia of C57BL/6 mice fed a normal or a high fat diet. Linear regression analysis of the expression of βlinc2 versus body weight (A), glycemia (C) and insulinemia (E). Linear regression analysis of the expression of βlinc3 versus body weight (B), glycemia (D) and insulinemia (F). Body weight, Insulinemia and Glycemia were measured at sacrifice. Fed blood glucose in HDR and ND mice was measured by a portable glucometer (Accucheck, Roche). Plasma insulin was measured by ELISA (ultrasensitive mouse insulin kit, ALPCO). The expression levels of the lncRNAs were measured by real-time PCR and normalized to Gapdh. F-test was used to determine significance at p < 0.05.
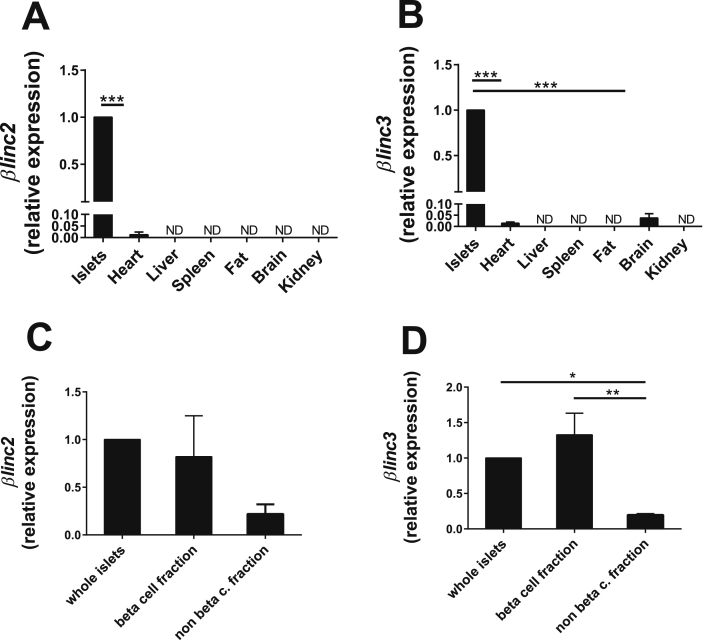
To assess whether the expression of these two novel lncRNAs is restricted to pancreatic islets, we analyzed their levels in a large panel of tissues. We found that βlinc2 is undetectable in the investigated tissues except in heart, where the expression is about 100 times lower compared to islets (Figure 4A). βlinc3 was only detectable in heart and brain, but again at much lower levels compared to islets (Figure 4B). To assess whether these lncRNAs are expressed in insulin-secreting cells, we measured their level in highly purified (∼99%) β-cell fractions obtained from FAC-sorted islet cells [23]. We found that βlinc2 and βlinc3 are indeed abundant in the β-cell fraction (Figure 4C–D).
Figure 4.
βlinc2 and βlinc3 are expressed in β-cells and their expression is higher in pancreatic islets compared to other tissues. A) Expression of βlinc2 and B) of βlinc3 in pancreatic islets and in different other tissues of C57BL/6 mice at 13 weeks of age. C) Expression of βlinc2 and D) of βlinc3 in whole islets and in FAC-sorted cells isolated from RIPYY mice. The expression levels were measured by real-time PCR and were normalized to the level detected in islets. T-test or ANOVA, Tukey post-hoc test. *P < 0.05 **P < 0.01 vs control (whole islets).
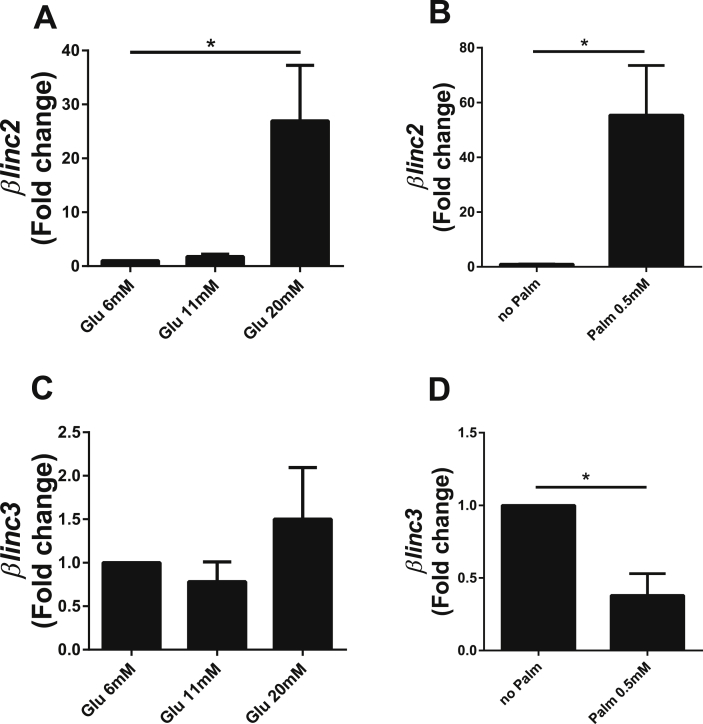
To determine the possible causes of the changes in lncRNA expression detected in the islets of obese mice, we exposed normal mouse islets to pathophysiological concentrations of glucose and palmitate. The expression of βlinc2 increased in the presence of high glucose (20 mM) and palmitate (0.5 mM), whereas βlinc3 was only modified by the presence of palmitate (Figure 5). These changes were not observed upon incubation of dissociated islet cells with pro-inflammatory cytokines (Supplemental Fig. 5).
Figure 5.
In vitro effects of chronically-elevated glucose and palmitate on the level of two lncRNAs differentially expressed in islets from mice fed a high fat diet. Isolated islets from C57BL/6 mice fed a regular chow diet were incubated for 48 h at 6, 11 or 20 mM glucose and at 6 mM glucose with or without 0.5 mM palmitate (RPMI supplemented with 5% FCS, 0.5% BSA and 11 mM glucose). LncRNA expression was measured by real-time PCR and normalized to Gapdh. Means ± SEM of 3–4 different experiments. t-test or ANOVA, Tukey post-hoc test, *P < 0.05 vs control, either glucose 6 mM or no palmitate.
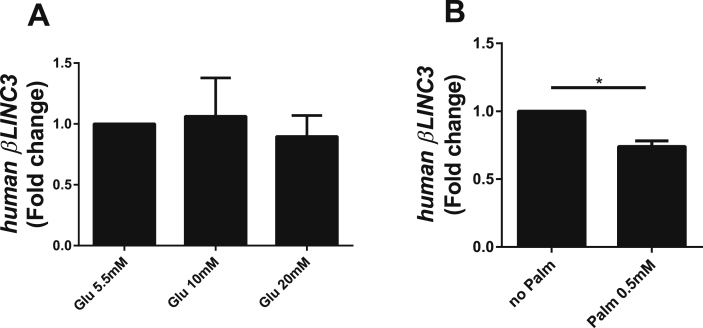
Subsequently, we searched for human orthologues of these two lncRNAs. We mapped the identified mouse lncRNA sequences to the human genome using TransMap. To validate the predicted orthologues we designed primers in the putative exons. This enabled us to detect the orthologue for βlinc3 (region shown in Supplemental Table 10) but not for βlinc2. As was the case for its mouse orthologue, the expression of human βLINC3 was not modified in the presence of 20 mM glucose but was decreased about 30% upon incubation with 0.5 mM palmitate (Figure 6).
Figure 6.
Effects of chronically-elevated glucose and palmitate on the levels of βLINC3 in human islets. Human islets were incubated for 48 h at 5.5, 10 or 20 mM glucose, and at 5.5 mM glucose with and without 0.5 mM palmitate (CMRL, 5% FCS, 0.5% BSA, 5.5 mM glucose). LncRNA expression was measured by real-time PCR and normalized to GAPDH. Means ± SEM of 3 different experiments. *P < 0.05 vs control, either glucose 5.5 mM or no palmitate.
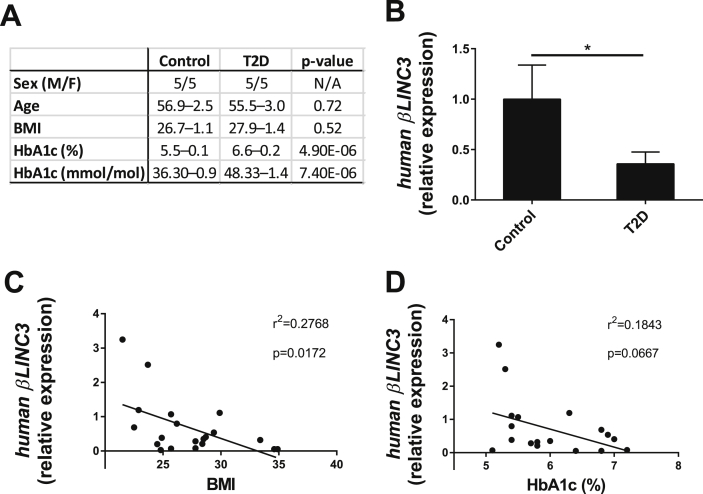
In view of these findings, we assessed whether the expression of human βLINC3 is diminished in the islets of T2D patients. The characteristics of the non-diabetic and diabetic donors are summarized in Figure 7A. Human βLINC3 expression was 75% lower in donors with T2D than in controls (Figure 7B). Furthermore, as was the case in mice, there was a negative correlation between the level of this lncRNA and the BMI of the subjects (Figure 7C). We also observed a trend for a negative correlation between the expression of human βLINC3 and HbA1c, but the values did not reach statistical significance (Figure 7D). There was no correlation with the age of the donors (data not shown).
Figure 7.
βLINC3 expression is decreased in islets from type 2 diabetes donors versus controls and inversely correlates to the BMI of the subjects. A. donor characteristics. B. LncRNA expression between control subjects and type 2 diabetes patients. LncRNA expression was measured by real-time PCR and normalized using three endogenous controls, 18s rRNA, CYCLOPHILINA and B2M. One tail t-test, *P < 0.05. Linear regression analysis of the expression of human βLINC3 versus BMI (C) and HbA1c (D). F-test was used to determine significance at p < 0.05.
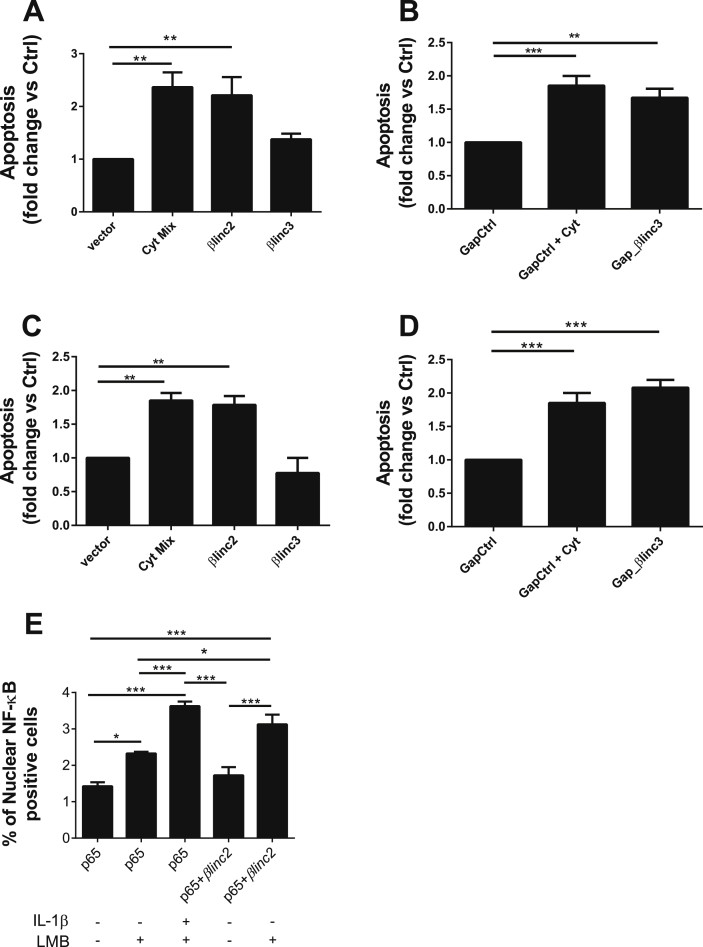
To assess the possible contribution of βlinc2 and βlinc3 to the regulation of specific β-cell functions, we modified their level in MIN6 and mouse islet cells. Overexpression of βlinc2 and down-regulation of βlinc3 in MIN6 cells (Supplemental Fig. 6) did not modify proinsulin mRNA levels, insulin content, or insulin release (Supplemental Fig. 7). As an increase in β-cell apoptosis and a consequent reduction in the β-cell mass can contribute to T2D development [4], we investigated the impact of changes in the expression of these lncRNAs on β-cell survival. We observed that the up-regulation of βlinc2 increases apoptosis of both MIN6 (Figure 8A) and dissociated mice islet cells (Figure 8C) to a level similar to the one seen upon 24 h exposure to pro-inflammatory cytokines (used as a positive control for apoptosis). Overexpression of βlinc3 did not affect cell survival (Figure 8A–C). However, downregulation of βlinc3 in MIN6 and dissociated mice islet cells, mimicking the conditions encountered under diabetic state, resulted in a rise in the number of apoptotic cells (Figure 8C–D). Similar results were obtained by TUNEL assay and using a different gapmer targeting βlinc3 (Supplemental Fig. 8 and 9).
Figure 8.
Overexpression and downregulation of the lncRNAs βlinc2 and βlinc3 promotes apoptosis in MIN6 β cells. (A) MIN6 cells were transfected for 48 h with a control plasmid (vector) or plasmids to induce the overexpression of the lncRNAs of interest. (B) The cells were transfected with a control gapmer or a gapmer targeting βlinc3 to knockdown this particular lncRNA. After 24 h of incubation, some of the cells were exposed to a mix of cytokines (0.1ng/ml IL-1β, 10ng/ml TNF-α and 30 ng/ml IFN-γ) as a positive control. The same experiments were repeated in dispersed mouse islet cells (C; D). The proportion of cells showing pyknotic nuclei was scored in at least 600 cells for condition. Fold changes were calculated by dividing the results by the values obtained in the control condition. The data represent the means ± SEM of 3–6 different experiments. E. MIN6 cells were transfected for 48 h with a plasmid expressing GFP-tagged p65 (Rela). They were then exposed for 3 h to either a high dose of IL-1β (10ng/ml), Leptomycin B (LMB) (22ng/ml) or a combination of the two. The number of cells displaying nuclear NF-κB localization were scored in at least 1500 cells for condition. Means ± SEM of six independent experiments. ANOVA, Tukey post-hoc test, *<0.05, **P < 0.01, ***p < 0.001.
Increasing evidence suggests an involvement of inflammatory processes in the pathogenesis of T2D and activation of the NF-κB pathway has been implicated in glucolipotoxic-induced β-cell apoptosis [36], [37], [38]. Among other mediators, hyperglycemia and hyperlipidemia increase the production and the release of IL-1β, a pro-inflammatory cytokine that activates the transcription factor NF-κB and that has been widely studied in the context of type 1 diabetes [39]. Since one of the initial events occurring shortly after β-cell exposure to IL-1β is the translocation of NF-κB to the nucleus [40], we transfected MIN6 cells with a GFP-tagged form of p65 (the main NF-κB subunit) [39], [41] and monitored its subcellular distribution after modulating the level of the lncRNAs. We found that incubation of the cells with leptomycin B, a compound that impedes the nuclear exit of NF-κB [42] was sufficient to increase the fraction of cells in which p65 was localized in the nucleus (Figure 8E). As expected, the localization of p65 in the nucleus was further increased when incubating the cells with high concentrations of IL-1β. No difference was seen by overexpressing βlinc2 alone, but, in the presence of leptomycin B, we observed an increase in the number of cells in which p65 is localized in the nucleus similar to the one seen in the presence of IL-1β (Figure 8 and Supplemental Fig. 10). These data suggest that at least part of the effect of βlinc2 on β-cell survival may be related to an increased shuttling of NF-κB to the nucleus. No differences were seen when up-regulating (Supplemental Fig. 11) or down-regulating βlinc3 (data not shown). We then assessed whether the changes in the level of βlinc2 or βlinc3 are directly affecting the expression of key apoptotic genes. As shown in Supplemental Fig. 12, none of the tested mRNAs was modified upon overexpression of βlinc2 or down-regulation of βlinc3.
4. Discussion
Human islets have recently been shown to express a large number of lncRNAs that, in concert with transcription factors, regulate the transcriptional landscape of β-cells [34], [43]. In this study, we used high-throughput RNA-sequencing to identify novel lncRNAs modified in a mouse model of diet-induced obesity and hyperglycemia that are potentially involved in the control of β-cell functions and β-cell failure. This T2D model integrates both genetic and environmental risk factors. Amongst the mice fed a HFD, the high responders (HDR) were chosen for the initial analysis since they correspond to the early diabetes situations in humans and display phenotypic features such as insulin resistance, hyperinsulinemia and hyperglycemia [21], typically encountered during the development of T2D.
The capacity to identify new transcripts is strongly influenced by the length and the depth of the sequencing and is more efficient if the sequencing is paired-ended. Our transcriptomic analysis was carried out with an unprecedented depth (100 nucleotide paired-end sequencing and 500 million reads per sample) and included also RNAs lacking a polyA tail. For comparison, in the other two main studies devoted to the identification of novel lncRNAs in mouse β-cells, Benner et al. [32] generated 30 million single reads per sample and Ku et al. [33] 150–371 million 82–85 paired-end sequencing reads per sample, resulting in the identification of 127 and 1359 non-annotated lncRNAs, respectively. Our comprehensive analysis led to the discovery of many novel transcripts with minimal protein-coding potential. This is in line with the view that lncRNA expression is more cell- and context-specific compared to that of protein-coding genes [11]. In agreement with other reports [11], [34], the level of the newly annotated islet lncRNAs was lower than that of protein-coding genes and overlapped that of previously annotated lncRNAs. We found that the expression of many of the newly annotated lncRNAs is modulated by the diet. We focused on two lncRNAs that are highly enriched in β-cells compared to other tissues. We found that the expression of these lncRNAs is altered also in 13–16 week-old diabetic db/db mice. The down-regulation of βlinc3 was similar in both models, whereas the expression of βlinc2 was more drastically affected in the islets of db/db mice than in HDR samples. This difference is associated with a more severe phenotype of β-cell failure and diabetes displayed in db/db mice compared to high-fat-fed mice [35].
The expression of βlinc2 was also positively correlated to body weight, glycemia, and insulinemia in ND and a HFD mice. However, its level was not significantly increased in the islets of low diet responder mice (LDR), suggesting that the changes may only occur under severe obesity and insulin resistant conditions with associated hyperglycemia. βlinc3 expression was negatively correlated to body weight and glycemia but not to insulinemia, suggesting that the decrease of this lncRNA is mainly associated with the development of obesity and less with the control of insulin release.
During the development of obesity-associated T2D, β-cells are chronically exposed to glucolipotoxic conditions [44]. Hence, we treated mouse islets with increasing concentrations of glucose or with the free fatty acid palmitate to investigate whether these pathophysiological conditions may explain the changes in lncRNA expression. Indeed, the level of βlinc2 was increased by both elevated glucose and palmitate concentrations, whereas βlinc3 was down-regulated by palmitate but not by glucose.
LncRNAs are less conserved than protein-coding genes [11] and may have implications in species-specific functions [45]. To confirm the relevance of our findings for human diabetes we search for potential orthologues. Unfortunately, although alignment tools indicated the existence of potential candidate regions, we could not formally identify a human orthologue of βlinc2. The function of lncRNAs can be preserved with sequence homologies as low as 21% [46]. Thus, computational tools based solely on sequence alignments may be inappropriate for the identification of lncRNA orthologues. In the future, a better understanding of the mode of action of lncRNAs will hopefully promote the design of new tools to search for orthologues facilitating the identification of human transcripts with functions analogous to mouse βlinc2. Using the available tools, we were able to identify the orthologue of βlinc3 in human islets and to confirm that its levels are also diminished upon treatment in the presence of elevated concentrations of palmitate. Since exposure of islet cells to elevated palmitate in vitro induces more rapid and harmful effects than those that may occur in vivo, we measured the level of the human βLINC3 in islets of control and T2D donors. Interestingly, the level of βLINC3 was lower in subjects with T2D, and, as was the case in mice, there was a negative correlation between its expression and the BMI of the subjects. Moreover, there was a trend for a negative correlation between the levels of βLINC3 and HbA1c, suggesting that lower amounts of βLINC3 may result in poorer glycemic control. The analysis of more subjects would be needed to confirm this assumption.
In genetically predisposed individuals, the progression of the disease coincides with a gradual deterioration in β-cell functions, in part associated with the loss of β-cells by apoptosis [3], [5]. The modulation of these two lncRNAs had no impact on insulin biosynthesis or release, but the increase of βlinc2 and the decrease of βlinc3 resulted in a rise in the number of apoptotic cells. Thus, altered expression of these lncRNAs cannot explain the secretory defects observed in db/db or HFD mice but may contribute to β-cell failure during the development and the progression of the disease.
The precise mechanisms underlying the effect of βlinc2 and βlinc3 on β-cell apoptosis remains to be determined. We found that overexpression of βlinc2, increases the rate of NF-κB nuclear translocation. Although basal NF-κB activity is required for normal insulin release [47], its prolonged activation plays a central role in cytokine- and in glucolipotoxic-induced β-cells death [37], [40]. One of the initial events leading to the activation of NF-κB, for instance in response to IL-1β [40], a cytokine produced in conditions of hyperglycemia and hyperlipidemia [48], [49], is its translocation to the nucleus. Since the extent of the induction of NF-κB target genes is influenced by different events including the speed of the nuclear translocation of the transcription factor and/or a more sustained activation of this pathway [39], [40], it is possible that an increased shuttling of NF-κB to the nucleus may promote the activation of this signaling cascade ultimately contributing to apoptosis. Despite the increased shuttling of p65 to the nucleus, we did not detect significant changes in the mRNA level of key apoptotic genes. This may be due to the fact that the fraction of cells in which the transcription factor is moving to the nucleus is too small to result in changes in gene expression detectable in the whole cell population. Alternatively, the translocation of p65 to the nucleus alone may not be sufficient to induce the expression of the target genes and may require additional convergent signals.
Since βlinc2 is not induced by pro-inflammatory cytokines, the effect of the lncRNA on NF-κB cannot reflect the release of IL-1β from β-cells. Thus, the induction of βlinc2 is likely to occur through a different, yet to be identified, mechanism. This alternative pathway may potentially synergize with the canonical cytokine-induced pathway leading to a more drastic activation of NF-κB. Additional studies will be needed to elucidate the mode of action of βlinc2 and βlinc3 and to dissect the molecular events through which these lncRNAs can affect the survival of β-cells. The lncRNAs Lethe and Nkila have been shown to interact directly with components of the NF-κB pathway [50], [51]. Future studies should determine whether this is also the case for βlinc2.
5. Conclusion
The discovery that mammalian genomes are extensively transcribed and generate thousands of transcripts lacking protein-coding potential has opened new perspectives in the study of the mechanisms regulating the activity of β-cells and of the causes of their failure under diabetic conditions. We have identified a large number of novel lncRNAs many of which are modulated under obesity and T2D conditions. At least two of them can affect the survival of β-cells and may potentially contribute to glucolipotoxic-mediated β-cell loss and to the manifestation and progression of T2D. The genome-wide data obtained in this study will provide the basis for future investigations on the involvement of other novel and potentially islet-specific lncRNAs in β-cell dysfunction and T2D development. This may pave the way to the identification of new therapeutic targets for diabetes prevention and treatment. Indeed, lncRNAs display a more restricted tissue distribution than protein-coding genes [11], [34], providing the ideal targets for highly specific therapeutic interventions. Examples already exist where the expression of these transcripts was manipulated in vivo in mice [17], [52].
Author contributions
AM conceived the experiments, generated the research, analyzed the data, wrote the manuscript, and approved its final version. SG, MLP, DRL, JLSE, AGR, FB, MI, LE, PG, and MP contributed to the acquisition of data, reviewed the manuscript, and approved its final version. RR conceived the experiments, analyzed the research data, wrote the manuscript, and approved its final version.
Acknowledgments
We would like to thank the Genomic Technologies Facility of our faculty (http://www.unil.ch/dafl) for performing the RNA-sequencing. We thank Nordic Network for islet transplantation (Prof Olle Korsgren) and HTL at Lund University Diabetes center coordinated by Ulrika Krus for providing human islets. This work was supported by the European Foundation for the Study of Diabetes and Lilly research fellowship (AM), by grants of the Swiss National Science Foundation (310030-146138 and 310030-169480 (RR), by the “Fondation Francophone pour la Recherche sur le Diabète” sponsored by the “Fédération Française des Diabétiques”, AstraZeneca, Eli Lilly, Merck Sharp & Dohme, Novo Nordisk and Sanofi (RR), the Swedish Research Council (B0338601 to LE), the Påhlsson foundation (LE and JLSE) and the Crafoord foundation (JLSE) and by grants from the Canadian Institutes of Health Research (MP). MP holds the Canada Research Chair in Diabetes and Metabolism.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2017.08.005.
Conflict of interests
The authors have no competing interests to declare.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Kahn S.E., Hull R.L., Utzschneider K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 2.Ferrannini E., Mari A. Beta cell function and its relation to insulin action in humans: a critical appraisal. Diabetologia. 2004;47(5):943–956. doi: 10.1007/s00125-004-1381-z. [DOI] [PubMed] [Google Scholar]
- 3.Prentki M., Nolan C.J. Islet beta cell failure in type 2 diabetes. Journal of Clinical Investigation. 2006;116(7):1802–1812. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lupi R., Del Prato S. Beta-cell apoptosis in type 2 diabetes: quantitative and functional consequences. Diabetes & Metabolism. 2008;34(Suppl 2):S56–S64. doi: 10.1016/S1262-3636(08)73396-2. [DOI] [PubMed] [Google Scholar]
- 5.Cnop M. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54(Suppl 2):S97–S107. doi: 10.2337/diabetes.54.suppl_2.s97. [DOI] [PubMed] [Google Scholar]
- 6.Guay C. Emerging roles of non-coding RNAs in pancreatic beta-cell function and dysfunction. Diabetes, Obesity and Metabolism. 2012;14(Suppl 3):12–21. doi: 10.1111/j.1463-1326.2012.01654.x. [DOI] [PubMed] [Google Scholar]
- 7.Guay C., Regazzi R. MicroRNAs and the functional beta cell mass: for better or worse. Diabetes & Metabolism. 2015;41(5):369–377. doi: 10.1016/j.diabet.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Bartel D.P. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carninci P. The transcriptional landscape of the mammalian genome. Science. 2005;309(5740):1559–1563. doi: 10.1126/science.1112014. [DOI] [PubMed] [Google Scholar]
- 10.Consortium, E.P. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 2007;447(7146):799–816. doi: 10.1038/nature05874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Derrien T. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Research. 2012;22(9):1775–1789. doi: 10.1101/gr.132159.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hangauer M.J., Vaughn I.W., McManus M.T. Pervasive transcription of the human genome produces thousands of previously unidentified long intergenic noncoding RNAs. PLoS Genet. 2013;9(6):e1003569. doi: 10.1371/journal.pgen.1003569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ulitsky I., Bartel D.P. lincRNAs: genomics, evolution, and mechanisms. Cell. 2013;154(1):26–46. doi: 10.1016/j.cell.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fatica A., Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nature Reviews Genetics. 2014;15(1):7–21. doi: 10.1038/nrg3606. [DOI] [PubMed] [Google Scholar]
- 15.Kung J.T., Colognori D., Lee J.T. Long noncoding RNAs: past, present, and future. Genetics. 2013;193(3):651–669. doi: 10.1534/genetics.112.146704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmitz S.U., Grote P., Herrmann B.G. Mechanisms of long noncoding RNA function in development and disease. Cellular and Molecular Life Sciences. 2016:1–19. doi: 10.1007/s00018-016-2174-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.You L. Downregulation of long noncoding RNA Meg3 affects insulin synthesis and secretion in mouse pancreatic beta cells. Journal of Cellular Physiology. 2015 doi: 10.1002/jcp.25175. [DOI] [PubMed] [Google Scholar]
- 18.Arnes L. betalinc1 encodes a long noncoding RNA that regulates islet beta-cell formation and function. Genes & Development. 2016;30(5):502–507. doi: 10.1101/gad.273821.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Motterle A. Involvement of long non-coding RNAs in beta cell failure at the onset of type 1 diabetes in NOD mice. Diabetologia. 2015;58(8):1827–1835. doi: 10.1007/s00125-015-3641-5. [DOI] [PubMed] [Google Scholar]
- 20.Motterle A., Sanchez-Parra C., Regazzi R. Role of long non-coding RNAs in the determination of beta-cell identity. Diabetes, Obesity and Metabolism. 2016;18(Suppl 1):41–50. doi: 10.1111/dom.12714. [DOI] [PubMed] [Google Scholar]
- 21.Peyot M.L. Beta-cell failure in diet-induced obese mice stratified according to body weight gain: secretory dysfunction and altered islet lipid metabolism without steatosis or reduced beta-cell mass. Diabetes. 2010;59(9):2178–2187. doi: 10.2337/db09-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan J.Y. Failure of the adaptive unfolded protein response in islets of obese mice is linked with abnormalities in beta-cell gene expression and progression to diabetes. Diabetes. 2013;62(5):1557–1568. doi: 10.2337/db12-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quoix N. The GluCre-ROSA26EYFP mouse: a new model for easy identification of living pancreatic alpha-cells. FEBS Lett. 2007;581(22):4235–4240. doi: 10.1016/j.febslet.2007.07.068. [DOI] [PubMed] [Google Scholar]
- 24.Gotoh M. Reproducible high yield of rat islets by stationary in vitro digestion following pancreatic ductal or portal venous collagenase injection. Transplantation. 1987;43(5):725–730. doi: 10.1097/00007890-198705000-00024. [DOI] [PubMed] [Google Scholar]
- 25.Roggli E. Changes in microRNA expression contribute to pancreatic beta-cell dysfunction in prediabetic NOD mice. Diabetes. 2012;61(7):1742–1751. doi: 10.2337/db11-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trapnell C. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nature Protocols. 2012;7(3):562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karolchik D. The UCSC genome browser Database. Nucleic Acids Research. 2003;31(1):51–54. doi: 10.1093/nar/gkg129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts A. Identification of novel transcripts in annotated genomes using RNA-Seq. Bioinformatics. 2011;27(17):2325–2329. doi: 10.1093/bioinformatics/btr355. [DOI] [PubMed] [Google Scholar]
- 29.Blanco E., Parra G., Guigo R. Using geneid to identify genes. Current Protocols in Bioinformatics. 2007 doi: 10.1002/0471250953.bi0403s18. Chapter 4: p. Unit 4 3. [DOI] [PubMed] [Google Scholar]
- 30.Benjamini Y., Hochberg Y. Controlling the false discovery rate - a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B-methodological. 1995;57(1):289–300. [Google Scholar]
- 31.Pepin E. Pancreatic beta-cell dysfunction in diet-induced obese mice: roles of AMP-kinase, protein kinase cepsilon, mitochondrial and cholesterol metabolism, and alterations in gene expression. PLoS One. 2016;11(4):e0153017. doi: 10.1371/journal.pone.0153017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benner C. The transcriptional landscape of mouse beta cells compared to human beta cells reveals notable species differences in long non-coding RNA and protein-coding gene expression. BMC Genomics. 2014;15:620. doi: 10.1186/1471-2164-15-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ku G.M. Research resource: RNA-Seq reveals unique features of the pancreatic beta-cell transcriptome. Molecular Endocrinology. 2012;26(10):1783–1792. doi: 10.1210/me.2012-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moran I. Human beta cell transcriptome analysis uncovers lncRNAs that are tissue-specific, dynamically regulated, and abnormally expressed in type 2 diabetes. Cell Metabolism. 2012;16(4):435–448. doi: 10.1016/j.cmet.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kobayashi K. The db/db mouse, a model for diabetic dyslipidemia: molecular characterization and effects of Western diet feeding. Metabolism. 2000;49(1):22–31. doi: 10.1016/s0026-0495(00)90588-2. [DOI] [PubMed] [Google Scholar]
- 36.Donath M.Y. Islet inflammation in type 2 diabetes: from metabolic stress to therapy. Diabetes Care. 2008;31(Suppl 2):S161–S164. doi: 10.2337/dc08-s243. [DOI] [PubMed] [Google Scholar]
- 37.Donath M.Y. Islet inflammation impairs the pancreatic beta-cell in type 2 diabetes. Physiology (Bethesda) 2009;24:325–331. doi: 10.1152/physiol.00032.2009. [DOI] [PubMed] [Google Scholar]
- 38.Choi H.J. Genome-wide identification of palmitate-regulated immediate early genes and target genes in pancreatic beta-cells reveals a central role of NF-kappaB. Molecular Biology Reports. 2012;39(6):6781–6789. doi: 10.1007/s11033-012-1503-5. [DOI] [PubMed] [Google Scholar]
- 39.Ortis F. Cytokine-induced proapoptotic gene expression in insulin-producing cells is related to rapid, sustained, and nonoscillatory nuclear factor-kappaB activation. Molecular Endocrinology. 2006;20(8):1867–1879. doi: 10.1210/me.2005-0268. [DOI] [PubMed] [Google Scholar]
- 40.Ortis F. Induction of nuclear factor-kappaB and its downstream genes by TNF-alpha and IL-1beta has a pro-apoptotic role in pancreatic beta cells. Diabetologia. 2008;51(7):1213–1225. doi: 10.1007/s00125-008-0999-7. [DOI] [PubMed] [Google Scholar]
- 41.Hayden M.S., Ghosh S. Signaling to NF-kappab. Genes Dev. 2004;18(18):2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 42.Huang T.T. A nuclear export signal in the N-terminal regulatory domain of IkappaBalpha controls cytoplasmic localization of inactive NF-kappaB/IkappaBalpha complexes. Proc Natl Acad Sci U S A. 2000;97(3):1014–1019. doi: 10.1073/pnas.97.3.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akerman I. Human pancreatic beta cell lncRNAs control cell-specific regulatory networks. Cell Metab. 2017;25(2):400–411. doi: 10.1016/j.cmet.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cnop M. Fatty acids and glucolipotoxicity in the pathogenesis of Type 2 diabetes. Biochem Soc Trans. 2008;36(Pt 3):348–352. doi: 10.1042/BST0360348. [DOI] [PubMed] [Google Scholar]
- 45.Esguerra J.L., Eliasson L. Functional implications of long non-coding RNAs in the pancreatic islets of Langerhans. Front Genet. 2014;5:209. doi: 10.3389/fgene.2014.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ulitsky I. Conserved function of lincRNAs in vertebrate embryonic development despite rapid sequence evolution. Cell. 2011;147(7):1537–1550. doi: 10.1016/j.cell.2011.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Norlin S., Ahlgren U., Edlund H. Nuclear factor-{kappa}B activity in {beta}-cells is required for glucose-stimulated insulin secretion. Diabetes. 2005;54(1):125–132. doi: 10.2337/diabetes.54.1.125. [DOI] [PubMed] [Google Scholar]
- 48.Maedler K. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. Journal of Clinical Investigation. 2002;110(6):851–860. doi: 10.1172/JCI15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boni-Schnetzler M. Free fatty acids induce a proinflammatory response in islets via the abundantly expressed interleukin-1 receptor I. Endocrinology. 2009;150(12):5218–5229. doi: 10.1210/en.2009-0543. [DOI] [PubMed] [Google Scholar]
- 50.Liu B. A cytoplasmic NF-kappaB interacting long noncoding RNA blocks IkappaB phosphorylation and suppresses breast cancer metastasis. Cancer Cell. 2015;27(3):370–381. doi: 10.1016/j.ccell.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 51.Rapicavoli N.A. A mammalian pseudogene lncRNA at the interface of inflammation and anti-inflammatory therapeutics. Elife. 2013;2:e00762. doi: 10.7554/eLife.00762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wheeler T.M. Targeting nuclear RNA for in vivo correction of myotonic dystrophy. Nature. 2012;488(7409):111–115. doi: 10.1038/nature11362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.