Abstract
Background
The recently proposed nomogram of Barcelona Clinic Liver Cancer (BCLC) lacks predictive accuracy for patients with stage D hepatocellular carcinoma (HCC). Tumor burden is crucial in prognostic prediction but is not included in the criteria of stage D HCC. This study aims to develop a nomogram with tumor burden as the core element for BCLC stage D patients.
Methods
A total of 386 patients were randomly grouped into derivation and validation sets (1:1 ratio). The multivariate Cox proportional hazards model was used to select factors with significant prognostic effect and generate the nomogram. Concordance indices and calibration plots were used to evaluate the performance of nomogram.
Results
Overall survival of study patients was significantly associated with tumor burden as well as hepatitis B, serum α-fetoprotein level, cirrhosis and performance status in multivariate Cox regression (all p<0.05). Beta-coefficients of these variables in derivation set were used to generate the nomogram. Each patient was assigned with a total nomogram point that predicted individualized 6-month and 1-year survival. The derivation and validation sets had a c-index of 0.759 (95% confidence interval [CI]: 0.552–0.923) and 0.741 (95% CI: 0.529–0.913), respectively. The calibration plots were close to the 45-degree line for 6-month and 1-year survival prediction for all quarters of patients in both derivation and validation sets.
Conclusion
Tumor burden is significantly associated with the outcome for patients with stage D HCC. The tumor burden-incorporated nomogram may serve as a feasible and easy-to-use tool in predicting survival on an individual level.
Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver cancer in the world. Major academic societies of liver disease recommend the Barcelona Clinic Liver Cancer (BCLC) staging system to be the prognostic model and allocating tool for treatment selection.[1, 2] Three major parameters including tumor burden, severity of cirrhosis and performance status (PS) have been used to predict the prognosis of HCC. Patients with Child-Turcotte-Pugh (CTP) class C or PS 3–4 are classified as terminal stage, or stage D, HCC because of very limited survival time after diagnosis with or without anti-cancer treatments. Tumor burden, including size and number of tumor nodule(s), vascular invasion and extra-hepatic involvement, may profoundly influence the outcome of HCC patients; however, it is not considered a criterion for BCLC stage D.[3, 4] So far, there is no comprehensive investigation regarding the prognostic effect of tumor burden for stage D HCC patients.
Recently, the nomogram of BCLC system, which provides individualized prediction of patient survival, has been proposed and externally validated.[5–7] The nomogram is a straightforward tool and does not require additional laboratory or imaging studies to accurately predict patient outcome except for those with BCLC stage D HCC.[7] The estimation of survival for stage D patients may need to be specifically designed because of their extremely poor prognosis resulting from advanced cirrhosis and/or debilitated general condition as well as various tumor burden (from a single small nodule to distant metastases). In addition, establishing an accurate prognostic model for late cancer stage has always been important for patients considering hospice care.[8, 9] Furthermore, the emergence and applications of immunotherapy show possible survival benefits for HCC, which also demands a feasible survival-predicting tool before large-scale clinical trials can be planned.[10, 11] This study aimed to investigate if tumor burden is related to the overall outcome in patients with terminal stage HCC, and to customize a nomogram for better prognostic stratification.
Patients and methods
Patients
During a 14-year period between 2002 and 2016, 386 newly diagnosed BCLC stage D patients in our hospital were prospectively collected and retrospectively analyzed. Etiology of underlying liver disease, number and size of tumor(s), serum biochemistry, PS, and liver cirrhosis were comprehensively recorded at the time of diagnosis. The survival status of all patients was checked every 3–4 months after enrollment and was confirmed by using the database of National Cancer Registry, Taiwan. Part of the study patients had been reported as described in our previous study.[7] This study complies with the standards of the Declaration of Helsinki and current ethical guidelines, and has been approved by the institutional review board (IRB; protocol number 2016-04-005AC) of Taipei Veterans General Hospital, Taiwan. The waiver of consent was obtained as justified by the IRB, and patient records/information was anonymized and de-identified prior to analysis.
Diagnosis and definitions
Findings of typical radiological features in at least two imaging modalities including contrast-enhanced dynamic computed tomography (CT), magnetic resonance imaging (MRI), ultrasound and hepatic arterial angiography, or by a single positive imaging study associated with serum α-fetoprotein (AFP) level ≥ 400 ng/mL or histological confirmation were used to diagnose HCC.[12] Patients who were seropositive for anti-hepatitis C virus (HCV) antibody were classified as HCV-related HCC. Hepatitis B virus (HBV)-related HCC was defined as seropositive for hepatitis B surface antigen. Daily consumption of at least 40 g of alcohol for 5 years or more was considered alcoholic liver disease.[13] Vascular invasion was diagnosed by the presence of thrombus adjacent to the tumor in portal system by at least two imaging modalities. Total tumor volume was calculated based on tumor diameter of every HCC nodule as previously described.[14] The Eastern Cooperative Oncology Group (ECOG) criteria were used to evaluate the overall physical status of study patients at the time of diagnosis.[15] Patients who are fully active were recorded as PS 0. Patients with some restriction of activity but still able to carry out work were documented as PS 1. Patients are ambulatory but unable to do any work activity were considered PS 2. Patients are capable of limited self-care and confined to bed or chair more than 50% of waking hours were classified as PS 3. Patients who are completely disabled and totally confined to bed or chair were recorded as PS 4. Patients with tumor burden within the Milan criteria (one nodule < 5 cm, or up to 3 nodules < 3 cm without vascular invasion or extra-hepatic involvement) were classified as tumor burden grade 1.[16] Patients were recorded as tumor burden grade 3 if lymph node involvement, vascular invasion, or distant metastasis were confirmed at the time of diagnosis. All remaining patients were coded as tumor burden grade 2. Chest CT scan was performed to detect metastatic lesion(s) and lymph node involvement. Bone metastasis from HCC was surveyed by bone scan and confirmed by MRI if indicated. All clinical data were recorded at the time of diagnosis.
Statistics
Categorical data were compared with the chi-squared or Fisher exact tests. Continuous characteristics were compared with the Mann-Whitney ranked sum test. The comparison of survival distributions was performed by using the Kaplan-Meier method with a log-rank test. All HCC-related variables were tested by the univariate survival analysis; variables with significant effect on prognosis were introduced into the multivariate Cox proportional regression model to generate beta coefficients (BETA). The ratios of calculated BETAs were used to determine the proportional prognostic effect in the nomogram. The efficiency of the nomogram model was examined by the concordance index,[17, 18] which estimates the probability that for two randomly selected patients, when one patient has an event after the other, this patient has fewer total points by the nomogram. Calibration was conducted by comparing the mean of nomogram-calculated survival with the survival distribution observed by the Kaplan-Meier method. A p value less than 0.05 was considered statistically significant. All statistical analyses were conducted with the SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
The baseline demographics of study patients are shown in Table 1. The mean age of enrolled patients was 66 years, and 22% of them were female. Hepatitis B (49%) was the predominant etiology of chronic liver disease, followed by hepatitis C and alcoholism. Forty-nine percent of patients had multiple tumors, and 70% of patients had a primary tumor diameter larger than 5 cm. There were 17%, 43% and 41% of patients who were classified as CTP class A, B, C respectively, and 0.25%, 9%, 7%, 55%, and 29% of patients had PS 0, 1, 2, 3, 4, respectively. Vascular invasion was found in 56% of patients, and 28% of patients had diabetes mellitus. A total of 103 (27%) patients were confirmed to have distant metastasis or lymph node involvement at diagnosis.
Table 1. Baseline demographics.
| Number of patients | 386 |
| Age (years, mean±standard deviation [SD]) | 66 ± 15 |
| Male/female (%) | 78/22 |
| Etiology of cirrhosis (%) | |
| Hepatitis B | 191 (49) |
| Hepatitis C | 112 (29) |
| Alcoholism | 88 (23) |
| Serum biochemistry (mean±SD) | |
| Albumin (g/dL) | 2.9 ± 0.6 |
| Bilirubin (mg/dL) | 4.4 ± 6 |
| Creatinine (mg/dL) | 1.4 ± 1.2 |
| Estimated glomerular filtration rate (ml/min/1.73m2) | 69 ± 41 |
| International normalized ratio of prothrombin time | 1.3 ± 0.3 |
| Child-Turcotte-Pugh class A/B/C (%) | 17/43/41 |
| Number and size of tumor (%) | |
| Single/multiple | 51/49 |
| ≤ 5 cm/ > 5 cm | 30/70 |
| Total tumor volume (cm3, mean±SD [median]) | 685 ± 1,055 (324) |
| Vascular invasion (%) | 215 (56) |
| Metastasis/lymph node | 103 (27) |
| α-fetoprotein (ng/mL, mean±SD [median]) | 359,623 ± 235,826 (441) |
| Tumor burden 1/2/3 (%) | 15/20/65 |
| Ascites (%) | 280 (73) |
| Performance status 0/1/2/3/4 (%) | 0.25/9/7/55/29 |
| Diabetes mellitus (%) | 107 (28) |
| Treatment modality (%) | |
| Resection | 13 (3) |
| Transplantation | 6 (2) |
| Ablation | 29 (8) |
| Transarterial chemoembolization | 53 (14) |
| Targeted therapy (sorafenib) | 13 (3) |
| Best supportive care | 272 (70) |
For the anti-cancer treatments, 3% of patients received surgical resection, and 2%, 8%, 14%, 3% and 70% of patients underwent transplantation, local ablation, transarterial chemoembolization (TACE), targeted therapy (sorafenib) and best supportive care, respectively.
Survival distribution of patients stratified by tumor burden
After an average follow-up period of 7.4 (median, 2) months, 349 (90%) patients died. As shown in Fig 1, patients with larger tumor burden had significantly worse overall survival (p< 0.001).
Fig 1. Survival distribution according to tumor burden for all patients.
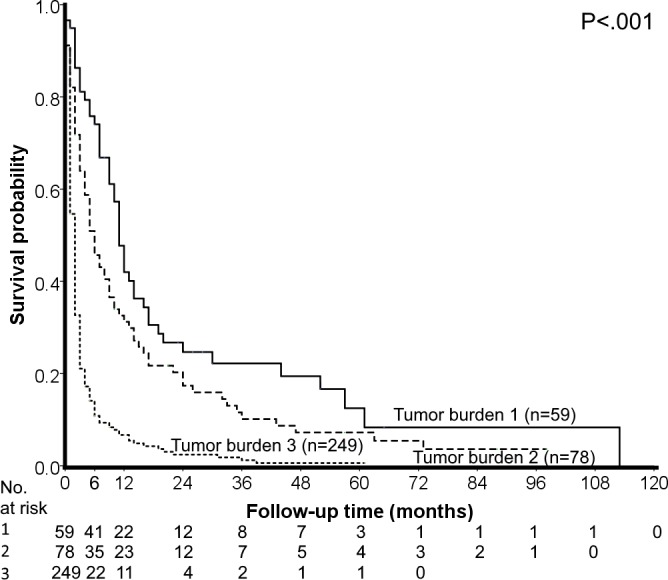
The survival of patients with smaller tumor burden is significantly better than that of patients with larger/more tumor nodule(s).
Univariate and multivariate survival analyses
Variables that were possibly linked with survival were investigated by using the Kaplan-Meier method (Table 2). Hepatitis B, alcoholism, larger tumor burden, advanced cirrhosis, poor PS and high serum AFP level were significantly associated with decreased survival of all study patients in univariate analysis (all p< 0.05). The multivariate model confirmed the independent prognostic effect of these variables except for alcoholism.
Table 2. Univariate and multivariate survival analyses.
| Univariate analysis of all patients | Multivariate analysis of all patients | Multivariate analysis of derivation set | ||||||
|---|---|---|---|---|---|---|---|---|
| N | 6-month survival (%) | 1-year survival (%) | P | BETA/hazard ratio | p | BETA/hazard ratio | p | |
| Sex (Male/Female) | 301/85 | 27/32 | 17/18 | 0.894 | ||||
| Age (<66/≥66 years) | 191/195 | 24/32 | 14/21 | 0.1 | ||||
| HBV (Neg/Pos) | 195/191 | 34/23 | 22/11 | 0.01 | 0.252/1.286 | 0.029 | 0.343/1.423 | 0.032 |
| HCV (Neg/Pos) | 274/112 | 28/28 | 17/19 | 0.953 | ||||
| Alcoholism (Neg/Pos) | 298/88 | 30/22 | 19/11 | 0.036 | ||||
| Tumor burden | < .001 | |||||||
| 1 | 59 | 74 | 42 | 0/1 | 0/1 | |||
| 2 | 78 | 46 | 31 | 0.406/1.501 | 0.036 | 0.601/1.823 | 0.036 | |
| 3 | 249 | 11 | 7 | 1.095/2.988 | < .001 | 1.064/2.899 | < .001 | |
| Child-Turcotte-Pugh | < .001 | |||||||
| A | 64 | 47 | 34 | 0/1 | 0/1 | |||
| B | 165 | 29 | 13 | 0.429/1.536 | 0.007 | 0.442/1.556 | 0.07 | |
| C | 157 | 30 | 15 | 0.593/1.81 | 0.001 | 0.823/2.277 | 0.003 | |
| Performance status | 0.014 | |||||||
| 0–2 | 62 | 49 | 26 | 0/1 | 0/1 | |||
| 3–4 | 324 | 25 | 16 | 0.374/1.453 | 0.044 | 0.613/1.846 | 0.023 | |
| α-fetoprotein (<400/≥400 ng/mL) | 187/199 | 40/17 | 28/8 | < .001 | 0.281/1.324 | 0.016 | 0.358/1.431 | 0.032 |
| Diabetes mellitus (Neg/Pos) | 279/107 | 27/31 | 17/19 | 0.293 | ||||
| eGFR (<60/≥60 ml/min/1.73m2) | 176/210 | 25/31 | 16/18 | 0.087 | ||||
BETA, beta coefficient; HBV, hepatitis B virus; HCV, hepatitis C virus; eGFR, estimated glomerular filtration rate
Characteristics of patients in derivation and validation sets
Patients were randomly split into derivation and validation sets based on 1:1 ratio. Comparison of these two patient groups showed no significant baseline differences (all p> 0.05; Table 3). The derivation group was used to evaluate the prognostic effect of variables which were significantly associated with survival in the univariate analysis to determine the BETAs. Hepatitis B (BETA = 0.343, p = 0.032), tumor burden 2 and 3 compared to tumor burden 1 (BETA = 0.601 and 1.064, p = 0.036 and < 0.001, respectively), CTP class B and C compared to class A (BETA = 0.442 and 0.823, p = 0.07 and 0.003, respectively), PS 3–4 compared to PS 0–2 (BETA = 0.613, p = 0.023), and serum AFP ≥ 400 ng/mL (BETA = 0.358, p = 0.032) were significantly associated with a decreased overall survival (Table 2), which were used to generated the nomogram.
Table 3. Comparison of demographics of the derivation and validation sets.
| Derivation set(n = 193) | Validation set (n = 193) | p value | |
|---|---|---|---|
| Age (years; mean ± SD) | 66 ± 15 | 66 ± 14 | 0.959 |
| Age ≥ 66 years | 98 (51) | 87 (50) | 0.262 |
| Male (n, %) | 153 (79) | 148 (77) | 0.539 |
| Liver disease (n, %) | |||
| Hepatitis B | 90 (47) | 101 (52) | 0.263 |
| Hepatitis C | 63 (33) | 49 (25) | 0.116 |
| Alcoholism | 42 (22) | 46 (24) | 0.628 |
| Tumor size > 5 cm (n, %) | 141 (73) | 131 (68) | 0.265 |
| Multiple tumors (n, %) | 90 (47) | 99 (51) | 0.360 |
| Metastasis/lymph node (n, %) | 51 (26) | 52 (27) | 0.908 |
| Total tumor volume (cm3, mean ± SD [median]) | 772 ± 999 (381) | 657 ± 1,110 (279) | 0.517 |
| Vascular invasion (n, %) | 105 (54) | 110 (57) | 0.608 |
| α-fetoprotein ≥ 400 ng/mL (n, %) | 97 (50) | 102 (53) | 0.611 |
| CTP class (n, %) | 0.214 | ||
| A | 27 (14) | 37 (19) | |
| B | 90 (47) | 75 (39) | |
| C | 76 (39) | 81 (42) | |
| Ascites (n, %) | 139 (72) | 141 (73) | 0.820 |
| Biochemistry (mean ± SD) | |||
| Albumin (g/dL) | 3 ± 0.6 | 2.9 ± 0.6 | 0.552 |
| Bilirubin (mg/dL) | 4 ± 5.2 | 4.6 ± 7 | 0.639 |
| INR of PT | 1.3 ± 0.3 | 1.3 ± 0.3 | 0.817 |
| eGFR ≥ 60 (mL/min/1.73 m2) (n, %) | 107 (55) | 103 (53) | 0.683 |
| Diabetes mellitus (n, %) | 50 (26) | 57 (30) | 0.426 |
| Performance status 0-2/3-4 (%) | 15/85 | 18/82 | 0.407 |
| Tumor burden (n, %) | 0.170 | ||
| 1 | 26 (13) | 33 (17) | |
| 2 | 46 (24) | 32 (17) | |
| 3 | 121 (63) | 128 (66) | |
| Treatment (n, %) | 0.785 | ||
| Surgical resection | 6 (3) | 7 (4) | |
| Ablation | 14 (7) | 15 (8) | |
| Transplantation | 2 (1) | 4 (2) | |
| TACE | 31 (16) | 22 (11) | |
| Targeted therapy | 6 (3) | 7 (4) | |
| Supportive care | 134 (69) | 138 (72) |
CTP, Child-Turcotte-Pugh; eGFR, estimated glomerular filtration rate; INR, international normalized ratio; PT, prothrombin time; SD, standard deviation; TACE, transarterial chemoembolization
Construction of the nomogram model
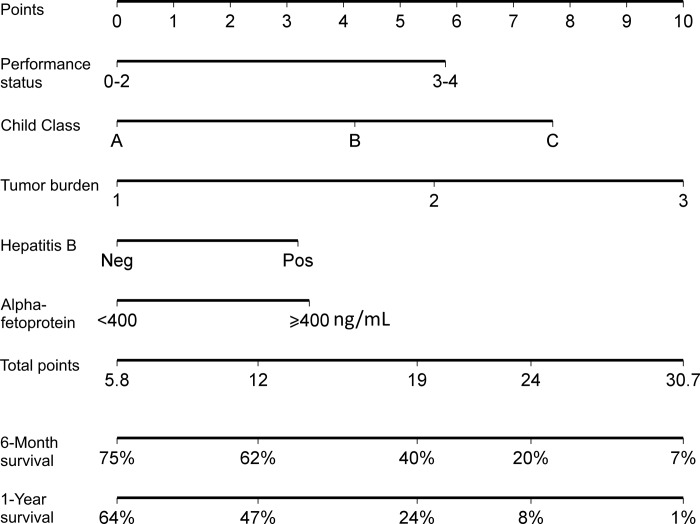
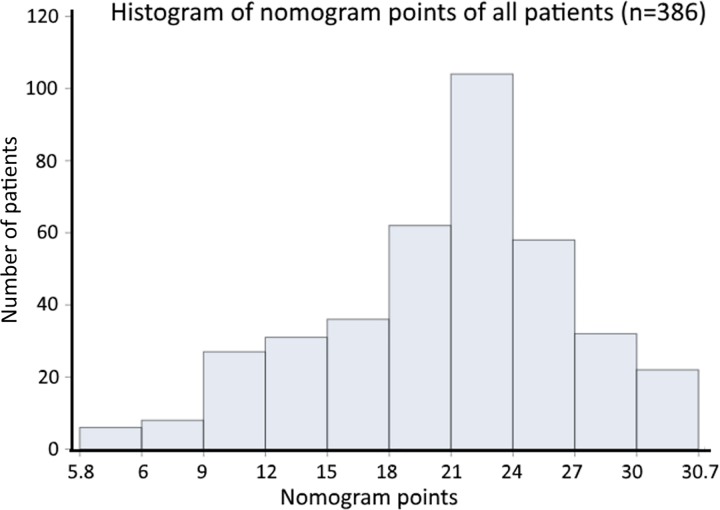
Tumor burden 3 had the highest BETA value in the model and was set as 10 points (Table 2). Sequentially, by using the ratios of BETAs between other prognostic factors and tumor burden 3, 7.7 (calculated as 0.823 divided by 1.064 and timed 10), 4.2, 5.6, 3.2, 5.8, 3.4 points were assigned to patients who were CTP class C, CTP class B, tumor burden 2, hepatitis B, PS 3–4 and AFP ≥ 400 ng/mL, respectively. Each patient had one individualized score from 5.8 to 30.7 by adding up the points from these five prognostic predictors. As shown in Fig 2, the projections from total points on the scales below indicate the estimated survival probability at 6 and 12 months. The histogram shows the majority of patients had a nomogram point between 18 to 27 (Fig 3).
Fig 2. Nomogram predicting 6- and 12-month survival of HCC patients.
The nomogram is used by adding up the points identified on the scale for the 5 parameters. The total points project downward to obtain the estimate 6- and 12-month survival.
Fig 3. The histogram of nomogram points of all enrolled patients.
Discrimination and calibration of nomogram in the derivation set
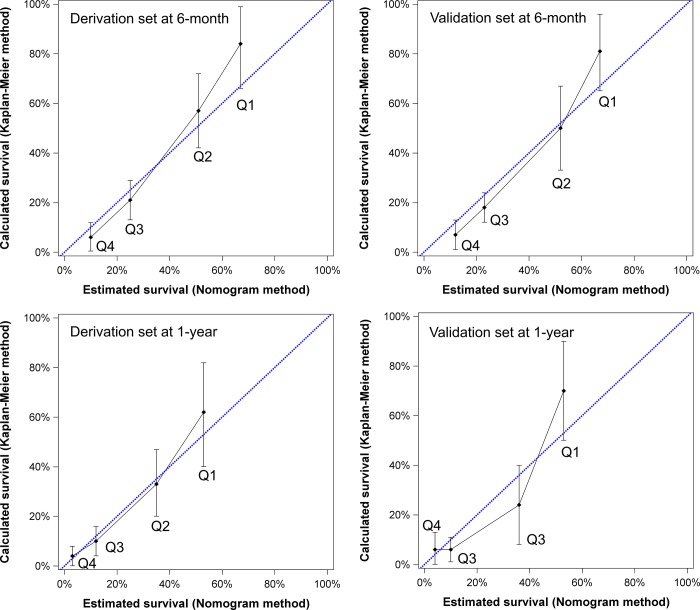
The nomogram generated from the derivation group had a concordance index of 0.759 (95% confidence interval [CI]: 0.552–0.923). Patients were divided into quarters by their specified points to investigate the accuracy of the model (nomogram points 5.8–12, 12.1–18, 18.1–24, 24.1–30.7). In the calibration plots (Fig 4), the mean and 95% CI of survival rates calculated by using the Kaplan-Meier method are shown on the Y-axis and the mean survival estimated by using the nomogram method is shown on the X-axis. The calibration plots for both 6-month and 1-year survival well matched the 45-degree line for derivation set patients.
Fig 4. The calibration plots of the nomogram in the derivation and validation sets for 6- and 12-month survival prediction.
The X-axis represents the nomogram-predicted survival and the Y-axis shows the mean survival and 95% confidence interval observed by the Kaplan-Meier method. By dividing patients into quarters based on nomogram points, the calibration line fits along with the 45-degree reference for both 6- and 12-month survival prediction in derivation and validation sets.
Discrimination and calibration of nomogram in the validation set
For the validation set, the nomogram had a concordance index of 0.741 (95% CI: 0.529–0.913). As shown in Fig 4, the nomogram-predicted mean survival is covered within the 95% CI of Kaplan-Meier method observed mean survival at 6 and 12 months for all quarters.
Discussion
HCC patients are classified as BCLC stage D due to CTP class C or PS 3–4, which are adapted to serve as sufficient criteria regardless of tumor burden. The arbitrary design of BCLC stage D results in remarkably complex compositions, and currently there are very few data focusing on the prediction of survival in BCLC stage D patients. In this study, we specifically investigated the prognostic effect of tumor burden, and proposed a new nomogram for patients with BCLC stage D HCC. By using clinically available parameters, our findings provide accurate survival estimation for terminal stage HCC based on the individual level, and may potentially improve the currently used staging systems for HCC.[1, 19]
The BCLC staging system is primarily determined by tumor burden, severity of cirrhosis and PS. Tumor burden (Okuda staging) had been a part of BCLC stage D when first published in 1999;[20, 21] however the HCC guidelines recommended by both European Association for the Study of the Liver and America Association for the Study of Liver Diseases removed tumor burden from the criteria of BCLC stage D.[1, 19] Patients classified into BCLC stage D could have very diverse clinical profiles; for example, patients with CTP class C, extra-hepatic metastases, and multiple co-morbidities are considered the same BCLC stage as patients with PS 3, minimal cirrhosis and a small resectable HCC nodule. With a well followed-up HCC cohort in our series, the baseline information of the study patients clearly showed that 15% (59/386) of patients had tumor burden within the Milan criteria. In addition, there were 17% and 9% of patients classified as CTP class A and PS 0–1, respectively. These findings disclose that a substantially high proportion of BCLC stage D patients had relatively small tumor burden, mild cirrhosis or relatively stable general condition at the time of diagnosis, indicating individualized prognostic prediction should be considered necessary from the clinical perspective.
BCLC stage D patients with mild cirrhosis and small tumor burden might potentially benefit from surgical resection or TACE. Similarly, selected patients with CTP class C and small tumor burden could choose liver transplantation or ablation to effectively prolong their survival.[22–24] Tumor burden has been shown an important survival predictor and is also highly related to treatment modalities.[25, 26] In this study, by dividing patients into three categories (within the Milan criteria, with distant involvement and vascular invasion, and the rest), both univariate and multivariate survival analyses showed the excellent discriminating power of tumor burden. Importantly, tumor burden 3 had the highest BETA value in the Cox regression model, which highlights the importance of tumor burden in predicting the clinical outcome. Consistently, the predominant prognostic power of tumor burden was also illustrated in our previous nomogram study for unselected HCC patients.[7] Although tumor burden is not considered a criterion for BCLC stage D HCC, our findings explicitly display the decisive role of tumor burden when the prognostic stratification is specifically evaluated within BCLC stage D.
In addition to tumor burden, cirrhosis and PS, the three parameters of original BCLC system, we found that hepatitis B and high serum AFP level were also associated with a poor prognosis as identified in the prognostic model. HBV infection was reported to associate with high tumor burden; notably, some studies showed HBV-related HCC patients had worse outcome compared to HCC patients without chronic viral hepatitis or patients with HCV-related HCC.[27–29] A multicenter study also pointed out HBV-related HCC patients suffered decreased survival compared to HCV-related HCC patients with matched clinical features.[30] The other factor, serum AFP at a level of > 400 ng/mL, was reported to have significantly discriminating ability for overall survival in HCC patients.[31] Abundant studies have associated aggressiveness of HCC and worse survival in patients with high serum AFP levels.[8, 32, 33] Altogether, these results suggest that our nomogram is a feasible and clinically accessible model in terms of outcome prediction.
The nomogram has concordance indices of 0.759 and 0.741 for derivation and validation sets, respectively. The interpretation of this finding is that if two HCC patients with different nomogram points are selected, the probability that the patient with higher nomogram score would die earlier is around 75%. Calibration plots showed nomogram-predicted survival covered by the 95% CI of mean survival observed by using the Kaplan-Meier method at 6 and 12 months for both derivation and validation sets. Clearly, this nomogram model shows patients with lower nomogram points (less severe cancer stage) had better survival distribution. With this nomogram, BCLC stage D patients could have individualized survival prediction, and candidates for future clinical trials can be more specifically identified.[34, 35]
This study has some limitations. First, the nomogram was generated from a cohort where hepatitis B is the main cause of chronic liver disease. External validation is required before it can be widely used in countries with high prevalence of alcoholic liver disease or hepatitis C. Second, anti-cancer treatments were not included in this study. Further study is needed to clarify the prognostic effect of variable treatment strategies for BCLC stage D patients. Also, only 2% of patients received transplantation in this cohort. For medical centers with a high volume of liver transplantation, this nomogram might not be suitable for survival prediction. Last, hepatitis B and C viral loads and specific anti-viral treatment may affect patient survival; this study does not include these factors because only a minority of patients received anti-viral treatment at different time periods and this nomogram was designed for all patients with different etiologies of HCC. Nomograms focusing on HCC patients with hepatitis B or hepatitis C are required to further investigate the influence of these variables.
In conclusion, contrary to the current BCLC scheme, this study indicates that tumor burden is a pivotal prognostic factor for patients with BCLC stage D HCC. With this easy-to-use nomogram, BCLC stage D patients can be better evaluated and stratified. An improved healthcare strategy can be planned according to the nomogram, which can also serve to identify candidates for anti-cancer treatments in future clinical trials.
Supporting information
The minimal anonymized data set.
(SAS7BDAT)
Abbreviations
- AFP
α-fetoprotein
- BCLC
Barcelona Clinic Liver Cancer
- CTP
Child-Turcotte-Pugh
- HCC
hepatocellular carcinoma
- eGFR
estimated glomerular filtration rate
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- PS
performance status
- TACE
transarterial chemoembolization
Data Availability
We have uploaded the minimal anonymized data set necessary to replicate our study findings as a Supporting Information file. Restrictions set by the Institutional Review Board of Taipei Veterans General Hospital prohibit the authors from making the full data set publicly available. For data access, please contact the Director of the IRB of Taipei Veterans General Hospital at d-mre@vghtpe.gov.tw.
Funding Statement
This study was supported by grants from the Center of Excellence for Cancer Research at Taipei Veterans General Hospital (MOHW106-TDU-B-211-144-003), Taiwan, from Taipei Veterans General Hospital (V106C-021, V105A-011, VN106-11), Taiwan.
References
- 1.EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee YH, Hsu CY, Huang YH, Hsia CY, Chiou YY, Su CW, et al. Vascular invasion in hepatocellular carcinoma: prevalence, determinants and prognostic impact. J Clin Gastroenterol. 2014;48:734–741. doi: 10.1097/MCG.0b013e3182a8a254 [DOI] [PubMed] [Google Scholar]
- 4.Liu PH, Lee YH, Hsia CY, Hsu CY, Huang YH, Chiou YY, et al. Surgical resection versus transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombosis: a propensity score analysis. Ann Surg Oncol. 2014;21:1825–1833. doi: 10.1245/s10434-014-3510-3 [DOI] [PubMed] [Google Scholar]
- 5.Adhoute X, Penaranda G, Raoul JL, Bourlière M. Nomogram of the Barcelona Clinic Liver Cancer System: external validation in European patients. Liver Int. 2016;36:1716–1717. doi: 10.1111/liv.13171 [DOI] [PubMed] [Google Scholar]
- 6.Hsu CY, Huo TI. Nomogram of the Barcelona clinic liver cancer system: on the go. Liver Int. 2016;36:1717–1718. doi: 10.1111/liv.13175 [DOI] [PubMed] [Google Scholar]
- 7.Hsu CY, Liu PH, Hsia CY, Lee YH, Al Juboori A, Lee RC, et al. Nomogram of the Barcelona Clinic Liver Cancer system for individual prognostic prediction in hepatocellular carcinoma. Liver Int. 2016;36:1498–1506. doi: 10.1111/liv.13114 [DOI] [PubMed] [Google Scholar]
- 8.Khalaf N, Ying J, Mittal S, Temple S, Kanwal F, Davila J, et al. Natural history of untreated hepatocellular carcinoma in a US cohort and the role of cancer surveillance. Clin Gastroenterol Hepatol. 2017;15:273–281.e1. doi: 10.1016/j.cgh.2016.07.033 [DOI] [PubMed] [Google Scholar]
- 9.Sanoff HK, Chang Y, Reimers M, Lund JL. Hospice utilization and its effect on acute care needs at the end of life in medicare beneficiaries with hepatocellular carcinoma. J Oncol Pract. 2017;13:e197–e206. doi: 10.1200/JOP.2016.017814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruix J, Tak WY, Gasbarrini A, Santoro A, Colombo M, Lim HY, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: multicentre, open-label, phase II safety study. Eur J Cancer. 2013;49:3412–3419. doi: 10.1016/j.ejca.2013.05.028 [DOI] [PubMed] [Google Scholar]
- 11.Luo CP, Mo HY, Wu LL, Ma Y, Peng NF. Soluble PD-L1 and prognosis of patients with hepatocellular carcinoma. Eur J Cancer. 2017;71:117–118. doi: 10.1016/j.ejca.2016.09.040 [DOI] [PubMed] [Google Scholar]
- 12.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. [DOI] [PubMed] [Google Scholar]
- 13.Lee YH, Hsu CY, Hsia CY, Huang YH, Su CW, Chiou YY, et al. Alcoholism worsens the survival of patients with hepatitis B virus and C virus-related hepatocellular carcinoma. Hepatol Int. 2013;7:645–654. doi: 10.1007/s12072-012-9375-2 [DOI] [PubMed] [Google Scholar]
- 14.Huo TI, Hsu CY, Huang YH, Su CW, Lin HC, Lee RC, et al. Prognostic prediction across a gradient of total tumor volume in patients with hepatocellular carcinoma undergoing locoregional therapy. BMC Gastroenterol. 2010;10:146 doi: 10.1186/1471-230X-10-146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu CY, Lee YH, Hsia CY, Huang YH, Su CW, Lin HC, et al. Performance status in patients with hepatocellular carcinoma: determinants, prognostic impact, and ability to improve the Barcelona Clinic Liver Cancer system. Hepatology. 2013;57:112–119. doi: 10.1002/hep.25950 [DOI] [PubMed] [Google Scholar]
- 16.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104 [DOI] [PubMed] [Google Scholar]
- 17.Harrell FE Jr., Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4 [DOI] [PubMed] [Google Scholar]
- 18.Pencina MJ, D'Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–2123. doi: 10.1002/sim.1802 [DOI] [PubMed] [Google Scholar]
- 19.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122 [DOI] [PubMed] [Google Scholar]
- 20.Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918–928. [DOI] [PubMed] [Google Scholar]
- 21.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933 [DOI] [PubMed] [Google Scholar]
- 22.Hsu CY, Liu PH, Lee YH, Hsia CY, Huang YH, Tsai YJ, et al. Active Treatments Prolong the Survival in Patients With Hepatocellular Carcinoma and Performance Status 3 or 4: A Propensity Score Analysis. J Clin Gastroenterol. 2015;49:878–884. doi: 10.1097/MCG.0000000000000300 [DOI] [PubMed] [Google Scholar]
- 23.Kudo M, Osaki Y, Matsunaga T, Kasugai H, Oka H, Seki T. Hepatocellular carcinoma in Child-Pugh C cirrhosis: prognostic factors and survival benefit of nontransplant treatments. Dig Dis. 2013;31:490–498. doi: 10.1159/000355259 [DOI] [PubMed] [Google Scholar]
- 24.Ruzzenente A, Capra F, Pachera S, Iacono C, Piccirillo G, Lunardi M, et al. Is liver resection justified in advanced hepatocellular carcinoma? Results of an observational study in 464 patients. J Gastrointest Surg. 2009;13:1313–1320. doi: 10.1007/s11605-009-0903-x [DOI] [PubMed] [Google Scholar]
- 25.Liu PH, Su CW, Hsu CY, Hsia CY, Lee YH, Huang YH, et al. Solitary large hepatocellular carcinoma: staging and treatment strategy. PLoS One. 2016;11:e0155588 doi: 10.1371/journal.pone.0155588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu CY, Huang YH, Hsia CY, Su CW, Lin HC, Loong CC, et al. A new prognostic model for hepatocellular carcinoma based on total tumor volume: the Taipei Integrated Scoring System. J Hepatol. 2010;53:108–117. doi: 10.1016/j.jhep.2010.01.038 [DOI] [PubMed] [Google Scholar]
- 27.Chen CH, Huang GT, Yang PM, Chen PJ, Lai MY, Chen DS, et al. Hepatitis B- and C-related hepatocellular carcinomas yield different clinical features and prognosis. Eur J Cancer. 2006;42:2524–2529. doi: 10.1016/j.ejca.2006.06.007 [DOI] [PubMed] [Google Scholar]
- 28.Zhou Y, Si X, Wu L, Su X, Li B, Zhang Z. Influence of viral hepatitis status on prognosis in patients undergoing hepatic resection for hepatocellular carcinoma: a meta-analysis of observational studies. World J Surg Oncol. 2011;9:108 doi: 10.1186/1477-7819-9-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huo TI, Huang YH, Hsia CY, Su CW, Lin HC, Hsu CY, et al. Characteristics and outcome of patients with dual hepatitis B and C-associated hepatocellular carcinoma: are they different from patients with single virus infection? Liver Int. 2009;29:767–773. doi: 10.1111/j.1478-3231.2008.01908.x [DOI] [PubMed] [Google Scholar]
- 30.Cantarini MC, Trevisani F, Morselli-Labate AM, Rapaccini G, Farinati F, Del Poggio P, et al. Effect of the etiology of viral cirrhosis on the survival of patients with hepatocellular carcinoma. Am J Gastroenterol. 2006;101:91–98. doi: 10.1111/j.1572-0241.2006.00364.x [DOI] [PubMed] [Google Scholar]
- 31.Hsu CY, Liu PH, Lee YH, Hsia CY, Huang YH, Lin HC, et al. Using serum α-fetoprotein for prognostic prediction in patients with hepatocellular carcinoma: what is the most optimal cutoff? PLoS One. 2015;10:e0118825 doi: 10.1371/journal.pone.0118825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tangkijvanich P, Anukulkarnkusol N, Suwangool P, Lertmaharit S, Hanvivatvong O, Kullavanijaya P, et al. Clinical characteristics and prognosis of hepatocellular carcinoma: analysis based on serum alpha-fetoprotein levels. J Clin Gastroenterol. 2000;31:302–308. [DOI] [PubMed] [Google Scholar]
- 33.Yamashita T, Ji J, Budhu A, Forgues M, Yang W, Wang HY, et al. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009;136:1012–1024. doi: 10.1053/j.gastro.2008.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen SC, Chao Y, Yang MH. Complete response to the combination of pembrolizumab and sorafenib for metastatic hepatocellular carcinoma: a case report. Am J Gastroenterol. 2017;112:659–660. doi: 10.1038/ajg.2017.1 [DOI] [PubMed] [Google Scholar]
- 35.Bruix Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The minimal anonymized data set.
(SAS7BDAT)
Data Availability Statement
We have uploaded the minimal anonymized data set necessary to replicate our study findings as a Supporting Information file. Restrictions set by the Institutional Review Board of Taipei Veterans General Hospital prohibit the authors from making the full data set publicly available. For data access, please contact the Director of the IRB of Taipei Veterans General Hospital at d-mre@vghtpe.gov.tw.