Abstract
Background
Babesia, usually found in wild and domestic mammals worldwide, have recently been responsible for emerging malaria-like zoonosis in infected patients. Human B. microti infection has been identified in China, primarily in the Southwest along the Myanmar border but little direct surveillance of B. microti infection in rodents has been carried out here (Yunnan province). In this region, a diverse topographic range combined with tropical moisture sustains a high biodiversity of small mammals, which might play important role on Babesia transmission.
Methods
Small mammals were captured in 141 sample locations from 18 counties located Yunnan Province, and screened for B. microti-like parasites infection by a nested PCR to target 18S rRNA gene of Babesia, plus directly sequencing for positive samples. Univariate and multivariate forward stepwise logistic regression analysis was used to access the association between infections and some related risk factors.
Results
Infection with Babesia microti was confirmed in 2.4% (53/ 2204) of small mammals. Significant differences in prevalence rates of B. microti were observed based on variations in forest, agricultural, and residential landscapes. Furthermore, adult small mammals had higher prevalence rates than younger, pubertal mammals. The near full-length 18S rRNA gene revealed that there were two types of B. microti, Kobe and Otsu, which demonstrate the genetic diversity and regional distribution.
Conclusions
There exists a wide distribution and genetic diversity of endemic B. microti in Southwestern China, warranting further investigations and monitoring of clinical disease in individuals presenting with Babesia like symptoms in these areas.
Author summary
Babesia spp are garnering more attention as causative agents of human disease, with B. microti responsible for most cases globally. Our study documents potential small mammal reservoir hosts, collected from a large of sample sites, with PCR and sequencing identifying the wide distribution and genetic diversity of endemic B. microti in Southwestern China. Our study adds to body of literature on Babesia in China, while focusing on potential rodent reservoirs of disease. This report will be important for public health, and for researchers in the field of parasitology.
Introduction
Intraerythrocytic protozoan parasites from the genus Babesia, found in wild and domestic mammals worldwide, have recently been responsible for emerging malaria-like zoonosis in infected patients. Transmitted by ticks, causative agents of human babesiosis vary by geographic region, with Babesia microti, maintained in rodent reservoirs, causing disease in the United States, whereas in Europe, the bovine pathogen B divergens is responsible for reported human cases [1]. Only a few human cases of babesiosis have been reported in Asia over the past two decades [2–6]. Recently, 61 confirmed cases of babesiosis have been reported in China; ten patients infected with B microti mainly in the Yunnan Province, two with B. divergens in Shandong Province, and 49 with B. venatorum (formerly called Babesia sp. EU1) in northern China [7–9]. There have been other reports of suspected Babesia-like cases, including the first reported case in the Yunnan Province, however they were not clearly identified for exact species [10]. B. microti has been identified in small wild rodents found in North America, Europe, and Japan [11], and occasionally in eastern China [12]. In southwestern China, the Yunnan province’s topographic range combined with tropical moisture, sustains a high biodiversity of small mammals that could potentially maintain Babesia spp. However, little direct surveillance on rodents has been conducted in this area, despite 16% (10/61) of recent reported cases of Babesiosis occurring here. This research aims to investigate the role small mammal play in Babesia transmission in the Yunnan province.
Materials and methods
Ethics statement
The research protocol involving with trapping wild small animals and collecting samples was approved by the Animal Subjects Research Review Boards at the Yunnan Institute of Endemic Diseases Control and Prevention (2013–003), in accordance with the medical research regulations of China and the Regulation of the People’s Republic of China for the Implementation of the Protection of Terrestrial Wildlife.
Collection of small mammal samples
During May 2011 to November 2015, small mammals were captured using animal snap traps set at agricultural, forest and residential landscapes in 141 sample locations from 18 counties located Yunnan Province (Table 1), with a total of 200 snap traps per sample location were placed for three consecutive nights. Mammal species was identified according to external morphology, fur color, measurements and visible characters of dentition [13], with recordings of sex, developmental stage, and environment taken at the time of sample processing. After identification of species, spleen tissues were removed from the animals and stored in liquid nitrogen until tested. For unidentified species in the field, the craniums were brought to the laboratory for further identification.
Table 1. Prevalence of B. microti in small mammals from different survey sites.
| Counties | No. of tested | No. of positive for B. microti (%) | Latitude | Longitude |
|---|---|---|---|---|
| Tengchong | 39 | 11(28.21) | 24.92 | 98.73 |
| Lushui | 114 | 22 (19.30) | 26.46 | 99.65 |
| Jinggu | 76 | 3 (3.95) | 23.58 | 100.68 |
| Shiping | 128 | 3 (2.34) | 23.79 | 102.36 |
| Weixi | 99 | 2 (2.02) | 27.31 | 99.28 |
| Menghai | 175 | 3 (1.71) | 22.02 | 100.49 |
| Yongde | 215 | 3 (1.40) | 24.12 | 99.40 |
| Mengla | 81 | 1 (1.23) | 21.49 | 101.58 |
| Deqin | 346 | 4 (1.16) | 28.08 | 99.20 |
| Shangri-La | 91 | 1 (1.10) | 28.45 | 99.80 |
| Yulong | 224 | 0 (0) | 26.96 | 100.31 |
| Gongshan | 100 | 0 (0) | 28.09 | 98.67 |
| Fugong | 134 | 0 (0) | 26.60 | 98.97 |
| Yunxian | 68 | 0 (0) | 24.73 | 100.34 |
| Ninger | 88 | 0 (0) | 23.06 | 101.05 |
| Yiliang | 94 | 0 (0) | 24.91 | 103.18 |
| Mile | 110 | 0(0) | 24.09 | 103.41 |
| Mengzi | 22 | 0(0) | 23.46 | 103.37 |
| Total | 2204 | 53 (2.40) |
DNA extraction and pcr analysis
DNA was extracted from spleen tissue using the DNA blood and tissue kit (Tiangen Biotechnique, Beijing, China) according to the manufacturer’s instructions. A nested PCR to target partial sequences within the 18S rRNA gene of B. microti-like parasites was done as previously described by Tsuji et al [11]. For B. microti PCR-positive samples, the nearly complete 18S rRNA gene sequence was amplified using primer pairs of Piro0F and Piro6R and of Piro1F and Piro6R designed by Kawabuchi et al [14]. Amplicons were directly sequenced with automated DNA sequence (ABI PRISM 373; Perkin-Elmer, Norwalk, CT). Sequences analysis was carried out using CLC and a FASTA search on the Genbank database. A Phylogenetic tree was constructed using MEGA software (version 6.06) [15].
Statistical analysis
Univariate analyses was used to access the association between rodents species, gender, developmental stage, sampling location, environment landscape as well as altitude and B. microti by using chi-square test or a Fisher’s exact test. All variables with a P-value of <0.05 from univariate analysis were entered into a multivariate forward stepwise logistic regression analysis. All analyses were conducted using SPSS (version 17.0, SPSS Inc. Chicago, IL).
Results
A total of 2204 small mammals belonging to 50 species, 26 genera and 9 families from four orders were collected at 141 sample locations across 18 counties of the Yunnan Province. (Table 1, Table 2). Rattus tanezumi made up 23.5% (n = 518) of all trapped small mammals. Fifty-three small mammals from 12 species, primarily Rattus brunneusculus (7.53%, 7/93), Apodemus draco (5.91%, 13/220), Eothenomys eleusis (14.30%, 5/35) and Rattus tanezumi (2.70%, 14/518), were actively infected with B. microti (Table 1). Positive rodents originated from ten counties including Tengchong, Lushui, Jinggu, Shiping, Weixi, Menghai, Yongde, Mengla, Deqin and Shangri-La (Table 1, Table 2). The prevalence of B. microti in small mammals in agricultural landscape, forest landscape and residential landscape were 1.79%, 3.37% and 0.93% respectively with significant difference (p = 0.043) (Table 3). There was no significant difference in prevalence of B. microti between male and female small mammals (χ2 = 0.022, p = 0.882, Table 3). However, the prevalence of B. microti in adult small mammals (2.69%) was significantly higher (χ2 = 5.486, p = 0.019) than that in the pubertal mammals (0.37%), as depicted in Table 3. The prevalence of B. microti in small mammals at the altitude classes of <1500 meters, 1500–2500 meters, >2500 meters were 2.76%, 5.04% and 0.77%, respectively (Table 3). The multivariate logistic regression analysis revealed that sampling at 1500–2500 meters, adult life stage, and forest landscape were risk factors associated with infection by B. microti (Table 4).
Table 2. Prevalence of B. microti in small mammals of different species.
| Orders | Families | Genera | Species | No. of tested | No. of positive (%) |
|---|---|---|---|---|---|
| Rodentia | Muridae | Rattus | R. brunneusculus | 93 | 7(7.53) |
| R. tanezumi | 518 | 14(2.70) | |||
| R. nitidus | 42 | 0(0) | |||
| R. norvegicus | 16 | 0(0) | |||
| R. turkestanicus | 7 | 0(0) | |||
| Apodemus | A. draco | 220 | 13(5.91) | ||
| A. latronum | 90 | 1(1.11) | |||
| A.chevrieri | 184 | 0(0) | |||
| Mus | M. pahari | 84 | 1(1.19) | ||
| M. caroli | 75 | 0(0) | |||
| M. musculus | 12 | 0(0) | |||
| Niviventer | N. confucianus | 108 | 1(0.93) | ||
| N. eha | 8 | 0(0) | |||
| N. fulvescens | 9 | 0(0) | |||
| N. andersoni | 24 | 0(0) | |||
| Vernaya | V. fulva | 2 | 0(0) | ||
| Micromys | M.minutus | 1 | 0(0) | ||
| Bandicota | B. indica | 2 | 0(0) | ||
| Berylmys | B. bowersi | 7 | 0(0) | ||
| Leopoldamys | L. edwardsi | 4 | 0(0) | ||
| Cricetidae | Eothenomys | E. eleusis | 35 | 5(14.30) | |
| E.custos | 65 | 2(3.08) | |||
| E.miletus | 88 | 0(0) | |||
| E. olitor | 4 | 0(0) | |||
| E.cachinus | 9 | 0(0) | |||
| E. proditor | 8 | 0(0) | |||
| Pitymys | P.leucurus | 35 | 1 | ||
| Volemys | V. clarkei | 13 | 0(0) | ||
| Sciuridae | Dremomys | D. pernyi | 20 | 0(0) | |
| Tamiops | T.swinhoei | 3 | 0(0) | ||
| Dipodidae | Eozapus | E.setchuanus | 2 | 0(0) | |
| Insectivora | Soricidae | Crocidura | C. russula | 18 | 1(5.56) |
| C. attenuate | 40 | 0(0) | |||
| C. dracula | 58 | 0(0) | |||
| C.horsfieldi | 4 | 0(0) | |||
| C.lasiura | 1 | 0(0) | |||
| Soriculus | S. leucops | 25 | 0(0) | ||
| S. nigrescens | 7 | 0(0) | |||
| Sorex | S. alpinus | 11 | 0(0) | ||
| S. unguiculatus | 5 | 0(0) | |||
| S.cylindricauda | 15 | 0(0) | |||
| Suneus | S. murinus | 54 | 1(1.85) | ||
| Anourosorex | A. squamipes | 48 | 0(0) | ||
| Erinaceidae | Neotetracus | N. sinensis | 15 | 6(40.00) | |
| Hylomys | H. suillus | 14 | 0(0) | ||
| Talpidae | Scaptonyx | S. fusicaudus | 3 | 0(0) | |
| Nasillus | N. gracilis | 13 | 0(0) | ||
| Lagomorpha | Ochotonidae | Ochotona | O. thibetana | 45 | 0(0) |
| O. gloveri | 3 | 0(0) | |||
| Scandentia | Tupaiidae | Tupaia | T.belangeri | 37 | 0(0) |
Table 3. Risk factors related to B. microti based on univariate analyses.
| Variable | Simple size | Babesia microti infection | |||
|---|---|---|---|---|---|
| cases | constituent ratio(%) | positive rate | χ2 | p | |
| altitude(m) | |||||
| ~1500 | 833 | 37.79 | 2.76 | ||
| 1500~2500 | 456 | 20.69 | 5.04 | 24.466 | <0.01 |
| 2500~ | 915 | 41.52 | 0.77 | ||
| gender | |||||
| male | 1059 | 48.05 | 2.46 | 0.022 | 0.882 |
| female | 1145 | 51.95 | 2.36 | ||
| age | |||||
| adult | 1932 | 87.66 | 2.69 | 5.486 | 0.019 |
| pubertal | 272 | 12.34 | 0.37 | ||
| landscape | |||||
| agricultural | 1176 | 53.36 | 1.79 | ||
| forest | 920 | 41.74 | 3.37 | 0.043a | |
| residential | 108 | 4.9 | 0.93 | ||
a) Fisher’s exact test
Table 4. Risk factors related to B. microti based on multivariate logistic regression.
| Variable | OR (95% CI) | p |
|---|---|---|
| altitude(m) | ||
| 2500~ | 1 | |
| 1500~2500 | 10.286(4.334~24.409) | <0.01 |
| ~1500 | 7.660(3.090~18.989) | <0.01 |
| age | 0.125 (0.017~0.917) | 0.041 |
| landscape | ||
| agricultural | 1 | |
| forest | 3.180(1.715~5.896) | <0.01 |
| residential | 0.446(0.059–3.364) | 0.434 |
Near full-length 18S rRNA gene sequences were recovered from the 21 out of 53 B. microti-positive samples. The sequence from four Neotetracus sinensis, which were collected from Lushui County (C7), were distinguished those from four Eothenomys eleusis collected at the same location. While the sequences from three R. tanezumi and three R. brunneusculus collected at Tengchong County were identical. Phylogenetic analyses based on different representative sequences (deposited in GenBank with accession nos. AY649342-AY649348) revealed that they were divided into two types of B. microti, with two belonging to Kobe type, and five belonging to Otsu type (Fig 1).
Fig 1. Neighbor-joining phylogenetic tree based on a comparison of Babesia microti 18S rRNA gene sequences obtained from Yunnan small mammals with Babesia microti reference strains.
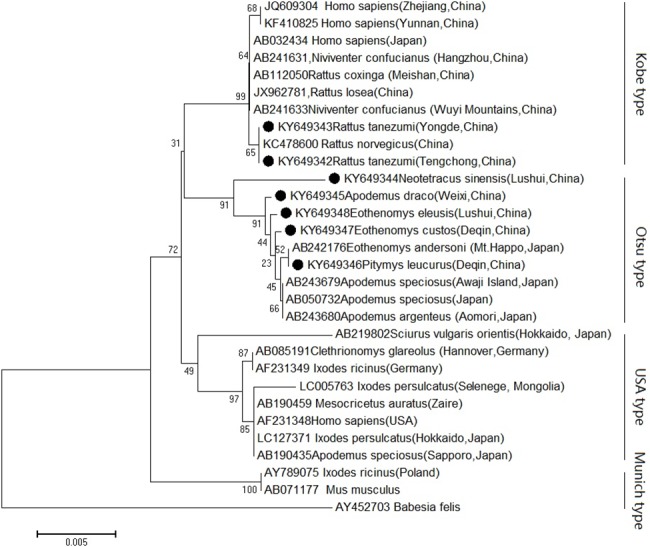
Babesia felis was included as the outgroup. The number on each branch shows the percent occurrence in 1,000 bootstrap replicates. Black circles stood for novel sequences identified in this study.
Discussion
Our present study shows the wide distribution and genetic diversity of Babesia microti in small mammals captured from the Yunnan Province based on the large number of samples tested and extent of geographic coverage. More than 100 Babesia species may infect a variety of both wild and domestic animals, but few have been confirmed to infect people, among which B. microti is the main species globally that causes human babesiosis [1]. B. microti infection was found in field rodents sampled in proximity to cases of human babesiosis in Taiwan and Zhejiang Province, China [12, 16]. However, until now, Babesia has not been found in small mammals from Yunnan Province. The prevalence of B. microti in small mammals in our study [2.40%) was in line with the low prevalence reported in the Dapan Mountains of Zhejiang Province (1.30%) [12]. However, the Rattus tanezumi and Nivivnter confucianus in Dapan Mountain had a much higher prevalence (5.6% and 20% respectively) than that of the same two species sampled in our study (2.70% and 0.93%). In China, there were four provinces (Zhejiang, Fujian, Beijing and Yunnan Provinces) which reported the B. microti infection in Nivivnter confucianus [12, 17, 18], which may be the main reservoir hosts in China. Our study found 12 species were positive for B. microti, which suggests a possible variety of reservoir hosts in the Yunnan Province.
The spleen is a secondary lymphoid organ specialized in filtering blood-borne pathogens and the splenic red pulp contains macrophages that trap and remove damaged red blood cells, which is good choice for diagnosis of Babesia infection [19]. The nested PCR is the method of choice for diagnosis of B. microti in the blood of rodent hosts and has a higher sensitivity than nested those for spleens [20]. However, in present research, all small mammals were collected by snap traps and died before we can get their blood. These are some limitations of our project.
The survey sites contained a broad range of altitudes from 500 meters to 4500 meters. Among the three altitude classes, small mammals with highest prevalence of B. microti distributed between 1500~2500 m. This result may be due to the temperature and humidity at this altitude range, which is conducive for tick growth and reproduction. Our findings suggests that the prevalence of B. microti in small mammals varies by landscape type, which is likely related to tick vector density and preferred habitat, with prevalence rates as from highest to lowest as follows; forest landscape > agricultural landscape > residential landscape. This study reiterates the need for individuals traveling into potential tick habitats, like the forest, to take proper protective measures to limit tick bite exposure. The prevalence of B. microti in adult small mammals was significantly higher than pubertal ones, which is likely due to dynamics of parasite carriage and the influence of age on likelihood of exposure.
This research found a high prevalence of B. microti in small mammals in Lushui County (Table 1) with different gene-types carried by different host species. Notably, the prevalence of B. microti in Tengchong (Table 1) was the highest among the survey sites, where six near full-length 18S rRNA gene identical sequences of B. microti were recovered from house rats, R. tanezumi and R. brunneusculus, which shared 99.9% identity with a human case of babesiosis reported from that region (GenBank KF410825). These findings suggest that there may be an important natural foci of B. microti in Tengchong and adjoining Lushui County. The ecology of B. microti in sampled wild animals from Yunnan province, suggests that Babesia infection in humans, livestock and ticks warrants further investigation.
Supporting information
(XLSX)
Acknowledgments
We thank Professor Zheng-Da Gong for identification of rodent species.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by State Key Research Development Program of China (2016YFC1201902, 2016YFC 1200301). National Natural Science Foundation of China (81360413, 81673235), and program of cultivation of technologically innovative talents of Yunnan Province (2014HB093). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Vannier E and Krause PJ. (2012) Human babesiosis. N Engl J Med 366:2397–407. doi: 10.1056/NEJMra1202018 [DOI] [PubMed] [Google Scholar]
- 2.Wei Q, Tsuji M, Zamoto A, Kohsaki M, Matsui T, Shiota T, et al. (2001) Human babesiosis in Japan: isolation of Babesia microti-like parasites from an asymptomatic transfusion donor and from a rodent from an area where babesiosis is endemic. J Clin Microbiol 39(6):2178–2183. doi: 10.1128/JCM.39.6.2178-2183.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim JY, Cho SH, Joo HN, Tsuji M, Cho SR, Park IJ, et al. (2007) First case of human babesiosis in Korea: detection and characterization of a novel type of Babesia sp. (KO1) similar to ovine babesia. J Clin Microbiol 45(6):2084–2087. doi: 10.1128/JCM.01334-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marathe A, Tripathi J, Handa V, and Date V. (2005) Human babesiosis—a case report. Indian J Med Microbiol 23(4):267–269. [PubMed] [Google Scholar]
- 5.Hong SH, Anu D, Jeong YI, Abmed D, Cho SH, Lee WJ, et al. (2014) Molecular Detection and Seroprevalence of Babesia microti among stock farmers in Khutul City, Selenge Province, Mongolia. Korean J Parasitol. 52:443–7. doi: 10.3347/kjp.2014.52.4.443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shih CM, Liu LP, Chung WC, Ong SJ, Wang CC. (1997) Human babesiosis in Taiwan: asymptomatic infection with a Babesia microti-like organism in a Taiwanese woman. J Clin Microbiol 35(2):450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou X, Li SG, Chen SB, Wang JZ, Xu B, Zhou HJ, et al. (2013) Co-infections with Babesia microti and Plasmodium parasites along the China-Myanmar border. Infect Dis Poverty 2(1):24 doi: 10.1186/2049-9957-2-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qi C, Zhou D, Liu J, Cheng Z, Zhang L, Wang L, et al. (2011) Detection of Babesia divergens using molecular methods in anemic patients in Shandong Province, China. Parasitol Res 109(1):241–245. doi: 10.1007/s00436-011-2382-8 [DOI] [PubMed] [Google Scholar]
- 9.Jiang JF, Zheng YC, Jiang RR, Li H, Huo QB, Jiang BG, et al. (2015) Epidemiological, clinical, and laboratory characteristics of 48 cases of "Babesia venatorum" infection in China: a descriptive study. Lancet Infect Dis 15:196–203. doi: 10.1016/S1473-3099(14)71046-1 [DOI] [PubMed] [Google Scholar]
- 10.Li JF, Meng DB, Wang QF. (1984) The discovery of human babesiosis. Chinese Journal of Veterinary Medicine. 10:19–20 (in Chinese). [Google Scholar]
- 11.Tsuji M, Wei Q, Zamoto A, Morita C, Arai S, Shiota T, et al. (2001) Human babesiosis in Japan: epizootiologic survey of rodent reservoir and isolation of new type of Babesia microti-like parasite. J Clin Microbiol 39(12):4316–4322. doi: 10.1128/JCM.39.12.4316-4322.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei YC, Jiang LP, Ye JL, Ying KM, Zheng BF. (2013) Molecular survey of Babesia in rodents from Dapan Mountain, Zhejiang. Chin Prev Med. 2013;14:949–52 (in Chinese). [Google Scholar]
- 13.Huang WJ, Chen YX, Wen YX, editors. (1995) Rodents of China. Shanghai: Fudan University Press;. [Google Scholar]
- 14.Kawabuchi T, Tsuji M, Sado A, Matoba Y, Asakawa M, Ishihara C (2005) Babesia microti-like parasites detected in feral raccoons (Procyon lotor) captured in Hokkaido, Japan. J Vet Med Sci 67(8):825–827. [DOI] [PubMed] [Google Scholar]
- 15.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28(10):2731–2739. doi: 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shih CM1, Liu LP, Chung WC, Ong SJ, Wang CC. 1997) Human babesiosis in Taiwan: asymptomatic infection with a Babesia microti-like organism in a Taiwanese women. J Clin Microbiol 35:450–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saitoito A, Takada N, Ishiguro F, Saito-Ito A1, Takada N, Ishiguro F, et al. (2008) Detection of Kobe-type Babesla microti associated with Japanese human babesiosis in field rodents in central Taiwan and southeastern mainland China. Parasitology.135:691–9. doi: 10.1017/S0031182008004356 [DOI] [PubMed] [Google Scholar]
- 18.Wang ZS, Wang XM, Dou XF, Li X, Ren HL, Liu J, et al. (2013) First report of Babesia microti infection in rodents in Miyun district of Beijing. Acta Parasitol Med Entomol Sin. 20:218–22. [Google Scholar]
- 19.Movilla R, Altet L, Serrano L, Tabar MD, Roura X. (2017) Molecular detection of vector-borne pathogens in blood and splenic samples from dogs with splenic disease. Parasites& Vectors 10 (1):131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Welc-Faleciak R, Bajer A, Bednarska M, Paziewska A, Siński E. (2007) Long term monitoring of Babesia microti infection in BALB/C mice using nested PCR. Ann Agri Environ Med 14(2):287–290]. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.


