Abstract
The helminth Schistosoma mansoni modulates the infected host’s immune system to facilitate its own survival, by producing excretory/secretory molecules that interact with a variety of the host’s cell types including those of the immune system. Herein, we characterise the S. mansoni adult male worm secretome and identify 111 proteins, including 7 vaccine candidates and several molecules with potential immunomodulatory activity. Amongst the molecules present in the secretome, a 17-19kDa protein analogous to human cyclophilin A was identified. Given the ability of cyclophilin A to modulate the immune system by regulating antigen presenting cell activity, we sought to determine whether recombinant S. mansoni Cyclophilin A (rSmCypA) is capable of modulating bone-marrow derived dendritic cell (BMDC) and T cell responses under in vitro conditions. rSmCypA was enzymatically active and able to alter the pro-inflammatory cytokine profile of LPS-activated dendritic cells. rSmCypA also modulated DC function in the induction of CD4+ T cell proliferation with a preferential expansion of Treg cells. This work demonstrates the unique protein composition of the S. mansoni male worm secretome and immunomodulatory activity of S. mansoni Cyclophilin A.
Author summary
Helminths are known for their ability to alter the host’s immune response in order to promote their survival. One such mechanism is the propensity of helminths to secrete molecules with immunomodulatory activity; such molecules alter various aspects of host immunity to the benefit of the parasite. Following detailed characterisation of the secretome, we have identified that Cyclophilin A is secreted from adult Schistosoma mansoni and that the protein has the capacity to alter dendritic cell and T cell function in vitro by inducing a T cell regulatory phenotype.
Introduction
Schistosomiasis is one of the most prevalent parasitic diseases, with approximately 230 million people being infected globally [1]. To develop improved schistosomiasis control strategies, including new drugs and vaccines, it is important to advance our understanding of how the parasite manipulates the host’s immune system to achieve the chronic infection state that is characteristic of helminth infections in man. The trematode Schistosoma mansoni is the second most common schistosome species responsible for cases of schistosomiasis in the tropics and subtropics [2, 3].
The co-evolution of parasitic helminths and mammals has led to the development of a spectrum of mechanisms whereby the immune system of the infected host is bypassed or modulated to facilitate the completion of the parasite’s life cycle [4, 5]. The mechanisms that govern the host’s immune system modulation during helminth infection include the release of excretory/secretory (ES) products from the helminth, that for an example drive a ‘modified’ T cell response as a mechanism of suppression of the immune system, to regulate protective host responses and in addition, prevent immunopathology [6, 7]. S. mansoni infection evokes a spectrum of cytokines, such as IL-4, IL-5, IL-10, IL-13, IL-25, IL-33 and TGF-β, as well as modulating the function of various immune cells, including regulatory T (Treg) and B cells, eosinophils, alternatively activated macrophages and tolerogenic dendritic cells (DC) [8, 9]. Indeed, helminth ES contains potent immunomodulatory molecules (IM) that have activities beyond their function in helminth immunity, and have been explored as potential therapeutic molecules [10–13].
Helminth infection modify the functions of T cells with the generation of Th2 CD4+ T cell responses and expansion of Treg cells to evoke a state of helminth-induced T cell hypo-responsiveness [9, 14–17]. These effects are not only caused by the direct activity of ES molecules on T cells, but also indirectly, through modulation of antigen presenting cell (APC) activity [18]. DCs form a heterogenic network of cells comprised of several subsets that are capable of responding to a variety of danger signals [19]. Interactions with certain pathogen pattern recognition receptors, including toll-like receptors (TLRs), drive DC maturation and antigen presentation, with elevated MHCII and co-stimulatory molecules (CD80, CD86) expressed on their surface. Additionally, TLR ligands can differentially influence DC cytokine production with LPS stimulation enabling pro-inflammatory cytokine (IFN-γ, IL-6 and IL-12) secretion [20]. In the course of a helminth infection, and despite the availability of TLR ligands, which include helminth-secreted components, DC subtypes demonstrate altered activity, with impaired cytokine release, decreased expression of co-stimulatory molecules and altered antigen presentation capacity that induces the development of a hypo-responsive T cell response [21].
S. mansoni male worm infections of mice induce a state of modified immunity in vivo, rendering mice refractory to anaphylaxis, allergic lung inflammation and experimental colitis [22–25]. Therefore, we sought to characterise the worm excretory/secretory (WES) proteome of adult male S. mansoni worms, and analyse the immunomodulatory potential of WES to identify novel anti-inflammatory IM and vaccine candidates. In the secretome, seven known vaccine candidates were present, and several potential IM were detected, including a secreted non-classical cyclophilin A (SmCypA). Enzymatic activity of the generated recombinant SmCypA (rSmCypA) was assessed, and the effect of SmCypA on bone marrow (BM) derived DC was investigated with regards to BMDC activation in response to TLR stimulation. We also determined the effect of rSmCypA on DC antigen presentation and subsequent T cell proliferation in vitro. Collectively, we demonstrate that the S. mansoni secretome has a unique composition that includes potential vaccine candidates and IM as well as a human CypA analogue. SmCypA modulates DC leading to the preferential expansion of Treg cells in vitro that may contribute to T cell hypo-responsiveness during S. mansoni infection.
Methods
Mice and infections
C57BL/6J and OT-II (TCROVA) transgenic mice were from Jackson Laboratory (Maine, USA) and bred in-house. All mice were bred in a specific pathogen-free barrier facility with male mice used at 8–10 weeks of age. A Puerto Rican strain of S. mansoni was maintained by passage in mice and albino Biomphalaria glabrata snails, as previously described [26].
Ethics statement
All animal care and experimental procedures were performed under an Irish Department of Health and Children Licence (holder Padraic Fallon, Licence Number B100/3250) in compliance with Irish Medicine Board regulations. Animal experiments received ethical approval from the Trinity College Dublin Bioresources Ethical Review Board (Reference: 121108).
Preparation of male adult worm excretory-secretory (WES) and adult worm (AW) molecules
Mice were infected with 200–300 S. mansoni cercariae and portally perfused, to recover worms, six to seven weeks after infection, as described previously [26]. Mice were perfused in Minimum Essential Media (MEM) supplemented with Earle’s Salts (Gibco) and 26.2 mM sodium bicarbonate and further washed several times; male worms were then selected via microscopic examination and any damaged, stunted or dead male worms were discarded. Male worms were subsequently washed thoroughly with 10 mL RPMI-1640 supplemented with 2 mM L-glutamine (Gibco), 50 IU Penicillin/Streptomycin (Gibco) and 5 μg/mL Gentamicin (Gibco) at 37°C under sterile conditions. Two hundred male worms were transferred to cellulose membrane dialysis tubing with a 10 kDa molecular weight cut-off (MWCO; Thermo Scientific) in incubation media; RPMI-1640 supplemented with 2 mM L-glutamine, 100 IU Penicillin/Streptomycin and 5 μg/mL Gentamicin. The tubing was placed in a T-75 cell culture flask containing nutrient media, incubation media with 10% Foetal Bovine Serum (FBS; Sigma) for up to 72 hrs (S1 Fig).
Worms were incubated at 37°C for seventy-two hours; the nutrient media was changed after 24 and 48 hours of incubation. Worm incubation media was harvested and concentrated using a stirred cell concentrator at 50 psi with a 10 kDa MWCO membrane (Amicon). The concentrated supernatant was dialyzed with Dulbecco’s Phosphate Buffered Saline (PBS; Biosera) at 4°C using a 10 kDa MWCO dialysis cassette. The WES preparation was then centrifuged and filtered through a 0.22 μm filter. All WES batches were subjected to a quality control analysis (QCA). A batch is defined as a specimen containing the concentrated WES products from 200 male worms over 72 hours, pooled together and stored at -80°C.
AW molecules were prepared as described previously [27]. In brief, live male worms were isolated, as above, and disrupted under liquid nitrogen with a percussion mortar. The resulting paste was sonicated and centrifuged (10,000 × g) for 1 h at 4°C. The supernatant was repeatedly clarified using a micro-centrifuge at 4°C followed by filtration through 0.45 μm and 0.22 μm filters. AW was stored at a stock concentration of 1 mg/ml at -80°C.
QCA entailed a sample from a batch being resolved on a 12% sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE) and visualized by silver staining. Serum from WES immunised rabbit was used in comparative Western blot analysis. Protein and endotoxin levels were assessed by BCA (Thermo Scientific) and LAL assays (Thermo Scientific), respectively.
WES protein separation, detection and mass spectrometry
300 μg of WES or AW proteins were precipitated using 10% w/v trichloroacetic acid (TCA; C2HCl3O2) and resuspended in rehydration buffer (8M urea, 40 mM Tris, 4% CHAPS, 2% v/v IPG Buffer, 0.002% w/v Bromophenol blue, 60 mM dithiothreitol; DTT). For first dimension separation, WES/AW products were loaded onto 13 cm IPG strips, pH 3–11 (GE Life Sciences), followed by reduction with 1% w/v DTT and alkylation with iodoacetamide (IAM, 2.5% w/v). Second dimension separation was performed on a 138 (W) x 130 (H) mm 12% SDS-PAGE using the ATTO Corporation electrophoresis system AE-6220 (ATTO Bioscience and Biotechnology). Running buffer was added to the buffer chamber and the gel was run at 25 mA and stained by Coomassie Brilliant Blue (Thermo Scientific). All visible spots were manually collected and identified by mass spectrometry.
Mass spectrometry was performed by the BSRC Mass Spectrometry and Proteomics facility at St. Andrews University (http://www.st-andrews.ac.uk/~bmsmspf/). The samples were analysed by a quadruple-time-of-flight mass spectrometer, the Q-STAR Pulsar XL (Applied Biosystems). Briefly, samples were digested with trypsin and loaded onto a capillary liquid chromatography system (nanoLC system). The peptides were separated by reverse phase chromatography and directly eluted into the mass spectrometer. The peptides were then subjected to electrospray ionization and tandem mass spectrometry (ESI-MS/MS) generating a mass (m) -to-charge (z) ratios (m/z) spectrum. The mass spectrometry data was analysed using the Mascot software (http://www.matrixscience.com/) searching the S. mansoni GeneDB sequence database (http://www.genedb.org/Homepage) for protein hits. Protein hits were identified through peptide mass fingerprints. Protein hits that showed at least 2 matched peptides with expectation values lower than 0.05 were considered positive hits.
Bioinformatic analyses of WES
The analysis for protein homologues was performed using the Protein-Protein Basic Local Alignment Search Tool (BLASTp) accessed at the National Centre for Biotechnology Information (NCBI) website (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Sequences alignments that showed a bit score higher than 30 and an expected value (E-value) lower than 1e-16 were considered homologues. Analysis of Gene Ontology (GO) was performed using the QuickGo web-based browser provided by the European Bioinformatics Institute (EBI, http://www.ebi.ac.uk/). GO terms associated with biological process and molecular function were addressed to each protein sequence by searching the UniProtKB-GOA database. Protein sequences were subjected to analysis by the web-based browser SignalP 4.0 Server (http://www.cbs.dtu.dk/services/SignalP/) for predictions of a secretory signal peptide. Protein sequences that did not contain a signal peptide were subjected to analysis by the web-based browser SecretomeP 2.0 Server for prediction of non-classical secretion i.e. not signal peptide triggered protein secretion. SecretomeP analysis was performed using predictions for mammalian sequences and proteins that showed a SecP score higher than 0.5 where considered non-classically secreted proteins. The TMHMM Server v. 2.0 (http://www.cbs.dtu.dk/services/TMHMM/) was used for the predictions of transmembrane helices in the protein sequences. The InterProScan (http://www.ebi.ac.uk/Tools/pfa/iprscan/) sequence search was used for assignment of protein signatures. This tool combines different protein signature recognition methods that search against specific databases. The protein signatures that describe the same protein family or domain are grouped into unique InterPro entries, with a unique accession number. Alignment of multiple sequences was performed using the ClustalW2 programme provided by the EBI (http://www.ebi.ac.uk/Tools/msa/clustalw2/).
Recombinant SmCypA production, purification and validation
For the production of rSmCypA a construct was designed to incorporate an N-terminal Honeybee melittin signal peptide and a C-terminal polyhistidine (His) affinity tag. Recombinant protein was expressed via baculovirus infection of SF9 insect cells and purified by nickel-NTA and size exclusion chromatography. Endotoxin was removed by Triton-X washing, with rSmCypA having <0.01 EU/mg as assessed by LAL assay.
For confirmation of purity, 5 μg of protein was loaded onto SDS-PAGE gels followed by western transfer for detection of the His-tag. 15% SDS-PAGE gels were visualized using Coomassie Brilliant Blue. For western transfer, proteins were immobilized onto a PVDF membrane (Millipore) at 30V for 18 hours at 4°C. Protein transfer was confirmed via Ponceau S (Sigma) staining. Membranes were blocked and probed with HisProbe-HRP (1:5000 dilution; Thermo Scientific) in Odessey Blocking Solution (Li-cor) for 1 hour. Membranes were developed using ECL Western Blotting Substrate (Thermo Scientific Pierce) and visualized via the ChemiDoc MP system (Bio-Rad). Gel images were acquired via HP Scanjet G4050.
The peptidyl-prolyl cis-trans isomerase (PPIase) activity of rSmCypA was measured using protease-coupled spectrophotometric assay [28], which follows the cleavage of the trans form of the chromogenic peptide substrate, N-succinyl-Ala-Ala-Pro-Phe-4-nitroanilide (Suc-AAPF-pNA; Sigma) by chymotrypsin. The serine protease inhibitor PMSF was added to reactions to block proteolytic activity. Reactions were carried out at 23°C in 200 μl of assay buffer (35 mM HEPES buffer, pH 7.9, 86 mM NaCl, and 0.015% Triton-X-100). Ice-cold chymotrypsin solution (added from a 2 mM stock prepared in 10 mM HCl; Sigma) was added immediately followed by rSmCypA and 4mM of substrate to initiate the reaction. The cis-trans isomerization of the Pro-Phe bond was measured by following the absorbance increases at 405 nm over 0–600s using a microplate reader (VersaMax tunable microplate reader; Molecular Devices, Sunnyvale, CA).
Detection of SmCypa expression by PCR
Following lysis of 200 adult male or female S. mansoni worms using Trizol reagent (Invitrogen), total RNA isolation was performed using the RNeasy kit (Qiagen) and was followed by reverse transcription with the Quantitect reverse transcription kit incorporating a genomic DNA elimination step (Qiagen), as per the manufacturer’s instructions. PCR for the detection of SmCypa and housekeeping gene (Pai1, Gapdh) expression was performed using the following primers, SmCypa (forward primer: ACGTCTATGCCACTGACGAC, reverse primer: ATGTGTCAGGGTGGCGATTT), Pai1 (forward primer: TAGCTCCGACAGAAGCACCT, reverse ACGACCTCG ACCAAACATTC), Gapdh (forward primer: ATCCCAGCCTTCGCATCAAA, reverse primer: CATCCCGTGGGATAAGGACG). Fold expression change was calculated by performing densitometry on images of agarose gel electrophoresis for SmCypa and housekeeping genes PCR product bands using ImageJ v1.51.
Bone marrow derived dendritic cell (BMDC) generation and culture
Femur bones were isolated from male C57BL/6 mice and BM was then flushed out using a 27G needle with RPM1-1640 (Gibco). Erythrocytes were lysed using PharmLyse solution according to manufacturer’s instructions (BD Biosciences), and the remaining cells were washed and counted. Cells were subsequently seeded at 2 x 106 cells/ml in untreated petri dishes in complete media (CM; RPMI-1640 supplemented with 10% heat-inactivated Fetal Bovine Serum (Sigma), 2 mmol L-glutamine, 50 IU/ml penicillin and 50 μg/ml streptomycin) supplemented with 20 ng/ml GM-CSF (R&D Systems). BM cells were cultured as described in detail by Lutz et al., [29]. Following 9 days of culture, 85% of the non-adherent cells expressed the DC marker CD11c (Clone #HL3; BD Biosciences) as assessed by flow cytometric analysis. For functional assays, 1 x 106 BMDCs per ml were treated or not with various concentrations of rSmCypA followed by stimulation for 24 hours with 100 ng/ml ultra-pure LPS (Invivogen) with or without rSmCypA.
CD4+ T cell culture
Spleen and lymph nodes (LN) were harvested from TCROVA mice and processed to generate single cell suspensions as described previously [30]. CD4+ T cells were isolated by magnetic bead negative selection (CD4+ T cell Isolation Kit II; Miltenyi Biotec) via AutoMACS. Cell purity was determined to be > 90% via flow cytometry. Cells were then labelled with Cell proliferation dye (eBioscience) as per manufacturer’s instructions before being utilized downstream. For T cell culture, 1 x 105 TCROVA CD4+ T cells were cultured with 2 x 104 BMDC previously treated or not with LPS and/or rSmCypA in the presence of 10μM OVA.
OVA323-339 uptake
To determine OVA323-339 uptake in BMDCs, OVA peptide (Cambridge Research Biochemicals) was labeled with the AlexaFluor647 microscale protein labeling kit (Life Technologies) as per manufacturer’s instructions. Isolated BMDCs, as described above, were treated with labeled peptide at 1μM with or without the presence of 100 ng/ml LPS. Cells were analyzed via flow cytometric analysis after 24 hours of incubation with labeled OVA for AlexaFluor647 positive cells.
Cytokine analysis
Concentrations of IL-12p70, TNF-α, IL-10, IL-4, IL-17A, IFN-γ (R&D Systems) from cell culture supernatants were determined via ELISA as per manufacturer’s instructions. Following Streptavidin-HRP treatment, ELISAs were developed using TMB substrate solution (eBioscience). Absorbance at wavelength 450 nm was read using a microplate reader (VersaMax tunable microplate reader; Molecular Devices).
Flow cytometric analysis
Single-cell suspensions from in vitro cultures or harvested LNs were analysed by flow cytometry. Cells were washed in flow cytometry staining buffer (PBS with 2% FCS and 0.02% sodium azide) followed by blocking with anti-mouse CD16/32 (2.4G2; BD Bioscience). The following mAbs from BD Biosciences, CD4-PE/V450 (RM4-5), CD80-PE-CF594 (16-10A1), CD40-PE (3/23), eBioscience; CD3-PE-eFluor-610 (145-2C11), CD11b-PerCP-Cy5.5/PE-Cy7 (M1/70), CD11c-PE/PE-Cy7 (N418), MHC-II-FITC/eFluor-450 (M5/114.15.2), Biolegend; PD-L1-PE/APC (10F.9G2), DEC-205-APC (NLDC-145), and Miltenyi Biotec; CD86-FITC/PE (PO3.3) were used at optimally titrated concentrations. For transcription factor staining, cells were fixed and permeabilized with a commercial transcription factor staining kit (eBioscience) according to the manufacturer's instructions, and the cells were stained with the following transcription factors from BD Bioscience—FoxP3-PE/ PE-CF594 (MF23), T-bet-FITC/APC (O4-46), RORγt-BV421 (Q31-378) and eBioscience GATA3-PE (TWAJ). Viable cells were distinguished using LIVE DEAD Aqua (Life Technologies). Populations of interest were gated according to appropriate “fluorescence minus one” controls. Samples were acquired on a CyAn ADP flow cytometer (Beckman Coulter) and were analyzed with FlowJo software (Tree Star).
Statistical analysis
Data are expressed as mean ± SEM and were analyzed by two-way analysis of variance (ANOVA) test or unpaired Student's t-tests (Prism 6; GraphPad Software). Significance for all statistical tests was shown in figures as P < 0.05 (*), P < 0.01 (**), P < 0.001 (***) and P < 0.0001 (****).
Results
Characterization of the S. mansoni worm secretome
A method for the collection of WES from live adult male S. mansoni worms in vitro [26] was adopted for bulk collection of WES (S1A Fig). Only adult male worms were utilized in WES preparations, to exclude any IM secretions from eggs that would contaminate the WES if female worms were included. Several independent batches of concentrated WES were analysed using one-dimensional SDS-PAGE to determine the spectrum of proteins being produced. The protein content of the WES batch was measured, with protein degradation and overall protein profile assessed. There was consistency, with little identifiable variation between the independent batches of WES (S1B Fig). WES Batches that showed no protein degradation, a similar protein profile of positive bands using rabbit polyclonal anti-WES antibody (S1C Fig) and a maximum of 0.5 endotoxin units per mg (EU/mg) were approved for further analysis. Protein yields were estimated to be equivalent to 110 ng of protein per worm within a 72-hour incubation period. Despite attempts to increase yield through elongated culture times there was little effect on the final protein yield.
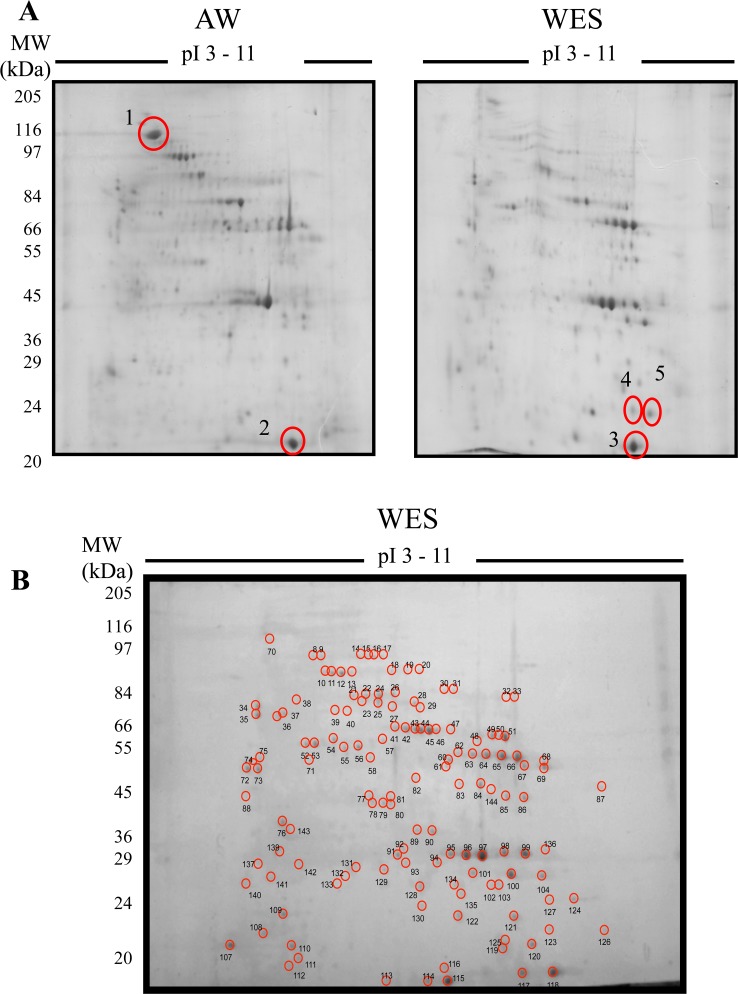
To address the question of whether male adult S. mansoni worms selectively secrete a unique proteome, we compared WES to a soluble adult male worm homogenate (AW) by two-dimensional (2D) SDS-PAGE. AW and WES preparations were resolved in parallel. The overall distribution and intensity of the spots revealed a differing protein composition between the WES and somatic preparations (Fig 1A). The AW preparation appears markedly more complex, supporting the hypothesis that the parasite actively secretes/excretes a specific fraction of molecules. To confirm this visual demarcation, one spot unique to the AW preparation (spot 1), two spots common to both preparations, with similar isoelectric points (pI) and molecular weights (spots 2 and 3), as well as two spots unique to the WES preparation (spots 4 and 5) were selected for identification by mass spectrometry (Fig 1B). Spot 1, which is unique to AW preparation, was identified as S. mansoni Paramyosin (Smp_021920.1), a major structural protein of schistosomes and other invertebrates. Spots 2 and 3, which are common to WES and AW preparations, were identified as S. mansoni Fatty acid-binding protein (Smp_095360.1). S. mansoni fatty acid protein, also known as Sm14, was previously identified in the worm tegument and the S. japonicum homologue had previously been detected in the adult worm excretory-secretory proteome [31]. Spots 4 and 5, which are unique to the WES preparation, were identified as S. mansoni Cyclophilin (Smp_040130), also known as Smp17.7. These results demonstrate that WES and AW preparations have common and unique molecules therefore demonstrating a clear demarcation of the protein profile between these preparations.
Fig 1. Proteomic analysis of WES molecules.
(A) Comparative 2D proteomic analysis between adult male worm somatic molecules (AW) and WES molecules. AW and WES (200 μg) were electrofused in a 3–11 IPG strip and then electrophoresed in a 12% SDS-PAGE stained with colloidal-coomassie blue. Five spots (circled and numbered 1–5) were selected for identification by mass spectrometry. Protein hits: 1 –SmParamyosin (Smp_021920.1); 2–3 –SmFatty acid-binding protein (Smp_095360.1); 4–5 –SmCyclophilin (Smp_040130). (B) A representative gel of S. mansoni WES molecules, analysed as in (A), showing individual spots that were analyzed by mass spectrometry.
To further identify the proteins present in WES, selected spots were picked from 2D gels and subjected to electrospray ionization and tandem mass spectrometry (ESI-MS/MS). A total of 170 spots were subjected to analysis by mass spectrometry, of which 136 (80%) showed positive hits when analysed against the S. mansoni GeneDB sequence database, resulting in the identification of 111 proteins (S1 Table). Homologues of some of the S. mansoni WES proteins identified were also present in two other human-infecting schistosome species, S. japonicum and S. haematobium as determined by BLAST analysis. S. japonicum homologues were detected for all S. mansoni WES proteins, with more than 70% of the homologues (80 proteins) detected having a similarity to their respective S. mansoni homologues higher than 80% (S2 Table). Furthermore, several schistosome antigens have been previously tested as vaccine candidates in different animal models [32]. A number of vaccine candidates were identified in our analysis of the S. mansoni WES proteins (Table 1). Three out of six potential candidates selected by the WHO in a study in the 1990’s were identified; TPI [33], Sm28GST [34] and Sm14 [35]. The ECL (200 kDa protein) [36], Sm21.7 [37], Sm-p80 [38] and Cu-Zn superoxide dismutase [39] were also detected. Two promising candidates, more recently tested, Sm29 [40] and Sm-TSP-2 [41] were not identified among WES molecules. Sm29 [40] and Sm-TSP-2 [41] have been characterized as membrane proteins highly expressed in the schistosome tegument and therefore are not expected to be excretory-secretory proteins [40].
Table 1. Vaccine candidates detected in the S. mansoni adult male worm secretome.
| ID | Protein description | Vaccine name | Vaccine type | Reference |
|---|---|---|---|---|
| Smp_003990 | Triosephosphate isomerase, putative | TPI | Transfer of anti-TPI monoclonal antibody | [33] |
| Smp_017730 | 200-kDa GPI-anchored surface glycoprotein | ECL (200 kDa protein) | DNA | [36] |
| Smp_054160 | Glutathione S-transferase 28 kDa (GST 28) (GST class-mu), putative | Sm28GST | DNA | [34] |
| Smp_086480 | Antigen Sm21.7, putative | Sm21.7 | Recombinant protein | [37] |
| Smp_095360.1 | Fatty acid binding protein | Sm14 | Recombinant protein | [35] |
| Smp_157500 | Calpain (C02 family) | Sm-p80 | DNA vaccine + recombinant protein boost | [38] |
| Smp_176200.2 | Cu-Zn superoxide dismutase | Cu-Zn superoxide dismutase | DNA | [39] |
ID–identification number at GeneDB.
WES molecules were analysed for predicted signal peptides, both classical N-terminal and non-classical internal. A small number of proteins had a classical signal peptide (6.3%; 8/111) while the majority of signal peptide containing proteins were non-classical (41.4%; 46/111). These proteins were classified as secretory. Three WES molecules were predicted to be trans-membrane (2.7%; 3/111) and the remaining contained no signal peptide (47.7%; 53/111), and were classified as excretory. The ratio however, of secreted to non-secreted proteins may have been influenced by such predictive software optimised using mammalian, rather than parasitic proteins [42].
S. mansoni WES proteins were screened for the presence of immunomodulatory candidates through homology analysis with other helminth proteins with known immunomodulatory activity (Table 2). Based on this analysis, five S. mansoni WES proteins were considered as potential immunomodulatory candidates: Calreticulin auto-antigen homologue precursor, Serpin, Peroxiredoxin 1 and two Cyclophilin proteins. S. mansoni Calreticulin auto-antigen homologue precursor contains a signal peptide and Serpin and Peroxiredoxin are predicted to be non-classically secreted with a SecP score of 0.601 and 0.597, respectively. The S. mansoni Cyclophilin A protein is predicted to be secreted via a non-classical pathway, while Cyclophilin B contains a signal peptide. Based on strong sequence homology we selected SmCypA to examine further for immunomodulatory activity.
Table 2. S. mansoni adult male WES proteins with immune-modulatory homologs.
| S. mansoni WES | Immunomodulatory helminth homologs | |||||
|---|---|---|---|---|---|---|
| ID | Protein description | GenBank ID | Description | Species | Immunomo—dulatory activity | Ref. |
| Smp_030370: Calreticulin autoantigen homolog precursor, putative | AAR99585.1 | Calreticulin-like protein | Haemonchus contortus | Binds to complement C1q inhibiting the classical complement pathway | [43] | |
| CAL30086.1 | Calreticulin precursor | Heligmosomoides polygyrus | Th2-skewing property | [44] | ||
| CAA07254.1 | Calreticulin | Necator americanus | Binds to complement C1q inhibiting the classical complement pathway | [45] | ||
| Smp_090080 | Serpin, putative | AAB65744.1 | Serpin precursor (Bm-spn-2) | Brugia malayi | Specific inhibition of neutrophil proteinases cathepsin G and neutrophil elastase | [46] |
| Smp_059480 | PeroxiredoxinPrx1 | AAB71727.1 | Peroxiredoxin | Fasciola hepatica | Induction of alternatively activated macrophages | [47] |
| Smp_040130 | Cyclophilin A | EPT31956.1 | Peptidyl-prolyl cis-trans isomerase | Toxoplasma gondii | Interacts with CCR5 receptor in DCs and Mϕ | [48] |
| Smp_040790 | Cyclophilin B, putative | |||||
ID–identification number at GeneDB or GenBank databases.
Production of recombinant SmCypA
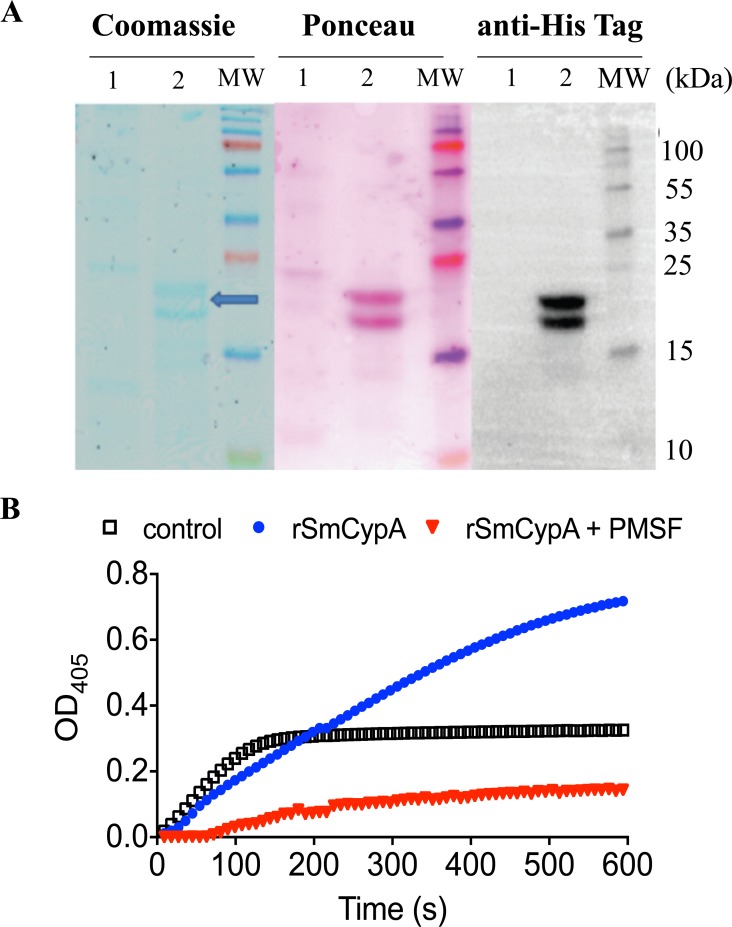
rSmCypA was expressed in insect cells and after Nickel and size chromatography purification, two bands, with a molecular weight of 18–22 kDa, which corresponds to predicted characteristics of the cyclophilin family [49, 50] were detected, following coomassie staining of SDS-PAGE resolved proteins. Western blot, using an anti-His tag, confirmed the predicted size of the recombinant protein (Fig 2A). To determine if rSmCypA was enzymatically active, the cis to trans isomerization of succinyl-Ala-Ala-Pro-Phe-4-nitroanilide was measured using the standard protease-coupled assay. rSmCypA was found to have PPIase activity, 30 μg/min, with increased Suc-AAPF-pNA cleavage to levels above that of the control reaction of chymotrypsin-α alone (Fig 2B). Furthermore, by inhibition of the signal using the chymotrypsin-α inhibitor phenylmethanesulfonylfluoride (PMSF), the PPIase activity was determined to be chymotrypsin-α dependent (Fig 2B). Therefore, the recombinant SmCypA protein generated purified as an enzymatically active protein.
Fig 2. Production of rSmCypA.
(A) Coomassie stain of SDS-PAGE gel containing WES (Lane 1) and rSmCypA (Lane 2), with a subsequent Ponceau stain following western transfer and Anti-His tag expression to identify recombinant protein. Molecular Weight (MW) markers are shown. (B) Quantification of PPIase activity of rSmCypA in the presence or absence of PMSF. Figure is representative of three independent experiments.
SmCypA alters BMDC function and LPS activation
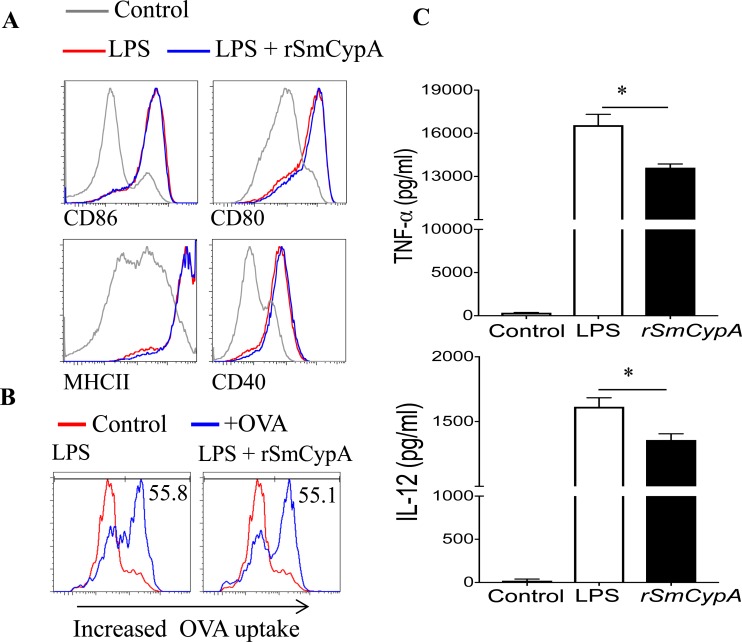
Given that cyclophilins from other species have been reported to modulate BMDC function [49], we sought to demonstrate whether rSmCypA has a similar activity. TLRs have been shown to play a key role in the recognition of helminth products by the host, and certain helminth products have been shown to modulate the activation of DC in response to LPS activation [51, 52]. Therefore, BMDC were treated with rSmCypA during simultaneous activation with LPS for 24 hours. Representative example of gating strategy for flow cytometry analysis of BMDC and the effect of LPS treatment on DC activation is shown (S2 Fig). Treatment with rSmCypA during LPS induced activation did not have an effect on cell surface expression levels of several molecules (CD80, CD86, MHCII, CD40) involved in DC antigen presentation (Fig 3A).
Fig 3. Immune modulatory effects of rSmCypA on BMDC.
(A) Representative histograms from flow cytometric analysis of LPS treated and LPS + rSmCypA co-treated BMDC for cell surface expression of CD86, CD80, MHCII and CD40. Data shown are representative of 5 independent experiments. (B) Representative histograms of BMDC for Alexa Fluor-647 labeled OVA323-339 uptake following LPS induced stimulation with or without simultaneous treatment with rSmCypA. Data shown are representative of 2 independent experiments. (C) ELISA for the quantification of TNF-α and IL-12 in the supernatant of BMDC treated with LPS only or co-treated with LPS and rSmCypA, n = 3 per group. Data shown are representative of 2 independent experiments. Data are presented as mean and SEM and statistical difference between groups was determined using Student's t test.
We then assessed the capacity of rSmCypA treated BMDC to uptake antigen. For that purpose, OVA323-339 was labeled with Alexa Fluor-647 and antigen uptake by LPS-only treated or LPS activated and rSmCypA co-treated BMDC was assessed by flow cytometric analysis. The uptake, by DC, of antigen and presentation capacity of labeled OVA323-339 was not altered following co-treatment with LPS and rSmCypA (Fig 3B). Interestingly, despite the inability of rSmCypA to alter BMDC cell surface expression of several co-stimulatory molecules, rSmCypA altered BMDC pro-inflammatory cytokine production, resulting in significantly (P<0.05) reduced expression of TNF-a and IL-12 (Fig 3C).
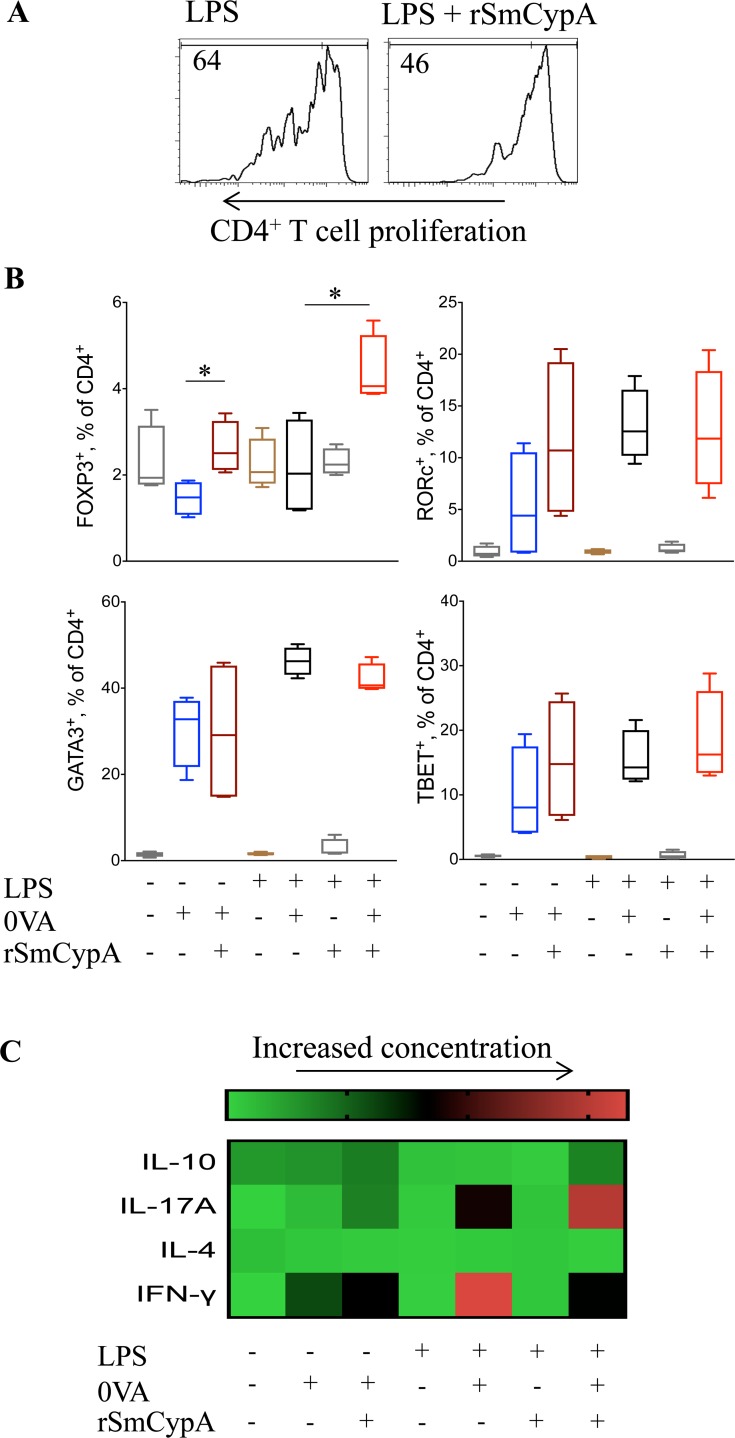
We next assessed the capacity of BMDC co-treated with LPS and rSmCypA to process and present antigens and subsequently drive T cell activation. CD4+ T cells isolated from TCROVA mice were labeled with CFSE and cultured in the presence of OVA with LPS + rSmCypA co-treated BMDC. rSmCypA treatment of BMDC did not alter the viability of TCROVA CD4+ T cells, but reduced their ability to evoke antigen-specific proliferation of T cells (Fig 4A). These results indicate that the reduced CD4+ T cell proliferation in response to rSmCypA treated BMDC, could potentially be the effect of altered T cell skewing with a preferential expansion of suppressive Treg cells. BMDC with LPS stimulation and rSmCypA treatment were cultured with TCROVA CD4+ T cells ± antigen (Fig 4B). Interestingly, rSmCypA-treated BMDC induced significantly (P<0.05) increased Foxp3+ Treg cell expansion but reduced Gata3+ Th2, while Rorc+ Th17 cell expansion had a modest increase (Fig 4B). The rSmCypA expansion of Treg cells was both dose and antigen dependent and it occurs irrespectively of LPS-induced stimulation (Fig 4B). In accordance with a preferential expansion of Foxp3+ Treg cells, when BMDC co-treated with rSmCypA and LPS were used as APC for the activation of TCROVA CD4+ T cells there is a significant (P<0.05) increase in secreted IL-10 and a decrease (P<0.05) in IFN-γ, marked elevated IL-17A, while IL-4 levels remained unchanged (Fig 4C).
Fig 4. Modulation of CD4+ T cells by SmCypA-treated BMDC.
(A) Representative histograms from flow cytometric analysis of CFSE labeled TCROVA CD4+ T cell cultures with OVA and LPS treated or LPS and rSmCypA co-treated BMDC as APC. (B) Flow cytometric analysis for the identification of Treg (FOXP3+), Th17 (RORc+), Th2 (GATA3+) and Th1 (TBET+) CD4+ T cells following culture with OVA and BMDC as APC, activated under the outlined conditions, n = 4 per group. (C) Heatmap of ELISA for the detection of IL-10, IL-17A, IFN-γ and IL-4 in the supernatant of TCROVA CD4+ T cells and BMDC co-cultures. BMDC were activated, or not, under the indicated conditions prior to their co-culture with the TCROVA CD4+ T cells ± antigen (OVA), n = 6 per group. Data are presented as mean and SEM and statistical difference between groups was determined using Student's t test.
Discussion
S. mansoni infection can modulate host immunity with the molecules that are excreted or secreted from adult worms acting at the interface of engagement with the immune system of the infected host. The characterisation of WES will potentially be of great significance in the identification of novel therapeutic targets for S. mansoni and other Schistosoma species’ infections of man [53, 54]. Several previously proposed vaccine candidates were found to be located in extracellular vesicles of adult S. mansoni worms [55]. In addition to the identification of vaccine candidates, adult S. mansoni worm proteome has previously been serologically screened for the successful detection of biomarkers and infection susceptibility markers [56]. In our study, 111 proteins of the S. mansoni male WES were identified by mass-spectometric analysis. This led to the identification of 7 previously proposed vaccine candidates and 5 molecules with potential immunomodulatory activity. Over 70% of the identified S. mansoni WES proteins share high homology with proteins described in S. japonicum. Our data show a clear demarcation of the WES and the AW of S. mansoni, revealing that only a fraction of the S. mansoni worm proteins are excreted/secreted. It should be noted that WES and AW used in this study were prepared from adult sexually mature male worms, with female worms not included in antigen preparations. This was to remove female worms and therefore presence of eggs, and release of egg secretions during in vitro culture, and prevent the potential confounding effects of the potent IM in eggs [57]. Further, infections of mice with male worms only have been shown to induce marked modulation of the immune system of the host [8]. Amongst the WES proteins, an enzymatically active homologue of human CypA was identified. SmCypA showed immunomodulatory activity in in vitro cell culture assays, with alterations in DC function and cytokine production leading to a DC mediated preferential expansion of CD4+ Treg cells.
Treg cells are important for limiting immunopathology during helminth infections, with significant expansion of these cells during helminth infections [9]. Evidence suggests that this effect is restricted to live helminths, indicating that Treg cell expansion is a result of released components found in the parasite’s WES, with TLR ligands playing a key role in DC modulation and subsequent Treg cell expansion [17, 58]. In this study, we identified SmCypA as a WES component that modulates DC to induce Treg cell expansion in vitro. It remains to fully characterise the FoxP3+ Treg cells that are expanded by SmCypA treated BMDC in vitro and further address the functionality of such cells in mediating the suppression of bystander T cells. Further, in the context of in vivo functions of SmCypA, it would be important to determine if modulation of DC by SmCypA induced Treg cells in vivo.
Cyclophilins are a group of proteins that have peptidyl-prolyl cis-trans isomerase activity, which have been identified in both prokaryotes and eukaryotes. These proteins are widely expressed and are found in several subcellular compartments at micromolar levels [59]. Cyclophilins have broad functionality, including roles as chaperones and cell signalling molecules [60, 61]. Initially identified as intracellular proteins, later studies showed that CypA and CypB could be secreted under conditions of stress. However, the exact mechanisms and degree of secretion of CypA and CypB are not fully understood [62, 63]. Elucidating these mechanisms would provide a greater understanding of the roles of cyclophilins during inflammation. The first member of the cyclophilins to be identified in mammals, cyclophilin A, is the major cellular target for the immunosuppressive drug cyclosporin A (CsA) as well as newly developed small molecule analogs of CsA that have been shown to limit inflammation and injury by inhibiting neutrophilia in a model of LPS induced acute lung injury [63].
Identification of SmCypA in the adult male worm secretome, highlights the different means by which species can produce similar proteins as part of their immune-suppressive stratagem (as demonstrated by S2 Table). Interestingly, a CypA homologue has been identified and shown to be present in all developmental stages of S. japonicum and in both male and female worms [64]. In S.mansoni CypA was detected by PCR in both adult male and females worms (S3 Fig). Furthermore, S.mansoni-infected patients have IgE responses against the protein [65], supporting that SmCypA is recognized by the infected host during infection. While these studies highlight the potential importance of schistosome CypA in modulation of the host’s immune system, in the context of human schistosomiasis we have not explored if SmCypA can modulate human DC and T cells.
Several recent studies show that helminths have the ability to alter DC function leading to the generation of DC that can significantly dampen immune responses. Adoptive transfer of BMDC exposed to helminth homogenates, resulted in CD4+ T cell IL-10 mediated disease suppression in an experimental model of colitis [66]. Chronic helminth infections have additionally been shown to evoke a preferential expansion of CD11clo DC that are less capable at inducing T cell proliferation and Th2 cytokine secretion in comparison to CD11chi DC [67]. Despite recent advances, the mechanisms that lead to alterations in DC function during S. mansoni infection are not fully understood. While previous studies show that predominantly helminth WES and not AW components are able to modulate DC function and result in the generation of a Th2/Treg skewed response [68, 69], limited individual WES components that induce Treg cells have been described, including a H. polygyrus TGF-β homologue [70]
We have not explored how rSmCypA interacts with BMDC and thereby modulates their function. Studies by Zhu et al., have highlighted that recombinant human CypA binds the CD147 receptor with ligation inducing elevated pERK, pStat3 and pAkt [71]. Whereas, CypA derived from Histoplasma capsulatum has been described as binding to the VLA-5 receptor on DC that modifies its adhesion properties [49]. While these studies showed extracellular CypA to have a preferentially pro-inflammatory effect [49, 71], in this study we show that rSmCypA has a DC immune-regulating capacity that attenuates the veracity of DC mediated T cell activation by specifically inducing a Treg cell response. It is possible therefore that rSmCypA competes with the abundant endogenous host CypA during infection to modulate the infected host’s immune system. This highlights that further work is required to elucidate the exact mechanism(s) of action for rSmCypA modulation of DC.
Whilst it is clear that Schistosoma species may utilize CypA as an immune modulator, it must be emphasized that this is just one of the potential IM that are produced by the parasite. A number of the other molecules in WES may also have immunomodulatory activity. In addition to this, understanding their composition, relative to each other, is key to elucidate how elegantly helminths modulate immune responses. Further work will be required to carefully delineate the vast array of molecules which helminths generate and to understand how this unique composition of IM act together for immune modulation.
Supporting information
(A) Infected mice were perfused 7 weeks post-infection for collection of adult worms. Males were carefully separated from females and transferred to a dialysis bag containing incubation media, in a cell culture flask with nutrient media. Worms were then incubated for 72 hours and the incubation media harvested. (B) Quality control of WES batches was performed by silver staining in three independent batches (B1, B2 and B3 above). (C) Western blot of preparations with polyclonal anti-WES rabbit serum (1:1400 dilution) and HRP-conjugated anti-rabbit IgG (1:2000 dilution). Serum collected prior to rabbit (normal rabbit serum—NRS) immunization with WES molecules was used as a negative control.
(TIFF)
Representative example of the gating strategy followed for the flow cytometry analysis of BMDC and following activation of BMDC with LPS.
(TIFF)
(A) Representative images of agarose gel electrophoresis of PCR products for expression of SmCypa (mRNA) and housekeeping genes Pai1 (mRNA) and Gapdh (mRNA) by male or female adult S. mansoni worms. (B) Fold expression change of SmCypa mRNA for male only and female only S. mansoni adult worms, compared to housekeeping genes Pai1 or Gapdh, n = 3, data are presented as mean and SEM.
(TIFF)
ID–identification number at GeneDB; MS–mowse score; CO—percentage of sequence coverage; SP–signal peptide; SecP–SecretomeP score, values above 0.5 indicate possible secretion; TM—number of transmembrane domains; (+)—signal peptide detected; (-) no signal peptide or transmembrane domain detected.
(DOCX)
S. mansoni WES proteins were subjected to similarity analysis using BLAST. Sequence alignments that showed a bit score higher than 30 and an E value lower than 1e-16 were considered homologs. ID–identification number at GeneDB or GenBank databases; (-)–no homologs found.
(DOCX)
Acknowledgments
We are grateful for assistance of Dr Catherine Botting, Emma Buchet and Emily Hams.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Science Foundation Ireland. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet. 2014;383(9936):2253–64. doi: 10.1016/S0140-6736(13)61949-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mott KE IMC, Lopez A, Mathers CD, editors. Schistosomiasis The Global Epidemiology of Infectious Diseases Geneva: World Health Organization; 2004. [Google Scholar]
- 3.Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, Jacobson J. Review series Helminth infections: the great neglected tropical diseases. 2008;118:1311–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maizels RM, Balic A, Gomez-Escobar N, Nair M, Taylor MD, Allen JE. Helminth parasites—masters of regulation. Immunological reviews. 2004;201:89–116. doi: 10.1111/j.0105-2896.2004.00191.x [DOI] [PubMed] [Google Scholar]
- 5.Khan AR, Fallon PG. Helminth therapies: Translating the unknown unknowns to known knowns. Int J Parasitol. 2013:4–11. [DOI] [PubMed] [Google Scholar]
- 6.Meurs L, Mbow M, Boon N, Vereecken K, Amoah AS, Labuda LA, et al. Cytokine Responses to Schistosoma mansoni and Schistosoma haematobium in Relation to Infection in a Co-endemic Focus in Northern Senegal. Plos Neglect Trop D. 2014;8(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pearce EJ, Caspar P, Grzych JM, Lewis FA, Sher A. Downregulation of Th1 Cytokine Production Accompanies Induction of Th2 Responses by a Parasitic Helminth, Schistosoma mansoni. J Immunol. 2012;189(3):1104–11. [PubMed] [Google Scholar]
- 8.Fallon PG, Mangan NE. Suppression of TH2-type allergic reactions by helminth infection. Nature reviews Immunology. 2007;7:220–30. doi: 10.1038/nri2039 [DOI] [PubMed] [Google Scholar]
- 9.Maizels RM, McSorley HJ. Regulation of the host immune system by helminth parasites. J Allergy Clin Immunol. 2016;138(3):666–75. doi: 10.1016/j.jaci.2016.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fallon PG, Alcami A. Pathogen-derived immunomodulatory molecules: future immunotherapeutics? Trends in immunology. 2006;27:470–6. doi: 10.1016/j.it.2006.08.002 [DOI] [PubMed] [Google Scholar]
- 11.Hewitson JP, Grainger JR, Maizels RM. Helminth immunoregulation: The role of parasite secreted proteins in modulating host immunity. Mol Biochem Parasit. 2009;167:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harnett W, Harnett MM. Helminth-derived immunomodulators: can understanding the worm produce the pill? Nature reviews Immunology. 2010;10:278–84. doi: 10.1038/nri2730 [DOI] [PubMed] [Google Scholar]
- 13.Adisakwattana P, Saunders SP, Nel HJ, Fallon PG. Helminth-derived immunomodulatory molecules. Adv Exp Med Biol. 2009;666:95–107. [DOI] [PubMed] [Google Scholar]
- 14.Fallon PG, Richardson EJ, Smith P, Dunne DW. Elevated type 1, diminished type 2 cytokines and impaired antibody response are associated with hepatotoxicity and mortalities during Schistosoma mansoni infection of CD4-depleted mice. Eur J Immunol. 2000;30(2):470–80. doi: 10.1002/1521-4141(200002)30:2<470::AID-IMMU470>3.0.CO;2-T [DOI] [PubMed] [Google Scholar]
- 15.Redpath SA, van der Werf N, Cervera AM, MacDonald AS, Gray D, Maizels RM, et al. ICOS controls Foxp3(+) regulatory T-cell expansion, maintenance and IL-10 production during helminth infection. Eur J Immunol. 2013;43(3):705–15. doi: 10.1002/eji.201242794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Werf N, Redpath SA, Azuma M, Yagita H, Taylor MD. Th2 cell-intrinsic hypo-responsiveness determines susceptibility to helminth infection. Plos Pathog. 2013;9(3):e1003215 doi: 10.1371/journal.ppat.1003215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McSorley HJ, Harcus YM, Murray J, Taylor MD, Maizels RM. Expansion of Foxp3+ regulatory T cells in mice infected with the filarial parasite Brugia malayi. J Immunol. 2008;181(9):6456–66. [DOI] [PubMed] [Google Scholar]
- 18.Ruyssers NE, De Winter BY, De Man JG, Loukas A, Pearson MS, Weinstock JV, et al. Therapeutic potential of helminth soluble proteins in TNBS-induced colitis in mice. Inflamm Bowel Dis. 2009;15(4):491–500. doi: 10.1002/ibd.20787 [DOI] [PubMed] [Google Scholar]
- 19.Villadangos JA, Schnorrer P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat Rev Immunol. 2007;7(7):543–55. doi: 10.1038/nri2103 [DOI] [PubMed] [Google Scholar]
- 20.Dearman R. Dendritic cell biology: Current state of the art. Toxicol Lett. 2009;189:S25–S. [Google Scholar]
- 21.Maizels RM, Hewitson JP. Myeloid Cell Phenotypes in Susceptibility and Resistance to Helminth Parasite Infections. Microbiol Spectr. 2016;4(6). [DOI] [PubMed] [Google Scholar]
- 22.Smith P, Walsh CM, Mangan NE, Fallon RE, Sayers JR, McKenzie AN, et al. Schistosoma mansoni worms induce anergy of T cells via selective up-regulation of programmed death ligand 1 on macrophages. J Immunol. 2004;173(2):1240–8. [DOI] [PubMed] [Google Scholar]
- 23.Mangan NE, Fallon RE, Smith P, van Rooijen N, McKenzie AN, Fallon PG. Helminth infection protects mice from anaphylaxis via IL-10-producing B cells. J Immunol. 2004;173(10):6346–56. [DOI] [PubMed] [Google Scholar]
- 24.Amu S, Saunders SP, Kronenberg M, Mangan NE, Atzberger A, Fallon PG. Regulatory B cells prevent and reverse allergic airway inflammation via FoxP3-positive T regulatory cells in a murine model. J Allergy Clin Immunol. 2010;125(5):1114–24 doi: 10.1016/j.jaci.2010.01.018 [DOI] [PubMed] [Google Scholar]
- 25.Smith P, Mangan NE, Walsh CM, Fallon RE, McKenzie AN, van Rooijen N, et al. Infection with a helminth parasite prevents experimental colitis via a macrophage-mediated mechanism. J Immunol. 2007;178(7):4557–66. [DOI] [PubMed] [Google Scholar]
- 26.Khan AR, Amu S, Saunders SP, Fallon PG. The generation of regulatory B cells by helminth parasites. Methods in molecular biology (Clifton, NJ). 2014;1190:143–62. [DOI] [PubMed] [Google Scholar]
- 27.Fallon PG, Smith P, Dunne DW. Type 1 and type 2 cytokine-producing mouse CD4+ and CD8+ T cells in acute Schistosoma mansoni infection. European journal of immunology. 1998;28:1408–16. doi: 10.1002/(SICI)1521-4141(199804)28:04<1408::AID-IMMU1408>3.0.CO;2-H [DOI] [PubMed] [Google Scholar]
- 28.Kofron JL, Kuzmic P, Kishore V, Colón-Bonilla E, Rich DH. Determination of kinetic constants for peptidyl prolyl cis-trans isomerases by an improved spectrophotometric assay. Biochemistry. 1991;30:6127–34. [DOI] [PubMed] [Google Scholar]
- 29.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223(1):77–92. [DOI] [PubMed] [Google Scholar]
- 30.Khan AR, Hams E, Floudas A, Sparwasser T, Weaver CT, Fallon PG. PD-L1hi B cells are critical regulators of humoral immunity. Nature Communications. 2015;6:5997 doi: 10.1038/ncomms6997 [DOI] [PubMed] [Google Scholar]
- 31.Liu F, Cui S-J, Hu W, Feng Z, Wang Z-Q, Han Z-G. Excretory/secretory proteome of the adult developmental stage of human blood fluke, Schistosoma japonicum. Molecular & cellular proteomics: MCP. 2009;8:1236–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tebeje BM, Harvie M, You H, Loukas A, McManus DP. Schistosomiasis vaccines: where do we stand? Parasit Vectors. 2016;9(1):528 doi: 10.1186/s13071-016-1799-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reynolds SR, Dahl CE, Harn DA. T and B epitope determination and analysis of multiple antigenic peptides for the Schistosoma mansoni experimental vaccine triose-phosphate isomerase. Journal of immunology 1994;152:193–200. [PubMed] [Google Scholar]
- 34.Dupré L, Kremer L, Wolowczuk I, Riveau G, Capron A, Locht C. Immunostimulatory effect of IL-18-encoding plasmid in DNA vaccination against murine Schistosoma mansoni infection. Vaccine. 2001;19:1373–80. [DOI] [PubMed] [Google Scholar]
- 35.Tendler M, Brito CA, Vilar MM, Serra-Freire N, Diogo CM, Almeida MS, et al. A Schistosoma mansoni fatty acid-binding protein, Sm14, is the potential basis of a dual-purpose anti-helminth vaccine. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nascimento EJM, Amorim RV, Cavalcanti A, Alves VF, Nakazawa M, Pereira VRA, et al. Assessment of a DNA vaccine encoding an anchored-glycosylphosphatidylinositol tegumental antigen complexed to protamine sulphate on immunoprotection against murine schistosomiasis. Memórias do Instituto Oswaldo Cruz. 2007;102:21–7. [DOI] [PubMed] [Google Scholar]
- 37.Francis P, Bickle Q. Cloning of a 21.7-kDa vaccine-dominant antigen gene of Schistosoma mansoni reveals an EF hand-like motif. Mol Biochem Parasit. 1992;50:215–24. [DOI] [PubMed] [Google Scholar]
- 38.Ahmad G, Zhang W, Torben W, Haskins C, Diggs S, Noor Z, et al. Prime-boost and recombinant protein vaccination strategies using Sm-p80 protects against Schistosoma mansoni infection in the mouse model to levels previously attainable only by the irradiated cercarial vaccine. Parasitol Res. 2009;105:1767–77. doi: 10.1007/s00436-009-1646-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shalaby KA, Yin L, Thakur A, Christen L, Niles EG, LoVerde PT. Protection against Schistosoma mansoni utilizing DNA vaccination with genes encoding Cu/Zn cytosolic superoxide dismutase, signal peptide-containing superoxide dismutase and glutathione peroxidase enzymes. Vaccine. 2003;22:130–6. [DOI] [PubMed] [Google Scholar]
- 40.Cardoso FC, Macedo GC, Gava E, Kitten GT, Mati VL, de Melo AL, et al. Schistosoma mansoni tegument protein Sm29 is able to induce a Th1-type of immune response and protection against parasite infection. Plos Neglect Trop D. 2008;2:e308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tran MH, Pearson MS, Bethony JM, Smyth DJ, Jones MK, Duke M, et al. Tetraspanins on the surface of Schistosoma mansoni are protective antigens against schistosomiasis. Nature medicine. 2006;12:835–40. doi: 10.1038/nm1430 [DOI] [PubMed] [Google Scholar]
- 42.Cass CL, Johnson JR, Califf LL, Xu T, Hernandez HJ, Stadecker MJ, et al. Proteomic analysis of Schistosoma mansoni egg secretions. Mol Biochem Parasit. 2007;155:84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naresha S, Suryawanshi A, Agarwal M, Singh BP, Joshi P. Mapping the complement C1q binding site in Haemonchus contortus calreticulin. Mol Biochem Parasit. 2009;166:42–6. [DOI] [PubMed] [Google Scholar]
- 44.Rzepecka J, Rausch S, Klotz C, Schnöller C, Kornprobst T, Hagen J, et al. Calreticulin from the intestinal nematode Heligmosomoides polygyrus is a Th2-skewing protein and interacts with murine scavenger receptor-A. Molecular immunology. 2009;46:1109–19. doi: 10.1016/j.molimm.2008.10.032 [DOI] [PubMed] [Google Scholar]
- 45.Kasper G, Brown A, Eberl M, Vallar L, Kieffer N, Berry C, et al. A calreticulin-like molecule from the human hookworm Necator americanus interacts with C1q and the cytoplasmic signalling domains of some integrins. Parasite Immunol. 2001;23:141–52. [DOI] [PubMed] [Google Scholar]
- 46.Zang X, Yazdanbakhsh M, Jiang H, Kanost MR, Maizels RM. A novel serpin expressed by blood-borne microfilariae of the parasitic nematode Brugia malayi inhibits human neutrophil serine proteinases. Blood. 1999;94:1418–28. [PubMed] [Google Scholar]
- 47.Donnelly S, Stack CM, O'Neill SM, Sayed AA, Williams DL, Dalton JP. Helminth 2-Cys peroxiredoxin drives Th2 responses through a mechanism involving alternatively activated macrophages. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2008;22:4022–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Golding H, Aliberti J, King LR, Manischewitz J, Andersen J, Valenzuela J, et al. Inhibition of HIV-1 infection by a CCR5-binding cyclophilin from Toxoplasma gondii. Blood. 2003;102:3280–6. doi: 10.1182/blood-2003-04-1096 [DOI] [PubMed] [Google Scholar]
- 49.Gomez FJ, Pilcher-Roberts R, Alborzi A, Newman SL. Histoplasma capsulatum cyclophilin A mediates attachment to dendritic cell VLA-5. Journal of immunology 2008;181:7106–14. [DOI] [PubMed] [Google Scholar]
- 50.Han H, Peng J, Hong Y. Molecular cloning and characterization of a cyclophilin A homologue from Schistosoma japonicum. 2012:807–17. doi: 10.1007/s00436-012-2903-0 [DOI] [PubMed] [Google Scholar]
- 51.Kane CM, Cervi L, Sun J, McKee AS, Masek KS, Shapira S, et al. Helminth antigens modulate TLR-initiated dendritic cell activation. J Immunol. 2004;173(12):7454–61. [DOI] [PubMed] [Google Scholar]
- 52.Cervi L, MacDonald AS, Kane C, Dzierszinski F, Pearce EJ. Cutting edge: dendritic cells copulsed with microbial and helminth antigens undergo modified maturation, segregate the antigens to distinct intracellular compartments, and concurrently induce microbe-specific Th1 and helminth-specific Th2 responses. J Immunol. 2004;172(4):2016–20. [DOI] [PubMed] [Google Scholar]
- 53.Curwen RS, Ashton PD, Johnston DA, Wilson RA. The Schistosoma mansoni soluble proteome: a comparison across four life-cycle stages. Mol Biochem Parasitol. 2004;138(1):57–66. doi: 10.1016/j.molbiopara.2004.06.016 [DOI] [PubMed] [Google Scholar]
- 54.Sotillo J, Toledo R, Mulvenna J, Loukas A. Exploiting Helminth-Host Interactomes through Big Data. Trends Parasitol. 2017. [DOI] [PubMed] [Google Scholar]
- 55.Sotillo J, Pearson M, Potriquet J, Becker L, Pickering D, Mulvenna J, et al. Extracellular vesicles secreted by Schistosoma mansoni contain protein vaccine candidates. Int J Parasitol. 2016;46(1):1–5. doi: 10.1016/j.ijpara.2015.09.002 [DOI] [PubMed] [Google Scholar]
- 56.Ludolf F, Patrocinio PR, Correa-Oliveira R, Gazzinelli A, Falcone FH, Teixeira-Ferreira A, et al. Serological screening of the Schistosoma mansoni adult worm proteome. PLoS Negl Trop Dis. 2014;8(3):e2745 doi: 10.1371/journal.pntd.0002745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meevissen MH, Yazdanbakhsh M, Hokke CH. Schistosoma mansoni egg glycoproteins and C-type lectins of host immune cells: molecular partners that shape immune responses. Exp Parasitol. 2012;132(1):14–21. doi: 10.1016/j.exppara.2011.05.005 [DOI] [PubMed] [Google Scholar]
- 58.Layland LE, Rad R, Wagner H, da Costa CU. Immunopathology in schistosomiasis is controlled by antigen-specific regulatory T cells primed in the presence of TLR2. Eur J Immunol. 2007;37(8):2174–84. doi: 10.1002/eji.200737063 [DOI] [PubMed] [Google Scholar]
- 59.Colgan J, Asmal M, Neagu M, Yu B, Schneidkraut J, Lee Y, et al. Cyclophilin A regulates TCR signal strength in CD4+ T cells via a proline-directed conformational switch in Itk. Immunity. 2004;21(2):189–201. doi: 10.1016/j.immuni.2004.07.005 [DOI] [PubMed] [Google Scholar]
- 60.Marin-Menendez A, Monaghan P, Bell A. A family of cyclophilin-like molecular chaperones in Plasmodium falciparum. Mol Biochem Parasitol. 2012;184(1):44–7. doi: 10.1016/j.molbiopara.2012.04.006 [DOI] [PubMed] [Google Scholar]
- 61.Saleh T, Jankowski W, Sriram G, Rossi P, Shah S, Lee KB, et al. Cyclophilin A promotes cell migration via the Abl-Crk signaling pathway. Nat Chem Biol. 2016;12(2):117–23. doi: 10.1038/nchembio.1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fearon P, Lonsdale-Eccles AA, Ross OK, Todd C, Sinha A, Allain F, et al. Keratinocyte secretion of cyclophilin B via the constitutive pathway is regulated through its cyclosporin-binding site. J Invest Dermatol. 2011;131(5):1085–94. doi: 10.1038/jid.2010.415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nishioku T, Dohgu S, Koga M, Machida T, Watanabe T, Miura T, et al. Cyclophilin A secreted from fibroblast-like synoviocytes is involved in the induction of CD147 expression in macrophages of mice with collagen-induced arthritis. J Inflamm (Lond). 2012;9(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li J, Zhuang W, Cong L, Shi W, Cai X, Huang F, et al. Cyclophilin A from Schistosoma japonicum promotes a Th2 response in mice. Parasite Vector. 2013;6:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Farnell EJ, Tyagi N, Ryan S, Chalmers IW, Moira APD, Jones FM, et al. Known allergen structures predict Schistosoma mansoni IgE-binding antigens in human infection. 2015;6:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matisz CE, Leung G, Reyes JL, Wang A, Sharkey KA, McKay DM. Adoptive transfer of helminth antigen-pulsed dendritic cells protects against the development of experimental colitis in mice. Eur J Immunol. 2015;45(11):3126–39. doi: 10.1002/eji.201545579 [DOI] [PubMed] [Google Scholar]
- 67.Smith KA, Hochweller K, Hammerling GJ, Boon L, MacDonald AS, Maizels RM. Chronic helminth infection promotes immune regulation in vivo through dominance of CD11cloCD103- dendritic cells. J Immunol. 2011;186(12):7098–109. doi: 10.4049/jimmunol.1003636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Segura M, Su Z, Piccirillo C, Stevenson MM. Impairment of dendritic cell function by excretory-secretory products: a potential mechanism for nematode-induced immunosuppression. Eur J Immunol. 2007;37(7):1887–904. doi: 10.1002/eji.200636553 [DOI] [PubMed] [Google Scholar]
- 69.McSorley HJ, O'Gorman MT, Blair N, Sutherland TE, Filbey KJ, Maizels RM. Suppression of type 2 immunity and allergic airway inflammation by secreted products of the helminth Heligmosomoides polygyrus. Eur J Immunol. 2012;42(10):2667–82. doi: 10.1002/eji.201142161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grainger JR, Smith KA, Hewitson JP, McSorley HJ, Harcus Y, Filbey KJ, et al. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-beta pathway. J Exp Med. 2010;207(11):2331–41. doi: 10.1084/jem.20101074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu D, Wang Z, Zhao J-J, Calimeri T, Meng J, Hideshima T, et al. The Cyclophilin A–CD147 complex promotes the proliferation and homing of multiple myeloma cells. Nature Medicine. 2015;21:572–80. doi: 10.1038/nm.3867 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Infected mice were perfused 7 weeks post-infection for collection of adult worms. Males were carefully separated from females and transferred to a dialysis bag containing incubation media, in a cell culture flask with nutrient media. Worms were then incubated for 72 hours and the incubation media harvested. (B) Quality control of WES batches was performed by silver staining in three independent batches (B1, B2 and B3 above). (C) Western blot of preparations with polyclonal anti-WES rabbit serum (1:1400 dilution) and HRP-conjugated anti-rabbit IgG (1:2000 dilution). Serum collected prior to rabbit (normal rabbit serum—NRS) immunization with WES molecules was used as a negative control.
(TIFF)
Representative example of the gating strategy followed for the flow cytometry analysis of BMDC and following activation of BMDC with LPS.
(TIFF)
(A) Representative images of agarose gel electrophoresis of PCR products for expression of SmCypa (mRNA) and housekeeping genes Pai1 (mRNA) and Gapdh (mRNA) by male or female adult S. mansoni worms. (B) Fold expression change of SmCypa mRNA for male only and female only S. mansoni adult worms, compared to housekeeping genes Pai1 or Gapdh, n = 3, data are presented as mean and SEM.
(TIFF)
ID–identification number at GeneDB; MS–mowse score; CO—percentage of sequence coverage; SP–signal peptide; SecP–SecretomeP score, values above 0.5 indicate possible secretion; TM—number of transmembrane domains; (+)—signal peptide detected; (-) no signal peptide or transmembrane domain detected.
(DOCX)
S. mansoni WES proteins were subjected to similarity analysis using BLAST. Sequence alignments that showed a bit score higher than 30 and an E value lower than 1e-16 were considered homologs. ID–identification number at GeneDB or GenBank databases; (-)–no homologs found.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.