Abstract
Suaeda monoica Forssk. ex J.F. Gmel (Chenopodiaceae), a mangrove herb, is distributed in tropical Africa, Arabian Peninsula, India, Pakistan, Palestine and Jordan. The plant parts are used to treat sore throat, hepatitis, wounds, rheumatism, paralysis, asthma, snakebites, skin disease and ulcer. Two new phytoconstituents characterized as 13,17-octahydropentalene-4,4,10,23-tetramethyl-17,21-diisopropyl-tetradecahydrocyclo-[a]-phenanthrene-(14), 20(23), 21(30)-trien-5α-ol (SMC-3) and [1,4,4-trimethyl-cyclopent-1(5)-enyl]-9,10,17,21-tetramethyl-9α-ol-16α (17α)-epoxy heptadecan-6,10-dione (SMC-4) belong to the class norsesquaterpenol and monocyclic triterpenoid, respectively, along with two known compounds 3-epi-lupeol (SMC-1) and 4-cyclopentylpyrocatechol (SMC-2) have been isolated from the ethanol extract of aerial parts of S. monoica using normal and reverse phase column as well as planar chromatography. The spectroscopic studies including 1D, 2D NMR (DEPT, COSY, HMBC and HSQC) aided by EIMS mass and IR spectra were used to establish their structures. All the four compounds were tested for cytotoxicity on cultured HepG2 cells and for cell proliferation activities. The results revealed no cytotoxicity even at highest (6.25–50 μg/ml) dose of all the four compounds. The compound SMC-1 showed prominent cell proliferative activity as compared to other SMC compounds.
Keywords: Suaeda monoica, Chenopodiaceae, Norsesquaterpenol, Triterpeniod, Cell-proliferation
1. Introduction
The genus Suaeda is a halophyte comprises about 75 species which usually grow in sandy and saline sea coast, salt marshes, desert soil and salt steppes and mainly distributed in the northern hemisphere but some are subcosmopolitan (Loutfy, 1991). Suaeda monoica Forssk. ex J. F. Gmel is a mangrove herb belongs to the family Chenopodiaceae. It is distributed in coasts of tropical Africa, southern part of the Arabian Peninsula, coastal regions of India and Pakistan and the Dead Sea region in Palestine and Jordan. The plant in appearance resembles to Suaeda maritima but is smaller in size and possesses simple edible leaves (Ravikumar et al., 2010, Ravikumar et al., 2011). Mangrove plants have already been proved for their usefulness in traditional medicines (Premnathan et al., 1992, Premnathan et al., 1996, Kokpal et al., 1990, Sundaram et al., 2011). The stems of S. monoica are mixed with henna (Lawsonia inermis) to obtain a black dye. In Kenya, a plant decoction is drunk to relieve sore throat. The leaves are edible and taken as a remedy for hepatitis and applied as an ointment to heal wounds (Bandaranayake, 1998, Padmakumar and Ayyakkannu, 1992). In folklore medicine the plant is used against rheumatism, paralysis, asthma, snake-bites, skin disease and ulcer (Kathiresan and Ramanathan, 1997, Muthazhagan et al., 2014).
The leaves possess antiviral and wound healing activities (Ravikumar et al., 2010, Ravikumar et al., 2011,). The plant also exhibited antiplasmodial effect against Plasmodium falciparum (Sundaram et al., 2011), insecticidal (Fahmy et al., 2003) and antimicrobial activities (Manivachagam et al., 2008). The preliminary phytochemical screening results of S. monoica showed the presence of a wide range of phytochemicals such as protein, resins, tannins, cardiac glycosides, terpenoids, flavonoids, phenols and glycosides (Lakshmanan et al., 2013). A series of fatty acids such as lauric, tridecanoic, myristic, pentadecanoic, palmitic, heptadecanoic, stearic, nonadecanoic, arachidic, heneicosanoic, behenic, oleic, linoleic and linolenic acids have been reported in S. monoica (Manivachagam et al., 2008). The presence of triterpenoids and sterols was also determined (Bandaranayake, 1998, Yezhelyev et al., 2006).
Many herbs are active against neoplastic diseases by inhibiting cell growth and proliferation. They are also used to treat diseases that result from decline of stem cell proliferation and utilized for regeneration and rehabilitation of tissues by promoting cell proliferation. The basic requirement of cell proliferation is in tissue regeneration, repair and aging. The best results of cell proliferation can be observed with the herbs that can promote categorical cell proliferation but at the same time not taking the peril of abnormal cell proliferation. The herbs involving cell proliferation showed beneficial effects of herbs on stem cell proliferation; few reports focus on hair growth, stimulation of the immune system and other positive side of herbal remediation but there is lack of scientific research exploring possible deleterious effects of herbs on cell proliferation and possibly cancer development (Hasan and Al Sorkhy, 2014). It has been found that Aconiti Lateralis Preparata Radix (ALR) promoted the proliferation rate of mouse bone marrow mesenchymal stem cells (m BMMSCs) up to 122.24% compared to untreated cells (Kim et al., 2013). Taking into consideration the demand of cytotoxic, anti cancer and cell proliferative agents this study was planned to explore these effects by the compounds isolated from the S. monoica. After completing this study we achieved our goals up to some extent of getting two new compounds as well as the biological properties of selected category.
2. Experimental methods
2.1. General
ATI Mattson genesis series Fourier transform (FT-IR) infrared spectrophotometer was used to record IR spectra. Bruker Avance DRX 500 MHz and 150 MHz spectrometer were used to record 1D and 2D NMR spectra for 1H and 13C, respectively. HR mass spectroscopic data were recorded using a Shimadzu GC-MS model QP2010 Plus spectrophotometer (Shimadzu Scientific Instruments, Kyoto, Japan). Bruker Bioapex FT–MS was used to scan the EI mass spectra. Silica gel (70–230 mesh) and Li Chroprep RP-18 [40–63 μm; octadecyl silica (ODS) gel] (Merck) were employed for column chromatography. The AR grade chemicals n-hexane, ethyl acetate, chloroform, methanol, ethanol, sulphuric acid and vanillin were procured from Faisal Zouman Al-Anazi Trading Est., Riyadh, Saudi Arabia. TLC was carried out on glass backed silica gel F254 plate (E. Merck) and derivatization of plates were carried out by using p-anisaldehyde solution as a spray reagent.
2.2. Plant material
The aerial parts of S. monoica were collected in March 2013 from Aqabaat Al-Makhwah, after tunnel No. 13, Saudi Arabia. The plant material was identified by a Field Taxonomist, Department of Pharmacognosy, College of Pharmacy, KSU, Riyadh. A voucher specimen (#15235) is deposited in herbarium of the Department.
2.3. Extraction and isolation
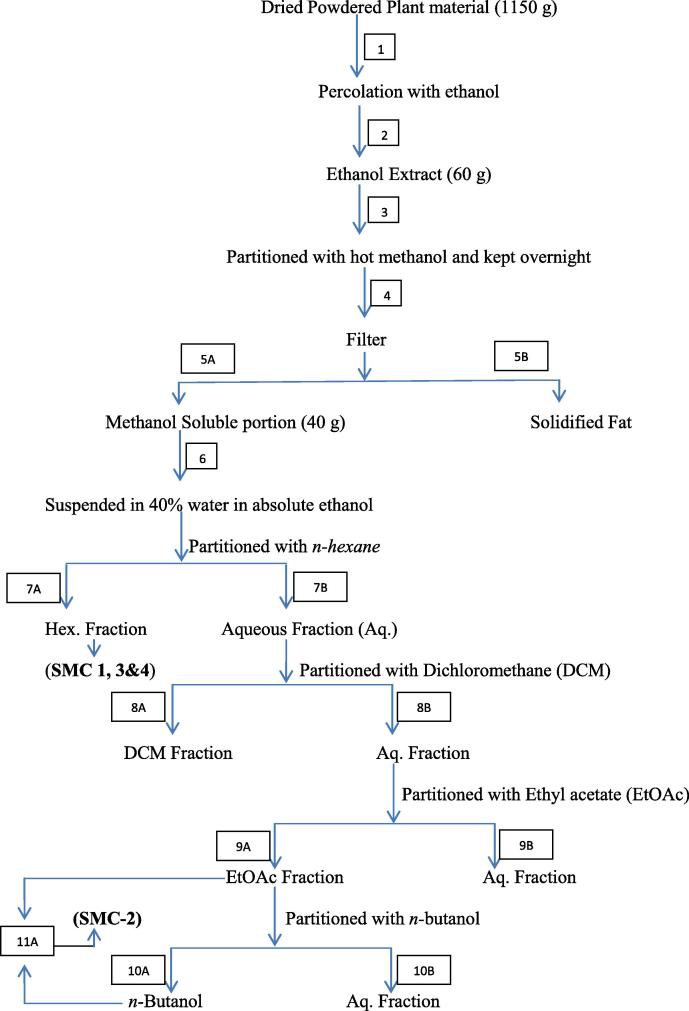
The collected aerial parts (1150 g) were air dried, powdered (1) and percolated with ethanol (2) (3 × 1 liter). After complete percolation the extracts were combined and concentrated to a semi-solid extract by rotary vacuum evaporator under reduced pressure to yield 60 g (3) which was partitioned with hot methanol (4) and kept overnight to get 40 g of extract (5A) and fat (5B) separately. This 40 g extract was suspended in 40% water in absolute ethanol (6) and then partitioned with n-hexane which had given a hexane fraction (7A) producing SMC-1, 3& 4 and an aqueous fraction (7B). The aqueous fraction was partitioned with dichloromethane (DCM), giving DCM (8A) and aqueous fraction (8B). Now aqueous fraction was again extracted with ethyl acetate giving EtOAc soluble portion (9A) and aqueous portion (9B). Ethyl acetate fraction was then extracted with n-butanol to obtain n-butanol fraction (10A) and aqueous fraction (10B). Based on TLC characteristics (9A) and (10A) were mixed together (11A) which produced SMC-2. First two fractions (1–2) of (7A) obtained after eluting with 10% EtOAc in hexane when subjected to separation by using chromatotron with mobile phase 5% acetone in hexane produced SMC-3 (30 mg) as viscous solid and another fractions (3–4) of (7A) when chromatographed on chromatotron by using 60% DCM in hexane produced SMC-4 (30 mg) as shiny yellowish brown liquid (Fig. 1).
Fig. 1.
Flow chart diagram of different fractions of ethanol extract of S. monoica.
2.3.1. (SMC-3): Normonisesquaterpenol
It was obtained as a yellow semi-solid; IR υmax (KBr): 3467, 2925, 2867, 1635, 1461, 1377, 1261, 1159, 1062 cm−1. 1H NMR (CDCl3): δ 2.58 (2H, t, J = 5.3 Hz, H2-7), 2.14 (3H, s, Me-33), 2.10 (3H, s, Me-31), 2.09 (3H, s, Me-32), 1.78 (1H, m, H-19), 1.75 (1H, m, H-9), 1.72 (1H, s, H2-22α), 1.67 (1H, s, H2-22β), 1.55 (1H, d, J = 5.6 Hz, H2-15α), 1.52 (1H, d, J = 9.5 Hz, H2-15β), 1.38 (2H, m, H2-1), 1.27 (1H, m, H-27), 1.24 (2H, m, H2-2), 1.23 (2H, m, H2-3), 1.21 (3H, s, Me-24), 1.14 (2H, m, H2-18), 1.12 (2H, m, H2-11), 1.08 (2H, m, H2-12), 1.05 (2H, m, H2-6), 0.87 (3H, s, Me-25),), 0.85 (3H, s, Me-26), 0.84 (3H, d, J = 5.6 Hz, Me-28), 0.82 (3H, d, J = 5.8 Hz, Me-29).
13C NMR (CDCl3): δ 24.88 (C-1), 24.51 (C-2), 28.04 (C-3), 29.79 (C-4), 74.53 (C-5), 16.07 (C-6), 20.82 (C-7), 118.63 (C-8), 32.86 (C-9), 37.49 (C-10), 21.10 (C-11), 37.35 (C-12), 32.89 (C-13), 145.59 (C-14), 37.54 (C-15), 37.52 (C-16), 25.76 (C-17), 39.44 (C-18), 32.76 (C-19), 144.59 (C-20), 121.18 (C-21), 39.86 (C-22), 122.62 (C-23), 22.70 (C-24), 22.80 (C-25), 23.84 (26), 31.62 (C-27), 19.73 (C-28), 19.82 (C-29), 117.35 (C-30), 12.27 (C-31), 11.83 (C-32), 11.33 (C-33); HR ESI MS: m/z 462.7163 (calcd. m/z 462.7159 for C33H50O); EI-MS m/z (rel. int.): 462 [M]+ (C33H50O) (50.4), 447 (9.8), 444 (32.6), 431 (89.2), 429 (33.8), 313 (60.5), 294 (6.5), 140 (9.2), 134 (43.7).
2.3.2. Suaedanortriterpenedione (SMC-4)
It was obtained as a shiny yellowish brown liquid; IR υmax (NaCl): 3483, 2925, 2858, 1692, 1677, 1635, 1461, 1377, 1193, 1128, 1031, 894, 738 cm−1. 1H NMR (CDCl3): δ 3.83 (1H, dd, J = 5.5, 6.0 Hz, H-16β), 2.04 (2H, m, H2-7), 2.02 (3H, s, Me-23), 1.97 (2H, m, H2-11), 1.94 (2H, m, H2-2), 1.84 (1H, m, H2-3), 1.78 (1H, m, H-13), 1.68 (92H, m, H2-8), 1.64 (2H, m, H2-15), 1.60 (2H,m, H2-12), 1.57 (1H, m, H2-8), 1.55 (1H, m, H-21), 1.52 (2H, m, H2-8), 1.48 (2H, m, H2-14), 1.37 (3H, brs, Me-26), 1.33 (3H, brs, Me-28), 1.27 (3H, d, J = 6.8 Hz, Me-22), 1.25 (3H, d, J = 6.2 Hz, Me-29), 1.22 (3H, s, Me-24), 1.18 (2H, m, H2-19), 1.10 (2H, m, H2-20), 0.87(3H, s, Me-25), 0.84 (3H, d, J = 6.1 Hz, Me-27). 13C NMR (CDCl3): δ 146.91 (C-1), 41.37 (C-2), 39.36 (C-3), 42.07 (C-4), 141.99 (C-5), 198.83 (C-6), 37.52 (C-7), 37.41 (C-8), 93.33 (C-9), 201.68 (C-10), 37.46 (C-11), 24.79 (C-12), 32.79 (C-13), 29.69 (C-14), 36.45 (C-15), 81.24 (C-16), 87.05 (C-17), 32.01 (C-18), 24.46 (C-19), 22.33 (C-20), 27.97 (C-21), 13.41 (C-22), 22.62 (C-23), 25.71 (C-24), 24.23 (C-25), 22.72 (26), 19.75 (C-27), 19.72 (C-28), 13.04 (C-29); HR ESI MS: m/z 462.7163 (calcd. m/z 462.7159 for C30H46O); EI-MS m/z (rel. int.): 462 [M]+ (C29H50O4) (6.8), 419 (97.2), 404 (100), 293 (2.6), 237 (12.7), 209 (2.6), 165 (5.8), 151 (54.2), 141 (3.8), 137 (54.2), 85 (17.2), 71 (29.8), 57 (14.1).
2.4. Cytotoxicity and cell proliferation assay
Human hepatoma cells (HepG2) were maintained in RPMI-1640 medium, supplemented with 10% bovine serum (Gibco, UAS), 1× penicillin-streptomycin mix (Invitrogen, USA), and 1× sodium pyruvate streptomycin (HyClone Laboratories, USA) at 37°C with 5% CO2 supply. HepG2 cells were seeded (0.5 × 105 cells/well, in triplicate) in a 96-well flat-bottom plate (Becton-Dickinson Labware, USA) and grown over night. Stocks of compounds (SMC-1 to SMC- 4, 1 mg each) were prepared by first dissolving in 50 μl of DMSO, and finally in RPMI (1 mg/ml). Stocks were then further diluted in RMPI to furnish four doses (50, 25, 12.5 and 6.25 μg/ml) of each. In the working doses, the final concentration of DMSO never exceeded >0.1%, and, therefore, showed non-cytotoxicity. The old culture media were replaced with the freshly prepared doses, including untreated control, and the cells were incubated for 48 h. MTT cell proliferation assay (TACS Kit, Tervigen) was performed as per the manufacturer’s instructions. Briefly, cells were treated with MTT reagent (10 μl/well) and incubated at room-temperature for 3–4 h in dark. Upon appearance of purple color, detergent solution (100 μl/well) was added and further incubated for 1.5 h. The optical density (OD) was recorded at 570 nm in a microplate reader (BioTek, ELx800). Non-linear regression analysis was performed in Excel software to determine the cell proliferation (%) using the following equation:
where the term ODs, ODb and ODc can be read as the absorbance of sample, blank and negative control, respectively.
3. Results and discussion
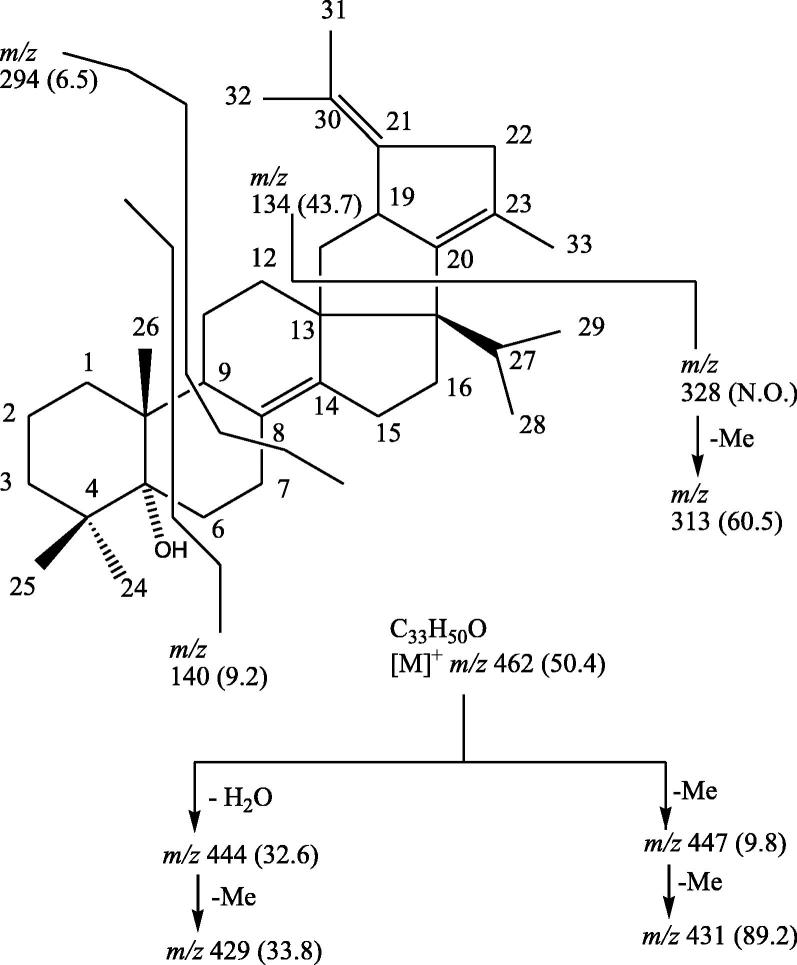
Compound SMC-3, named Normonisesquaterpenol, had IR absorption bands for a hydroxyl group (3467 cm−1) and unsaturation (1635 cm−1). On the basis of mass and 13C NMR spectra the molecular ion peak of SMC-3 was determined at m/z 462 consistent with a molecular formula of a norsesquaterpenol C33H50O (Fig. 2). The ion peaks arising at m/z 444 [M–H2O]+, 429 [444–Me]+, 447 [M–Me]+, 431 [447–Me]+ and 140 [C5, 6–C9,10 fission, C9H16O]+ suggested the existence of the hydroxyl group at C-5. The ion fragments generated at m/z 294 [C7,8–C9,10 fission, C22H30]+, 134 [C13,18–C17,20 fission, C10H14]+ and 313 [M–134–Me]+ indicated the saturated nature of the rings A and B and existence of the vinylic linkages in the rings C and F (Fig. 3). The 1H NMR spectrum of SMC-3 exhibited three singlets at δ 2.14, 2.10 and 2.08, integrating for three protons each, assigned to methyl C-33, C-31 and C-32 protons, respectively, located on the vinylic carbons C-23 and C-30, two three-proton doublets at δ 0.84 (J = 5.6 Hz) and 0.82 (J = 5.8 Hz) ascribed to secondary C-28 and C-29 methyl protons and two three-proton singlets at δ 1.21, 0.87 and 0.85 accounted to tertiary C-24, C-25 and C-26 tertiary methyl protons, respectively. A two-proton triplet at δ 2.58 (J = 5.3 Hz) was due to methylene H2-7 protons nearby to the vinylic carbons. The other methylene and methine protons appeared from δ 1.78 to 1.05. The absence of any proton signal beyond δ 2.58 suggested tetra-substituted vinylic bonds and existence of the hydroxyl function on a quaternary carbon. The 13C NMR spectrum of SMC-3 displayed the presence of vinylic carbons between δ 145.59 and 117.35, oxygenated carbon at δ 74.53 (C-5), and methyl carbons at δ 23.84 (C-26), 22.80 (C-25), 22.70 (C-24), 19.82 (C-29), 19.73 (C-28), 12.27 (C-31), 11.83 (C-32) and 11.33 (C-33). Its DEPT spectrum showed the presence of eight methyl, eleven methylene and three methine carbons. The 1H-1H COSY spectrum of SMC-3 exhibited correlation of H-9 with H2-7, H2-1, H2-11 and Me-26; H2-18 with H-19 and H2-12; H-27 with H2-16, Me-27 and Me-29; and Me-33 with H2-22. The HMBC spectrum of SMC-3 showed interactions of that H2-3, H2-6, Me-25 and Me-26 with C-5; H-9, H2-7, and H2-15 with C-8; H2-16, Me-28, Me-29 and H-27 with C-17; H2-22 and Me-33 with C-23; and Me-31 and Me-32 with C-30. The HSQC spectrum of SMC-3 displayed interactions of methyl C-24.C-25, C-26, C-28, C-29, C-31, C-32 and C-33 protons with their respective carbon signals. On the basis of above discussion the structural formula of SMC-3 was established as 13,17-octahydropentalene-4,4,10,23-tetramethyl-17,21-diisopropyl-tetradecahydrocyclo-[a]-phenanthrene-(14), 20(23), 21(30)- trien-5α-ol, a new norsesquaterpenol.
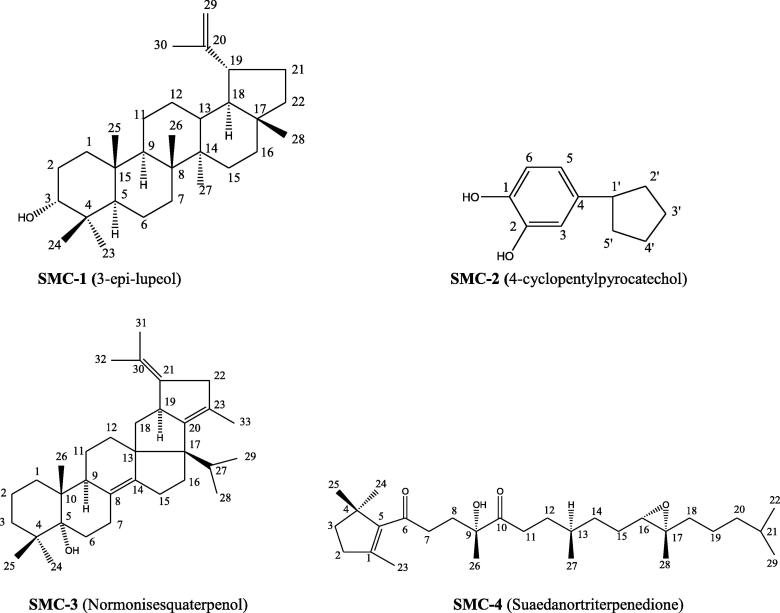
Fig. 2.
Chemical Structures of compounds (SMC 1–4) isolated from S. monoica.
Fig. 3.
Mass fragmentation pattern of SMC-3.
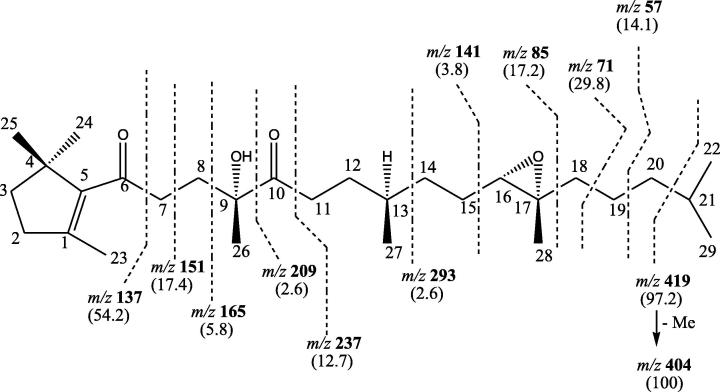
Compound SMC-4, named suaedanortriterpenedione (Fig. 2), showed IR absorption bands for hydroxyl group (3483 cm−1), carbonyl functions (1692, 1677 cm−1) and unsaturation (1635 cm−1). Its molecular ion peak was determined at m/z 462 on the basis of mass and 13C NMR spectra corresponding to a molecular formula of a monocyclic nortriterpenoid, C29H50O4. The ion peaks arising at m/z 137 [C6-C7 fission]+, 151 [C7-C8 fission]+ and 165 [C8-C9 fission]+ indicated the presence of a trimethyl substituted cyclopentene ring at one of the terminal of the molecule and one of the carbonyl group at C-6. The ion fragments generated at m/z 209 [C9-C10 fission]+, 237 [C10-C11 fission]+ and 293 [C13-C14 fission]+ supported the existence of the hydroxyl group at C-9. The ion peaks produced at m/z 141 [C15-C16 fission]+, 85 [C17-C18 fission]+, 71 [C18-C19 fission]+, 57 [C19-C20 fission]+, 419 [C20-C21 fission, Me-C3H7]+ and 404 [419-Me]+ suggested location of an epoxy ring at C17-C16 position (Fig. 4). The 1H NMR spectrum of SMC-4 displayed a one-proton double doublet at δ 3.83 with coupling interactions of 5.5, 6.0 Hz assigned to oxymethine H-16 β proton, a three - proton singlet at δ 2.02 ascribed to C-23 methyl protons located on a vinylic C-1 carbon, four three-proton singlets at δ 1.37, 1.33, 1.22 and 0.87 attributed to tertiary C-26, C-28, C-24 and C-25 methyl protons and three doublets at δ 1.27 (J = 6.8 Hz), 1.25 (J = 6.2 Hz) and 0.84 (J = 6.1 Hz) integrating for three protons each associated with secondary carbon at C-22, C-29 and C-21 protons. The absence of any proton signal beyond δ 3.83 indicated the existence of a tetrasubstituted vinylic linkage in the molecule. The 13C NMR spectrum of SMC-4 exhibited the signals for carbonyl carbons at δ 198.83 (C-6) and 201.68 (C-10), vinylic carbons at δ 146.91 (C-1) and 141.99 (C-5), oxygenated carbons at δ 93.33 (C-9), 81.24 (C-16) and 87.05 (C-17) and methyl carbons between δ 25.71 and 13.04. The DEPT spectrum of SMC-4 showed the presence of eight methyl, eleven methylene, three methine and seven quaternary carbons. The 1H-1H COSY spectrum of SMC-4 displayed correlations of H2-2 and H2-3 with Me-23; H2-8 with Me-26; H-16 and H2-18 with Me-28; and H2-20, H-21 and Me-22 with Me-29. The HMBC spectrum of SMC-4 showed interactions of H2-2, H2-3 and Me-23 with C-1; H2-3 and Me-25 with C-5; H2-7 and H2-8 with C-8; Me-26, H2-8 and H2-11 with C-10; H2-15, H-16, H2-18 and Me-28 with C-17; and H2-20, Me-22 and Me-29 with C-21. The HSQC spectrum of SMC-4 exhibited that 1H NMR signals of the methyl protons correlated with their respective carbon signals and H-16 at δ. 3.83 interacted with C-16 at δ 81.24. On the basis of these evidences the structure of SMC-4 has been formulated as [1,4,4-trimethyl-cyclopent-1(5)-enyl]-9,10,17,21-tetramethyl-9α-ol-16α (17α)-epoxy heptadecan-6,10-dione (Fig. 2). This is a new monocyclic triterpenoid.
Fig. 4.
Mass fragmentation pattern of SMC-4.
Our MTT assay showed non-cytotoxicity of all the four isolated compounds (SMC-1 to SMC-4) on cultured HepG2 cells. The four compounds (SMC-1 to SMC-4) isolated from S. monoica tested with four doses (6.25 to 50 μg/ml) on cultured HepG2 cells, showed no toxicity, even at the highest dose. Therefore, the compounds (50 μg/ml) were further evaluated for cell proliferative activities. Of these, SMC-1 showed the maximum cells growth activity (34.5%), followed by SMC-2 (28.7%), SMC-4(27.5%) and SMC-3 (24.3%) as compared to untreated control (Fig. 5). Our results therefore, warrant the therapeutic value of SMC-1 and its source plant S. monoica as a promising cell proliferating agent.
Fig. 5.
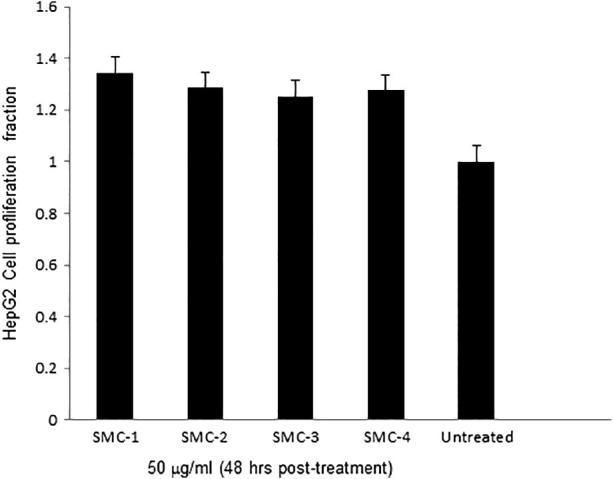
MTT assay showing cell proliferative or growth stimulatory activities of compounds (SMC 1–4) on cultured HepG2 cells.
Regeneration or cell proliferative potential of plant extract can be exploited in many ways such as disease prevention and curative measures. There are several plant extracts and isolated compounds which have shown their usefulness as cell proliferating agents. Some of these phytoconstituents including polysaccharides from Astragalus and Isatis root, flavones from Epimedium and Propolis, astragalosides and ginsenosides were found to enhance lymphocyte proliferation and antibody titer, while Epimedium polysaccharide mainly stimulated cellular immune responses (Kong et al., 2004). As far as curative measures are concerned wound healing is one of the most frequently used effect of the plant extracts which included Wrightia tinctoria, Aloe vera, Terminalia chebula and Curcuma longa (Krishnamoorthy et al., 2012). Root bark of Persea americana includes chemical compounds with estrogen-like activity and validates its potential use as anticancer agent, particularly against breast carcinoma in Nigerian traditional medicine (Engel et al., 2011). The present study describes no cytotoxicity of all the four isolated compounds (SMC-1 to SMC-4) instead showed cell proliferation on cultured HepG2 cells. Among all the four compounds SMC-1 (3-epi-lupeol) showed the maximum cells growth activity (34.5%). It can be concluded that SMC-1 can be used as cell proliferating compound and S. monoica may be recommended for cellular regeneration. Further studies are needed to explore the therapeutic potential of S. monoica and its chemical constituents as immunostimulators as well as mechanism of the cellular immunity by exploiting its cell proliferative effect.
4. Conclusion
Cell proliferation is one of the major healing mechanisms in human body in which the newly generated cells replace the damaged cells and thus restore the normal physiology of the organ as well as the whole system. Natural products have always been in demand for restoration of normal functions of the various organs of our body. The traditional use of S. monoica for sore throat and wound healing justifies its use for cell proliferative potential and its healing power for a large spectrum of ailments.
Acknowledgement
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding of this research project through the Research Group Project No. RG-1435-053.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Mansour S. Al-Said, Email: msalsaid@ksu.edu.sa.
Nasir A. Siddiqui, Email: nasiratksu@gmail.com, nsiddiqui@ksu.edu.sa.
Mohamed Ahmed Mukhair, Email: mmukhair@ksu.edu.sa.
Mohammad K. Parvez, Email: khalid_parvez@yahoo.com.
Perwez Alam, Email: alamperwez007@gmail.com.
Mohd. Ali, Email: maliphyto@gmail.com.
Anzarul Haque, Email: anzarulhaqque@gmail.com.
References
- Bandaranayake W.M. Traditional and medicinal uses of mangroves. Mangroves Salt Marshes. 1998;2:133–148. [Google Scholar]
- Engel N., Oppermann C., Falodun A., Kragl U. Proliferative effects of five traditional Nigerian medicinal plant extracts on human breast and bone cancer cell lines. J. Ethnopharmacol. 2011;137(2):1003–1010. doi: 10.1016/j.jep.2011.07.023. [DOI] [PubMed] [Google Scholar]
- Fahmy A.K., Ibrahim A.M.H., El-Shazly A.M., El-Sayed A.M. Plant derived pest control agents: III- insecticidal and biochemical studies of different plant extracts against Culex pipiens larvae. J. Agric. Environ. Sci. 2003;2(1):56–68. [Google Scholar]
- Hasan N.M., Al Sorkhy M.K. Herbs that promote cell proliferation. Int. J. Herbal Med. 2014;1(6):18–21. [Google Scholar]
- Kathiresan K., Ramanathan T. Tamil Nadu, India, Annamalai University; 1997. Medicinal Plants of Parangipettai Coast; pp. 72–76. [Google Scholar]
- Kim D.R., Kim H.Y., Park J.K., Park S.K., Chang M.S., Jeon J.Y. Aconiti lateralis preparata radix activates the proliferation of mouse bone marrow mesenchymal stem cells and induces osteogenic lineage differentiation through the bone morphogenetic protein-2/smad-dependent runx2 pathway. Evid. Based Complement. Alternat. Med. 2013;2013:24–34. doi: 10.1155/2013/586741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokpal V., Miles D.H., Payne A.M., Chittarwong V. Chemical constituents and bioactive compounds from mangrove plants. Stud. Nat. Prod. Chem. 1990;7:175–199. [Google Scholar]
- Kong X., Hu Y., Rui R., Wang D., Li X. Effects of Chinese herbal medicinal ingredients on peripheral lymphocyte proliferation and serum antibody titer after vaccination in chicken. Int. Immunopharmaco. 2004;4(7):975–982. doi: 10.1016/j.intimp.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy J.R., Sumitira S., Ranjith M.S. An in vitro study of wound healing effect of a poly-herbal formulation as evidenced by enhanced cell proliferation and cell migration. Egypt. Dermatol. Online J. 2012;8(1):1–7. [Google Scholar]
- Lakshmanan G., Rajeshkannan C., Kavitha A., Mekala B., Kamaladevi N. Preliminary screening of biologically active constituents of Suaeda monoica and Sesuvium portulacastrum from palayakayal mangrove forest of Tamilnadu. J. Pharmacog. Phytochem. 2013;2(3):149–152. [Google Scholar]
- Loutfy B. Notes on Suaeda Forssk. ex Scop. Studies in the Chenopodiaceae of Arabia: 2. Kew. Bull. 1991;46(2):291–296. [Google Scholar]
- Manivachagam C., Kannathasan K., Venkatesalu V. Antimicrobial activity of fatty acid methyl esters of some members of chenopodiaceae. Z. Naturforsch. 2008;63(5–6):331–336. doi: 10.1515/znc-2008-5-604. [DOI] [PubMed] [Google Scholar]
- Muthazhagan K., Thirunavukkarasu P., Ramanathan T., Kannan D. Studies on phytochemical screening, antimicrobial and anti radical scavenging effect coastal salt mash plant of a Suaeda monoica. Res. J. Phytochem. 2014;8:102–111. [Google Scholar]
- Padmakumar K., Ayyakkannu K. Antiviral activity of marine plants. Indian J. Virol. 1992;13:33–36. [Google Scholar]
- Premnathan M., Chandra K., Bajpai S.K., Kathiresan K. A survey of some Indian marine plants for antiviral activity. Bot. Mar. 1992;35(4):321–324. [Google Scholar]
- Premnathan M., Nakashima H., Kathiresan K., Rajendra N., Yamamoto N. In Vitro antihuman immunodeficiency virus activity of mangrove plants. Ind. J Med. Res. 1996;103:278–281. [PubMed] [Google Scholar]
- Ravikumar S., Gnanadesigan M., Serebiah J.S., Inbaneson S.J. Hepatoprotective effect of an Indian salt marsh herb Suaeda monoica Forrsk ex. Gmel against concanavalin-A induced toxicity in rats. Life Sci. Med. Res. 2010;2:1–9. [Google Scholar]
- Ravikumar S., Gnanadesigan M., Inbaneson S.J., Kalaiarasi A. Hepatoprotective and antioxidant properties of Suaeda maritima (L.) Dumort ethanolic extract on concanavalin-A induced hepatotoxicity in rats. Ind. J Exp. Biol. 2011;49:455–460. [PubMed] [Google Scholar]
- Sundaram R., Ganesan R., Inbaneson J.S., Andy R. Antiplasmodial activity of two marine polyherbal preparations from Chaetomorpha antennina and Aegiceras corniculatum against Plasmodium falciparum. Parasitol. Res. 2011;108(1):107–113. doi: 10.1007/s00436-010-2041-5. [DOI] [PubMed] [Google Scholar]
- Yezhelyev MV., Gao X., Xing Y., Al-Hajj A., Nie S., O'Regan RM. Emerging use of nanoparticles in diagnosis and treatment of breast cancer. Lancet Oncol. 2006;7(8):657–667. doi: 10.1016/S1470-2045(06)70793-8. [DOI] [PubMed] [Google Scholar]