Abstract
Objective
Fructose consumption has been implicated in the development of obesity and insulin resistance. Emerging evidence shows that fibroblast growth factor 21 (FGF21) has beneficial effects on glucose, lipid, and energy metabolism and may also mediate an adaptive response to fructose ingestion. Fructose acutely stimulates circulating FGF21 consistent with a hormonal response. We aimed to evaluate whether fructose-induced FGF21 secretion is linked to metabolic outcomes in obese humans before and after bariatric surgery-induced weight loss.
Methods
We recruited 40 Roux-en-Y gastric bypass patients and assessed the serum FGF21 response to fructose (75-g fructose tolerance test) and basal and insulin-mediated glucose and lipid fluxes during a 2-step hyperinsulinemic-euglycemic clamp with infusion of [6,6-2H2] glucose and [1,1,2,3,3-2H5] glycerol. Liver biopsies were obtained during bariatric surgery. Nineteen subjects underwent the same assessments at 1-year follow-up.
Results
Serum FGF21 increased 3-fold at 120 min after fructose ingestion and returned to basal levels at 300 min. Neither basal FGF21 nor the fructose-FGF21 response correlated with liver fat content or liver histopathology, but increased levels were associated with elevated endogenous glucose production, increased lipolysis, and peripheral/muscle insulin resistance. At 1-year follow-up, subjects had lost 28 ± 6% of body weight and improved in all metabolic outcomes, but fructose-stimulated FGF21 dynamics did not markedly differ from the pre-surgical state. The association between increased basal and stimulated FGF21 levels with poor metabolic health was no longer present after weight loss.
Conclusions
Fructose ingestion in obese humans stimulates FGF21 secretion, and this response is related to systemic metabolism. Further studies are needed to establish if FGF21 signaling is (patho)physiologically involved in fructose metabolism and metabolic health.
Keywords: Fructose, FGF21, Insulin resistance, Hyperinsulinemic-euglycemic clamp, Obesity, Translational study
Abbreviations: AUC, area under the curve; ChREBP, carbohydrate response element-binding protein; EGP, endogenous glucose production; FFA, free fatty acid; FGF21, fibroblast growth factor 21; GLP1, glucagon-like peptide 1; IQR, interquartile range; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; Ra, rate of appearance; Rd, rate of disappearance; SD, standard deviation
Highlights
-
•
High fructose consumption may contribute to metabolic disease.
-
•
Fructose ingestion acutely stimulates FGF21 secretion in obese humans.
-
•
Higher FGF21 levels are associated with poor metabolic health.
-
•
The FGF21 response to fructose persists after bariatric surgery-induced weight loss.
-
•
The role of FGF21 signaling in fructose metabolism needs further investigation.
1. Introduction
Fructose is the sweetest of all naturally-occurring carbohydrates. It is one of the main monosaccharides in our diet [1], and many humans worldwide consume fructose on a daily basis [2]. Nearly 10% of the energy in an average Western diet comes from fructose [3]. Recently, however, concerns about the consumption of fructose have been raised as high fructose intake may contribute to the current epidemics of obesity and its metabolic complications [4], [5]. Humans commonly consume fructose together with glucose in the form of sucrose or high-fructose corn syrup, and sugar (in general) has evident health implications: its overconsumption is associated with chronic increased energy intake and weight gain [6], [7]. However, several lines of evidence also implicate fructose (specifically) to be a particularly harmful sugar [8]. Firstly, animal models consistently develop obesity and insulin resistance when exposed to high-fructose diets [9], [10], [11]. Secondly, human fructose consumption is epidemiologically linked with weight gain, insulin resistance, and other components of the metabolic syndrome [5], [6], [12], [13], [14]. Thirdly, isocaloric intervention trials (comparing high-fructose diets vs energy-matched control diets) demonstrate that fructose promotes visceral adiposity, de novo lipogenesis, dyslipidemia, and hepatic insulin resistance, and more so than glucose [15], [16], [17].
Some of the worrying observations regarding the adverse health effects of high-fructose exposure may be the result of its unique hepatic metabolism. Glucose ingestion raises systemic blood glucose levels, which results in an appropriate insulin response in order to coordinate systemic glucose handling. Fructose, on the other hand, is almost completely extracted from portal blood upon first-pass through the liver, and its metabolism is therefore mostly hepatic [18]. Hepatic fructolysis rapidly metabolizes fructose into triose phosphates, thereby providing substrate to the glycolysis, glycogenesis, gluconeogenesis, and/or lipogenesis pathways. In addition, carbohydrate metabolites in the liver activate carbohydrate response element-binding protein (ChREBP), a key transcription factor for enzymes in the glycolysis and lipogenesis pathways [19]. Thus, since fructose is preferentially metabolized by the liver, fructose more than glucose acutely raises intrahepatic carbohydrate intermediaries, activating ChREBP and promoting hepatic glycolysis and lipogenesis gene programs [20].
Recent rodent studies also demonstrate that sugar-activated ChREBP can transactivate hepatic expression of fibroblast growth factor 21 (FGF21) [21]. This novel metabolic hormone is synthesized by multiple tissues, including the liver, adipose tissue, and pancreas [22]. However, the liver is the major contributor to serum levels, and FGF21 in the liver also responds to dietary manipulations [23], [24]. It has important beneficial effects on whole-body carbohydrate and lipid metabolism as well as energy balance and body weight [25], [26], [27]. In rodents, FGF21 helps to coordinate the physiological adaptations to fasting and ketogenic diets [23], [28]. In humans, it is not meaningfully regulated by short-term fasting or ketogenic diets [21]. Instead, circulating FGF21 levels rise after short-term high-carbohydrate overfeeding [29]. In fact, the acute ingestion of an oral fructose load leads to an increase in FGF21 secretion and a return to baseline within 4–5 h [30]. This is the only known – to our knowledge – acute hormonal response to fructose ingestion in humans, and this raises the intriguing hypothesis that FGF21 is involved in hepatic fructose metabolism or the whole-body metabolic response to fructose ingestion. Although translational evidence in humans is limited, we have recently demonstrated that, in rodents, the ChREBP-FGF21 axis is essential for physiological hepatic fructose metabolism, including the shuttling of fructose carbon into the lipogenesis pathway [21].
Although pharmacological FGF21 administration to animals has evident beneficial effects [25], [26], [27], elevated circulating FGF21 levels are, surprisingly, associated with poor metabolic health in humans and animals. In fact, FGF21 levels are increased with obesity, insulin resistance, type 2 diabetes, and non-alcoholic fatty liver disease (NAFLD) [31], [32]. Moreover, obese mice display an attenuated signaling response and diminished metabolic improvements upon exogenous FGF21 treatment, indicating that obesity is associated with resistance to FGF21 [33]. It is currently unknown what mechanism is responsible for this phenomenon and also whether it is reversible with weight loss.
Therefore, to investigate the relevance of the fructose-FGF21 paradigm in relation to human metabolic disease, we performed a clinical study designed to evaluate whether fructose-induced FGF21 secretion is related to basal or insulin-mediated metabolic fluxes in obese humans before and after bariatric surgery-induced weight loss.
2. Material and methods
2.1. Design
This multicenter observational intervention study was part of RESOLVE, a European research program on the metabolic syndrome (www.resolve-diabetes.org). The study was designed to evaluate the fructose-FGF21 axis in obese humans before and after bariatric surgery-induced weight loss and its relation to metabolic outcomes. The protocol was approved by the Academic Medical Center medical ethics committee, and all subjects provided written informed consent in accordance with the Declaration of Helsinki. The study was prospectively registered in the Netherlands Trial Registry (www.trialregister.nl: NTR4666).
2.2. Subjects
Forty morbidly obese subjects were recruited from the outpatient clinics of two obesity centers in the Amsterdam metropolitan area. Subjects were eligible to participate if they i) were aged >18 years, ii) met the criteria for bariatric surgery in accordance with current national guidelines [34], iii) were scheduled to undergo laparoscopic Roux-en-Y gastric bypass surgery, and iv) had stable weight (<5% weight change) for at least 3 months prior to the study assessments. Exclusion criteria were i) substance abuse (alcohol >2 units/day, recreational drugs), ii) use of lipid-lowering drugs, exogenous insulin, incretin mimetics, antipsychotics, or antidepressants, iii) childhood-onset obesity, or iv) any somatic disorder except for common obesity-related conditions (for instance, dyslipidemia, hypertension, or obstructive sleep apnea). All subjects completed a medical evaluation including history, physical examination, and blood tests. Body composition was determined by bioelectrical impedance analysis (Maltron BF-906; Rayleigh, UK).
2.3. Fructose challenge
Fructose tolerance tests were performed as described [30]. Briefly, after an overnight fast, subjects ingested an oral dose of 75 g fructose dissolved in 225 ml water. Blood samples were collected regularly for 5 h following the ingestion of fructose.
2.4. Liver fat content
We assessed the percentage of liver volume comprised of fat using proton magnetic resonance spectroscopy as described [35]. This method has high diagnostic accuracy and high precision with low variability for assessment of hepatic steatosis in the context of NAFLD [36].
2.5. Hyperinsulinemic-euglycemic clamp protocol
Basal glucose and lipolysis fluxes and tissue-specific parameters of insulin sensitivity were assessed during a two-step hyperinsulinemic-euglycemic clamp study, which has been described in detail [37], [38]. This experimental protocol allowed us to accurately measure i) the basal rate of endogenous glucose production (EGP), ii) the basal rate of appearance (Ra) of glycerol (reflecting whole-body lipolysis), iii) the insulin-mediated suppression of EGP (reflecting hepatic insulin sensitivity), iv) the insulin-mediated suppression of glycerol Ra (reflecting adipose tissue insulin sensitivity), and v) the insulin-stimulated rate of disappearance (Rd) of glucose (reflecting peripheral/muscle insulin sensitivity).
Briefly, after an overnight fast, subjects received primed continuous infusions of the stable isotope-labeled metabolic tracers [6,6-2H2] glucose and [1,1,2,3,3-2H5] glycerol (>99% enriched; Cambridge Isotopes, Andover, MA, USA). Basal fluxes were determined after 2 h of tracer equilibration. Insulin-mediated suppressions of EGP and glycerol Ra were assessed after 2 h of low-dose (step 1) insulin infusion [Actrapid 20 mU·(m2 body surface area)−1 min−1; Novo Nordisk Farma, Alphen aan de Rijn, The Netherlands]. Insulin-stimulated glucose Rd was assessed after 2 h of high-dose (step 2) insulin infusion (60 mU m−2 min−1). During hyperinsulinemia, plasma glucose was maintained constant at 5.0 mmol/l by frequent bedside monitoring of glucose levels and variable infusion of exogenous glucose (enriched with [6,6-2H2] glucose to approximate plasma enrichment).
2.6. Surgery and liver histology
Subjects underwent scheduled laparoscopic Roux-en-Y gastric bypass surgery 1–6 weeks after baseline study assessments. Laparoscopic subcapsular liver biopsies were taken from the lower left lobe (segment III) by an experienced surgeon at the start of the procedure. Liver histopathology was evaluated by an experienced liver pathologist who was blinded to all subject data and scored in accordance with non-alcoholic steatohepatitis (NASH) Clinical Research Network recommendations [39], [40]. Subjects were instructed to maintain stable weight through consumption of a weight-maintenance diet in the preoperative period.
2.7. Follow-up
Weight loss after gastric bypass surgery is maximal at 1-year follow-up and body weight usually stabilizes at this time [41]. Therefore, we invited subjects to participate in follow-up study assessments 1 year after the bariatric surgery. All baseline study assessments except for the liver biopsy were repeated. Subjects were only eligible for the follow-up study if they had undergone a Roux-en-Y gastric bypass procedure. All subjects in the follow-up study provided additional written informed consent.
2.8. Laboratory analyses
Plasma glucose was determined with the glucose oxidase method using a Biosen C-line plus glucose analyzer (EKF Diagnostics, Barleben/Magdeburg, Germany). Plasma insulin and cortisol were determined by immunoassay on an Immulite 2000 system (Diagnostic Products, Los Angeles, CA, USA), with intra-assay variation of 4–5% and 3–6%, respectively, and inter-assay variation of 5% and 5–7%, respectively. Plasma glucagon was determined by radioimmunoassay (Linco Research, St Charles, MO, United States), with intra-assay variation of 4–8% and inter-assay variation of 6–11%. Plasma free fatty acids (FFAs) were determined by enzymatic colorimetric method (NEFA C test kit; Wako Chemicals, Neuss, Germany), with intra-assay variation of 1% and inter-assay variation of 4–15%. Serum FGF21 was determined by commercially-available enzyme-linked immunosorbent assay (R&D Systems Europe, Abingdon, UK), with intra-assay variation of 3–4% and inter-assay variation of 5–11%. Plasma enrichments of [6,6-2H2] glucose and [1,1,2,3,3-2H5] glycerol (tracer-to-tracee ratios) were determined by gas chromatography-mass spectrometry [42].
2.9. Calculations
Metabolic fluxes (EGP, glycerol Ra, and glucose Rd) were calculated using modified versions of the Steele equations for the steady state (basal fluxes) or non-steady state (during insulin infusion) [43], [44]. Basal EGP and glycerol Ra were expressed as μmol·(fat-free body mass [FFM])−1 min−1 and μmol·(kg total body mass)−1 min−1, respectively. Insulin-mediated effects on EGP, glycerol Ra, and glucose Rd were expressed as percentage relative to basal. Area under the curve (AUC) was calculated by trapezoidal method. The FGF21 response to fructose ingestion was expressed as the serum FGF21 AUC from 0 to 300 min.
2.10. Statistical analyses
Data are presented as count (%), mean ± standard deviation (SD) or median [interquartile range (IQR)], depending on type and distribution. Post-ingestion hormone and metabolite levels were compared to basal levels using two-tailed paired t tests at each time point with Bonferroni correction for multiple comparisons. Correlations were evaluated by linear regression analysis. Within-subject comparisons before vs after bariatric surgery were evaluated by two-tailed paired t or Wilcoxon signed-rank tests, depending on distribution. For before vs after comparisons of post-ingestion hormone and metabolite levels, we used two-tailed paired t tests at each time point with Bonferroni correction for multiple comparisons. Findings were considered significant if p < 0.05. Statistical analyses were performed using IBM SPSS Statistics v23 (Armonk, NY, USA) and GraphPad Prism v6 (La Jolla, CA, USA).
3. Results
3.1. Fructose ingestion acutely stimulated circulating FGF21, glucose, and insulin levels
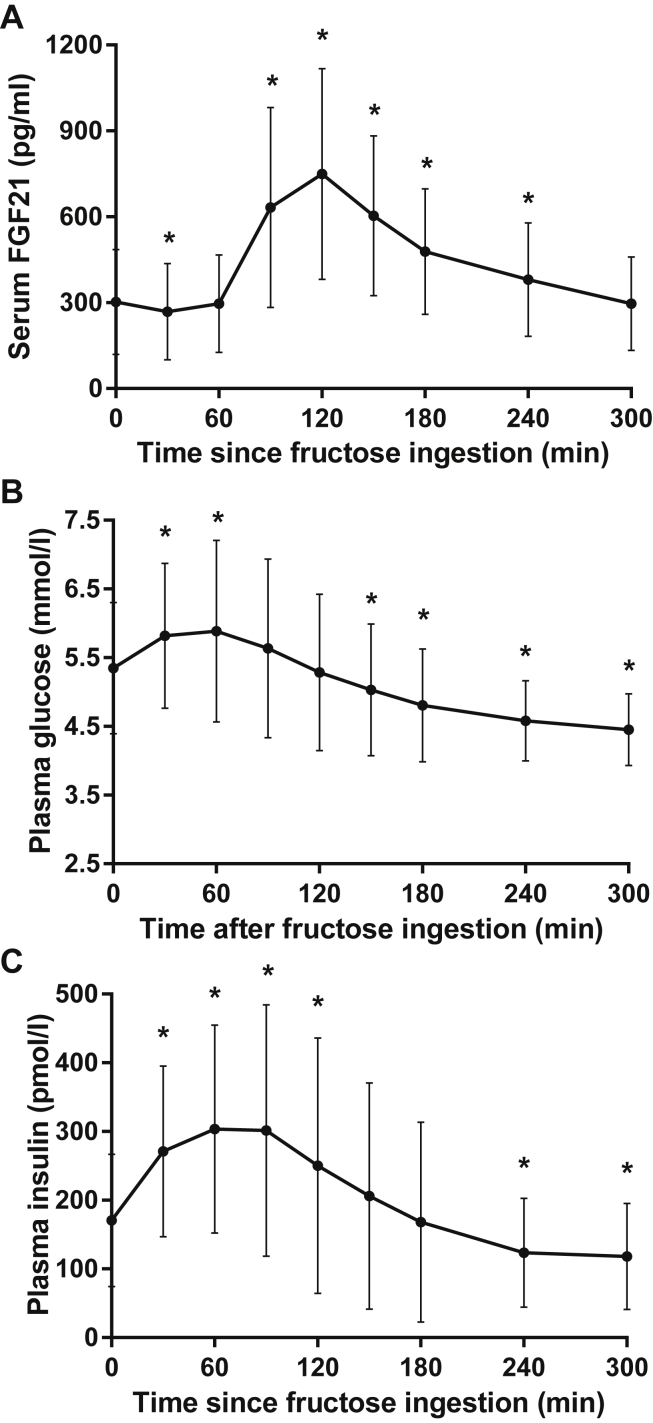
Baseline characteristics of the included subjects prior to bariatric surgery are presented in Table 1. In these obese subjects, we first evaluated the humoral responses to a 75-g oral fructose load. Consistent with previous reports [30], serum FGF21 levels initially decreased by 9 ± 11% at 30 min, then rapidly increased to 311 ± 200% of basal levels at 120 min, and returned to basal levels at 300 min (Figure 1A). Although there was substantial between-subject variation in basal FGF21 levels as well as in the magnitude of the FGF21 response, each subject displayed strongly stimulated FGF21 levels at 90–120 min. Fructose ingestion also stimulated a small and transient increase in plasma glucose levels at 30–60 min (Figure 1B). This was accompanied by a modest increase in plasma insulin levels at 30–120 min (Figure 1C).
Table 1.
Baseline characteristics of included subjects (n = 40).
| Clinical parameters | |
| Male sex (%) | 18 (45) |
| Age (years) | 49 (37–55) |
| Length (cm) | 174 ± 8 |
| Weight (kg) | 131 (112–147) |
| BMI (kg/m2) | 43.4 ± 6.1 |
| Waist circumference (cm) | 133 ± 15 |
| Body fat (%) | 46 ± 6 |
| Liver fat (%) | 9.5 (3.6–17.3) |
| Biochemical parameters | |
| Glucose (mmol/l) | 5.1 ± 0.6 |
| Triglycerides (mmol/l) | 1.18 (0.82–1.70) |
| Total cholesterol (mmol/l) | 4.78 ± 1.03 |
| LDL (mmol/l) | 2.94 ± 0.94 |
| HDL (mmol/l) | 1.21 ± 0.31 |
| CRP (mg/l) | 4.6 (2.0–10.4) |
| ALT (U/l) | 37 ± 24 |
| Liver histology | |
| Steatosis grade | 1 (1–2) |
| NAFLD activity score | 2 (1–3) |
| Global NASH score | 4 (2–4) |
| Hyperinsulinemic-euglycemic clamp | |
| Basal insulin (pmol/l)a | 159 ± 107 |
| Basal EGP (μmol kgFFM−1 min−1) | 13.0 ± 1.7 |
| Basal glycerol Ra (μmol kg−1min−1) | 2.7 ± 1.0 |
| Step 1 insulin (pmol/l)b | 378 ± 113 |
| Step 1 suppression of EGP (% of basal) | 73 ± 13 |
| Step 1 suppression of glycerol Ra (% of basal) | 57 (39–65) |
| Step 2 insulin (pmol/l)c | 881 ± 220 |
| Step 2 stimulation of glucose Rd (% of basal) | 354 (250–483) |
Data are count (%), mean ± SD, or median (IQR).
After an overnight fast.
After 2 h of low-dose insulin infusion.
After 2 h of high-dose insulin infusion.
Figure 1.
Fructose ingestion acutely stimulated (A) serum FGF21, (B) plasma glucose, and (C) plasma insulin levels in treatment-naive obese subjects. Data are mean ± SD (n = 40). *p < 0.05 vs basal with Bonferroni correction.
3.2. Basal and fructose-stimulated FGF21 levels are not related to hepatic steatosis or NASH activity
Fructose is preferentially metabolized by the liver [18], and circulating FGF21 is mostly derived from the liver [45]. Therefore, to evaluate whether variation in circulating FGF21 levels may reflect changes in synthesis/secretion in the context of NAFLD/NASH, we assessed liver fat content by magnetic resonance spectroscopy and histopathological features of NAFLD/NASH in liver biopsies. Liver histology confirmed the radiologic assessment of hepatic steatosis (liver fat content on spectroscopy vs percentage of steatotic hepatocytes on histology: r = 0.64, p < 0.001). Neither basal FGF21 nor the FGF21 AUC after fructose ingestion correlated with liver fat content (r = 0.26, p = 0.131 and r = 0.17, p = 0.337, respectively). In addition, although only seven subjects had global NASH scores ≥5 (histology indicative of NASH [39]), these subjects did not present with differences in basal FGF21 (310 ± 108 vs 311 ± 205 pg/ml, p = 0.991) nor FGF21 AUC (1.4 ± 0.5 vs 1.3 ± 0.6 pg/ml min 105, p = 0.746) compared to subjects with lower NASH scores.
3.3. Elevated basal and fructose-stimulated FGF21 levels are associated with parameters of metabolic disease
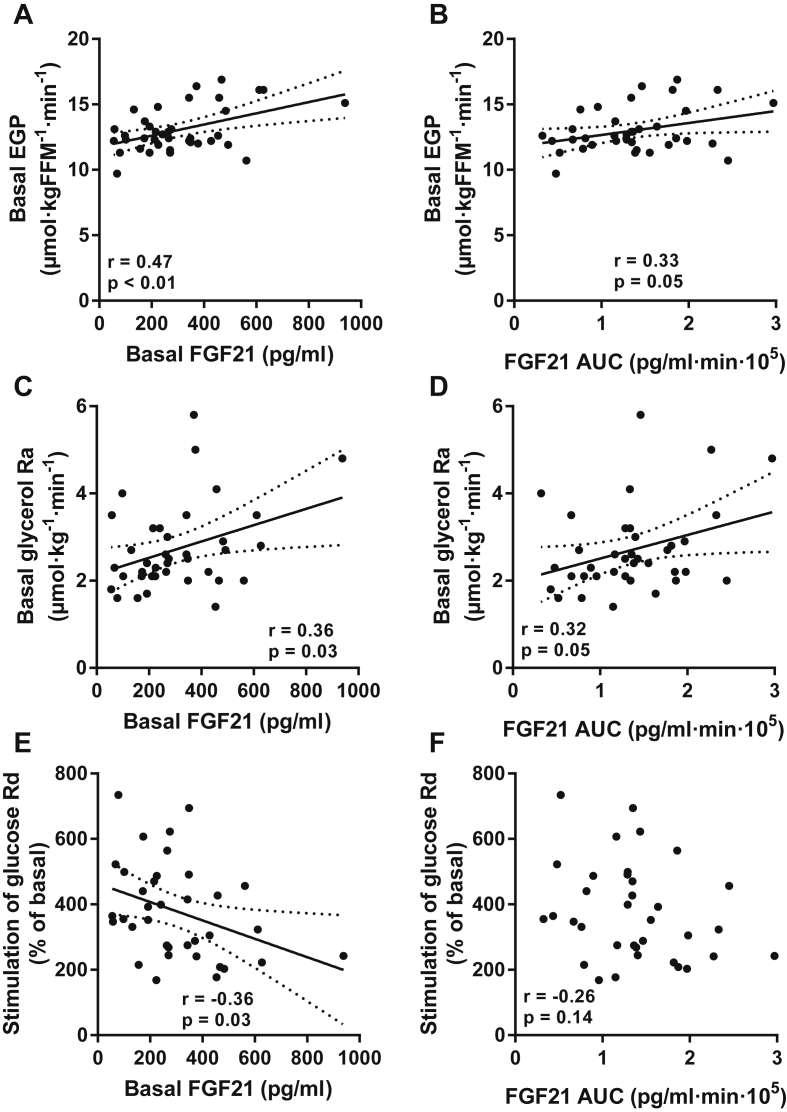
To determine whether FGF21 dynamics relate to basal metabolic fluxes or parameters of insulin action, we measured these fluxes using the glucose clamp technique and infusions of stable isotope-labeled metabolic tracers. Both basal FGF21 and FGF21 AUC correlated positively with basal EGP (Figure 2A–B) and basal glycerol Ra (Figure 2C–D). Basal FGF21, but not the FGF21 AUC, correlated negatively with insulin-stimulated glucose Rd (Figure 2E–F). In these subjects, circulating FGF21 levels did not correlate with insulin suppression of EGP or insulin suppression of glycerol Ra (not shown). These results indicate that higher basal and stimulated FGF21 levels are associated with distinct features of metabolic disease in obese humans, specifically with elevated basal EGP, increased basal lipolysis, and peripheral insulin resistance.
Figure 2.
Correlations between basal (left panels) or fructose-stimulated (right panels) serum FGF21 levels and (A–B) basal EGP, (C–D) basal lipolysis, or (E–F) peripheral insulin sensitivity. Lines are best fit (solid) and 95% CI (between dashed lines).
3.4. Bariatric surgery-induced weight loss does not affect basal FGF21 and the FGF21 response to fructose
Thirty-six (out of 40 included) subjects were eligible to participate in the follow-up study: one subject did not have surgery and three subjects had sleeve gastrectomy (instead of the planned gastric bypass surgery). Seventeen (out of 36 eligible) subjects declined follow-up participation. Therefore, a total of 19 subjects completed follow-up at 1 year. In these subjects, bariatric surgery was associated with improvements in body weight, adiposity, hepatic steatosis, and clinical biochemistry (Table 2). In addition, and consistent with previous reports [46], all clamp-derived parameters of glucose metabolism and insulin action, aside from basal EGP, were markedly improved at 1 year (Table 2), indicating increased insulin sensitivity in all tissues.
Table 2.
Clinical and metabolic characteristics of subjects before and after bariatric surgery (n = 19).
| Before surgery | After surgery (364 ± 18 days) | p | |
|---|---|---|---|
| Clinical parameters | |||
| Weight (kg) | 126 (110–145) | 91 (83–100) | <0.001 |
| BMI (kg/m2) | 43.5 ± 6.9 | 31.5 ± 5.4 | <0.001 |
| Waist circumference (cm) | 132 ± 16 | 104 ± 14 | <0.001 |
| Body fat (%) | 47 ± 7 | 33 ± 10 | <0.001 |
| Liver fat (%) | 8.6 (3.0–21.6) | 3.2 (2.0–5.0) | 0.023 |
| Biochemical parameters | |||
| Glucose (mmol/l) | 4.9 ± 0.5 | 4.5 ± 0.2 | 0.001 |
| Triglycerides (mmol/l) | 1.05 (0.78–1.68) | 0.68 (0.54–0.98) | <0.001 |
| Total cholesterol (mmol/l) | 4.98 ± 0.83 | 4.17 ± 0.82 | <0.001 |
| LDL (mmol/l) | 3.13 ± 0.69 | 2.2 ± 0.68 | <0.001 |
| HDL (mmol/l) | 1.30 ± 0.27 | 1.58 ± 0.44 | <0.001 |
| CRP (mg/l) | 8.7 (2.9–10.4) | 1.0 (0.6–2.8) | <0.001 |
| ALT (U/l) | 33 ± 18 | 20 ± 4 | 0.010 |
| Clamp, basala | |||
| Insulin (pmol/l) | 142 ± 79 | 40 ± 21 | <0.001 |
| Glucagon (ng/l) | 99 (77–120) | 68 (55–87) | 0.001 |
| Cortisol (nmol/l) | 179 ± 51 | 184 ± 55 | 0.812 |
| FFA (mmol/l) | 0.75 (0.49–0.83) | 0.65 (0.52–0.75) | 0.064 |
| EGP (μmol kgFFM−1 min−1) | 12.7 ± 1.3 | 12.0 ± 1.7 | 0.151 |
| Glycerol Ra (μmol kg−1 min−1) | 2.7 ± 0.9 | 3.9 ± 1.3 | 0.002 |
| Clamp, step1b | |||
| Insulin (pmol/l) | 377 ± 117 | 279 ± 56 | 0.003 |
| Glucagon (ng/l) | 90 ± 18 | 59 ± 15 | <0.001 |
| Cortisol (nmol/l) | 212 ± 77 | 188 ± 45 | 0.233 |
| FFA (mmol/l) | 0.13 ± 0.09 | 0.03 ± 0.02 | <0.001 |
| Suppression of EGP (% of basal) | 75 ± 16 | 94 ± 18 | 0.006 |
| Suppression of glycerol Ra (% of basal) | 57 (39–65) | 80 (72–88) | <0.001 |
| Clamp, step2c | |||
| Insulin (pmol/l) | 890 ± 186 | 666 ± 117 | <0.001 |
| Glucagon (ng/l) | 78 ± 22 | 52 ± 12 | <0.001 |
| Cortisol (nmol/l) | 162 (137–214) | 149 (124–172) | 0.080 |
| FFA (mmol/l) | 0.02 (0.01–0.05) | 0.01 (0.01–0.01) | 0.003 |
| Stimulation of glucose Rd (% of basal) | 355 (271–527) | 590 (486–649) | 0.004 |
Data are mean ± SD or median (IQR) and compared by 2-tailed paired t or Wilcoxon signed-rank tests, respectively.
After an overnight fast.
After 2 h of low-dose insulin infusion.
After 2 h of high-dose insulin infusion.
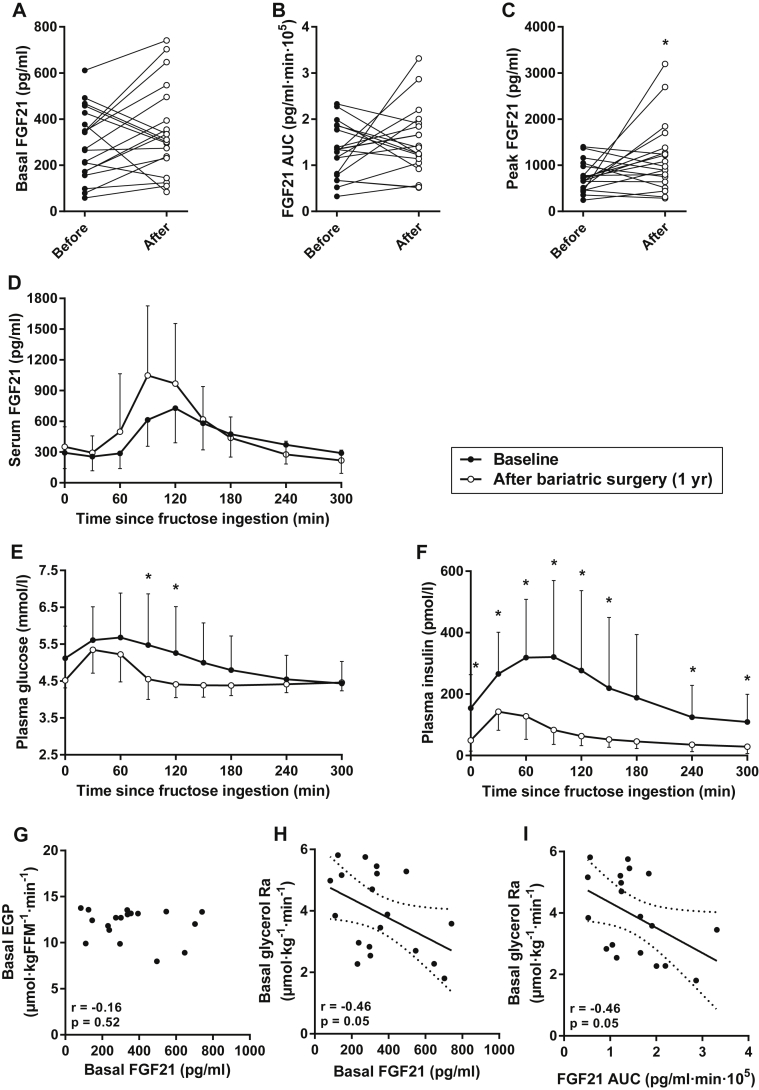
Large intra-individual differences in serum FGF21 were observed before and after bariatric surgery-induced weight loss, but weight loss did not produce a consistent directional effect on their basal FGF21 levels or the FGF21 AUC after fructose (Figure 3A–B). However, peak fructose-stimulated FGF21 levels were slightly higher in subjects after bariatric surgery (Figure 3C). Post-ingestion FGF21 tended to peak earlier [that is, at 90 min instead of 120 min (Figure 3D)]. In addition, bariatric surgery-induced weight loss was associated with reduced glucose and insulin levels after fasting and following fructose ingestion (Figure 3E–F). There were no hypoglycemic events after fructose ingestion.
Figure 3.
The effect of bariatric surgery-induced weight loss on (A–D) FGF21 dynamics, (E) glucose, and (F) insulin following fructose ingestion. (G) Basal FGF21 and EGP did not correlate in post-bariatric subjects. (H–I) Basal and fructose-stimulated serum FGF21 levels correlated negatively with the rate of lipolysis at 1-year follow-up in post-bariatric subjects. (D–F) Data are mean ± SD (n = 19). (H–I) Lines are best fit (solid) and 95% CI (between dashed lines). *p < 0.05 for before vs after with Bonferroni correction.
In obese subjects prior to bariatric surgery, the strongest correlation we observed was between basal FGF21 and EGP (Figure 2A). We also observed a strong correlation between basal FGF21 and EGP when we restricted the pre-bariatric analysis to the 19 subjects who were followed up (r = 0.51, p = 0.026), whereas basal FGF21 and EGP did not correlate in these 19 subjects at 1-year follow-up (Figure 3G). At this time, surprisingly, both basal and fructose-stimulated FGF21 levels were negatively correlated with basal glycerol Ra (Figure 3H–I). In contrast to our results in obese subjects prior to bariatric surgery, these observations indicate that higher basal and post-ingestion FGF21 levels are associated with lower rates of lipolysis in post-bariatric subjects. This was also confirmed by multiple linear regression analyses, including insulin and/or parameters of adiposity (BMI, waist circumference, or body fat content) as covariates, where FGF21 was an independent (and inverse) predictor of basal glycerol Ra. Post-bariatric FGF21 dynamics did not correlate with other metabolic parameters (not shown), although this follow-up study was not powered to detect weak correlations.
4. Discussion
We here demonstrate a relationship between fructose-FGF21 dynamics and human metabolism. Firstly, we confirm that fructose ingestion acutely stimulates FGF21 secretion in morbidly obese humans, thereby extending the potential of FGF21 as a marker of fructose metabolism to this high-risk population. We previously reported that the FGF21 response to a 75-g oral fructose dose varied widely among healthy humans and that excursions were greater in overweight subjects [30]. In the present study, we also observed substantial variation in FGF21 dynamics between obese subjects. Moreover, this variation could not be explained by differences in liver fat content or histopathological features of NAFLD/NASH in liver biopsies. Therefore, instead, the variation may reflect individual differences in intestinal fructose absorption [47], differences in sensitivity of the ChREBP-FGF21 axis to intrahepatic fructose metabolites [21], or differences in sensitivity to paracrine or endocrine FGF21 actions [33]. Further studies may elucidate whether FGF21 signaling is physiologically involved in human fructose metabolism and metabolic health, as is the case for rodents [21].
Secondly, basal and fructose-induced FGF21 levels correlated with several features of metabolic disease in treatment-naive morbidly obese subjects: increased levels were associated with elevated hepatic glucose production, increased whole-body lipolysis, and peripheral insulin resistance. Although these results are consistent with the possibility that FGF21 causally contributes to fructose-mediated metabolic pathology, that interpretation is not in agreement with emerging preclinical evidence. This includes consistently beneficial effects of treatment with recombinant human FGF21 in animal models of obesity, diabetes, and NAFLD [25], [26], [27]. Therefore, in line with the phenomenon of FGF21 resistance in murine obesity [33], these results possibly reflect epiphenomenal associations between obesity-related FGF21 resistance and obesity-related insulin resistance/metabolic syndrome. In addition, we have recently identified the ChREBP-FGF21 as an essential signaling axis in hepatic fructose physiology [21]. Following this paradigm, humans with FGF21 resistance would have to compensate by increasing FGF21 secretion in order to metabolize ingested fructose.
Thirdly, we demonstrate that fructose-FGF21 responsiveness is conserved in obese subjects after bariatric surgery. At 1-year follow-up, subjects had lost 28 ± 6% of their starting body weight and presented with substantial improvements in most measured metabolic parameters. In fact, post-bariatric subjects were metabolically healthy with regards to both hepatic steatosis [48] and insulin sensitivity [37]. Nevertheless, neither basal FGF21 levels nor the serum FGF21 response to fructose was markedly different from the pre-bariatric state, and the associations between FGF21 levels and indices of poor metabolic health were no longer present at this time. Thus, post-bariatric patients are characterized by persistently high fructose-FGF21 excursions in light of markedly reduced body weight and the resolution of hepatic steatosis and other metabolic demise. One possible explanation for this observation is persistent FGF21 resistance, because the subjects' post-bariatric body mass index (BMI) was still in the overweight/obese range. To summarize, although the clinical relevance of these findings remains to be determined, they indicate that i) high serum FGF21 levels are not the result of fatty liver, as has been suggested [24], and ii) the fructose-FGF21 axis, which is likely mediated by hepatic ChREBP activation [21], is conserved in obese patients after bariatric weight loss. It will now be of interest to determine whether persistently elevated FGF21 levels in post-bariatric patients reflect sustained (or irreversible) obesity-related FGF21 resistance.
Several factors may have contributed to the altered fructose-stimulated plasma glucose and insulin dynamics after bariatric surgery-induced weight loss. Ingestion of fructose raises blood glucose, in part because it provides gluconeogenic substrate and induces the expression of hepatic gluconeogenic genes [8], [20]. In healthy subjects, 29–54% of an oral fructose dose is converted to glucose within 3–6 h after ingestion [49], but gluconeogenesis from fructose is decreased in Roux-en-Y gastric bypass patients [50]. This suggests that fructose carbon may instead be diverted into other metabolic pathways, such as lipogenesis or lactate production [51]. Moreover, although the initial increment in plasma glucose at 30–60 min was comparable, subsequent dynamics suggest that glucose clearance is particularly increased after bariatric surgery-induced weight loss. Glucose clearance is primarily dependent on insulin stimulation of peripheral glucose Rd [52], and our clamp results also demonstrate greatly improved peripheral insulin sensitivity at 1-year follow-up. In accordance, the magnitude of the insulin response to fructose ingestion was greatly reduced after surgery. Note that FGF21, glucose, and insulin levels peaked earlier in post-bariatric patients. This is consistent with the known increased rate of nutrient absorption and rapidity of insulin secretion after gastric bypass surgery [50], [53], [54]. The time frame in which insulin is released also suggests that another (non-glycemic) signal, such as fructose-induced incretin secretion [55], mediates part of the insulin response. In fact, fructose ingestion stimulated prolonged glucagon-like peptide 1 (GLP1) release in humans [56], and liraglutide administration to mice increased hepatic and circulating FGF21 levels [57], [58], suggesting that GLP1 may be involved in the FGF21 response to fructose. If that is the case, then further studies may also evaluate how these hormones are related in individuals after bariatric surgery, who are characterized by increased postprandial GLP1 release [59], but no marked changes in FGF21 dynamics.
One particularly interesting observation is that basal FGF21 levels strongly correlated with basal glucose production in treatment-naive obese subjects, but not in post-bariatric surgery subjects. We have recently demonstrated that, in the setting of high-fructose feeding to rodents, activation of ChREBP is a major determinant of basal glucose production independently of hepatic insulin signaling [20]. As hepatic ChREBP activity is increased in obese subjects [60] and ChREBP appears to be a major regulator of circulating FGF21 [21], the strong correlation between basal glucose production and FGF21 in pre-treatment obese subjects may be driven by ChREBP. In contrast, after bariatric surgery-induced weight loss, we would expect hepatic ChREBP activity to decline. Thus, it may no longer be a major driver of either basal glucose production or FGF21, leading to the loss of correlation between these two parameters.
Another finding that raises an interesting hypothesis is the observation that basal and stimulated FGF21 levels correlated positively with basal lipolysis in treatment-naive obese subjects and negatively in post-bariatric subjects. However, FGF21 is known to have pleiotropic effects on adipose tissue function [61], and our results suggest that the actions of FGF21 on adipose tissue may be dependent on the metabolic state. This may seem surprising, but the possibility that FGF21 has distinct effects in different metabolic states has previously been suggested [24], [62]. Potentially consistent with this, it has been reported that exogenous FGF21 suppresses adipose tissue lipolysis (which reduces FFA release) in some [63], [64], but not in all studies [65].
The acute increase in circulating FGF21 following fructose ingestion suggests that FGF21 may mediate whole-body homeostatic actions in response to a nutritional challenge. Notably, fructose ingestion also suppresses plasma FFA levels in healthy humans, suggesting inhibition of adipose tissue lipolysis, but the signaling mechanism is unknown [66]. Since very little absorbed fructose enters the systemic circulation after first-pass through the liver [18], [67] and the circulating insulin response to ingested fructose is relatively small [30], it is likely that another unknown signal mediates this antilipolytic effect of fructose. Further studies are needed to establish if fructose-induced FGF21 secretion might participate in this. Nevertheless, our results are consistent with the hypothesis that treatment-naive obese subjects are resistant to FGF21's antilipolytic action, whereas the link between FGF21 and lipolysis may be restored in post-bariatric subjects.
We acknowledge that the observational design of our study does not allow us to make causal conclusions regarding the effects of FGF21. In this regard, we highlight that fructose tolerance tests and hyperinsulinemic-euglycemic clamp studies reflect vastly different metabolic states. The observed associations between results from these discrete states do not imply a direct relationship. In addition, gastric bypass surgery is designed so nutrients bypass the duodenum and proximal jejunum [68]. Although the intestinal fructose transporter GLUT5 is strongly expressed in the distal small intestine [69], we cannot rule out that fructose absorption was enhanced or reduced after surgery. We also note that plasma insulin concentrations during step 1 and 2 of the clamp were lower after bariatric surgery, likely due to increased insulin clearance after surgery [70]. Our results, however, did not differ when all analyses were repeated using insulin-corrected fluxes.
5. Conclusions
We show that fructose ingestion stimulates FGF21 secretion in an endocrine pattern in morbidly obese humans. This fructose-FGF21 responsiveness is exaggerated in subjects with poor metabolic health as reflected by the associations with elevated EGP, increased lipolysis, and insulin resistance. Finally, we demonstrate that the FGF21 response to fructose persists in post-bariatric subjects. This work adds to the growing body of evidence that links fructose ingestion to FGF21 signaling and systemic metabolism. Further studies are needed to establish if FGF21 signaling is physiologically involved in fructose metabolism and metabolic health.
Acknowledgements
We thank Dr. Aart J. Nederveen (Department of Radiology, Academic Medical Center, Amsterdam, The Netherlands) for assistance with magnetic resonance spectroscopy experiments. The authors declare no conflicts of interest related to this work. K.W.t.H. and P.W.G. contributed equally to data acquisition and analysis, discussions about the results, and preparation of the manuscript. A.D. and B.A.v.W. performed surgeries. M.T.A. was responsible for laboratory analyses. J.V. assessed liver histopathology. J.A.R., M.N., E.M.F., and M.A.H. contributed to discussions about the results. M.J.S. designed and supervised the study and has final responsibility. All authors critically reviewed and approved the final manuscript. This study was funded in part by a grant from the EU (FP7-EU 305707) to M.J.S. M.N. is supported by grants VIDI 2013 (016.146.327) and CVON 2012 young talent (IN-CONTROL). M.A.H. is supported by a grant from the NIH (R01DK100425).
Conflict of interest/author agreement statement
-
•
The final manuscript has been read and approved for submission by all authors.
-
•
We confirm that the manuscript has not been published or is under consideration for publication elsewhere.
-
•
We also acknowledge that no specific grant from any funding agency in the public, commercial or not-for-profit sectors was received for this study.
-
•
The authors have no conflict of interest to disclose.
-
•
We further confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
References
- 1.Hanover L.M., White J.S. Manufacturing, composition, and applications of fructose. American Journal of Clinical Nutrition. 1993;58:724s–732s. doi: 10.1093/ajcn/58.5.724S. [DOI] [PubMed] [Google Scholar]
- 2.Tappy L., Le K.A. Metabolic effects of fructose and the worldwide increase in obesity. Physiological Reviews. 2010;90:23–46. doi: 10.1152/physrev.00019.2009. [DOI] [PubMed] [Google Scholar]
- 3.Marriott B.P., Cole N., Lee E. National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. Journal of Nutrition. 2009;139:1228s–1235s. doi: 10.3945/jn.108.098277. [DOI] [PubMed] [Google Scholar]
- 4.Lustig R.H., Schmidt L.A., Brindis C.D. Public health: the toxic truth about sugar. Nature. 2012;482:27–29. doi: 10.1038/482027a. [DOI] [PubMed] [Google Scholar]
- 5.Bray G.A. Soft drink consumption and obesity: it is all about fructose. Current Opinion in Lipidology. 2010;21:51–57. doi: 10.1097/MOL.0b013e3283346ca2. [DOI] [PubMed] [Google Scholar]
- 6.Bray G.A., Nielsen S.J., Popkin B.M. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. American Journal of Clinical Nutrition. 2004;79:537–543. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- 7.Tappy L., Le K.A. Health effects of fructose and fructose-containing caloric sweeteners: where do we stand 10 years after the initial whistle blowings? Current Diabetes Reports. 2015;15:627. doi: 10.1007/s11892-015-0627-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herman M.A., Samuel V.T. The sweet path to metabolic demise: fructose and lipid synthesis. Trends in Endocrinology and Metabolism. 2016;27:719–730. doi: 10.1016/j.tem.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bremer A.A., Stanhope K.L., Graham J.L., Cummings B.P., Wang W., Saville B.R. Fructose-fed rhesus monkeys: a nonhuman primate model of insulin resistance, metabolic syndrome, and type 2 diabetes. Clinical and Translation Science. 2011;4:243–252. doi: 10.1111/j.1752-8062.2011.00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez F.J., Rizza R.A., Romero J.C. High-fructose feeding elicits insulin resistance, hyperinsulinism, and hypertension in normal mongrel dogs. Hypertension. 1994;23:456–463. doi: 10.1161/01.hyp.23.4.456. [DOI] [PubMed] [Google Scholar]
- 11.Tran L.T., Yuen V.G., McNeill J.H. The fructose-fed rat: a review on the mechanisms of fructose-induced insulin resistance and hypertension. Molecular and Cellular Biochemistry. 2009;332:145–159. doi: 10.1007/s11010-009-0184-4. [DOI] [PubMed] [Google Scholar]
- 12.Malik V.S., Popkin B.M., Bray G.A., Després J.P., Hu F.B. Sugar sweetened beverages, obesity, type 2 diabetes and cardiovascular disease risk. Circulation. 2010;121:1356–1364. doi: 10.1161/CIRCULATIONAHA.109.876185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu T., Giovannucci E., Pischon T., Hankinson S.E., Ma J., Rifai N. Fructose, glycemic load, and quantity and quality of carbohydrate in relation to plasma C-peptide concentrations in us women. American Journal of Clinical Nutrition. 2004;80:1043–1049. doi: 10.1093/ajcn/80.4.1043. [DOI] [PubMed] [Google Scholar]
- 14.Kelishadi R., Mansourian M., Heidari-Beni M. Association of fructose consumption and components of metabolic syndrome in human studies: a systematic review and meta-analysis. Nutrition. 2014;30:503–510. doi: 10.1016/j.nut.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 15.ter Horst K.W., Schene M.R., Holman R., Romijn J.A., Serlie M.J. Effect of fructose consumption on insulin sensitivity in nondiabetic subjects: a systematic review and meta-analysis of diet-intervention trials. American Journal of Clinical Nutrition. 2016;104:1562–1576. doi: 10.3945/ajcn.116.137786. [DOI] [PubMed] [Google Scholar]
- 16.Schwarz J.M., Noworolski S.M., Wen M.J., Dyachenko A., Prior J.L., Weinberg M.E. Effect of a high-fructose weight-maintaining diet on lipogenesis and liver fat. Journal of Clinical Endocrinology & Metabolism. 2015;100:2434–2442. doi: 10.1210/jc.2014-3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stanhope K.L., Schwarz J.M., Keim N.L., Griffen S.C., Bremer A.A., Graham J.L. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. Journal of Clinical Investigation. 2009;119:1322–1334. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayes P.A. Intermediary metabolism of fructose. American Journal of Clinical Nutrition. 1993;58:754s–765s. doi: 10.1093/ajcn/58.5.754S. [DOI] [PubMed] [Google Scholar]
- 19.Iizuka K., Miller B., Uyeda K. Deficiency of carbohydrate-activated transcription factor ChREBP prevents obesity and improves plasma glucose control in leptin-deficient (ob/ob) mice. American Journal of Physiology. Endocrinology and Metabolism. 2006;291:E358–E364. doi: 10.1152/ajpendo.00027.2006. [DOI] [PubMed] [Google Scholar]
- 20.Kim M.S., Krawczyk S.A., Doridot L., Fowler A.J., Wang J.X., Trauger S.A. ChREBP regulates fructose-induced glucose production independently of insulin signaling. Journal of Clinical Investigation. 2016;126:4372–4386. doi: 10.1172/JCI81993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fisher F.M., Kim M., Doridot L., Cunniff J.C., Parker T.S., Levine D.M. A critical role for ChREBP-mediated FGF21 secretion in hepatic fructose metabolism. Molecular Metabolism. 2017;6:14–21. doi: 10.1016/j.molmet.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shenoy V.K., Beaver K.M., Fisher F.M., Singhal G., Dushay J.R., Maratos-Flier E. Elevated serum fibroblast growth factor 21 in humans with acute pancreatitis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0164351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Badman M.K., Pissios P., Kennedy A.R., Koukos G., Flier J.S., Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metabolism. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Fisher F.M., Maratos-Flier E. Understanding the physiology of FGF21. Annual Review of Physiology. 2016;78:223–241. doi: 10.1146/annurev-physiol-021115-105339. [DOI] [PubMed] [Google Scholar]
- 25.Kharitonenkov A., Shiyanova T.L., Koester A., Ford A.M., Micanovic R., Galbreath E.J. FGF-21 as a novel metabolic regulator. Journal of Clinical Investigation. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu J., Lloyd D.J., Hale C., Stanislaus S., Chen M., Sivits G. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58:250–259. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kharitonenkov A., Wroblewski V.J., Koester A., Chen Y.F., Clutinger C.K., Tigno X.T. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology. 2007;148:774–781. doi: 10.1210/en.2006-1168. [DOI] [PubMed] [Google Scholar]
- 28.Potthoff M.J., Inagaki T., Satapati S., Ding X., He T., Goetz R. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:10853–10858. doi: 10.1073/pnas.0904187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lundsgaard A.M., Fritzen A.M., Sjoberg K.A., Myrmel L.S., Madsen L., Wojtaszewski J.F. Circulating FGF21 in humans is potently induced by short term overfeeding of carbohydrates. Molecular Metabolism. 2017;6:22–29. doi: 10.1016/j.molmet.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dushay J., Toschi E., Mitten E.K., Fisher F.M., Herman M.A., Maratos-Flier E. Fructose ingestion acutely stimulates circulating FGF21 levels in humans. Molecular Metabolism. 2014;4:51–57. doi: 10.1016/j.molmet.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X., Yeung D.C., Karpisek M., Stejskal D., Zhou Z.G., Liu F. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes. 2008;57:1246–1253. doi: 10.2337/db07-1476. [DOI] [PubMed] [Google Scholar]
- 32.Dushay J., Chui P.C., Gopalakrishnan G.S., Varela-Rey M., Crawley M., Fisher F.M. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology. 2010;139:456–463. doi: 10.1053/j.gastro.2010.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fisher F.M., Chui P.C., Antonellis P.J., Bina H.A., Kharitonenkov A., Flier J.S. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes. 2010;59:2781–2789. doi: 10.2337/db10-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verdam F.J., de Jonge C., Greve J.W. [Practice guideline for the treatment of morbid obesity] Nederlands Tijdschrift voor Geneeskunde. 2012;156:A4630. [PubMed] [Google Scholar]
- 35.van der Valk F., Hassing C., Visser M., Thakkar P., Mohanan A., Pathak K. The effect of a diiodothyronine mimetic on insulin sensitivity in male cardiometabolic patients: a double-blind randomized controlled trial. PLoS One. 2014;9 doi: 10.1371/journal.pone.0086890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dulai P.S., Sirlin C.B., Loomba R. MRI and MRE for non-invasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: clinical trials to clinical practice. Journal of Hepatology. 2016;65:1006–1016. doi: 10.1016/j.jhep.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.ter Horst K.W., Gilijamse P.W., Koopman K.E., de Weijer B.A., Brands M., Kootte R.S. Insulin resistance in obesity can be reliably identified from fasting plasma insulin. International Journal of Obesity. 2015;39:1703–1709. doi: 10.1038/ijo.2015.125. [DOI] [PubMed] [Google Scholar]
- 38.de Weijer B.A., Aarts E., Janssen I.M., Berends F.J., van de Laar A., Kaasjager K. Hepatic and peripheral insulin sensitivity do not improve 2 weeks after bariatric surgery. Obesity. 2013;21:1143–1147. doi: 10.1002/oby.20220. [DOI] [PubMed] [Google Scholar]
- 39.Kleiner D.E., Brunt E.M., van Natta M., Behling C., Contos M.J., Cummings O.W. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 40.Brunt E.M., Kleiner D.E., Wilson L.A., Belt P., Neuschwander-Tetri B.A. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology. 2011;53:810–820. doi: 10.1002/hep.24127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sjostrom L., Narbro K., Sjostrom C.D., Karason K., Larsson B., Wedel H. Effects of bariatric surgery on mortality in Swedish obese subjects. New England Journal of Medicine. 2007;357:741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 42.Ackermans M.T., Pereira Arias A.M., Bisschop P.H., Endert E., Sauerwein H.P., Romijn J.A. The quantification of gluconeogenesis in healthy men by (2)H2O and [2-(13)C] glycerol yields different results: rates of gluconeogenesis in healthy men measured with (2) H2O are higher than those measured with [2-(13)C] glycerol. Journal of Clinical Endocrinology &Metabolism. 2001;86:2220–2226. doi: 10.1210/jcem.86.5.7383. [DOI] [PubMed] [Google Scholar]
- 43.Finegood D.T., Bergman R.N., Vranic M. Estimation of endogenous glucose production during hyperinsulinemic-euglycemic glucose clamps. Comparison of unlabeled and labeled exogenous glucose infusates. Diabetes. 1987;36:914–924. doi: 10.2337/diab.36.8.914. [DOI] [PubMed] [Google Scholar]
- 44.Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Annals of the New York Academy of Sciences. 1959;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- 45.Markan K.R., Naber M.C., Ameka M.K., Anderegg M.D., Mangelsdorf D.J., Kliewer S.A. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes. 2014;63:4057–4063. doi: 10.2337/db14-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rubino F. Bariatric surgery: effects on glucose homeostasis. Current Opinion in Clinical Nutrition and Metabolic Care. 2006;9:497–507. doi: 10.1097/01.mco.0000232914.14978.c5. [DOI] [PubMed] [Google Scholar]
- 47.Gibson P.R., Newnham E., Barrett J.S., Shepherd S.J., Muir J.G. Review article: fructose malabsorption and the bigger picture. Alimentary Pharmacology & Therapeutics. 2007;25:349–363. doi: 10.1111/j.1365-2036.2006.03186.x. [DOI] [PubMed] [Google Scholar]
- 48.Szczepaniak L.S., Nurenberg P., Leonard D., Browning J.D., Reingold J.S., Grundy S. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. American Journal of Physiology – Endocrinology and Metabolism. 2005;288:E462–E468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 49.Sun S.Z., Empie M.W. Fructose metabolism in humans – what isotopic tracer studies tell us. Nutrition & Metabolism. 2012;9:89. doi: 10.1186/1743-7075-9-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Surowska A., De Giorgi S., Theytaz F., Campos V., Hodson L., Stefanoni N. Effects of Roux-en-Y gastric bypass surgery on postprandial fructose metabolism. Obesity. 2016;24:589–596. doi: 10.1002/oby.21410. [DOI] [PubMed] [Google Scholar]
- 51.Teff K.L., Grudziak J., Townsend R.R., Dunn T.N., Grant R.W., Adams S.H. Endocrine and metabolic effects of consuming fructose- and glucose-sweetened beverages with meals in obese men and women: influence of insulin resistance on plasma triglyceride responses. Journal of Clinical Endocrinology & Metabolism. 2009;94:1562–1569. doi: 10.1210/jc.2008-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abdul-Ghani M.A., DeFronzo R.A. Pathogenesis of insulin resistance in skeletal muscle. Journal of Biomedical & Biotechnology. 2010;2010:476279. doi: 10.1155/2010/476279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jacobsen S.H., Bojsen-Moller K.N., Dirksen C., Jorgensen N.B., Clausen T.R., Wulff B.S. Effects of gastric bypass surgery on glucose absorption and metabolism during a mixed meal in glucose-tolerant individuals. Diabetologia. 2013;56:2250–2254. doi: 10.1007/s00125-013-3003-0. [DOI] [PubMed] [Google Scholar]
- 54.Andrade H.F., Pedrosa W., Diniz Mde F., Passos V.M. Adverse effects during the oral glucose tolerance test in post-bariatric surgery patients. Archives of Endocrinology and Metabolism. 2016;60:307–313. doi: 10.1590/2359-3997000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kong M.F., Chapman I., Goble E., Wishart J., Wittert G., Morris H. Effects of oral fructose and glucose on plasma GLP-1 and appetite in normal subjects. Peptides. 1999;20:545–551. doi: 10.1016/s0196-9781(99)00006-6. [DOI] [PubMed] [Google Scholar]
- 56.Teff K.L., Elliott S.S., Tschöp M., Kieffer T.J., Rader D., Heiman M. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. Journal of Clinical Endocrinology & Metabolism. 2004;89:2963–2972. doi: 10.1210/jc.2003-031855. [DOI] [PubMed] [Google Scholar]
- 57.Yang M., Zhang L., Wang C., Liu H., Boden G., Yang G. Liraglutide increases FGF-21 activity and insulin sensitivity in high fat diet and adiponectin knockdown induced insulin resistance. PLoS One. 2012;7 doi: 10.1371/journal.pone.0048392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nonogaki K., Hazama M., Satoh N. Liraglutide suppresses obesity and hyperglycemia associated with increases in hepatic fibroblast growth factor 21 production in KKAy mice. BioMed Research International. 2014;2014:751930. doi: 10.1155/2014/751930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meek C.L., Lewis H.B., Reimann F., Gribble F.M., Park A.J. The effect of bariatric surgery on gastrointestinal and pancreatic peptide hormones. Peptides. 2016;77:28–37. doi: 10.1016/j.peptides.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 60.Eissing L., Scherer T., Tödter K., Knippschild U., Greve J.W., Buurman W.A. De novo lipogenesis in human fat and liver is linked to ChREBP-β and metabolic health. Nature Communications. 2013;4:1528. doi: 10.1038/ncomms2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ge X., Wang Y., Lam K.S.L., Xu A. Metabolic actions of FGF21: molecular mechanisms and therapeutic implications. Acta Pharmaceutica Sinica. 2012;2:350–357. [Google Scholar]
- 62.Yu H., Xia F., Lam K.S., Wang Y., Bao Y., Zhang J. Circadian rhythm of circulating fibroblast growth factor 21 is related to diurnal changes in fatty acids in humans. Clinical Chemistry. 2011;57:691–700. doi: 10.1373/clinchem.2010.155184. [DOI] [PubMed] [Google Scholar]
- 63.Park J.G., Xu X., Cho S., Hur K.Y., Lee M.S., Kersten S. CREBH-FGF21 axis improves hepatic steatosis by suppressing adipose tissue lipolysis. Scientific Reports. 2016;6:27938. doi: 10.1038/srep27938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hotta Y., Nakamura H., Konishi M., Murata Y., Takagi H., Matsumura S. Fibroblast growth factor 21 regulates lipolysis in white adipose tissue but is not required for ketogenesis and triglyceride clearance in liver. Endocrinology. 2009;150:4625–4633. doi: 10.1210/en.2009-0119. [DOI] [PubMed] [Google Scholar]
- 65.Zhao C., Liu Y., Xiao J., Liu L., Chen S., Mohammadi M. FGF21 mediates alcohol-induced adipose tissue lipolysis by activation of systemic release of catecholamine in mice. Journal of Lipid Research. 2015;56:1481–1491. doi: 10.1194/jlr.M058610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tappy L., Randin J.P., Felber J.P. Comparison of thermogenic effect of fructose and glucose in normal humans. American Journal of Physiology. Endocrinology & Metabolism. 1986;250:13–16. doi: 10.1152/ajpendo.1986.250.6.E718. [DOI] [PubMed] [Google Scholar]
- 67.Tran C., Jacot-Descombes D., Lecoultre V., Fielding B.A., Carrel G., Le K.A. Sex differences in lipid and glucose kinetics after ingestion of an acute oral fructose load. British Journal of Nutrition. 2010;104:1139–1147. doi: 10.1017/S000711451000190X. [DOI] [PubMed] [Google Scholar]
- 68.Abell T.L., Minocha A. Gastrointestinal complications of bariatric surgery: diagnosis and therapy. American Journal of the Medical Sciences. 2006;331:214–218. doi: 10.1097/00000441-200604000-00008. [DOI] [PubMed] [Google Scholar]
- 69.Davidson N.O., Hausman A.M., Ifkovits C.A., Buse J.B., Gould G.W., Burant C.F. Human intestinal glucose transporter expression and localization of GLUT5. American Journal of Physiology. 1992;262:C795–C800. doi: 10.1152/ajpcell.1992.262.3.C795. [DOI] [PubMed] [Google Scholar]
- 70.Bojsen-Moller K.N., Dirksen C., Jorgensen N.B., Jacobsen S.H., Hansen D.L., Worm D. Increased hepatic insulin clearance after Roux-en-Y gastric bypass. Journal of Clinical Endocrinology & Metabolism. 2013;98:E1066–E1071. doi: 10.1210/jc.2013-1286. [DOI] [PubMed] [Google Scholar]