Abstract
The synthesis of 3-[3/4-(2-aryl-2-oxoethoxy)arylidene]chroman/thiochroman-4-one derivatives (1–34) and evaluation of their anticancer activities were aimed in this work. Final compounds were obtained in multistep synthesis reactions using phenol/thiophenol derivatives as starting materials. For anticancer activity evaluation, all compounds were offered to National Cancer Institute (NCI), USA and selected ones were tested against sixty human tumor cell lines derived from nine neoplastic diseases. The activity results were evaluated according to the drug screening protocol of the institute. Compounds containing thiochromanone skeleton exhibited higher anticancer activity.
Keywords: Chromanone; Thiochromanone; Benzopyranone; Benzothiopyranone; Flavone; α,β-Unsaturated carbonyl; Anticancer activity
1. Introduction
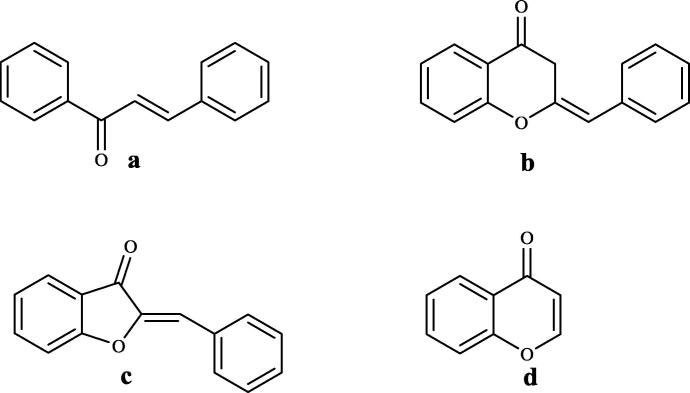
Natural sourced flavones are known with a large scale of biological activities which attribute to subclasses divided according to their molecular structures: These subclasses are flavonols, flavones, flavanones, flavan-3-ols, anthocyanidins, isoflavones, proanthocyanidins, homoisoflavonoids, aurones and chalcones (Mahapatra et al., 2015, Kupcewicz et al., 2014). Among these groups, synthetic derivatives of chalcones, homoisoflavonoids and aurones (Fig. 1) constitute our subjects of study which they fall apart possessing a common α,β-unsaturated carbonyl as pharmacophoric group along with high anticancer activity reported in many studies (Modranka et al., 2012).
Fig. 1.
The chemical structures of (a) chalcone, (b) homoisoflavonoid, (c) aurone, (d) chromenone.
Flavon derivatives are essential compounds, such as their corresponding chalcone derivatives that they act as flavon precursors which are very important scaffolds in terms of anticancer effects (Boumendjel et al., 2008, Kupcewicz et al., 2014, Brien et al., 2012, Cotelle, 2001, Lopez-Lazaro, 2002, Gul et al., 2007). The significant advantage of chalcone derivatives as cytotoxic agents is the low propensity to interact with DNA and to decrease the risk of mutagenicity as a common side effect of current chemotherapeutic agents (Noushini et al., 2013). Studies on chalcone derivatives are mostly based on the replacement of the phenyl rings with heterocyclic rings and poly-aromatic groups, introduction of different substituents on the phenyl moieties and cyclization of the chalcone to give rigid analogs such as tetralone, benzofuranone (aurone), benzopyranones (chromanone) and 3-benzylidene-4-benzothiopyranones(thiochromanone) were reported to exhibit promising cytotoxic activities (Letafat et al., 2013, Okombi et al., 2006, Pouget et al., 2002, Zheng-yue et al., 2011).
Chromanone and its analogs are also important pharmacophores and privileged structures in medicinal chemistry and have featured in a number of clinically used drugs (Keri et al., 2014, Emami and Ghanbarimasir, 2015), especially, as anticancer agents (Seifert et al., 2014, Lampronti et al., 2003, Gamal-Eldeen et al., 2009, Alizadeh et al., 2015). Furthermore, various chromenes (Fig. 1) moiety-containing compounds which are structural analogs of chromanones were also reported with high anticancer activity (Kwon et al., 2015, Alizadeh et al., 2008, Venkateswararao et al., 2014). Homoisoflavonoids (3-benzylidene-4-chromanones) are the other molecular framework in similar structure and increasing anticancer activity potency (Nafisi and Namdar, 2012, Yen et al., 2010, Perjési et al., 2008, Ivanova et al., 2013, Alipour et al., 2014, Thapa et al., 2011).
3-Arylidenechroman-4-one/thiochromanon-4-one, 2-aryl-1-indanone, 2-aryl-1-tetralone are known as structural analogs and bioisosteres of aurones and possess anticancer activities (Hartmann et al., 1994, Chen et al., 1994, Hartmann et al., 1996, Dimmock et al., 1999, Ohtsu et al., 2002, Dimmock et al., 2002, Boulamwini and Assefa, 2002, Gupta et al., 2004).
In this study, using molecular modification strategy which would provide modifying selectivity profile, activating in different or dual modes and/or reducing undesired side effects (Sandhu et al., 2014) and considering literature findings on α,β-unsaturated carbonyl as pharmacophore group for anticancer activity and arylidene moiety as a synthon (El-Gohary, 2014) some new 3-arylidenechroman-4-one and 3-arylidenethiochroman-4-one derivatives were designed and synthesized as a continuation of our previous and present studies containing 2-arylidene-1-indanon, 2-benzylideneaurone and 2-arylidene-1-tetralone (Gundogdu-Karaburun et al., 2014, Demirayak et al., 2015, Demirayak et al., 2016) molecules. The antiproliferative activity of the compounds were evaluated against various cancer cell lines comparing with standard drugs.
2. Materials and methods
2.1. General
All reagents and solvents were obtained from Sigma-Aldrich Chemical Co (Sigma-Aldrich Corp., St. Louis, MO, USA) and Merck KGaA (Darmstadt, Germany). Melting points were determined using a Electrothermal 9100 digital melting point apparatus (Essex, UK) and are uncorrected. All reactions were monitored by thin layer chromatography (TLC) using Silica Gel 60 F254 TLC plates (Merck KGaA, Darmstadt, Germany). Petroleum ether and ethyl acetate were used as mobile phase prepared in various ratios for TLC. Spectroscopic data were recorded as follows: IR spectra were IR Shimadzu 8400S FT-IR spectrometer (Tokyo, Japan), 1H-NMR spectra on a Bruker 500 MHz spectrometer (Billerica, MA, USA) in DMSO-d6 with TMS as internal standard 13C NMR spectra on a Bruker 75 MHz spectrometer (Billerica, MA, USA) and mass spectra using a Agilent 110 MSD spectrometer (Santa Clara, CA, USA) instruments.
2.2. Synthesis of chroman/thiochroman-4-one derivatives (IIIa-IIIe)
Chroman/thiochroman-4-one derivatives (IIIa-IIIe) were obtained by gradual synthetic reaction. Firstly, phenol/thiophenol derivative was reacted with acrylonitrile in tetrahydrofuran (THF) using benzyltrimethylammonium hydroxide (Triton-B) as a catalyst to gain 3-aryloxy/arylthioxy-propionitrile derivatives (Ia-Ie). Then, the obtained nitrile intermediates were hydrolyzed in acetic acid-hydrochloric acid mixture to acquire corresponding carboxylic acids (IIa-IIe). Subsequently, these intermediates were heated to 50–100 °C with excess polyphosphoric acid (PPA) for 3–4 h to reach chromanone and thiochromanones (IIIa-IIIe) with cyclization reaction. Later, this viscous mixture was reacted with ice-water and neutralized with sodium bicarbonate, the collapsed portion was get by filtering and it was crystallised from ethanol.
2.3. Synthesis of 3-(3/4-hydroxyarylidene)chroman/thiochroman-4-one derivatives (IVa-IVj)
Chromanone (IIIa, IIIb) and thiochromanone (IIIc-IIIe) derivatives (20 mmol) were refluxed with 3- or 4-hydroxybenzaldehyde (22 mmol) in 70 mL n-butanol for 30 min using hydrochloric acid as a catalyst (1 mL). The precipitate formed upon cooling was filtered and crystallised. The chemical properties of the obtained ten derivatives (IVa-IVj) were given in Table 1.
Table 1.
3-(3/4-Hydroxyarylidene)chroman/thiochroman-4-one derivatives.  .
.
| C. | Chemical name | X | OH | R | M.P. (°C) |
|---|---|---|---|---|---|
| IVa | 3-(3-Hydroxyarylidene)chroman-4-one | O | 3 | H | 203–204 (Mulvagh et al., 1979) |
| IVb | 3-(4-Hydroxyarylidene)chroman-4-one | O | 4 | H | 230–232 (Kirkiacharian et al., 1989) |
| IVc | 6-Chloro-3-(3-hydroxyarylidene)chroman-4-one | O | 3 | Cl | 192–194 (Siddiah et al., 2006) |
| IVd | 6-Chloro-3-(4-hydroxyarylidene)chroman-4-one | O | 4 | Cl | 238–240 (Kirkiacharian et al., 1989) |
| IVe | 3-(3-Hydroxyarylidene)thiochroman-4-one | S | 3 | H | 147–148 |
| IVf | 3-(4-Hydroxyarylidene)thiochroman-4-one | S | 4 | H | 192–194 (Levai and Schag, 1979) |
| IVg | 6-Methyl-3-(3-hydroxyarylidene)thiochroman-4-one | S | 3 | CH3 | 147–148 |
| IVh | 6-Methyl-3-(4-hydroxyarylidene)thiochroman-4-one | S | 4 | CH3 | 222–223 |
| IVi | 6-Chloro-3-(3-hydroxyarylidene)thiochroman-4-one | S | 3 | Cl | 156–158 |
| IVj | 6-Chloro-3-(4-hydroxyarylidene)thiochroman-4-one | S | 4 | Cl | 202–204 |
2.4. General procedure of the synthesis 3-[3/4-(2-aryl-2-oxoethoxy)arylidene]chroman/thiochroman-4-one derivatives (1–34)
Equimolar quantities (3 mmol) of 3-(3/4-hydroxyarylidene)chroman/thiochroman-4-one (IVa-IVj) derivative and brominated acetophenone derivative were refluxed for 6 h in acetone in the presence of potassium carbonate. After TLC check, the reaction solvent was evaporated and the obtained residue was washed with water and crystallised from ethanol to achieve final compounds (1–34). The chemical properties of the obtained compounds (1–34) were given in Table 2.
Table 2.
3-[3/4-(2-Aryl-2-oxoethoxy)arylidene]chroman/thiochroman-4-one derivatives.  .
.
| Comp. | X | —R | —R’ | Position | M.P. (°C) | M.F. |
|---|---|---|---|---|---|---|
| 1 | O | —H | —H | 3 | 119 | C24H18O4 |
| 2 | O | —H | —CH3 | 3 | 146 | C25H20O4 |
| 3 | O | —H | —OCH3 | 3 | 161 | C25H20O5 |
| 4 | O | —H | —Cl | 3 | 140 | C24H17ClO4 |
| 5 | O | —H | —H | 4 | 176 | C24H18O4 |
| 6 | O | —H | —CH3 | 4 | 179 | C25H20O4 |
| 7 | O | —H | —OCH3 | 4 | 200 | C25H20O5 |
| 8 | O | —H | —Cl | 4 | 193 | C24H17ClO4 |
| 9 | O | —Cl | —H | 3 | 130 | C24H17ClO4 |
| 10 | O | —Cl | —OCH3 | 3 | 157 | C25H19ClO5 |
| 11 | O | —Cl | —Cl | 3 | 133 | C24H16Cl2O4 |
| 12 | O | —Cl | —H | 4 | 185 | C24H17ClO4 |
| 13 | O | —Cl | —OCH3 | 4 | 160 | C25H19ClO5 |
| 14 | O | —Cl | —Cl | 4 | 188 | C24H16Cl2O4 |
| 15 | S | —H | —H | 3 | 148 | C24H18O3S |
| 16 | S | —H | —CH3 | 3 | 136 | C25H20O3S |
| 17 | S | —H | —OCH3 | 3 | 137 | C25H20O4S |
| 18 | S | —H | —Cl | 3 | 137 | C24H17ClO3S |
| 19 | S | —H | —H | 4 | 146 | C24H18O3S |
| 20 | S | —H | —CH3 | 4 | 140 | C25H20O3S |
| 21 | S | —H | —OCH3 | 4 | 130 | C25H20O4S |
| 22 | S | —H | —Cl | 4 | 176 | C24H17ClO3S |
| 23 | S | —CH3 | —H | 3 | 110 | C25H20O3S |
| 24 | S | —CH3 | —OCH3 | 3 | 165 | C26H22O4S |
| 25 | S | —CH3 | —Cl | 3 | 153 | C25H19ClO3S |
| 26 | S | —CH3 | —H | 4 | 151 | C25H20O3S |
| 27 | S | —CH3 | —OCH3 | 4 | 158 | C26H22O4S |
| 28 | S | —CH3 | —Cl | 4 | 200 | C25H19ClO3S |
| 29 | S | —Cl | —H | 3 | 134 | C24H17ClO3S |
| 30 | S | —Cl | —OCH3 | 3 | 155 | C25H19ClO4S |
| 31 | S | —Cl | —Cl | 3 | 148 | C24H16Cl2O3S |
| 32 | S | —Cl | —H | 4 | 173 | C24H17ClO3S |
| 33 | S | —Cl | —OCH3 | 4 | 177 | C25H19ClO4S |
| 34 | S | —Cl | —Cl | 4 | 212 | C24H16Cl2O3S |
2.4.1. 3-[3-(2-Phenyl-2-oxoethoxy)benzylidene]chroman-4-one (1)
IR (KBr) νmax (cm−1): 3071, 3044 (Ar—H and CH—), 2956, 2921, 2863 (Aliphatic C—H), 1703, 1687 (C O), 1600–1489 (C C), 1276, 1220 (C—O). 1H NMR δ (ppm): 5.44 (2H, d, J: 1.82 Hz, Chromanone C2—H), 5.71 (2H, s, O—CH2—), 7.06–7.19 (5H, m, Ar—H), 7.65 (1H, t, J:7.03 Hz, Ar—H), 7.61–7.65 (3H, m, Ar—H), 7.75–7.77 (2H, m, Ar—H), 7.92 (1H, dd, J: 1.66 Hz, J: 7.85 Hz, Ar—H), 8.08 (2H, d, J: 8.20 Hz, Ar—H). 13C NMR δ (ppm): 67.84, 70.64, 116.59, 116.93, 118.41, 121.92, 122.48, 123.18, 127.74, 128.34, 129.29, 130.30, 131.48, 134.28, 134.84, 135.53, 136.77, 136.95, 158.53, 161.12, 194.86. For C24H18O4 calculated: 77.82% C, 4.90% H; found: 77.76% C, 4.82% H. MS [M+1]+: m/z 371.
2.4.2. 3-[3-(2-(4-Methylphenyl)-2-oxoethoxy)benzylidene]chroman-4-one (2)
IR (KBr) νmax (cm−1): 3082, 3033 (Ar—H and CH—), 2964, 2920, 2860 (Aliphatic C—H), 1697, 1668 (C O), 1606–1479 (C C), 1286, 1230 (C—O). 1H NMR δ (ppm): 2.40 (3H, s, Ar—CH3), 5.42 (2H, d, J: 1.71 Hz, Chromanone C2—H), 5.62 (2H, s, O—CH2—), 7.04–7.18 (5H, m, Ar—H), 7.40–7.46 (3H, m, Ar—H), 7.63 (1H, d, J: 8.65 Hz, Ar—H), 7.75 (1H, s, CH—), 7.91 (1H, dd, J: 1.66 Hz, J: 7.85 Hz, Ar—H), 7.95 (2H, d, J: 8.16 Hz, Ar—H). For C25H20O4 calculated: 78.11% C, 5.24% H; found: 78.08% C, 5.32% H. MS [M+1]+: m/z 385.
2.4.3. 3-[3-(2-(4-Methoxyphenyl)-2-oxoethoxy)benzylidene]chroman-4-one (3)
IR (KBr) νmax (cm−1): 3080, 3032 (Ar—H and CH—), 2961, 2920, 2871 (Aliphatic C—H), 1698, 1669 (C O), 1608–1475 (C C), 1282, 1234 (C—O). 1H NMR δ (ppm): 3.88 (3H, s, Ar—OCH3), 5.43 (2H, d, J: 1.73 Hz, Chromanone C2—H), 5.63 (2H, s, O—CH2—), 7.11–7.15 (2H, d, J: 7.80 Hz Ar—H), 7.38–7.44 (3H, m, Ar—H), 7.65 (2H, d, J: 8.55 Hz, Ar—H), 7.76 (1H, s, CH—), 7.88–7.91 (3H, m, Ar—H), 7.97 (2H, d, J: 8.24 Hz, Ar—H). For C25H20O5 calculated: 74.99% C, 5.03% H; found: 75.04% C, 5.10% H. MS [M+1]+: m/z 401.
2.4.4. 3-[3-(2-(4-Chlorophenyl)-2-oxoethoxy)benzylidene]chroman-4-one (4)
IR (KBr) νmax (cm−1): 3074, 3026 (Ar—H and CH—), 2960, 2917, 2876 (Aliphatic C—H), 1697, 1668 (C O), 1611–1470 (C C), 1287, 1231 (C—O). 1H NMR δ (ppm): 5.44 (2H, d, J: 1.75 Hz, Chromanone C2—H), 5.65 (2H, s, O—CH2—), 7.13–7.16 (2H, d, J: 7.84 Hz Ar—H), 7.42–7.48 (3H, m, Ar—H), 7.69 (2H, d, J: 8.64 Hz, Ar—H), 7.78 (1H, s, CH—), 7.89–7.93 (3H, m, Ar—H), 7.99 (2H, d, J: 8.20 Hz, Ar—H). For C24H17ClO4 calculated: 71.20% C, 4.23% H; found: 71.27% C, 4.25% H. MS [M+1]+: m/z 404.5.
2.4.5. 3-[4-(2-Phenyl-2-oxoethoxy)benzylidene]chroman-4-one (5)
IR (KBr) νmax (cm−1): 3077, 3054 (Ar—H and CH—), 2987, 2863 (Aliphatic C—H), 1711, 1687 (C O), 1603–1479 (C C), 1276, 1228 (C—O). 1H NMR δ (ppm): 5.45 (2H, d, J: 1.70 Hz, Chromanone C2—H), 5.74 (2H, s, O—CH2—), 7.09 (1H, d, J: 8.29 Hz, Ar—H), 7.13–7.18 (3H, m, Ar—H), 7.48 (2H, d, J: 8.75 Hz, Ar—H), 7.63 (3H, t, J: 7.01 Hz, Ar—H), 7.73–7.76 (2H, m, CH— and Ar—H), 7.91 (1H, dd, J: 1.61 Hz, J: 7.83 Hz, Ar—H), 8.08 (2H, d, J: 7.20 Hz, Ar—H). 13C NMR δ (ppm): 67.96, 70.66, 115.54, 118.32, 122.04, 122.38, 127.12, 127.68, 128.36, 129.15, 129.32, 132.86, 134.36, 136.53, 159.74, 160.96, 181.51, 194.66. For C24H18O4 calculated: 77.82% C, 4.90% H; found: 77.72% C, 4.86% H. MS [M+1]+: m/z 371.
2.4.6. 3-[4-(2-(4-Methylphenyl)-2-oxoethoxy)benzylidene]chroman-4-one (6)
IR (KBr) νmax (cm−1): 3059, 3030 (Ar—H and CH—), 2904, 2950 (Aliphatic C—H), 1711, 1649 (C O), 1589–1508 (C C), 1294, 1269, 1249 (C—O). 1H NMR δ (ppm): 2.43 (3H, s, Ar—CH3), 5.46 (2H, d, J: 1.70 Hz, Chromanone C2—H), 5.67 (2H, s, O—CH2—), 7.09 (1H, d, J: 8.39 Hz, Ar—H), 7.11–7.18 (3H, m, Ar—H), 7.43 (2H, d, J: 8.05 Hz, Ar—H), 7.48 (2H, t, J: 8.76 Hz, Ar—H), 7.62 (1H, d, J: 8.15 Hz, Ar—H), 7.76 (1H, s, CH—), 7.91 (1H, dd, J: 1.64, 1.66 Hz, J: 7.84 Hz, Ar—H), 7.98 (2H, d, J: 7.2 Hz, Ar—H). 13C NMR δ (ppm): 21.72, 67.96, 70.56, 115.52, 118.32, 122.03, 122.38, 127.08, 127.68, 128.46, 129.13, 129.85, 132.26, 132.86, 136.53, 136.85, 144.87, 159.77, 181.51, 194.14. For C25H20O4 calculated: 78.11% C, 5.24% H; found: 78.14% C, 5.34% H. MS [M+1]+: m/z 385.
2.4.7. 3-[4-(2-(4-Methoxyphenyl)-2-oxoethoxy)benzylidene]chroman-4-one (7)
IR (KBr) νmax (cm−1): 3076, 3031 (Ar—H and CH—), 2972, 2924, 2873 (Aliphatic C—H), 1696, 1665 (C O), 1611–1474 (C C), 1275, 1229 (C—O). 1H NMR δ (ppm): 3.88 (3H, s, Ar—OCH3), 5.42 (2H, d, J: 1.70 Hz, Chromanone C2—H), 5.62 (2H, s, O—CH2—), 7.12–7.16 (2H, d, J: 7.78 Hz Ar—H), 7.44–7.46 (3H, m, Ar—H), 7.67 (2H, d, J: 8.52 Hz, Ar—H), 7.74 (1H, s, CH—), 7.85–7.90 (3H, m, Ar—H), 7.98 (2H, d, J: 8.20 Hz, Ar—H). 13C NMR δ (ppm): 56.13, 67.96, 70.38, 114.56, 115.52, 118.32, 122.38, 127.05, 129.11, 130.74, 132.86, 136.54, 136.85, 159.82, 181.51, 192.96. For C25H20O5 calculated: 74.99% C, 5.03% H; found: 75.08% C, 5.07% H. MS [M+1]+: m/z 401.
2.4.8. 3-[4-(2-(4-Chlorophenyl)-2-oxoethoxy)benzylidene]chroman-4-one (8)
IR (KBr) νmax (cm−1): 3067, 3034 (Ar—H and CH—), 2978, 2814 (Aliphatic C—H), 1704, 1682 (C O), 1615–1479 (C C), 1275, 1224 (C—O). 1H NMR δ (ppm): 5.48 (2H, d, J: 1.71 Hz, Chromanone C2—H), 5.73 (2H, s, O—CH2—), 7.09 (1H, d, J: 8.26 Hz, Ar—H), 7.13–7.18 (3H, m, Ar—H), 7.48 (2H, d, J: 8.77 Hz, Ar—H), 7.62 (1H, d, J: 8.05 Hz, Ar—H), 7.71 (2H, d, J: 8.84 Hz, Ar—H), 7.76 (1H, s, CH—), 7.91 (1H, dd, J: 1.70 Hz, J: 7.82 Hz, Ar—H), 8.09 (2H, d, J: 8.56 Hz, Ar—H). For C24H17ClO4 calculated: 71.20% C, 4.23% H; found: 71.25% C, 4.29% H. MS [M+1]+: m/z 404.5.
2.4.9. 3-[3-(2-Phenyl-2-oxoethoxy)benzylidene]-6-chlorochroman-4-one (9)
IR (KBr) νmax (cm−1): 3067, 3058 (Ar—H and CH—), 2982, 2860 (Aliphatic C—H), 1713, 1685 (C O), 1604–1481 (C C), 1274, 1231 (C—O). 1H NMR δ (ppm): 5.43 (2H, d, J: 1.75 Hz, Chromanone C2—H), 5.73 (2H, s, O—CH2—), 7.12 (1H, d, J: 8.24 Hz, Ar—H), 7.15–7.22 (3H, m, Ar—H), 7.52 (2H, d, J: 8.20 Hz, Ar—H), 7.69 (2H, t, J: 7.26 Hz, Ar—H), 7.79–7.84 (2H, m, CH— and Ar—H), 7.96 (1H, dd, J: 1.73 Hz, J: 7.86 Hz, Ar—H), 8.14 (2H, d, J: 7.32 Hz, Ar—H). For C24H17ClO4 calculated: 71.20% C, 4.23% H; found: 71.15% C, 4.31% H. MS [M+Na]+: m/z 427.1.
2.4.10. 3-[3-(2-(4-Methoxyphenyl-2-oxoethoxy)benzylidene]-6-chlorochroman-4-one (10)
IR (KBr) νmax (cm−1): 3082, 3035 (Ar—H and CH—), 2976, 2925, 2872 (Aliphatic C—H), 1695, 1667 (C O), 1613–1475 (C C), 1276, 1232 (C—O). 1H NMR δ (ppm): 3.89 (3H, s, Ar—OCH3), 5.43 (2H, d, J: 1.65 Hz, Chromanone C2—H), 5.63 (2H, s, O—CH2—), 7.16–7.19 (2H, d, J: 7.74 Hz Ar—H), 7.52–7.59 (2H, m, Ar—H), 7.71 (2H, d, J: 8.12 Hz, Ar—H), 7.75 (1H, s, CH—), 7.82–7.88 (3H, m, Ar—H), 7.91 (2H, d, J: 8.10 Hz, Ar—H). 13C NMR δ (ppm): 56.12, 68.19, 70.37, 114.55, 115.55, 117.30, 120.73, 123.00, 126.41, 126.52, 126.90, 127.62, 128.19, 130.74, 133.04, 136.05, 137.73, 159.61, 160.00, 164.11, 180.51, 192.91. For C25H19ClO5 calculated: 69.05% C, 4.40% H; found: 69.09% C, 4.32% H. MS [M+1]+: m/z 435.1.
2.4.11. 3-[3-(2-(4-Chlorophenyl-2-oxoethoxy)benzylidene]-6-chlorochroman-4-one (11)
IR (KBr) νmax (cm−1): 3072, 3036 (Ar—H and CH—), 2979, 2815 (Aliphatic C—H), 1705, 1680 (C O), 1618–1477 (C C), 1273, 1227 (C—O). 1H NMR δ (ppm): 5.47 (2H, d, J: 1.70 Hz, Chromanone C2—H), 5.73 (2H, s, O—CH2—), 7.10 (1H, d, J: 8.12 Hz, Ar—H), 7.16–7.18 (2H, m, Ar—H), 7.54 (2H, d, J: 8.70 Hz, Ar—H), 7.65 (1H, d, J: 8.23 Hz, Ar—H), 7.75 (2H, d, J: 8.84 Hz, Ar—H), 7.76 (1H, s, CH—), 7.91 (1H, dd, J: 1.74 Hz, J: 7.62 Hz, Ar—H), 8.11 (2H, d, J: 8.54 Hz, Ar—H). For C24H16Cl2O4 calculated: 65.62% C, 3.67% H; found: 65.65% C, 3.61% H. MS [M+Na]+: m/z 461.0.
2.4.12. 3-[4-(2-Phenyl-2-oxoethoxy)benzylidene]-6-chlorochroman-4-one (12)
IR (KBr) νmax (cm−1): 3055, 3015 (Ar—H and CH—), 2968, 2905 (Aliphatic C—H), 1717, 1666 (C O), 1602–1473 (C C), 1284, 1271, 1228 (C—O). 1H NMR δ (ppm): 5.48 (2H, d, J: 1.80 Hz, Chromanone C2—H), 5.72 (2H, s, O—CH2—), 7.12 (3H, t, J: 8.73 Hz, Ar—H), 7.46 (2H, d, J: 8.60 Hz, Ar—H), 7.58–7.65 (3H, m, Ar—H), 7.62 (1H, t, J: 7.54 Hz, Ar—H), 7.75 (1H, bs, CH—), 7.88 (1H, d, J: 7.82 Hz, Ar—H), 8.05 (2H, d, J: 8.64 Hz, Ar—H). 13C NMR δ (ppm): 29.00, 70.64, 115.51, 126.31, 127.62, 128.36, 129.32, 130.17, 131.22, 132.21, 132.43, 133.72, 134.34, 134.75, 137.09, 137.38, 140.94, 159.29, 185.46, 194.71. For C24H17ClO4 calculated: 71.20% C, 4.23% H; found: 71.16% C, 4.34% H. MS [M+1]+: m/z 404.5.
2.4.13. 3-[4-(2-(4-Methoxyphenyl-2-oxoethoxy)benzylidene]-6-chlorochroman-4-one (13)
IR (KBr) νmax (cm−1): 3076, 3045 (Ar—H and CH—), 2988, 2864 (Aliphatic C—H), 1705, 1689 (C O), 1606–1474 (C C), 1278, 1226 (C—O). 1H NMR δ (ppm): 3.88 (3H, s, Ar—OCH3), 5.48 (2H, s, Chromanone C2—H), 5.72 (2H, s, O—CH2—), 7.15 (3H, d, J: 8.74 Hz, Ar—H), 7.46 (2H, d, J: 8.32 Hz, Ar—H), 7.63 (1H, dd, J: 2.75 Hz, J: 8.84 Hz, Ar—H), 7.67 (2H, d, J: 8.53 Hz, Ar—H), 7.75 (1H, s, CH—), 7.80 (1H, d, J: 2.69 Hz, Ar—H), 8.06 (2H, d, J: 8.53 Hz, Ar—H). For C25H19ClO5 calculated: 69.05% C, 4.40% H; found: 69.11% C, 4.33% H. MS [M+1]+: m/z 435.1.
2.4.14. 3-[4-(2-(4-Chlorophenyl-2-oxoethoxy)benzylidene]-6-chlorochroman-4-one (14)
IR (KBr) νmax (cm−1): 3078, 3044 (Ar—H and CH—), 2987, 2865 (Aliphatic C—H), 1712, 1684 (C O), 1602–1477 (C C), 1275, 1222 (C—O). 1H NMR δ (ppm): 5.47 (2H, s, Chromanone C2—H), 5.70 (2H, s, O—CH2—), 7.13 (3H, d, J: 8.74 Hz, Ar—H), 7.46 (2H, d, J: 8.32 Hz, Ar—H), 7.63 (1H, dd, J: 2.73 Hz, J: 8.80 Hz, Ar—H), 7.68 (2H, d, J: 8.50 Hz, Ar—H), 7.74 (1H, s, CH—), 7.84 (1H, d, J: 2.72 Hz, Ar—H), 8.12 (2H, d, J: 8.50 Hz, Ar—H). For C24H16Cl2O4 calculated: 65.62% C, 3.67% H; found: 65.55% C, 3.58% H. MS [M+1]+: m/z 439.
2.4.15. 3-[3-(2-Phenyl-2-oxoethoxy)benzylidene]thiochroman-4-one (15)
IR (KBr) νmax (cm−1): 3065, 3039 (Ar—H and CH—), 2969, 2950 (Aliphatic C—H), 1706, 1658 (C O), 1603–1490 (C C), 1285, 1221 (C—O). 1H NMR δ (ppm): 4.27 (2H, s, Thiochromanone C2—H), 5.69 (2H, s, O—CH2—), 7.08 (2H, d, J: 8.79 Hz, Ar—H), 7.32 (1H, d, J: 7.98 Hz, Ar—H), 7.40–7.422 (1H, m, Ar—H), 7.49–7.53 (3H, m, Ar—H), 7.57–7.63 (3H, m, Ar—H and CH—), 7.70–7.73 (1H, m, Ar—H), 8.03–8.05 (3H, m, Ar—H). For C24H18O3S calculated: 74.59% C, 4.69% H; found: 74.55% C, 4.59% H. MS [M+1]+: m/z 387.
2.4.16. 3-[3-(2-(4-Methylphenyl)-2-oxoethoxy)benzylidene]thiochroman-4-one (16)
IR (KBr) νmax (cm−1): 3076, 3024 (Ar—H and CH—), 2965, 2850 (Aliphatic C—H), 1698, 1650 (C O), 1599–1474 (C C), 1274, 1222 (C—O). 1H NMR δ (ppm): 2.09 (3H, s, Ar—CH3), 4.28 (2H, s, Thiochromanone C2—H), 5.69 (2H, s, O—CH2—), 7.06 (2H, d, J: 8.73 Hz, Ar—H), 7.32–7.35 (1H, m, Ar—H), 7.41–7.43 (1H, m, Ar—H), 7.49–7.53 (3H, m, Ar—H), 7.57–7.61 (2H, m, Ar—H and CH—), 7.69–7.73 (1H, m, Ar—H), 8.03–8.05 (3H, m, Ar—H). 13C NMR δ (ppm): 28.67, 123.99, 124.73, 126.43, 128.43, 130.25, 130.79, 132.01, 134.59, 135.52, 136.39, 136.50, 141.19, 185.32. For C25H20O3S calculated: 74.98% C, 5.03% H; found: 74.93% C, 5.08% H. MS [M+1]+: m/z 401.
2.4.17. 3-[3-(2-(4-Methoxyphenyl)-2-oxoethoxy)benzylidene]thiochroman-4-one (17)
IR (KBr) νmax (cm−1): 3075, 3020 (Ar—H and CH—), 2969, 2857 (Aliphatic C—H), 1695, 1655 (C O), 1594–1471 (C C), 1272, 1221 (C—O). 1H NMR δ (ppm): 3.89 (3H, s, Ar—OCH3), 4.29 (2H, s, Thiochromanone C2—H), 5.66 (2H, s, O—CH2—), 7.10 (2H, d, J: 8.70 Hz, Ar—H), 7.30–7.32 (1H, m, Ar—H), 7.43–7.45 (1H, m, Ar—H), 7.51–7.54 (3H, m, Ar—H), 7.59–7.63 (2H, m, Ar—H and CH—), 7.75–7.78 (1H, m, Ar—H), 8.10–8.13 (3H, m, Ar—H). 13C NMR δ (ppm): 28.67, 123.98, 124.72, 126.42, 128.42, 130.25, 132.00, 134.09, 135.51, 136.37, 141.19, 148.50, 185.31. For C25H20O4S calculated: 72.09% C, 4.84% H; found: 72.03% C, 4.88% H. MS [M+1]+: m/z 417.
2.4.18. 3-[3-(2-(4-Chlorophenyl)-2-oxoethoxy)benzylidene]thiochroman-4-one (18)
IR (KBr) νmax (cm−1): 3056, 3027 (Ar—H and CH—), 2976, 2860 (Aliphatic C—H), 1692, 1659 (C O), 1592–1470 (C C), 1274, 1225 (C—O). 1H NMR δ (ppm): 4.29 (2H, s, Thiochromanone C2—H), 5.68 (2H, s, O—CH2—), 7.12 (2H, d, J: 8.60 Hz, Ar—H), 7.35–7.37 (1H, m, Ar—H), 7.41–7.44 (1H, m, Ar—H), 7.50–7.52 (3H, m, Ar—H), 7.61–7.63 (2H, m, Ar—H and CH—), 7.74–7.76 (1H, m, Ar—H), 8.11–8.14 (3H, m, Ar—H). 13C NMR δ (ppm): 28.99, 70.63, 116.56, 116.96, 117.18, 117.36, 118.41, 121.59, 121.90, 122.49, 123.23, 127.74, 129.41, 130.31, 131.49, 133.52, 135.54, 136.77, 136.93, 137.23, 139.15, 158.45, 161.12, 181.61, 193.99. For C24H17ClO3S calculated: 68.48% C, 4.07% H; found: 68.53% C, 4.12% H. MS [M+1]+: m/z 420.5.
2.4.19. 3-[4-(2-Phenyl-2-oxoethoxy)benzylidene]thiochroman-4-one (19)
IR (KBr) νmax (cm−1): 3075, 3039 (Ar—H and CH—), 2951, 2867 (Aliphatic C—H), 1696, 1650 (C O), 1599–1474 (C C), 1272, 1224 (C—O). 1H NMR δ (ppm): 4.25 (2H, s, Thiochromanone C2—H), 5.69 (2H, s, O—CH2—), 7.06 (2H, d, J: 8.73 Hz, Ar—H), 7.32–7.35 (1H, m, Ar—H), 7.40–7.42 (1H, m, Ar—H), 7.49–7.53 (3H, d, J: 8.73 Hz, Ar—H), 7.57–7.61 (3H, m, Ar—H and CH—), 7.69–7.73 (1H, m, Ar—H), 8.03–8.05 (3H, m, Ar—H). For C24H18O3S calculated: 74.59% C, 4.69% H; found: 74.53% C, 4.58% H. MS [M+1]+: m/z 387.
2.4.20. 3-[4-(2-(4-Methylphenyl)-2-oxoethoxy)benzylidene]thiochroman-4-one (20)
IR (KBr) νmax (cm−1): 3063, 3039 (Ar—H and CH—), 2963, 2950 (Aliphatic C—H), 1709, 1658 (C O), 1608–1490 (C C), 1286, 1252, 1221 (C—O). 1H NMR δ (ppm): 2.40 (3H, s, Ar-CH3), 4.27 (2H, s, Thiochromanone C2—H), 5.63 (2H, s, O—CH2—), 7.07 (2H, d, J: 8.80 Hz, Ar—H), 7.32 (1H, d, J: 1.20 Hz, J: 7.50 Hz, Ar—H), 7.37–7.41 (3H, m, Ar—H), 7.48–7.52 (3H, m, Ar—H), 7.61 (1H, s, CH—), 7.93 (2H, d, J: 8.18 Hz, Ar—H), 8.15 (1H, dd, J: 1.30 Hz and 8.73 Hz, Ar—H). For C25H20O3S calculated: 74.98% C, 5.03% H; found: 74.94% C, 5.11% H. MS [M+1]+: m/z 401.
2.4.21. 3-[4-(2-(4-Methoxyphenyl)-2-oxoethoxy)benzylidene]thiochroman-4-one (21)
IR (KBr) νmax (cm−1): 3075, 3039 (Ar—H and CH—), 2950, 2867 (Aliphatic C—H), 1696, 1650 (C O), 1599–1474 (C C), 1275, 1224 (C—O). 1H NMR δ (ppm): 3.87 (2H, s, Ar—OCH3), 4.27 (2H, s, Thiochromanone C2—H), 5.61 (2H, s, O—CH2—), 7.06 (2H, d, J: 8.93 Hz, Ar—H), 7.12 (2H, d, J: 8.81 Hz, Ar—H), 7.31 (1H, dd, J: 7.96, 8.63 Hz, J: 15.92 Hz, Ar—H), 7.41 (1H, d, J: 7.62 Hz, Ar—H), 7.49–7.53 (3H, m, Ar—H), 7.61 (1H, s, CH—), 8.01–8.08 (3H, m, Ar—H), 8.24 (1H, d, J: 2.06 Hz Ar—H). For C25H20O4S calculated: 72.09% C, 4.84% H; found: 72.01% C, 4.89% H. MS [M+1]+: m/z 417.
2.4.22. 3-[4-(2-(4-Chlorophenyl)-2-oxoethoxy)benzylidene]thiochroman-4-one (22)
IR (KBr) νmax (cm−1): 3062, 3030 (Ar—H and CH—), 2957, 2880 (Aliphatic C—H), 1704, 1675 (C O), 1599–1474 (C C), 1272, 1224 (C—O). 1H NMR δ (ppm): 4.27 (2H, s, Thiochromanone C2—H), 5.67 (2H, s, O—CH2—), 7.09 (2H, d, J: 8.93 Hz, Ar—H), 7.32 (1H, d, J: 7.45 Hz, Ar—H), 7.40–7.42 (1H, m, Ar—H), 7.49–7.53 (3H, m, Ar—H), 7.61 (1H, s, CH—), 7.42 (2H, d, J: 8.0 Hz, Ar—H), 8.03–8.06 (3H, m, Ar—H). For C24H17ClO3S calculated: 68.48% C, 4.07% H; found: 68.54% C, 4.13% H. MS [M+1]+: m/z 420.5.
2.4.23. 3-[3-(2-Phenyl-2-oxoethoxy)benzylidene]-6-methylthiochroman-4-one (23)
IR (KBr) νmax (cm−1): 3074, 3042 (Ar—H and CH—), 2964, 2869 (Aliphatic C—H), 1699, 1660 (C O), 1598–1476 (C C), 1273, 1226 (C—O). 1H NMR δ (ppm): 2.42 (3H, s, Ar-CH3), 4.26 (2H, s, Thiochromanone C2—H), 5.70 (2H, s, O—CH2—), 7.09 (2H, d, J: 8.56 Hz, Ar—H), 7.30–7.33 (1H, m, Ar—H), 7.48–7.52 (1H, m, Ar—H), 7.58–7.60 (3H, d, J: 8.56 Hz, Ar—H), 7.61–7.63 (2H, m, Ar—H and CH—), 7.65–7.72 (1H, m, Ar—H), 8.04–8.06 (3H, m, Ar—H). For C25H20O3S calculated: 74.98% C, 5.03% H; found: 75.04% C, 5.10% H. MS [M+1]+: m/z 415.
2.4.24. 3-[3-(2-(4-Methoxyphenyl)-2-oxoethoxy)benzylidene]-6-methylthiochroman-4-one (24)
IR (KBr) νmax (cm−1): 3078, 3054 (Ar—H and CH—), 2971, 2854 (Aliphatic C—H), 1698, 1662 (C O), 1592–1470 (C C), 1275, 1220 (C—O). 1H NMR δ (ppm): 2.42 (3H, s, Ar—CH3), 3.87 (3H, s, Ar—OCH3), 4.26 (2H, s, Thiochromanone C2—H), 5.72 (2H, s, O—CH2—), 7.15 (2H, d, J: 8.34 Hz, Ar—H), 7.32–7.34 (1H, m, Ar—H), 7.49–7.51 (1H, m, Ar—H), 7.58–7.60 (3H, d, J: 8.56 Hz, Ar—H), 7.60–7.62 (2H, m, Ar—H and CH—), 7.68–7.70 (1H, m, Ar—H), 8.04–8.06 (2H, m, Ar—H). For C26H22O4S calculated: 72.54% C, 5.15% H; found: 72.51% C, 5.21% H. MS [M+1]+: m/z 431.
2.4.25. 3-[3-(2-(4-Chlorophenyl)-2-oxoethoxy)benzylidene]-6-methylthiochroman-4-one (25)
IR (KBr) νmax (cm−1): 3065, 3039 (Ar—H and CH—), 2969, 2950 (Aliphatic C—H), 1706, 1658 (C O), 1603–1490 (C C), 1285, 1221 (C—O). 1H NMR δ (ppm): 2.33 (3H, s, Ar—CH3), 4.19 (2H, s, Thiochromanone C2—H), 5.65 (2H, s, O—CH2—), 7.06 (1H, dd, J: 2.36 Hz, J: 8.25 Hz, Ar—H), 7.12–7.13 (2H, m, Ar—H), 7.30 (1H, d, J: 7.99 Hz, Ar—H), 7.35 (1H, dd, J:1.68 Hz, J: 8.05 Hz, Ar—H), 7.40 (1H, t, J: 7.35 Hz, Ar—H), 7.60 (1H, s, CH—), 7.66 (2H, d, J: 8.61 Hz, Ar—H), 7.86 (1H, bs, Ar—H), 8.05 (2H, d, J: 8.59 Hz, Ar—H). For C25H19ClO3S calculated: 69.04% C, 4.40% H; found: 69.13% C, 4.50% H. MS [M+1]+: m/z 435.1.
2.4.26. 3-[4-(2-Phenyl-2-oxoethoxy)benzylidene]-6-methylthiochroman-4-one (26)
IR (KBr) νmax (cm−1): 3065, 3039 (Ar—H and CH—), 2951, 2867 (Aliphatic C—H), 1696, 1650 (C O), 1599–1474 (C C), 1272, 1224 (C—O). 1H NMR δ (ppm): 2.34 (3H, s, Ar—CH3), 4.24 (2H, s, Thiochromanone C2—H), 5.69 (2H, s, O—CH2—), 7.08 (2H, d, J: 8.81 Hz, Ar—H), 7.29–7.38 (2H, m, Ar—H), 7.52 (2H, d, J: 8.77 Hz, Ar—H), 7.57–7-58 (2H, m, Ar—H), 7.60 (1H, s, CH—), 7.68–7.76 (1H, m, Ar—H), 7.89 (1H, bs, Ar—H), 8.05 (2H, d, J: 8.60 Hz, Ar—H). For C25H20O3S calculated: 74.98% C, 5.03% H; found: 74.88% C, 5.08% H. MS [M+1]+: m/z 401.
2.4.27. 3-[4-(2-(4-Methoxyphenyl)-2-oxoethoxy)benzylidene]-6-methylthiochroman-4-one (27)
IR (KBr) νmax (cm−1): 3067, 3045 (Ar—H and CH—), 2952, 2921, 2860 (Aliphatic C—H), 1711, 1691 (C O), 1610–1472 (C C), 1273, 1211 (C—O). 1H NMR δ (ppm): 2.34 (3H, s, Ar—CH3), 3.87 (3H, s, Ar—OCH3), 4.23 (2H, s, Thiochromanone C2—H), 5.61 (2H, s, O—CH2—), 7.06 (2H, d, J: 8.73 Hz, Ar—H), 7.11 (2H, d, J: 8.80 Hz, Ar—H), 7.27–7.35 (2H, m, Ar—H), 7.51 (2H, d, J: 8.61 Hz, Ar—H), 7.60 (1H, s, CH—), 7.85 (1H, bs, Ar—H), 8.02 (2H, d, J: 8.95 Hz, Ar—H). For C26H22O4S calculated: 72.54% C, 5.15% H; found: 72.62% C, 5.08% H. MS [M+1]+: m/z 431.
2.4.28. 3-[4-(2-(4-Chlorophenyl)-2-oxoethoxy)benzylidene]-6-methylthiochroman-4-one (28)
IR (KBr) νmax (cm−1): 3105, 3035 (Ar—H and CH—), 2975, 2866 (Aliphatic C—H), 1704, 1680 (C O), 1601–1475 (C C), 1275, 1230 (C—O). 1H NMR δ (ppm): 2.34 (3H, s, Ar—CH3), 4.23 (2H, s, Thiochromanone C2—H), 5.67 (2H, s, O—CH2—), 7.09 (2H, d, J: 8.73 Hz, Ar—H), 7.28–7.35 (2H, m, Ar—H), 7.52 (2H, d, J: 8.61 Hz, Ar—H), 7.60 (1H, s, CH—), 7.67 (2H, d, J: 8.21 Hz, Ar—H), 7.86 (1H, bs, Ar—H), 8.06 (2H, d, J: 8.95 Hz, Ar—H). For C25H19ClO3S calculated: 69.04% C, 4.40% H; found: 69.09% C, 4.52% H. MS [M+1]+: m/z 435.
2.4.29. 3-[3-(2-Phenyl-2-oxoethoxy)benzylidene]-6-chlorothiochroman-4-one (29)
IR (KBr) νmax (cm−1): 3105, 3040 (Ar—H and CH—), 2958, 2922, 2870 (Aliphatic C—H), 1688, 1649 (C O), 1601–1490 (C C), 1275, 1260, 1221 (C—O). 1H NMR δ (ppm): 4.26 (2H, s, Thiochromanone C2—H), 5.67 (2H, s, O—CH2—), 7.07 (1H, dd, J: 2.19 Hz, J: 8.26 Hz, Ar—H), 7.12–7.15 (2H, m, Ar—H), 7.41 (1H, t, J: 7.92 Hz, Ar—H), 7.47 (1H, d, J: 8.47 Hz, Ar—H), 7.58–7.60 (3H, m, Ar—H), 7.64 (1H, s, CH—), 7.71 (1H, t, J: 7.41, 7.42 Hz, Ar—H), 7.97 (1H, d, J: 2.46 Hz, Ar—H), 8.05 (2H, d, J: 7.13 Hz, Ar—H). 13C NMR δ (ppm): 28.99, 70.64, 115.52, 126.31, 127.68, 128.35, 129.43, 130.17, 131.25, 132.21, 132.43, 133.71, 137.07, 139.23, 140.94, 159.21, 185.45, 193.86. For C24H17ClO3S calculated: 68.48% C, 4.07% H; found: 68.57% C, 4.14% H. MS [M+1]+: m/z 420.5.
2.4.30. 3-[3-(2-(4-Methoxyphenyl)-2-oxoethoxy)benzylidene]-6-chlorothiochroman-4-one (30)
IR (KBr) νmax (cm−1): 3077, 3045 (Ar—H and CH—), 2950, 2921, 2860 (Aliphatic C—H), 1702, 1689 (C O), 1587–1470 (C C), 1273, 1231 (C—O). 1H NMR δ (ppm): 3.86 (3H, s, Ar—OCH3), 4.27 (2H, s, Thiochromanone C2—H), 5.58 (2H, s, O—CH2—), 7.05 (1H, dd, J: 1.84 Hz, J: 8.44 Hz, Ar—H), 7.09 (2H, d, J: 9.0 Hz, Ar—H), 7.12 (2H, d, J: 8.57 Hz, Ar—H), 7.40 (1H, t, J: 8.18 Hz, Ar—H), 7.46 (1H, d, J: 8.41 Hz, Ar—H), 7.58 (1H, dd, J: 2.36 Hz, J: 8.71 Hz, Ar—H), 7.64 (1H, s, CH—), 7.97 (1H, d, J: 2.47 Hz, Ar—H), 8.02 (2H, d, J: 8.77 Hz, Ar—H). For C25H19ClO4S calculated: 66.59% C, 4.25% H; found: 66.63% C, 4.17% H. MS [M+1]+: m/z 450.5.
2.4.31. 3-[3-(2-(4-Chlorophenyl)-2-oxoethoxy)benzylidene]-6-chlorothiochroman-4-one (31)
IR (KBr) νmax (cm−1): 3075, 3020 (Ar—H and CH—), 2965, 2850 (Aliphatic C—H), 1698, 1650 (C O), 1599–1474 (C C), 1274, 1222 (C—O). 1H NMR δ (ppm): 4.62 (2H, s, Thiochromanone C2—H), 5.65 (2H, s, O—CH2—), 7.07 (1H, dd, J: 2.22 Hz, J: 8.12 Hz, Ar—H), 7.13 (2H, d, J: 8.67 Hz, Ar—H), 7.41 (1H, t, J: 8.0 Hz, Ar—H), 7.47 (1H, d, J: 8.77 Hz, Ar—H), 7.59 (1H, dd, J: 1.77 Hz, J: 8.43 Hz, Ar—H), 7.64 (1H, s, CH—), 7.67 (2H, d, J: 8.52 Hz, Ar—H), 7.97 (2H, d, J: 2.28 Hz, Ar—H), 8.06 (1H, d, J: 8.57 Hz, Ar—H). For C24H16Cl2O3S calculated: 63.30% C, 3.54% H; found: 63.33% C, 3.47% H. MS [M+1]+: m/z 455.
2.4.32. 3-[4-(2-Phenyl-2-oxoethoxy)benzylidene]-6-chlorothiochroman-4-one (32)
IR (KBr) νmax (cm−1): 3108, 3044 (Ar—H and CH—), 2952, 2931, 2872 (Aliphatic C—H), 1687, 1654 (C O), 1608–1485 (C C), 1273, 1262, 1220 (C—O). 1H NMR δ (ppm): 4.25 (2H, s, Thiochromanone C2—H), 5.66 (2H, s, O—CH2—), 7.10 (1H, dd, J: 2.19 Hz, J: 8.26 Hz, Ar—H), 7.18–7.20 (2H, m, Ar—H), 7.47 (1H, t, J: 7.92 Hz, Ar—H), 7.52 (1H, d, J: 8.47 Hz, Ar—H), 7.59–7.61 (3H, m, Ar—H), 7.65 (1H, s, CH—), 7.74 (1H, t, J: 7.42 Hz, Ar—H), 7.98 (1H, d, J: 2.47 Hz, Ar—H), 8.07 (2H, d, J: 7.23 Hz, Ar—H). For C24H17ClO3S calculated: 68.49% C, 4.07% H; found: 68.54% C, 4.15% H. MS [M+1]+: m/z 420.1.
2.4.33. 3-[4-(2-(4-Methoxyphenyl)-2-oxoethoxy)benzylidene]-6-chlorothiochroman-4-one (33)
IR (KBr) νmax (cm−1): 3074, 3046 (Ar—H and CH—), 2956, 2924, 2866 (Aliphatic C—H), 1705, 1684 (C O), 1583–1474 (C C), 1276, 1238 (C—O). 1H NMR δ (ppm): 3.85 (3H, s, Ar—OCH3), 4.26 (2H, s, Thiochromanone C2—H), 5.57 (2H, s, O—CH2—), 7.05 (1H, dd, J: 1.80 Hz, J: 8.42 Hz, Ar—H), 7.11 (2H, d, J: 8.90 Hz, Ar—H), 7.15 (2H, d, J: 8.54 Hz, Ar—H), 7.44 (1H, t, J: 8.28 Hz, Ar—H), 7.47 (1H, d, J: 8.42 Hz, Ar—H), 7.59 (1H, dd, J: 2.32 Hz, J: 8.70 Hz, Ar—H), 7.66 (1H, s, CH—), 7.98 (1H, d, J: 2.49 Hz, Ar—H), 8.05 (2H, d, J: 8.70 Hz, Ar—H). For C25H19ClO4S calculated: 66.59% C, 4.25% H; found: 66.65% C, 4.14% H. MS [M+Na]+: m/z 473.1.
2.4.34. 3-[4-(2-(4-Chlorophenyl)-2-oxoethoxy)benzylidene]-6-chlorothiochroman-4-one (34)
IR (KBr) νmax (cm−1): 3076, 3027 (Ar—H and CH—), 2968, 2856 (Aliphatic C—H), 1695, 1656 (C O), 1587–1472 (C C), 1270, 1221 (C—O). 1H NMR δ (ppm): 4.63 (2H, s, Thiochromanone C2—H), 5.65 (2H, s, O—CH2—), 7.09 (1H, dd, J: 2.20 Hz, J: 8.10 Hz, Ar—H), 7.15 (2H, d, J: 8.63 Hz, Ar—H), 7.40 (1H, t, J: 8.12 Hz, Ar—H), 7.48 (1H, d, J: 8.70 Hz, Ar—H), 7.64 (1H, dd, J: 1.75 Hz, J: 8.40 Hz, Ar—H), 7.65 (1H, s, CH—), 7.64 (2H, d, J: 8.50 Hz, Ar—H), 7.99 (2H, d, J: 2.24 Hz, Ar—H), 8.07 (1H, d, J: 8.56 Hz, Ar—H). 13C NMR δ (ppm): 28.87, 70.63, 115.53, 127.45, 128.36, 129.20, 129.32, 130.30, 130.39, 130.95, 132.01, 132.37, 133.30, 134.36, 134.74, 138.04, 139.99, 159.46, 184.44, 194.68. For C24H16Cl2O3S calculated: 63.30% C, 3.54% H; found: 63.38% C, 3.46% H. MS [M+Na]+: m/z 477.0.
2.5. Anticancer activity
The evaluation of anticancer activity was performed at the National Cancer Institute (NCI) of Bethesda, USA. To determine anticancer activity of the selected compounds were tested against sixty human tumor cell lines derived from nine neoplastic diseases namely; leukaemia (L, 4 or 6 cell lines), non-small cell lung cancer (NSCLC, 9 cell lines), colon cancer (CC, 7 cell lines), central nervous system cancer (CNSC, 6 cell lines), melanoma (M, 8 or 9 cell lines), ovarian cancer (OC, 6 or 7 cell lines), renal cancer (RC, 8 cell lines), prostate cancer (PC, 2 cell lines), breast cancer (BC, 6 or 8 cell lines). The cytotoxic and/or growth inhibitory effects of the compounds were evaluated at one concentration as a first stage. According to the in vitro screening program of the institute promising compounds were tested at lower five concentrations ranging from 10−4 to 10−8 M. The percentage growth was evaluated spectrophotometrically versus controls not treated with test agents. Three dose response parameters (GI50, TGI and LC50) were calculated for each experimental agent (Boyd, 1989, Boyd and Paull, 1995).
3. Results and discussion
New thirty-four 3-[3/4-(2-aryl-2-oxoethoxy)arylidene]chroman/thiochroman-4-one derivatives (1–34) were synthesized, in this study. The synthesis of the compounds was carried out in a multi-step reaction as shown Scheme 1. In the first step, appropriate phenol or thiophenol derivative was reacted with acrylonitrile in THF using Triton-B as a catalyst. The resulting 3-aryloxy/arylthioxy propionitrile compounds (Ia-Ie) were hydrolyzed by acetic acid/hydrochloric acid mixture to get corresponding carboxylic acid derivatives (IIa-IIe) which were heated with polyphosphoric acid (PPA) to acquire ring closure products, chromanon-4-one or thiochroman-4-one derivatives (IIIa-IIIe). The obtained cyclic ketone compounds were reacted with 3-/4-hydroxybenzaldehyde derivatives catalyzed by hydrochloric acid in n-butanol to get hydroxyarylidene intermediates (IVa-IVj) which were then reacted with appropriate 2-bromoacetophenone derivatives in acetone and in a basic medium provided by K2CO3. The structures of the final compounds (1–34) were proved by IR, 1H NMR, MS spectral data and elemental analysis data. In the IR spectra of the compounds, characteristic stretching bands were observed at about 1700–1680 cm−1 and 1680–1650 cm−1 belong to two ketone C O bonds. In finger print region, the stretching bands for C—O bonds were seen at about 1270–1225 cm−1. In the NMR spectra of the compounds, all protons were seen at estimated fields of the spectrum. The signals at about 4.19–5.48, 5.57–5.74 and 7.60–7.78 ppm were assigned to —CH2C—, COCH2— and —C CH— groups, respectively and they were observed as common peaks in all spectrums. The protons of 1,4-disubstituted phenyl moiety were seen as doublet peaks and they were determined in accordance with splitting pattern of AB system. In the 13C NMR spectra of the compounds, as common to all compounds carbon atom of the acetyl group was seen at about 70.37–71.66 ppm. C-2 carbon atom of the chroman-4-one ring was given signal at about 67.84–68.19 ppm, whereas C-2 carbon atom of the thiochroman-4-one was given 28.67–29.00 ppm field. The carbonyl carbons were resonated at ppm. Besides, all other aromatic carbons were observed at estimated regions. In the MS spectra of the compounds, M+1 peaks or M+Na peaks were observed consistent with calculated molecular weights. Also, elemental analysis results gave confirmative molecular formulas.
Scheme 1.
The synthesis of the 3-[3/4-(2-aryl-2-oxoethoxy)arylidene]chroman/thiochroman-4-one derivatives (1–34). Reactants/reagents and reaction conditions. i : THF, Triton-B, reflux, 30 min; ii : AcOH/HCl, 160–170 °C; iii : PPA, 50–100 °C, 3–4 h; iv : n-butanol, catalytic HCl, reflux, 1 h; v : K2CO3, acetone, reflux, 6 h.
The experimental procedure was realized at National Cancer Institute of USA (NCI) for determining anticancer activity of the compounds. All final compounds (1–34) were offered to the institute, fourteen of them was selected to be tested. According to the drug screening protocol of the institute, compounds 1, 7, 8, 11, 17, 18, 21, 22, 24, 25, 27, 28, 30 and 31 were screened against 60 tumor cell lines derived from nine cancer disease at one dose (10−5 M) as a first stage study and the results were given as percent cell growth promotion (Table 3). Among them, compounds 1, 11, 17, 18, 24, 25, 30 and 31 which exhibited growth percentage lower than 80% on tumor cells as a mean value were passed through to second stage and tested at five concentrations (0.01, 0.1, 1, 10 and 100 µM). The results were given as log10 GI50 which is the log value of growth inhibitory activity that corresponds to the concentration of the compounds causing 50% decrease in net cell growth and they were shown in Table 4. The growth inhibition of half percentage of tumor cells (GI50) calculated from the equation [(Ti - Tz)/(C - Tz)] × 100 = 50 where abbreviations Tz, C, and Ti state absorbance measurements at time zero, (Tz), control growth (C) and test growth in the presence of sample at the five concentration levels (Ti) (Husain et al., 2013). Besides, dose-response graphics plotted according to the results were identified for all compounds against all cancer types (some of them were shown as Supplementary Data).
Table 3.
60 human tumor cell lines’ anticancer screening data at single dose assay as percent cell growth promotion of selected compounds.
| Com | NSCLC | CC | BC | OC | L | RC | M | PC | CNSC | Mean |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 73.59 | 21.64 | 37.84 | 69.31 | −18.47 | 79.67 | 65.3 | 101.03 | 87.66 | 54.70 |
| 7 | 93.34 | 94.38 | 82.99 | 93.86 | 66.72 | 94.15 | 88.47 | 105.63 | 97.01 | 89.11 |
| 8 | 99.17 | 101.43 | 91.74 | 98.33 | 90.97 | 100.15 | 99.22 | 108.54 | 89.01 | 96.37 |
| 11 | 89.09 | 83.49 | 67.44 | 81.33 | 19.71 | 92.74 | 86.03 | 124.48 | 91.17 | 79.53 |
| 17 | 52.23 | −49.79 | −36.15 | 59.22 | −64.04 | 11.74 | −58.79 | 101.83 | 36.77 | −0.10 |
| 18 | 38.66 | −46.31 | −10.45 | 51.54 | −61.46 | −12.38 | −82.43 | 27.66 | 18.30 | −11.63 |
| 21 | 99.93 | 104.05 | 96.42 | 103.53 | 88.65 | 101.41 | 95.23 | 120.25 | 95.24 | 98.34 |
| 22 | 107.89 | 107.6 | 103.38 | 105.13 | 90.68 | 100.19 | 104.18 | 113.32 | 106.74 | 103.44 |
| 24 | 92.05 | 68.76 | 79.47 | 90.17 | 4.50 | 93.42 | 90.58 | 108.79 | 99.49 | 79.08 |
| 25 | 83.42 | 69.59 | 73.49 | 98.21 | 11.81 | 78.70 | 92.49 | 108.30 | 102.65 | 77.49 |
| 27 | 97.49 | 80.77 | 96.64 | 105.17 | 64.31 | 99.53 | 94.86 | 104.51 | 106.06 | 95.19 |
| 28 | 100.28 | 100.93 | 106.49 | 109.23 | 89.76 | 102.74 | 102.74 | 113.20 | 102.95 | 101.83 |
| 30 | 82.47 | 57.61 | 63.97 | 83.82 | 16.03 | 81.84 | 74.95 | 61.92 | 92.11 | 69.47 |
| 31 | 65.29 | 30.40 | 45.10 | 81.01 | 5.01 | 75.46 | 73.05 | 43.72 | 78.90 | 56.67 |
NSCLC Non-small cell lung cancer, CC Colon cancer, BC breast cancer, OC ovarian cancer, L leukaemia, RC renal cancer, M melanoma, PC prostate cancer, CNSC central nervous system cancer.
Table 4.
Log10 GI50 Values.
| Comp | L | NSLC | CC | CNS | M | OC | RC | PC | BC | MG_MID |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | −5.59 | −5.15 | −5.69 | −5.28 | −5.44 | −5.43 | −5.64 | −5.45 | −5.49 | −5.45 |
| 11 | −5.41 | −5.01 | −5.25 | −4.88 | −4.95 | −5.11 | −4.91 | −4.87 | −5.20 | −5.08 |
| 17 | −6.26 | −5.46 | −6.00 | −5.68 | −5.72 | −5.66 | −5.55 | −5.74 | −6.00 | −5.78 |
| 18 | −6.39 | −5.48 | −6.05 | −5.75 | −5.77 | −5.66 | −5.80 | −5.76 | −5.82 | −5.83 |
| 24 | −5.57 | −4.48 | −5.25 | −4.83 | −4.79 | −4.75 | −4.52 | −4.71 | −5.03 | −4.84 |
| 25 | −5.63 | −4.89 | −5.53 | −5.11 | −5.22 | −5.11 | −4.99 | −5.09 | −5.42 | −5.22 |
| 30 | −5.79 | −4.92 | −5.43 | −5.15 | −5.03 | −4.98 | −4.88 | −5.36 | −5.18 | −5.17 |
| 31 | −6.13 | −5.18 | −5.14 | −5.33 | −5.23 | −5.48 | −5.50 | −6.18 | −5.27 | −5.42 |
| A | −5.48 | −5.17 | −5.11 | −5.12 | −5.08 | −5.18 | −4.99 | −4.49 | −4.79 | −5.09 |
| B | −6.39 | −6.20 | −6.14 | −6.18 | −6.08 | −6.45 | −6.17 | −6.41 | −6.05 | −6.20 |
A: Melphalan, B: Cisplatin
As can be shown in Table 3, compounds caused growth percentages of tumor cells ranging from −11.63 to 103.44%. Compounds 17 and 18 exhibited the highest antiproliferative activity with values of −0.10 and −11.63% and these two compounds differ from other compounds which have growth percentages even more than 50%. Furthermore, compounds 1, 37, 30, 25, 24 and 11 have also showed remarkable values of 54.70, 56.67, 69.47, 77.49, 79.08 and 79.53% which are lower than 80% thus they are acceptable to be test in the second stage. These eight compounds have attracted attention with carrying 1,3-benzylidene moiety considering 1,4-benzylidene bearing derivatives could not exhibit higher activities. Besides, it appears six of these eight compounds (17, 18, 24, 25, 30 and 31) have thiochromanone skeleton. In previous studies, thiochromanone containing compounds were determined to have many potential biological activities; in particular, the ring was introduced some unprecedented benefits to anticancer drug discovery in low doses (Gao et al., 2010). Also, several compounds, based primarily on the benzophenone or thiochromanone thiosemicarbazone molecular structure, were identified as lead compound inhibitors of cathepsin L which increases during cancer progression and aids in cancer metastasis (Song et al., 2012). Additionally, the studies in synthetic flavonoid derivatives showed that the presence of heterocyclic thioether feature will profit the antitumor activities of flavonoids (Huang et al., 2007). Other remarkable situation was determined that two of these compounds possessed two chlorine atoms and three others possessed one chlorine atom. Also, six thiochromanone compounds were attracted attention with bearing 4-methoxy and 4-chloro phenyl acetyloxy moieties bonded to third position of main structure (3-(hydroxyarylidene) thiochroman-4-one derivatives). A similar finding was determined in our previous study that methoxy and chloro substituents on various molecules have provided increase of anticancer activity (Yurttas et al., 2013, Yurttas et al., 2015).
Among the cancer types, leukaemia, melanoma and colon cancer cells were found as the most sensitive cells against the tested compounds. The lowest growth value (−100.00%) was obtained against the type of cell lines S (leukaemia), LOX IMVI (melanoma) and SW-620 (colon cancer) for compounds 17 and 18.
Compounds with growth values under 80% were subjected to further testing and log10 GI50 and MG-MID values were determined as a result of this test. MG-MID value was calculated as mean graph midpoint for each subpanels of cancer types for the tested compounds and standard drugs cisplatin and melphalan which are two of the commonly used chemotherapeutic agents by giving log10 GI50 (Table 4). The test method states that the compounds having log10 GI50 values greater than −4 were considered as inactive. It can be seen that both of the compounds log10 GI50 values are smaller than −4. Accordingly, when we considered MG-MID values of standard drugs and selected compounds; all compounds except 11 and 24 exhibited higher activity than melphalan (−5.09), however all compounds showed lower activity than cisplatin (−6.20).
The lowest concentrations (MG_MID) were reached for compounds 17 (−5.73) and 18 (−5.83) These values which are obtained according to concentration, were found proportional with previously described growth percentage values. The lowest growth percentage values were determined consistent with the MG_MID of these compounds. The lowest log10 GI50 value was obtained against leukaemia cells as −6.39 which was also equals to the value found for the standard used cisplatin and this value was the lowest value obtained for the standard. The obtained average log10 GI50 and MG_MID values against nine types of cancer were represented, collectively in Table 4.
According to the reported literature, compounds possessing chromanone and/or thiochromanone as a main structure and an aryl group/arylidene group with one or more free hydroxyl groups are known to have very high anticancer effects as specified. Considering the results obtained in our study, thiochromanone compounds containing 1,3-disubstituted benzylidene residue were observed to have extremely high anticancer activity. In light of this information, arylidene thiochromanone compounds carrying phenolic groups will be studied in the next part of our work. Furthermore, it will also aimed to examine the change of anticancer effects when the phenacyloxy residue brought into ortho position on benzylidene moiety.
4. Conclusion
In this study, 3-[3/4-(2-aryl-2-oxoethoxy)arylidene]chroman/thiochroman-4-one derivatives (1–34) and anticancer activity of the selected compounds were investigated by NCI. According to the drug screening protocol of the institute, mostly final compounds (15–34) containing thiochromanone skeletone caused lower growth percentages in various cancer cell lines. Additionally, compound 18 namely 3-[3-(2-(4-chlorophenyl)-2-oxoethoxy)benzylidene]thiochroman-4-one was determined as the most potent molecule which showed higher anticancer activity than standard drug melphalan.
Conflict of interest
The authors confirm that this article content has no conflict of interest.
Acknowledgements
This work was supported by Anadolu University, Turkey (BAP Project No: 050301). The authors present their thanks to NCI (USA) and Anadolu University BIBAM (Turkey) for anticancer test results and NMR spectra, respectively.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jsps.2017.04.040.
Appendix A. Supplementary material
References
- Alipour E., Mousavi Z., Safaei Z., Pordeli M., Safavi M., Firoozpour L., Mohammadhosseini N., Saeedi M., Kabudanian Ardestani S., Shafiee A., Foroumadi A. Synthesis and cytotoxic evaluation of some new[1,3]dioxolo[4,5-g]chromen-8-one derivatives. Daru J. Pharmaceut. Sci. 2014;22:41. doi: 10.1186/2008-2231-22-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alizadeh B.H., Saeedi M., Dehghan G., Foroumadi A., Shafiee A. Synthesis of some novel pyrano[2,3-f]chromenone derivatives. J. Iran Chem. Soc. 2015;12:605–612. [Google Scholar]
- Alizadeh B.H., Ostad S.N., Foroumadi A., Amini M., Dowlatabadi R., Navidpour L., Shafiee A. Synthesis and cytotoxic activity of novel chromenes. ARKIVOC. 2008:45–56. xiii. [Google Scholar]
- Boulamwini J.K., Assefa H. CoMFA and CoMSIA 3D QSAR and docking studies on conformationally-restrained cinnamyl HIV-1 integrase inhibitors: exploration of a binding mode at the active site. J. Med. Chem. 2002;45:841–852. doi: 10.1021/jm010399h. [DOI] [PubMed] [Google Scholar]
- Boumendjel A., Boccard J., Carrupt P., Nicolle E., Blanc M., Geze A., Choisnard L., Wouessidjewe D., Matera E., Dumontet C. Antimitotic and antiproliferative activities of chalcones: Forward structure–activity relationship. J. Med. Chem. 2008;51:2307–2310. doi: 10.1021/jm0708331. [DOI] [PubMed] [Google Scholar]
- Boyd M.R. Enantioselective heterocyclic synthesis of spiro chromanone–thiochroman complexes catalyzed by a bifunctional indane catalyst. Princip. Prac. Oncol. 1989;3:1–42. doi: 10.1039/c0cc03489d. [DOI] [PubMed] [Google Scholar]
- Boyd M.R., Paull K.D. Synthesis and biochemical evaluation of thiochromanone thiosemicarbazone analogues as inhibitors of cathepsin L. Drug Dev. Res. 1995;34:91–109. [Google Scholar]
- Brien K.A., Bandi R.K., Behera A.K., Mishra B.K., Majumdar P., Satam V., Savagian M., Tzou S., Lee M., Zeller M., Robles A.J., Mooberry S., Pati H., Lee M. Design, synthesis and cytotoxicity of novel chalcone analogs derived from 1-cyclohexylpyrrolidin-2-one and 2,3-dihydrobenzo[f]chromen-1-one. Arch. Pharm. Chem. Life Sci. 2012;345:341–348. doi: 10.1002/ardp.201100265. [DOI] [PubMed] [Google Scholar]
- Chen H., Ji Z., Wong L.K., Siuda J.F., Narayanan V.L. 2-(1-Cyclopenten-1-yl)-2-[2-(dimethylamino)ethyl]-5-(E)-benzylidene cyclopentanone hydrochlorides: A new series of moderate cytotoxic agents. Bioorg. Med. Chem. Lett. 1994;4:2701–2704. [Google Scholar]
- Cotelle N. Role of flavonoids in oxidative stress. Curr. Topics Med. Chem. 2001;1:569–590. doi: 10.2174/1568026013394750. [DOI] [PubMed] [Google Scholar]
- Demirayak S., Yurttas L., Gundogdu-Karaburun N., Karaburun A.C., Kayagil İ. Synthesis and anti-cancer activity evaluation of new aurone derivatives. J. Enzyme Inhib. Med. Chem. 2015;30:816–825. doi: 10.3109/14756366.2014.976568. [DOI] [PubMed] [Google Scholar]
- Demirayak S., Yurttas L., Gundogdu-Karaburun N., Karaburun A.C., Kayagil İ. Synthesis and antiproliferative activity of 2-arylidene 6-(2-aryl-2-oxoethoxy)benzofuran-3-one derivatives. Lett. Drug Des. Discov. 2016;13:563–569. [Google Scholar]
- Dimmock J.R., Padmanilyam M.P., Zello G.A., Quail J.W., Oloo E.O., Prisciak J.S., Kraatz H.B., Cherkasov A., Lee J.S., Allen T.M., Santos C.L., Manavathu E.K., De Clercq E., Balzarini J., Stables J.P. Cytotoxic 1,3-diarylidene-2-tetralones and related compounds. Eur. J. Med. Chem. 2002;37:813–824. doi: 10.1016/s0223-5234(02)01402-2. [DOI] [PubMed] [Google Scholar]
- Dimmock J.R., Kandepu N.M., Nazarali A.J., Kowalchuk T.P., Motaganahalli N., Quail J.W., Mykytiuk P.A., Audette G.F., Prasad L., Parjesi P., Allen T.M., Santos C.L., Szydlowski J., De Clercq E., Balzarini J. Conformational and quantitative structure-activity relationship study of cytotoxic 2-arylidenebenzocycloalkanones. J. Med. Chem. 1999;42:1358–1366. doi: 10.1021/jm9806695. [DOI] [PubMed] [Google Scholar]
- El-Gohary N.S. Arylidene derivatives as synthons in heterocyclic synthesis. Open Access Library J. 2014;1:1–47. [Google Scholar]
- Emami S., Ghanbarimasir Z. Recent advances of chroman-4-one derivatives: Synthetic approaches and bioactivities. Eur. J. Med. Chem. 2015;26:539–563. doi: 10.1016/j.ejmech.2015.02.048. [DOI] [PubMed] [Google Scholar]
- Gamal-Eldeen A.M., Abdel-Lateff A., Okino T. Modulation of carcinogen metabolizing enzymes by chromanone A; a new chromone derivative from algicolous marine fungus Penicillium sp. Environ. Toxicol. Pharmacol. 2009;28:317–322. doi: 10.1016/j.etap.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Gao Y., Ren Q., Wu H., Li M., Wang J. Status of the NCI preclinical antitumor drug discovery screen, principles and practice of oncology. Chem. Commun. (Camb) 2010;46(48):9232–9234. doi: 10.1039/c0cc03489d. [DOI] [PubMed] [Google Scholar]
- Gul H.I., Yerdelen K.O., Gul M., Das U., Pandit B., Li P.K., Secen H., Sahin F. Synthesis of 4’-hydroxy-3’-piperidinomethylchalkone derivatives and their cytotoxicity against PC-3 cell lines. Arch. Pharm. 2007;340:195–201. doi: 10.1002/ardp.200600072. [DOI] [PubMed] [Google Scholar]
- Gundogdu-Karaburun N., Karaburun A.C., Demirayak S., Kayagil İ., Yurttas L. Synthesis and anticancer activity of some 2-[3/4-(2-substituted phenyl-2- oxoethoxy)benzylidene]-6-substituted-2,3-dihydro-1H-inden-1-one derivatives. Lett. Drug Des. Discov. 2014;11:578–585. [Google Scholar]
- Gupta R., Jindal D.P., Jit B., Narang G., Palusczak A., Hartmann R.W. Synthesis and evaluation of a dimer of 2-(4-pyridylmethyl)-1-indanones as a novel nonsteroidal aromatase inhibitor. Arch. Pharm. 2004;337:398–401. doi: 10.1002/ardp.200400853. [DOI] [PubMed] [Google Scholar]
- Hartmann R.W., Bayer H., Grun G. Aromatase Inhibitors. Syntheses and structure-activity studies of novel pyridyl-substituted indanones, indans, and tetralins. J. Med. Chem. 1994;37:1275–1281. doi: 10.1021/jm00035a007. [DOI] [PubMed] [Google Scholar]
- Hartmann R.W., Frostcher M., Ledergerber D., Wachter G.A., Grun G.L., Sergejew T.M. Synthesis and evaluation of azole-substituted tetrahydronaphthalenes as inhibitors of P450 Arom, P450 17, and P450 TxA2. Arch. Pharm. 1996;329:251–261. doi: 10.1002/ardp.19963290506. [DOI] [PubMed] [Google Scholar]
- Huang W., Liu M.Z., Li Y., Tana Y., Yang G. Design and synthesis of 3-substitutedmethylenethiochroman-4-ones as anticancer agents. Bioorg. Med. Chem. 2007;15:5191–5197. doi: 10.1016/j.bmc.2007.05.022. [DOI] [PubMed] [Google Scholar]
- Husain A., Rashis M., Shaharyar M., Siddiqui A.A., Mishra R. Benzimidazole clubbed with triazolo-thiadiazoles and triazolo-thiadiazines: New anticancer agents. Eur. J. Med. Chem. 2013;62:785–798. doi: 10.1016/j.ejmech.2012.07.011. [DOI] [PubMed] [Google Scholar]
- Ivanova L., Varinska L., Pilatova M., Gal P., Solar P., Perjesi P., Smetana K., Jr., Ostro A., Mojzis J. Cyclic chalcone analogue KRP6 as a potent modulator of cell proliferation: an in vitro study in HUVECs. Mol. Biol. Rep. 2013;40:4571–4580. doi: 10.1007/s11033-013-2547-x. [DOI] [PubMed] [Google Scholar]
- Keri R.S., Budagumpi S., Pai R.K., Balakrishna R.G. Chromones as a privileged scaffold in drug discovery: A review. Eur. J. Med. Chem. 2014;78:340–374. doi: 10.1016/j.ejmech.2014.03.047. [DOI] [PubMed] [Google Scholar]
- Kirkiacharian S., Gomis M., Koutsourakis P. Rearrangements of 3-benzylidenechroman-4-ones. Eur. J. Med. Chem. 1989;24:309–312. [Google Scholar]
- Kupcewicz B., Jarzecki A.A., Małecka M., Krajewska U., Rozalski M. Cytotoxic activity of substituted chalcones in terms of molecular electronic properties. Bioorg. Med. Chem. Lett. 2014;24:4260–4265. doi: 10.1016/j.bmcl.2014.07.027. [DOI] [PubMed] [Google Scholar]
- Kwon H.B., Park C., Jeon K.H., Lee E., Park S.E., Jun K.Y., Kadayat T.M., Thapa P., Karki R., Na Y., Park M.S., Rho S.B., Lee E.S., Kwon Y. A series of novel terpyridine-skeleton molecule derivants inhibit tumor growth and metastasis by targeting topoisomerases. J. Med. Chem. 2015;58:1100–1122. doi: 10.1021/jm501023q. [DOI] [PubMed] [Google Scholar]
- Lampronti I., Martello D., Bianchi N., Borgatti M., Lambertini E., Piva R., Jabbar S., Choudhuri M.S.K., Khan M.T.H., Gambari R. In vitro antiproliferative effects on human tumor cell lines of extracts from the Bangladeshi medicinal plant Aegle marmelos Correa. Phytomedicine. 2003;10:300–308. doi: 10.1078/094471103322004794. [DOI] [PubMed] [Google Scholar]
- Letafat B., Shakeri R., Emami S., Noushini S., Mohammadhosseini N., Shirkavand N., Ardestani S.K., Safavi M., Samadizadeh M., Letafat A., Shafiee A., Foroumadi A. Synthesis and in vitro cytotoxic activity of novel chalcone-like agents. Iran J. Basic Med. Sci. 2013;16:1155–1162. [PMC free article] [PubMed] [Google Scholar]
- Levai A., Schag J.B. Synthesis, stereochemical assignments, and biological activities of homoisoflavanoids. Pharmazie. 1979;34:749. [Google Scholar]
- Lopez-Lazaro M. Flavonoids as anticancer agents: Structure-activity relationship study. Curr. Med. Chem. Anticanc. Agent. 2002;2:691–714. doi: 10.2174/1568011023353714. [DOI] [PubMed] [Google Scholar]
- Mahapatra D.K., Bharti S.K., Asati V. Anti-cancer chalcones: Structural and molecular target perspectives. Eur. J. Med. Chem. 2015;98:69–114. doi: 10.1016/j.ejmech.2015.05.004. [DOI] [PubMed] [Google Scholar]
- Modranka J., Albrecht A., Jakubowski R., Krawczyk H., Rózalski M., Krajewska U., Janecka A., Wyrebska A., Rózalska B., Janecki T. Synthesis and biological evaluation of a-methylidene-d-lactones with 3,4-dihydrocoumarin skeleton. Bioorg. Med. Chem. Lett. 2012;20:5017–5026. doi: 10.1016/j.bmc.2012.06.022. [DOI] [PubMed] [Google Scholar]
- Mulvagh D., Meegan M.J., Donnelly D. Design, syntheses, and antitumor activity of novel chromone and aurone derivatives. J. Chem. Res. Synopses. 1979:137–139. [Google Scholar]
- Nafisi S., Namdar R. Molecular aspects on the specific interaction of homoisoflavonoids to DNA. J. Photochem. Photobiol. B. 2012;117:207–213. doi: 10.1016/j.jphotobiol.2012.09.016. [DOI] [PubMed] [Google Scholar]
- Noushini S., Alipour E., Emami S., Safavi M., Ardestani S.K., Gohari A.R., Shafiee A., Foroumadi A. Synthesis and cytotoxic properties of novel (E)-3-benzylidene-7-methoxychroman-4-one derivatives. Daru J. Pharmaceut. Sci. 2013;21:1–10. doi: 10.1186/2008-2231-21-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsu H., Xiao Z., Ishida J., Nagai M., Wang H.K., Itokawa H., Su C.Y., Shih C., Chiang T., Chang E., Lee Y., Tsai M.Y., Chang C., Lee K.H. Curcumin analogues as novel androgen receptor antagonists with potential as anti-prostate cancer agents. J. Med. Chem. 2002;45:5037–5042. doi: 10.1021/jm020200g. [DOI] [PubMed] [Google Scholar]
- Okombi S., Rival D., Bonnet S., Mariotte A., Perrier E., Boumendjel A. Discovery of benzylidenebenzofuran-3(2H)-one (aurones) as inhibitors of tyrosinase derived from human melanocytes. J. Med. Chem. 2006;49:329–333. doi: 10.1021/jm050715i. [DOI] [PubMed] [Google Scholar]
- Perjési P., Das U., Clercq E.D., Balzarini J., Kawase M., Sakagami H., Stables J.P., Lorand T., Rozmer Z., Dimmock J.R. Design, synthesis and antiproliferative activity of some 3-benzylidene-2,3-dihydro-1-benzopyran-4-ones which display selective toxicity for malignant cells. Eur. J. Med. Chem. 2008;43:839–845. doi: 10.1016/j.ejmech.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouget C., Fagnere C., Basly J., Habrioux G., Chulia A. Synthesis and inhibitory activity of pyridinyl-substituted flavanone derivatives. Bioorg. Med. Chem. Lett. 2002;12:1059–1061. doi: 10.1016/s0960-894x(02)00072-0. [DOI] [PubMed] [Google Scholar]
- Sandhu S., Bansal Y., Silakari O., Bansal G. Coumarin hybrids as novel therapeutic agents. Bioorg. Med. Chem. 2014;22:3806–3814. doi: 10.1016/j.bmc.2014.05.032. [DOI] [PubMed] [Google Scholar]
- Seifert T., Malo M., Kokkola T., Engen K., Fridén-Saxin M., Wallén E.A.A., Kakkonen L.M., Jarho E.M., Luthman K. Chroman-4-one- and chromone-based sirtuin 2 inhibitors with antiproliferative properties in cancer cells. J. Med. Chem. 2014;57:9870–9888. doi: 10.1021/jm500930h. [DOI] [PubMed] [Google Scholar]
- Siddiah V., Rao C.V., Venkatesvarlu S., Krishnaraju A.V., Subbaraju G.V. Synthése et activité de nouveaux oxyisobutyrates d’éthyle de benzylidéne-2 benzo[b]cyclanones, de benzylidéne-3 chromanones-et de benzylidéne-3 camphre apparentés au clofibrate. Bioorg. Med. Chem. 2006;14:2545–2551. doi: 10.1016/j.bmc.2005.11.031. [DOI] [PubMed] [Google Scholar]
- Song J., Jones L.M., Kumar G.D.K., Conner E.S., Bayeh L., Chavarria G.E., Charlton- Sevcik A.K., Chen S.E., Chaplin D.J., Trawick M.L., Pinney K.G. Some practical considerations and applications of the National Cancer Institute in vitro anticancer drug discovery screen. ACS Med. Chem. Lett. 2012;3:450–453. doi: 10.1021/ml200299g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapa U., Thapa P., Karki R., Yun M., Choi J.H., Jahng Y., Lee E., Jeon K.H., Na Y., Ha E.M., Cho W.J., Kwon Y., Lee E.S. Synthesis of 2,4-diaryl chromenopyridines and evaluation of their topoisomerase I and II inhibitory activity, cytotoxicity, and structure–activity relationship. Eur. J. Med. Chem. 2011;46:3201–3209. doi: 10.1016/j.ejmech.2011.04.029. [DOI] [PubMed] [Google Scholar]
- Venkateswararao E., Sharma V.K., Manickam M., Yun J., Jung S. Synthesis and SAR studies of bis-chromenone derivatives for anti-proliferative activity against human cancer cells. Bioorg. Med. Chem. Lett. 2014;24:5256–5259. doi: 10.1016/j.bmcl.2014.09.057. [DOI] [PubMed] [Google Scholar]
- Yen C., Nakagawa-Goto K., Hwang T., Wu P., Morris-Natschke S.L., Lai W., Bastow K.F., Chang F., Wu Y.C., Lee K.H. Antitumor agents. 271: Total synthesis and evaluation of brazilein and analogs as anti-inflammatory and cytotoxic agents. Bioorg. Med. Chem. Lett. 2010;20:1037–1039. doi: 10.1016/j.bmcl.2009.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurttas L., Demirayak S., Akalın Çiftçi G., Ulusoylar-Yıldırım Ş., Kaplancıklı Z.A. Synthesis and biological evaluation of some 1,2- disubstituted benzimidazole derivatives as new potential anticancer agents. Arch. Pharm. Chem. Life Sci. 2013;346:403–414. doi: 10.1002/ardp.201200452. [DOI] [PubMed] [Google Scholar]
- Yurttas L., Demirayak S., Akalın Çiftçi G. Cytotoxic, antiproliferative and apoptotic effects of new benzimidazole derivatives on A549 lung carcinoma and C6 glioma cell lines. Anticancer Agents Med. Chem. 2015;15:1174–1184. doi: 10.2174/1871520615666150703122625. [DOI] [PubMed] [Google Scholar]
- Zheng-yue M., Xing-hua Z., Chun-na L., Ya-jun Z., Geng-liang Y., Shi-kui W., Yang H.E. Design and synthesis of 3-substitutedmethylenethiochroman-4-ones as anticancer agents. Chem. Res. Chin. Univ. 2011;27:787–791. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.