Abstract
Lyophilization is used to ensure an increased shelf-life of liposomes, by preserving them in dry state, more stable than the aqueous dispersions. When stored as aqueous systems, the encapsulated drugs are released and the liposomes might aggregate or fuse. The aim of this study was to develop and optimize a lyophilized formulation of simvastatin (SIM) loaded into long circulating liposomes using the Quality by Design (QbD) approach. Pharmaceutical development by QbD aims to identify characteristics that are critical for the final product quality, and to establish how the critical process parameters can be varied to consistently produce a product with the desired characteristics. In the case of lyophilized liposomes, the choice of the optimum formulation and technological parameters has to be done, in order to protect the integrity of the liposomal membrane during lyophilization. Thus, the influence of several risk factors (3 formulation factors: PEG proportion, cholesterol concentration, the cryoprotectant to phospholipids molar ratio, and 2 process parameters: the number of extrusions through 100 nm polycarbonate membranes and the freezing conditions prior lyophilization) over the critical quality attributes (CQAs) of lyophilized long circulating liposomes with simvastatin (lyo-LCL-SIM), i.e. the size, the encapsulated SIM concentration, the encapsulated SIM retention, the Tm change and the residual moisture content, was investigated within the current study using the design of experiments tool of QbD. Moreover, the design space for lyo-LCL-SIM was determined, in which the established quality requirements of the product are met, provided that the risk factors vary within the established limits.
Keywords: Lyophilization, Liposomes, Simvastatin, QbD
1. Introduction
Statins, 3-hydroxyl-3-methylglutaryl coenzyme A reductase inhibitors, are therapeutic agents clinically used for their serum cholesterol-reducing activity, being recommended as first-line medications for the prevention and treatment of coronary artery diseases (Stone et al., 2014). In recent years, besides the lipid-lowering activity, many studies have shown that statins have anti-cancer actions, being studied in colorectal, prostate, breast and lung tumors (Cho et al., 2008, Kochuparambil et al., 2011, Cardwell et al., 2015, Campbell et al., 2006, Afzali et al., 2016). Moreover, statins have been reported to reduce the overall risk of cancer and the risk of specific cancers such as colon cancer (Liu et al., 2016). Despite the evidences regarding the benefits of statins in cancer, the doses required for cancer treatment and prevention are very high, even toxic after systemic administration (Thibault et al., 1996). Taking all these into account, the development of stable carriers, able to deliver therapeutic doses of statins to tumors, should be a promising approach.
Polyethylene glycolated (PEG-ylated) liposomes are therapeutic nanocarriers with demonstrated potential for prolonging the circulation time of drugs in vivo and for passive tumor accumulation, these benefits being exploited in the development of clinically used liposomal products (Immordino et al., 2006, Fan and Zhang, 2013). However, liposomes are aqueous dispersions with limited physical and chemical stability upon long-term storage (Guan et al., 2011), the reduced stability of these formulations being a barrier for their development as marketable products (Chaudhury et al., 2012). In this context, lyophilization has been proposed as a suitable technique for the improvement of physical stability of liposomal drug delivery systems, by preserving them in dried form as lyophilized cakes to be reconstituted with water for injection prior administration (EL-Nesr et al., 2010).
The results of a previous study showed that the optimum conditions for the preparation of SIM-loaded PEG-ylated liposomes (LCL-SIM), in order to get the maximum SIM concentration in vesicles having the size of 180 ± 20 nm, are met for a composition of 70 mM phospholipids, 7 mM cholesterol and 18 mM SIM (Porfire et al., 2015). However, due to the increased interest regarding the stabilization of liposomes through lyophilization, other critical formulation and process parameters were explored within the current work, in order to establish their influence on the quality of the lyophilized liposomes. Moreover, the design space for lyophilized liposomes has been defined through this study, by implementing the Quality by Design (QbD) strategy as a systematic approach to liposomal development, in order to improve the product quality, by understanding and controlling formulation and manufacturing variables (Yu, 2008, Xu et al., 2012). This strategy has been recommended in the latest years by the drug regulatory agencies for the development of better quality products (ICH, 2005, ICH, 2009). According to these recommendations, several key steps of the QbD approach, i.e. the identification of the critical quality attributes (CQAs), the risk assessment and the determination of the design space (Pramod et al., 2016), were followed within the current paper, for a better understanding of the formulation and preparation process of lyophilized LCL-SIM (lyo-LCL-SIM).
2. Materials and methods
2.1. Materials
The chemicals used for liposomes preparation and characterization were the following: simvastatin from Biocon Limited (India); 1,2-Dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) and N-(Carbonyl-methoxypolyethylenglycol-2000)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine (Na-salt) (MPEG-2000-DSPE), from Lipoid GmbH (Germany); cholesterol (CHO) from sheep wool (>92.5%, GC) and trehalose from Sigma-Aldrich (Germany). All the solvents and other reagents used were of analytic grade purity, commercially available.
2.2. Preparation of liposomes
Simvastatin-loaded long-circulating liposomes (LCL-SIM) were prepared using the film hydration method described before (Porfire et al., 2015). Briefly, phospholipids (a mixture of DPPC and MPEG-2000-DSPE), CHO and SIM were dissolved in ethanol in a round-bottomed flask and the solvent was evaporated under reduced pressure at 40 °C in a rotary evaporator, leading to the formation of a thin film on the surface of the flask. The dry lipids were then hydrated with 5 ml phosphate buffered saline (PBS, pH = 7.8) for 15 min at 45 °C. The liposomes were separated by centrifugation at 10,000g, 15 min (Sigma 3–30 K), and were subsequently diluted to 5 ml with the hydration buffer, after removal of the supernatant containing non-encapsulated drug. Liposomal suspension was subsequently homogenized under high pressure three times through a 0.8 μm polycarbonate membrane, three times through a 0.2 μm polycarbonate membrane and between 1 and 3 times through 0.1 μm polycarbonate membrane, according to the experimental design, using LiposoFast LF-50 equipment (Avestin Europe GmbH, Germany). Finally, LCL-SIM were either lyophilized immediately after preparation, or frozen prior to lyophilization at −40 °C or −80 °C, based on the experimental design, in order to obtain the lyophilized LCL-SIM (lyo-LCL-SIM).
2.3. Freeze-drying of the liposomes
Liposomes were freeze-dried using an VirTis AdVantage PLUS equipment (SP Scientific). A volume of 1 ml liposomal dispersion was transferred into glass vials and each vial was placed on the shelf inside the equipment. Shelf temperature was then lowered and maintained to −40 °C and the chamber pressure at 1000 mbars for 145 min, in order to allow the complete freezing of the liposomes. Sublimation of the solvent was then initiated by decreasing the pressure to 150 mbars and increasing the temperature to −30 °C for the next 23 h and 20 min. Secondary drying was performed in order to remove absorbed water from the product. To achieve this, shelf temperature was raised and maintained at 20 °C for approximately 20 h. At the end of the process, the vials were closed with rubber caps and stored at 4 °C until further analysis.
In order to study the effect of the addition and concentration of lyoprotectant on the properties of lyophilized liposomes, freshly prepared liposomal suspensions were freeze-dried with/without trehalose as cryoprotectant. For this purpose, the required amount of trehalose was dissolved in the liposomal suspension immediately after preparation, to achieve the desired molar carbohydrate to lipid ratios, according to the experimental design.
2.4. Liposomes' characterization
The characterization of liposomes was performed immediately after preparation (size, simvastatin concentration, the gel to liquid phase transition temperature (Tm)), on the freeze dried cakes (residual moisture content) as well as after reconstitution of the cakes by addition of distilled water (size, simvastatin concentration, encapsulated simvastatin retention (ESR), phase transition temperature).
2.4.1. Liposomal size
Liposomal size was determined by dynamic light scattering method, using Zetasizer Nano ZS analyzer (Malvern Instruments Co., Malvern, UK). The measurement was performed at 25 °C with a scattering angle of 90°. The dynamic light scattering data was collected using a helium laser source and mean results were provided by photon correlation spectroscopy (PCS).
2.4.2. Simvastatin concentration and encapsulated solute retention (ESR)
Liposomal simvastatin content was determined by using a HPLC/UV method, after complete dissolution of freshly prepared liposomes and reconstituted liposomes in methanol. The analyses were performed using a liquid chromatography (Agilent 1100, Agilent Technologies, Santa Clara, CA), equipped with an ultraviolet (UV) detector. The mobile phase consisted of a mixture of 72% acetonitrile and 28% acetate buffer solution and was delivered isocratically at a flow rate of 0.8 ml/min through the Gemini C18 column (100 × 3 mm, internal diameter 3 µm) from Phenomenex (Phenomenex, Torrance, CA), preceded by a 0.2 mm online filter. All chromatographic separations were performed at room temperature and detection was carried out at 238 nm with a UV detector (Porfire et al., 2015). Liposomal SIM was expressed both as concentration (µg/ml) and ESR (%). For the determination of SIM concentration in reconstituted liposomes, lyophilized cakes were rehydrated with distilled water, the leaked SIM during lyophilization was removed by centrifugation, and the initial volume of the liposomal dispersion was reconstituted with distilled water. The ESR was expressed as the percentage of entrapped drug retained after lyophilization, and was calculated using the equation:
| (1) |
2.4.3. Differential scanning calorimetric study
Freshly prepared liposome samples, as well as lyophilized samples after reconstitution to their initial volume with distilled water, were sealed in aluminum pans and heated in the differential scanning calorimeter (Mettler-Toledo, GmbH, Switzerland), from 20 to 60 °C, at a rate of 10 °C/min, under dynamic nitrogen atmosphere at a flow of 50 mL/minute, using an empty aluminum pan as a reference. Temperature was calibrated using indium (melting point of 156.6 °C) as standard. The phase transition temperature (Tm) was calculated as midpoint of the transition for each formulation. The Tm change was calculated as the difference between the Tm of the freshly prepared liposomes and the Tm of the reconstituted sample.
2.4.4. Thermogravimetric analysis (TGA)
For TGA, accurately weighted liposomal samples (15–20 mg) were heated at 10 °C/min from 25 up to 250 °C, under dynamic nitrogen atmosphere at a flow of 50 ml/minute in a Mettler Toledo TGA/SDTA851 equipment (Switzerland). Moisture content (%) was determined, with Mettler Star universal analysis software, by the stable weight loss (%) at a temperature around 100 °C.
2.5. Quality by design (QbD) approach to freeze-dried liposomes development
2.5.1. Determination of Critical Quality Attributes (CQAs) of lyophilized liposomes and identification of the potential risk factors
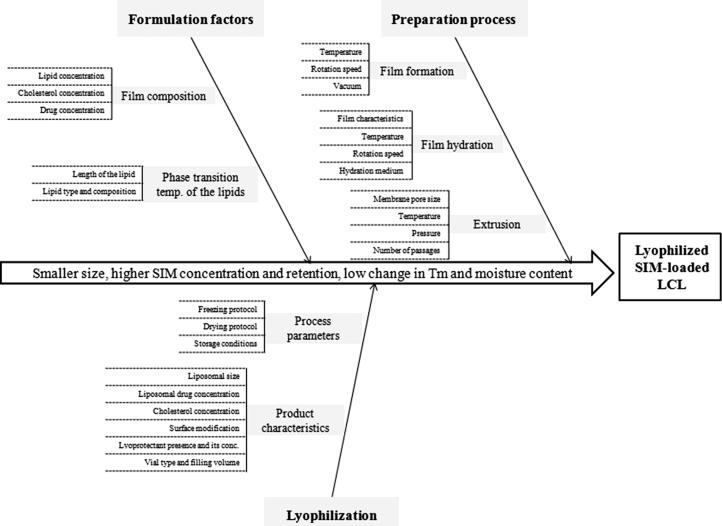
The identification of the CQAs of lyophilized liposomes was performed based on preliminary studies and review of the literature, as the first step of the quality by design approach. According to these, the CQAs of a lyophilized liposomal formulation are the size of the vesicles and aggregation formation, the drug concentration, the encapsulated solute retention after lyophilization, the residual moisture content, the change in Tm between the hydrated and dehydrated state, the cake elegance and reconstitution time. Among these, five CQAs were selected to be investigated within this study, i.e. the size, the encapsulated SIM concentration and ESR, the Tm change and the residual moisture content. The potential risk factors which might influence the quality of the product were identified through risk analysis, by the use of an Ishikawa diagram (Fig. 1) (Patel et al., 2016). After the risk assessment, five variables were chosen to be further studied and were included in an experimental design.
Fig. 1.
Ishikawa diagram illustrating CPP affecting the CQAs of lyophilized SIM-loaded LCL.
2.5.2. Experimental design
The Design of Experiments (DoE) was used in the subsequent step as tool of QbD, in order to study the influence of risk factors on the CQAs of lyo-LCL-SIM. Thus, an experimental design with 5 factors at 3 levels and 3 central points (resulting in a total of 21 experimental runs) was constructed in order to study the influence of formulation and process parameters (independent variables) on the properties (dependent variables) of lyo-LCL-SIM, as shown in Table 1. The design was developed using Modde 11 Pro software (Umetrics, Sweden). The independent variables were selected based on risk analysis, and they were represented by 3 formulation factors, i.e. PEG proportion (X1), cholesterol concentration (X2), the cryoprotectant to phospholipids molar ratio (X3), and 2 process parameters, i.e. the number of extrusions through 100 nm polycarbonate membranes (X4) and the freezing conditions prior lyophilization (X5). The total phospholipids content and the initial simvastatin concentration were kept constant for all experiments at 70 mM and 18 mM, respectively, based on the results of a previous optimization study which showed best simvastatin encapsulation efficiency for this preparation formula (Porfire et al., 2015). The responses of the experimental design were the quality attributes of the lyophilized liposomes: liposomal size (Y1; nm), drug concentration in the freeze-dried product (Y2; µg/ml), the encapsulated simvastatin retention (Y3; %), the residual moisture content (Y4; %) and the change in the phospholipid’s transition temperature (Y5; °C). The matrix of experimental design is presented in Table 2.
Table 1.
Independent and dependent variables of experimental design evaluated for freeze-dried liposomes development.
| Variable | Level used |
||
|---|---|---|---|
| Low (−1) | Medium (0) | High (+1) | |
| Independent variables | |||
| X1 = PEG proportion (%) | 0 | 2.5 | 5 |
| X2 = Cholesterol concentration (mM) | 5 | 10 | 15 |
| X3 = The cryoprotectant to phospholipids molar ratio | 0 | 2.5 | 5 |
| X4 = The number of extrusions through 100 nm polycarbonate membranes | 1 | 2 | 3 |
| X5 = The freezing conditions prior lyophilization | −80 °C | −20 °C | None |
| Dependent variables (CQAs) | |||
| Y1 = Liposomal size (nm) | |||
| Y2 = Liposomal SIM concentration (µg/ml) Y3 = The encapsulated simvastatin retention (%) | |||
| Y4 = The residual moisture content (%) | |||
| Y5 = The change in the phospholipid’s transition temperature (°C) | |||
Table 2.
Matrix of experimental design.
| Formulation code | Run order | X1 (%) | X2 (mM) | X3(mM) | X4 | X5 |
|---|---|---|---|---|---|---|
| N1 | 16 | 0 | 5 | 0 | 1 | −80 °C |
| N2 | 8 | 0 | 10 | 2.5 | 1 | −20 °C |
| N3 | 7 | 0 | 15 | 5 | 1 | None |
| N4 | 20 | 2.5 | 5 | 0 | 1 | −20 °C |
| N5 | 9 | 2.5 | 10 | 2.5 | 1 | None |
| N6 | 11 | 2.5 | 15 | 5 | 1 | −80 °C |
| N7 | 15 | 5 | 5 | 2.5 | 1 | −80 °C |
| N8 | 14 | 5 | 10 | 5 | 1 | −20 °C |
| N9 | 2 | 5 | 15 | 0 | 1 | None |
| N10 | 19 | 0 | 5 | 5 | 3 | None |
| N11 | 6 | 0 | 10 | 0 | 3 | −80 °C |
| N12 | 13 | 0 | 15 | 2.5 | 3 | −20 °C |
| N13 | 12 | 2.5 | 5 | 2.5 | 3 | None |
| N14 | 4 | 2.5 | 10 | 5 | 3 | −80 °C |
| N15 | 18 | 2.5 | 15 | 0 | 3 | −20 °C |
| N16 | 1 | 5 | 5 | 5 | 3 | −20 °C |
| N17 | 5 | 5 | 10 | 0 | 3 | None |
| N18 | 17 | 5 | 15 | 2.5 | 3 | −80 °C |
| N19 | 10 | 2.5 | 10 | 2.5 | 2 | None |
| N20 | 21 | 2.5 | 10 | 2.5 | 2 | None |
| N21 | 3 | 2.5 | 10 | 2.5 | 2 | None |
X1, PEG proportion (%); X2, cholesterol concentration (mM); X3, the cryoprotectant to phospholipids molar ratio; X3, the number of extrusions through 100 nm polycarbonate membranes; X5, the freezing conditions prior lyophilization.
2.5.3. Data processing
The experimental data were fitted with the chosen experimental design using the statistical module from Modde 11 Pro software. The same software was used to calculate the statistical parameters. Data fitting and calculation of statistical parameters were performed by partial least squares (PLS) method.
The experimental design used in this study allowed fitting the data with a linear regression interaction model. The statistical parameters determined were R2, representing the explained variation, and Q2, the fraction of the variation of the response that can be predicted by the model. Moreover, the validity of the experimental design was checked by the analysis of variance (ANOVA) test.
3. Results
3.1. Liposomes preparation and characterization
All the formulations of SIM loaded long circulating liposomes were prepared by film hydration method, keeping the total phospholipid amount and the initial simvastatin concentration constant, while other formulation factors (PEG and cholesterol content) were varied according to the experimental design. Regarding the process parameters, the evaporation of organic solvent and the hydration steps were performed always in the same conditions (temperature, rotation speed, vacuum pressure), while the extrusion steps and the storage conditions before lyophilization were variable. Additionally, after preparation, trehalose was dissolved in the liposomal dispersion as lyoprotectant, in variable concentration.
The freshly prepared liposomes were characterized in terms of size, simvastatin concentration and phase transition temperature. These physico-chemical properties are presented in Table 3. As shown in this table, immediately after preparation the size of liposomal vesicles was between 125.3 and 173.7 nm, which falls in the desired size region for liposomes. In terms of simvastatin concentration, the values were between 270.7 and 3493.8 µg/ml, corresponding to an encapsulation efficiency in the range 3.4–46.2%. Regarding the phase transition temperature, the values were between 36.3 and 37.3 for all the formulations, several degrees below the transition temperature of the major phospholipid, DPPC (Leonenko et al., 2004).
Table 3.
The physico-chemical properties of freshly-prepared LCL-SIM.
| Formulation code | Run order | Size (nm) | SIM concentration (µg/ml) | Tm (°C) |
|---|---|---|---|---|
| N1 | 16 | 130.1 | 765.39 | 37.32 |
| N2 | 8 | 127.23 | 436.11 | 36.79 |
| N3 | 7 | 173.7 | 270.70 | 36.96 |
| N4 | 20 | 142.1 | 1258.57 | 40.95 |
| N5 | 9 | 173.7 | 606.82 | 37.51 |
| N6 | 11 | 152.1 | 356.82 | 37.24 |
| N7 | 15 | 147.2 | 1051.43 | 38.15 |
| N8 | 14 | 148.26 | 740.36 | 37.82 |
| N9 | 2 | 172.5 | 327.23 | 37.69 |
| N10 | 19 | 130.26 | 2411.45 | 36.15 |
| N11 | 6 | 126.03 | 1051.05 | 36.43 |
| N12 | 13 | 137.53 | 359.10 | 36.3 |
| N13 | 12 | 128.1 | 3493.77 | 37.49 |
| N14 | 4 | 127.9 | 1301.05 | 37.31 |
| N15 | 18 | 166.9 | 379.58 | 36.77 |
| N16 | 1 | 125.3 | 3290.81 | 37.26 |
| N17 | 5 | 157.2 | 1123.89 | 36.54 |
| N18 | 17 | 160.76 | 516.53 | 37.18 |
| N19 | 10 | 134.4 | 676.62 | 36.74 |
| N20 | 21 | 135.33 | 934.21 | 37.29 |
| N21 | 3 | 150.2 | 1137.17 | 35.15 |
3.2. CQAs of lyophilized liposomes and risk analysis
The current work is focused on the study of several CQAs of lyophilized liposomes, selected based on the results of previous studies and literature research. These CQAs were: the size, the encapsulated SIM concentration and retention, the Tm change and the residual moisture content. According to ICH Q8 definition, these properties should be within an appropriate limit, range, or distribution to ensure the desired product quality (ICH Q8 (R2), 2009). The results obtained at the physico-chemical characterization of lyophilized SIM-loaded LCL, in terms of selected CQAs, are presented in Table 4.
Table 4.
The physico-chemical properties of lyo-LCL-SIM (the matrix of responses of the experimental design).
| Formulation code | Run order | Size (nm) | SIM concentration (µg/ml) | ESR (%) | Tm change | Moisture content (%) |
|---|---|---|---|---|---|---|
| N1 | 16 | 242.4 | 343.62 | 44.89 | 1.34 | 2.67 |
| N2 | 8 | 269 | 177.84 | 40.78 | 0.65 | 4.13 |
| N3 | 7 | 233.7 | 129.96 | 48.01 | 0.74 | 5.4 |
| N4 | 20 | 218.4 | 510.16 | 40.54 | 4.71 | 4.77 |
| N5 | 9 | 245.8 | 431.26 | 71.07 | 1.56 | 4.27 |
| N6 | 11 | 499.3 | 168.17 | 47.13 | −1.92 | 5.07 |
| N7 | 15 | 182.4 | 434.86 | 41.36 | 1.34 | 4.19 |
| N8 | 14 | 204.4 | 300.75 | 40.62 | 1.66 | 5.8 |
| N9 | 2 | 1201.1 | 121.32 | 37.07 | −0.96 | 1.56 |
| N10 | 19 | 334.5 | 714.45 | 29.63 | 1.99 | 3.15 |
| N11 | 6 | 1088.2 | 341.05 | 32.45 | 1.13 | 2.24 |
| N12 | 13 | 821 | 138.96 | 38.7 | −2.12 | 3.56 |
| N13 | 12 | 380.9 | 795.44 | 22.77 | 3.6 | 6.53 |
| N14 | 4 | 213.9 | 432.2 | 33.22 | 0.84 | 4.17 |
| N15 | 18 | 811.9 | 161.72 | 42.6 | −2.22 | 3.12 |
| N16 | 1 | 163.4 | 994.04 | 30.21 | 1.78 | 5.2 |
| N17 | 5 | 1177 | 588.69 | 52.38 | 0.89 | 2.21 |
| N18 | 17 | 696.4 | 302.46 | 58.56 | −2.78 | 4.29 |
| N19 | 10 | 270 | 304.17 | 44.95 | 0.43 | 4.02 |
| N20 | 21 | 237 | 362.21 | 38.77 | 2.09 | 4.73 |
| N21 | 3 | 300 | 466.92 | 41.06 | 1.05 | 3.46 |
The desired size for liposomal vesicles is usually within the range of 20–200 nm, especially if an enhanced permeation and retention effect (EPR) is of interest, or if the final product has to be sterilized by filtration. The EPR is a passive accumulation of nanovesicles into tumors or inflamed area due to their extravasation through the endothelial lining of blood vessel wall and accumulation inside the interstitial space (Torchilin, 2000). A vesicles size within the mentioned range can easily be obtained by extruding the liposomal formulation through polycarbonate membranes with desired pore size, at a temperature above the Tm of the lipid. However, even if this is the case for freshly prepared liposomes, liposome particle size usually increases during lyophilization due to the fusion/aggregation of vesicles. Taking into account that vesicles size changes are related to the leak of the encapsulated solute, the size change has to be well controlled and prevented (Crowe and Crowe, 1988). The risk analysis for size showed that film composition, the size of the freshly prepared liposomes and the lyoprotectant concentration are the factors with significant influence on the size of lyophilized liposomes.
The encapsulated SIM concentration is important for both the manufacturer and the patients, because an increased drug concentration in the final formulation allows a greater flexibility in dosing, increased dosing intervals and reduced manufacturing costs. Even if a high concentration of encapsulated drug can be achieved by optimizing the encapsulation efficiency, drug retention inside liposomes during lyophlization is also a critical attribute, the leakage of encapsulated drug being one of the factors limiting the development of commercial liposome products. Thus, ESR is one of the most important CQAs of a lyophilized liposomal formulation, being considered as the most sensitive parameter reflecting the damage caused during lyophilization (Hays et al., 2003). In order to achieve an acceptable ESR after lyophilization, the liposome bilayer composition, the lyoprotectant concentration and freeze-drying protocol need to be optimized. Regarding the bilayer composition, the nature of the phospholipid and the cholesterol content are the most important parameters influencing the ESR. DPPC is probably the most used synthetic phospholipid, because determines increased stability and less drug leakage due to its relatively high Tm (about 41 °C) (Sulkowski et al., 2005). The presence of cholesterol in the membrane has usually a reducing effect on membrane fluidity, with positive effect on ESR, but it was observed that lyophilization with trehalose in the presence of cholesterol could alter the Tm of the phospholipid membrane (Ohtake et al., 2005). Modification of the liposomal surface by PEGylation may also impact on ESR, due to the potential interactions between the PEG moieties and cryoprotectant.
The Tm change between the hydrated and dehydrated state determines whether a phase transition occurred during lyophilization and rehydration (Chen et al., 2010). Thus, to estimate the quality of the lyophilized product, the phase transition has to be taken into consideration as CQA. The major risk factor for the Tm change during lyophilization is the presence and the nature of cryoprotectant. Thus, the cryoprotectant prevents the phase transition during lyophilization, this being very important because at the phase transition temperature, when the change from the gel phase to the liquid crystalline phase occurs, the encapsulated drug is released from the liposomes (Chen et al., 2010).
The residual moisture content is another indicator of the quality of the freeze-dried cakes. It is considered that the ESR is dependent on the residual moisture content during lyophilization, being higher with the reduction in the residual water (Van Winden and Crommelin, 1997). Moreover, a reduced moisture content is desired for improved stability. The most important factors influencing the residual moisture content, derived from the risk analysis, are the type and concentration of cryoprotectant and the lyophilization process parameters.
After performing the risk analysis for all the selected quality attributes, the following risk factors were chosen to be studied within the DoE approach: the liposomes bilayer composition (PEG proportion, cholesterol concentration); the cryoprotectant to phospholipids molar ratio; the number of extrusions through 100 nm polycarbonate membranes and the freezing conditions prior lyophilization.
3.3. The influence of various factors on the size of lyo-LCL-SIM
According to the data presented in Table 4, the size of lyo-LCL-SIM varied from 160 to 1200 nm. Thus, it can be concluded that aggregation/fusion of liposomal vesicles occurred during lyophilization, evidenced by up to ten times increase in liposomal size as compared to the values before lyophilization. A good correlation between the observed and predicted values was obtained for size, since R2 had a value of 0.802, and good predictive ability of the model, shown by Q2 value of 0.646. The results of ANOVA test (Table 5) showed a significant influence of the studied factors on liposomal size, since the p value for the regression was lower than 0.05, and that the model did not present a significant lack of fit (p = 0.108).
Table 5.
Analysis of variance for prediction of lyo-LCL-SIM CQAs.
| CQA | Source | Degrees of freedom | Sum of squares | Mean square | F value | P value |
|---|---|---|---|---|---|---|
| Liposomal size | Total corrected | 20 | 1.6488 | 0.08244 | ||
| Regression | 4 | 1.3252 | 0.3313 | 16.378 | <0.001 | |
| Residual | 16 | 0.3236 | 0.0202 | |||
| Lack of fit | 14 | 0.3184 | 0.0227 | 8.647 | 0.108 | |
| Pure error | 2 | 0.00526 | 0.00263 | |||
| Liposomal SIM concentration | Total corrected | 20 | 1,077,280 | 53863.80 | ||
| Regression | 6 | 969,918 | 161,653 | 21.080 | <0.001 | |
| Residual | 14 | 107,359 | 7668.47 | |||
| Lack of fit | 12 | 93751.80 | 7812.65 | 1.148 | 0.557 | |
| Pure error | 2 | 13606.80 | 6803.40 | |||
| ESR | Total corrected | 19 | 0.1660 | 0.00874 | ||
| Regression | 5 | 0.1255 | 0.0251 | 8.662 | 0.001 | |
| Residual | 14 | 0.0406 | 0.0029 | |||
| Lack of fit | 12 | 0.0385 | 0.0032 | 3.056 | 0.273 | |
| Pure error | 2 | 0.00209 | 0.00105 | |||
| Tm change | Total corrected | 20 | 70.325 | 3.516 | ||
| Regression | 5 | 59.393 | 11.878 | 16.299 | <0.001 | |
| Residual | 15 | 10.931 | 0.729 | |||
| Lack of fit | 13 | 9.524 | 0.733 | 1.041 | 0.592 | |
| Pure error | 2 | 1.407 | 0.704 | |||
| Residual moisture content | Total corrected | 19 | 24.958 | 1.314 | ||
| Regression | 5 | 18.072 | 3.614 | 7.348 | 0.001 | |
| Residual | 14 | 6.887 | 0.492 | |||
| Lack of fit | 12 | 6.076 | 0.506 | 1.250 | 0.528 | |
| Pure error | 2 | 0.810 | 0.405 | |||
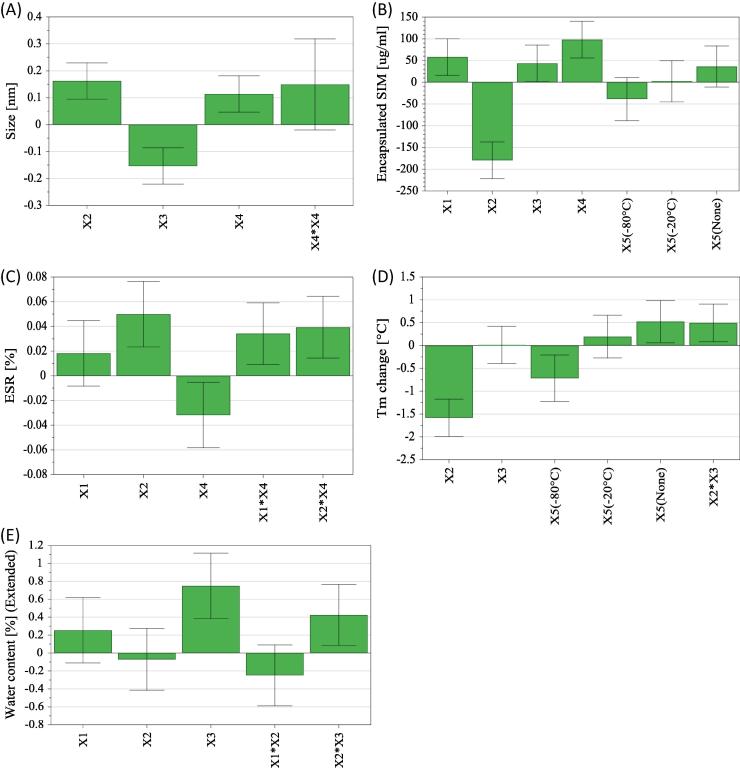
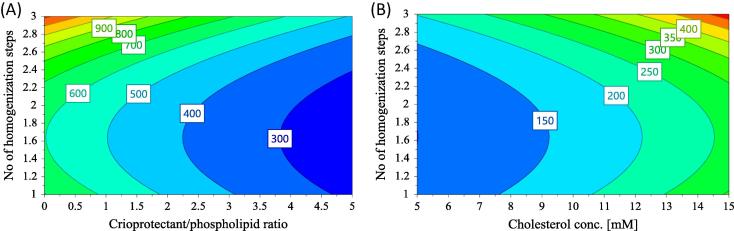
The coefficients of the equation describing the influence of formulation and process parameters on liposomal size are shown in Fig. 2(A). According to this figure, the size of liposomes after liophylization is significantly influenced by cholesterol concentration (X2), the concentration of cryoprotectant (X3) and the number of homogenization steps through 100 nm filter (X4). Thus, a significant increase of liposomal size is obtained if the cholesterol concentration and the number of homogenization steps increase, while the increase in the concentration of cryoprotectant results in the reduction of liposomal size. The reduction of vesicles size with the increase of cryoprotectant’s concentration can be explained by the physical stabilizing effect of trehalose, the ability to prevent vesicle’s aggregation being higher for higher concentration, in accordance with previously mentioned effect of cryoprotectants in maintaining the same particle distribution (EL-Nesr et al., 2010). Moreover, the presence of the quadratic term X4 × X4 in the regression equation, shows that the number of homogenization steps through 100 nm filter has a nonlinear influence on this response, though the influence is not always significant. This nonlinear influence of the number of homogenization steps over size is also illustrated by the contour plot for size (Fig. 3). According to the figure, if the liposomes are passed once through 100 nm filters, the size of the lyo-LCL-SIM decrease for increased cryoprotectant concentration and lower cholesterol content. For 2 or 3 passages through 100 nm filter, there is a marked increase in liposomal size with the number of passages, at low cryoprotectant/phospholipid ratio, but the size decreases with the number of passages for high cryoprotectant/phospholipid ratio. Actually, the nonlinear effect of the number of extrusions through 100 nm filter is related with the impact of this process parameter on the size of the freshly prepared liposomes, larger liposomes being reported to be more resistant to fusion and aggregation of vesicles than smaller ones (Ueno and Sriwongsitanont, 2005).
Fig. 2.
The scaled and centered coefficients of the regression equations describing the influence of formulation and process parameters over the critical quality attributes of lyophilized liposomes: size (A); liposomal SIM concentration (B); ESR (C); Tm change (D) and residual moisture content (E).
Fig. 3.
Contour plot for size (A) with respect to cryoprotectant/phospholipids ratio and the number of homogenization steps (PEG proportion is 4.7; cholesterol concentration is 14.2 and freezing is set at −80 °C); and (B) with respect to cholesterol concentration and the number of homogenization steps (PEG proportion is 4.7; cryoprotectant/phospholipids ratio is 5 and freezing is set at −80 °C).
3.4. The influence of various factors on liposomal SIM concentration
As shown in Table 4, liposomal SIM concentration ranged from 121 to 994 µg/ml, after lyophilization. Statistical analysis has shown a very good fitting of the data with the proposed model for prediction of liposomal SIM concentration, with R2 = 0.900 and Q2 = 0.759. Moreover, the ANOVA test evidenced a significant influence of the studied factors over this response, since p value for the regression was lower than 0.001, and that the model did not present a significant lack of fit (p = 0.557). The overall results of ANOVA for liposomal SIM concentration are presented in Table 5.
The influence of studied factors on liposomal SIM concentration is shown as coefficients of the regression equation plot in Fig. 2(B). According to the mentioned plot, PEG proportion (X1), the concentration of cryoprotectant (X3) and the number of homogenization steps through 100 nm filter (X4) had a significant, positive influence on liposomal SIM concentration. At the same time, cholesterol concentration (X2) had the biggest impact on liposomal SIM concentration, the influence of this factor being negative. Thus, the concentration of SIM in lipososmes will be lower at high cholesterol content, probably due to a reduction in the encapsulation efficiency of SIM in the presence of a high cholesterol content, as shown in a previous study (Porfire et al., 2015).
3.5. The influence of various factors on ESR
The ESR varied from 23 to 71%, as shown in Table 4. A good fitting of the data with the proposed regression model has been shown through the calculation of statistical parameters, since R2 was 0.756 and Q2 was 0.488. Additionally, the ANOVA test showed that this result is significantly influenced by the studied factors, since the p value for the regression model was 0.001 and the p value for the lack of fit was 0.273, as seen in Table 5.
Fig. 2(C) shows the coefficients of the regression equation for ESR, illustrating the influence of the factors on ESR. According to the figure, ESR is significantly influenced by cholesterol concentration (X2) and by the number of homogenization steps through 100 nm filter (X4). Moreover, there are significant interactions between the PEG content and the number of homogenization (X1 × X4) steps as well as between cholesterol content and the number of homogenization steps (X2 × X4). Thus, an increase in cholesterol concentration leads to an increase in ESR. Based on this effect, we can say that an increase in cholesterol content is not favorable for the encapsulation of SIM in liposomes, but, on the other hand, the presence of cholesterol prevents the leak of the encapsulated SIM during lyophilization, and increase the ESR. The PEG content has also a positive effect on ESR, but the influence is not statistically significant. Regarding the number of homogenization steps, a high number of passages through 100 nm filter determines the reduction of ESR, probably due to an increase of the fragility of liposomal membrane.
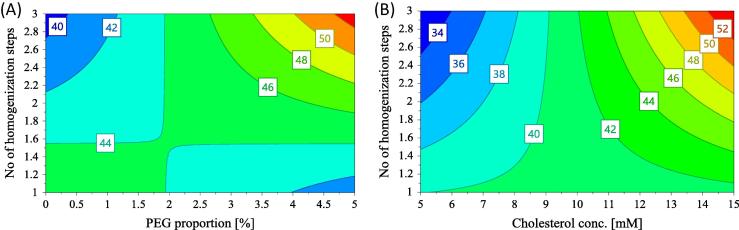
Regarding the interactions between factors with significant impact on ESR, they are illustrated by the contour plots for ESR in Fig. 4. As seen in Fig. 4(A), for PEG proportion lower than 2, the ESR decreases with the increase of the number of homogenization steps. On the contrary, for PEG proportion between 2 and 5, the ESR increases with the number of homogenization steps. In Fig. 4(B), showing the interaction between cholesterol concentration and the number of homogenization steps, we can see that the ESR is higher for low number of homogenization steps at low cholesterol content, and increases with the number of homogenization steps for cholesterol content higher than 1.
Fig. 4.
Contour plot for ESR (A) with respect to PEG proportion and the number of homogenization steps (cholesterol concentration is 14.2; cryoprotectant/phospholipids ratio is 5 and freezing is set at −80 °C) and (B) with respect to cholesterol concentration and the number of homogenization steps (PEG proportion is 4.7; cryoprotectant/phospholipids ratio is 5 and freezing is set at −80 °C).
3.6. The influence of various factors on Tm change
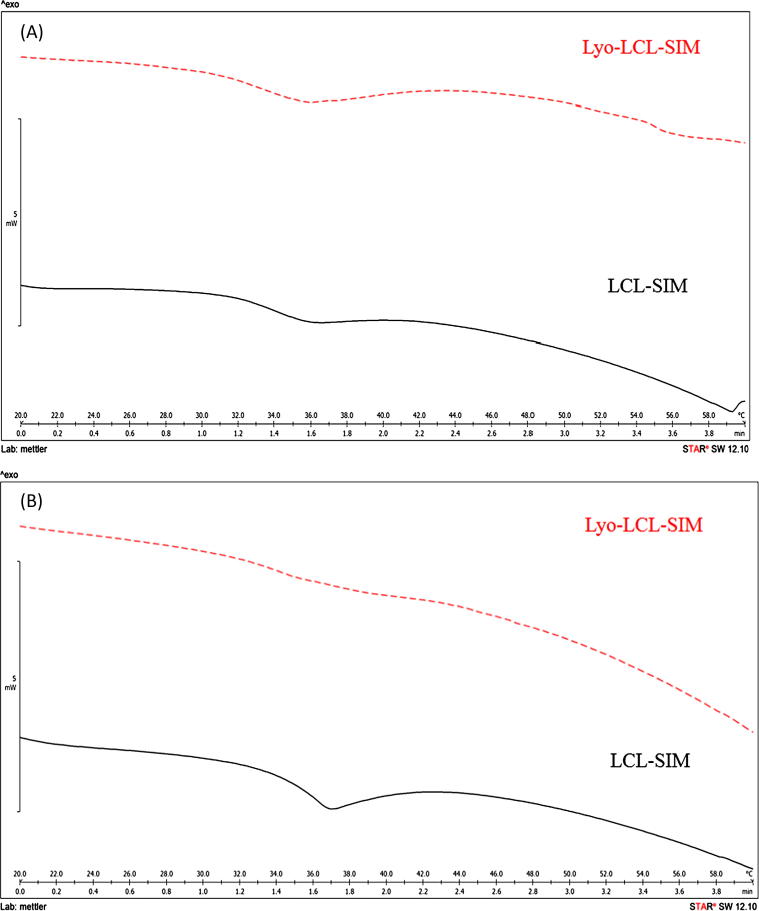
The Tm change calculated for the formulations in the experimental design was in the range between −2.8 and 4.7 °C, according with the results presented in Table 4, so both decrease as well as increase in Tm value occurred during lyophilization, depending on the formulation. Fig. 5 shows the DSC overlaid thermograms of fresh and reconstituted liposomal formulations, displaying the phase transition temperature occurring around 36 °C in all the cases, with almost no change in Tm value (A) or an increase of Tm value (B) between the fresh and rehydrated liposomes. It was interesting to note that, whatever the Tm change, the Tonset shifted to lower values for reconstituted liposomes compared with fresh liposomes. The proposed regression model performed a very good correlation between the observed and predicted values of Tm change, with correlation coefficient of 0.845, and good predictive ability of the model, reflected by Q2 value of 0.715. The results of ANOVA (Table 5) showed that the Tm change is significantly influenced by the studied factors, because the p value for the regression model is lower than 0.001 and the p value for the lack of fit is 0.592.
Fig. 5.
Differential scanning calorimetry thermograms of fresh (LCL-SIM) and reconstituted lyophilized liposomes (Lyo-LCL-SIM), showing minor Tm change (A) or an increase in Tm during lyophilization/rehydration (B).
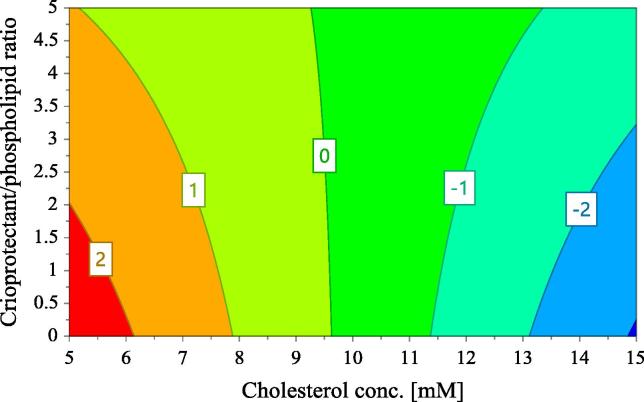
The influence of the evaluated factors on Tm change is expressed in terms of the coefficients of the regression equation in Fig. 2(D). As seen in this figure, the factors with significant influence on Tm change are cholesterol concentration (X2) and the freezing conditions prior lyophilization (X5). Thus, a decrease of Tm is obtained when cholesterol content is higher, and when the samples are kept at −80 °C before lyophilization, while drying of the samples immediately after preparation determines the increase of Tm. Besides the mentioned influence of factors, there is an interaction between cholesterol concentration (X2) and the cryoprotectant to phospholipids molar ratio (X3) with significant, positive influence on Tm change. This interaction is illustrated by the contour plot in Fig. 6. According to this plot, if cholesterol concentration is around 10 mM, the change of transition temperature during lyophilization is close to 0, whatever the concentration of trehalose is. If the concentration of cholesterol is between 5 and 10 mM, a decrease of Tm occurs during lyophilization, because the Tm change has positive values. Regarding the interaction between cholesterol concentration and cryoprotectant, higher cryoprotectant/phospholipid ratio are required for stability (lower Tm change) when the concentration of cholesterol decreases from 10 to 5 mM. On the other hand, if the concentration of cholesterol is higher than 10 mM, the Tm change has positive values, so the Tm decreases during lyophilization, this effect being in agreement with previous observation of other authors (Ohtake et al., 2005). Concerning the discussed interaction, the changes in Tm are higher when the cholesterol concentration is high and the trehalose concentration is low. An important consequence of this interaction is that we can determine, based on the cholesterol content of our formulation, the required cryoprotectant content, in order to prevent a phase transition during lyophilization, which is undesirable for stability.
Fig. 6.
Contour plot for Tm change with respect to cholesterol concentration and the cryoprotectant/phospholipids ratio (PEG proportion is 4.7; the number of homogenization steps is 2 and freezing is set at −80 °C).
3.7. The influence of various factors on residual moisture content
The residual moisture content of the samples prepared during the current study was between 1.5 and 6.5%, according to the calculations performed following the thermogravimetric study and shown in Table 4. Statistical analysis has shown a good fitting of the model proposed for prediction of residual moisture content, since R2 was 0.6266 and Q2 was 0.500. The ANOVA test (Table 5) showed that the residual moisture content is significantly influenced by the studied factors, because the p value for the regression model is 0.001 and the p value for the lack of fit is 0.528.
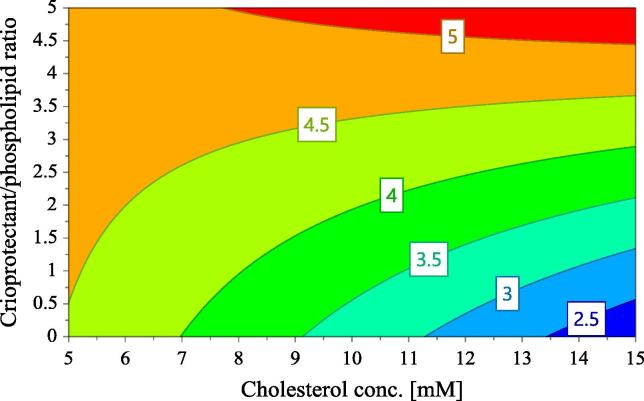
Fig. 2(E) shows the influence of the studied factors on residual moisture content. According to this figure, the moisture content of lyophilized liposomes increases significantly with the cryoprotectant/phospholipids ratio, probably due to the ability of anhydrous trehalose to absorb water molecules and to transform into a dihydrate. Additionally, the moisture content is positively influenced by the interaction between cholesterol concentration (X2) and the cryoprotectant to phospholipids molar ratio (X3), this interaction being shown by the contour plot in Fig. 7. According to the contour plot for residual moisture content, the water content of the lyophilisates is high for the formulations with 5 mM cholesterol content, whatever the cryoprotectant to phospholipid ratio. For higher cholesterol concentration, the residual moisture content increases in direct relationship with the increase of trehalose concentration.
Fig. 7.
Contour plot for the residual moisture content with respect to cholesterol concentration and the cryoprotectant/phospholipids ratio (PEG proportion is 4.7; the number of homogenization steps is 2 and freezing is set at −80 °C).
3.8. Establishing and evaluation of the design space
The design space for lyo-LCL-SIM was constructed using the factors which significantly influenced the CQAs of the product evaluated in this study. Among all the studied factors, cholesterol concentration has been found to have significant influence on all the responses, except the residual moisture content. Thus, cholesterol concentration (X2) had positive influence on size and ESR and negative influence on SIM concentration and Tm change. In other words, cholesterol had a stabilizing role in the formulation, confirmed by the increase of ESR and the decrease of Tm change with increased concentration of cholesterol, although the size increase promoted by cholesterol is not desirable. The other factors with important influence on CQAs were PEG proportion (X1), the cryoprotectant to phospholipids molar ratio (X3) and the number of extrusions through 100 nm polycarbonate membranes (X4). According to the results presented above, the increase of PEG proportion increased liposomal SIM concentration, ESR and the residual moisture content. On the other hand, the increase of cryoprotectant to phospholipids molar ratio decreased the size (prevented the aggregation of vesicles), increased SIM concentration but also increased the moisture content of the lyophilizates. Among the selected process parameters, the number of extrusions through 100 nm polycarbonate membranes was the most significant, and exerted a positive influence on size and SIM concentration but decreased the ESR.
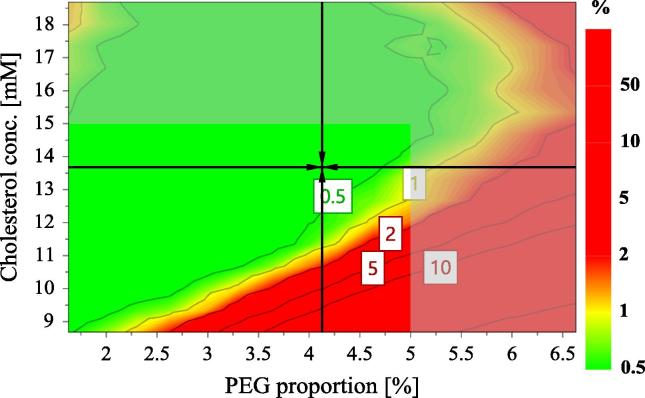
The design space for lyo-LCL-SIM was determined by the use of Design Space Explorer option from the optimization module of Modde 11 Pro software. Thus, the design space is the green region shown in Fig. 8, and shows the combination of factors for which the LCL-SIM formulations will meet the specifications in terms of CQAs (Table 6), with a probability of failure less than 1%. The combination inside the design space which is pointed out by the black arrows indicates the robust setpoint, corresponding to the formulation for which the prediction errors are the lowest.
Fig. 8.
The design space for lyo-LCL-SIM that meet the specifications in terms of CQAs, expressed as the probability of failure (%).
Table 6.
The desired CQAs of lyo-LCL-SIM.
| Response | Criterion | Min | Target | Max | Pred. min | Pred. max |
|---|---|---|---|---|---|---|
| Size (nm) | Minimize | 200 | 800 | 104.87 | 683.644 | |
| Liposomal SIM concentration (µg/ml) | Excluded | 20.22 | 790.79 | |||
| ESR (%) | Maximize | 40 | 45 | 32.88 | 47.69 | |
| Tm change (C) | Minimize | −2 | 2 | −3.08 | 4.13 | |
| Water content (%) | Target | 0 | 3 | 4 | 2.13 | 5.49 |
In order to confirm the validity of the design space, the formulation corresponding to the robust setpoint (13.7 mM cholesterol concentration; 4.13% PEG; 0.92 cryoprotectant to phospholipid molar ratio; 2 homogenizations through 100 nm polycarbonate membranes; freezing at −80 °C before lyophilization) was prepared in triplicate and the CQAs were determined, the practical values being compared with the theoretical ones, predicted by the model. As shown in Table 7, the actual CQAs were always inside the prediction interval of the model for each response, thus confirming that the design space was accurately defined.
Table 7.
The predicted and determined CQAs of the formulation corresponding to the robust setpoint inside the design space for lyo-LCL-SIM.
| Response | Predicted | Min | Max | Actual |
|---|---|---|---|---|
| Size (nm) | 514.73 | 328.8 | 805.75 | 710.03 |
| Liposomal SIM concentration (µg/ml) | 271.72 | 165.4 | 377.97 | 288.9 |
| ESR (%) | 44.90 | 40.55 | 49.74 | 42.23 |
| Tm change (C) | −0.72 | −1.65 | 0.21 | −1.18 |
| Water content (%) | 2.87 | 2.16 | 3.58 | 3.15 |
4. Conclusions
The current work brings an important innovative contribution in the field of lyophilized liposomes, through the successful application of the QbD approach in the development of lyo-LCL-SIM. In this manner, the influence of both formulation and process parameters on the CQAs of lyo-LCL-SIM was determined through the use of DoE. Thus, among the formulation factors, the cholesterol content had the most significant influence on the CQAs of lyo-LCL-SIM, while the number of extrusions through polycarbonate membranes was the most important process parameter for the quality of the final product. This modern, scientifically based approach of pharmaceutical development, enabled us to determine the design space for lyo-LCL-SIM, in which the established quality requirements of the product are met, provided that the risk factors vary within the established limits. We could conclude that QbD is a useful, time-effective strategy for the development of lyophilized liposome's having controlled, predictable quality.
Acknowledgement
This work was supported by Iuliu Hatieganu University of Medicine and Pharmacy Cluj-Napoca through project no. 1495/4/28.01.2014.
Footnotes
Peer review under responsibility of King Saud University.
References
- Afzali M., Vatankhah M., Ostad S.N. Investigation of simvastatin-induced apoptosis and cell cycle arrest in cancer stem cells of MCF-7. J. Cancer Res. Ther. 2016;12(2):725–730. doi: 10.4103/0973-1482.146127. [DOI] [PubMed] [Google Scholar]
- Campbell M.J., Esserman L.J., Zhou Y., Shoemaker M., Lobo M. Breast cancer growth prevention by statins. Cancer Res. 2006;66:8707–8714. doi: 10.1158/0008-5472.CAN-05-4061. [DOI] [PubMed] [Google Scholar]
- Cardwell C.R., Mc Menamin U., Hughes C.M., Murray L.J. Statin use and survival from lung cancer: a population-based cohort study. Cancer Epidemiol. Biomarkers Prev. 2015;24(5):833–841. doi: 10.1158/1055-9965.EPI-15-0052. [DOI] [PubMed] [Google Scholar]
- Chaudhury A., Das S., Lee R.F.S., Tan K.B., Ng W.K. Lyophilization of cholesterol-free PEGylated liposomes and its impact on drug loading by passive equilibration. Int. J. Pharm. 2012;430:167–175. doi: 10.1016/j.ijpharm.2012.04.036. [DOI] [PubMed] [Google Scholar]
- Chen C., Han D., Cai C., Tang X. An overview of liposome lyophilization and its future potential. J. Contr. Rel. 2010;142:299–311. doi: 10.1016/j.jconrel.2009.10.024. [DOI] [PubMed] [Google Scholar]
- Cho S.J., Kim J.S., Kim J.M., Lee J.Y., Jung H.C. Simvastatin induces apoptosis in human colon cancer cells and in tumor xenografts, and attenuates colitis-associated colon cancer in mice. Int. J. Cancer. 2008;123:951–957. doi: 10.1002/ijc.23593. [DOI] [PubMed] [Google Scholar]
- Crowe J.H., Crowe L.M. Factors affecting the stability of dry liposomes. Biochim. Biophys. Acta. 1988;939:327–334. doi: 10.1016/0005-2736(88)90077-6. [DOI] [PubMed] [Google Scholar]
- EL-Nesr O.H., Yahiya S.A., EL-Gazayerly O.N. Effect of formulation design and freeze-drying on properties of fluconazole multilamellar liposomes. Saudi Pharm. J. 2010;18:217–224. doi: 10.1016/j.jsps.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y., Zhang Q. Development of liposomal formulations: from concept to clinical investigations. Asian J. Pharm. Sci. 2013;8(2):81–87. [Google Scholar]
- Guan T., Miao Y., Xu L., Yang S., Wang J. Injectable nimodipine-loaded nanoliposomes: preparation, lyophilization and characteristics. Int. J. Pharm. 2011;410(1–2):180–187. doi: 10.1016/j.ijpharm.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Hays L.M., Crowe J.H., Wolkers W., Rudenko S. Factors affecting leakage of trapped solutes from phospholipid vesicles during thermotropic phase transitions. Cryobiology. 2003;42:88–102. doi: 10.1006/cryo.2001.2307. [DOI] [PubMed] [Google Scholar]
- ICH Q9, 2005. Quality risk management, 2005 [cited 2016 Oct 10]. Available from: <http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q9/Step4/Q9_Guideline.pdf>.
- ICH Q8 (R2), 2009. Pharmaceutical development, 2009 [cited 2016 Oct 10]. Available from: <http://www.fda.gov/downloads/Drugs/Guidances/ucm073507.pdf>.
- Immordino M.L., Dosio F., Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006;1(3):297–315. [PMC free article] [PubMed] [Google Scholar]
- Kochuparambil S.T., Al-Husein B., Goc A., Soliman S., Somanath P.R. Anticancer efficacy of simvastatin on prostate cancer cells and tumor xenografts is associated with inhibition of Akt and reduced prostate-specific antigen expression. J. Pharmacol. Exp. Ther. 2011;336:496–505. doi: 10.1124/jpet.110.174870. [DOI] [PubMed] [Google Scholar]
- Leonenko Z.V., Finot E., Ma H., Dahms T.E.S., Cramb D.T. Investigation of temperature-induced phase transitions in DOPC and DPPC phospholipid bilayers using temperature-controlled scanning force microscopy. Biophys. J. 2004;86(6):3783–3793. doi: 10.1529/biophysj.103.036681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.C., Hao W.R., Hsu Y.P., Sung L.C., Kao P.F. Statins dose-dependently exert a significant chemopreventive effect on colon cancer in patients with chronic obstructive pulmonary disease: a population-based cohort study. Oncotarget. 2016;7(40):65270–65283. doi: 10.18632/oncotarget.11263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtake S., Schebor C., Palecek S.P., de Pablo J.J. Phase behavior of freeze-dried phospholipid-cholesterol mixtures stabilized with trehalose. Biochim. Biophys. Acta. 2005;1713:57–64. doi: 10.1016/j.bbamem.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Patel G.M., Shelat P.K., Lalwani A.N. QbD based development of proliposome lopinavir for improved oral bioavailability. Eur. J. Pharm. Biopharm. 2016;80:347–357. doi: 10.1016/j.ejps.2016.08.057. [DOI] [PubMed] [Google Scholar]
- Porfire A., Tomuta I., Muntean D., Luca L., Licarete E. Optimizing long-circulating liposomes for delivery of simvastatin to C26 colon carcinoma cells. J. Lipos. Res. 2015;25(4):261–269. doi: 10.3109/08982104.2014.987787. [DOI] [PubMed] [Google Scholar]
- Pramod K., Tahir M.A., Charoo N.A., Ansari S.H., Ali J. Pharmaceutical product development: a quality by design approach. Int. J. Pharm. Investig. 2016;6(3):129–138. doi: 10.4103/2230-973X.187350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone N.J., Robinson J.G., Lichtenstein A.H., Bairey Merz C.N., Blum C.B. ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014;63:288–934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Sulkowski W.W., Pentak D., Nowak K., Sulkowska A. The influence of temperature, cholesterol content and pH on liposome stability. J. Mol. Struct. 2005;744:737–747. [Google Scholar]
- Thibault A., Samid D., Tompkins A.C., Figg W.D., Cooper M.R. Phase I study of lovastatin, an inhibitor of the mevalonate pathway, in patients with cancer. Clin. Cancer Res. 1996;2:483–491. [PubMed] [Google Scholar]
- Torchilin V.P. Drug targeting. Eur. J. Pharm. Sci. 2000;11(Suppl 2):S81–91. doi: 10.1016/s0928-0987(00)00166-4. [DOI] [PubMed] [Google Scholar]
- Ueno M., Sriwongsitanont S. Effect of PEG lipid on fusion and fission of phospholipid vesicles in the process of freeze-thawing. Polymer. 2005;46:1257–1267. doi: 10.1248/cpb.52.641. [DOI] [PubMed] [Google Scholar]
- Van Winden E.C., Crommelin D.J. Long term stability of freeze-dried, lyoprotected doxorubicin liposomes. Eur. J. Pharm. Biopharm. 1997;43:295–307. [Google Scholar]
- Xu X., Costa A.P., Khan M.A., Burgess D.J. Application of quality by design to formulation and processing of protein liposomes. Int. J. Pharm. 2012;434(1–2):349–359. doi: 10.1016/j.ijpharm.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Yu L.X. Pharmaceutical quality by design: product and process development, understanding, and control. Pharm. Res. 2008;25(4):781–791. doi: 10.1007/s11095-007-9511-1. [DOI] [PubMed] [Google Scholar]