Abstract
OBJECTIVE
Traumatic brain injury (TBI) is a leading cause of death and disability. Patients with TBI in low and middle-income countries have worse outcomes than patients in high-income countries. We evaluated important clinical indicators associated with mortality for patients with TBI at University Teaching Hospital of Kigali, Kigali, Rwanda.
METHODS
A prospective consecutive sampling of patients with TBI presenting to University Teaching Hospital of Kigali Accident and Emergency Department was screened for inclusion criteria: reported head trauma, alteration in consciousness, headache, and visible head trauma. Exclusion criteria were age <10 years, >48 hours after injury, and repeat visit. Data were assessed for association with death using logistic regression. Significant variables were included in a multivariate logistic regression model and refined via backward elimination.
RESULTS
Between October 7, 2013, and April 6, 2014, 684 patients were enrolled; 14 (2%) were excluded because of incomplete data. Of patients, 81% were male with mean age of 31 years (range, 10–89 years; SD 11.8). Most patients (80%) had mild TBI (Glasgow Coma Scale [GCS] score 13–15); 10% had moderate (GCS score 9–12) and 10% had severe (GCS score 3–8) TBI. Multivariate logistic regression determined that GCS score <13, hypoxia, bradycardia, tachycardia, and age >50 years were significantly associated with death.
CONCLUSIONS
GCS score <13, hypoxia, bradycardia, tachycardia, and age >50 years were associated with mortality. These findings inform future research that may guide clinicians in prioritizing care for patients at highest risk of mortality.
Keywords: Rwanda, Traumatic brain injury
INTRODUCTION
Worldwide each year, 10 million people sustain traumatic brain injury (TBI). Globally, TBI is the leading cause of disability in people <40 years old, causing severe disability in 150–200 people per million each year and resulting in loss of the most productive years of life.1 Low- and middle-income countries (LMIC) have the greatest burden of TBI.2 Of the 3.9 million deaths and 138 million disability-adjusted life years lost attributable to unintentional injury, >90% of these occur in LMIC.3 Road traffic injuries (RTIs) are the leading cause of TBIs; thus, as countries develop and motor vehicle use increases, rates of RTIs and TBIs will increase. Despite challenges in data gathering in sub-Saharan Africa, rates of TBI due to RTIs already exceed both the global rate and the rate for other LMIC.4
In addition to a higher incidence of TBI in LMIC, the mortality rate is higher in LMIC for the same injury compared with high-income settings. Challenges to quality care in a setting with limited resources are numerous, including limited prehospital care or access to care, less equipment, fewer trained acute and intensive care personnel, and limited neurosurgical capacity.5 An analysis of >8000 hospitalized patients with TBI showed that patients with severe TBI in LMIC have more than twice the odds of death compared with patients treated in high-income countries.6 Quality audits in TBI care in LMIC have found no global standardization of TBI care, limited local standardization of care, and poor control of secondary brain injury, which compound the resource and personnel challenges in this setting.7
The University Teaching Hospital of Kigali (UTHK) is 1 of 2 hospitals with the capacity to provide the highest levels of TBI care with computed tomography (CT), critical care, and neurosurgical services. UTHK is public and available to all Rwandans regardless of their financial resources. Similar to other LMIC, Rwandan patients with TBI have high mortality rates. Unpublished data from a trauma registry at UTHK documented the mortality rate of patients with severe TBI to be 58% (R.T. Petroze, oral communication, September 2012). In 2008, Rwandan patients with severe TBI who had been involved in RTIs had an 89% mortality rate.8 Critical factors that contributed to these TBI-related deaths, particularly injuries in the acute care setting that are amenable to prompt medical intervention, are largely unknown. We systematically evaluated the presentation of TBI at UTHK to understand the epidemiology, acute care, and stabilization of these patients. Our objective was to evaluate important clinical indicators associated with mortality through prospective sampling of patients with acute TBI presenting for care. Understanding these predictors could guide clinicians in developing clinical practice guidelines to provide appropriate care to the most critically ill patients.
MATERIALS AND METHODS
Study Design and Ethics
Between October 7, 2013, and April 6, 2014, we conducted an observational prospective cohort study of all patients with acute TBI who presented to UTHK Accident and Emergency Department (A&E) for care. This 6-month period included an approximately equal portion of days in both dry and rainy seasons in Rwanda, and the duration of data collection represents the limits of available funding for this study. The study protocol was approved by the UTHK ethics committee and the Rwandan National Health Research Council. It was determined to meet criteria as a quality improvement project by the Duke University Medical Center Institutional Review Board (Pro00044873).
Setting
Rwanda is a small, densely populated country in East Africa with a total population of approximately 11 million; approximately 1.15 million reside in the capital city of Kigali.9 The decentralized public health care system includes nurse-staffed health centers, district-level hospitals, and referral hospitals and tertiary care centers. UTHK, centrally located in Kigali with approximately 500 patient beds, is the largest public referral center in Rwanda. UTHK A&E includes 30 patient beds, and the hospital has a 5-bed intensive care unit with ventilators available, has a 64-slice CT scanner, and is staffed primarily by general practitioners with on-call neurosurgical care. At the time of this study, there was 1 neurosurgeon on staff at UTHK who was supported by 2 general practitioners, whom he trained to be part of the neurosurgery service. This neurosurgeon and the 2 general practitioners would accept consultations at any time when they were in Kigali and not otherwise occupied; however, their practical availability was limited by the size of the service, and critical patients could not always be assessed immediately. Plans were being formed during our data collection for formal residency training in neurosurgery at UTHK. Limited operating rooms were available around the clock for emergent craniotomies when not otherwise occupied. Rwanda is engaged in a large Human Resources for Health program to improve health care provider skills and capacity. Funding and support for Human Resources in Health is coordinated by the Rwandan Ministry of Health by reallocation of funds from The U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) and The Global Fund to Fight AIDS, Tuberculosis and Malaria.10
Population
Inclusion criteria included signs and symptoms of TBI (headache, reported trauma to the head, visible trauma to the head, or any alteration in consciousness in the context of an injury), ≥10 years of age, sustaining an injury <48 hours before presentation for care at UTHK, first visit to UTHK for this injury (patients may have received care for this injury at other hospitals before arrival at UTHK) and not dead on arrival. Our patient cohort was continuously enrolled during the data collection period.
Data Collection Procedures
At UTHK A&E, there is a single entrance where patients present to the triage desk and nurses seeking care. Trained research assistants (RAs) sat near this desk and evaluated every patient that arrived at UTHK A&E for care and further assessed any injured patient for study inclusion criteria. These RAs were hired locally and were fluent in the local language of Kinyarwanda, French, and English. They all completed formal education and worked clinically as nurses in Rwanda before full-time employment on this study team. RAs collected patient demographics, injury characteristics, and treatment rendered by closely observing each patient during a 4-hour observation period while the enrolled patient was cared for in A&E (the period ended at 4 hours, death, discharge or admission). A case report form (Appendix A) and data log were used to collect data on individual patients. Study coordinators subsequently located all enrolled patients in the hospital to collect follow-up data by a variety of means, including direct observation, discussions with nurses and physicians, or reviewing the patient’s chart (Appendix B). These assessments continued daily until the patient’s death or discharge from UTHK.
Measures
In-hospital mortality defines the dependent variable of death in this study; patients were observed until the time of their death or discharge from UTHK. On initial presentation, RAs assessed airway patency; an unstable airway was defined as the presence of any snoring, gurgling, or blood or secretions in the mouth. The Glasgow Coma Scale (GCS) score was determined at the earliest possible point after patient arrival by physicians and by the trained RAs. RAs observed and recorded the time and values of the first assessed vital signs on arrival at UTHK A&E, including heart rate, respiratory rate, blood pressure, and oxygen saturation. Respiratory rate was dichotomized (<20 or ≥20 breaths per minute) while heart rate, systolic blood pressure, and oxygen saturation by noninvasive pulse oximetry were categorized as follows: <60 beats per minute, 60–89 beats per minute, 90–99 beats per minute, or >100 beats per minute (heart rate), <90 mm Hg, 90–100 mm Hg, and >100 mm Hg (systolic blood pressure), and <90%, 90%–94%, and >94% (oxygen saturation).11–17 RAs documented mechanisms of injury and any safety-related factors including alcohol use (determined by staff suspicion, smell, or patient report of alcohol use). Age was categorized into 5 groups of <20 years, 20–30 years, 31–40 years, 41–50 years, and >50 years, and each decade was evaluated for crude associations with mortality. Based on findings of a steep jump in mortality, age was dichotomized to ≥50 or >50 in the final models. Other severe injuries, defined as any injury other than TBI that required hospital admission or any kind of surgery performed in the operating room, were counted. Follow-up information was collected by the study coordinator and included date and time of any surgical procedures, head CT scan interpretation, and dates of discharge or death.
Quality Control
RAs were extensively trained in patient assessment and determination of GCS score, which included multiple evaluations of their assessment skills. Intermittent dual data collection, especially GCS score evaluation, was performed to evaluate accuracy of the RAs. Study staff evaluated study registers and patient registers daily to ensure no patients were excluded or overlooked. Data were handwritten on the case report and follow-up forms and checked for errors or missing data. Data were then entered into a Research Electronic Data Capture (REDCAP) database and checked by an investigator (E.K.) for completeness and errors.
Data Analysis
Descriptive statistics (frequency, percentages) were used to compare survivors with discharge and deaths. To identify significant differences between the groups, χ2 tests were performed. Univariate logistic regression models were used to examine the crude associations between potential predictors and mortality. A multivariate logistic model included all variables with P ≤ 0.10 in univariate analysis, adjusted using backward elimination. We report the adjusted, multivariate logistic regression model with better performance; thus, some variables were eliminated from the final model. Significance was set at P < 0.05. All statistical analyses were performed using Stata 12.2 (StataCorp LLC, College Station, Texas, USA).
RESULTS
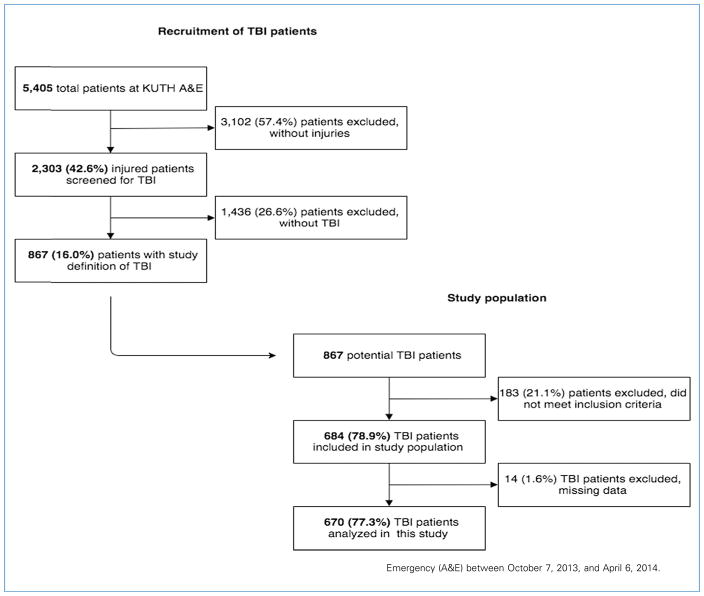
Between October 7, 2013, and April 6, 2014, of 5405 patients seen at UTHK A&E, 2303 (42.6%) sustained an injury, of which 867 (16%) had sustained a TBI. Of patients who met the case definition of TBI, 183 (21.1%) were excluded for not meeting the inclusion criteria, and 14 (1.6%) were excluded for missing data. During the entire study period, 5 of 5404 patients (0.1%) were missed for enrollment. An enrollment chart is shown in Figure 1.
Figure 1.
Prospective enrollment of patients with traumatic brain injury (TBI) from University Teaching Hospital of Kigali (UTHK) Accident and Emergency (A&E) between October 7, 2013, and April 6, 2014.
Demographics
Mean patient age was 31 years (SD 11.8), and most patients (70.7%) were between 20 and 40 years old (Table 1). Most (540; 81%) patients with TBI were male. RTIs occurred in 414 (63.3%) patients; 153 (37%) were on a motorcycle, 118 (28.5%) were in a car, and 102 (24.6%) were pedestrians. Of the motorcyclists, 23 (15.2%) were not wearing a helmet. Moderate TBI occurred in 68 (10.2%) patients, and severe TBI occurred in 66 (9.8%) patients. Clinical characteristics are described in Table 2. In the univariate associations, factors that were associated with mortality included age, sex, alcohol use, whether or not the patient was transferred for care, GCS score, airway status, oxygen saturation, heart rate, blood pressure, and “other severe injuries.”
Table 1.
Demographic Characteristics of Patients with Traumatic Brain Injury at University Teaching Hospital of Kigali Accident and Emergency
| Total Patients | Discharged | Died | ||
|---|---|---|---|---|
| Characteristic | (N = 670) | (n = 608) | (n = 62) | P Value |
| Age | ||||
| <20 years | 89 (13.1) | 84 (94.4) | 5 (5.6) | <0.05* |
| 20–30 years | 283 (41.7) | 260 (91.9) | 23 (8.1) | |
| 31–40 years | 197 (29.0) | 179 (90.9) | 18 (9.1) | |
| 41–50 years | 62 (9.1) | 57 (91.9) | 5 (8.1) | |
| >50 years | 48 (7.1) | 37 (77.1) | 11 (22.9) | |
| Sex | ||||
| Male | 540 (81.0) | 484 (89.6) | 56 (10.4) | <0.05* |
| Female | 127 (19.0) | 121 (95.3) | 6 (4.7) | |
| Alcohol use | ||||
| Yes | 152 (22.8) | 145 (95.4) | 7 (4.6) | <0.05* |
| No | 514 (77.2) | 461 (89.7) | 53 (10.3) | |
| Mechanism of injury | ||||
| RTC | 414 (63.3) | 375 (90.6) | 39 (9.4) | 0.57 |
| Fall | 77 (11.8) | 71 (92.2) | 6 (7.8) | |
| Assault | 163 (24.9) | 152 (93.3) | 11 (6.7) | |
| RTC vehicle | ||||
| Pedestrian | 102 (24.6) | 93 (91.2) | 9 (8.8) | 0.17 |
| Bicycle | 31 (7.5) | 27 (87.1) | 4 (12.9) | |
| Motorcycle | 153 (37.0) | 142 (92.8) | 11 (7.2) | |
| Car | 118 (28.5) | 106 (89.8) | 12 (10.2) | |
| Bus | 10 (2.4) | 7 (70.0) | 3 (30.0) | |
| Helmet use | ||||
| Yes | 128 (84.8) | 120 (93.8) | 8 (6.2) | 0.25 |
| No | 23 (15.2) | 20 (87.0) | 3 (13.0) | |
Data are presented as number (%). RTC, road traffic crash.
Statistically significant P value.
Table 2.
Clinical Characteristics of Patients with Traumatic Brain Injury at University Teaching Hospital of Kigali Accident and Emergency
| Characteristic | Total Patients (N = 670) | Discharged (n = 608) | Died (n = 62) | P Value |
|---|---|---|---|---|
| GCS score | ||||
| 3–8 | 66 (9.8) | 29 (43.9) | 37 (56.1) | <0.01* |
| 9–12 | 68 (10.2) | 57 (83.8) | 11 (16.2) | |
| 13–15 | 536 (80.0) | 522 (97.4) | 14 (2.6) | |
| Patient transferred | ||||
| Yes | 196 (29.2) | 157 (80.1) | 39 (19.9) | <0.01* |
| No | 474 (70.8) | 451 (95.2) | 23 (4.8) | |
| Airway condition | ||||
| Normal | 573 (86.6) | 535 (93.4) | 38 (6.6) | <0.01* |
| Abnormal | 89 (13.4) | 70 (78.7) | 19 (21.3) | |
| Hypoxia | ||||
| No hypoxia (>94%) | 472 (82.7) | 446 (94.5) | 26 (5.5) | <0.01* |
| Mild hypoxia (90%–94%) | 76 (13.3) | 63 (82.9) | 13 (17.1) | |
| Hypoxic (<90%) | 23 (4.0) | 10 (43.5) | 13 (56.5) | |
| Tachypnea | ||||
| No tachypnea (<20 breaths/min) | 317 (54.9) | 293 (92.4) | 24 (7.6) | 0.24 |
| Tachypnea (≥20 breaths/min) | 260 (45.1) | 233 (89.6) | 27 (10.4) | |
| Heart rate | ||||
| <60 beats/min | 23 (3.9) | 15 (65.2) | 8 (34.8) | <0.01* |
| 60–89 beats/min | 339 (57.9) | 327 (96.5) | 12 (3.5) | |
| 90–100 beats/min | 105 (17.9) | 101 (96.2) | 4 (3.8) | |
| >100 beats/min | 119 (20.3) | 91 (76.5) | 28 (23.5) | |
| Blood pressure | ||||
| <90 mm Hg | 19 (3.3) | 13 (68.4) | 6 (31.6) | <0.01* |
| 90–99 mm Hg | 20 (3.4) | 17 (85.0) | 3 (15) | |
| 100–149 mm Hg | 463 (79.4) | 436 (94.2) | 27 (5.8) | |
| ≥150 mm Hg | 81 (13.9) | 66 (81.5) | 15 (18.5) | |
| Other injuries | ||||
| None | 573 (85.5) | 531 (92.7) | 42 (7.3) | <0.01* |
| 1 | 65 (9.7) | 54 (83.1) | 11 (16.9) | |
| 2 | 32 (4.8) | 23 (71.9) | 9 (28.1) | |
Data are presented as number (%). GCS, Glasgow Coma Scale.
Statistically significant P value.
Univariate Analysis
Alcohol use was suspected in 152 patients (22.8%), more commonly in male patients (139 [25.9%]) than female patients (13 [10.8%]). Among 48 patients >50 years old with TBI, 26 (54.2%) were transferred to UTHK from other hospitals for care, whereas 168 (27.1%) of 619 patients ≤50 years old were transferred. The overall mortality rate was 9.3% (62 of 670 patients) with a mortality rate of 16.2% (11 of 68 patients) for moderate TBI and 56.1% (37 of 66 patients) for severe TBI. Male patients had a crude mortality rate of 10.4% compared with 4.7% in female patients. All patients >50 years old with TBI (48 [7.2%]) had a crude mortality of nearly 23% compared with 8% in all patients ≤50 years old. Only 26 (3.9%) patients in this cohort underwent surgery for TBI, including 10 (1.9%) patients with mild TBI, 8 (11.8%) patients with moderate TBI, and 8 (12.1%) patients with severe TBI. Of the patients with TBI who underwent surgery, only 4 died, and crude mortality breakdown was 25% for patients with moderate and severe TBI and 0% for patients with mild TBI.
Multivariate Analysis
Final, backward elimination multivariate model adjusted for age >50, GCS score ≤13, hypoxia, bradycardia, and tachycardia. GCS score ≤8, bradycardia, and hypoxia had the strongest association with death (odds ratios of 41.6, 11.29, and 7.36) (Table 3).
Table 3.
Association of Demographic and Clinical Characteristics with Mortality of Patients with Traumatic Brain Injury at University Teaching Hospital of Kigali Accident and Emergency
| Characteristic | Crude OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value |
|---|---|---|---|---|
| Age >50 years | 3.46 (1.66, 7.20) | <0.01 | 3.80 (1.21, 11.89) | <0.05 |
| Male sex | 2.33 (0.98, 5.54) | 0.06 | ||
| Alcohol use | 0.42 (0.19, 0.94) | <0.05 | ||
| Patient transferred | 4.87 (2.82, 8.41) | <0.01 | ||
| GCS score | ||||
| 13–15 | — | — | ||
| 9–12 | 7.20 (3.12, 16.60) | <0.01 | 6.54 (2.30,11.60) | <0.01 |
| 3–8 | 47.57 (23.16, 97.71) | <0.01 | 41.60 (14.97, 115.59) | <0.01 |
| Abnormal airway condition | 3.81 (2.09, 6.99) | <0.01 | ||
| Hypoxia | ||||
| No hypoxia (>94%) | — | |||
| Mild hypoxia (90%–94%) | 3.54 (1.73, 7.24) | <0.01 | ||
| Hypoxia (<90%) | 22.33 (8.94, 55.64) | <0.01 | 7.36 (2.20, 14.57) | <0.01 |
| Heart rate | ||||
| Normal* (60–89 beats/min) | — | |||
| Bradycardia (<60 beats/min) | 14.53 (5.17, 40.85) | <0.01 | 11.29 (2.20, 14.57) | <0.01 |
| Mild tachycardia* (90–100 beats/min) | 1.08 (0.34, 3.42) | 0.13 | ||
| Tachycardia (>100 beats/min) | 8.38 (4.10, 17.14) | <0.01 | 5.20 (2.23, 12.12) | <0.01 |
| Blood pressure | ||||
| Normotensive (100–149 mm Hg) | — | |||
| Hypotension (<90 mm Hg) | 7.45 (2.63, 21.14) | <0.01 | ||
| Mild hypotension (90–99 mm Hg) | 2.85 (0.79, 10.35) | <0.01 | ||
| Hypertension (≥150 mm Hg) | 3.67 (1.86, 7.26) | <0.01 | ||
| Other injuries | ||||
| None | — | |||
| 1 | 2.58 (1.25, 5.29) | <0.05 | ||
| 2 | 4.95 (2.15, 11.37) | <0.01 |
OR, odds ratio; CI, confidence interval; GCS, Glasgow Coma Scale.
Owing to small sample sizes, normal heart rate and mild tachycardia were merged for the adjusted OR.
DISCUSSION
In this prospective observational cohort of patients with acute TBI presenting to a large public referral hospital in an urban African setting, we describe the epidemiology of TBI and predictors of mortality. Included in the evaluations were systematic assessments for the involvement of alcohol and the use of safety equipment, such as helmets among motorcycle users. In addition, we describe key clinical indicators associated with mortality, which may prove to be useful targets for quality improvement in the care of severe TBIs in such settings. These findings are novel in the context of the unique setting of Rwanda, with high rates of helmet use and an organized prehospital care system. Results of this analysis may help direct design of interventions to improve TBI mortality in this context that can be applied to other LMIC, as they also improve helmet use compliance.
Age and Sex
Most (70.7%) TBIs occurred in young men 20–40years old with a mean age of 31 (SD 11.8), which is in agreement with the international, regional, and Rwandan literature.8 Globally, injuries occur predominantly among young, economically productive men 20–40 years old.18 The CRASH (Corticosteroid Randomisation After Significant Head Injury) trial, a multinational study on TBI treatment that enrolled 10,008 patients, showed for LMIC the mean age was 35.8 (SD 16).19 Regional data also support this trend; 75% of patients with TBI in Moshi, Tanzania, were 15–45 years old,20 and 60% of patients with severe TBI in Uganda were 15–45 years old.21 This trend was not observed in recent data from high-income countries. An analysis of studies including data from the years 1984–2004 found a consistent increase in the median age and the absolute number of patients with TBI >50 years old, likely related to longer life spans and increased mobility among elderly adults.19 The male predominance described in the present study has also been described in other settings in East Africa; 80% of all TBIs in Moshi and 82% of severe TBIs in Uganda occur in men. These associations are not unexpected based on other global estimates and are likely linked to gender roles and high-risk behaviors.20,21
Mechanism of Injury
The most common mechanism of injury in our study was road traffic injuries (63.3%), of which 24.6% of patients were pedestrians and 37% were motorcyclists, highlighting a characteristically high burden of vulnerable road users among injured patients in LMIC. Similar data are found in other LMIC such as Tanzania (65.7% RTIs with 20% involving pedestrians and 48.8% involving motorcyclists) and Uganda (79.2% RTIs with 31% involving pedestrians and 45.6% involving motorcyclists).20,21 Alternatively, TBI epidemiology based on 2001 data from Wisconsin in the United States reported 65.8% of TBIs were due to RTIs, with 12.5% involving pedestrians and 20.3% involving motorcyclists.22 Another analysis of patients with TBI seen in emergency departments across the United States found that in 2010 only 15.7% were caused by motor vehicle traffic, and most (46.3%) were attributable to falls.23 These findings demonstrate the increasing and profound prevalence of road traffic crashes especially among vulnerable road users who are more predominant in LMIC, where road infrastructure, safety police, and enforcement are limited. Alternatively, in high-income countries where road safety policy is improved and where life expectancy is higher, elderly individuals are at risk of falls, and the proportions of falls naturally increase.24 The increasing age of patients with TBI in high-income countries likely contributes to this shift toward falls as a predominant mechanism of injury.
Alcohol
Our study highlights several risk factors for injury. For instance, these data showed evidence for potential alcohol involvement in 23% of patients with TBI. Potential alcohol involvement was observed primarily among young men. Alcohol use among patients sustaining injury has been shown to be a prevalent problem; in Tanzania, approximately 27% of patients with TBI used alcohol at the time of injury.20 International literature has found that injured patients seeking care have rates of alcohol use of 6%–45%; the African countries included in this study were South Africa with 45% and 18% in Mozambique.25 In the crude analysis, alcohol was a significant protector against death; this was not significant in the adjusted analysis (adjusted odds ratio 0.31, 95% confidence interval [0.07, 1.29], P = 0.11). This finding is not unique, and other reports have found a significant association between objectively assessed, elevated blood alcohol concentration and survival after TBI.26 The cause of this association is unclear, but animal models have demonstrated a potential physiologic relationship associated with reduction of the local neuroinflammatory response to injury.27 Also noteworthy are data associating alcohol use with increased severity of injury28 and decreased physiologic ability to compensate for shock.29 Moreover, alcohol-related deaths make up a large share of prehospital injury mortality, which are impossible to assess in hospital-based studies such as ours.30 Alcohol may increase the risk of being injured and may exogenously decrease the GCS score, but it remains unclear how it impacts outcomes in patients with TBI.
Motorcycle Helmet Use
Among motorcyclists with TBI, we collected information on helmet use, documenting that 84.8% of such motorcyclists wore a helmet. Our prospective assessment found lower helmet use compared with a retrospective analysis performed at UTHK in 2011 of 269 patients with motorcycle injury that reported helmet use in 92.3%.31 These rates of helmet use are very high compared with surrounding countries. One study in Nigeria found that among patients with TBI who sustained their injury on a motorcycle, the rate of helmet use was 1.2%.32 Rwanda has been a pioneer in Africa in implementing and enforcing a helmet use policy despite challenges with quality equipment and enforcement. In 2001, national legislation that included motorcycle helmet use was approved, and a follow-up public awareness campaign occurred in 2003.33 Finding a 15% helmet nonuse rate among patients with TBI and a slightly lower proportion of motorcyclists (37% vs. 45%–48% in surrounding countries) sustaining TBI are likely due to this successful helmet implementation.34
Resources
The observed mortality rate of severe TBI in Kigali was 56.1%. A study on severe TBI from Kampala, Uganda, showed that 25.8% sustained fatal injuries, and in Moshi, Tanzania, 46.9% died of TBI.20,21 Each of these settings has unique combinations of health infrastructure assets and constraints that contribute to these statistics. For instance, of these 3 countries, Rwanda is the only one with an organized and available prehospital care system. This resource could result in more patients presenting to UTHK close to death and cause some selection bias toward higher mortality than the aforementioned hospitals in Kampala and Moshi. Although Uganda does not have a prehospital system, in Kampala, there is a neurosurgery residency training program, a well-equipped intensive care unit, and operating rooms dedicated to neurosurgery.21 Tanzania has no prehospital system and no training program in neurosurgery and lacks adequate intensive care unit and operating room equipment required for advanced neurosurgery. Although comparing outcomes in different countries is difficult because of health system differences, we tried to compare clinical risk factors; in our multivariate analysis, severe TBI, bradycardia, and hypoxia had the strongest association with mortality. In Tanzania, hypotension and moderate and severe TBI were the only indicators associated with mortality.20 These differences are likely due to access to care differences in Rwanda given the presence of a prehospital care system.35–37 To understand reasons for this disparity, some African studies conducted quality audits and found that important causes of secondary injury (hypoxia and hypotension) are underrecognized and under-treated.15 These causes of secondary injury have been shown, mostly in high-income settings, to increase TBI mortality by 20%–35% among patients who experience hypoxia and by even more among patients who experience hypotension.13–17,38
Limitations
This study has several limitations. Although this is one of the largest (N = 670) prospective registries evaluating patients with TBI in a low- and middle-income setting, some of our subgroups were relatively small in number; thus, determining associations with mortality was difficult in these groups. For example, there were only 22 hypoxic patients (oxygen saturation <90%) and 19 hypotensive patients (systolic blood pressure <90 mm Hg). To mitigate this impact, we limited the number of variables analyzed to avoid finding spurious associations. We determined alcohol use through subjective means owing to a lack of objective testing at UTHK; this should be considered in evaluating results associated with alcohol use. Although we intentionally defined TBI in our population broadly with the goal of ensuring patients were not missed, this came with the consequence of most of the cohort having mild TBI, and we were unable to include CT head findings in the analysis. For reasons beyond the scope of this article, many patients with TBI did not have a CT scan of the head performed. The radiologist wrote results on a slip of paper, and this was often lost from the chart, so we had this data point on only a limited number of patients. Future studies should find an alternative data collection methodology if pursuing an analysis that includes CT results. Additionally, these findings were from a single urban, teaching hospital, which limits generalizability to other settings given the unique health system challenges faced in each low- and middle-income setting. Given the very limited amount of data available to describe TBI epidemiology in sub-Saharan Africa, we believe these data still make an important contribution to the literature. Our results show that hypoxia and heart rate alterations are associated with worse outcomes, but it remains unclear if mortality could be improved by a targeted intervention. Although the frequency and timeliness of such interventions were collected as part of this study, insufficient data were available to analyze their impact on patient outcomes.
CONCLUSIONS
A large proportion of trauma patients presenting to UTHK, Rwanda, sustained TBI, and initial GCS score of ≤13, bradycardia or tachycardia, age >50, and hypoxia were associated with mortality. Overall, 56% of patients with severe TBI died. Future research on treatment of secondary injury and impact of acute stabilization is needed to further delineate why patients in low-and middle-income settings have a higher mortality rate compared with patients in high-income settings.
Acknowledgments
We thank the physicians and staff at UTHK A&E, Dr. Severien Muneza for his neurosurgical expertise, Assumpta Muzayire for her work as study coordinator and Cecile Kateretse and Eliane Maria Spiecker who both provided administrative support. EK acknowledges support from the National Institutes of Health Office of the Director, Fogarty International Center, Office of AIDS Research, National Cancer Center, National Eye Institute, National Heart, Blood, and Lung Institute, National Institute of Dental and Craniofacial Research, National Institute on Drug Abuse, National Institute of Mental Health, National Institute of Allergy and Infectious Diseases, and National Institutes of Health Office of Women’s Health and Research through the Fogarty International Clinical Research Scholars and Fellows Program at Vanderbilt University (R24 TW007988) and the American Recovery and Reinvestment Act. CAS acknowledges salary support funding from the Fogarty International Center (K01 TW010000-01A1) and the Duke University Division of Emergency Medicine.
Abbreviations and Acronyms
- A&E
Accident and Emergency Department
- CRASH
Corticosteroid Randomisation After Significant Head Injury
- CT
Computed tomography
- GCS
Glasgow Coma Scale
- LMIC
Low- and middle-income countries
- PEPFAR
The U.S. President’s Emergency Plan for AIDS Relief
- RA
Research assistant
- RTI
Road traffic injury
- TBI
Traumatic brain injury
- UTHK
University Teaching Hospital of Kigali
APPENDIX A
APPENDIX B
Footnotes
Conflict of interest statement: The authors declare that the article content was composed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Fleminger S, Pondsford J. Long term outcome after traumatic brain injury. BMJ. 2005;331:1419–1420. doi: 10.1136/bmj.331.7530.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Georgoff P, Meghan S, Mirza K, Stein SC. Geographic variation in outcomes from severe traumatic brain injury. World Neurosurg. 2010;74:331–345. doi: 10.1016/j.wneu.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 3.Chandran A, Hyder AA, Peek-Asa C. The global burden of unintentional injuries and an agenda for progress. Epidemiol Rev. 2010;32:110–120. doi: 10.1093/epirev/mxq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC. The impact of traumatic brain injuries: a global perspective. Neuro-Rehabilitation. 2007;22:341–353. [PubMed] [Google Scholar]
- 5.Razzak JA, Sasser SM, Kellermann AL. Injury prevention and other international public health initiatives. Emerg Med Clin North Am. 2005;23:85–98. doi: 10.1016/j.emc.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 6.De Silva MJ, Roberts I, Perel P, Edwards P, Kenward MG, Fernandes J, et al. Patient outcome after traumatic brain injury in high-, middle- and low-income countries: analysis of data on 8927 patients in 46 countries. Int J Epidemiol. 2009;38:452–458. doi: 10.1093/ije/dyn189. [DOI] [PubMed] [Google Scholar]
- 7.Alexander T, Fuller G, Hargovan P, Clarke DL, Muckart DJ, Thomas SR. An audit of the quality of care of traumatic brain injury at a busy regional hospital in South Africa. S Afr J Surg. 2009;47:120–126. [PubMed] [Google Scholar]
- 8.Twagirayezu E, Teteli R, Bonane A, Rugwizangoga E. Road traffic injuries at Kigali University Central Teaching Hospital, Rwanda. East Cent Afr J Surg. 2008;13:73–76. [Google Scholar]
- 9.World Bank. [Accessed April 1, 2015];World Development Report 2012: Gender Equality and Development. 2011 Available at: http://elibrary.worldbank.org/doi/book/10.1596/978-0-8213-8810-5.
- 10.MOH. [Accessed April 1, 2015];Human Resources for Health Program Rwanda. 2012 Available at: http://www.hrhconsortium.moh.gov.rw/
- 11.Brasel KJ, Guse C, Gentilello LM, Nirula R. Heart rate: is it truly a vital sign? J Trauma. 2007;62:812–817. doi: 10.1097/TA.0b013e31803245a1. [DOI] [PubMed] [Google Scholar]
- 12.Malone DL, Kuhls D, Napolitano LM, McCarter R, Scalea T. Back to basics: validation of the admission systemic inflammatory response syndrome score in predicting outcome in trauma. J Trauma. 2001;51:458–463. doi: 10.1097/00005373-200109000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Chesnut RM, Marshall LF, Klauber MR, Blunt BA, Baldwin N, Eisenberg HM, et al. The role of secondary brain injury in determining outcome from severe head injury. J Trauma. 1993;34:216–222. doi: 10.1097/00005373-199302000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Steyerberg EW, Mushkudiani N, Perel P, Butcher I, Lu J, McHugh GS, et al. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med. 2008;5:1251–1261. doi: 10.1371/journal.pmed.0050165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reed AR, Welsh DG. Secondary injury in traumatic brain injury patients—a prospective study. S Afr Med J. 2002;92:221–224. [PubMed] [Google Scholar]
- 16.Chi JH, Knudson MM, Vassar MJ, McCarthy MC, Shapiro MB, Mallet S, et al. Prehospital hypoxia affects outcome in patients with traumatic brain injury: a prospective multicenter study. J Trauma. 2006;61:1134–1141. doi: 10.1097/01.ta.0000196644.64653.d8. [DOI] [PubMed] [Google Scholar]
- 17.Stocchetti N, Furlan A, Volta F. Hypoxemia and arterial hypotension at the accident scene in head injury. J Trauma. 1996;40:764–767. doi: 10.1097/00005373-199605000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Hitimana J, Perez M, Kinasha A, Kakande I. Clinical presentation and outcome of neurosurgical conditions at Butare Teaching Hospital, Rwanda. East Cent Afr J Surg. 2009;14:50–56. [Google Scholar]
- 19.Roozenbeek B, Maas AIR, Menon DK. Changing patterns in the epidemiology of traumatic brain injury. Nat Rev Neurol. 2013;9:231–236. doi: 10.1038/nrneurol.2013.22. [DOI] [PubMed] [Google Scholar]
- 20.Staton CA, Msilanga D, Kiwango G, Vissoci JR, de Andrade L, Lester R, et al. A prospective registry evaluating the epidemiology and clinical care of traumatic brain injury patients presenting to a regional referral hospital in Moshi, Tanzania: challenges and the way forward. Int J Inj Contr Saf Promot. 2017;24:69–77. doi: 10.1080/17457300.2015.1061562. [DOI] [PubMed] [Google Scholar]
- 21.Tran TM, Fuller AT, Kiryabwire J, Mukasa J, Muhumuza M, Ssenyojo H, et al. Distribution and characteristics of severe traumatic brain injury at Mulago National Referral Hospital in Uganda. World Neurosurg. 2015;83:269–277. doi: 10.1016/j.wneu.2014.12.028. [DOI] [PubMed] [Google Scholar]
- 22.Tives KS, Yang H, Layde PM. The epidemiology of traumatic brain injury in Wisconsin, 2001. WMJ. 2005;104:22–25. 54. [PubMed] [Google Scholar]
- 23.Marin JR, Weaver MD, Yealy DM, Mannix RC. Trends in visits for traumatic brain injury to emergency departments in the United States. JAMA. 2014;311:1917–1919. doi: 10.1001/jama.2014.3979. [DOI] [PubMed] [Google Scholar]
- 24.Faul M, Xu L, Wald MM, Coronado VG. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. [Google Scholar]
- 25.World Health Organization. [Accessed June 1, 2016];Alcohol and injury in emergency departments: summary of the report from the WHO Collaborative Study on Alcohol and Injuries. 2007 Available at: http://apps.who.int/iris/handle/10665/43581.
- 26.Raj R, Mikkonen ED, Siironen J, Hernesniemi J, Lappalainen J, Skrifvars MB. Alcohol and mortality after moderate to severe traumatic brain injury: a meta-analysis of observational studies. J Neurosurg. 2016;124:1684–1692. doi: 10.3171/2015.4.JNS141746. [DOI] [PubMed] [Google Scholar]
- 27.Goodman MD, Makley AT, Campion EM, Friend LA, Lentsch AB, Pritts TA. Preinjury alcohol exposure attenuates the neuro-inflammatory response to traumatic brain injury. J Surg Res. 2013;184:1053–1058. doi: 10.1016/j.jss.2013.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stübig T, Petri M, Zeckey C, Brand S, Müller C, Otte D, et al. Alcohol intoxication in road traffic accidents leads to higher impact speed difference, higher ISS and MAIS, and higher preclinical mortality. Alcohol. 2012;46:681–686. doi: 10.1016/j.alcohol.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Hu TM, Lee RP, Lee CJ, Subeq YM, Lin NT, Hsu BG. Heavy ethanol intoxication increases proinflammatory cytokines and aggravates hemorrhagic shock-induced organ damage in rats. Mediators Inflamm. 2013;2013:121786. doi: 10.1155/2013/121786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zink BJ, Maio RF, Chen B. Alcohol, central nervous system injury, and time to death in fatal motor vehicle crashes. Alcohol Clin Exp Res. 1996;20:1518–1522. doi: 10.1111/j.1530-0277.1996.tb01693.x. [DOI] [PubMed] [Google Scholar]
- 31.Allen Ingabire JC, Petroze RT, Calland F, Okiria JC, Byiringiro JC. Profile and economic impact of motorcycle injuries treated at a university referral hospital in Kigali, Rwanda. Rwanda Med J. 2015;72:5–11. [Google Scholar]
- 32.Ogbonna M, Nnadi N, Bankole OB, Gbalipre FB. Motorcycle-related traumatic brain injuries: helmet use and treatment outcome. Neurosci J. 2015;2015:1–6. doi: 10.1155/2015/696787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown H. Rwanda’s road-safety transformation. [Accessed April 1, 2015];Bulletin of the World Health Organization. 2007 85 doi: 10.2471/BLT.07.010607. Available at: http://www.who.int/bulletin/volumes/85/6/07-010607.pdf?ua=1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization. [Accessed June 1, 2016];Helmet use saves lives: increasing helmet use promoted as an effective method of reducing road injuries and deaths. 2010 Available at: http://www.who.int/mediacentre/news/releases/2006/pr44/en/
- 35.Henry JA, Reingold AL. Prehospital trauma systems reduce mortality in developing countries: a systematic review and meta-analysis. J Trauma Acute Care Surg. 2012;73:261–268. doi: 10.1097/TA.0b013e31824bde1e. [DOI] [PubMed] [Google Scholar]
- 36.Husum H, Gilbert M, Wisborg T, Van Heng Y, Murad M. Rural prehospital trauma systems improve trauma outcome in low-income countries: a prospective study from North Iraq and Cambodia. J Trauma. 2003;54:1188–1196. doi: 10.1097/01.TA.0000073609.12530.19. [DOI] [PubMed] [Google Scholar]
- 37.Murad MK, Issa DB, Mustafa FM, Hassan HO, Husum H. Prehospital trauma system reduces mortality in severe trauma: a controlled study of road traffic casualties in Iraq. Prehosp Disaster Med. 2012;27:36–41. doi: 10.1017/S1049023X11006819. [DOI] [PubMed] [Google Scholar]
- 38.McHugh GS, Engel DC, Butcher I, Steyerberg EW, Lu J, Mushkudiani N, et al. Prognostic value of secondary insults in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24:287–293. doi: 10.1089/neu.2006.0031. [DOI] [PubMed] [Google Scholar]