Abstract
Objective
Obesity-induced accumulation of ectopic fat in the liver is thought to contribute to the development of insulin resistance, and increased activity of hepatic CB1R has been shown to promote both processes. However, lipid accumulation in liver can be experimentally dissociated from insulin resistance under certain conditions, suggesting the involvement of additional mechanisms. Obesity is also associated with pro-inflammatory changes which, in turn, can promote insulin resistance. Kupffer cells (KCs), the liver's resident macrophages, are the major source of pro-inflammatory cytokines in the liver, such as TNF-α, which has been shown to inhibit insulin signaling in multiple cell types, including hepatocytes. Here, we sought to identify the role of CB1R in KCs in obesity-induced hepatic insulin resistance.
Methods
We used intravenously administered β-D-glucan-encapsulated siRNA to knock-down CB1R gene expression selectively in KCs.
Results
We demonstrate that a robust knock-down of the expression of Cnr1, the gene encoding CB1R, results in improved glucose tolerance and insulin sensitivity in diet-induced obese mice, without affecting hepatic lipid content or body weight. Moreover, Cnr1 knock-down in KCs was associated with a shift from pro-inflammatory M1 to anti-inflammatory M2 cytokine profile and improved insulin signaling as reflected by increased insulin-induced Akt phosphorylation.
Conclusion
These findings suggest that CB1R expressed in KCs plays a critical role in obesity-related hepatic insulin resistance via a pro-inflammatory mechanism.
Keywords: CB1 receptors, Kupffer cells, Insulin resistance, Inflammation, siRNA
Highlights
-
•
CB1R signaling promotes hepatic insulin resistance by promoting hepatic steatosis and hepatic inflammation.
-
•
CB1R knock-down in liver macrophages (Kupffer cells, KCs) improves global insulin resistance and glucose homeostasis.
-
•
CB1R expressed in KCs play a critical role in hepatic insulin resistance independent of ectopic fat in the liver or adipose tissue inflammation.
1. Introduction
Obesity is a risk factor for developing insulin resistance, which is defined as the inability of cells to respond normally to insulin. A commonly held view is that in a subset of obese, insulin-resistant individuals, β-cell dysfunction ensues, leading to decreased insulin production, poor blood glucose regulation, and ultimately type 2 diabetes (T2D) [1], [2]. The endocannabinoid system (ECS) is comprised of G-protein coupled cannabinoid 1 and 2 receptors (CB1/2R), their endogenous lipid ligands or endocannabinoids, and synthesizing and degrading enzymes. The discovery of the ECS has triggered an avalanche of experimental studies implicating it in a growing number of physiological/pathological functions [3], [4]. Modulation of ECS activity both in the peripheral and central nervous systems and in various peripheral organs with specific antagonists holds therapeutic promise for a broad range of diseases such as inflammatory disorders or obesity/metabolic syndrome, among others [4]. The mechanisms that link obesity and insulin resistance are the subject of intensive research, with increasing evidence for a major role of inflammation. Specifically, the development of excess, including ectopic, adipose tissue (AT) is strongly associated with chronic inflammation caused by infiltration of activated immune cells and overproduction of pro-inflammatory cytokines. Pro-inflammatory cytokines, such as TNF-α, can block insulin receptor signaling in multiple cell types, including adipocytes and hepatocytes [5], [6]. It is well known that nonalcoholic fatty liver disease is a strong risk factor for insulin resistance and type 2 diabetes [7], but lipid accumulation in liver can be experimentally dissociated from insulin resistance under certain conditions [8], suggesting that other mechanisms are also involved. Liver resident macrophages, called Kupffer cells (KCs), are thought to be the major source of hepatic inflammation [9] and appear to be involved in the regulation of multiple aspects of liver biology [10]. We have previously established a pro-inflammatory function of CB1R in macrophages [11] and CB1R signaling is strongly involved in the development of fatty liver [12] and insulin resistance [13], [14]. Here, using a method to silence gene expression selectively in Kupffer cells in vivo [11], [15], [16], [17], [18], [19], we demonstrate that knock-down of CB1R in Kupffer cells leads to improved global insulin sensitivity by reducing inflammation, ROS production and promoting mitochondria uncoupling through an increase in UCP2 activity.
2. Material & methods
2.1. Animals and diet
The experimental protocol was approved by the Institutional Animal Care and Use Committee of the National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health (NIH). Six-week-old male wild-type C57BL/6 mice were obtained from Taconic Inc. (Taconic, Germantown, NY). Cnr1−/− mice on a C57Bl/6J background were generated by heterozygote breeding. Mice were maintained under a 12:12-h light–dark cycle and fed ad libitum either a high fat diet (HFD, D12492; 60% of calories from fat, 20% from protein, and 20% from carbohydrates; Research Diets, New Brunswick, NJ) or standard mouse chow (NIH-31 rodent diet) for 15 weeks prior to treatment. Food intake was assessed as the cumulative amount eaten over 7 days.
2.2. GeRPS administration by intravenous injection in vivo
The β1,3-d-glucan-encapsulated siRNA particles (GeRPs) were prepared as previously described [16], [17]. Briefly, diet-induced obese mice were treated with 6 doses of FITC-GeRPs by intravenous injections every 3rd day for 15 days. Each dose of FITC-glucan shells contained 0.33 mg of fluorescently labeled GeRPs loaded with 1 nmol (13.3ug) control (GCAUCAAGUCGACUGUUAA) or Cnr1 (GCAUCAAGAGCACUGUUAA) siRNA and 16.6 nmol Endoporter.
2.3. Glucose homeostasis
Glucose tolerance and insulin sensitivity tests (GTTs and ISTs) were performed 24 h after the last GeRPs injection as described in [20]. A dose of 1.5 g/kg glucose or 0.75 U/kg of insulin was injected intraperitoneally, and blood glucose levels were measured as previously described [20].
2.4. Isolation of Kupffer cells and hepatocytes
Liver cells were isolated as described in [17]. Briefly, after anesthesia, the liver was first perfused with calcium-free Hanks balanced salt solution (HBSS, Gibco #14185-052, Gaithersburg, MD) then followed by collagenase digestion (0.6 mg/mL collagenase from Clostridium histolyticum [Sigma #C6885, St. Louis, MO] in HBBS containing 1 mM CaCl2). After digestion, liver cells were released by dissociation from the lobes and underwent several steps of filtration through a 100 μm cell strainer using ice-cold HBSS-CaCl2. Cell suspension was then centrifuged at a speed of 50 g for 3 min at 4 °C. The supernatant from the first centrifugation of hepatocytes was loaded on a Percoll gradient (25% and 50%) and centrifuged for 30 min at 2300 rpm and 4 °C. The interphase ring with Kupffer cells was collected and washed 2 times with PBS. The hepatocyte pellet obtained after the first centrifugation was washed 3 times in the same conditions in order to obtain the enriched hepatocyte fraction. Cells were cultured overnight in RPMI-1640 medium (ThermoFisher Scientific, 11875093, Waltham, MA) supplemented with 10% FBS (ThermoFischer Scientific, 10082147, Waltham, MA), 100 nM dexamethasone (Sigma #D-4902, St. Louis, MO), 100 nM insulin (Gibco #12585-014, Gaithersburg, MD), and 1% Penicillin/Streptomycin (ThermoFischer Scientific, 15140122, Waltham, MA) at 37 °C and 5% CO2. The following day, primary cells were used for subsequent analyses.
2.5. Preparation of conditioned medium
Hepatocyte conditioned medium (CM) was prepared by incubating primary hepatocytes isolated from lean or diet-induced obese (DIO) mice for 48 h in the condition described above. KCs CM was obtained by incubating primary KCs isolated from WT or global Cnr1−/− mice for 48 h in the same medium described above in the presence of 20 μg/mL of LPS.
2.6. Serum parameters
Serum insulin was measured using the STELLUX™ Chemi Rodent Insulin ELISA (ALPCO, Salem, NH), c-peptide and adiponectin were quantified using ELISA kits according to manufacturer's instruction (ALPCO, Salem, NH). Circulating ALT, AST, triglycerides and total cholesterol were quantified by colorimetric kits from BioAssay Systems (Hayward, CA).
2.7. Liver parameters
Intrahepatic triglyceride content was determined as previously described [11] whereas glycogen content was determined based on the enzymatic reaction described in [21]. Tissue and cells extraction for endocannabinoids measurement by liquid chromatography–tandem mass spectrometry (LC-MS/MS) was performed as previously described [11].
2.8. Immunohistochemistry
KCs were identified in liver and adipose tissue sections using antibodies against F4/80 (AbD Serotec, Raleigh, NC) or Iba-1 (Wako, 019-1974) and analyzed using a Zeiss LSM700 confocal microscope. Immunopositivity was quantified using Image J software.
2.9. Reactive Oxygen Species (ROS) detection
ROS production in isolated KCs was determined with the DCFDA – Cellular Reactive Oxygen Species Detection Assay Kit (Abcam, Ab113851, Cambridge, UK) according to manufacturer's instructions.
2.10. Assays for NF-κB p65 phosphorylation
Phosphorylation of NF-κB p65 in KCs was assessed by a phospho-RelA/NF-κB p65 ELISA kit (R&D Systems, KVB7226, Minneapolis, MN) according to the manufacturer's instructions.
2.11. Immunoblotting
Cell lysis was performed in 1x RIPA buffer (Thermo-Scientific) supplemented with phosphatase and protease inhibitor tablets (Roche), 2 mM Na3VO4, and 2 mM NaF. Total protein concentration was determined with BCA assay (Thermo-Scientific) and adjusted to the same concentration with additional lysis buffer. Proteins were separated on SDS-PAGE (4–12% Bis-Tris or Tris-Glycin gradient gel, Bio-Rad, Hercules, CA) and transferred onto nitrocellulose membranes. Membranes were blocked in 3% non-fat milk in Tris-buffered saline (TBS) with 0.1% Tween-20 (TBST) and incubated overnight at 4 °C in 5% BSA TBST with primary antibody against phospho-p65 (RelA), p65, phospho-AKTSer473, AKT (Cell Signaling #9271, #9272, 1/1000, Danvers, MA, USA), UCP2 (Cell Signaling #89326, Danvers, MA), FABP4 (Abcam, Ab66682), CB1R (rabbit polyclonal antibody, Immunogenes, Hungary), or HRP-conjugated mouse monoclonal antibody anti β-actin (Abcam ab49900, 1/20, 000) as a loading control. Appropriate secondary antibody was diluted in 5% non-fat milk TBST and incubated for 1 h at room temperature. Luminescent signal was generated with Super Signal West Pico chemiluminescent substrate (Thermo-Scientific) and detected with a membrane imager (G:BOX, Syngene). Quantification was performed using the analysis options of NIH Image J software.
2.12. Real-time PCR
Total RNA extraction from liver, hepatocytes and Kupffer cells was reverse transcribed, and real-time PCR were performed as previously described [11]. QuantiTect Primer Assays (Qiagen, Germantown, MD) were used to detect gene expression. Expression of a gene of interest is reported as a relative value comparing it to the geometric average of 18S, L19, L38, and TATA box binding protein expression.
2.13. Statistics
Values are expressed as means ± SEM. Data were analyzed by Student's t-test (GraphPad Prism v6 for Windows). Significance was set at P < 0.05.
3. Results
3.1. In vivo Cnr1 knock-down in KCs improves glucose tolerance and insulin sensitivity without affecting body weight or liver fat content in obese mice
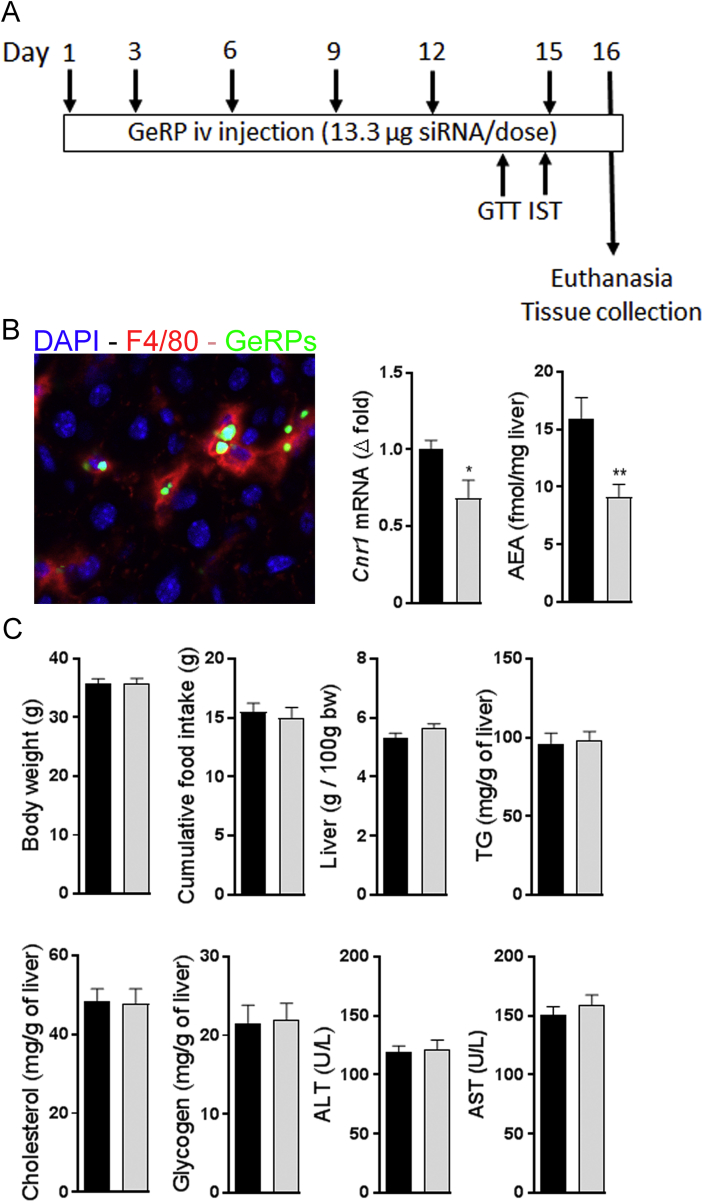
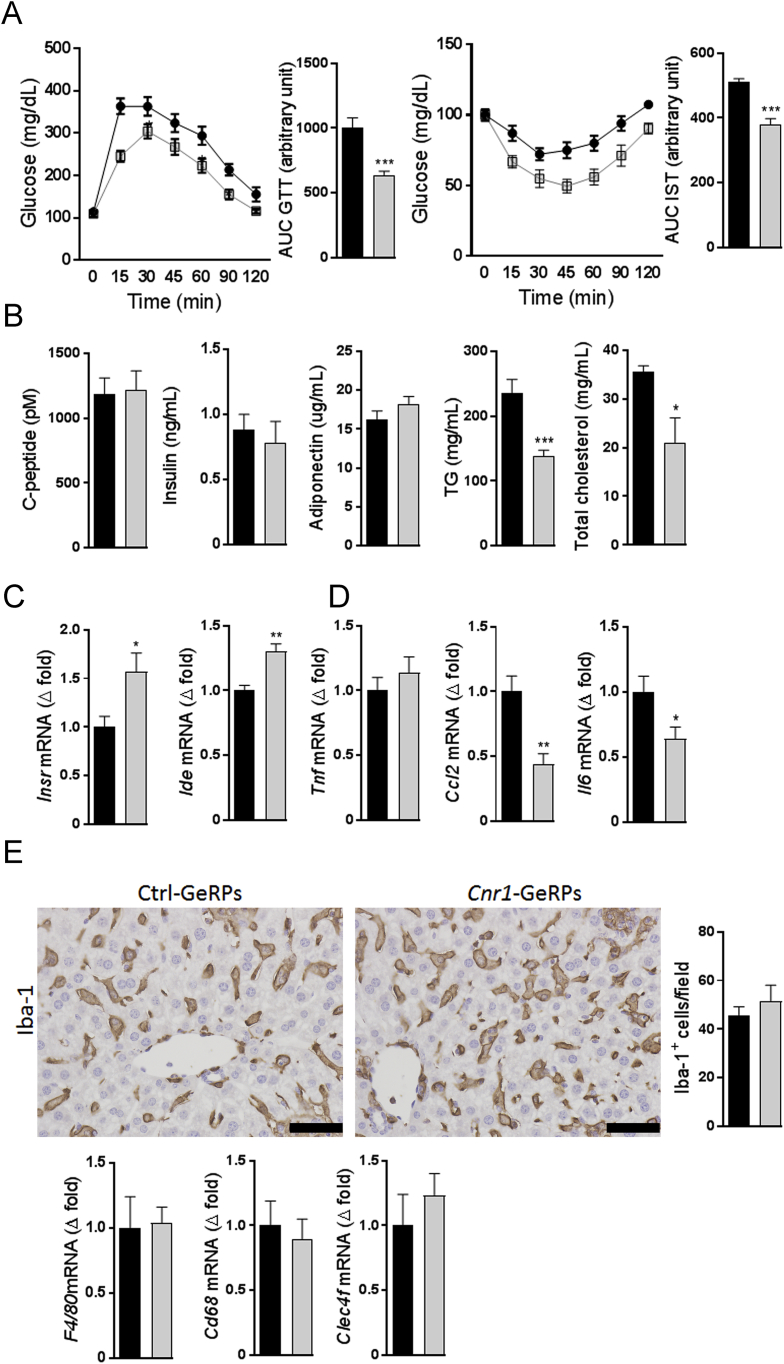
Diet-induced obese (DIO) mice were treated with fluorescently labeled GeRPs via intravenous injections every 3rd day for 15 days (Figure 1A), resulting in their selective uptake by KCs (Figure 1B). In agreement with previous observations [17], Using GeRPs containing a siRNA against Cnr1 resulted in a significant, ∼33% decrease in global liver Cnr1 expression and a ∼50% decrease in hepatic anandamide levels (Figure 1B). This treatment had no impact on body weight, food intake, liver mass, or hepatic triglycerides, cholesterol, and glycogen content (Figure 1C). Similarly, no differences were observed in terms of circulating ALT and AST levels between groups (Figure 1C). However, mice receiving Cnr1 siRNA displayed improved glucose tolerance and insulin sensitivity (Figure 2A), whereas no differences between the groups were noted regarding circulating insulin, c-peptide and adiponectin (Figure 2B). Interestingly, siRNA-mediated Cnr1 knock-down in KCs led to a significant reduction in circulating triglycerides and total cholesterol levels (Figure 2B) and also increased hepatic insulin receptor (Insr) and insulin degrading enzyme (Ide) gene expression (Figure 2C). Moreover, this treatment was associated with a lower expression of Ccl2 and Il6 but not Tnf, suggesting a decrease in liver inflammation (Figure 2D). Interestingly, the number of Iba-1 positive hepatic macrophages was similar in the 2 groups. Similarly, the gene expression of three KCs markers, F4/80, Cd68 and C-type lectin domain family 4 member F (Clec4f), remained unaffected (Figure 2E), suggesting that the reduced inflammation was not due to a decrease in hepatic macrophage content. Also, intravenous injection of GeRPs did not influence the number of macrophages present in adipose tissue (Supplementary Figure 1A) and did not influence Cnr1 expression is this tissue (Supplementary Figure 1B).
Figure 1.
GeRP-mediated selective knock-down of Cnr1 in Kupffer cells in obese mice does not influence hepatic steatosis. A Protocol for 15-day GeRP treatment in obese mice. B KCs staining (red) in liver section from an obese mouse 24 h after intravenous injection with FITC-labeled GeRPs (green) (magnification ×200). Cnr1 gene expression and AEA content in liver from DIO mice treated with control (black columns, n = 10) or Cnr1-GeRPs (light grey columns, n = 10). C Body weight, cumulative food intake, liver weight, and liver TG, cholesterol, and glycogen content in obese mice treated with control or Cnr1-GeRPs. Columns and bars represent means ± SEM. Significant differences from values in control-GeRPs treated obese mice *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 2.
Cnr1 knock-down in Kupffer cells of DIO mice improves glucose tolerance, insulin sensitivity and reduces hepatic inflammation. A Glucose tolerance (GTT) and insulin sensitivity test (IST) from DIO mice treated with control (black columns, n = 10) or Cnr1-GeRPs (light grey columns, n = 10). Points and bars are means ± SEM from 2 independent experiments. Areas under the curve (AUC) from each experiment were used for statistical analyses. B Plasma concentrations of c-peptide, insulin, adiponectin triglycerides, and total cholesterol. C Whole liver gene expression of insulin receptor (Insr) and insulin-degrading enzyme (Ide). D Whole liver gene expression of the pro-inflammatory cytokines Tnf, Ccl2 and Il-6. E Hepatic macrophage histology as assessed by Iba-1 immuno-staining (scale bars: 50 μm) and whole liver gene expression of macrophage markers F4/80, Cd68, and Clec4f. Columns and bars represent means ± SEM. Significant differences from values in control-GeRPs treated obese mice *P < 0.05, **P < 0.01, ***P < 0.001.
3.2. In vivo Cnr1 knock-down in KCs reduces endocannabinoid tone selectively in KCs
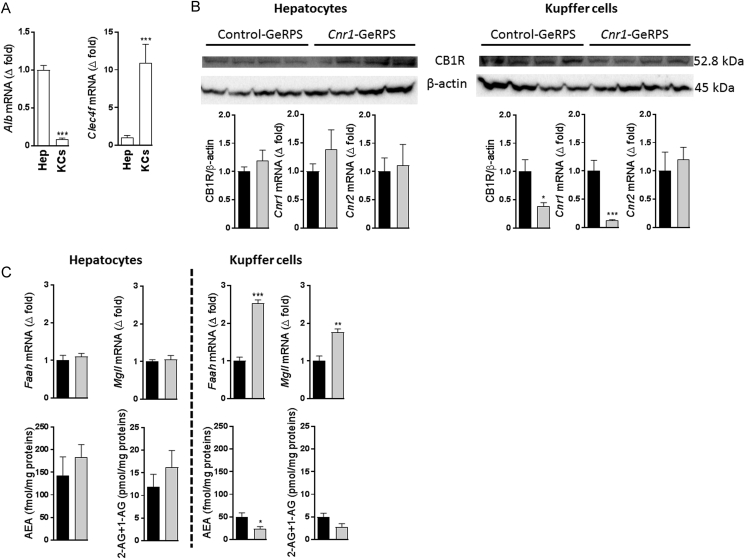
Hepatocyte and KC fractions were isolated from the liver of mice sacrificed 24 h following the administration of GeRPs. The purity of the fractions was analyzed by the expression of the hepatocyte marker albumin (Alb) and the KC marker Clec4f [17], which showed an enrichment of over 80% for the respective fractions (Figure 3A). As expected, Cnr1 expression remained unchanged by GeRP treatment in hepatocytes as these cells did not contain GeRPs, whereas it was reduced by 80% in KCs, which was paralleled by a nearly 60% reduction in CB1R protein levels as compared to KCs isolated from mice treated with control GeRPs (Figure 3B). The much greater reduction of Cnr1 in KCs compared to whole liver (see Figure 1B) is likely due to the expression of Cnr1 in other liver cell types such as hepatocytes [22], stellate cells [23], cholangiocytes [24], and liver vascular endothelial cells [25], which remained unaffected due to lack of uptake of GeRPs by these cells [15], [17]. Additionally, Cnr1 knock-down was associated with an increase in Faah and Mgll mRNA, which encode endocannabinoid degrading enzymes, along with a reduced anandamide and 2-arachidonoyl-glycerol content in KCs but not in hepatocytes (Figure 3C).
Figure 3.
In vivo Cnr1 knock-down in Kupffer cells reduces the endocannabinoid tone in these cells. A Relative expression of Alb and Clec4f in hepatocyte and Kupffer cell fractions, with levels in hepatocytes defined as 1.0. B CB1R protein quantification by western blot and Cnr1/Cnr2 gene expression in hepatocytes or Kupffer cells isolated from DIO mice treated with control (black columns) or Cnr1-GeRPs (grey columns). C Gene expression for Faah and Mgll and endocannabinoid (AEA and 2-AG) quantification in hepatocyte and Kupffer cell fractions from DIO mice treated either with control (black columns) or Cnr1-GeRPs (grey columns). Columns and bars represent means ± SEM. Significant differences from values in DIO mice treated with control-GeRPs, *P < 0.05, **P < 0.01, ***P < 0.001.
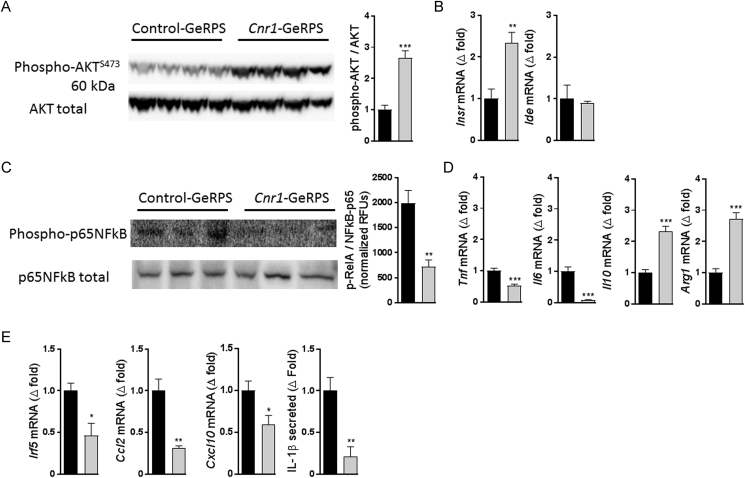
3.3. In vivo Cnr1 knock-down in KCs restores insulin signaling and induces a shift from pro-inflammatory M1 to anti-inflammatory M2 profile
Similar to the improved insulin sensitivity following the treatment of DIO mice with peripheral CB1R antagonists [20], [26], insulin-stimulated AktS473 phosphorylation was increased in KCs of mice treated with Cnr1-GeRPs compared to control GeRPs (Figure 4A). KCs with Cnr1 knock-down displayed an increase in insulin receptor (Insr) but not in insulin degrading enzyme (Ide) mRNA expression (Figure 4B). Cnr1 knock-down was associated with an inhibition of NF-kB activity, as reflected by the reduced p65 phosphorylation observed by western blotting and by cell-based ELISA (Figure 4C). In parallel, a shift from a pro-to anti-inflammatory phenotype was indicated by lower Tnf and IL6 as well as higher Il10 and Arginase (Arg1) expression (Figure 4D). Additionally, KCs with reduced Cnr1 expression also had a marked reduction in Irf5, Ccl2, and Cxcl10 expression along with reduced Il-β protein secretion (Figure 4E).
Figure 4.
In vivo Cnr1 knock-down in KCs restores insulin signaling and promotes an anti-inflammatory profile. A Representative Akt phosphorylation by western blot after insulin stimulation and its quantification. B Gene expression of insulin receptor (Insr) and insulin degrading enzyme (Ide) in isolated Kupffer cells. C Representative NF-κB phosphorylation analysis by western blot and by cell-based ELISA kit. D Gene expression for TNFα (Tnf), IL-6 (Il6), IL-10 (Il10), and arginase 1 (Arg1) in isolated Kupffer cells. E Gene expression for interferon regulatory factor 5 (Irf5), CCL2 (Ccl2), CXCL10 (Cxcl10), and secretion of IL-1β from isolated Kupffer cells from obese mice treated either with control (black columns) or Cnr1-GeRPs (grey columns). Columns and bars represent means ± SEM. Significant differences from values in control-GeRPs treated DIO mice *P < 0.05, **P < 0.01, ***P < 0.001.
3.4. In vivo Cnr1 knock-down reduces oxidative stress markers and increases mitochondria uncoupling in KCs
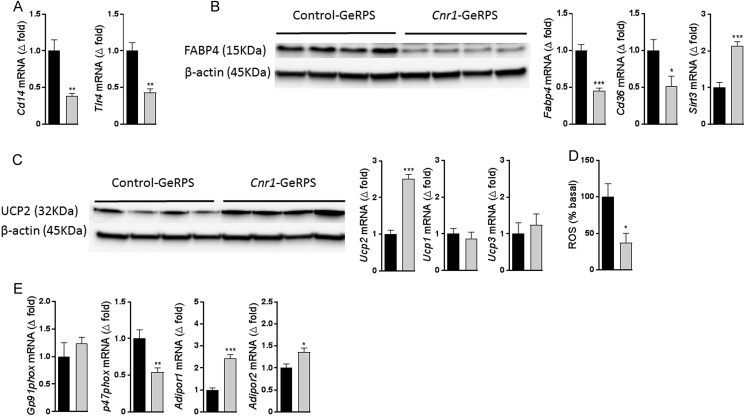
Cnr1 knock-down in KCs resulted in reduced expression of Cd14 and Tlr4, genes encoding two receptors involved in NF-κB activation (Figure 5A), along with downregulation of the fatty acid binding protein-4 both at the mRNA and protein levels as well as a downregulation of Cd68 and upregulation in sirtuin 3 (Sirt3) (Figure 5B). In parallel, uncoupling protein 2 (UCP2) mRNA and protein were elevated in KCs with Cnr1 knock-down, whereas Ucp1 and Ucp3 expression did not change (Figure 5C). Together, these data suggest a link between CB1R signaling and mitochondria uncoupling in KCs. Knock-down of Cnr1 also led to a significant decrease in reactive oxygen species (ROS) activity, as quantified using 2′,7′–dichlorofluorescin diacetate (DCFDA) as a probe (Figure 5D). Moreover, Cnr1 knock-down reduced the expression of Gp91phox and p47phox, 2 subunits of the multi-protein complex NADPH oxidase 2, and increased Adipor1 and Adipor2 mRNA expression, suggesting a decrease in oxidative stress (Figure 5E).
Figure 5.
In vivo Cnr1 knock-down in KCs reduces oxidative stress. A Gene expression for CD14 and TLR4 in Kupffer cells. B Representative western blot showing FABP4 protein levels in Kupffer cells isolated from obese mice treated with either control or Cnr1-GeRPs and gene expression for Fabp4 and Sirtuin-3 (Sirt3). C UCP-2 protein and gene expression along with Ucp1 and Ucp3 gene expression in Kupffer cells. D Reactive Oxygen Species (ROS) produced by Kupffer cells isolated from obese mice treated with either control or Cnr1-GeRPs. E Gene expression for Gp91phox, p47phox, AdipoR1, and Adipor2 in isolated Kupffer cells from obese mice treated either with control (black columns) or Cnr1-GeRPs (grey columns). Columns and bars represent means ± SEM. Significant differences from values in control-GeRPs treated obese mice *P < 0.05, **P < 0.01, ***P < 0.001.
3.5. Hepatocyte-derived endocannabinoids regulate cytokine secretion by KCs
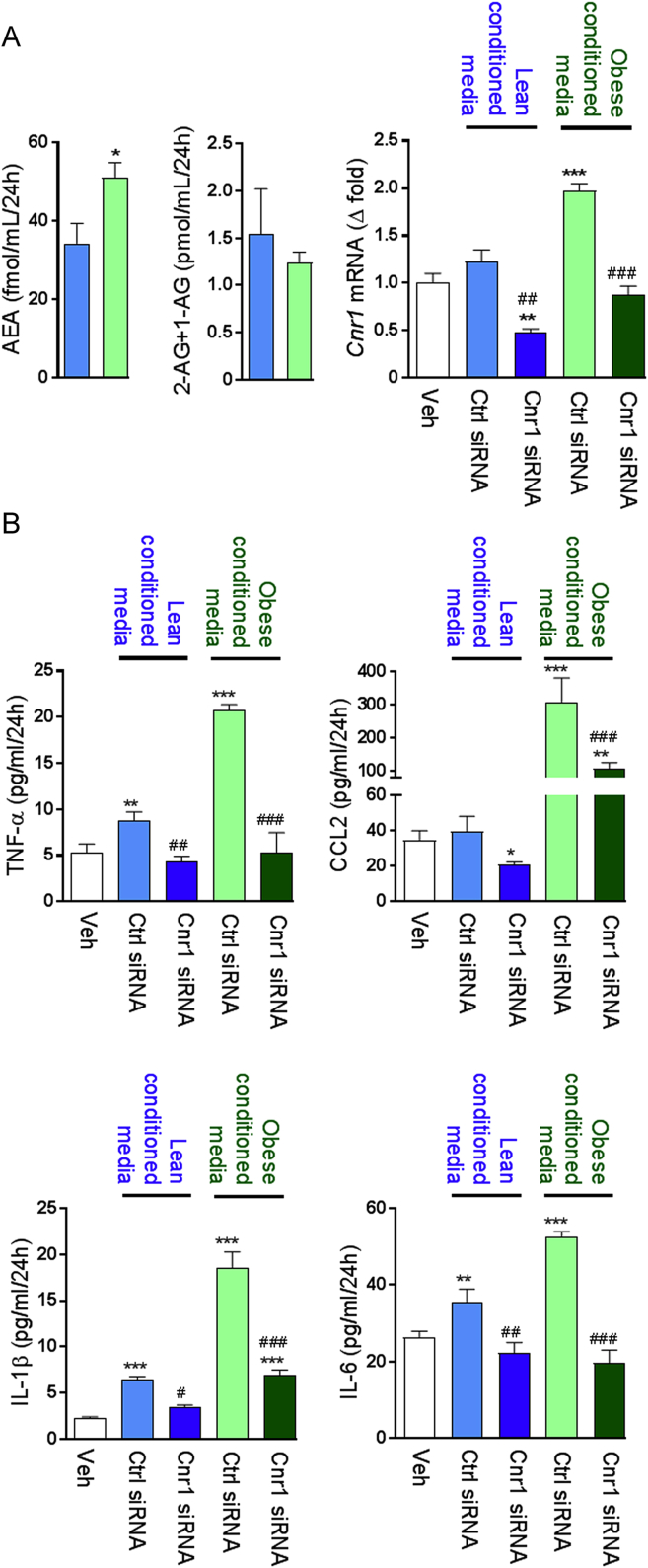
In order to investigate the stimuli involved in the inflammatory response of KCs, we incubated KCs isolated from lean mice in presence of conditioned medium (CM) from hepatocytes isolated from lean or obese mice. The CM from obese hepatocytes contained more anandamide (AEA) but not 2-arachidonoyl glycerol (2-AG) than CM from lean hepatocytes, and it triggered an increase in Cnr1 in lean KCs which could be prevented by Cnr1-siRNA pre-treatment (Figure 6A). KCs incubated with the CM from obese hepatocytes secreted increased amounts of TNFα, CCL2, IL-1β, and IL-6 compared to KCs incubated with CM from lean hepatocytes (Figure 6B), and these changes were CB1R-dependent as they were abrogated by Cnr1 knock-down (Figure 6B).
Figure 6.
KCs secretary response is influenced by changes in endocannabinoid tone. A Endocannabinoid content in conditioned medium from hepatocytes isolated from lean (blue columns) or obese mice (green columns) and Cnr1 expression in Kupffer cells incubated for 24 h in these conditioned media in presence of either control or Cnr1 siRNA. B Effect of conditioned medium on Kupffer cells secretion of TNF-α, CCL2, IL-1β, and IL-6 after 24 h. Columns and bars represent means ± SEM from 3 individual experiments with n = 4 per condition. Significant differences from values in Veh-treated Kupffer cells (white columns) *P < 0.05, **P < 0.01, ***P < 0.001 or control siRNA treated cells (blue or green columns) #P < 0.05, ##P < 0.01, ###P < 0.001.
3.6. Changes in inflammatory tone directly influence hepatocytes response to insulin
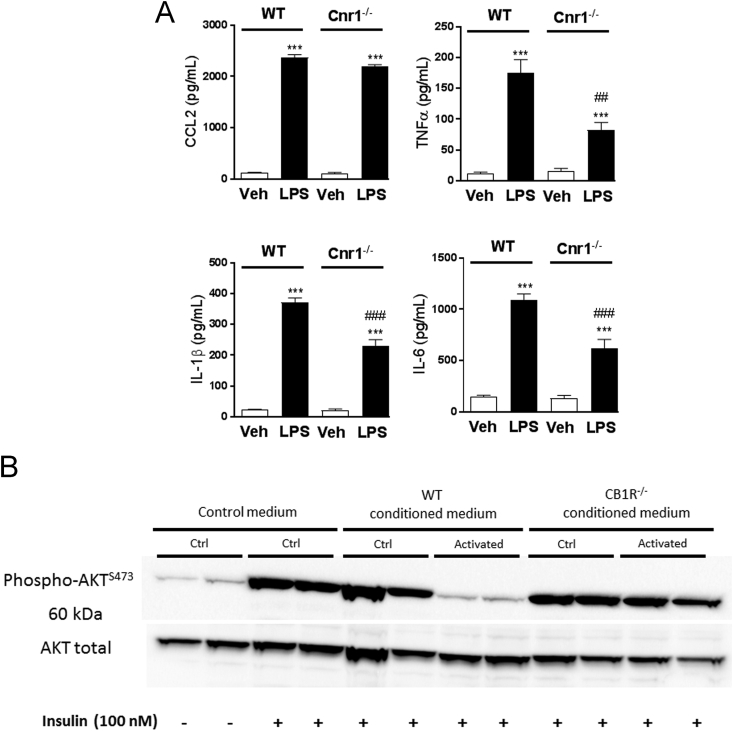
In view of the EC-mediated increase in pro-inflammatory cytokine secretion by KCs, we investigated if this could impair the insulin response of hepatocytes. KCs isolated from WT and CB1R−/– mice were incubated with vehicle or LPS (50 ng/mL) for 48 h. LPS triggered an increase in TNFα, IL-1β, and IL6 but not in CCL2 secretion, which was much stronger in WT compared to CB1R-deficient KCs (Figure 7A). We then incubated primary hepatocytes from lean mice with regular medium or KC conditioned medium and tested their response to insulin. Insulin induced a robust increase in AKT phosphorylation on Ser473 in hepatocytes maintained in regular medium or in CM from CB1R-deficient KCs, but its effect was abrogated in hepatocytes incubated with CM from WT KCs (Figure 7B), suggesting that a CB1R-mediated cytokine secretion by KCs inhibits hepatocyte insulin signaling.
Figure 7.
Changes in inflammatory tone directly influence hepatocyte response to insulin. A Cytokine and chemokine production by Kupffer cells isolated from wildtype (WT, empty columns) or CB1R−/– mice (Cnr1−/−, black columns) after 48 h of treatment with saline or LPS (50 ng/mL). Columns and bars represent means ± SEM from 3 individual experiments with n = 3 replicates per condition. B Representative western blot showing Akt phosphorylation in response to insulin challenge (100 nM, 30 min) in hepatocytes incubated for 24 h in conditioned medium obtained from LPS-treated Kupffer cells as described in A. Columns and bars represent means ± SEM. Significant differences from values in vehicle treated cells *P < 0.05, **P < 0.01, ***P < 0.001 or LPS treated cells #P < 0.05, ##P < 0.01, ###P < 0.001.
4. Discussion
In the present study, we demonstrated that selective silencing Cnr1 in KCs improves hepatic insulin sensitivity in DIO mice, independent of ectopic fat deposition. We used the GeRP technology to deliver siRNA to KCs without affecting other hepatic cells [15], [16], [17], [18], [19] by taking advantage of the micrometer-size and Dectin 1 receptor-mediated recognition of the glucan shells [16]. Further specificity for hepatic macrophages was ensured by using the intravenous route for the administration of GeRPs, which limits their distribution to the liver without affecting macrophages in other tissues including adipose tissue [17]. As resident macrophages, KCs are the major source of pro-inflammatory cytokines in the liver [27], [28], and steatotic hepatocytes influence the M1/M2 balance of KCs by promoting the apoptosis of alternatively-activated M2 KCs, thus shifting their balance toward the pro-inflammatory M1 phenotype [29]. CD14 has been reported as a potential marker for necrotic liver inflammation, and its expression correlates with the phagocytic function of KCs [30]. Moreover, binding of LPS to CD14 on the KC membrane activates IKK kinases, thus relieving the IκB-mediated inhibition of NF-κB, leading to an increase in TNF-α secretion [31]. This, in turn, triggers monocyte infiltration through the expression of C-X-C motif chemokine 10 (CXCL10) and Chemokine ligand 2 (CCL2) [32]. In addition, increases in the levels of such pro-inflammatory cytokines in patients with non-alcoholic steatohepatitis (NASH) are related to the level of CD14+ KCs [27], [33], [34]. In view of the above, the decreased expression of Cd14, Tnf, Cxcl10, and Ccl2 in KCs following Cnr1 knock-down reflects a decrease in their pro-inflammatory polarization.
KCs are also activated by the binding of LPS or FFA to TLRs resulting in the release of cytokines and chemokines via NF-κB signaling. The activation of the NF-κB signaling pathway is also directly induced by oxidative or endoplasmic reticulum (ER) stress. Several pro-inflammatory cytokines, such as TNF-α, IL-1β, IL-6, C-C chemokine receptors 2 (CCR-2), macrophage inflammatory protein 1 (MIP-1), COX-2, CCL2, and intercellular adhesion molecule/vascular cell adhesion molecule (ICAM/VCAM) are produced by the activated NF-κB pathway [35], [36], and systemic inhibition of NF-κB reduces hepatic inflammation and improves insulin resistance [37]. Here, we found a drastic inhibition of NFκB activation after Cnr1-knockdown in KCs, partially explaining the associated decrease in the expression of pro-inflammatory markers such as Tnf, Ccl2, Cxcl10, Il6, and IL-1β. The degree of NF-κB inhibition and the associated reduction in cytokine release and insulin sensitization following Cnr1 knock-down were similar to those reported following a KC-specific knock-down of NF-κB [17]. This suggests that CB1Ris a major upstream signal involved in NF-κB activation in KCs.
The transcription factor interferon regulatory factor 5 (Irf5) plays an important role in polarizing macrophages towards an inflammatory phenotype and promoting insulin resistance and hepatic fibrosis [38], [39], [40], [41]. Thus, the 50% decrease in Irf5 mRNA expression induced by Cnr1 knockdown in KCs likely contributes to their M1→M2 shift and to the improved in vivo insulin sensitivity. This is in agreement with our previous findings showing that IRF5 is a downstream target of CB1R in macrophages, in which it drives the CB1R-mediated increase in TNF-α secretion, and its in vivo knock-down in macrophages prevents the loss of pancreatic islet β-cells and the development of type 2 diabetes [42]. Our observations linking Irf5 expression and CB1R signaling are also in agreement with findings that IRF5 expression in obese subjects is negatively correlated with insulin sensitivity [38], [39], [40].
Adiponectin is known to stimulate the production of IL-10 and IL-1R antagonist, decrease phagocytic activity, and suppress pro-inflammatory cytokine production in macrophages by inhibiting NF-κB [43], [44], [45]. Adiponectin can also promote macrophage polarization toward an anti-inflammatory M2 phenotype [46], [47] similarly to Cnr1 knock-down. In addition, this effect can be either direct [46] or indirect through IL-4-mediated M2 polarization [48]. Accordingly, we found that Cnr1 knockdown in KCs led to increased expression of both Adipor1 and Adipor2, which was associated with increased Il10 and Arg1 expression. AdipoR1 is abundantly expressed in macrophages [44], [49], [50], whereas AdipoR2 is predominantly expressed in the liver [51]. AdipoR1 signals via activating AMP-activated kinase (AMPK) while AdipoR2 activates PPARα, both contributing to increased insulin sensitivity [52]. Simultaneous disruption of both AdipoR1 and AdipoR2 abolished adiponectin binding and actions, resulting in increased liver triglyceride content, inflammation and oxidative stress in adipose tissue, and consequent insulin resistance and glucose intolerance [53]. A similar mechanism could be at play in KCs following Cnr1 knock-down, as indicated by the increase in both Adipor1 and Adipor2 expression. Indeed, macrophage-specific AdipoR1 transgenic mice (AdR1-TG) exhibit enhanced whole-body glucose tolerance and insulin sensitivity with reduced pro-inflammatory cytokines, MCP-1 and TNF-α, both in the serum and in the insulin target metabolic tissues [54].
Inflammation is often coupled to increased ROS production and a pivotal source of ROS in inflammatory cells is the NADPH oxidase or NOX [55]. Rapid release of ROS in response to LPS and other microbial stimuli in KCs and other macrophages occurs mainly through the isoform NOX2 [56]. Upon activation, NOX2 will produce superoxide, a major form of ROS that signals to redox-sensitive targets [57], [58], [59]. UCP2 has been considered in the pathogenesis of NAFLD since its identification [60], [61]. In this condition, KCs and other macrophages have diminished UCP2, which could be attributed to the increased oxidative stress seen in fatty liver [62]. In our study, we observed that Cnr1 knock-down in KCs led to an increase in Ucp2 expression without any changes in Ucp1 or Ucp3 expression, suggesting that the reduction in ROS production we observed was due, at least partially, to the uncoupling of the mitochondrial respiratory chain, especially since macrophages isolated from Ucp2−/− mice were reported to generate more ROS than those from wild-type mice [63]. The observed reduction in the expression of Fabp4 in CB1R-deficient KCs can also contribute to reduced oxidative stress, as it was recently shown that inhibition or genetic deletion of FABP4/aP2 in macrophages caused an increase in UCP2, resulting in decreased ER stress and a UCP2-dependent reduction in ROS production [64]. This is in agreement with previous findings where Leishmania donovani infection in macrophages was associated with a strong upregulation of UCP2 and a strong reduction in ROS generation [65]. Furthermore, in agreement with our findings, it was recently shown that lower FABP4 can induce Sirt3 expression, which was linked to a decrease in ROS production and an anti-inflammatory phenotype in macrophages [66].
Finally, experiments using conditioned media from LPS-stimulated KCs implicated endocannabinoids in triggering the secretion of pro-inflammatory cytokines by KCs, which then could act on neighboring hepatocytes to inhibit insulin signaling. Previously, we have demonstrated that LPS causes a robust, >10-fold increase in anandamide synthesis in RAW264.7 macrophages [67], which could act as autocrine mediator on CB1R located on the same cell. Indeed, the involvement of CB1R in cytokine release by KCs is supported by the reduced cytokine release and corresponding loss of biological activity of medium conditioned with CB1R-deficient KCs, as illustrated in Figure 7. These observations suggest a cross-talk between KCs and hepatocytes, which could trigger hepatic insulin resistance.
To conclude, the data presented demonstrate that CB1R signaling in KCs plays an important role in hepatic insulin resistance independently of ectopic fat in the liver or adipose tissue inflammation. Given the documented role of hepatocyte CB1R in insulin resistance [14], our study reinforces the importance CB1R expressed by different types of liver cells in glycemic control and further highlights the therapeutic potential of peripheral CB1R blockade in the metabolic complications of obesity.
Author contributions
T.J. and G.K. designed the study, analyzed results and wrote the manuscript; T.J. performed most of the experiments; Z.Z. performed all liver perfusion and helped isolating hepatocytes and Kupffer cells; S.N. and Y.S. prepared, provided, designed, and tested the GeRPs; J.L. and N.J.C. assisted with cell culture, western-blot and PCR analysis; R.C. conducted the endocannabinoids measurements; G.G. assisted with animal care and in vivo experiments; M.A. and M.P.C. provided insights and critical review of the manuscript; B.G. provided helpful and critical comments on the manuscript. All authors had access to the manuscript and agreed with the final version.
Acknowledgments
All authors declare no conflict of interest. We thank Judith Harvey-White (National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institutes of Health (NIH)) for technical assistance and Raouf Kechrid (National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institutes of Health (NIH)) for assistance with the animal studies.
This study was supported by intramural NIH funds to G.K and by the NIH grant DK103407 to M.P.C.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.molmet.2017.08.011.
Contributor Information
Tony Jourdan, Email: tony.jourdan@nih.gov.
George Kunos, Email: george.kunos@nih.gov.
Conflict of interest
None declared.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Muoio D.M., Newgard C.B. Molecular and metabolic mechanisms of insulin resistance and [beta]-cell failure in type 2 diabetes. Nature Reviews Molecular Cell Biology. 2008;9:193–205. doi: 10.1038/nrm2327. [DOI] [PubMed] [Google Scholar]
- 2.Prentki M., Nolan C.J. Islet β cell failure in type 2 diabetes. The Journal of Clinical Investigation. 2006;116:1802–1812. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Marzo V. Targeting the endocannabinoid system: to enhance or reduce? Nature Reviews Drug Discovery. 2008;7:438–455. doi: 10.1038/nrd2553. [DOI] [PubMed] [Google Scholar]
- 4.Pacher P., Bátkai S., Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacological Reviews. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirosumi J., Tuncman G., Chang L., Gorgun C.Z., Uysal K.T., Maeda K. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 6.Yuan M., Konstantopoulos N., Lee J., Hansen L., Li Z.-W., Karin M. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkβ. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 7.Perry R.J., Samuel V.T., Petersen K.F., Shulman G.I. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature. 2014;510:84–91. doi: 10.1038/nature13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun Z., Lazar M.A. Dissociating fatty liver and diabetes. Trends in Endocrinology and Metabolism. 2013;24:4–12. doi: 10.1016/j.tem.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramadori G., Armbrust T. Cytokines in the liver. European Journal of Gastroenterology and Hepatology. 2001;13:777–784. doi: 10.1097/00042737-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Jager J., Aparicio-Vergara M., Aouadi M. Liver innate immune cells and insulin resistance: the multiple facets of Kupffer cells. Journal of Internal Medicine. 2016;280:209–220. doi: 10.1111/joim.12483. [DOI] [PubMed] [Google Scholar]
- 11.Jourdan T., Godlewski G., Cinar R., Bertola A., Szanda G., Liu J. Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates beta cell loss in type 2 diabetes. Nature Medicine. 2013;19:1132–1140. doi: 10.1038/nm.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mallat A., Teixeira-Clerc F., Lotersztajn S. Cannabinoid signaling and liver therapeutics. Journal of Hepatology. 2013;59:891–896. doi: 10.1016/j.jhep.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 13.Cinar R., Godlewski G., Liu J., Tam J., Jourdan T., Mukhopadhyay B. Hepatic cannabinoid-1 receptors mediate diet-induced insulin resistance by increasing de novo synthesis of long-chain ceramides. Hepatology. 2014;59:143–153. doi: 10.1002/hep.26606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J., Zhou L., Xiong K., Godlewski G., Mukhopadhyay B., Tam J. Hepatic cannabinoid Receptor-1 mediates diet-induced insulin resistance via inhibition of insulin signaling and clearance in mice. Gastroenterology. 2012;142:1218–1228. doi: 10.1053/j.gastro.2012.01.032. e1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aouadi M., Tesz G.J., Nicoloro S.M., Wang M., Chouinard M., Soto E. Orally delivered siRNA targeting macrophage Map4k4 suppresses systemic inflammation. Nature. 2009;458:1180–1184. doi: 10.1038/nature07774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tesz Gregory J., Aouadi M., Prot M., Nicoloro Sarah M., Boutet E., Amano Shinya U. Glucan particles for selective delivery of siRNA to phagocytic cells in mice. Biochemical Journal. 2011;436:351–362. doi: 10.1042/BJ20110352. [DOI] [PubMed] [Google Scholar]
- 17.Tencerova M., Aouadi M., Vangala P., Nicoloro S.M., Yawe J.C., Cohen J.L. Activated Kupffer cells inhibit insulin sensitivity in obese mice. The FASEB Journal. 2015;29:2959–2969. doi: 10.1096/fj.15-270496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aouadi M., Tencerova M., Vangala P., Yawe J.C., Nicoloro S.M., Amano S.U. Gene silencing in adipose tissue macrophages regulates whole-body metabolism in obese mice. Proceedings of the National Academy of Sciences. 2013;110:8278–8283. doi: 10.1073/pnas.1300492110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aouadi M., Vangala P., Yawe J.C., Tencerova M., Nicoloro S.M., Cohen J.L. Lipid storage by adipose tissue macrophages regulates systemic glucose tolerance. American Journal of Physiology – Endocrinology and Metabolism. 2014;307:E374–E383. doi: 10.1152/ajpendo.00187.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tam J., Cinar R., Liu J., Godlewski G., Wesley D., Jourdan T. Peripheral cannabinoid-1 receptor inverse agonism reduces obesity by reversing leptin resistance. Cell Metabolism. 2012;16:167–179. doi: 10.1016/j.cmet.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Degrace P., Demizieux L., Du Z.Y., Gresti J., Caverot L., Djaouti L. Regulation of lipid flux between liver and adipose tissue during transient hepatic steatosis in carnitine-depleted rats. Journal of Biological Chemistry. 2007;282:20816–20826. doi: 10.1074/jbc.M611391200. [DOI] [PubMed] [Google Scholar]
- 22.Osei-Hyiaman D., Liu J., Zhou L., Godlewski G., Harvey-White J., Jeong W.I. Hepatic CB(1) receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. Journal of Clinical Investigation. 2008;118:3160–3169. doi: 10.1172/JCI34827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teixeira-Clerc F., Julien B., Grenard P., Tran Van Nhieu J., Deveaux V., Li L. CB1 cannabinoid receptor antagonism: a new strategy for the treatment of liver fibrosis. Nature Medicine. 2006;12:671–676. doi: 10.1038/nm1421. [DOI] [PubMed] [Google Scholar]
- 24.DeMorrow S., Francis H., Gaudio E., Ueno Y., Venter J., Onori P. Anandamide inhibits cholangiocyte hyperplastic proliferation via activation of thioredoxin 1/redox factor 1 and AP-1 activation. American Journal of Physiology Gastrointestinal and Liver Physiology. 2008;294:G506–G519. doi: 10.1152/ajpgi.00304.2007. [DOI] [PubMed] [Google Scholar]
- 25.Batkai S., Jarai Z., Wagner J.A., Goparaju S.K., Varga K., Liu J. Endocannabinoids acting at vascular CB1 receptors mediate the vasodilated state in advanced liver cirrhosis. Nature Medicine. 2001;7:827–832. doi: 10.1038/89953. [DOI] [PubMed] [Google Scholar]
- 26.Tam J., Vemuri V.K., Liu J., Batkai S., Mukhopadhyay B., Godlewski G. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. Journal of Clinical Investigation. 2010;120:2953–2966. doi: 10.1172/JCI42551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baffy G. Kupffer cells in non-alcoholic fatty liver disease: the emerging view. Journal of Hepatology. 2009;51:212–223. doi: 10.1016/j.jhep.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ono M., Saibara T. Is impaired Kupffer cell function really important to the pathogenesis of nonalcoholic steatohepatitis? Journal of Gastroenterology and Hepatology. 2012;27:622–624. doi: 10.1111/j.1440-1746.2012.07084.x. [DOI] [PubMed] [Google Scholar]
- 29.Wan J., Benkdane M., Teixeira-Clerc F., Bonnafous S., Louvet A., Lafdil F. M2 Kupffer cells promote M1 Kupffer cell apoptosis: a protective mechanism against alcoholic and nonalcoholic fatty liver disease. Hepatology. 2014;59:130–142. doi: 10.1002/hep.26607. [DOI] [PubMed] [Google Scholar]
- 30.Tonan T., Fujimoto K., Qayyum A., Morita Y., Nakashima O., Ono N. CD14 expression and Kupffer cell dysfunction in non-alcoholic steatohepatitis: superparamagnetic iron oxide-magnetic resonance image and pathologic correlation. Journal of Gastroenterology and Hepatology. 2012;27:789–796. doi: 10.1111/j.1440-1746.2011.07057.x. [DOI] [PubMed] [Google Scholar]
- 31.Ogawa Y., Imajo K., Yoneda M., Kessoku T., Tomeno W., Shinohara Y. Soluble CD14 levels reflect liver inflammation in patients with nonalcoholic steatohepatitis. PLoS One. 2013;8:e65211. doi: 10.1371/journal.pone.0065211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tosello-Trampont A.C., Landes S.G., Nguyen V., Novobrantseva T.I., Hahn Y.S. Kuppfer cells trigger nonalcoholic steatohepatitis development in diet-induced mouse model through tumor necrosis factor-α production. Journal of Biological Chemistry. 2012;287:40161–40172. doi: 10.1074/jbc.M112.417014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Day C.P. Genes or environment to determine alcoholic liver disease and non-alcoholic fatty liver disease. Liver International. 2006;26:1021–1028. doi: 10.1111/j.1478-3231.2006.01323.x. [DOI] [PubMed] [Google Scholar]
- 34.Diehl A.M. IV. Nonalcoholic fatty liver disease abnormalities in macrophage function and cytokines. American Journal of Physiology – Gastrointestinal and Liver Physiology. 2002;282:G1–G5. doi: 10.1152/ajpgi.00384.2001. [DOI] [PubMed] [Google Scholar]
- 35.Oeckinghaus A., Hayden M.S., Ghosh S. Crosstalk in NF-[kappa]B signaling pathways. Nature Immunology. 2011;12:695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- 36.Ruland J. Return to homeostasis: downregulation of NF-[kappa]B responses. Nature Immunology. 2011;12:709–714. doi: 10.1038/ni.2055. [DOI] [PubMed] [Google Scholar]
- 37.Cai D., Yuan M., Frantz D.F., Melendez P.A., Hansen L., Lee J. Local and systemic insulin resistance resulting from hepatic activation of IKK-[beta] and NF-[kappa]B. Nature Medicine. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dalmas E., Toubal A., Alzaid F., Blazek K., Eames H.L., Lebozec K. Irf5 deficiency in macrophages promotes beneficial adipose tissue expansion and insulin sensitivity during obesity. Nature Medicine. 2015;21:610–618. doi: 10.1038/nm.3829. [DOI] [PubMed] [Google Scholar]
- 39.Krausgruber T., Blazek K., Smallie T., Alzabin S., Lockstone H., Sahgal N. IRF5 promotes inflammatory macrophage polarization and TH1–TH17 responses. Nature Immunology. 2011;12:231–238. doi: 10.1038/ni.1990. [DOI] [PubMed] [Google Scholar]
- 40.Weiss M., Blazek K., Byrne A.J., Perocheau D.P., Udalova I.A. IRF5 is a specific marker of inflammatory macrophages in vivo. Mediators of Inflammation. 2013;2013:9. doi: 10.1155/2013/245804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alzaid F., Lagadec F., Albuquerque M., Ballaire R., Orliaguet L., Hainault I. IRF5 governs liver macrophage activation that promotes hepatic fibrosis in mice and humans. JCI Insight. 2016;1:e88689. doi: 10.1172/jci.insight.88689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jourdan T., Szanda G., Cinar R., Godlewski G., Holovac D.J., Park J.K. Developmental role of macrophage Cannabinoid-1 receptor signaling in type 2 diabetes. Diabetes. 2017;66:994. doi: 10.2337/db16-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolf A.M., Wolf D., Rumpold H., Enrich B., Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochemical and Biophysical Research Communications. 2004;323:630–635. doi: 10.1016/j.bbrc.2004.08.145. [DOI] [PubMed] [Google Scholar]
- 44.Yamaguchi N., Argueta J.G.M., Masuhiro Y., Kagishita M., Nonaka K., Saito T. Adiponectin inhibits toll-like receptor family-induced signaling. FEBS Letters. 2005;579:6821–6826. doi: 10.1016/j.febslet.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 45.Yokota T., Oritani K., Takahashi I., Ishikawa J., Matsuyama A., Ouchi N. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000;96:1723–1732. [PubMed] [Google Scholar]
- 46.Ohashi K., Parker J.L., Ouchi N., Higuchi A., Vita J.A., Gokce N. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. Journal of Biological Chemistry. 2010;285:6153–6160. doi: 10.1074/jbc.M109.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fukushima J., Kamada Y., Matsumoto H., Yoshida Y., Ezaki H., Takemura T. Adiponectin prevents progression of steatohepatitis in mice by regulating oxidative stress and Kupffer cell phenotype polarization. Hepatology Research. 2009;39:724–738. doi: 10.1111/j.1872-034X.2009.00509.x. [DOI] [PubMed] [Google Scholar]
- 48.Lovren F., Pan Y., Quan A., Szmitko P.E., Singh K.K., Shukla P.C. Adiponectin primes human monocytes into alternative anti-inflammatory M2 macrophages. American Journal of Physiology – Heart and Circulatory Physiology. 2010;299:H656–H663. doi: 10.1152/ajpheart.00115.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tian L., Luo N., Zhu X., Chung B.H., Garvey W.T., Fu Y. Adiponectin-AdipoR1/2-APPL1 signaling axis suppresses human foam cell formation: differential ability of AdipoR1 and AdipoR2 to regulate inflammatory cytokine responses. Atherosclerosis. 2012;221:66–75. doi: 10.1016/j.atherosclerosis.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chinetti G., Zawadski C., Fruchart J.C., Staels B. Expression of adiponectin receptors in human macrophages and regulation by agonists of the nuclear receptors PPARα, PPARγ, and LXR. Biochemical and Biophysical Research Communications. 2004;314:151–158. doi: 10.1016/j.bbrc.2003.12.058. [DOI] [PubMed] [Google Scholar]
- 51.Yamauchi T., Kamon J., Ito Y., Tsuchida A., Yokomizo T., Kita S. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 52.Yamauchi T., Iwabu M., Okada-Iwabu M., Kadowaki T. Adiponectin receptors: a review of their structure, function and how they work. Best Practice and Research Clinical Endocrinology and Metabolism. 2014;28:15–23. doi: 10.1016/j.beem.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 53.Yamauchi T., Nio Y., Maki T., Kobayashi M., Takazawa T., Iwabu M. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nature Medicine. 2007;13:332–339. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 54.Luo N., Chung B.H., Wang X., Klein R.L., Tang C.-K., Garvey W.T. Enhanced adiponectin actions by overexpression of adiponectin receptor 1 in macrophages. Atherosclerosis. 2013;228:124–135. doi: 10.1016/j.atherosclerosis.2013.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pollock J.D., Williams D.A., Gifford M.A.C., Li L.L., Du X., Fisherman J. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nature Genetics. 1995;9:202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- 56.Lambeth J.D. NOX enzymes and the biology of reactive oxygen. Nature Reviews Immunology. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 57.Forman H.J., Torres M. Reactive oxygen species and cell signaling. American Journal of Respiratory and Critical Care Medicine. 2002;166:S4–S8. doi: 10.1164/rccm.2206007. [DOI] [PubMed] [Google Scholar]
- 58.Jay Forman H., Torres M. Redox signaling in macrophages. Molecular Aspects of Medicine. 2001;22:189–216. doi: 10.1016/s0098-2997(01)00010-3. [DOI] [PubMed] [Google Scholar]
- 59.Gostner J.M., Becker K., Fuchs D., Sucher R. Redox regulation of the immune response. Redox Report. 2013;18:88–94. doi: 10.1179/1351000213Y.0000000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Toda C., Diano S. Mitochondrial UCP2 in the central regulation of metabolism. Best Practice and Research Clinical Endocrinology and Metabolism. 2014;28:757–764. doi: 10.1016/j.beem.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 61.Baffy G. Uncoupling protein-2 and non-alcoholic fatty liver disease. Frontiers in Bioscience. 2005;10:2082–2096. doi: 10.2741/1683. [DOI] [PubMed] [Google Scholar]
- 62.Fülöp P., Derdák Z., Sheets A., Sabo E., Berthiaume E.P., Resnick M.B. Lack of UCP2 reduces fas-mediated liver injury in ob/ob mice and reveals importance of cell-specific UCP2 expression. Hepatology. 2006;44:592–601. doi: 10.1002/hep.21310. [DOI] [PubMed] [Google Scholar]
- 63.Arsenijevic D., Onuma H., Pecqueur C., Raimbault S., Manning B.S., Miroux B. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nature Genetics. 2000;26:435–439. doi: 10.1038/82565. [DOI] [PubMed] [Google Scholar]
- 64.Xu H., Hertzel A.V., Steen K.A., Wang Q., Suttles J., Bernlohr D.A. Uncoupling lipid metabolism from inflammation through fatty acid binding protein-dependent expression of UCP2. Molecular and Cellular Biology. 2015;35:1055–1065. doi: 10.1128/MCB.01122-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Basu Ball W., Kar S., Mukherjee M., Chande A.G., Mukhopadhyaya R., Das P.K. Uncoupling protein 2 negatively regulates mitochondrial reactive oxygen species generation and induces phosphatase-mediated anti-inflammatory response in experimental visceral leishmaniasis. The Journal of Immunology. 2011;187:1322–1332. doi: 10.4049/jimmunol.1004237. [DOI] [PubMed] [Google Scholar]
- 66.Xu H., Hertzel A.V., Steen K.A., Bernlohr D.A. Loss of fatty acid binding protein 4/aP2 reduces macrophage inflammation through activation of SIRT3. Molecular Endocrinology. 2016;30:325–334. doi: 10.1210/me.2015-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu J., Batkai S., Pacher P., Harvey-White J., Wagner J.A., Cravatt B.F. Lipopolysaccharide induces anandamide synthesis in macrophages via CD14/MAPK/phosphoinositide 3-kinase/NF-kappaB independently of platelet-activating factor. Journal of Biological Chemistry. 2003;278:45034–45039. doi: 10.1074/jbc.M306062200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.