Abstract
Objective
Ribosomal protein S6 Kinase-1 (S6K1) has been linked to resistance exercise-mediated improvements in glycemia. We hypothesized that S6K1 may also play a role in regulating glycemic control in response to endurance exercise training.
Methods
S6k1-knockout (S6K1KO) and WT mice on a 60 cal% high-fat diet were trained for 4 weeks on treadmills, metabolically phenotyped, and compared to sedentary controls.
Results
WT mice showed improved glucose tolerance after training. In contrast, S6K1KO mice displayed equally high glucose tolerance already in the sedentary state with no further improvement after training. Similarly, training decreased mitochondrial ROS production in skeletal muscle of WT mice, whereas ROS levels were already low in the sedentary S6K1KO mice with no further decrease after training. Nevertheless, trained S6K1KO mice displayed an increased running capacity compared to trained WT mice, as well as substantially reduced triglyceride contents in liver and skeletal muscle. The improvements in glucose handling and running endurance in S6K1KO mice were associated with markedly increased ketogenesis and a higher respiratory exchange ratio.
Conclusions
In high-fat fed mice, loss of S6K1 mimics endurance exercise training by reducing mitochondrial ROS production and upregulating oxidative utilization of ketone bodies. Pharmacological targeting of S6K1 may improve the outcome of exercise-based interventions in obesity and diabetes.
Keywords: S6K1, Exercise, Glycemic control, Metabolic phenotyping, Reactive oxidative species
Abbreviations: HFD, high-fat diet; ROS, reactive oxygen species; TCA, tricarboxylic acid cycle; mTOR, mammalian target of rapamycin
Highlights
-
•
Absence of S6k1 upregulates oxidative substrate utilization under HFD consumption.
-
•
S6k1 knockout mice show enhanced running performance and improved glycemia already in the sedentary state.
-
•
Aerobic endurance exercise training of S6k1 knockout mice further improves running performance but not glycemia.
-
•
Metabolic improvements are associated with lower rates of TCA-linked mitochondrial H2O2 production and increased ketogenesis.
1. Introduction
Physical exercise training is associated with increased insulin sensitivity, improved glycemic control, and reduced risk for developing type 2 diabetes mellitus. While the beneficial effect of exercise has been attributed to metabolic adaptation in energy substrate preference, mitochondrial density, and skeletal muscle function, the regulatory network responsible for the diabetes-protective effect of exercise is not well understood.
The ribosomal protein S6 kinase beta-1 (S6K1), also known as p70S6 kinase, is part of the mTORC1 (mammalian target of rapamycin complex 1) signaling pathway that regulates cell growth, motility, and survival [1]. Chronic resistance training that implies high-intensity muscle contraction activates the mTORC1 pathway, thereby increasing protein synthesis and muscle mass, as evidenced by the positive correlation between the exercise-induced increase in S6K1 phosphorylation and skeletal muscle hypertrophy [2]. Conversely, muscle atrophy resulting from muscle unloading decreased mTORC1 signaling, respectively [3], [4]. Moreover, rapamycin, an inhibitor of mTORC1, blunted protein synthesis and muscle growth in response to increased mechanical loading [5] but did not affect endurance exercise mediated muscle myofibrillar and mitochondrial protein synthesis [6]. On the other hand, endurance training that induces low-intensity high-volume muscle contraction, increases muscle oxidative capacity and glucose uptake [7], [8] through the activation of AMPK [9], [10]. The disruption of the mTORC1 pathway through the absence of S6k results in enhanced lipid utilization in mice consuming a high fat diet (HFD), and protection from HFD-induced insulin resistance mediated by phosphorylation of IRS-1 at Ser-1101 [11], [12], [13]. Hence, both increased lipid oxidation and lower inhibitory Ser-1101 phosphorylation of IRS-1 may improve glucose metabolism and insulin sensitivity, and protect against type 2 diabetes mellitus. Mice deficient in S6k1 as well as S6k1/S6k2 double knockouts show higher expression of Ppargc1a in plantaris muscle and higher AMPK activation in hepatocytes and adipose tissue [12], [14], which could lead to increased mitochondrial biogenesis. Therefore, it is possible that the absence of S6k1 ameliorates the decrease in AMPK activation induced by HFD consumption [15], thus facilitating synergistic improvements of glucose homeostasis through changes in mitochondrial activity.
The aim of our present study was to investigate the impact of S6k1 deletion on chronic endurance exercise, glucose metabolism, and running performance in mice on a HFD and whether the effects relate to lipid utilization and mitochondrial activity in skeletal muscle.
2. Materials and methods
2.1. Animals
Age- and sex-matched S6k1 knockout (S6K1KO) mice were generated as described [11], [12]. Male S6K1KO mice and the corresponding wild type (WT) littermate controls were originally generated in a mixed 129/SveJ × C57Bl/6 line [16]. All mice were housed under a 12:12 h light–dark cycle (lights on at 6 a.m.) with ad libitum access to a chow diet (V153x R/M-H, Ssniff, Soest, Germany) and tap water for three months. Subsequently, the mice were placed on a HFD (60% kcal from fat [D12492], Research Diets Inc., New Brunswick, NJ, USA) for one month before and during the four weeks of the exercise training intervention. All experiments were approved by the Ethics Committee of the State Ministry of Agriculture, Nutrition and Forestry (State of North Rhine-Westphalia, Germany).
2.2. Genotyping
Isolation of DNA from mouse tail tips was performed with the InViSorb Genomic DNA Kit II (Invitek, Berlin, Germany). Genotyping of mice was performed by a two-step PCR with primers for the S6k1 knockout. Isolated genomic DNA served as template for the first PCR (WT outside fwd: 5′- GATGGGGCAGGGCTTAGGAGGC -3′, WT outside Rev: 5′- CGCTGTGTCCCTTCCTCTCCC -3′, Neo outside: 5′- GAGCTTGGCGGCGAATGGGCTG -3′). For the subsequent second PCR, the product of the first PCR was used as template (WT inside forward: 5′- GAAGGAGCTACTGGCTATTGGGG -3′, WT inside reverse: 5′- CTCCCCCCTCTGCCCCCCTC -3′, Neo inside: 5′- GGGCTGACCGCTTCCTCGTGC -3′).
2.3. Exercise training protocol
Four months old male mice consuming HFD for one month were subsequently trained on 4-lane treadmills (TSE systems, Bad Homburg, Germany) for 4 weeks, 1 h per day, 5 days per week followed by a two-day recovery phase. During these 4 weeks, training parameters were gradually increased from low intensities in the first week to higher intensities in the last week (i.e. maximum speed up to 18 m/min, running time up to 45 min and treadmill slope from 0° to 10° in order to simulate uphill running). Glucose tolerance and running performance were analyzed in the last week of the training intervention. Tissues were harvested after 2 h fasting (1 h after the last training bout) for subsequent analyses.
2.4. Running performance test
In the 4th week of training, mice performed a running performance test on the treadmill until exhaustion. At a 10° slope, the mice underwent a 15 min adaptation and warm-up phase and the subsequent measurement phase, which spanned over a time course of max. 2 h and gradually increased speed up to 18 m/min. Mice were motivated by gentle tail tapping. Exhaustion was considered when an animal did not respond to motivation stimuli and broke a light barrier at the rear of the system three times within 15 s. Mice refusing to exercise were also considered exhausted.
2.5. Measurements of body weight and body composition
Body weight was measured with an electronic scale (Sartorius, Göttingen, Germany) and body composition (fat and lean mass) was analyzed using nuclear magnetic resonance technology (Whole Body Composition Analyzer; Echo MRI, TX, USA) at the beginning and the end of the training intervention.
2.6. Intraperitoneal glucose tolerance test (i.p. GTT)
Mice were fasted for 6 h, and glucose was injected intraperitoneally with 2 g glucose per kg body weight (BW) (Glucose 20%, B. Braun Melsungen AG, Melsungen, Germany). Tail-blood glucose levels (mg/dl) were measured with a glucometer and standard glucose strips (Bayer Vital GmbH, Leverkusen, Germany) before (0 min) and at 15, 30, 45, 60, and 120 min after injection.
2.7. Indirect calorimetry
In the 3rd week of the exercise protocol, the animals were placed in a customized calorimetric cage system (TSE PhenoMaster, TSE systems, Bad Homburg, Germany) for the assessment of spontaneous physical activity (SPA), respiratory exchange ratio (RER), and energy expenditure (EE) as described [17]. Measurements were taken on line for 3 consecutive days, every 30 min starting 1 h after the exercise intervention. During this time, the animals were not removed from the cages or further trained, since the measurements were taken in the protocols recovery phase. SPA measurements were taken with a non-invasive infrared based light beam system and the RER and EE measurements through O2 consumption and CO2 production quantification at a cage in-flow rate of 0.4 l/min and a sampling flow rate of 0.38 l/min. Total food intake (FI) was quantified manually in parallel. Whole-body carbohydrate and fat oxidation rates (g/min) were calculated using the following equations: carbohydrate oxidation rate = 4.585 × VCO2 (l/min) − 3.226 × VO2 (l/min); fat oxidation rate = 1.695 × VO2 (l/min) − 1.701 × VCO2 (l/min) [18].
2.8. Plasma analysis
Mice were euthanized by decapitation at the end of the study; blood was collected and added to an anticoagulant cocktail (for 50 ml: 25 ml 0.5 M EDTA, 92 mg aprotinin dissolved in 21 ml saline [0.15 M], 4 ml heparin [10,000 U/ml], 21.6 mg diprotin A) and subsequently centrifuged (15 min, 3000×g, 4 °C). Cholesterol and non-esterified fatty acid (NEFA) levels were measured using enzymatic assay kits (Biolatest, Erba Lachema, Karasek, Czech Republic and Autokit NEFA HR(2), Wako, Neuss, Germany), triglyceride levels were determined using Triacylglycerols Liquid 1000 (Erba Lachema, Karásek, Brno, Czech Republic), plasma glucose was measured using LabAssay Glucose (Wako, Neuss, Germany), lactate was measured using Lactate Assay Kit (Sigma-Aldrichm St. Louis, MO, USA), and ketone bodies were assessed with Autokit Total Ketone Bodies, Autokit 3-hydroxybutyrate and ketone body calibrator 300 as standard (all: Wako, Neuss, Germany). The samples were run in technical duplicates and within the same assay according to the manufacturer's instructions.
Plasma corticosterone levels were measured using a [125I] radioimmunoassay kit from MP Biomedicals (Orangeburg, NY, USA). Two multiplex bead based immunoassays (Bio-Plex Pro™ Mouse Diabetes 8-plex, Diabetes adiponectin, Biorad, Hercules, CA, USA) were used to determine plasma levels of adiponectin, leptin, insulin, resistin, glucagon, plasminogen activator inhibitor-1 (PAI-1), glucagon-like peptide-1 (GLP-1), gastric inhibitory polypeptide (GIP), and ghrelin. Analysis was performed using a Bioplex 200 suspension array system (Biorad, Hercules, CA, USA) according to the manufacturer's instructions. Protein concentrations were calculated from the appropriate optimized standard curves using Bio-Plex Manager Software version 6.0 (Biorad, Hercules, CA, USA).
2.9. Muscle mitochondrial respiration and reactive oxygen species (ROS) production
Ex vivo mitochondrial respiration was measured in freshly permeabilized Soleus muscle using the Oxygraph-2k (Oroboros Instruments, Innsbruck, Austria) as described [19]. Defined respiratory states were obtained by the following protocols: (i) tricarboxylic acid cycle (TCA)-linked respiration using 2 mM malate, 10 mM pyruvate (state 2, complex I), 2.5 mM ADP, 10 mM glutamate and (state 3, complex I), 10 mM succinate (state 3, complex I + II), 10 μM cytochrome c (mitochondrial membrane integrity check), and carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP) (stepwise increments of 0.25 μM up to the final concentration of max. 1.25 μM, uncoupled state) and (ii) β-oxidation-linked respiration using 2 mM malate, 1 mM octanoyl-carnitine (state 2, complex I + II), 2.5 mM ADP (state 3, complex I + II), 10 μM cytochrome c (mitochondrial membrane integrity check), and FCCP (stepwise increments of 0.25 μM up to the final concentration of max. 1.25 μM, uncoupled state). No increase in oxygen consumption upon addition of cytochrome c indicated integrity of the outer mitochondrial membrane after saponin permeabilization. Furthermore, ROS production was assessed simultaneously with respiration by measuring the H2O2 levels fluorometrically (O2k-Fluorescence Modul, Oroboros Instruments) using Amplex Red (Amplex® Red, Invitrogen, Karlsruhe, Germany) [20]. The same protocols as for respiration were used except from addition of 5 nM oligomycin instead of cytochrome C and FCCP, to induce state 4. Citrate synthase activity was measured spectrophotometrically using a commercial kit (Citrate Synthase Assay Kit, Sigma–Aldrich, MO, USA) [20].
2.10. RNA extraction, cDNA synthesis, and Quantitative Real-Time PCR
RNA was extracted and cDNA was synthesized as described previously [21]. Quantitative Real-time PCR was performed with a 7500 Fast Real-Time PCR System using SYBR® Green Real-Time PCR (Thermo Fisher Scientific, Waltham, MA, USA). Expression of genes was normalized to Gapdh according to the ΔCt method [22]. Primer sequences are listed in Supplementary Table 2.
2.11. SDS-PAGE and western blotting
Tissues were homogenized and lysed in lysis buffer (20 mM Tris–HCl, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% (v/v) Triton-X 100), including proteinase and phosphatase inhibitors (Complete™ and PhosSTOP™, Roche Diagnostics, Mannheim, Germany), using an automatic tissue lyser (TissueLyser II, Qiagen, Hilden, Germany) for 4 min at 25 Hz. After centrifugation (13,500×g, 10 min, 4 °C), supernatants were collected, and protein content was determined using BCA Protein Assay Kit (Pierce, Rockford, IL, USA). Equal amounts of protein from each sample diluted with Laemmli sample buffer were separated by SDS-PAGE and transferred to nitrocellulose membranes (BioRad, München, Germany). Membranes were probed with primary antibodies (Supplementary Table 3), followed by HRP-conjugated goat anti-rabbit IgG (Dianova, Hamburg, Germany) diluted 1:10,000 in 1× TTBS + 5% non-fat dry milk as secondary antibody for 1 h at room temperature. Specific protein bands were visualized using enhanced chemiluminescence reagents for Western blot analysis (Perkin Elmer, Waltham, MA, USA). Protein bands were quantified using Quantity One software (BioRad, Munich, Germany).
2.12. Measurement of triglycerides and glycogen in liver and muscle
Thirty mg frozen tissue (wet weight) was pestled and analyzed using the Triglycerides (TRIGS) GPO-PAP Kit (Randox, Crumlin, UK). Glycogen was determined by the amyloglucosidase method [23]. Briefly, tissue homogenates were incubated with 1 M KOH at 70 °C for 30 min. Subsequently, samples were supplemented with acetic acid and assay buffer containing sodium acetate and amyloglucosidase and incubated O/N at 37 °C. Glucose was then measured enzymatically by a glucose oxidase-based colorimetric detection kit (Glucose liquicolor, Human, Taunusstein, Germany).
2.13. Statistical analysis
Quantitative data are presented as mean ± standard error of the mean (SEM). Data were compared by two-tailed unpaired Student's t-test and two-way analysis of variance with post-hoc Bonferroni test (GraphPad Prism software). The significance level was set at p < 0.05.
3. Results
3.1. S6K1KO mice are lean despite increased energy intake
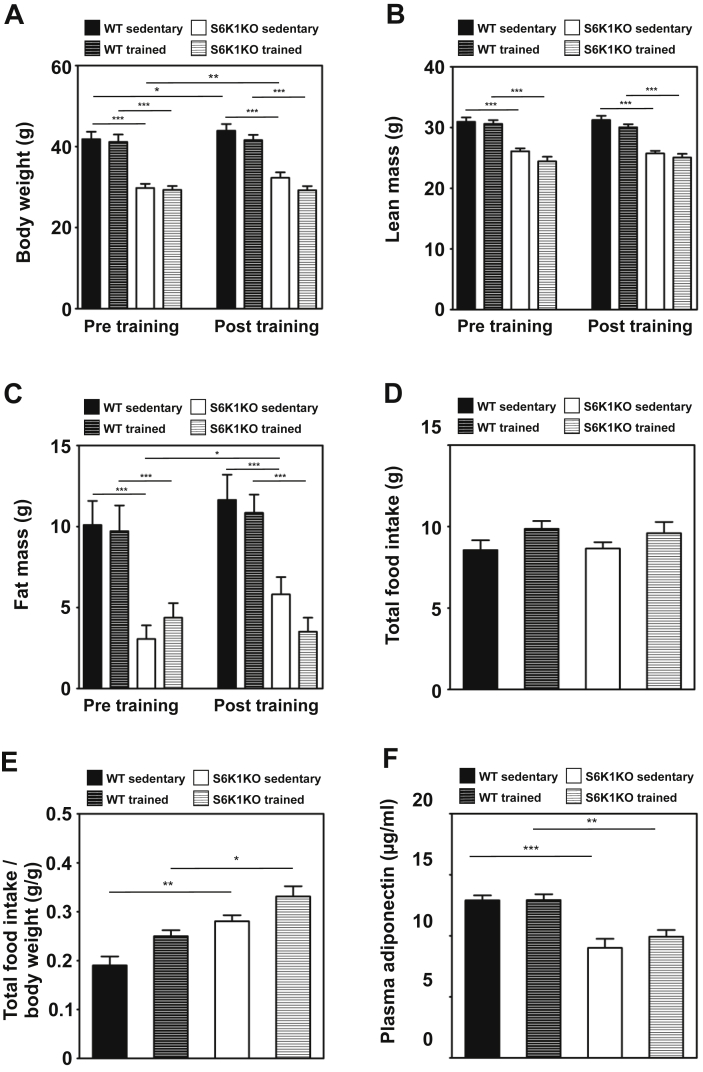
At an age of 14–16 weeks, male S6k1 knockout mice (S6K1KO) and wildtype littermates (WT) were divided into two groups, sedentary and trained, and placed on a HFD. After one month and prior to exercise training, S6K1KO mice had ∼30% lower body weight compared to WT (S6K1KO vs. WT: 29.55 ± 0.46 g vs. 41.49 ± 1.26; p < 0.05), due to lower gain of both lean and fat mass (Figure 1A–C). Exercise training resulted in a modest reduction in body weight in both S6K1KO and WT mice (Figure 1A) and prevented an increase in fat mass in S6K1KO mice (Figure 1C).
Figure 1.
Endurance exercise training prevents HFD-induced gain of body weight in wildtype and S6K1KO mice. Body composition in terms of (A) Body weight, (B) lean mass, (C) fat mass, (D) total food intake, (E) food intake/body weight of 3 consecutive days (n = 5–6), (F) final plasma adiponectin of WT and S6K1KO mice before (pre) and after (post) 4 weeks of chronic endurance exercise compared to respective sedentary littermate controls. Mice were fasted for 2 h before euthanization. Data presented as mean ± SEM. Statistical analyses were done by two-way ANOVA with Bonferroni's multiple comparison test. n = 9–11; *p < 0.05, **p < 0.01, ***p < 0.001.
Total food intake was not different between WT and S6K1KO mice in both sedentary and trained states (Figure 1D). However, normalized to body weight S6K1KO mice displayed increased food intake in both sedentary (WT vs. S6K1KO: 0.10 ± 0.018 vs. 0.280 ± 0.012 g/g; p < 0.05) and trained states (WT vs. S6K1KO: 0.249 ± 0.012 vs. 0.331 ± 0.021 g/g; p < 0.05) (Figure 1E). Plasma levels of adiponectin were lower in S6K1KO than in WT mice (Figure 1F, Supplementary Table 1).
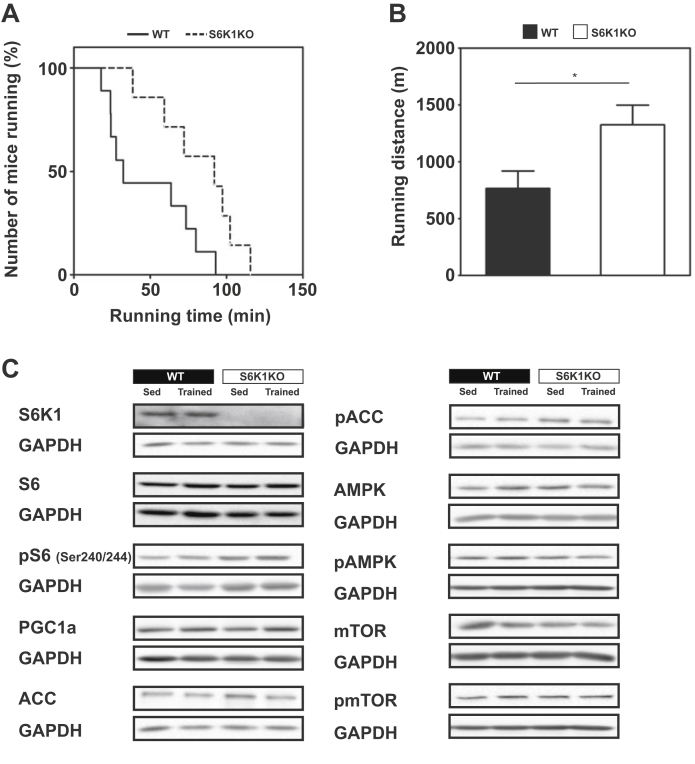
3.2. S6K1KO mice show enhanced running performance and improved glycemia after training
Compared to WT, S6K1KO mice displayed markedly increased running performance after training on treadmills. Both running time (WT vs. S6K1KO: 48.41 ± 9.05 vs. 82.44 ± 9.45 min; p < 0.05) and running distance (WT vs. S6K1KO: 764.9 ± 145.5 vs. 1324.8 ± 159.5 m; p < 0.05) were increased approx. 1.7-fold in the knockout mice (Figure 2A and B). The increase in exercise performance was not accompanied by changes in the abundance or phosphorylation of proteins involved in skeletal muscle energy metabolism, including ribosomal protein S6, peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α), acetyl-CoA carboxylase (ACC), 5′-AMP-activated protein kinase (AMPK), eukaryotic translation initiation factor 2 (eIF2α), PRKR-like endoplasmic reticulum kinase (PERK), C-Jun-amino-terminal kinase (SAPK/JNK), and mTOR (Figure 2C; Supplementary Figure S1). Furthermore, running performance assessed by distance and time did not correlate with body weight or fat mass of the animals (Supplementary Figure S2).
Figure 2.
S6K1KO mice show an improved running performance and increased respiratory control ratio after 4 weeks of chronic endurance exercise. Running performance was tested after 4 weeks of chronic endurance exercise as described in the methods section. (A) Kaplan–Meier plot and (B) total running distance of trained S6K1KO animals. (C) Representative Western Blots of S6K1, (p-)S6, PGC1α, (p-)ACC in red gastrocnemius muscle and (p-)AMPK and (p-)mTOR in quadriceps muscle of sedentary and trained S6K1KO mice compared to respective WT controls (n = 4–6). Data presented as mean ± SEM. Statistical analyses were done by two-tailed unpaired Student's t-test. n=7–9; *p < 0.05.
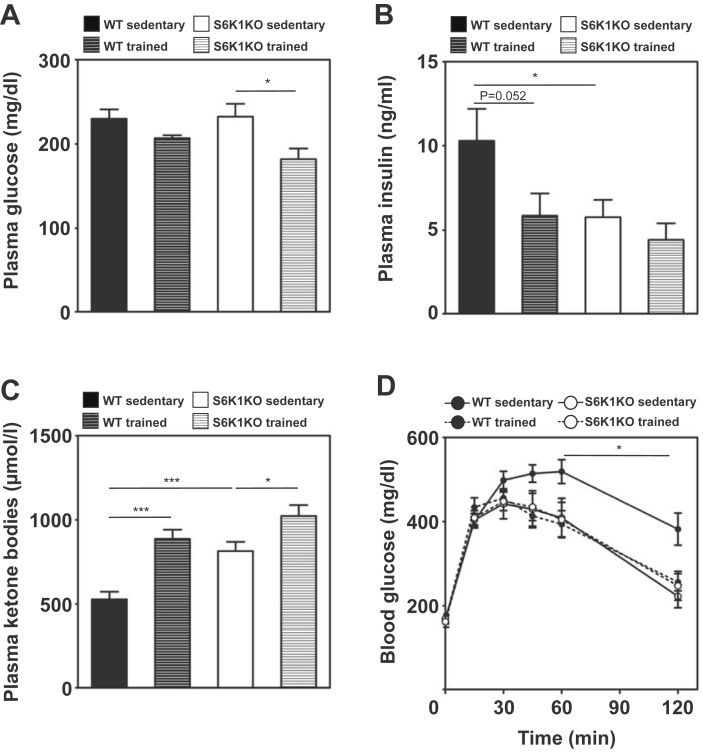
Sedentary WT and S6K1KO mice exhibited similar fasted (6 h) plasma glucose levels (Figure 3A). Only in S6K1KO mice exercise training decreased fasting plasma glucose levels by ∼20% (Sedentary vs. trained: 232.57 ± 15.14 vs. 181.95 ± 12.78 mg/dl; p < 0.05; Figure 3A, Supplementary Table 1). Consistent with improved glycemia, WT mice showed a trend towards lower fasting plasma insulin after training (Sedentary vs. trained: 10.29 ± 1.91 vs. 5.86 ± 1.91; p = 0.052), whereas S6K1KO mice displayed lower insulin levels in both sedentary and trained states compared to WT (Figure 3B).
Figure 3.
Glucose tolerance of sedentary S6K1KO mice is comparable to trained WT mice in and does not further improve by endurance training. (A) Final plasma glucose, (B) final plasma insulin, and (C) final plasma ketone bodies (n = 9–11) concentration after 2 h fasting and 1 h after the last exercise intervention. In the 4th week of the chronic endurance exercise protocol mice were subjected to intraperitoneal tolerance test for glucose (GTT). (D) Time course of blood glucose concentrations of sedentary and trained S6K1KO and WT mice after intraperitoneal injection of glucose (2 mg/kg) (n = 8–10). Data presented as mean ± SEM, Statistical analyses were done by two-way ANOVA with Bonferroni's multiple comparison test. *p < 0.05, ***p < 0.001.
Plasma ketone bodies almost doubled in response to training in WT mice (Figure 3C). Sedentary S6K1KO mice showed higher ketone bodies which further increased by training (Figure 3C). In WT mice, exercise training markedly improved glucose tolerance (Figure 3D). Sedentary S6K1KO mice showed already better glucose tolerance than WT that was not further enhanced by training (Figure 3D).
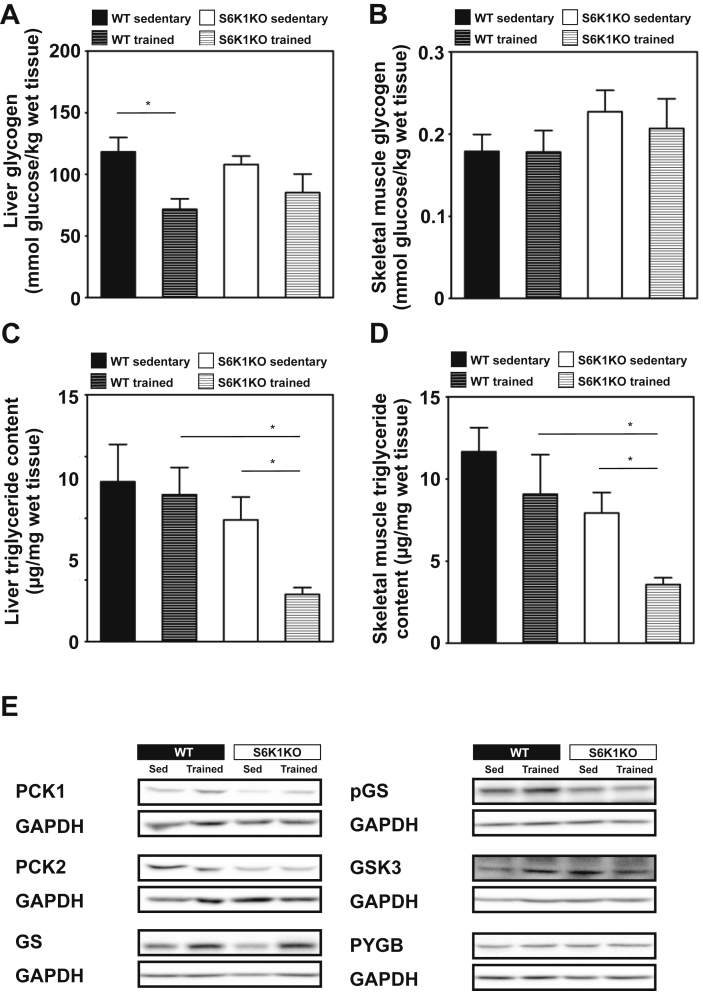
3.3. S6K1KO mice display reduced triglycerides in liver and skeletal muscle after training
Glycogen levels in liver and skeletal muscle were similar in both WT and S6K1KO mice (Figure 4A and B). However, exercise training reduced hepatic glycogen in WT mice more than in S6K1KO mice (ΔWT vs. ΔS6K1KO: −51.71 ± 11.25 vs. −25.20 ± 6.06 mmol glucose/kg; p < 0.05; Figure 4A and B). Triglyceride content of liver and skeletal muscle was similar in sedentary WT and S6K1KO mice and did not change in WT mice after training. Conversely, exercise training reduced triglycerides in both liver (ΔWT vs. ΔS6K1KO: −2.08 ± 0.87 vs. −12.05 ± 4.03 μg/mg; p < 0.05) and muscle in S6K1KO mice (ΔWT vs. ΔS6K1KO: −2.59 ± 1.01 vs. −4.36 ± 1.19 μg/mg; p < 0.05; Figure 4C and D). Expression of peroxisome proliferator activated receptor alpha (Ppara) was not changed by genotype or exercise (data not shown). Despite a trend for increased protein abundance of glycogen synthase (GS) in the liver in response to exercise, hepatic phosphoenolpyruvate carboxy-kinase (PCK1, PCK2), glycogen synthase kinase 3 (GSK3), and glycogen phosphorylase B (PYGB) were not different between genotypes or interventions, respectively (Figure 4E and F, Supplementary Figure S1).
Figure 4.
S6K1KO mice show no exercise mediated depletion of liver glycogen but reduced triglyceride content in liver and skeletal muscle compared to WT controls. (A) Liver and (B) skeletal muscle glycogen, (C) liver and (D) skeletal muscle triglyceride content. (E) Representative Western Blots of PCK1, PCK2, Glycogen Synthase (GS) phospho-GS, GSK3 and PY in liver (n = 4–6). Data presented as mean ± SEM. Statistical analyses were done by two-way ANOVA with Bonferroni's multiple comparison test. *p < 0.05.
3.4. S6K1KO mice show enhanced substrate utilization after training
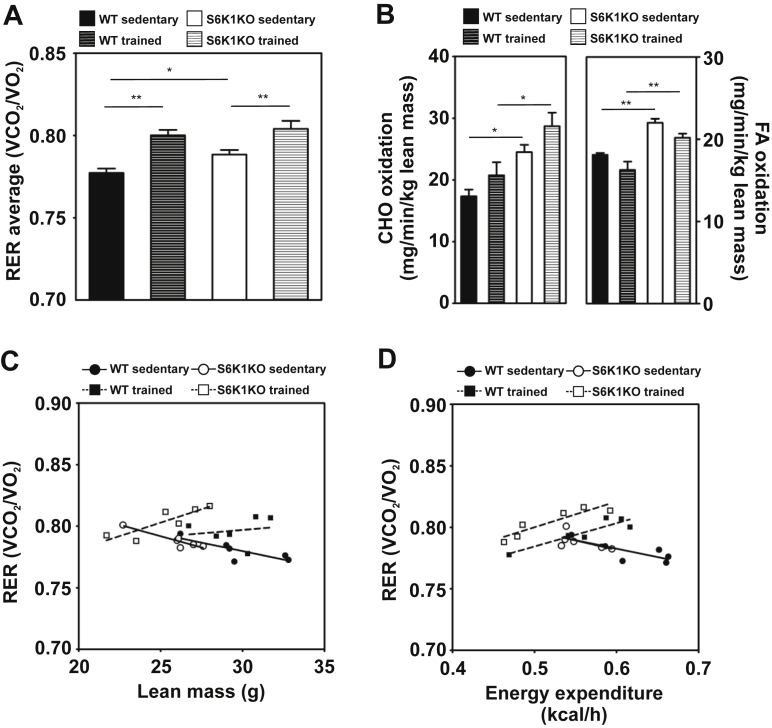
S6K1KO mice exhibited higher respiratory exchange ratio (RER) compared to WT in the sedentary state (WT vs. S6K1KO: 0.777 ± 0.003 vs. 0.789 ± 0.003; p < 0.05; Figure 5A). After exercise training, both WT and S6K1KO mice showed higher RER than their sedentary counterparts, (WT 0.800 ± 0.003; S6K1KO: 0.804 ± 0.005; Figure 5A). S6K1KO mice showed substantially increased (41%) whole-body carbohydrate oxidation rates and increased (22%) whole-body fat oxidation rates compared to WT controls (Figure 5B). Exercise training further increased carbohydrate oxidation and a concomitant reduction in fat oxidation in both WT and S6K1KO mice (Figure 5B). While both WT and S6K1KO mice showed a training-associated shift in energy substrate preference towards substrates yielding higher RER (Figure 5C), S6K1KO mice showed a markedly improved substrate utilization in response to training (Figure 5D).
Figure 5.
S6K1KO mice have increased energy substrate utilization. (A) 3 days average of respiratory exchange ratio (RER), (B) Whole-body carbohydrate (CHO) oxidation and whole-body fatty acid oxidation (FAO), (C) Correlation analysis of RER and lean mass, (D) Correlation analysis of RER and energy expenditure. Data presented as mean ± SEM. Statistical analyses were done by two-way ANOVA with Bonferroni's multiple comparison test. n = 5–6; *p < 0.05, **p < 0.01.
3.5. S6K1KO mice show reduced hydrogen peroxide emission in the Soleus muscle
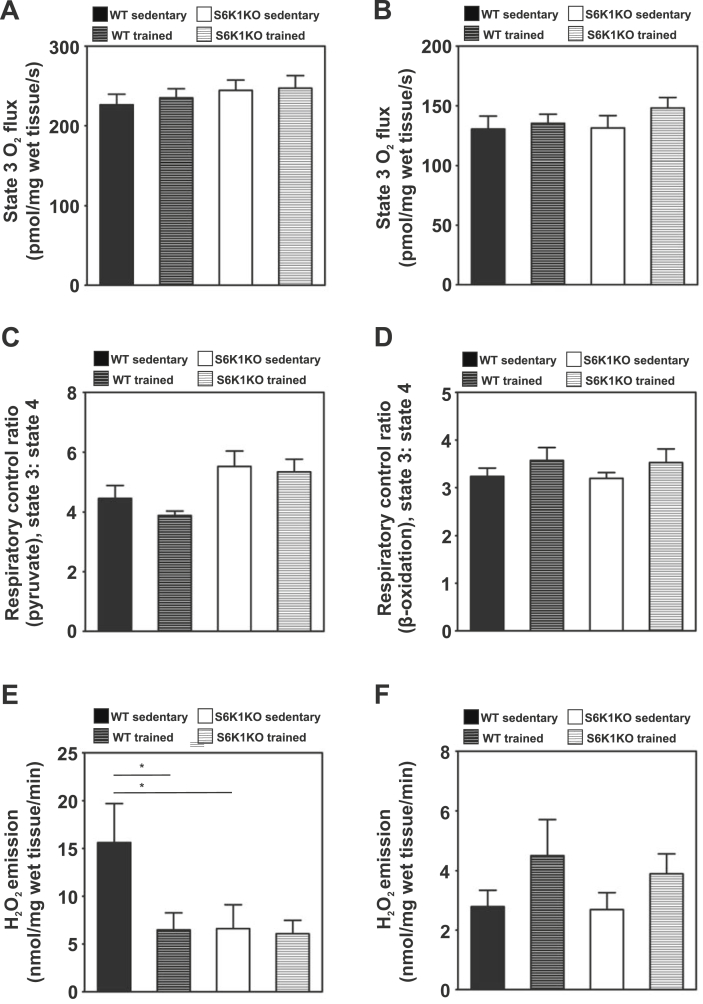
Mitochondrial oxidative capacity was not affected by training or genotype at any of the respiratory conditions (Figure 6A and B and Supplementary Figure S3). This was paralleled by comparable mitochondrial density as assessed from the citrate synthase activity assay (Supplementary Figure S3). Respiratory control ratio (RCR), an indicator of the mitochondrial coupling, was also similar among the groups (Figure 6C and D). However, the rates of hydrogen peroxide emission stimulated by TCA substrates were decreased after training in WT mice (Sedentary vs trained: 15.62 ± 4.07 vs 6.49 ± 1.78; p < 0.05) and remained lower in S6K1KO mice (Sedentary vs trained: 6.62 ± 2.49 vs 6.08 ± 1.41; p > 0.05; Figure 6E), independent of the training status. On the other hand, β-oxidation-linked hydrogen peroxide emission was similar among the groups (Figure 6F) and transcriptional markers for ER-stress were not altered through the ablation of S6K1KO nor due to chronic endurance exercise (Supplementary Figure S4).
Figure 6.
Hydrogen peroxide production is reduced but not mitochondrial oxidative capacity and coupling in the Soleus muscle of sedentary and trained WT and S6K1KO mice. Oxygen flux at state 3 (ADP-stimulated) respiration (A, B); respiratory control ratio (RCR) (C, D); and hydrogen peroxide emission (E, F) were assessed with substrates for tricarboxylic acid (TCA) cycle (pyruvate, glutamate and succinate) (A, C, E) as well as β-oxidation (octanoyl-carnitine) (B, D, F). Data presented as mean ± SEM. Statistical analyses were done by two-way ANOVA with Bonferroni's multiple comparison test. n = 8–10; *p < 0.05.
4. Discussion
Chronic exercise training is associated with improvements in glycemic control, but the precise regulatory circuits involved in metabolic adaptation to physical activity and diabetes protection are not fully understood. Our results show that deletion of S6k1 mimics the effects of chronic endurance exercise on glucose tolerance and running endurance, implicating an important role of S6k1 in aerobic exercise metabolism. Exercise training resulted in the expected better glucose tolerance in WT mice on a high fat diet. However, while training did not further improve glucose tolerance in S6K1KO mice, running performance was further increased in the knockouts, independently of body or fat mass weight. This indicates the involvement of different rate limiting pathways in training-induced improvements in glucose handling and exercise performance.
S6K1KO mice are lean and protected from diet-induced obesity [12]. Interestingly, compared to WT littermates, S6K1KO mice showed higher food intake adjusted to body weight, and higher whole-body oxidation rates for both glucose and fatty acids, indicating altered energy efficiency of these mice on a high fat diet. Nevertheless, steady state glycogen or triglyceride concentrations in skeletal muscle and liver were not different between S6K1KO and WT mice indicating unaltered energy stores. In line with increased lipid oxidation, S6K1KO mice exhibited a better glucose tolerance and lower insulin in the sedentary state, thus reflecting protection from HFD-induced insulin resistance. This protective effect has been attributed to both higher fatty acid oxidation and improved insulin signaling downstream of the insulin receptor [11], [12], [13]. We found that the levels of plasma ketone bodies in the sedentary state were higher in HFD-fed S6K1KO than in WT mice. Ketone bodies are generated during fatty acid oxidation thereby reflecting states of increased lipolysis and/or starvation [24]. Interestingly, in chow-fed S6K1KO, 6-h fasting levels of ketone bodies were reportedly unchanged [12], emphasizing the requirement of dietary lipid oversupply to stimulate ketogenesis in S6K1KO mice.
Inhibition of mTOR was reported to promote ketogenesis through the activation of peroxisome proliferator-activated receptor α (PPARα) [25]. Likewise, knockout of the close S6k1 homolog S6k2 has been described to increase ketone body production via activation of PPARα [26]. However, we did not observe changes in mTOR abundance or Ppara expression in our study, suggesting that elevated substrate availability per se is sufficient to drive ketone body formation in S6K1KO mice on a high fat diet.
Exercise training markedly reduced triglyceride content in liver and skeletal muscle in S6K1KO, whereas glycogen content in these tissues was unaltered. In contrast, WT mice had lower hepatic glycogen, but no changes in triglycerides after training. This demonstrates a preferential use of lipids in S6K1KO animals and sparing of glycogen reserves in response to the exercise challenge. In WT mice, we did not observe a correlation of ectopic fat with glucose tolerance. However, measurements of lipid metabolites such as diacylgycerols and ceramides might be required to further assess the role of cellular lipids in exercise-mediated improvements of glucose tolerance [27].
Although exercise-mediated ketogenesis was not significantly different between trained WT and trained S6K1KO mice, plasma cholesterol levels were higher in the exercised WT mice than in the exercised S6K1KO animals. As ketone bodies serve as precursors in the biosynthesis of cholesterol under non-oxidative conditions [28], we speculate that S6K1KO animals have an elevated oxidative flux of ketones in response to exercise which could explain their higher exercise performance when compared to the controls. The tendency of higher RER values of S6K1KO mice could reflect elevated oxidation of ketone bodies as fuel [29]. Nevertheless, the extent to which S6K1KO mice rely on oxidation of ketones under acute exercise conditions remains to be determined.
Our analysis of mitochondrial function did not reveal any changes in oxygen fluxes or respiratory control linked to exercise or genotype of the mice. Likewise, mitochondrial density (CSA) was not significantly different between genotypes or sedentary and exercised mice. Remarkably, mitochondrial H2O2 (TCA) production was substantially reduced only in WT mice in response to training, while it was already low in sedentary S6K1KO mice and did not decrease further after training. As a result, the decreased insulin levels in trained WT mice and S6k1 knockouts and the improvements in glucose tolerance parallel the mitochondrial H2O2 production. Consistent with a role of S6K1 in regulating mitochondrial function, knockdown of S6K1 or inhibition of mTOR by rapamycin in endothelial cells reduced mitochondrial superoxide generation whereas overexpression of a constitutively active S6K1 mutant had the opposite effect in cultured cells [30].
While moderate levels of ROS during exercise are part of the natural adaptive response, high superoxide production may be harmful and impair insulin action [31], [32]. Reduction of mitochondrial H2O2 has been shown to improve glucose tolerance in mice and to protect against high-fat diet induced insulin resistance [33]. A more recent study showed that in obese women, aerobic exercise training reduced mitochondrial H2O2 production and improved insulin sensitivity [34]. Interestingly, exercise-induced alterations in mitochondrial H2O2 production were restricted to obese individuals; however, the levels of S6K1 activation were not determined in this study. Given the established role of S6k1 as a nutritionally regulated kinase, we speculate that S6k1 may constitute a regulator of mitochondrial H2O2 production affecting skeletal muscle insulin sensitivity in particular under conditions of lipid-induced mitochondrial stress [35]. Several mitochondrial sites of H2O2 generation are known, but their contributions in vivo and their respective regulatory network are not well understood. A recent study found that in isolated rat skeletal muscle mitochondria, H2O2 generation was reduced under conditions mimicking mild and intense aerobic exercise, thus supporting our findings [36].
5. Conclusion
Activation of the mTORC1/S6K pathway may play an important role in metabolic adaptation to endurance exercise training that relates to improvements in glucose homeostasis. The absence of S6k1 upregulates ketogenesis and improves oxidative substrate utilization and conservation of carbohydrate reserves under a HFD consumption, thus mimicking the condition of chronic endurance exercise. Exercise-induced improvements in glycemic control are associated with lower rates of TCA-linked mitochondrial H2O2 production. Since this study is limited to whole-body knockout of S6k1, further studies using tissue-specific knockout mice are necessary to elucidate the possible influence of prenatal development as well as tissue-specific links between reactive oxygen species production and changes in insulin sensitivity and exercise.
Author contributions
C. B., T. J., D. G. K., A. C., T. R. C., M. R., and H. A-H. wrote the manuscript and analyzed and interpreted the data. C. B., T. J., A. P., M. D., S. M-L., S. H., S. K., and S.L. performed the experiments and analyzed data. C. B., T. J., D. G. K., A. C., M. R., T. R. C., and H. A-H. were involved in the study design and contributed to data interpretation. H. A.-H. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgements
We thank Angelika Horrighs, Anette Kurowski, Ilka Römer, Annette Schober, Peter Herdt, and Cornelia Köllmer for expert technical assistance and Annette Schürmann and Matthias H. Tschöp for generously providing reagents and mice. This work was supported by the Ministry of Innovation, Science and Research of the State of North Rhine-Westphalia (MIWF NRW) and the German Federal Ministry of Health (BMG) and was funded in part by grants from the Deutsche Forschungsgemeinschaft (SFB1116) and the German Academic Exchange Service (DAAD).
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2017.08.008.
Conflict of interest
The authors declare no duality of interests associated with this manuscript.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Magnuson B., Ekim B., Fingar D.C. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. The Biochemical Journal. 2012;441(1):1–21. doi: 10.1042/BJ20110892. [DOI] [PubMed] [Google Scholar]
- 2.Edgett B.A., Fortner M.L., Bonen A., Gurd B.J. Mammalian target of rapamycin pathway is up-regulated by both acute endurance exercise and chronic muscle contraction in rat skeletal muscle. Applied Physiology, Nutrition, and Metabolism. 2013;38(8):862–869. doi: 10.1139/apnm-2012-0405. [DOI] [PubMed] [Google Scholar]
- 3.Baar K., Esser K. Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. The American Journal of Physiology. 1999;276(1 Pt 1):C120–C127. doi: 10.1152/ajpcell.1999.276.1.C120. [DOI] [PubMed] [Google Scholar]
- 4.Hornberger T.A., Hunter R.B., Kandarian S.C., Esser K.A. Regulation of translation factors during hindlimb unloading and denervation of skeletal muscle in rats. American Journal of Physiology. Cell Physiology. 2001;281(1):C179–C187. doi: 10.1152/ajpcell.2001.281.1.C179. [DOI] [PubMed] [Google Scholar]
- 5.Bodine S.C., Stitt T.N., Gonzalez M., Kline W.O., Stover G.L., Bauerlein R. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nature Cell Biology. 2001;3(11):1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 6.Philp A., Schenk S., Perez-Schindler J., Hamilton D.L., Breen L., Laverone E. Rapamycin does not prevent increases in myofibrillar or mitochondrial protein synthesis following endurance exercise. The Journal of Physiology. 2015;593(18):4275–4284. doi: 10.1113/JP271219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ploug T., Stallknecht B.M., Pedersen O., Kahn B.B., Ohkuwa T., Vinten J. Effect of endurance training on glucose transport capacity and glucose transporter expression in rat skeletal muscle. The American Journal of Physiology. 1990;259(6 Pt 1):E778–E786. doi: 10.1152/ajpendo.1990.259.6.E778. [DOI] [PubMed] [Google Scholar]
- 8.Egan B., Zierath J.R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metabolism. 2013;17(2):162–184. doi: 10.1016/j.cmet.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Winder W.W., Holmes B.F., Rubink D.S., Jensen E.B., Chen M., Holloszy J.O. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. Journal of Applied Physiology. 2000;88(6):2219–2226. doi: 10.1152/jappl.2000.88.6.2219. [DOI] [PubMed] [Google Scholar]
- 10.Winder W.W., Taylor E.B., Thomson D.M. Role of AMP-activated protein kinase in the molecular adaptation to endurance exercise. Medicine and Science in Sports and Exercise. 2006;38(11):1945–1949. doi: 10.1249/01.mss.0000233798.62153.50. [DOI] [PubMed] [Google Scholar]
- 11.Castaneda T.R., Abplanalp W., Um S.H., Pfluger P.T., Schrott B., Brown K. Metabolic control by S6 kinases depends on dietary lipids. PLoS One. 2012;7(3) doi: 10.1371/journal.pone.0032631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Um S.H., Frigerio F., Watanabe M., Picard F., Joaquin M., Sticker M. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431(7005):200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 13.Tremblay F., Brule S., Hee Um S., Li Y., Masuda K., Roden M. Identification of IRS-1 Ser-1101 as a target of S6K1 in nutrient- and obesity-induced insulin resistance. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(35):14056–14061. doi: 10.1073/pnas.0706517104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aguilar V., Alliouachene S., Sotiropoulos A., Sobering A., Athea Y., Djouadi F. S6 kinase deletion suppresses muscle growth adaptations to nutrient availability by activating AMP kinase. Cell Metabolism. 2007;5(6):476–487. doi: 10.1016/j.cmet.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Choi K.M., Lee Y.S., Kim W., Kim S.J., Shin K.O., Yu J.Y. Sulforaphane attenuates obesity by inhibiting adipogenesis and activating the AMPK pathway in obese mice. The Journal of Nutritional Biochemistry. 2014;25(2):201–207. doi: 10.1016/j.jnutbio.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Shima H., Pende M., Chen Y., Fumagalli S., Thomas G., Kozma S.C. Disruption of the p70(s6k)/p85(s6k) gene reveals a small mouse phenotype and a new functional S6 kinase. The EMBO Journal. 1998;17(22):6649–6659. doi: 10.1093/emboj/17.22.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chadt A., Immisch A., de Wendt C., Springer C., Zhou Z., Stermann T. Deletion of both Rab-GTPase-activating proteins TBC1D1 and TBC1D4 in mice eliminates insulin- and AICAR-stimulated glucose transport. Diabetes. 2015;64(3):746–759. doi: 10.2337/db14-0368. [DOI] [PubMed] [Google Scholar]
- 18.Peronnet F., Massicotte D. Table of nonprotein respiratory quotient: an update. Canadian Journal of Sport Sciences. 1991;16(1):23–29. [PubMed] [Google Scholar]
- 19.Jelenik T., Sequaris G., Kaul K., Ouwens D.M., Phielix E., Kotzka J. Tissue-specific differences in the development of insulin resistance in a mouse model for type 1 diabetes. Diabetes. 2014;63(11):3856–3867. doi: 10.2337/db13-1794. [DOI] [PubMed] [Google Scholar]
- 20.Jelenik T., Kaul K., Sequaris G., Flogel U., Phielix E., Kotzka J. Mechanisms of insulin resistance in primary and secondary non-alcoholic fatty liver. Diabetes. 2017;66(8):2241–2253. doi: 10.2337/db16-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chadt A., Leicht K., Deshmukh A., Jiang L.Q., Scherneck S., Bernhardt U. Tbc1d1 mutation in lean mouse strain confers leanness and protects from diet-induced obesity. Nature Genetics. 2008;40(11):1354–1359. doi: 10.1038/ng.244. [DOI] [PubMed] [Google Scholar]
- 22.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Passonneau J.V., Lauderdale V.R. A comparison of three methods of glycogen measurement in tissues. Analytical Biochemistry. 1974;60(2):405–412. doi: 10.1016/0003-2697(74)90248-6. [DOI] [PubMed] [Google Scholar]
- 24.Newman J.C., Verdin E. beta-hydroxybutyrate: much more than a metabolite. Diabetes Research and Clinical Practice. 2014;106(2):173–181. doi: 10.1016/j.diabres.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sengupta S., Peterson T.R., Laplante M., Oh S., Sabatini D.M. mTORC1 controls fasting-induced ketogenesis and its modulation by ageing. Nature. 2010;468(7327):1100–1104. doi: 10.1038/nature09584. [DOI] [PubMed] [Google Scholar]
- 26.Kim K., Pyo S., Um S.H. S6 kinase 2 deficiency enhances ketone body production and increases peroxisome proliferator-activated receptor alpha activity in the liver. Hepatology. 2012;55(6):1727–1737. doi: 10.1002/hep.25537. [DOI] [PubMed] [Google Scholar]
- 27.Ritter O., Jelenik T., Roden M. Lipid-mediated muscle insulin resistance: different fat, different pathways? Journal of Molecular Medicine (Berlin, Germany) 2015;93(8):831–843. doi: 10.1007/s00109-015-1310-2. [DOI] [PubMed] [Google Scholar]
- 28.Cotter D.G., Schugar R.C., Crawford P.A. Ketone body metabolism and cardiovascular disease. American Journal of Physiology. Heart and Circulatory Physiology. 2013;304(8):H1060–H1076. doi: 10.1152/ajpheart.00646.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frayn K.N. Calculation of substrate oxidation rates in vivo from gaseous exchange. Journal of Applied Physiology: Respiratory, Environmental and Exercise Physiology. 1983;55(2):628–634. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- 30.Rajapakse A.G., Yepuri G., Carvas J.M., Stein S., Matter C.M., Scerri I. Hyperactive S6K1 mediates oxidative stress and endothelial dysfunction in aging: inhibition by resveratrol. PLoS One. 2011;6(4) doi: 10.1371/journal.pone.0019237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Houstis N., Rosen E.D., Lander E.S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440(7086):944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 32.Di Meo S., Iossa S., Venditti P. Skeletal muscle insulin resistance: role of mitochondria and other ROS sources. The Journal of Endocrinology. 2017;233(1):R15–R42. doi: 10.1530/JOE-16-0598. [DOI] [PubMed] [Google Scholar]
- 33.Chen L., Na R., Gu M., Salmon A.B., Liu Y., Liang H. Reduction of mitochondrial H2O2 by overexpressing peroxiredoxin 3 improves glucose tolerance in mice. Aging Cell. 2008;7(6):866–878. doi: 10.1111/j.1474-9726.2008.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Konopka A.R., Asante A., Lanza I.R., Robinson M.M., Johnson M.L., Dalla Man C. Defects in mitochondrial efficiency and H2O2 emissions in obese women are restored to a lean phenotype with aerobic exercise training. Diabetes. 2015;64(6):2104–2115. doi: 10.2337/db14-1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koves T.R., Ussher J.R., Noland R.C., Slentz D., Mosedale M., Ilkayeva O. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metabolism. 2008;7(1):45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 36.Goncalves R.L., Quinlan C.L., Perevoshchikova I.V., Hey-Mogensen M., Brand M.D. Sites of superoxide and hydrogen peroxide production by muscle mitochondria assessed ex vivo under conditions mimicking rest and exercise. The Journal of Biological Chemistry. 2015;290(1):209ss–227. doi: 10.1074/jbc.M114.619072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.