Abstract
Advanced head and neck squamous cell carcinoma (HNSCC) remains a therapeutic challenge due to the development of therapy resistance. Several studies have implicated the development of cancer stem cells as a possible mechanism for therapy resistance in HNSCC. Heat shock protein 90’s (Hsp90’s) molecular chaperone function is implicated in pathways of resistance in HNSCC. Therefore, in the present study, we investigated the efficacy of novel C-terminal Hsp90 inhibitors (KU711 and KU757) in targeting HNSCC cancer stem cells (CSCs). Treatment of HNSCC human cell lines MDA1986, UMSCC 22B, and UMSCC 22B cisplatin-resistant cells with the KU compounds indicated complete blockage of self-renewal for the resistant and parent cell lines starting from 20 μM KU711 and 1 μM KU757. Dose-dependent decrease in the cancer stem cell markers CD44, ALDH, and CD44/ALDH double-positive cells was observed for all cell lines after treatment with KU711 and KU757. When cells were treated with either drug, migration and invasion were downregulated greater than 90% even at the lowest concentrations of 20 μM KU711 and 1 μM KU757. Western blot showed >90% reduction in client protein “stemness” marker BMI-1 and mesenchymal marker vimentin, as well as increase in epithelial marker E-cadherin for both cell lines, indicating epithelial to mesenchymal transition quiescence. Several CSC-mediated miRNAs that play a critical role in HNSCC therapy resistance were also downregulated with KU treatment. In vivo, KU compounds were effective in decreasing tumor growth with no observed toxicity. Taken together, these results indicate that KU compounds are effective therapeutics for targeting HNSCC CSCs.
Abbreviations: ALDH, aldehyde dehydrogenase; BMI1, B lymphoma Mo-MLV insertion region 1 homolog; CSC, cancer stem cell; EMT, epithelial to mesenchymal transition; HNSCC, head and neck squamous cell carcinoma; HSP, heat shock protein (Hsp90, Hsp70); KU, Kansas University (compounds KU757, KU711); SCC, squamous cell carcinoma; UMSCC, University of Michigan Squamous Cell Carcinoma (cell lines UMSCC 22, UMSCC 22B-cis); WB, Western blot
Introduction
Despite the introduction of newer therapeutic protocols, mortality rates associated with head and neck squamous cell carcinoma (HNSCC) have remained largely unchanged over the last four decades [1]. Concurrent radiation and chemotherapy have become the standard for adjuvant therapy after surgical ablation, as well as the definitive treatment of HNSCC in select cases. Systemic platinum-based chemotherapy, namely, cisplatin, remains a first-line agent due to its radiosensitizing and cytotoxic effects [2], [3]. Unfortunately, chemotherapy and radiation-based treatments have been associated with significant toxicity, particularly in patients receiving concurrent chemoradiotherapy [4]. HNSCC has shown marked resistance to radiation and cisplatin in many cases, and treatment resistance requiring dose escalation and resultant toxicities continues to be problematic, highlighting a need for the development of novel therapies that effectively treat this disease and its cisplatin resistance [5], [6].
Cancer stem cells (CSCs) represent a subpopulation of cells within a tumor that have the ability of self-renewal and regeneration. Recent literature suggests that this population of cells is thought to significantly contribute to tumor proliferation, invasion, metastatic potential, and resistance to drug therapy [7], [8], [9], [10], [11]. This subpopulation may lie quiescent for periods of time as well as harbor protective mechanisms against cellular damage, and is felt to be responsible for the majority of tumor growth and metastatic potential in HNSCC [11], [12]. Furthermore, CSCs have been shown to contribute to resistance against chemotherapeutic agents, including platinum-based regimens along with external beam radiation [5], [13]. Nor et al. using an HNSCC xenograft model observed that cisplatin treatment increased the fraction of CSCs as defined by the ALDHhigh/CD44high populations, again implicating this cellular subpopulation in resistance to current standard-of-care therapies [14]. Thus, it seems to reason that in order to more effectively treat or abrogate chemo- and drug resistance in HNSCC, this process should involve some level of targeting of the CSC population.
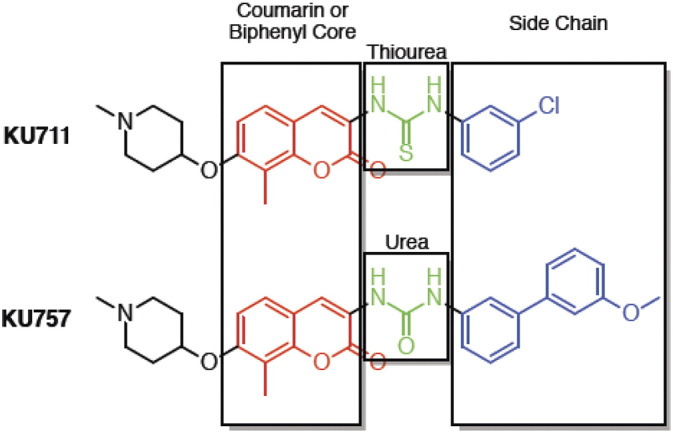
Heat shock protein (Hsp) 90 is a molecular chaperone protein that regulates several “client” proteins involved in cancer development, including proteins involved in pathways critical for cell growth, invasiveness, and survival [15]. Numerous proteins implicated as critical for CSCs’ development are also dependent on Hsp90. This suggests a high therapeutic potential for Hsp90 inhibitors as they can simultaneously suppress multiple oncogenic pathways involving the bulk tumor cell population of a cancer as well as its CSCs. Use of first- and second-generation Hsp90 inhibitors targeting the N-terminal domain of the chaperone was restricted due to dose-limiting toxicity, resulting mainly from activation of the heat shock response leading to induction of compensatory proteins (e.g., Hsp70) with prosurvival effects [16]. Thus, early-generation N-terminal Hsp90 inhibitors have not progressed beyond early-phase clinical trials despite showing potent anticancer effects. To address limitations of N-terminal Hsp90 inhibitors, our group has developed potent, novel Hsp90 inhibitors targeting the carboxy terminus of the chaperone which blocks Hsp90 chaperone function without concurrently upregulating Hsp70 and its prosurvival effects, thus avoiding this key limitation of N-terminal inhibitors [17], [18], [19], [20], [21]. These compounds have potential to act synergistically with current standard-of-care therapies and prolong or prevent development of drug resistance [16], [22]. Hence, we hypothesized that C-terminal Hsp90 inhibitors (especially our lead compounds KU711 and KU757, chosen for their potency and selectivity for cancer cells; structures in Supplemental Figure 1) can inhibit key CSC functions including migration, invasion, self-renewal, and epithelial to mesenchymal transition (EMT); can target the miRNAs involved in CSC function; and can reduce tumor growth of HNSCC xenografts.
Supplemental Figure 1.
Materials and Methods
Cell Culture
The validated HNSCC cell line UMSCC 22B was generated at the Michigan Otolaryngology and Translational Oncology Laboratory and graciously donated by Dr. Thomas Carey. UMSCC 22B-cis, a kind gift from Dr. Jacques Nor, University of Michigan, Ann Arbor, is a cisplatin-resistant line generated by co-culturing UMSCC 22B with increasing concentrations of cisplatin in vitro up to 12 μM concentration, as previously described [14]. The validated HNSCC cell line MDA-1986 was graciously donated by Dr. Jeffrey Myers (University of Texas, M.D. Anderson Cancer Center, Houston, TX). Cells were grown and maintained in Dulbecco's modified Eagle's medium (Sigma-Aldrich, St. Louis, MO) supplemented with 10% fetal bovine serum (Sigma-Aldrich) and 1% combination of penicillin and streptomycin (Sigma-Aldrich) in a 37°C humidified atmosphere of 5% CO2 in air. Drug compounds used in these experiments included two C-terminal Hsp90 inhibitors, KU711 and KU757, and these were obtained from Dr. Brian S. J. Blagg (University of Notre dame, Indiana, IN). Finally, a standard N-terminal Hsp90 inhibitor, 17-AAG, was obtained from Sigma-Aldrich.
Orosphere Formation Assay
MDA-1986, UMSCC 22B, and UMSCC 22B-cis cells were plated at 100 cells/well in a 96-well ultralow attachment plate (Corning, Corning, NY) in low-glucose DMEM (Life Technologies) with varying concentrations of KU711, KU757, 17-AAG, and cisplatin [up to five times their half-maximal inhibitory concentration (IC50) values[. Cells were cultured for 10 to 14 days, and orosphere formation (>25 cells) was assessed in each well using light microscopy.
ALDEFLOUR Assay/Flow Cytometry
UMSCC 22B, UMSCC 22B-cis, and MDA-1986 cells were treated and collected as described for Western blot analysis and evaluated for ALDH activity using the ALDEFLOUR assay kit as per the manufacturer's instructions (Stem Cell Technologies, Vancouver, BC, Canada). CD44-positive populations were detected by flow cytometry using APC conjugated CD44. Flow cytometric analysis was conducted on a CyAn ADP analyzer (Beckman Coulter, Brea, CA). DAPI staining was used to exclude dead cells from analysis.
Western Blot/EMT
Cells were grown to 60% to 80% confluence and treated for 24 hours at 20 to 40 μM KU711 or 1 to 2.5 μM KU757 (1-2× and 1-2.5× IC50), 1 μM 17-AAG, or 2 μM cisplatin. Treated cells were collected and resuspended in lysis buffer, and protein concentrations were determined using the BSA protein assay (Thermo Fisher Scientific, Waltham, MA). Immunoblotting was performed by methodology published previously [23]. BMI-1, E-cadherin, and vimentin antibodies were purchased from Cell signaling Technology (CST, Danvers, MA), β-actin from Millipore (EMD Millipore, Billerica, MA), and donkey anti-rabbit IgG HRP (1: 3000) and goat anti-mouse IgG HRP (1: 3000) secondary antibodies from Santa-Cruz. Membranes were developed with either SuperSignal West PICO or FEMTO (Thermo Fisher Scientific, Waltham, MA) for 5 minutes and visualized by enhanced chemiluminescence and captured on autoradiography film (Molecular Technologies, St. Lewis, MO) on a Konica Minolta SRX 101A developer (Ramsey, NJ). Actin levels were assessed as a housekeeping gene to ensure equal loading and transfer of proteins. Studies were replicated for accuracy.
Migration and Invasion Assay
UMSCC 22B and UMSCC 22B-cis cell lines were collected and resuspended in serum-free DMEM with penicillin/streptomycin and 20 to 40 μM KU711 or 1 to 2.5 μM KU757 or 1 μM 17-AAG or 2 μM cisplatin. Equal numbers of cells were plated onto either standard or Matrigel-coated upper wells of the 8-μm polycarbonate Boyden chambers (Corning) with DMEM supplemented with 10% fetal bovine serum as lower wells’ chemoattractant. Chambers were incubated for 24 hours at 37°C; migrated cells were fixed in 2% paraformaldehyde and stained with 1% crystal violet in 20% methanol for 20 minutes. After staining, membranes were washed in water, and the remaining upper-well cells were removed using a cotton swab. Migration using standard chambers and invasion with Matrigel-coated chambers were quantified using light microscopy as number of cells per high-powered field.
miRNA Analysis
UMSCC 22B and 22B cisplatin cells were treated with either KU711 at 20 μM or KU757 at 1 μM for 24 hours, and RNA was isolated using Qiagen miRNeasy kit as per the manufacturer's protocol. The RNA was quantified using NanoDrop, and approximately 500 ng of the RNA was reverse transcribed using the miScript II RT Kit. The cDNA was used as a template in real-time PCR with a human miScript miRNA cancer stem cell PCR Array and the miScript SYBR Green PCR Kit (Qiagen). The data were normalized to global CT mean of expressed miRNA, and the fold difference of the target genes’ expression compared to the control group was calculated by the 2−ΔΔCT method.
HNSCC Xenograft and Immunohistochemistry
In vivo efficacy of the Ku compounds KU711 and KU757 was assessed using a murine buccal tumor model. The buccal mucosa was injected with 1 × 106 cells, and tumor treatment was initiated when the tumors reached the size of approximately 4 mm2 in diameter; the mice were randomized into control, KU711, KU757, 17-AAG, and cisplatin groups. Mice were dosed intraperitoneally with 5 mg/kg of KU757 and 50 mg/kg of 17-AAG twice weekly and with KU711 and cisplatin at 5 mg/kg every day for 3 weeks. Tumor burden was measured using caliper measurement of greatest tumor dimension at twice-per-week intervals. All procedures were performed in accordance with the University Committee for the Use and Care of Animals–approved protocol.
Histologic evaluation was performed by a pathologist. To reduce bias, each group was blinded and randomly assigned with a group number (1-5). Signs suggestive of toxicity, such as chronic inflammation (defined by the presence of lymphocytes and plasma cells), cellular necrosis, and steatohepatitis, were assessed in the liver and kidney. “Normal” was assigned to specimens with essentially no pathology except for a small degree of inflammation comparable to what is seen physiologically. “Mild, moderate, or severe” was assigned to specimens with a less than 10%, 10% to 50%, and greater than 50% increase in inflammatory cells compared to the physiologic state, respectively. Percent proliferation was determined via positive nuclear staining by immunohistochemical stain Ki67. Ki67-positive neoplastic cells and Ki67-negative cells were counted in five representative high-power microscopic fields in each specimen, and the percent positivity was calculated for each high-power field. The final percent was calculated by taking the average of the five values.
Statistical Analysis
Significance (set as 95%, P < .05) between treatment groups in flow cytometry, orosphere assay, and Boyden chamber assays was identified using Student’s 2-tailed, unpaired t test. Migration and invasion data were proportionally adjusted for treatment-related cell death using corresponding viability data from DAPI exclusion by flow cytometry. Each experiment was done in triplicate and was replicated to ensure accuracy. The data values are presented as mean ± standard deviation.
Results
Hsp90 Inhibitors Block Self-Renewal
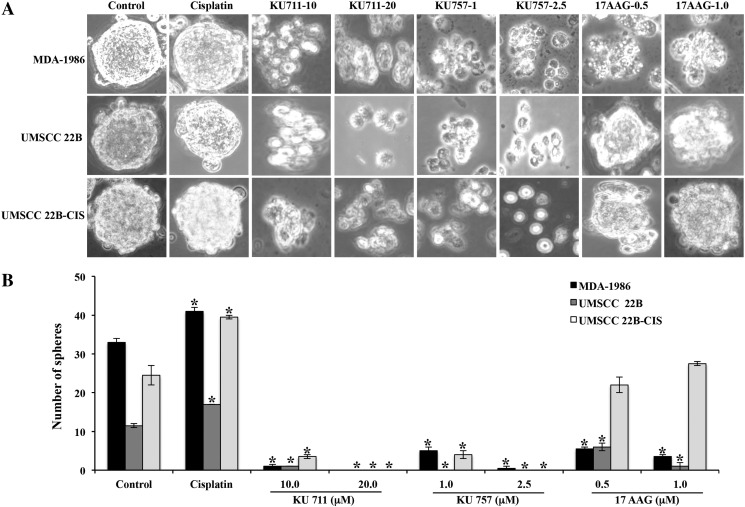
Self-renewal of tumors, a function attributed to CSCs, was analyzed by orosphere formation assay (Figure 1, A and B). MDA1986, UMSCC 22B, and UMSCC 22B-cis experienced inhibition of orosphere formation after treatment with both C-terminal Hsp90 inhibitors. Significant inhibition was observed even with the starting concentrations of 10 μM KU711 and 1 μM KU757 (P < .001 vs control), and complete blockage of orosphere formation was seen at concentrations of 20 μM KU711 and 2.5 μM KU757. Orosphere formation was increased when both the parent and cisplatin-resistant UMSCC 22B cell lines were treated with cisplatin, relative to control (P < .001) (Figure 1B). Similar trends were observed when the same agents were used to treat the oral cavity squamous cell carcinoma cell line MDA-1986 (P < .001) (Figure 1, A and B). N-terminal Hsp90 inhibitor 17-AAG treatment resulted in inhibition of orosphere formation to a lesser extent in lines MDA-1986 and UMSCC 22B, and inhibition was not statistically significant in UMSCC 22B-cis cells (Figure 1B).
Figure 1.
(A and B) Orosphere formation assay. HNSCC cells were treated with varying concentrations of Hsp90 inhibitors in an ultralow attachment plates. Formation of spheres was counted by light microscopy, and representative sphere images are presented.
Hsp90 Inhibitors Target Cancer Stem Cell Markers
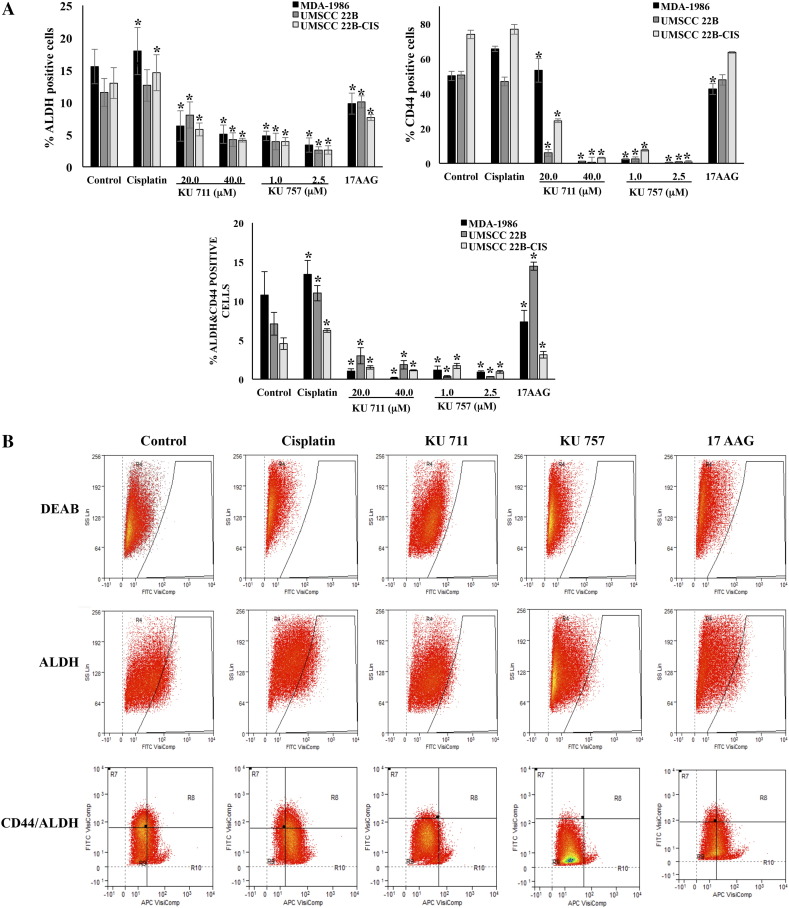
HNSCC cells from each cell line were evaluated for CSC markers using CD44 expression and ALDH activity. ALDH activity was assessed by ALDEFLOUR assay. Cell lines UMSCC 22B, UMSCC 22B-cis, and MDA-1986 were treated with two C-terminal Hsp90 inhibitors, KU711 and KU757, as well as one N-terminal Hsp90 inhibitor, 17-AAG. As seen from Figure 2, A and B, a dose-dependent decrease in ALDH+ cells, CD44+ cells, and double-positive (ALDH+/CD44+) cells was observed for all cell lines treated with 20 to 40 μM KU711 and 1.0 to 2.5 μM KU757 (P < .001 relative to control), indicating decreased CSC cell populations following treatment. In contrast, each of these cell populations was increased or unchanged when treated with cisplatin alone (Figure 2, A and B), in agreement with previously described findings by Nor et al. [14]. Finally, ALDH+, CD44+, double-positive population changes relative to control were variable when cells were treated with 17-AAG. Even though 17-AAG treatment decreased the ALDH-positive population, the levels of CD44-positive or double-positive cells did not show significant difference compared to control cells (Figure 2, A and B).
Figure 2.
(A) Analysis of cancer stem cell markers CD44 and ALDH after treatment of HNSCC cells with HSp90 inhibitors KU711, KU757, and 17-AAG for 24 hours by flow cytometry analysis. (B) Representative flow cytometry data.
BMI1, a known marker of “stemness,” was evaluated for inhibition by Hsp90 inhibitors since it is known to be a client protein of Hsp90 chaperone function (Figure 4). In MDA-1986 cells treated with KU711 and KU757, BMI1 expression was decreased by up to 65% and 31%, respectively. BMI1 concentration decreased by 58% when treated with 1 μM 17-AAG. Finally, cisplatin treatment resulted in slight increase in BMI1 expression relative to control (consistent with what has been reported by Nor et al.) [14].
Figure 4.
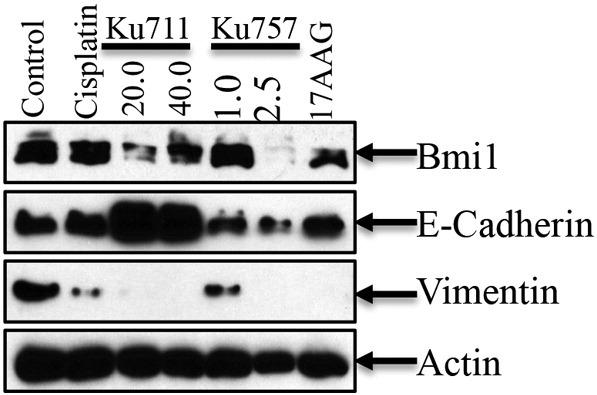
EMT and changes in the levels of BMI-1 were evaluated by immunoblot analysis. Actin was used as a loading control.
Hsp90 Inhibitors Decrease Migration, Invasion, and EMT
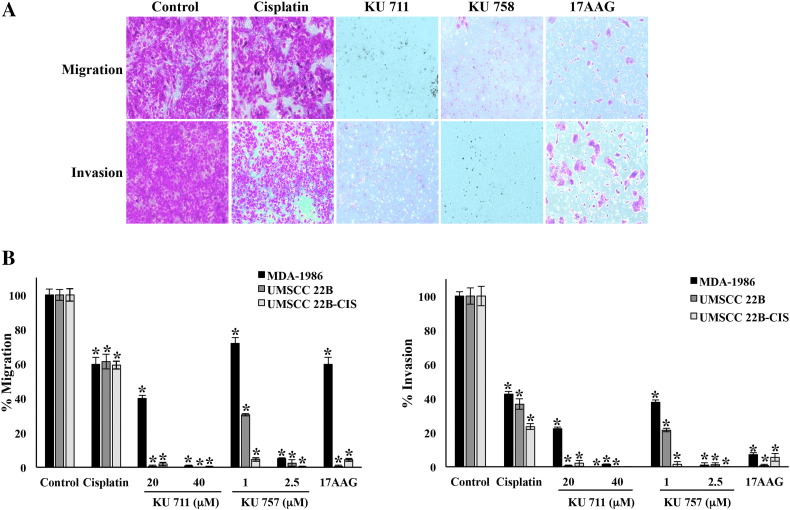
To examine whether inhibition of this HNSCC CSC population can lead to decreases in invasive potential, we next analyzed migration and invasion by Boyden chamber assay. Additionally, we evaluated EMT transition by Western blotting for epithelial and mesenchymal markers such as vimentin and E-cadherin. Boyden chambers were used to determine migration and invasion after 24 hours of treatment each with Hsp90 inhibitors (KU711, KU757, or 17-AAG). In all HNSCC cell lines tested, there was a significant decrease in both migration and invasion after treatment with KU711 or KU757 (Figure 3, A and B). In UMSCC 22B and UMSCC 22B-cis, Hsp90 inhibition by 20 μM KU711, 2.5 μM KU757, and 1 μM 17-AAG each resulted in a greater than 90% decrease in EMT (P < .001 vs control). Statistically significant inhibition of migration and invasion was seen with cisplatin therapy as well, though to a lesser degree of approximately 40% and 60%, respectively. Migration and invasion were inhibited by >90% in MDA-1986 cells when treated at higher concentrations of 40 μM KU711 and 2.5 μM KU757, while 17-AAG resulted in only a 40% decrease in migration and >90% decrease in invasion (P < .001).
Figure 3.
(A and B) Migration and invasion of HNSCC cells using Boyden chamber assay after 24-hour drug treatment with HSP90 inhibitors. Cell line MDA-1986 is used in panel A.
We next evaluated E-cadherin expression as a marker of epithelial differentiation. In MDA-1986 cells treated with KU711, E-cadherin expression was increased by 50% to 53%, while there was no significant change observed with cisplatin, 17-AAG, or KU757 treatment compared to controls (Figure 4). Likewise, vimentin expression was evaluated as a marker of mesenchymally derived cells. Vimentin was decreased under all treatment conditions relative to control, indicating decreased EMT. This trend was greater with Hsp90 inhibitor treatment relative to cisplatin treatment as most of the vimentin expression was attenuated completely at higher concentrations of KU711, KU757, and 17-AAG (Figure 4).
Downregulation of miRNAs Implicated in Treatment Resistance Pathways
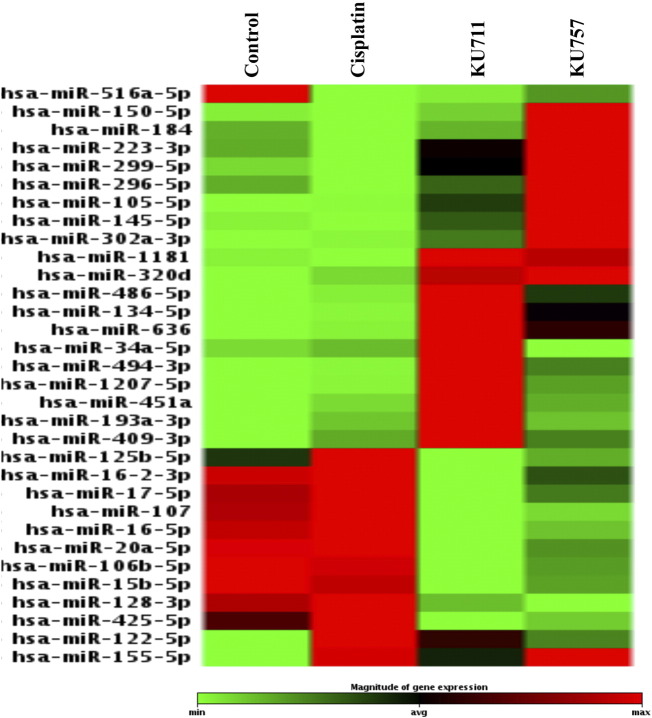
Aberrant miRNA expressions are implicated in CSC functions such as self-renewal, differentiation, metastasis development, and tumor recurrence [24].Hence, in an effort to identify the CSC-related miRNAs that are modulated by our novel HSP90 inhibitors, KU711 and KU757, we have used miScript miRNA array for identification of cancer stem cells (Qiagen, Germantown, MD). The results from our study showed that several miRNAs that are known to be upregulated in HNSCC such as miRNA 15b, 16, 17, 107,20a, 106b, 128, and 425 are all downregulated after treatment with our C-terminal Hsp90 inhibitors but are not significantly affected by cisplatin treatment (Figure 5) [25], [26].Similarly, several of the downregulated miRNAs in HNSCC such as 223, 299, 145, 302a, 494, 409, and 128 are upregulated only when these cancer cells are treated with KU711 and KU757 but not with cisplatin treatment (Figure 5) [25], [26]. The miRNA 516 and miRNA 122 and 155 implicated in HNSCC are modulated by cisplatin as well as by KU compounds. These results indicate that cisplatin is able to modify only three miRNAs that are implicated in HNSCC, whereas our novel c-terminal Hsp90 inhibitors KU711 and KU757 are able to alter several miRNAs that are dysregulated in HNSCC. Further validation of these miRNAs is needed to better understand the exact mechanisms through which these miRNAs target cancer stem cells following treatment with KU711 and KU757. Additionally, analysis of the genes regulated by the miRNAs by TargetScan indicates that proteins such as MAPK, FOXX2, SOX11, SMAD4, BCL11A, and E2F2 are regulated by these miRNAs after treatment with cisplatin, whereas treatment with KU711 and KU757 regulates several genes involved in cell cycle, proliferation, MAPK, and β-catenin pathways (Table 1).
Figure 5.
UMSCC 22B cells were treated with cisplatin, KU711, or KU757 for 24 hours. The cDNA from the cells was used in miScript miRNA array for cancer stem cells. Greater than two-fold changes in miRNA expression compared to controls are presented in the figure.
Table 1.
(A) Genes Regulated by miRNAs That are Modulated After Treatment of UMSCC22B Cells With Cisplatin; (B) Genes Regulated by miRNAs That are Modulated After Treatment of UMSCC22B Cells With KU Compounds
| A | |
| SOX11 | hsa-miR-296-5p, hsa-miR-137, hsa-miR-141-3p |
| MAP3K12 | hsa-miR-130a-3p, hsa-miR-122-5p, 146b-5p |
| FOXK2 | hsa-miR-146b-5p, hsa-miR-155-5p, hsa-miR-125b-5p, hsa-miR-130a-3p, hsa-miR-409-3p |
| SMAD4 | hsa-miR-130a-3p, hsa-miR-125b-5p, hsa-miR-409-3p, hsa-miR-146b-5p |
| BCL11A | hsa-miR-146b-5p, hsa-miR-130a-3p, hsa-miR-125b-5p |
| E2F2 | hsa-miR-125b-5p, hsa-miR-409-3p, hsa-miR-146b-5p |
| B | |
| CCND2 | hsa-miR-299-5p, hsa-miR-302a-3p, hsa-miR-494-3p, hsa-miR-145-5p, hsa-miR-320d |
| IGF1R | hsa-miR-223-3p, hsa-miR-145-5p, hsa-miR-494-3p, hsa-miR-320d |
| CDK6 | hsa-miR-320d, hsa-miR-494-3p, hsa-miR-145-5p |
| CTNND2 | hsa-miR-636, hsa-miR-494-3p, hsa-miR-122-5p |
| CUL3 | hsa-miR-302a-3p, hsa-miR-636, hsa-miR-494-3p |
| XIAP | hsa-miR-105-5p, hsa-miR-320d, hsa-miR-494-3p |
| BCL2L11 | hsa-miR-302a-3p, hsa-miR-494-3p, hsa-miR-105-5p |
| IGF2BP1 | hsa-miR-494-3p, hsa-miR-302a-3p, hsa-miR-150-5p |
| APC | hsa-miR-494-3p, hsa-miR-150-5p, hsa-miR-223-3p |
| EIF5 | hsa-miR-494-3p, hsa-miR-302a-3p |
| TCF4 | hsa-miR-105-5p, hsa-miR-155-5p, hsa-miR-145-5p, hsa-miR-636 |
| KRAS | hsa-miR-105-5p, hsa-miR-155-5p, hsa-miR-134-5p, hsa-miR-409-3p |
| SMAD2 | hsa-miR-302a-3p, hsa-miR-486-5p, hsa-miR-105-5p, hsa-miR-155-5p |
| XIAP | hsa-miR-105-5p, hsa-miR-320d, hsa-miR-494-3p |
| BCL2L11 | hsa-miR-302a-3p, hsa-miR-494-3p, hsa-miR-105-5p |
| SLC12A2 | hsa-miR-636, hsa-miR-105-5p |
| TP53INP2 | hsa-miR-105-5p, hsa-miR-302a-3p |
| BCL2L2 | hsa-miR-1207-5p, hsa-miR-105-5p |
| NOVA1 | hsa-miR-107, hsa-miR-146b-5p, hsa-miR-96-5p, hsa-miR-128-3p, hsa-let-7c-5p, hsa-let-7a-5p, hsa-let-7f-5p, hsa-miR-222-3p, hsa-miR-221-3p |
| NRAS | hsa-miR-146b-5p, hsa-let-7c-5p, hsa-let-7a-5p, hsa-let-7f-5p |
| ZNRF3 | hsa-miR-146b-5p, hsa-miR-107, hsa-miR-16-5p, hsa-miR-15b-5p |
| BCL11A | hsa-miR-146b-5p, hsa-miR-107, hsa-miR-96-5p |
Antitumor Effects with Decreased Toxicities
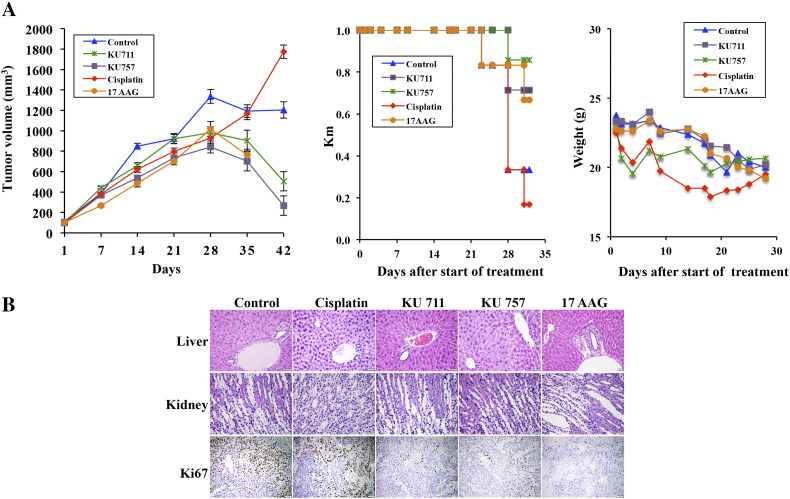
In vivo treatment using a murine buccal tumor model showed strong antitumor effects for KU711 and KU757 treatment, with significant decrease in tumor burden up to 6 weeks of treatment (Figure 6A). Steady and continued tumor growth was seen in the control and cisplatin treatment groups (Figure 6A). Mice treated with 17-AAG did show early response to treatment but did not survive beyond week 5 (Figure 6A). In each group, treatment effects were seen around 4 weeks of treatment, presumably when drug concentrations had reached an effective systemic concentration.
Figure 6.
(A and B) HNSCC xenograft was developed using MDA-1986. (A) Tumor volume curves, Kaplan-Meier survival curves, and changes in mouse weight during treatment. (B) Histopathology evaluation of HNSCC xenografts showing improved toxicity profile with KU compound treatment.
On histopathology, KU711, KU757, and 17-AAG showed decrease in the proliferation marker Ki67 by 15%, 15%, and 5% to 10% from the control value of 30%, indicating the efficacy of the drugs in preventing proliferation. But ki67 percentage was increased to 50 from the control value of 30 for cisplatin treatment which is consistent with our tumor growth studies (Figure 6B and Table 2). 17-AAG experimental group alone showed a mild increase (less than 10%) in periportal lymphoplasmacytic infiltration, while all other groups showed a small, physiologic amount of inflammation in the liver. Similarly, cisplatin showed less than 10% increase in lymphocytic inflammation in the kidney, predominately in the inner medulla, while other groups showed no changes compared to control groups (Figure 6B and Table 2).
Table 2.
Histopathology Evaluation of HNSCC Xenografts Showing Improved Toxicity Profile with KU Compound Treatment
| Group | Control | Cisplatin | KU711 | KU757 | 17-AAG |
|---|---|---|---|---|---|
| Liver | Normal | Normal | Normal | Normal | Mild periportal lymphoplasmacytic infiltration |
| Kidney | Normal | MILD lymphocytic inner medullary infiltrate | Normal | Normal | Normal |
| Ki67 (%) | 30 | 50 | 15 | 15 | 5-10 |
Discussion
Despite introduction of platinum-based chemotherapy regimens, survival outcomes for advanced HNSCC remain poor over the last few decades [1]. Cisplatin remains the mainstay of most commonly used chemotherapeutic regimens in HNSCC, though with well-recognized toxicity to the peripheral nerves, kidneys, and hearing. Treatment failures in advanced HNSCC treated with primary chemoradiation or surgery with adjuvant chemoradiotherapy are attributable at least in part to tumor resistance. This study builds on previous evidence that the CSC population is likely implicated in HNSCC aggressiveness (invasion and migration), tumorgenicity, and resistance mechanisms (e.g., cisplatin resistance). We now demonstrate that novel C-terminal Hsp90 inhibitors target this CSC subpopulation of cells as noted by inhibition of orosphere formation and expression of the cancer stem cell markers ALDH and CD44 as well as blocking migration, invasion, and tumor growth without significant toxicity associated with cisplatin or 17-AAG.
Hsp90 inhibitors were initially developed with the rationale that Hsp90 is overexpressed in tumor cells and serves as a molecular chaperone to many “client” proteins that are overexpressed in models of carcinogenesis [27]. Indeed, these inhibitors accumulate considerably more efficiently in tumor cells relative to normal tissue, further suggesting that these novel inhibitors are likely to preferentially target the tumor cell population and CSC subpopulation [15]. Our group focused on two novel C-terminal inhibitors, KU711 and KU757, which have shown potency and selectivity for HNSCC in vitro as well as recently in vivo. While cisplatin has been shown to increase the CSC population in HNSCCs in addition to other proposed mechanisms of resistance [14], our observation with novel C-terminal Hsp90 inhibitors in this study shows the opposite effect on CSCs. In this study, a dose-dependent decrease in ALDH+ cells, CD44+ cells, and double-positive (ALDH+/CD44+) cells was observed for all cell lines treated with both C-terminal Hsp90 inhibitors, while results were more variable with 17-AAG. Of note, populations of CSCs (as measured by CD44+/ALDH+ double-positive cells) varied across cell lines when treated with the N-terminal inhibitor 17-AAG, and this could represent variable upregulation of the prosurvival heat shock response upon 17-AAG treatment at the chosen dose. As noted, early-generation N-terminal Hsp90 inhibitors have failed to progress beyond phase II clinical trials due to dose-related toxicity from induction of the compensation Hsp70-mediated prosurvival heat shock response [17]. We have noted that our C-terminal Hsp90 inhibitors avoid upregulation of the heat shock response, as indicated by expression of Hsp70 [23]. Additionally, our group has previously shown that C-terminal Hsp90 inhibitors had both antitumor efficacy and improved toxicity profiles compared with standard agents in in vitro and in vivo (orthotopic) HNSCC models [28]. HNSCC cell treatment was shown to induce apoptosis and downregulate oncogenic client proteins (such as Akt and Raf-1), and animals treated with Hsp90 inhibitors showed comparable tumor response with decreased toxicity (body score and weight loss) compared to cisplatin chemotherapy. Furthermore, we had shown that our novel C- terminal Hsp90 inhibitors effectively target CSCs in HNSCC cell lines in vitro for differentiated and anaplastic thyroid cancer models, resulting in decreased CSC function and inhibition of migration/invasion [23]. Mclaughlin et al. recently proposed that Hsp90 inhibition (drug AUY922) sensitizes HNSCC to platin-based agents via modulation of DNA damage response mechanisms [29]. Taken together, these findings suggest that novel c-terminal Hsp90 inhibitors like KU711 and KU757 may have a potential adjunct role to current HNSCC treatments, especially in cases where cisplatin resistance warrants the use of alternatives with comparable efficacy.
EMT is a critical step in a primary tumor's initiation of metastasis and progression. Our results demonstrate that C-terminal Hsp90 inhibition also led to quiescence of EMT, as predicted by increased E-cadherin expression with concurrent suppression of vimentin expression. Likewise, marked dose-dependent inhibition of migration and invasion was seen with both of our C-terminal Hsp90 inhibitors (KU711 and KU757). Taken together, these C-terminal inhibitors may have an important function to lower HNSCC's propensity toward development of regional and distant metastasis. This will require more in vivo studies for validation and further translation.
Expression of Bmi1, a transcriptional regulator, CSC functional marker, and known client protein of Hsp90, was downregulated following treatment with C-terminal Hsp90 inhibitors. This downregulation was dose dependent with inhibitor KU757 and highly significant compared to controls (P < .0001). These findings are consistent with the hypothesis that these C-terminal Hsp90 inhibitor drugs may be effective in overcoming mechanisms of cisplatin chemoresistance in HNSCC, as Nor et al. observed that cisplatin treatment resulted in subsequently induced expression of Bmi-1 [14]. Furthermore, Chen et al. showed that targeting Bmi1+ CSCs sensitized HNSCC tumors to cisplatin-based chemotherapy, further validating Bmi1 inhibition as a potential mechanistic approach to overcoming cisplatin resistance mechanisms [30]. Hsp90 inhibitors have also demonstrated additive or synergistic effects when used in combination with other chemotherapeutic agents [27]. Together, these findings further implicate the role the CSC population plays in the mechanisms of cisplatin resistance in HNSCC, suggesting that combination therapies with C-terminal Hsp90 inhibitors, particularly those with chemoradiotherapy-sensitizing effects, may be critical in overcoming cisplatin treatment resistance in HNSCC.
Several of the miRNAs that are implicated in the pathogenesis of HNSCC as well as in cancer stem cell function are all modulated by our novel C-terminal Hsp90 inhibitors and not by cisplatin. This suggests that compounds like KU757 or KU711 may have a role as potential therapeutics for targeting cancer stem cell populations that are responsible for therapy resistance and metastasis. Analysis of the pathways specifically targeted by miRNAs modulated by KU757 and KU711 (MAPK, cell cycle, and β-catenin) indicates that these C-terminal Hsp90 inhibitors target several CSC pathways involved in migration, invasion, and proliferation. Finally, the above data are promising in the context of concurrent in vivo experiments showing both antitumor effects and decreased treatment toxicity. Specifically, novel Hsp90 inhibitors avoid the well-described renal and hepatic toxicities associated with cisplatin and first-generation Hsp90 inhibitors, respectively. Even though, treatment effects and toxicity were studied in the MDA-1986 cell line where additional cisplatin resistance was not generated, in vivo, the MDA-1986 tumor did show inherent cisplatin resistance as indicated by lack of response to cisplatin treatment (Figure 6). Overall, the results from our study clearly demonstrate that KU757 and KU711 may be an effective therapeutic strategy to targeting CSCs in HNSCC and to overcome cisplatin therapy resistance. Validation of these important findings with xenograft model using cisplatin-resistant cell lines may be warranted to advance these promising compounds translationally toward clinical applications in the future for this challenging disease.
Conflict of Interests
None of the authors have any conflict of interest related to this work. Dr. Cohen has equity share interest in Hylapharm, LLC, and MedGuider, LLC, and is a consultant for Acousys Biodevices, Inc.
The following is the supplementary data related to this article.
Acknowledgments
Acknowledgements
The authors would like to acknowledge the following funding sources for support of the project in part. These include National Institute of Health grants through the National Cancer Institute: R01 CA120458 (B.S.J.B. and M.S.C.), R01 CA216919 (B.S.J.B. and M.S.C.), and R01 CA213566 (B.S.J.B. and M.S.C.). We would additionally like to acknowledge support from the Department of Surgery (M.S.C. and C.S.) and the Department of Otolaryngology Head & Neck Surgery at the University of Michigan (K.J.K., T.E.C., M.E.P.P.). The authors would also like to acknowledge Jacquelyn Sanchez for her help with the project.
References
- 1.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 2.Sacco AG, Cohen EE. Current treatment options for recurrent or metastatic head and neck squamous cell carcinoma. J Clin Oncol. 2015;33(29):3305–3313. doi: 10.1200/JCO.2015.62.0963. [DOI] [PubMed] [Google Scholar]
- 3.Forastiere AA. Chemotherapy in the treatment of locally advanced head and neck cancer. J Surg Oncol. 2008;97(8):701–707. doi: 10.1002/jso.21012. [DOI] [PubMed] [Google Scholar]
- 4.Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefebvre JL, Greiner RH, Giralt J, Maingon P, Rolland F, Bolla M. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350(19):1945–1952. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 5.Prince ME, Ailles LE. Cancer stem cells in head and neck squamous cell cancer. J Clin Oncol. 2008;26(17):2871–2875. doi: 10.1200/JCO.2007.15.1613. [DOI] [PubMed] [Google Scholar]
- 6.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7(8):573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 7.Al-Hajj M, Clarke MF. Self-renewal and solid tumor stem cells. Oncogene. 2004;23(43):7274–7282. doi: 10.1038/sj.onc.1207947. [DOI] [PubMed] [Google Scholar]
- 8.Cho RW, Clarke MF. Recent advances in cancer stem cells. Curr Opin Genet Dev. 2008;18(1):48–53. doi: 10.1016/j.gde.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 9.Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007;23:675–699. doi: 10.1146/annurev.cellbio.22.010305.104154. [DOI] [PubMed] [Google Scholar]
- 10.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 11.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104(3):973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chinn SB, Darr OA, Owen JH, Bellile E, McHugh JB, Spector ME, Papagerakis SM, Chepeha DB, Bradford CR, Carey TE. Cancer stem cells: mediators of tumorigenesis and metastasis in head and neck squamous cell carcinoma. Head Neck. 2015;37(3):317–326. doi: 10.1002/hed.23600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winquist RJ, Furey BF, Boucher DM. Cancer stem cells as the relevant biomass for drug discovery. Curr Opin Pharmacol. 2010;10(4):385–390. doi: 10.1016/j.coph.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Nor C, Zhang Z, Warner KA, Bernardi L, Visioli F, Helman JI, Roesler R, Nor JE. Cisplatin induces Bmi-1 and enhances the stem cell fraction in head and neck cancer. Neoplasia. 2014;16(2):137–146. doi: 10.1593/neo.131744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearl LH, Prodromou C, Workman P. The Hsp90 molecular chaperone: an open and shut case for treatment. Biochem J. 2008;410(3):439–453. doi: 10.1042/BJ20071640. [DOI] [PubMed] [Google Scholar]
- 16.Jhaveri K, Ochiana SO, Dunphy MP, Gerecitano JF, Corben AD, Peter RI, Janjigian YY, Gomes-DaGama EM, Koren J, 3rd, Modi S. Heat shock protein 90 inhibitors in the treatment of cancer: current status and future directions. Expert Opin Investig Drugs. 2014;23(5):611–628. doi: 10.1517/13543784.2014.902442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sidera K, Patsavoudi E. HSP90 inhibitors: current development and potential in cancer therapy. Recent Pat Anticancer Drug Discov. 2014;9(1):1–20. [PubMed] [Google Scholar]
- 18.Donnelly A, Blagg BS. Novobiocin and additional inhibitors of the Hsp90 C-terminal nucleotide-binding pocket. Curr Med Chem. 2008;15(26):2702–2717. doi: 10.2174/092986708786242895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marcu MG, Chadli A, Bouhouche I, Catelli M, Neckers LM. The heat shock protein 90 antagonist novobiocin interacts with a previously unrecognized ATP-binding domain in the carboxyl terminus of the chaperone. J Biol Chem. 2000;275(47):37181–37186. doi: 10.1074/jbc.M003701200. [DOI] [PubMed] [Google Scholar]
- 20.Zhao H, Donnelly AC, Kusuma BR, Brandt GE, Brown D, Rajewski RA, Vielhauer G, Holzbeierlein J, Cohen MS, Blagg BS. Engineering an antibiotic to fight cancer: optimization of the novobiocin scaffold to produce anti-proliferative agents. J Med Chem. 2011;54(11):3839–3853. doi: 10.1021/jm200148p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao J, Zhao H, Hall JA, Brown D, Brandes E, Bazzill J, Grogan PT, Subramanian C, Vielhauer G, Cohen MS. Triazole containing novobiocin and biphenyl amides as Hsp90 C-terminal inhibitors. Medchemcomm. 2014;5(9):1317–1323. doi: 10.1039/C4MD00102H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camphausen K, Tofilon PJ. Inhibition of Hsp90: a multitarget approach to radiosensitization. Clin Cancer Res. 2007;13(15 Pt 1):4326–4330. doi: 10.1158/1078-0432.CCR-07-0632. [DOI] [PubMed] [Google Scholar]
- 23.White PT, Subramanian C, Zhu Q, Zhang H, Zhao H, Gallagher R, Timmermann BN, Blagg BS, Cohen MS. Novel HSP90 inhibitors effectively target functions of thyroid cancer stem cell preventing migration and invasion. Surgery. 2016;159(1):142–151. doi: 10.1016/j.surg.2015.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garg M. Emerging role of microRNAs in cancer stem cells: Implications in cancer therapy. World J Stem Cells. 2015;7(8):1078–1089. doi: 10.4252/wjsc.v7.i8.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Courthod G, Franco P, Palermo L, Pisconti S, Numico G. The role of microRNA in head and neck cancer: current knowledge and perspectives. Molecules. 2014;19(5):5704–5716. doi: 10.3390/molecules19055704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sethi N, Wright A, Wood H, Rabbitts P. MicroRNAs and head and neck cancer: reviewing the first decade of research. Eur J Cancer. 2014;50(15):2619–2635. doi: 10.1016/j.ejca.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 27.Scaltriti M, Dawood S, Cortes J. Molecular pathways: targeting Hsp90—who benefits and who does not. Clin Cancer Res. 2012;18(17):4508–4513. doi: 10.1158/1078-0432.CCR-11-2138. [DOI] [PubMed] [Google Scholar]
- 28.Cohen SM, Mukerji R, Samadi AK, Zhao HP, Blagg BSJ, Cohen MS. Novel C-terminal Hsp90 inhibitor for head and neck squamous cell cancer (HNSCC) with in vivo efficacy and improved toxicity profiles compared with standard agents. Ann Surg Oncol. 2012;19:S483–S490. doi: 10.1245/s10434-011-1971-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLaughlin M, Barker HE, Khan AA, Pedersen M, Dillon M, Mansfield DC, Patel R, Kyula JN, Bhide SA, Newbold KL. HSP90 inhibition sensitizes head and neck cancer to platin-based chemoradiotherapy by modulation of the DNA damage response resulting in chromosomal fragmentation. BMC Cancer. 2017;17 doi: 10.1186/s12885-017-3084-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen DM, Wu MS, Li Y, Chang I, Yuan Q, Ekimyan-Salvo M, Deng P, Yu B, Yu YX, Dong JQ. Targeting BMI1(+) cancer stem cells overcomes chemoresistance and inhibits metastases in squamous cell carcinoma. Cell Stem Cell. 2017;20(5):621–634. doi: 10.1016/j.stem.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]