Abstract
Δ9-Tetrahydrocannabinol (Δ9-THC) shows its effects by activating cannabinoid receptors which are on some tissues and neurons. Cannabinoid systems have role on cell proliferation and development of neurons. Furthermore, it is interesting that cannabinoid system and rho/rho-kinase signalization pathway, which have important role on cell development and proliferation, may have role on neuron proliferation and development together. Thus, a study is planned to investigate rhoA and rho-kinase enzyme expressions and their activities in the brain of chronic Δ9-THC treated mice. One group of mice are treated with Δ9-THC once to see effects of acute treatment. Another group of mice are treated with Δ9-THC three times per day for one month. After this period, rhoA and rho-kinase enzyme expressions and their activities in mice brains are analyzed by ELISA method. Chronic administration of Δ9-THC decreased the expression of rhoA while acute treatment has no meaningful effect on it. Administration of Δ9-THC did not affect expression of rho-kinase on both chronic and acute treatment. Administration of Δ9-THC increased rho-kinase activity on both chronic and acute treatment, however, chronic treatment decreased its activity with respect to acute treatment. This study showed that chronic Δ9-THC treatment down-regulated rhoA expression and did not change the expression level of rho-kinase which is downstream effector of rhoA. However, it elevated the rho-kinase activity. Δ9-THC induced down-regulation of rhoA may cause elevation of cypin expression and may have benefit on cypin related diseases. Furthermore, use of rho-kinase inhibitors and Δ9-THC together can be useful on rho-kinase related diseases.
Keywords: Brain, Mice, RhoA, Rho-kinase, Δ9-Tetrahydrocannabinol
1. Introduction
Δ9-Tetrahydrocannabinol (Δ9-THC), which is a major component of Cannabis sativa, has psychoactive properties. It binds to endocannabinoid system receptors. Endogenous cannabinoids (endocannabinoids), their receptors and enzymes that degrade/synthesize these cannabinoids form the endocannabinoid system. Two types of cannabinoid receptors, CB(1) and CB(2), are defined up to date. Both of these receptor types are G protein-coupled receptors (GPRCs). CB(1) receptors are highly localized in the brain and cannabinoids show their psychoactive properties through these receptors (Chen and Firestein, 2007). Distribution of CB(2) receptors are more restricted and they are mostly found in immune cells and in some neurons (Galve-Roperh et al., 2006). Some studies showed that endocannabinoid system plays important role on proliferation and regulation of neurons of the central nervous system (Ahnert-Hilger et al., 2004, Maccarrone and Finazzi-Agro, 2003, Mechoulam et al., 2002, Nagayama et al., 1999, Piomelli, 2003). Rho/rho-kinase signalization pathway, which we evaluated in our study, is also important for cell proliferation and development (Charest and Firtel, 2007, Hall, 1998, Nagayama et al., 1999).
RhoA is a member of small GTPases and it transduce signals to intracellular downstream effectors (Vogt et al., 2003). RhoA’s downstream effector is rho-kinase. Two rho-kinase isoforms are defined which are Rho-kinase β (ROCK1) and Rho kinase α (ROCK2) (Julian and Olson, 2014). They play important roles on smooth muscle contraction, cell migration, apoptosis and cell proliferation (Julian and Olson, 2014).
Since endocannabinoid system plays role on cell proliferation and development in neurons as well as rho/rho-kinase pathway, it is interesting whether chronic Δ9-THC treatment affects rho/rho-kinase signalization pathway or not.
2. Material and method
Albino male mice (8 week old, balb/c) obtained from the Experimental Animal Center are used in study. This study was approved by the Animal Care Committee and Ethics Committee.
Mice are divided into three groups which are; control group, acute Δ9-THC treated group and chronic Δ9-THC treated group. All groups contained 8 mice. Δ9-THC (15 mg/kg) is applied by gavage once to acute Δ9-THC treated group after water is applied for 29 days by gavage. Δ9-THC (15 mg/kg) is applied by gavage three times a day to chronic Δ9-THC treated group for 30 days. Water is applied by gavage to control group under same experimental conditions for 30 days. At the end of the protocol described above, cervical dislocation is applied to the mice 1 h later after last gavage. Brain of the mice are stored in eppendorf tubes at −80 °C for later use in quantitative analysis.
Synthetic Δ9-THC Marinol (Unimed Pharmaceuticals Inc., Marietta, USA), that was dissolved at 0.1 mg/ml in 5.5% fat milk (w/v) in water, is used as the Δ9-THC source.
2.1. Quantitative analysis
2.1.1. Tissue homogenization
3 ml/gr Radio-immunoprecipitation Assay (RIPA) buffer, 30 μl phenylmethanesulfonyl fluoride (PMSF), 30 μl sodyum vanadate, 30 μl protease inhibitor is applied on frozen tissue samples that are stored in Eppendorf tubes then homogenates are obtained by using ultrosonication on those tubes on ice. Homogenates are then centrifuged at 10.000 RPM for 10 min and supernatants are taken and pellets are discarded.
2.1.2. Protein quantification
Bradford method is used to quantify the protein in homogenized tissues. By using Bovine serum albumin (1 μg/ml), 1, 2, 3, 5, 7, 8, 10 (μg/ml) standards are prepared. 10 μl is taken from every sample and completed to 100 μl by adding distilled water. 1 ml Bradford solution is added to standards and samples, vortexed and absorbances at 595 nm are measured manually. Protein quantification (μg/μl) is done according to the standard curve drawn in GraphPad Prism software (GraphPad Software, Inc., San Diego, USA).
2.1.3. Enzyme-Linked ImmunoSorbent Assay (ELISA) test
ELISA test is used to examine the expression and of RhoA (CUSABIO Inc., Maryland, USA), rho-kinase II (CUSABIO Inc., Maryland, USA) and the activity of rho-kinase (Cell Biolabs Inc., San Diego, USA).
2.2. Statistic analyzes
Results were expressed as means ± Standard Error of Mean (S.E.M.), and n refers to the number of animals used for each experiment. Differences in results between tissues were tested by one-way analysis of variance (one-way ANOVA) corrected for multiple comparisons (Bonferroni corrections). P values less than 0.05 were considered to be significant.
3. Results
3.1. ELISA RhoA protein quantification
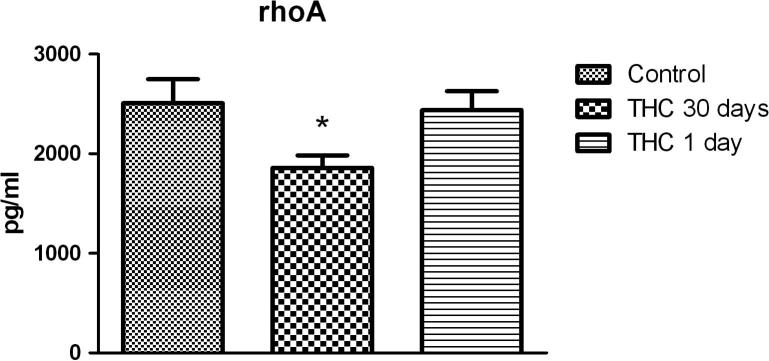
Chronic Δ9-THC treatment decreased expression of rhoA while acute Δ9-THC treatment had no meaningful effect (Fig. 1). Mean values of rhoA concentrations for control, chronic and acute Δ9-THC treated groups are found to be 2508 pg/ml (S.E.M. 240.3), 1858 pg/ml (S.E.M. 124.4), 2438 pg/ml (S.E.M. 191.4) respectively.
Fig. 1.
Effect of chronic and acute Δ9-THC treatment on rhoA expression (n = 8). Statistical analysis: one-way ANOVA. Post hoc: Bonferroni. (*: For control P < 0.05).
3.2. ELISA Rho-kinase II enzyme quantification
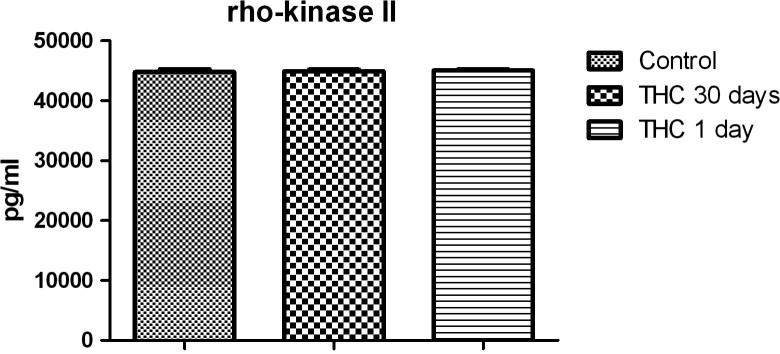
Chronic and acute Δ9-THC treatment did not change expression of rho-kinase II (Fig. 2). Mean values of rho-kinase II concentrations for control, chronic and acute Δ9-THC treated groups are found to be 44,809 pg/ml (S.E.M. 473.5), 44,958 pg/ml (S.E.M. 283.7), 45,081 pg/ml (S.E.M. 209.3) respectively.
Fig. 2.
Effect of chronic and acute Δ9-THC treatment on rho-kinase II expression (n = 8). Statistical analysis: one-way ANOVA. Post hoc: Bonferroni.
3.3. ELISA Rho-kinase activity quantification
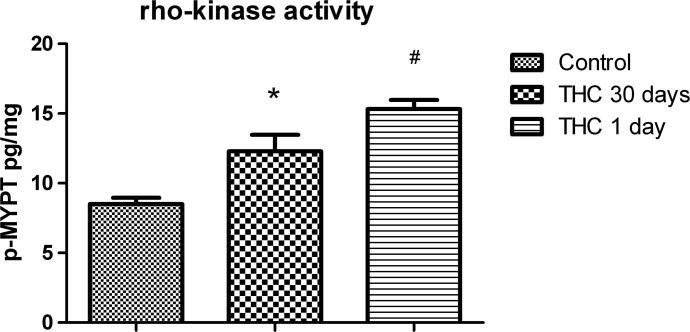
Both Chronic and acute Δ9-THC treatment elevated activity of rho-kinase while acute Δ9-THC treatment increased the rho-kinase activity more than chronic Δ9-THC treatment (Fig. 3). Mean values of rho-kinase II concentrations for control, chronic and acute Δ9-THC treated groups are found to be 8.50 p-MYPT pg/mg (S.E.M. 0.46), 12.29 p-MYPT pg/mg (S.E.M. 1.19), 14.21 p-MYPT pg/mg (S.E.M. 1.05).
Fig. 3.
Effect of chronic and acute Δ9-THC treatment on rho-kinase activity (n = 8). Statistical analysis: one-way ANOVA. Post hoc: Bonferroni. (*: For control P < 0.05. # For 30 days P < 0.05).
4. Discussion
In this study, Rho/rho-kinase signalization pathway, which is important for intracellular signal transduction, is evaluated (Charest and Firtel, 2007, Hall, 1998, Nagayama et al., 1999). Authors showed that chronic Δ9-THC treatment decreased the expression of rhoA in the brain. This situation may be explained by chronic Δ9-THC treatment down-regulated rhoA expression by activating CB1 and CB2 continuously. Since CB1 and CB2 receptors are G protein coupled, change in rhoA level is expected. While chronic Δ9-THC treatment down-regulated rhoA expression, it did not change the level of rho-kinase which is downstream effector of rhoA. Both acute and chronic Δ9-THC treatment elevated the rho-kinase activity with respect to control group. However, chronic Δ9-THC treatment decreased the rho-kinase activity with respect to acute Δ9-THC treatment due to down regulation of the rhoA expression in the long term. A study showed that CB receptors also activate rac, ras and cdc42 proteins (Kurihara et al., 2006). In the same study, it is also stated that active CB receptors caused the increase of phosphorylation of myosin light chains which are substrates of rho-kinase (Kurihara et al., 2006). Also, same study emphasized the increase of rho-kinase activity as found in this study (Kurihara et al., 2006).
Moreover, the reason for the increased activity of rho-kinase in the groups that are treated with acute and chronic Δ9-THC could be due to the stimulation of CB receptors with Δ9-THC. Since there is no Δ9-THC in the control group, CB receptors could be in an inactivated or low activity state.
Studies have shown that chronic Δ9-THC administration causes downregulation of CB1 receptors (Hirvonen et al., 2012). The reduction in RhoA may be due to the downregulation of Cb1 receptors, but further studies are needed to determine this. However, the downregulation of CB1 receptors and of RhoA may have had an additive effect on the reduction of the activity of the rho-kinase enzyme. Receptors such as dopamine (D) and serotonin (5ht) also function by activating RhoA (Li et al., 2015, Quilter et al., 2012). In addition, chronic Δ9-THC administration changes the activation and expression of these receptors (Franklin and Carrasco, 2013, Tournier et al., 2016).
CB receptors also causes elevation of intracellular calcium (Ayman et al., 2003, Rao and Kaminski, 2006). rhoA independent increase of rho-kinase activity may be caused by the elevation of intracellular calcium since it activates rho-kinase (Ayman et al., 2003). Furthermore, this study did not evaluate rhoA activity. Although its expression was decreased, its activity might be increased. To determine this, further studies are needed. Previous studies showed that rhoA acts to inhibit expression of cypin protein which has role on dendrite formation. Cypin is a newly identified protein in neurons. Chronic Δ9-THC treatment induced down-regulation of rhoA may cause elevation of cypin expression and may have benefit on cypin related diseases (Chen and Firestein, 2007). Furthermore, use of rho-kinase inhibitors and Δ9-THC together can be useful on rho-kinase related diseases. Since Δ9-THC treatment induces increase of rho-kinase activity, recent studies are also carried out to show the therapeutic benefits of Δ9-THC on some diseases like Alzheimer (Cao et al., 2014).
This study showed that chronic Δ9-THC treatment down-regulated rhoA expression and did not change the expression level of rho-kinase which is downstream effector of rhoA. However, it elevated the rho-kinase activity.
Acknowledgements
Authors thank to Cukurova University for funding this study (TSA-2014-2430). The authors declare that they have no conflict of interest.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahnert-Hilger G., Holtje M., Grosse G., Pickert G., Mucke C., Nixdorf-Bergweiler B., Boquet P., Hofmann F., Just I. Differential effects of Rho GTPases on axonal and dendritic development in hippocampal neurones. J. Neurochem. 2004;90:9–18. doi: 10.1111/j.1471-4159.2004.02475.x. [DOI] [PubMed] [Google Scholar]
- Ayman S., Wallace P., Wayman C.P., Gibson A., McFadzean I. Receptor-independent activation of Rho-kinase-mediated calcium sensitisation in smooth muscle. Br. J. Pharmacol. 2003;139:1532–1538. doi: 10.1038/sj.bjp.0705394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C., Li Y., Liu H., Bai G., Mayl J., Lin X., Sutherland K., Nabar N., Cai J. The potential therapeutic effects of THC on Alzheimer's disease. J. Alzheimer's Disease: JAD. 2014;42:973–984. doi: 10.3233/JAD-140093. [DOI] [PubMed] [Google Scholar]
- Charest P.G., Firtel R.A. Big roles for small GTPases in the control of directed cell movement. Biochem. J. 2007;401:377–390. doi: 10.1042/BJ20061432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Firestein B.L. RhoA regulates dendrite branching in hippocampal neurons by decreasing cypin protein levels. J. Neurosci.: Off. J. Soc. Neurosci. 2007;27:8378–8386. doi: 10.1523/JNEUROSCI.0872-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin J.M., Carrasco G.A. Cannabinoid receptor agonists upregulate and enhance serotonin 2A (5-HT(2A)) receptor activity via ERK1/2 signaling. Synapse. 2013;67:145–159. doi: 10.1002/syn.21626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galve-Roperh I., Aguado T., Rueda D., Velasco G., Guzman M. Endocannabinoids: a new family of lipid mediators involved in the regulation of neural cell development. Curr. Pharm. Des. 2006;12:2319–2325. doi: 10.2174/138161206777585139. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hirvonen J., Goodwin R.S., Li C.T., Terry G.E., Zoghbi S.S., Morse C., Pike V.W., Volkow N.D., Huestis M.A., Innis R.B. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol. Psychiatry. 2012;17:642–649. doi: 10.1038/mp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian L., Olson M.F. Rho-associated coiled-coil containing kinases (ROCK): structure, regulation, and functions. Small GTPases. 2014;5:e29846. doi: 10.4161/sgtp.29846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara R., Tohyama Y., Matsusaka S., Naruse H., Kinoshita E., Tsujioka T., Katsumata Y., Yamamura H. Effects of peripheral cannabinoid receptor ligands on motility and polarization in neutrophil-like HL60 cells and human neutrophils. J. Biol. Chem. 2006;281:12908–12918. doi: 10.1074/jbc.M510871200. [DOI] [PubMed] [Google Scholar]
- Li J., Gu J., Wang B., Xie M., Huang L., Liu Y., Zhang L., Xue J., Guo F., Zhang L., Zhang L. Activation of dopamine D1 receptors regulates dendritic morphogenesis through Rac1 and RhoA in prefrontal cortex neurons. Mol. Neurobiol. 2015;51:1024–1037. doi: 10.1007/s12035-014-8762-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M., Finazzi-Agro A. The endocannabinoid system, anandamide and the regulation of mammalian cell apoptosis. Cell Death Differ. 2003;10:946–955. doi: 10.1038/sj.cdd.4401284. [DOI] [PubMed] [Google Scholar]
- Mechoulam, R., Spatz, M., Shohami, E., 2002. Endocannabinoids and Neuroprotection. Science's STKE: Signal Transduction Knowledge Environment, 2002, re5. [DOI] [PubMed]
- Nagayama T., Sinor A.D., Simon R.P., Chen J., Graham S.H., Jin K., Greenberg D.A. Cannabinoids and neuroprotection in global and focal cerebral ischemia and in neuronal cultures. J. Neurosci.: Off. J. Soc. Neurosci. 1999;19:2987–2995. doi: 10.1523/JNEUROSCI.19-08-02987.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat. Rev. Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Quilter C.R., Bagga M., Moinie A., Junaid F., Sargent C.A. Gene structure and expression of serotonin receptor HTR2C in hypothalamic samples from infanticidal and control sows. BMC Neurosci. 2012;13:37. doi: 10.1186/1471-2202-13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao G.K., Kaminski N.E. Cannabinoid-mediated elevation of intracellular calcium: a structure-activity relationship. J. Pharmacol. Exper. Therapeut. 2006;317:820–829. doi: 10.1124/jpet.105.100503. [DOI] [PubMed] [Google Scholar]
- Tournier B.B., Tsartsalis S., Dimiziani A., Millet P., Ginovart N. Time-dependent effects of repeated THC treatment on dopamine D2/3 receptor-mediated signalling in midbrain and striatum. Behav. Brain Res. 2016;311:322–329. doi: 10.1016/j.bbr.2016.05.045. [DOI] [PubMed] [Google Scholar]
- Vogt S., Grosse R., Schultz G., Offermanns S. Receptor-dependent RhoA activation in G12/G13-deficient cells: genetic evidence for an involvement of Gq/G11. J. Biol. Chem. 2003;278:28743–28749. doi: 10.1074/jbc.M304570200. [DOI] [PubMed] [Google Scholar]