Abstract
The objective of the present work was to formulate, optimize and evaluate the potential of novel soft nanovesicles i.e. nano-transfersomes, containing eprosartan mesylate (EM) for transdermal delivery. Nano-transfersomes of EM were developed using Phospholipon 90G, Span 80 (SP) and sodium deoxycholate (SDC) and characterized for vesicle size, shape, entrapment efficiency, in vitro skin permeation study and confocal laser scanning microscopy. The optimized nano-transfersomes formulation showed vesicles size of 108.53 ± 0.06 nm and entrapment efficiency of 63.00 ± 2.76%. The optimized nano-transfersomes provided an improved transdermal flux of 27.22 ± 0.29 µg/cm2/h with an enhancement ratio of 16.80 over traditional liposomes through Wistar rat skin. Confocal laser microscopy of rat skin treated with the optimized formulation showed that the formulation was eventually distributed and permeated deep into the rat skin. The present investigation has shown that the nature and concentration of surfactants (edge activators) influence immense control on the characteristics of nano-transfersomes. It was concluded that the developed nano-transfersomes surmount the limitation of low penetration ability of the traditional liposomes across the rat skin. Improved drug delivery presented by nano-transfersomes establishes this system as an encouraging dosage form for the delivery of EM via skin route.
Keywords: Eprosartan mesylate, Transdermal, Nano-transfersomes, Confocal laser scanning microscopy
1. Introduction
Hypertension is one of the most important causes for raising the chances of renal disease, blindness, stroke and arteriosclerosis. Hypertension affects 78 million people in the US and around 1 billion people globally (Ahad et al., 2016, Go et al., 2014). The incidences of hypertension are bound to increase further, unless broad and efficient precautionary steps are enforced. The published reports from the Framingham Heart Study proposed that people who are having normal blood pressure at the age of 55 years have a 90% threat of acquiring hypertension in their life span (Ahad et al., 2016, Vasan et al., 2002, Vasan et al., 2001).
The incidence of hypertension is also rapidly increasing in the Kingdom of Saudi Arabia, and reports have revealed that approximately one-quarter of the adult Saudi population is affected by hypertension. In 2007, the approximate prevalence of hypertensive cases in Saudi Arabia was 26.1%. Male cases were more in numbers as compared to the female cases (28.6 vs 23.9, respectively). Individuals living in the major cities exhibited a higher occurrence of high blood pressure (around 27.9%) in comparison with people living in the rural areas (occurrence of 22.4%). It has also been reported that as an individual’s age increases, the risk of hypertension also increases proportionally, even if extensive and efficient preventive measures have been taken (Al-Nozha et al., 2007). Cases with high blood pressure require long-term management of hypertension. In some certain cases, a lifetime treatment is suggested (Lake and Pinnock, 2000; Saroha et al., 2011). Among the antihypertensive agents, angiotensin II receptor blockers are the most commonly prescribed medications for hypertensive patients. Nevertheless, the majority of the angiotensin II receptor blockers showed a low oral bioavailability; some examples are given here (EM 13%, candesartan 15%, valsartan 10–35%, olmesartan 28.6%, losartan 30%, and telmisartan 42%). They may be expected to be administered more frequently in a day due to their short half-life (losartan 1.5–2 h, EM 5–9 h, valsartan 7.5 h, candesartan 9 h, olmesartan 10–15 h and telmisartan 24 h) and this may result in patient non-compliance (Ahad et al., 2016). There is a great need for improvement of drug delivery systems for antihypertensive drugs believing the significant of their application and drawbacks related to their conventional dosage forms (Ahad et al., 2015a, Ahad et al., 2013, Ahad et al., 2016; Gungor and Ozsoy, 2012).
It has been previously reported that when drugs are administered via oral route, approximately 74% of orally administered actives are not found to be as effective as desired (Marwah et al., 2016). The efficacy of orally administered drugs can be improved by utilizing other routes of drug delivery. Delivery of drugs via transdermal route could be the answer to this problem. Transdermal route can also improve quality of life. Transdermal route is more established than the oral dosage forms. Thus, actives delivered by means of transdermal route are perfectly suited for disorders such as hypertension, which require continual action (Ahad et al., 2015a, Ahad et al., 2013, Ahad et al., 2016, Ahad et al., 2015c, Bairwa et al., 2013). Hence, anti-hypertensive drugs have been subjugated for transdermal investigation in the present work.
The utmost challenge with delivery of actives across transdermal route is the hindrance properties of the stratum corneum that limits the absorptions of the majority of drugs (Malakar et al., 2012, Naga Sravan Kumar Varma et al., 2014). Several advances have been attempted to surmount the hurdle of stratum corneum to achieve enhanced transdermal permeation of drugs. Lipid-based formulations provide improved delivery of drug via skin route (El Maghraby et al., 2015). Their advantages being superior compatibility with living system and ease of mixing with lipids of the skin (Chourasia et al., 2011). Liposomes transdermal delivery systems have been investigated since 1980s and have elicited a substantial attention. Nevertheless, the liposomes don't profoundly permeate within the rats’ skin and stay restricted to the upper layer of the skin (Gillet et al., 2011). Newer categories of lipid-based formulations viz. transfersomes have been formulated as novel kind of liposomes (Song et al., 2012). Nano-transfersomes were listed as the first generation of elastic vesicular formulations acquainted by Cevc et al. (1998). Transfersomes are reportedly more capable in delivering drugs via skin in terms of amount and deepness in comparison with conventional liposomes (Ahad et al., 2012, Jain et al., 2003). Transfersomes are highly flexible vesicular formulations that contain phospholipids (lipid bilayer forming substance) and edge activators in optimal ratio to contribute elasticity to vesicle membrane of nano-transfersomes (El Maghraby et al., 2004, Maheshwari et al., 2012). By virtue of their flexibleness, they can permeate intact via the skin holes, which are practically minor in comparison with transfersomes own diameters and they are able to convey therapeutic concentrations of applied actives when used under non-occluded circumstances (Bendas and Tadros, 2007). The hydration sensitivity of nano-transfersomes plus its exclusive driving force produces a new opening to regulate the carriers’ penetration movement. In addition to the above advantages, nano-transfersomes could efficiently shield the drug and counter the unwanted quick clearance through the dermal blood vessels. Also, they are competent to hold the drug for sufficiently adequate time, on, in and underneath the skin barrier. In addition, nano-transfersomes can cross the obstacle layer of skin, independent of drug concentration. Nano-transfersomes have been applied as carriers for a variety of substances, including anticancer drugs, ketoprofen, insulin, corticosteroids and proteins (El Zaafarany et al., 2010). These nano-transfersomes formulations were colloidal dispersions having average diameter in range of 100–200 nm (Jain et al., 2005a).
EM is an angiotensin II receptor blocker having low oral bioavailability of only 13% in humans. It is a BCS (Biopharmaceutics Classification System) class II drug with log partition coefficient of 3.9, and half-life of 5–9 h (Israili, 2000). These characteristic properties of EM make it an appropriate candidate for transdermal formulation for the management of hypertension.
2. Materials and methods
2.1. Materials
EM was procured from BASF, Germany. Phospholipon 90G was obtained as gift sample from Phospholipid GmbH (Nattermannallee, Germany). SDC and cholesterol were bought from AppliChem Panreac, Darmstadt, Germany. SP was obtained from Fluka Chemica (Buch, Switzerland). Rhodamine 6G was sourced from Sigma-Aldrich (St. Louis, MO, USA). Methanol and chloroform were purchased from BDH, England, and Sigma-Aldrich, USA, respectively.
2.2. Preparation of nano-transfersomes and liposomes
Edge activators with different HLB values [SP (HLB-4.3) and SDC (HLB-16.7)] were employed to prepare EM nano-transfersomes vesicles using Thin film hydration technique (Ahad et al., 2012). In brief, Phospholipid 90G and edge activator were accurately weighed and placed into a flask with round bottom and dissolved in 20 mL mixture of chloroform–methanol (2:1). The organic phase was gradually evaporated with reduced pressure, by a rotary evaporator (Buchi™ Rotavapor®-210, Zurich, Switzerland) such that a thin dry film of the components was formed on the inner wall of the flask. The temperature of the rotary evaporator was set above the lipid transition temperature of the phospholipids used. The dried lipid film was maintained under reduced pressure to remove traces of solvent (Dai et al., 2013). The dried lipid film was then hydrated with phosphate buffer saline (pH 7.4, 20 ml) using rotary evaporator under normal pressure. The prepared vesicles were allowed to swell, in order to produce large multi-lamellar vesicles. Smaller vesicles were prepared by sonication using a probe sonicator (Bandelin Electronic GmbH & Co. KG, Berlin). The prepared smaller vesicles were then passed through 450 nm and 200 nm polycarbonate membranes (Chromafil® Xtra, Macherey-Nagel GmbH & Co. KG, Germany). Formulations were stored at 4 °C and evaluated for various characterization parameters (Al-Mahallawi et al., 2014). Similarly, EM loaded liposomes formulations were also prepared using Phospholipon® 90G and cholesterol in 75:25 ratio that was used as a control formulation. The same method was applied for the preparation of Rhodamine 6G (0.03%, w/v) loaded transfersomes and liposomes for the confocal laser scanning microscopy study.
2.3. Characterization of the prepared nano-transfersomes and liposomes
2.3.1. Vesicles size, polydispersity index and zeta potential
The vesicles size, polydispersity index and zeta potential of all prepared transfersomes and liposomes formulations were characterized by Dynamic Light Scattering method using Zetasizer Nano ZS (Malvern Instruments, United Kingdom) at 25 ± 1 °C. Samples were appropriately diluted with filtered phosphate buffer saline and characterized at scattering angle of 90° (Ahad et al., 2014, Dragicevic-Curic et al., 2009). Surface charge of drug loaded vesicles was determined using Zetasizer Nano ZS (Malvern Instruments, Malvern, UK). Analysis time was kept 60 s, and average zeta potential of the vesicles was determined.
2.3.2. Nano-transfersomes morphology
Nano-transfersomes were visualized using transmission electron microscopy (JEM-1011, JEOL, Tokyo, Japan), set at voltage of 80 kV. A thin film of nano-transfersomes drop was allowed to form on copper grid and allowed to dry properly. The samples were then viewed under transmission electron microscope (Madheswaran et al., 2014).
2.3.3. Determination of entrapment efficiency (%)
The entrapment efficiency of EM loaded nano-transfersomes vesicles was determined by ultracentrifugation at 40,000 rpm and 4 °C for 1 h using OptimaTM Max-E, Ultra Centrifuge (Beckman Coulter, Pasadena, CA) (Meng et al., 2013). When samples were centrifuged, the supernatant was withdrawn with proper care and quantified by HPLC method (Ahad et al., 2015b). The entrapment efficiency was calculated by using the following equation.
where T is the amount of total EM and C is the amount of EM detected only in the supernatant.
2.3.4. Preparation of rat skin
The hair on Wistar Albino rats (180–200 g) skin was shaved and the remaining hypodermic tissues were detached. The dermis part was cleaned gently with the help of a cotton swab having isopropyl alcohol to get rid of the remaining fat. The prepared rat skin was further cleaned using phosphate buffer saline, sealed in aluminum sheet and kept in a deep freezer at −20 °C until use (used within 2 weeks of preparation) (Ahad et al., 2011a, Ahad et al., 2011b; Narishetty and Panchagnula, 2004).
2.3.5. In vitro skin permeation study
On the experiment day, the rat skin samples were thawed and inspected for any cuts and holes. The skin samples were cut to proper dimensions and put on a vertical type Franz cell of the SFDC-6 transdermal diffusion cell instrument (Logan, USA) facing stratum corneum toward the donor compartment, while dermis side toward the receiver cell. Nano-transfersomes formulations were applied in non-occlusive condition to the rat skin surface. The receptor medium was 12 ml ethanolic phosphate buffer saline (pH 7.4, 20:80). The receiver cell vehicle was maintained at 37 °C and agitated with the help of a small magnetic bead rotating at a speed of 100 rpm. Sample of 1 ml of receiver medium was taken and refilled with equal volume of fresh vehicle. The withdrawn samples were analyzed with HPLC method. Various parameters such as cumulative amount of drug permeated, lag time (Tlag), permeability coefficient (Kp) and enhancement ratio (ER) were calculated.
2.3.6. Confocal laser scanning microscopy
For confocal laser scanning microscopy study, rat abdominal skin was excised and mounted on Franz diffusion cell. The optimized nano-transfersomes and liposomes loaded with Rhodamine 6G (0.03%) were placed in donor compartment of Franz diffusion cell under non-occlusive condition (Guo et al., 2015, Zeb et al., 2016). On completion of skin permeation study for 10 h, the excessive residual formulation that contained dye was carefully cleaned from the surface of the excised rat skin and the required treated area was cut into small sections and positioned on the glass slide with stratum corneum facing upward. The samples were visualized using ×10 objective lens system of an inverted Zeiss LSM 780 microscope (Carl Zeiss, Jena, Germany). The rat’s skin depth was optically examined at various increments through the z-axis of a confocal microscope. The excitation wavelength used was 528 nm, while emission wavelength was 551 nm (Ma et al., 2015, Shi et al., 2012).
3. Results and discussion
The main component of nano-transfersomes constitutes phospholipid and edge activators. Numerous edge activators have been tried in various formulation developments of nano-transfersomes. Among them, the most widely reported are sodium cholate, sodium deoxycholate, potassium glycyrrhizinate and various grades of Spans and Tweens (Mura et al., 2009). In the present study we have applied two surfactants: SP and SDC. The biosurfactant SDC was used because of its biocompatibility. SP was particularly chosen because of its biocompatible nature, which provides optimum elasticity to the vesicle membrane (Jain et al., 2005a). SP also has high acceptability in different types of pharmaceutical formulations (El Maghraby et al., 1999). Several formulations of nano-transfersomes were prepared with SP, SDC and Phospholipon 90G using thin layer evaporation procedure (Ahad et al., 2012). Liposome was used as control, and was prepared by a similar method used for the preparation of nano-transfersomes.
3.1. Characterization of nano-transfersomes
3.1.1. Effect of formulation composition on vesicles size, polydispersity index and zeta potential
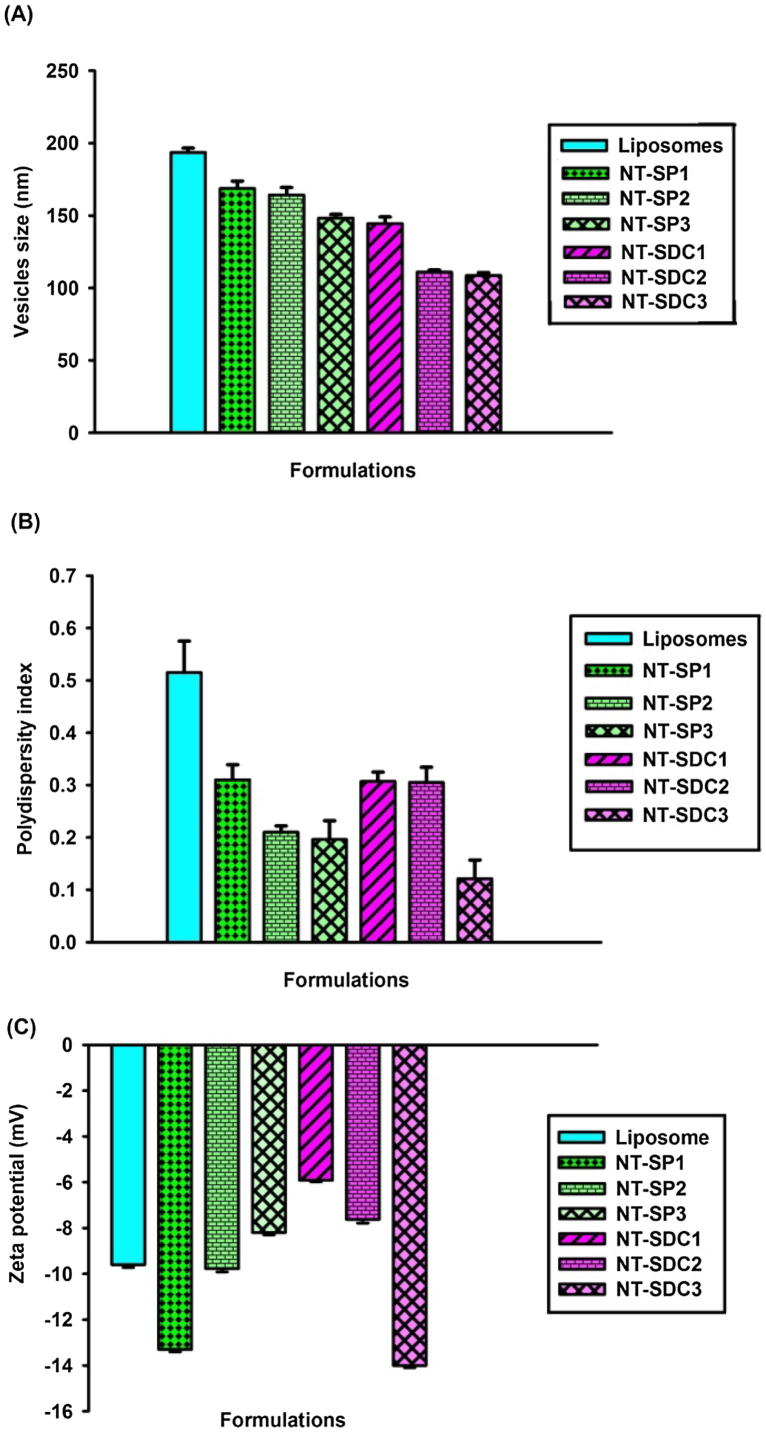
There were significant differences in vesicles size among the nano-transfersomes formulation comprising different surfactants. Moreover, a decrease in vesicle size was detected whenever each surfactant concentration was raised up to 25% w/w. The effect of formulations composition on vesicle size, polydispersity index, and zeta potential of nano-transfersomes containing EM is presented in Fig. 1.
Fig. 1.
Effect of formulations composition on (A) vesicles size, (B) polydispersity index and (C) zeta potential of EM loaded nano-transfersomes (mean ± SD).
The characterization of the developed formulations for their vesicles size demonstrated that the vesicles size of the investigated nano-transfersomes lied in the range of 108–168 nm (Fig. 1a), representing that the prepared formulations had vesicles of small size which is necessary for their topical uses. Study by Verma et al. (2003) exhibited that by reducing the vesicles' size, the permeation of entrapped drug(s) into rooted skin lamina is enhanced (Verma et al., 2003).
The polydispersity index of the prepared formulation vesicles had values ranging from 0.121 to 0.310, showing homogenous population of the vesicles (Fig.1b). It was detected that on increasing the concentration of surfactant, the index of polydispersity decreased. The size of vesicles of liposomes formulation (control) was found to be 193.5 nm with polydispersity index of 0.515.
It was observed that the vesicles size of the traditional liposomes reduced significantly by adding edge activator in place of cholesterol (Fig. 1a). The zeta potentials of all nano-transfersomes formulations were found to be negative and in the range of −5.91 mV to −14 mV owing to the net charge of the lipid content in the nano-formulations. Phosphatidylcholine present in Phospholipid 90G is a zwitterionic compound with an isoelectric point between 6 and 7 (Chain and Kemp, 1934; Duangjit et al., 2013). It has been reported that the negatively charged transfersomes formulations greatly enhance the skin permeation of drug(s) in transdermal delivery (Sinico et al., 2005).
3.1.2. Effect of formulation composition on entrapment efficiency
The entrapment efficiency for all developed nano-transfersomes was found in the range of 63.0 ± 2.76% (NT-SDC3) to 95 ± 5.36% for formulation NT-SP1 (Table 1), while liposomes depicted the entrapment efficiency of 93.50% (Table 1). It was observed that entrapment efficiency is found to be inversely proportional to the concentration of surfactants. Formulations with both types of surfactant (SP and SDC) show reduction in entrapment efficiency on increment in surfactant concentration (Table 1). This could be happen owing to the presence of mixed micelles in the formulated vesicles at higher concentration of surfactant, with the consequence of lower drug entrapment in mixed micelles (Jain et al., 2003).
Table 1.
Effect of formulations composition on entrapment efficiency of EM loaded nano-transfersomes (mean ± SD).
| Formulation code | Entrapment efficiency (%) |
|---|---|
| NT-SP1 | 95.00 ± 5.36 |
| NT-SP2 | 94.21 ± 3.20 |
| NT-SP3 | 89.78 ± 2.60 |
| NT-SDC1 | 74.01 ± 1.08 |
| NT-SDC2 | 63.02 ± 2.24 |
| NT-SDC3 | 63.00 ± 2.76 |
| Liposome | 93.49 ± 3.03 |
EM, eprosartan mesylate; NT-SDC, nano-transfersomes with sodium deoxycholate; NT-SP, nano-transfersomes with Span 80; SD, standard deviation.
Our results are well corroborated with the published work of Mishra et al., 2007, who have reported similar pattern of decrease in entrapment efficiency of propranolol hydrochloride in transfersomes containing soya phosphatidylcholine and various types of surfactants (Mishra et al., 2007). The lowering of entrapment efficiency too depended on the type of surfactant used. SP presented a slighter consequence in comparison with SDC (Table 1).
3.1.3. Effect of formulation composition on EM transdermal flux
It is well established that the nano-transfersomes are well permeated via skin with respect to liposomes (van den Bergh et al., 2001). Antecedently, Ahad et al., 2012 evaluated the skin permeation ability of nano-transfersomes using lipophilic drug valsartan and found superiority of nano-transfersomes over liposomes in terms of skin permeation via rat skin (Ahad et al., 2012).
Other researchers have also reported superior skin permeation capability of transfersomes in contrast to traditional liposomes; for example, enhanced permeation of pergolide was documented by Honeywell-Nguyen and Bouwstra (2003). In another study, skin permeation of 5-Fluorouracil as well as estradiol was found to be better in comparison to the liposomes formulation (El Maghraby et al., 2000, El Maghraby et al., 2001). In another study, authors concluded a higher delivery of dipotassium glycyrrhizinate via skin (Trotta et al., 2002). In the year 2000, Guo et al. and Hofer et al. reported better systemic delivery of transfersomes loaded with cyclosporin A and Interleukin-2 and Interferon-a, respectively via transdermal route (Guo et al., 2000).
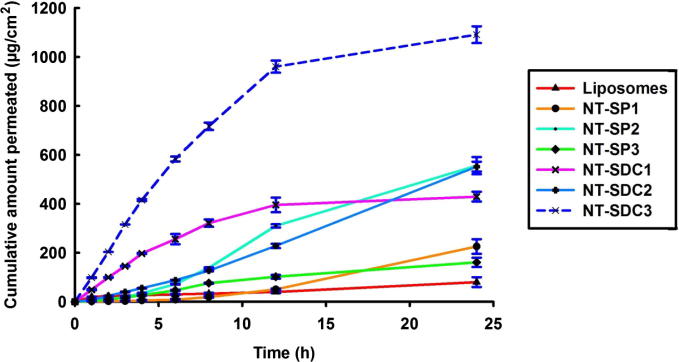
In the present study, transdermal delivery potential of developed EM formulations was assessed using the rat’s skin by means of Franz diffusion cells. In vitro skin permeation profile of different EM loaded nano-transfersomes formulations across rat skin is presented in Fig. 2. It was observed that transdermal flux of EM enhanced linearly with an increment in the concentration of SDC. As the concentration of SDC increased from 5% to 15%, the transdermal flux of EM enhanced from 10.46 µg/cm2/h to 13.66 µg/cm2/h. With an additional rise in the concentration of SDC (25%), the transdermal flux was further increased from 13.66 µg/cm2/h to 27.22 µg/cm2/h (Table 2). With respect to edge activator SP, the transdermal flux enhanced initially with an increase in the concentration of SP and then reduced. It was noticed that on increasing the surfactant concentration, the transdermal flux of EM also increased from 5.44 µg/cm2/h to 14.64 µg/cm2/h, but on further increase in SP concentration, the flux of EM reduced from 14.64 µg/cm2/h to 4.19 µg/cm2/h (Table 2).
Fig. 2.
In vitro skin permeation profile of different EM loaded nano-transfersomes formulations across rat skin (mean ± SD).
Table 2.
Composition and permeation parameters for EM loaded nano-transfersomes and liposome formulations (mean ± SD).
| Formulation codes | Composition phospholipid: edge activator (%w/w) | Flux (µg/cm2/h ± SD) | KP × 10−3 (cm/h ± SD) | Tlag (h ± SD) | Enhancement ratio (ER) |
|---|---|---|---|---|---|
| NT-SP1 | 95:5 | 5.44 ± 0.21 | 1.81 ± 0.07 | 5.97 ± 0.15 | 3.36 |
| NT-SP2 | 85:15 | 14.64 ± 0.26 | 4.88 ± 0.09 | 3.07 ± 0.12 | 9.04 |
| NT-SP3 | 75:25 | 4.19 ± 0.28 | 1.40 ± 0.09 | 6.03 ± 0.15 | 2.59 |
| NT-SDC1 | 95:5 | 10.46 ± 0.28 | 3.49 ± 0.09 | 4.13 ± 0.12 | 6.46 |
| NT-SDC2 | 85:15 | 13.66 ± 0.06 | 4.55 ± 0.02 | 4.10 ± 0.10 | 8.43 |
| NT-SDC3 | 75:25 | 27.22 ± 0.29 | 9.07 ± 0.10 | 2.0 ± 0.10 | 16.80 |
| Liposome | – | 1.62 ± 0.21 | 0.54 ± 0.07 | 7.83 ± 0.76 | – |
EM, eprosartan mesylate; NT-SDC, nano-transfersomes with sodium deoxycholate; NT-SP, nano-transfersomes with Span 80; KP, permeability coefficient; Tlag, lag time; h, hours; SD, standard deviation.
The pattern of transdermal flux depicted that too low or too high SP concentration is not favorable in formulation development of vesicular system which is intended to pass via skin. It also showed that the possible permeation enhancing consequence of surfactants is not primarily accountable for enhanced EM skin delivery from nano-transfersomes vesicles. These results are in correspondence to reports published earlier (Jain et al., 2003, Jain et al., 2005b, Mishra et al., 2007). Reduced drug delivery at higher SP concentration could be explained due to the presence of prepared vesicles that exit side-by-side with mixed micelles (edge activator concentration 25% w/w of phospholipid). However solely mixed micelles be present at surfactant concentration of more than 25% w/w of phospholipid. These mixed micelles are described as fewer efficient in comparison with transfersomes for drug delivery via transdermal route, since micelles are considerably not as great sensitive to water activity gradient than nano-transfersomes. Talking about surfactants, the potential reasoning for ameliorated presentation of SDC in the case of transdermal flux, is its lower vesicular size value (p < 0.05) than vesicles size of nano-transfersomes prepared with SP, which provides better enhancement of drug across the rat skin (Mishra et al., 2007).
3.1.4. Nano-transfersomes morphology
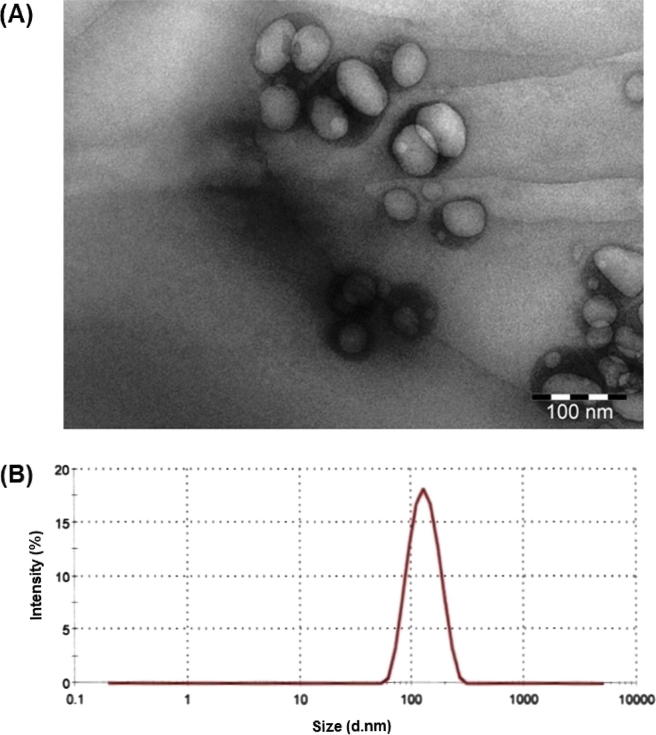
The morphology of the nano-transfersomes vesicles was assessed by transmission electron microscopy (Fig.3a), justifying the vesicular characteristics. EM loaded nano-transfersomes formulated from Phospholipon 90G and SDC were well-identified as spherical vesicles with uniform size distribution, displaying sealed unilamellar nano-vesicular structure. The optimized nano-transfersomes formulation (NT-SDC3) showed vesicles size of 108.53 ± 0.06 nm (Fig.3b).
Fig. 3.
(A) Transmission electron microscopy and (B) vesicles size distribution of optimized EM nano-transfersomes formulation (NT-SDC3).
3.1.5. Confocal laser scanning microscopy
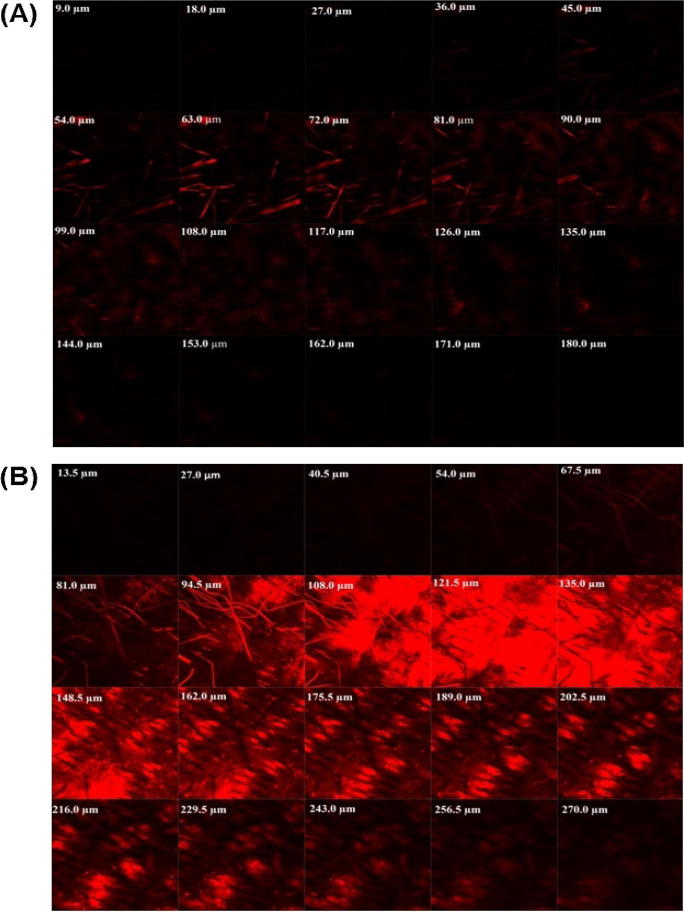
The probe Rhodamine 6G was utilized to investigate the skin permeation ability of developed nano-transfersomes into previously shaved rat’s skin after its application in non-occlusive conditions. The thickness of rat’s skin was optically scanned for 20 fragments from the surface of the skin (left to right) (Fig. 4). The confocal microscopy investigation confirmed the permeation of EM-loaded nano-transfersomes to the deeper layers of the skin (Fig. 4).
Fig. 4.
Confocal laser microscopy image of rat skin treated with Rhodamine 6G loaded (A) liposomes and (B) optimized EM nano-transfersomes formulation (NT-SDC3).
It was observed that traditional liposomes formulations did not enable Rhodamine 6G to permeate into deeper layers of rat skin; instead, it ensued to hold on to the upper layer of the rat’s skin (Fig.4a). On the other hand, nano-transfersomes formulation loaded with Rhodamine 6G demonstrated enhanced depth of dye permeation up to 270 μm (Fig.4b). The confocal laser scanning microscopy study following application of the optimized NT-SDC3 comprising Rhodamine 6G clearly defined the transdermal potential of the developed formulation (Honeywell-Nguyen et al., 2002, Jain et al., 2008, van den Bergh et al., 1999).
4. Conclusion
It was concluded that developed nano-transfersomes demonstrated vesicles size in nano range. Good entrapment of EM was observed in the soft lipid-based nano-transfersomes. The optimized formulation depicted better transdermal flux of EM across rat skin as compared to liposomes formulation. Confocal laser microscopy depicted that the transfersomes permeated into deeper layer of rat skin. Further studies are in progress in our laboratory to establish the antihypertensive effectiveness of the above optimized formulations.
Conflict of interest
All authors have approved the final manuscript, and the authors declare that they have no conflict of interests to disclose.
Acknowledgement
This Project was funded by the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, Award Number (13-NAN1268-02).
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahad A., Aqil M., Kohli K., Sultana Y., Mujeeb M., Ali A. Interactions between novel terpenes and main components of rat and human skin: mechanistic view for transdermal delivery of propranolol hydrochloride. Curr. Drug Deliv. 2011;8:213–224. doi: 10.2174/156720111794479907. [DOI] [PubMed] [Google Scholar]
- Ahad A., Aqil M., Kohli K., Sultana Y., Mujeeb M., Ali A. Role of novel terpenes in transcutaneous permeation of valsartan: effectiveness and mechanism of action. Drug Dev. Ind. Pharm. 2011;37:583–596. doi: 10.3109/03639045.2010.532219. [DOI] [PubMed] [Google Scholar]
- Ahad A., Aqil M., Kohli K., Sultana Y., Mujeeb M., Ali A. Formulation and optimization of nanotransfersomes using experimental design technique for accentuated transdermal delivery of valsartan. Nanomedicine. 2012;8:237–249. doi: 10.1016/j.nano.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Ahad A., Al-Jenoobi F.I., Al-Mohizea A.M., Aqil M., Kohli K. Transdermal delivery of calcium channel blockers for hypertension. Expert Opin. Drug Deliv. 2013;10:1137–1153. doi: 10.1517/17425247.2013.783562. [DOI] [PubMed] [Google Scholar]
- Ahad A., Raish M., Al-Mohizea A.M., Al-Jenoobi F.I., Alam M.A. Enhanced anti-inflammatory activity of carbopol loaded meloxicam nanoethosomes gel. Int. J. Biol. Macromol. 2014;67:99–104. doi: 10.1016/j.ijbiomac.2014.03.011. [DOI] [PubMed] [Google Scholar]
- Ahad A., Al-Jenoobi F.I., Al-Mohizea A.M., Akhtar N., Raish M., Aqil M. Systemic delivery of beta-blockers via transdermal route for hypertension. Saudi Pharm. J. 2015;23:587–602. doi: 10.1016/j.jsps.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahad A., Al-Mohizea A.M., Al-Saleh A.A., Alwabel A.S., Aqel A.J., Al-Qahtani K.M., Al-Jenoobi F.I. Validation of a rapid and sensitive HPLC-UV method for the quantification of eprosartan mesylate in bulk drug, teventenTM and ultradeformable lipid based vesicular system. Curr. Pharm. Anal. 2015;11:1–6. [Google Scholar]
- Ahad A., Al-Saleh A.A., Akhtar N., Al-Mohizea A.M., Al-Jenoobi F.I. Transdermal delivery of antidiabetic drugs: formulation and delivery strategies. Drug Discov. Today. 2015;20:1217–1227. doi: 10.1016/j.drudis.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Ahad A., Al-Mohizea A.M., Al-Jenoobi F.I., Aqil M. Transdermal delivery of angiotensin II receptor blockers (ARBs), angiotensin-converting enzyme inhibitors (ACEIs) and others for management of hypertension. Drug Deliv. 2016;23:579–590. doi: 10.3109/10717544.2014.942444. [DOI] [PubMed] [Google Scholar]
- Al-Mahallawi A.M., Khowessah O.M., Shoukri R.A. Nano-transfersomal ciprofloxacin loaded vesicles for non-invasive trans-tympanic ototopical delivery: in-vitro optimization, ex-vivo permeation studies, and in-vivo assessment. Int. J. Pharm. 2014;472:304–314. doi: 10.1016/j.ijpharm.2014.06.041. [DOI] [PubMed] [Google Scholar]
- Al-Nozha M.M., Abdullah M., Arafah M.R., Khalil M.Z., Khan N.B., Al-Mazrou Y.Y., Al-Maatouq M.A., Al-Marzouki K., Al-Khadra A., Nouh M.S., Al-Harthi S.S., Al-Shahid M.S., Al-Mobeireek A. Hypertension in Saudi Arabia. Saudi Med. J. 2007;28:77–84. [PubMed] [Google Scholar]
- Bairwa M., Pilania M., Gupta V., Yadav K. Hypertension vaccine may be a boon to millions in developing world. Hum. Vaccin. Immunother. 2013:10. doi: 10.4161/hv.27520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendas E.R., Tadros M.I. Enhanced transdermal delivery of salbutamol sulfate via ethosomes. AAPS PharmSciTech. 2007;8:E107. doi: 10.1208/pt0804107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cevc G., Gebauer D., Stieber J., Schatzlein A., Blume G. Ultraflexible vesicles, transfersomes, have an extremely low pore penetration resistance and transport therapeutic amounts of insulin across the intact mammalian skin. Biochim. Biophys. Acta. 1998;1368:201–215. doi: 10.1016/s0005-2736(97)00177-6. [DOI] [PubMed] [Google Scholar]
- Chain E., Kemp I. The isoelectric points of lecithin and sphingomyelin. Biochem. J. 1934;28:2052–2055. doi: 10.1042/bj0282052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chourasia M.K., Kang L., Chan S.Y. Nanosized ethosomes bearing ketoprofen for improved transdermal delivery. Results Pharm. Sci. 2011;1:60–67. doi: 10.1016/j.rinphs.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y., Zhou R., Liu L., Lu Y., Qi J., Wu W. Liposomes containing bile salts as novel ocular delivery systems for tacrolimus (FK506): in vitro characterization and improved corneal permeation. Int. J. Nanomed. 2013;8:1921–1933. doi: 10.2147/IJN.S44487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragicevic-Curic N., Scheglmann D., Albrecht V., Fahr A. Development of different temoporfin-loaded invasomes-novel nanocarriers of temoporfin: characterization, stability and in vitro skin penetration studies. Colloids Surf. B Biointerfaces. 2009;70:198–206. doi: 10.1016/j.colsurfb.2008.12.030. [DOI] [PubMed] [Google Scholar]
- Duangjit S., Opanasopit P., Rojanarata T., Ngawhirunpat T. Evaluation of meloxicam-loaded cationic transfersomes as transdermal drug delivery carriers. AAPS PharmSciTech. 2013;14:133–140. doi: 10.1208/s12249-012-9904-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Maghraby G.M., Williams A.C., Barry B.W. Skin delivery of oestradiol from deformable and traditional liposomes: mechanistic studies. J. Pharm. Pharmacol. 1999;51:1123–1134. doi: 10.1211/0022357991776813. [DOI] [PubMed] [Google Scholar]
- El Maghraby G.M., Williams A.C., Barry B.W. Oestradiol skin delivery from ultradeformable liposomes: refinement of surfactant concentration. Int. J. Pharm. 2000;196:63–74. doi: 10.1016/s0378-5173(99)00441-x. [DOI] [PubMed] [Google Scholar]
- El Maghraby G.M., Williams A.C., Barry B.W. Skin delivery of 5-fluorouracil from ultradeformable and standard liposomes in-vitro. J. Pharm. Pharmacol. 2001;53:1069–1077. doi: 10.1211/0022357011776450. [DOI] [PubMed] [Google Scholar]
- El Maghraby G.M., Williams A.C., Barry B.W. Interactions of surfactants (edge activators) and skin penetration enhancers with liposomes. Int. J. Pharm. 2004;276:143–161. doi: 10.1016/j.ijpharm.2004.02.024. [DOI] [PubMed] [Google Scholar]
- El Maghraby G.M., Ahmed A.A., Osman M.A. Penetration enhancers in proniosomes as a new strategy for enhanced transdermal drug delivery. Saudi Pharm. J. 2015;23:67–74. doi: 10.1016/j.jsps.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Zaafarany G.M., Awad G.A., Holayel S.M., Mortada N.D. Role of edge activators and surface charge in developing ultradeformable vesicles with enhanced skin delivery. Int. J. Pharm. 2010;397:164–172. doi: 10.1016/j.ijpharm.2010.06.034. [DOI] [PubMed] [Google Scholar]
- Gillet A., Lecomte F., Hubert P., Ducat E., Evrard B., Piel G. Skin penetration behaviour of liposomes as a function of their composition. Eur. J. Pharm. Biopharm. 2011;79:43–53. doi: 10.1016/j.ejpb.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Go A.S., Mozaffarian D., Roger V.L., Benjamin E.J., Berry J.D., Blaha M.J., Dai S., Ford E.S., Fox C.S., Franco S., Fullerton H.J., Gillespie C., Hailpern S.M., Heit J.A., Howard V.J., Huffman M.D., Judd S.E., Kissela B.M., Kittner S.J., Lackland D.T., Lichtman J.H., Lisabeth L.D., Mackey R.H., Magid D.J., Marcus G.M., Marelli A., Matchar D.B., McGuire D.K., Mohler E.R., 3rd, Moy C.S., Mussolino M.E., Neumar R.W., Nichol G., Pandey D.K., Paynter N.P., Reeves M.J., Sorlie P.D., Stein J., Towfighi A., Turan T.N., Virani S.S., Wong N.D., Woo D., Turner M.B. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gungor S., Ozsoy Y. Systemic delivery of antihypertensive drugs via skin. Ther. Deliv. 2012;3:1101–1116. [PubMed] [Google Scholar]
- Guo J., Ping Q., Sun G., Jiao C. Lecithin vesicular carriers for transdermal delivery of cyclosporin A. Int. J. Pharm. 2000;194:201–207. doi: 10.1016/s0378-5173(99)00361-0. [DOI] [PubMed] [Google Scholar]
- Guo F., Wang J., Ma M., Tan F., Li N. Skin targeted lipid vesicles as novel nano-carrier of ketoconazole: characterization, in vitro and in vivo evaluation. J. Mater. Sci. Mater. Med. 2015;26:175. doi: 10.1007/s10856-015-5487-2. [DOI] [PubMed] [Google Scholar]
- Honeywell-Nguyen P.L., Bouwstra J.A. The in vitro transport of pergolide from surfactant-based elastic vesicles through human skin: a suggested mechanism of action. J. Control Release. 2003;86:145–156. doi: 10.1016/s0168-3659(02)00415-7. [DOI] [PubMed] [Google Scholar]
- Honeywell-Nguyen P.L., de Graaff A.M., Groenink H.W., Bouwstra J.A. The in vivo and in vitro interactions of elastic and rigid vesicles with human skin. Biochim. Biophys. Acta. 2002;1573:130–140. doi: 10.1016/s0304-4165(02)00415-4. [DOI] [PubMed] [Google Scholar]
- Israili Z.H. Clinical pharmacokinetics of angiotensin II (AT1) receptor blockers in hypertension. J. Hum. Hypertens. 2000;14(Suppl 1):S73–86. doi: 10.1038/sj.jhh.1000991. [DOI] [PubMed] [Google Scholar]
- Jain S., Jain P., Umamaheshwari R.B., Jain N.K. Transfersomes – a novel vesicular carrier for enhanced transdermal delivery: development, characterization, and performance evaluation. Drug Dev. Ind. Pharm. 2003;29:1013–1026. doi: 10.1081/ddc-120025458. [DOI] [PubMed] [Google Scholar]
- Jain S., Jain N., Bhadra D., Tiwary A.K., Jain N.K. Transdermal delivery of an analgesic agent using elastic liposomes: preparation, characterization and performance evaluation. Curr. Drug Deliv. 2005;2:223–233. doi: 10.2174/1567201054368020. [DOI] [PubMed] [Google Scholar]
- Jain S., Sapre R., Tiwary A.K., Jain N.K. Proultraflexible lipid vesicles for effective transdermal delivery of levonorgestrel: development, characterization, and performance evaluation. AAPS PharmSciTech. 2005;6:E513–E522. doi: 10.1208/pt060364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S.K., Gupta Y., Jain A., Rai K. Enhanced transdermal delivery of acyclovir sodium via elastic liposomes. Drug Deliv. 2008;15:141–147. doi: 10.1080/10717540801952407. [DOI] [PubMed] [Google Scholar]
- Lake Y., Pinnock S. Improved patient acceptability with a transdermal drug-in-adhesive oestradiol patch. Aust. N Z J. Obstet. Gynaecol. 2000;40:313–316. doi: 10.1111/j.1479-828x.2000.tb03341.x. [DOI] [PubMed] [Google Scholar]
- Ma M., Wang J., Guo F., Lei M., Tan F., Li N. Development of nanovesicular systems for dermal imiquimod delivery: physicochemical characterization and in vitro/in vivo evaluation. J. Mater. Sci. Mater. Med. 2015;26:191. doi: 10.1007/s10856-015-5524-1. [DOI] [PubMed] [Google Scholar]
- Madheswaran T., Baskaran R., Yong C.S., Yoo B.K. Enhanced topical delivery of finasteride using glyceryl monooleate-based liquid crystalline nanoparticles stabilized by cremophor surfactants. AAPS PharmSciTech. 2014;15:44–51. doi: 10.1208/s12249-013-0034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheshwari R.G., Tekade R.K., Sharma P.A., Darwhekar G., Tyagi A., Patel R.P., Jain D.K. Ethosomes and ultradeformable liposomes for transdermal delivery of clotrimazole: a comparative assessment. Saudi Pharm. J. 2012;20:161–170. doi: 10.1016/j.jsps.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malakar J., Sen S.O., Nayak A.K., Sen K.K. Formulation, optimization and evaluation of transferosomal gel for transdermal insulin delivery. Saudi Pharm. J. 2012;20:355–363. doi: 10.1016/j.jsps.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marwah H., Garg T., Goyal A.K., Rath G. Permeation enhancer strategies in transdermal drug delivery. Drug deliv. 2016;23:564–578. doi: 10.3109/10717544.2014.935532. [DOI] [PubMed] [Google Scholar]
- Meng S., Chen Z., Yang L., Zhang W., Liu D., Guo J., Guan Y., Li J. Enhanced transdermal bioavailability of testosterone propionate via surfactant-modified ethosomes. Int. J. Nanomed. 2013;8:3051–3060. doi: 10.2147/IJN.S46748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra D., Garg M., Dubey V., Jain S., Jain N.K. Elastic liposomes mediated transdermal delivery of an anti-hypertensive agent: propranolol hydrochloride. J. Pharm. Sci. 2007;96:145–155. doi: 10.1002/jps.20737. [DOI] [PubMed] [Google Scholar]
- Mura S., Manconi M., Sinico C., Valenti D., Fadda A.M. Penetration enhancer-containing vesicles (PEVs) as carriers for cutaneous delivery of minoxidil. Int. J. Pharm. 2009;380:72–79. doi: 10.1016/j.ijpharm.2009.06.040. [DOI] [PubMed] [Google Scholar]
- Naga Sravan Kumar Varma V., Maheshwari P.V., Navya M., Reddy S.C., Shivakumar H.G., Gowda D.V. Calcipotriol delivery into the skin as emulgel for effective permeation. Saudi Pharm. J. 2014;22:591–599. doi: 10.1016/j.jsps.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narishetty S.T., Panchagnula R. Transdermal delivery of zidovudine: effect of terpenes and their mechanism of action. J. Control Release. 2004;95:367–379. doi: 10.1016/j.jconrel.2003.11.022. [DOI] [PubMed] [Google Scholar]
- Saroha K., Yadav B., Sharma B. Transdermal patch: a discrete dosage form. Int. J. Curr. Pharm. Res. 2011;3:98–108. [Google Scholar]
- Shi J., Wang Y., Luo G. Ligustrazine phosphate ethosomes for treatment of Alzheimer's disease, in vitro and in animal model studies. AAPS PharmSciTech. 2012;13:485–492. doi: 10.1208/s12249-012-9767-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinico C., Manconi M., Peppi M., Lai F., Valenti D., Fadda A.M. Liposomes as carriers for dermal delivery of tretinoin: in vitro evaluation of drug permeation and vesicle-skin interaction. J. Control Release. 2005;103:123–136. doi: 10.1016/j.jconrel.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Song C.K., Balakrishnan P., Shim C.K., Chung S.J., Chong S., Kim D.D. A novel vesicular carrier, transethosome, for enhanced skin delivery of voriconazole: characterization and in vitro/in vivo evaluation. Colloids Surf. B Biointerfaces. 2012;92:299–304. doi: 10.1016/j.colsurfb.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Trotta M., Peira E., Debernardi F., Gallarate M. Elastic liposomes for skin delivery of dipotassium glycyrrhizinate. Int. J. Pharm. 2002;241:319–327. doi: 10.1016/s0378-5173(02)00266-1. [DOI] [PubMed] [Google Scholar]
- van den Bergh B.A., Vroom J., Gerritsen H., Junginger H.E., Bouwstra J.A. Interactions of elastic and rigid vesicles with human skin in vitro: electron microscopy and two-photon excitation microscopy. Biochim. Biophys. Acta. 1999;1461:155–173. doi: 10.1016/s0005-2736(99)00176-5. [DOI] [PubMed] [Google Scholar]
- van den Bergh B.A., Wertz P.W., Junginger H.E., Bouwstra J.A. Elasticity of vesicles assessed by electron spin resonance, electron microscopy and extrusion measurements. Int. J. Pharm. 2001;217:13–24. doi: 10.1016/s0378-5173(01)00576-2. [DOI] [PubMed] [Google Scholar]
- Vasan R.S., Larson M.G., Leip E.P., Kannel W.B., Levy D. Assessment of frequency of progression to hypertension in non-hypertensive participants in the Framingham Heart Study: a cohort study. Lancet. 2001;358:1682–1686. doi: 10.1016/S0140-6736(01)06710-1. [DOI] [PubMed] [Google Scholar]
- Vasan R.S., Beiser A., Seshadri S., Larson M.G., Kannel W.B., D'Agostino R.B., Levy D. Residual lifetime risk for developing hypertension in middle-aged women and men: the Framingham heart study. JAMA. 2002;287:1003–1010. doi: 10.1001/jama.287.8.1003. [DOI] [PubMed] [Google Scholar]
- Verma D.D., Verma S., Blume G., Fahr A. Liposomes increase skin penetration of entrapped and non-entrapped hydrophilic substances into human skin: a skin penetration and confocal laser scanning microscopy study. Eur. J. Pharm. Biopharm. 2003;55:271–277. doi: 10.1016/s0939-6411(03)00021-3. [DOI] [PubMed] [Google Scholar]
- Zeb A., Qureshi O.S., Kim H.S., Cha J.H., Kim J.K. Improved skin permeation of methotrexate via nanosized ultradeformable liposomes. Int. J. Nanomed. 2016;11:3813–3824. doi: 10.2147/IJN.S109565. [DOI] [PMC free article] [PubMed] [Google Scholar]