Abstract
Background
Curative surgery is performed for a foot with ulcers and loss of protective sensation to heal the wound and prevent amputation. Evidence supports that patients with diabetes have decreased concentrations of growth factors in their tissues, notably epidermal growth factor (EGF). Injecting EGF deep into the bottom of the wound and its contours encourages a more effective response in terms of granulation tissue growth and wound closure.
Objective
To assess the effectiveness and safety of curative metatarsal bone surgery combined with intralesional administration of human recombitant EGF in neuropathic diabetic forefoot ulceration.
Methods
A prospective, open-label study of the effectiveness and safety of curative metatarsal bone surgery combined with intralesional administration of human recombitant EGF in neuropathic ulceration of the forefoot in patients with diabetes was conducted on a convenience sample of 212 patients with diabetes who had a total of 231 neuropathic ulcerations of the forefoot. The eligibility criteria included normal physical activity without a history of minor amputation and meeting the inclusion criteria without meeting any of the exclusion criteria in the Vascular Surgery Service of the Clinic Surgical Hospital “José R. López Tabrane” from January 2009 to May 2015. The follow-up process ended in November 2015, which was based on nonprobability consecutive sampling of 128 patients with diabetes who had a total of 131 foot ulcers in the treatment group and 84 patients with diabetes who had a total of 100 foot ulcers in the control group.
Results
The groups had comparable demographic and baseline characteristics. In the recombitant human EGF study group, there was a 2.1-fold shorter time of re-epithelization (healing), less recidivism, and a 2.3-fold decrease in lesions, which favored the selected treatment. The safety profile was appropriate according to the low frequency of complications and the light or moderate characteristics of the complications. Only shivering and fever were more frequent in the recombitant human EGF-treated group.
Conclusions
The combination of curative metatarsal bone surgery with intralesional administration of recombinant human EGF resulted in a significant reduction in the re-epithelization time, recidivism, and development of new diabetic lesions. The safety profile was appropriate. However, more randomized, triple-blind, and placebo trials are needed to evaluate the efficacy and safety of this new therapy.
Key words: diabetic foot surgery, diabetic foot ulcer, epidermal growth factor, neuropathic ulceration, wound healing
Introduction
The incidence of diabetic foot disease is on the rise throughout the world.1 The prevalence of foot ulceration among patients with diabetes mellitus ranges from 1.3% to 4.8% in community-dwelling populations2 to 12% among hospitalized patients.3 Diabetic foot ulcers (DFUs) have a major influence on a patient as well as on the health care system.4 These ulcers tend to heal slowly and require intensive care, and healing can be complicated by infection and gangrene, leading to long-term hospitalization and/or amputation.5
Although the pathophysiology of DFU is multifactorial, peripheral vascular disease, neuropathy, and infection are the 3 primary pathologic components that contribute to diabetic foot complications, and they frequently occur simultaneously as an etiologic trio. Important factors that contribute to these conditions include altered biomechanics and poor-quality shoes.6, 7 Each of these components is usually insufficient to cause ulceration, but the combination of 2 or more factors typically results in a poorly healing foot ulcer.8
Several lines of evidence suggest that, apart from repetitive biomechanical stress and impaired tissue perfusion, DFUs are intrinsically defective for wound healing.9 Recruitment of leukocytes is an important early event in wound healing,10 and several lines of evidence suggest that the formation of advanced glycation end products may contribute to impaired wound healing.11 Advanced glycation end products result from a defective inflammatory response to tissue damage as well as glycation of skin collagen and are possibly components of factors that impair matrix degradation.12 In addition, the reduced levels of active growth factors in the wound environment may partially explain why some wounds fail to heal.13
Chronic ulcers are known to have reduced levels of platelet-derived growth factor, basic fibroblast growth factor, epidermal growth factor, and transforming growth factor compared with acute wounds. It has been suggested that growth factors in DFUs may become trapped by extracellular matrix molecules or may be excessively degraded by proteases, resulting in nonhealing. Ulcerations may result from enhanced degradation of growth factors. Abnormalities have also been observed in the proliferative capacity of the fibroblasts of patients with diabetes when comparing fibroblasts derived from normal skin with those from foot ulcers.14, 15 The mechanism of this decreased fibroblast proliferation is unclear, but could be caused by impaired cellular responsiveness to 1 or more growth factors.16
The morphologic and functional changes demonstrated in diabetic populations are more frequent in a neuropathic foot and increase the shear stress of the foot.17 Pressure is a factor in 90% of diabetic plantar ulcers, and the pressure must be modified or removed. Pressure-induced ischemia occurs in tissues over weight-bearing bony areas during ambulation and standing. Neuropathy prevents the perception of protective pain, increasing the potential for tissue breakdown.18
Chronic wounds represent a major health burden and drain on resources. Recent advances in our understanding of chronic wound biology have led to the development of several new treatments that offer renewed hope to patients with ulcers and other chronic wounds.19
The principal objective of treatment is to close the wound.14, 15, 16, 17 Curative surgery is performed on a foot with ulcers and loss of protective sensation with the objective of achieving healing and avoiding amputation.20 The adjuvant therapies act through different mechanisms to re-establish normal wound conditions.13 Growth factor therapy is considered to be an adjuvant therapy and key element in the maintenance of tissue integrity and intercellular communication. Preclinical and clinical studies have suggested that intralesional administration of rh-EGF is safe and efficacious for normal wound healing in DFUs.20, 21, 22, 23
Whereas curative metatarsal bone surgery focuses on accelerating the wound healing process of DFU, there is insufficient information to support the combination of this surgical procedure with the intralesional administration of rh-EGF. Therefore, we assessed the effectiveness and safety of this novel procedure for healing DFUs and avoiding amputation.
Materials and Methods
Study Design
A curative surgery trial was conducted on a convenience sample of 212 patients with diabetes who had a total of 231 neuropathic ulcerations of the forefoot. The eligibility criteria included normal physical activity with no history of minor amputation. Eligible patients were referred to the Vascular Surgery Service of the Clinic Surgical Hospital “José R. Lopez Tabrane” from January 2009 to May 2015 and were followed through November 2015. There were 128 patients with diabetes with a total of 131 foot ulcers included in the treatment group and 84 patients with diabetes with a total of 100 foot ulcers in the control group.
To be eligible to participate, patients needed to provide informed consent for the surgical procedure, be at least age 18 years, have a documented diagnosis of type 1 or type 2 diabetes according to the criteria of the Latin-American Association of Diabetes 2000 Guide, have a documented diagnosis of neuropathic ulceration of the forefoot, and lack clinical manifestations of peripheral arterial disease or soft tissue infection.
Patients were not eligible to participate if they had a history of chronic uncompensated diseases, including cardiopathy with myocardial infarction, unstable angina, or cardiac insufficiency with edema during the past 3 months; diabetic coma; hepatic insufficiency; moderate to grave renal function or renal failure (creatinine >200 mmol/L and oliguria); hemoglobin <100 g/L; antecedents or suspicion of malignant diseases; psychiatric diseases that compromise treatment or evaluations; pregnancy or currently breastfeeding; or hypersensitivity to the product or any of its components. In the assessment of efficacy, the main response variable was the proportion of patients with complete healing (defined as epithelization and complete closure of the lesion without secretion or the need for dressing). Secondary variables for the effectiveness included recidivism and the development of new lesions.
The safety variables included the type, duration, intensity, seriousness, and treatment. The assessments of safety included a physical examination, interview, and clinical laboratory parameters. The severity of adverse events was classified as mild if no therapy was necessary; moderate if a specific treatment was needed; and severe in case of death, life-threatening disease, or hospitalization or its prolongation.
The control variables included age, gender, ethnicity, type of diabetes mellitus (type 1 or 2), time of evolution of the diabetes mellitus, current treatment for the diabetes (oral hypoglycemic drugs or insulin), stage according to the Wagner Grading System for Diabetic Foot Infections; stage according to the perfusion, extent/size, depth/tissue loss, infection, and sensation (PEDIS) classification; presence of osteomyelitis; time of evolution of the current ulcer (in weeks); and type of curative metatarsal bone surgery.
Treatment Protocol
After patients were selected for enrollment in the study, they were fully informed of the nature of the study and provided written informed consent. During the primary assessment, a detailed past medical history was taken that identified the duration since the diagnosis of diabetes, previous ulcers, treatments used, and allergies to drugs.
A comprehensive, thorough physical examination of the lower extremities and ulcers was performed by trained physicians. Photographs of the wounds were taken, and the exact surface area of the wound was measured. Baseline laboratory tests were performed to determine the complete blood count; erythrocyte sedimentation rate; fasting blood sugar; lipid profile; serum phosphorus, calcium, sodium, potassium, and amylase; and liver and renal function tests as well as a vascular evaluation were performed.
After the evaluation, the methodology of treatment was similar for the 231 neuropathic forefoot ulcerations included in the investigation, which involved the following steps:
-
1.
Surgical or sharp debridement of all of the hyperkeratosis or necrotic tissue.
-
2.
Group A underwent curative metatarsal bone surgery (according the specific indication) and intralesional application of hr-EGF (Heberprot-P, Center for Genetic Engineering and Biotechnology (CIGB), Havana, Cuba) for the standard method. The lyophilized formulation of 75 µg was given 3 times per week until the wound healed (re-epithelization; defined as the absence of need for a local dressing and bandages) or until 24 doses (8 weeks of treatment).
-
3.
Group B, the control group, underwent curative metatarsal bone surgery alone (according the specific indication).
The following indications were established for each surgery type:
Osteotomy
Performed in the presence of neuropathic ulceration in the projection of the metatarsal head without evidence of osteomyelitis according to the criteria of the Diabetic Foot Study Group from the Europe Association for the Study of the Diabetes.
Decapitation
Performed in the case of neuropathic ulceration in the projection of the metatarsal head with evidence of osteomyelitis according to the criteria of the Diabetic Foot Study Group from the Europe Association for the Study of the Diabetes.
Statistical Analyses
Statistical analyses of the results were performed using SPSS version 19.0 for windows (IBM-SPSS, Inc, Armonk, NY). Exploratory analyses for each variable (main, secondary, and control) were performed to evaluate their global behaviors and evaluate the hypothesis of applying proper statistical tests in the assessment stage. With the quantitative variables, the measurements of the central tendency and dispersion were estimated.
For all variables (quantitative and qualitative), the logistic regression model was adjusted to study the influence of each variable and their interactions on the response to the treatment and occurrence of serious adverse events (at 2 weeks). In case any statistically significant dependence(s) is/are detected, confirmatory analysis with the main variable should envisage it/them as covariable(s) or stratum (strata). The hypothesis that there was a difference between independent qualitative and dependent quantitative variables was established by calculating the odds ratio (OR) with a probability of P < 0.05 indicating statistical significance.
Ethics
The protocol was approved by the institutional review board of the Clinic Surgical Hospital “José R. Lopez Tabrane,” Matanzas City, Cuba. The protocol was also approved by the institutional review board of the University of Medical Sciences, Matanzas City, Cuba. Patients were fully informed about the aim of the study and they were told that their participation was optional. Written informed consent was obtained from each participant.
Results
Basic Characteristics
Two hundred twelve subjects with diabetes with a total of 231 forefoot neuropathic ulcerations who met the eligibility criteria (inclusion and exclusion) were included in this study (Table I). The mean age of participants was 56.5 years in the treated group and 56.7 in the control group. In both groups, there was a predominance of women, the ethnicity was mixed, and most had type 2 diabetes with a time of evolution of 13.8 years in Group A versus 14.2 years in Group B. The characteristics of the wound were a time of evolution of 7.3 weeks in the treatment group and 7.4 weeks in the control group. According to the Wagner Grading System, grade 2 was the most prevalent and he infection was PEDIS grade 1. According to this finding, it is possible to establish that the groups were comparable according to their demographic and baseline characteristics.
Table I.
Demographic characteristics and baseline clinical findings, 2009–2015.*
| Variable | Group A: Treatment (n = 131) | Group B: Control (n = 100) | ||
|---|---|---|---|---|
| Age, y | 56.4 (5.3) | 56.7 (5.8) | ||
| Gender | Female | 73 (55.7) | 56 (56) | |
| Male | 58 (44.3) | 44 (44) | ||
| Ethnicity | Asian | 8 (6.1) | 3 (3) | |
| White | 32 (24.4) | 23 (23) | ||
| Mestizo | 58 (44.3) | 45 (45) | ||
| African descent | 33 (25.2) | 29 (29) | ||
| Type of DM | Type 1 | 4 (3) | 3 (3) | |
| Type 2 | 127 (97) | 97 (97) | ||
| Time of evolution of diabetes mellitus, y | 13.8 (5.5) | 14.2 (5.1) | ||
| Time of evolution of the DFU, wk | 7.3 (2.1) | 7.4 (1.9) | ||
| Wagner grade | 2 | 86 (65.6) | 62 (62.6) | |
| 3 | 45 (34.4) | 38 (37.4) | ||
| PEDIS | 1 | 86 (58.1) | 62 (56.5%) | |
| 2 | 45 (41.9) | 38 (43.5) | ||
DM = diabetes mellitus; DFU = diabetic foot ulcer; PEDIS = perfusion, extension, deep, infection and sensibility classification.
Values for age, time of evolution of diabetes mellitus, and time to diabetic foot ulcer are presented as mean (SD). Other values are presented as n (%). Values in boldface type are significant at P < 0.05, based on χ2 test.
Efficacy Assessment
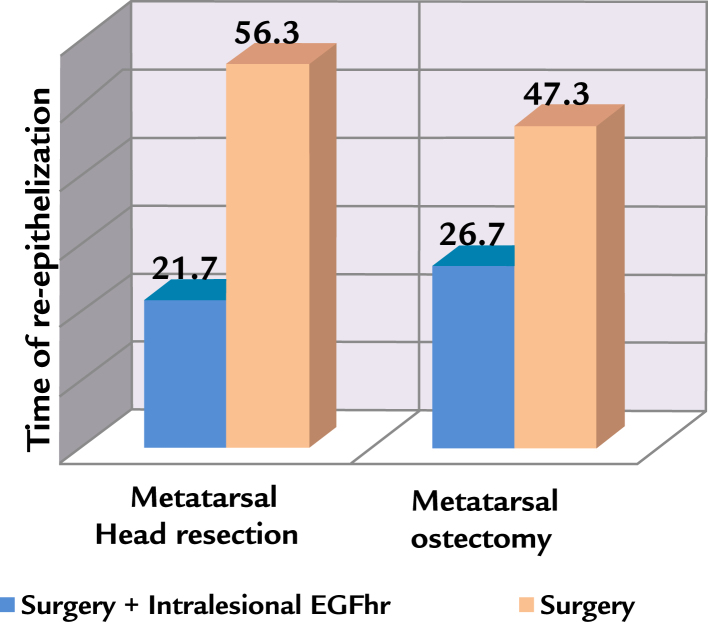
Evaluation of the time of re-epithelization (healing) shows a significant reduction in favor of the treatment group from 56.3 to 21.7 days (Fig. 1) for the patients who underwent metatarsal head resection and from 47.3 to 26.7 days for patients who underwent metatarsal osteotomy. The logistic regression model (Table III) exhibits a bad prognosis factor as the presence of osteomyelitis (OR, 2.3)
Figure 1.
Time of re-epithelialization (days) in the metatarsal bone surgery in combination or not with the administration of intralesional human recombinant epidermal growth factor (EGFhr), Matanzas, Cuba, 2009–2015.
Table III.
Logistic models results in the predictive variables. Matanzas, Cuba, 2009–2015.*
| Predictive variables | Healing time (re-epithelization) | Recidivism | New lesson | Adverse drug reactions |
|---|---|---|---|---|
| Age <45 y | 0.32 | 0.53 | 0.51 | 0.29 |
| Age 45–55 y | 0.50 | 0.45 | 0.39 | 0.56 |
| Age 56–65 y | 0.37 | 0.62 | 0.18 | 0.72 |
| Age >65 y | 0.61 | –0.15 | 0.36 | 0.56 |
| Male | 0.93 | 0.43 | 0.42 | 0.29 |
| Female | 0.13 | 0.52 | 0.19 | –0.885 |
| Ethnicity white | 1.00 | 0.32 | 0.61 | 0.18 |
| Ethnicity black | 0.32 | 0.29 | 0.57 | 0.62 |
| Ethnicity mixed | 0.92 | 0.72 | 0.62 | 0.57 |
| Type 1 diabetes mellitus | 0.19 | 0.11 | 0.21 | 0.79 |
| Type 2 diabetes mellitus | 0.62 | 0.92 | 0.34 | 0.28 |
| <5 y evolution of diabetes mellitus | 0.72 | –0.12 | –0.07 | 0.53 |
| 5–10 y evolution of diabetes mellitus | 0.39 | 0.62 | 0.45 | 0.02 |
| 11–15 y evolution of diabetes mellitus | 0.41 | 0.72 | 0.62 | 0.29 |
| More than 15 y evolution of the diabetes mellitus | 0.32 | 0.82 | 0.61 | 0.51 |
| Current treatment for the diabetes (oral drugs) | 0.27 | 0.62 | 0.59 | 0.82 |
| Current treatment for the diabetes (insulin) | 0.22 | 0.32 | 0.91 | –0.479 |
| 3 Wagner’s grade | 0.51 | 1.32 | 0.36 | 0.52 |
| 4 Wagner’s grade | 0.37 | 0.34 | 0.61 | 0.67 |
| PEDIS 1 | 0.32 | 0.23 | 0.47 | 0.61 |
| PEDIS 2 | 0.61 | 0.82 | 0.62 | 0.52 |
| Osteomyelitis | 2.3 | 6.2 | 2.7 | 1.8 |
| <6 wk evolution of the current ulcer | 0.29 | 0.25 | 0.63 | 0.43 |
| More than 6 wk evolution of the current ulcer | 0.19 | 0.52 | 0.47 | 0.52 |
| Metatarsal osteotomy | 0.37 | 0.29 | 0.28 | 0.39 |
| Metatarsal decapitation | 0.25 | 0.58 | 0.25 | 0.18 |
PEDIS = perfusion, extension, deep, infection and sensibility classification
Values are presented as odds ratio (95% CI). Values presented in boldface type are significant at P < 0.05.
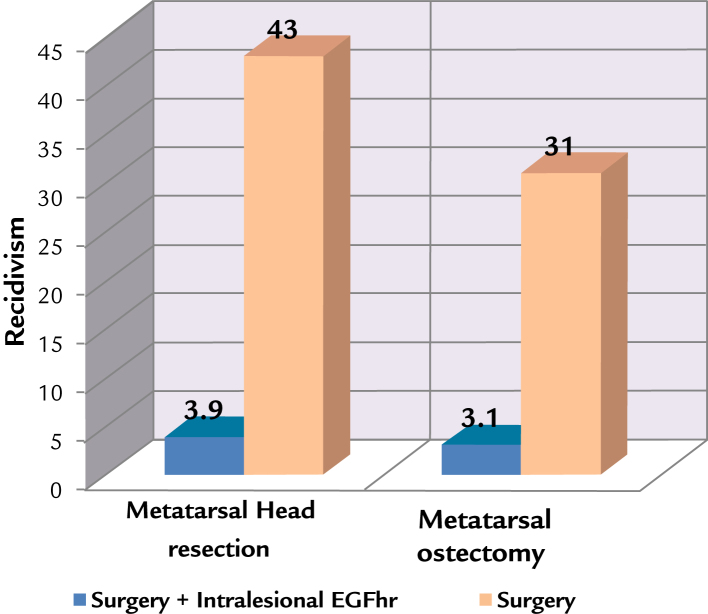
The recidivism (Fig. 2) for metatarsal head resection showed a reduction from 43% in the control group to 3.9% in the treated group, and there were similar results in the metatarsal osteotomy group, which diminished from 31% to 3.1%. The logistic regression model (Table III) shows a bad prognosis for the presence of osteomyelitis (OR, 6.22), Wagner grade 3 (OR, 1.32), and a protective minor time of evolution of diabetes mellitus (OR, 0.12) and older people (OR, 0.15).
Figure 2.
Recidivism in the metatarsal bone surgery in combination or not with the administration of intralesional human recombinant epidermal growth factor (EGFhr), Matanzas, Cuba, 2009–2015.
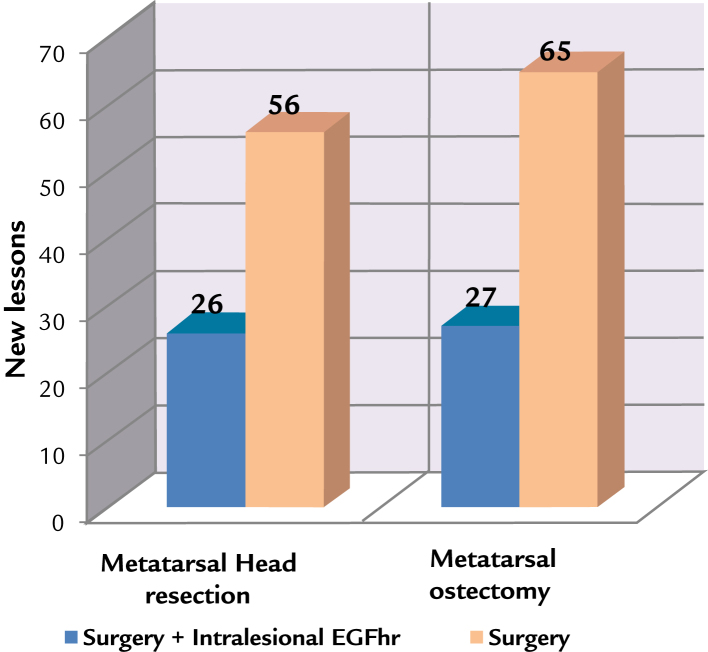
The rate of new lesions (Fig. 3) in the metatarsal head resection group reduced from 56% to 26%, whereas it reduced from 65% to 27% in the metatarsal osteotomy group. Multivariate analysis (Table III) established the risk factors as the presence of osteomyelitis (OR, 2.7) and the minor time of evolution of diabetes as protective (OR, –0.07).
Figure 3.
New lesions in the metatarsal bone surgery in combination or not with the administration of intralesional human recombinant epidermal growth factor (EGFhr), Matanzas, Cuba, 2009–2015.
Assessment of the Safety Results
Safety was monitored daily during treatment and over 6 months after the beginning of the study. The patients were also evaluated for adverse drug reactions (Table II). The most frequent complications in the treatment group was a hematoma, which was observed in 5.6% (n = 9), followed by local infections 1.9% (n = 4), local pain 1.6% (n = 3), shivering 1.2% (n = 2), border necrosis 0.6% (n = 1), and fever 0.6% (n = 1). The control group had a hematoma in 10.2% (n = 10), local infection in 3.6% (n = 6), local pain in 2.4% (n = 4), and border necrosis in 2.2% (n = 2).
Table II.
Adverse events in both groups, Matanzas, Cuba, 2009–2015.
| Complication | Group A: Treatment |
Group B: Control |
||
|---|---|---|---|---|
| No | % | No | % | |
| Hematomas | 9 | 5.6 | 10 | 10.2 |
| Local infections | 4 | 1.9 | 6 | 3.6 |
| Local pain | 3 | 1.6 | 4 | 2.4 |
| Shivering | 2 | 1.2 | – | – |
| Border necrosis | 1 | 0.6 | 2 | 1.2 |
| Fever | 1 | 0.6 | – | – |
Multivariate analysis showed risk factors of osteomyelitis (OR, 1.8) and PEDIS grade 2 (OR, 1.7). The protective factors were female sex (OR, –0.885) and insulin therapy (OR, –0.479). There were no minor or major amputations reported as an outcome of treatment.
Discussion
This clinical trial evaluates patients with chronic ulcers, involving exposure of the subcutaneous tissue and/or tendons and/or a joint capsule. They were Wagner grade 2 or 3, with osteomyelitis and light to moderate baseline infection of the wound as well as hyperkeratosis that had to be sharpened, removed, or surgically manipulated. The demographic and baseline characteristics of the patients in both groups are homogenous and similar to those in the DFU clinical trials in Cuba with a median age of 65 years, a greater proportion of women, and a mixed ethnic distribution. The groups generally had type 2 diabetes.2, 20, 21, 22, 23
Surgical intervention is now accepted as a form of treatment and prevention of chronic ulcerations.24 Prophylactic surgery is performed to prevent a more serious event. This implies the presence of a deformity and a history of chronically recurrent ulceration that puts the limb at risk.25 The goals of surgery in this scenario are to eliminate the deformity and reduce the risk of reulceration and amputation.26
The evidence supports treatment of long nonhealing ulcers with deep tissue damage (Wagner grade 3 or 4) with hr-EGF Heberprot-P in addition to standard diabetic foot syndrome treatment.25 The pharmacologically active ingredient is a peptide that is produced using recombinant DNA, and it has a biological mechanism that is similar to endogenous epidermal growth factor in terms of stimulating the migration and proliferation of fibroblasts, keratinocytes, and endothelial and other cells; actively participating in the healing of injuries; assisting with epithelialization; healing; and recovery of tissue elasticity.27
There is evidence of an advantage for add-on therapy with epidermal growth factors in DFUs concerning complete wound closure and the time to complete wound healing. Evidence based on medical practice in more than 2000 patients treated with rh-EGF in Cuba showed a 75% probability of a full granulation response in treated patients, 61% probability of wound healing, and 71% reduction in the relative risk of amputation, as well as positive risk-benefit coefficient (5.40).21 Prospective and active pharmaco-vigilance for 1788 patients with a total of 1835 DFUs revealed complete granulation in 76% of the DFU cases in 5 weeks (35 days).22 These results made it possible to presume the following: intervention with Heberprot-P reduces the risk of amputation by 71% and the outcome of patients treated with this therapy occurs within 45 days with Heberprot-P: healing (71%) or amputation (29%).21
With the intralesional application of hr-EGF, the re-epithelization (healing) time is 2.1-fold shorter, recidivism is less frequent, and new lesions occur 2.3-fold less often. These results have statistical significance (P < 0.05), justifying the clinical relevance of this observation. There were few recurrences in the rh-EGF-treated patients who had complete ulcer healing. It seems as if the tissue has a sort of memory of the treatment, which is not transferable to untreated zones. By contrast, no effect was seen on new DFUs in other locations (especially on the contralateral limb).21
The efficacy of this product, as demonstrated in the reduced time for complete filling of the ulcer defect with granulating tissue as well as its complete epithelialization, has been demonstrated in randomized clinical studies.20, 21, 22, 23
There is a good tolerance to intralesional rh-EGF. Approximately half of patients (63.1% in clinical trials and 46.2% in postmarketing pharmacovigilance) reported adverse events.21 The most frequent adverse reactions during Heberprot-P administration are pain and burning at the injection site, shivering, trembling, local infection, and fever.22 The rate with which the main adverse reactions manifest, as observed in the course of various clinical trials, is as follows: number of patients = 297: pain at the injection site = 75 (25.2%), burning at the injection site = 51 (17.2%), trembling = 57 (19.2%), shivering = 50 (16.8%), local infection = 39 (13.1%), and body temperature increase (fever) = 27 (9%).20 The proportion of patients with adverse events are 69.7% versus 54.4% in the clinical trials and 51.9% versus 40.0% during pharmacovigilance in Cuba for 75 μg and 25 μg, respectively, which means that the safety profiles in the clinical trials and postmarketing pharmacovigilance were similar.2
Risks arise from the short- and long-term adverse event profiles. More than 90% of the adverse events were mild or moderate, and they were easily manageable. Therefore, the benefit–risk balance seems favorable.21, 22
Conclusions
A combination of curative metatarsal bone surgery associated with intralesional administration of hr-EGF is associated with a reduction in the re-epithelization time, recidivism, and presence of new lesions. The safety profile is considered to be acceptable given the low frequency of complications as well the moderate characteristics of adverse events. The most frequent complications were surgical hematoma, local infection, and local pain and shivering with injection. The logistic regression model shows that osteomyelitis an important risk factor for the development of repeat ulcers, new lesions, and complications; the presence of a Wagner grade 2 DFU is associated with recidivism, and DFU infection PEDIS classification 2 is associated with complications. The protective factors are a short time of having diabetes mellitus, lower duration of ulceration, female gender, and older age. This therapeutic approach is effective and safe for treating neuropathic ulceration of the forefoot.
Conflicts of Interest
The authors have indicated that they have no conflicts of interest regarding the content of this article.
Acknowledgments
The Matanzas Diabetic Foot Study Group includes Arístides L. Garcia Herrera, MD, PhD; Ridel J. Febles Sanabria, MD, MS; Elaine Castaneiras Jorge, MD, MS; Odalis Vazquez Diaz, MD; Liliana Acosta Cabadilla MD, MS; EdelFleitas Perez MD, MS; Santiago Cantero Calderon, MD; Vania M Peña Hernandez, MD; Leydis Rodríguez Hernández, MD; Marta J Jimenez Perez, MD; Omnia Perez Martin, MD; Isis de la C. Jiménez Abreu, MD; Cristobal Pancorbo Sandoval, MD; Alejandro Piedrafita Tejeda, MD; and Yutdelys Robens Aguilar, MS.
References
- 1.Yazdanpanah L., Nasiri M., Adarvishi S. Literature review on the management of diabetic foot ulcer. World Journal of Diabetes. 2015;6(1):37–53. doi: 10.4239/wjd.v6.i1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cychosz C.C., Phisitkul P., Belatti D.A., Wukich D.K. Preventive and Therapeutic Strategies for Diabetic Foot Ulcers. Foot Ankle Int. 2016;37(3):334–343. doi: 10.1177/1071100715611951. [DOI] [PubMed] [Google Scholar]
- 3.Bakker K., Apelqvist J., Lipsky B.A., Van Netten J.J., Schaper N.C. The 2015 IWGDF guidance on the prevention and management of foot problems in diabetes. Int Wound J. 2015;8:453–464. doi: 10.1111/iwj.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.García-Herrera A.L., Fernández-Montequín J.I., Rodríguez Fernández R. ELSEVIER; Madrid: 2004. El pie diabético; pp. 38–69. [Google Scholar]
- 5.Yusuf M.K., Mahadi S.I., Mahmoud S.M., Widatalla A.H., Ahmed M.E. Diabetic neuropathic forefoot and heel ulcers: management, clinical presentation and outcomes. J Wound Care. 2015;24(9):420–425. doi: 10.12968/jowc.2015.24.9.420. [DOI] [PubMed] [Google Scholar]
- 6.Alavi A., Sibbald R.G., Mayer D., Goodman L., Botros M., Armstrong D.G., Woo K., Boeni T., Ayello E.A., Kirsner R.S. Diabetic foot ulcers: Part II. Management. J Am Acad Dermatol. 2014;70(1):21. doi: 10.1016/j.jaad.2013.07.048. e1-24; quiz 45-6. [DOI] [PubMed] [Google Scholar]
- 7.Game F.L., Attinger C., Hartemann A., Hinchliffe R.J., Löndahl M., Price P.E., Jeffcoate W.J., International Working Group on the Diabetic Foot (IWGDF) IWGDF guidance on use of interventions to enhance the healing of chronic ulcers of the foot in diabetes. Diabetes Metab Res Rev. 2016;32(Suppl 1):75–83. doi: 10.1002/dmrr.2700. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong D.G., Lavery L.A., Frykberg R.G., Wu S.C., Boulton A.J. Validation of a diabetic foot surgery classification. Int Wound J. 2006;3:240–246. doi: 10.1111/j.1742-481X.2006.00236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis J., Lipp A. Pressure-relieving interventions for treating diabetic foot ulcers. Cochrane Database Syst Rev. 2013;1:CD002302. doi: 10.1002/14651858.CD002302.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Sanz-Corbalán I., Lázaro-Martínez J.L., Aragón-Sánchez J., García-Morales E., Molines-Barroso R., Alvaro-Afonso F.J. Analysis of Ulcer Recurrences after Metatarsal Head Resection in Patients Who Underwent Surgery to Treat Diabetic Foot Osteomyelitis. Int J Low Extrem Wounds. 2015;14(2):154–159. doi: 10.1177/1534734615588226. [DOI] [PubMed] [Google Scholar]
- 11.Podskubka A., Stĕdrý V., Kafunĕk M. Distal shortening osteotomy of the metatarsals using the Weil technique: surgical treatment of metatarsalgia and dislocation of the metatarsophalangeal joint. Acta Chir Orthop Traumatol Cech. 2002;69(2):79–84. [PubMed] [Google Scholar]
- 12.Marx R.C., Mizel M.S. What׳s New in Foot and Ankle Surgery? J Bone Joint Surg Am. 2015;97(10):862–868. doi: 10.2106/JBJS.O.00126. [DOI] [PubMed] [Google Scholar]
- 13.Berlanga J. Diabetic lower extremity wounds: the rationale for growth factors-based infiltration treatment. Int Wound J. 2011;8:612–620. doi: 10.1111/j.1742-481X.2011.00840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.lraiyah T., Tsapas A., Prutsky G., Domecq J.P., Hasan R., Firwana B., NabhanM, Prokop L., Hingorani A., Claus P.L., Steinkraus L.W., Murad M.H. A systematic review and meta-analysis of adjunctive therapies in diabetic foot ulcers. J Vasc Surg. 2016;63(2 Suppl):46S–58S. doi: 10.1016/j.jvs.2015.10.007. e2. [DOI] [PubMed] [Google Scholar]
- 15.Lee K.M., Kim W.H., Lee J.H., Choi M.S. Risk factors of treatment failure in diabetic foot ulcer patients. Arch Plast Surg. 2013;40:123–128. doi: 10.5999/aps.2013.40.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molines-Barroso R.J., Lázaro-Martínez J.L., Aragón-Sánchez J., García- Morales E., Beneit-Montesinos J.V., Álvaro-Afonso F.J. Analysis of transfer lesions in patients who underwent surgery for diabetic foot ulcers located on the plantar aspect of the metatarsal heads. Diabet Med. 2013;30(8):973–976. doi: 10.1111/dme.12202. [DOI] [PubMed] [Google Scholar]
- 17.Oh T.S., Lee H.S., Hong J.P. Diabetic foot reconstruction using free flaps increases 5-year-survival rate. J Plast Reconstr Aesthet Surg. 2013;66:243–250. doi: 10.1016/j.bjps.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 18.Patel V.G., Wieman T.J. Effect of metatarsal head resection for diabetic foot ulcers on the dynamic plantar pressure distribution. Am J Surg. 1994;167(3):297–301. doi: 10.1016/0002-9610(94)90203-8. [DOI] [PubMed] [Google Scholar]
- 19.Evans K.K., Attinger C.E., Al-Attar A., Salqado C., Chu C.K., Mardini S., Neville R. The importance of limb preservation in the diabetic population. J Diabetes Complications. 2011;25:227–231. doi: 10.1016/j.jdiacomp.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Fernández-Montequín J.I., Valenzuela-Silva C.M., González-Díaz O., Savigne W., Sancho-Soutelo N., Rivero-Fernández F. Intralesional injections of recombinant human epidermal growth factor promote granulation and healing in advanced diabetic foot ulcers. Multicenter, randomized, placebo-controlled, double blind study. Int Wound J. 2009;6:432–443. doi: 10.1111/j.1742-481X.2009.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.López-Saura P.A., Yera-Alos I.B., Valenzuela-Silva C., González-Díaz O., Río-Martín Ad. Medical Practice Confirms Clinical Trial Results of the Use of Intralesional Human Recombinant Epidermal Growth Factor in Advanced Diabetic Foot Ulcers. Adv Pharmacoepidem Drug Safety. 2013;2:2. [Google Scholar]
- 22.Yera-Alos I., Alonso-Carbonell L., Valenzuela-Silva C.M., Tuero-Iglesias A.D., Moreira-Martínez M., Marrero-Rodríguez I. Active post-marketing surveillance of the intralesional administration of human recombinant epidermal growth factor in diabetic foot ulcers. BMC Pharmacology and Toxicology. 2013;14:44. doi: 10.1186/2050-6511-14-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.García Herrera A.L., Rodríguez Fernández R., Ruiz V.M., Rodríguez Hernández L., Acosta Cabadilla L., Febles Sanabria R. Reduction in the amputation rate with Heberprot P in the local treatment of diabetic foot. Spanish Journal of Surgical Research. 2011;XIV:21–26. (in Spanish) [Google Scholar]
- 24.Garcia Herrera A.L., Febles Sanabria R., Cabadilla Acosta L., Moliner Cartaya M. Tratamiento quirúrgico curativo combinado con Heberprot-P® en las úlceras neuropáticas del antepié. Rev Cubana Angiol Cir Vasc, Dic. 2015;vol.16(2):125–138. [Google Scholar]
- 25.Valenzuela-Silva C.M., Tuero-Iglesias A.D., García-Iglesias E., González-Díaz O., Del Río-Martín A. Granulation Response and Partial Wound Closure predict Healing in Clinical Trials on Advanced Diabetes Foot Ulcers Treated with Recombinant, Human Epidermal Growth Factor. Diabetes Care. 2013;36:210–215. doi: 10.2337/dc12-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berlanga J., Fernández-Montequin J.I., López Mola E., López-Saura P.A., del Río A., Valenzuela C. Heberprot-P: A Novel Product for Treating Advanced Diabetic Foot Ulcer MEDICC Review. 2013;Vol 15(No 1) doi: 10.37757/MR2013V15.N1.4. January. [DOI] [PubMed] [Google Scholar]
- 27.Berlanga J., Fernández J.I., Valdés C., Franco N., Rojas I., Santana H., inventors; Centro de Ingeniería Genética Biotechnología, assignee Use of a pharmaceutical composition containing epidermal growth factor (EGF) for diabetic foot amputation prevention. World patent WO PCT/CU2002/000011. 2008 Dec 18. [Google Scholar]