Graphical abstract
Keywords: Nitrofurazone analogues, Hydrazide-hydrazones, Antibacterial activity, Antifungal activity, MIC
Abstract
In this research we synthesized and tested for in vitro antimicrobial activity 21 nitrofurazone analogues. The compounds we obtained were identified on the basis of 1H NMR and 13C NMR spectroscopy. The in vitro screening of antimicrobial properties of synthesized compounds revealed a wide spectrum of antimicrobial activity. Compounds 28, 29, 32–43, and 45–48 showed very high bactericidal effect towards Staphylococcus spp. ATTC and Bacillus spp. ATTC (MIC = 0.002–7.81 µg/ml and MBC = 0.002–31.25 µg/ml). The levels of activity of several compounds were far better than those of nitrofurantoin, ciprofloxacin or cefuroxime.
1. Introduction
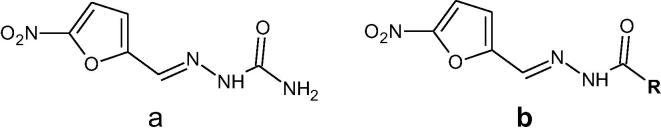
The increasing number of cases of multidrug-resistant infections difficult to diagnose and treat pose a major concern to public health care. To overcome these problems, developing new and safe antimicrobial agents with better effectiveness is required (Coates et al., 2002). One of several routes to find new chemotherapeutic agents is to modify the chemical structure of existing medicines which could result in broadering the spectrum of their activity and reducing their toxicity to human body (Moellering, 2011). In our research we decided to synthesized nitrofurazone analogues, because nitrofurazone is important antibacterial agent (McCalla et al., 1970) and in its structure we found the hydrazide-hydrazone moiety which is of our interest due to its promising biological activity (Fig. 1) (Rollas and Kűçűkgűzel, 2007, Bala et al., 2013).
Fig. 1.
Chemical structure of nitrofurazone (A) and synthesized analogues (B).
Recently we have published interesting results concerning antibacterial activity of hydrazide-hydrazone derivatives (Popiołek et al., 2014, Popiołek et al., 2016a, Popiołek et al., 2016b, Popiołek and Biernasiuk, 2016a, Popiołek and Biernasiuk, 2016b). Hydrazide-hydrazones of 3-methoxybenzoic acid showed significant antibacterial activity against Gram-positive bacterial strains, especially against Bacillus spp. ATTC (Popiołek and Biernasiuk, 2016a). In addition to this we have reported that hydrazide-hydrazones of 2-substituted acetic acid displayed potent bactericidal activity against Gram-positive and Gram-negative bacterial strains (Popiołek and Biernasiuk, 2016b).
It is worth to add that beside antibacterial activity (Küçükgüzel et al., 2002, Küçükgüzel et al., 2003, Özkay et al., 2010, Deep et al., 2010, Rasras et al., 2010, Kumar et al., 2011, Rutkauskas et al., 2013, Pieczonka et al., 2013, Cukurovali and Yilmaz, 2014, Satyanarayana et al., 2014, Morjan et al., 2014, Rambabu et al., 2015), hydrazide-hydrazone derivatives have attracted much attention thanks to their usability as intermediates in organic synthesis (Rollas and Kűçűkgűzel, 2007, Bala et al., 2013) and they display a wide spectrum of such interesting biological properties as antifungal (Loncle et al., 2004, Backes et al., 2014), antitubercular (Koçyiğit-Kaymakçıoğlu et al., 2006, Koçyiğit-Kaymakçıoğlu et al., 2009, Pavan et al., 2010, Velezheva et al., 2016), antiviral (Şenkardes et al., 2016), anticancer (Kumar et al., 2012, Çıkla et al., 2013, Wardakhan et al., 2013, Nasr et al., 2014, Küçükgüzel et al., 2015, He et al., 2016, Mukherjee et al., 2016), anti-inflammatory (Moldovan et al., 2011) and analgesic activity (Mohareb et al., 2010).
Based on the afore mentioned facts, and in an attempt to find new potent antimicrobial agents thanks to this research we synthesized and evaluated for their in vitro antimicrobial activity 21 analogues of nitrofurazone and we discovered that they showed very high bactericidal activity, particularly against Staphylococcus spp. ATTC and Bacillus spp. ATTC.
2. Materials and methods
2.1. Chemistry
Reagents and solvent used in this research were purchased from Sigma-Aldrich (Munich, Germany) and Merck Co. (Darmstadt, Germany) and were used without further purification. Melting points were determined on Fisher-Johns blocks melting point apparatus (Fisher Scientific, Germany) and left uncorrected. The 1H NMR and 13C NMR spectra were recorded on the BRUKER AVANCE 300 apparatus (Bruker BioSpin GmbH, Germany) in DMSO-d6 with TMS as the internal standard. Chemical shifts are reported in ppm (δ) with the use of TMS as the standard reference. The coupling constants (J) are given in Hertz. The progress of the reaction and purity of obtained compounds were monitored by TLC, using pre-coated aluminum sheet 60 F254 plates (Merck Co. USA), in a CHCl3/C2H5OH (10:1, v/v) solvent system. The spots were detected by exposure to the UV lamp at 254 nm. The elemental analysis of obtained compounds was carried out with the AMZ 851 CHX analyser (PG, Gdańsk, Poland). The results of elemental analysis (C, H, N) were within ±0.4% of the calculated values.
2.1.1. Preparation of carboxylic acid hydrazides (9–13, 20)
The compounds 11, 12, 13 were prepared using the procedures reported earlier (Popiołek et al., 2016b). Compound 9, 10, 20 were synthesized by following procedure: 0.01 mol of appropriate ethyl ester of carboxylic acid was dissolved in ethanol and heated under reflux with 0.011 mol of 100% hydrazine monohydrate for 2 h. After that the solution was cooled to room temperature and the precipitate formed. Then it was filtered off, dried and recrystallized from ethanol.
Physico-chemical and spectral data of compounds 9–13, 20 are presented in Supplementary Materials.
2.1.2. Preparation of nitrofurazone analogues (28–48)
2.1.2.1. General procedure
0.01 mol of previously obtained carboxylic acid hydrazides (9–13, 20) or commercially available hydrazides (7, 8, 14–19, 21–27) were dissolved in 10–20 ml of ethanol and then 0.011 mol of 5-nitro-2-furaldehyde was added. The mixture was heated under reflux for 3 h. After that the solution was allowed to cool at room temperature and then was placed in refrigerator for 12 h. Subsequently the precipitation created was filtered off and recrystallized from ethanol.
Physico-chemical and spectral data of compounds 28–48 are presented in Supplementary Materials.
2.2. Microbiology
2.2.1. In vitro antimicrobial assay
The examined compounds were screened in vitro for antibacterial and antifungal activities using the broth microdilution method based on European Committee on Antimicrobial Susceptibility Testing (EUCAST) (EUCAST discussion document E. Dis 5.1, 2003) and Clinical and Laboratory Standards Institute guidelines (M27-S4, 2012).
In this research a panel of reference and clinical or saprophytic strains of microorganisms was used. This included Gram-positive bacteria (Staphylococcus aureus ATCC 25923, Staphylococcus aureus ATCC 43300, Staphylococcus aureus ATCC 6538, Staphylococcus epidermidis ATCC 12228, Bacillus subtilis ATCC 6633, Bacillus cereus ATCC 10876, Micrococcus luteus ATCC 10240), Gram-negative bacteria (Escherichia coli ATCC 25922, Klebsiella pneumoniae ATCC 13883, Proteus mirabilis ATCC 12453, Bordetella bronchiseptica ATCC 4617, Salmonella typhimurium ATCC 14028, Pseudomonas aeruginosa ATCC 9027) and fungi belonging to yeasts (Candida albicans ATCC 10231, Candida parapsilosis ATCC 22019).
The antimicrobial assays were performed like in our previous research concerning in vitro screening of hydrazide-hydrazone derivatives (Popiołek and Biernasiuk, 2016a, Popiołek and Biernasiuk, 2016b). Nitrofurantoin, ciprofloxacin, and cefuroxime (Sigma-Aldrich) were used as reference antibacterial compounds. Fluconazole (Sigma-Aldrich) was used as reference antifungal positive control. All the experiments were repeated three times and representative data were presented.
The MBC/MIC or MFC/MIC ratios were used to determine bactericidal/fungicidal (MBC/MIC ≤ 4, MFC/MIC ≤ 4) or bacteriostatic/fungistatic (MBC/MIC > 4, MFC/MIC > 4) effect of the tested compounds (Wiegand et al., 2008).
3. Results and discussion
3.1. Chemistry
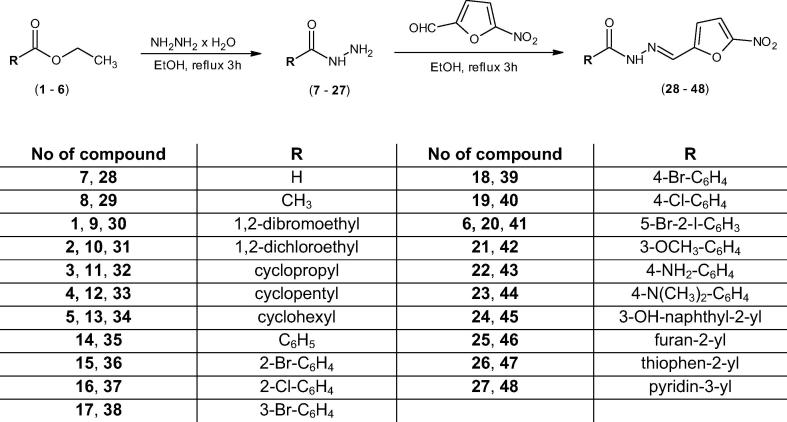
Nitrofurazone analogues analyzed in this study were obtained in the condensation reaction of appropriate carboxylic acid hydrazides (7–27) with 5-nitro-2-furaldehyde. The reactions were performed by heating substrates under reflux for 3 h. In the case of the synthesis of nitrofurazone analogues 28, 29, 35–40, 42–48 commercially available hydrazides of carboxylic acids (7, 8, 14–19, 21–27) were used. Whereas for the synthesis of nitrofurazone analogues (30–34, 41) we initially conduced the synthesis of hydrazides (9–13, 20) by the reaction of appropriate ethyl esters (1–6) with hydrazine monohydrate.
Chemical structures of synthesized compounds were confirmed on the basis of 1H NMR and 13C NMR spectroscopy. The spectra of the compounds we obtained gave satisfactory results and confirmed the formation of expected products. In the 1H NMR spectra of compounds (28–48) two singlet signals for CH and NH groups appeared at δ 7.92–8.74 ppm and δ 10.95–12.40 ppm, respectively. As for the 13C NMR spectra, signals for CH group were found in the range of δ 151.6–152.8 ppm, and for the carbonyl group (C O) at δ 160.1–174.9 ppm. Signals for other aliphatic and aromatic groups in compounds (28–48) were observed at expected regions. Reactions conducted in this study were performed according to the steps presented in the Scheme 1.
Scheme 1.
Synthesis pathway to nitrofurazone analogues (28–48).
3.2. In vitro antimicrobial assay
The results of our study indicated that examined compounds (28–48) exhibited a wide spectrum of antimicrobial activity against tested reference bacteria and yeasts (Table 1A, Table 1B). Among these compounds, 28, 29, 32–43, and 45–48 showed very strong, mainly bactericidal effect towards Staphylococcus spp. ATTC and Bacillus spp. ATTC (MIC = 0.002–7.81 µg/ml and MBC = 0.002–31.25 µg/ml). Substances 38 and 45 were especially potent because of MIC < 1 µg/ml (0.002–0.98 µg/ml) against these bacteria.
Table 1A.
The activity data expressed as MIC (MBC or MFC) [µg/ml] and {MBC/MIC or MFC/MIC ratio} against the reference strains of microorganisms. The standard chemotherapeutics agents: nitrofurantoin (NIT), ciprofloxacin (CIP), cefuroxime (CFX) and fluconazole (FLU) were used as positive control.
| Species | MIC (MBC or MFC) [µg/ml] and {MBC/MIC or MFC/MIC ratio} of the tested compounds |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | NIT | CIP | CFX | FLU | ||
| Gram-positive bacteria |
Staphylococcus aureus ATCC 25923 |
7.81 (7.81) {1} |
7.81 (7.81) {1} |
15.62 (125) {8} |
31.25 (250) {8} |
3.91 (7.81) {2} |
1.95 (3.91) {2} |
1.95 (15.62) {8} |
3.91 (7.81) {2} |
3.91 (3.91) {1} |
3.91 (7.81) {2} |
0.061 (7.81) {128} |
15.62 (15.62) |
0.488 | 0.49 | na |
|
Staphylococcus aureus ATCC 6538 |
7.81 (7.81) {1} |
7.81 (7.81) {1} |
31.25 (62.5) {2} |
31.25 (62.5) {2} |
3.91 (7.81) {2} |
3.91 (3.91) {1} |
0.98 (3.91) {4} |
3.91 (7.81) {2} |
1.95 (3.91) {2} |
7.81 (15.62) {2} |
0.98 (1.95) {2} |
15.62 (15.62) |
0.244 | 0.98 | na | |
|
Staphylococcus aureus ATCC 43300 |
0.98 (3.91) {4} |
1.95 (3.91) {2} |
7.81 (15.62) {2} |
7.81 (15.62) {2} |
0.98 (1.95) {2} |
0.244 (0.488) {2} |
0.244 (0.488) {2} |
1.95 (3.91) {2} |
0.488 (0.98) {2} |
7.81 (15.62) {2} |
0.122 (0.244) {2} |
7.81 (15.62) |
0.244 | nd | na | |
|
Staphylococcus epidermidis ATCC 12228 |
0.98 (1.95) {2} |
0.98 (1.95) {2} |
3.91 (15.62) {4} |
3.91 (15.62) {4} |
0.061 (0.244) {4} |
0.061 (0.122) {2} |
0.244 (0.488) {2} |
0.48 (0.98) {2} |
0.061 (0.122) {2} |
0.244 (0.244) {1} |
0.002 (0.002) {1} |
3.91 (7.81) |
0.122 | 0.24 | na | |
|
Micrococcus luteus ATCC 10240 |
62.5 (125) {2} |
62.5 (250) {4} |
250 (1000) {4} |
62.5 (500) {8} |
62.5 (62.5) {1} |
500 (>1000) {>2} |
250 (>1000) {>4} |
250 (>1000) {>4} |
62.5 (125) {2} |
1000 (>1000) {>1} |
125 (125) {1} |
62.5 (62.5) |
0.976 | 0.98 | na | |
|
Bacillus subtilis ATCC 6633 |
0.98 (0.98) {1} |
1.95 (1.95) {1} |
3.91 (7.81) {2} |
3.91 (7.81) {2} |
0.002 (0.008) {1} |
0.244 (7.81) {32} |
0.98 (3.91) {4} |
1.95 (1.95) {1} |
3.91 (3.91) {1} |
1.95 (1.95) {1} |
0.244 (0.98) {4} |
3.91 (3.91) |
0.031 | 15.63 | na | |
|
Bacillus cereus ATCC 10876 |
7.81 (7.81) {1} |
7.81 (7.81) {1} |
31.25 (31.25) {1} |
62.5 (125) {2} |
0.98 (3.91) {4} |
3.91 (7.81) {2} |
0.98 (15.62) {16} |
3.91 (3.91) {1} |
1.95 (3.91) {2} |
1.95 (1.95) {1} |
0.488 (0.98) {2} |
7.81 (15.62) |
0.061 | 31.25 | na | |
| Gram-negative bacteria |
Bordetella bronchiseptica ATCC 4617 |
31.25 (125) {4} |
62.5 (500) {8} |
125 (500) {4} |
62.5 (250) {4} |
500 (>1000) {>2} |
500 (>1000) {>2} |
– | – | 125 (1000) {8} |
– | 1000 (>1000) {>1} |
125 (>1000) |
0.976 | nd | na |
|
Klebsiella pneumoniae ATCC 13883 |
7.81 (15.62) {2} |
7.81 (7.81) {1} |
125 (250) {2} |
125 (125) {1} |
31.25 (>1000) {>32} |
1000 (>1000) {>1} |
– | – |
125 (500) {4} |
– | – | 15.62 (31.25) |
0.122 | nd | na | |
|
Proteus mirabilis ATCC 12453 |
31.25 (62.5) {2} |
62.5 (125) {2} |
500 (1000) {2} |
250 (500) {2} |
250 (>1000) {>4} |
250 (>1000) {>4} |
– | – |
62.5 (250) {4} |
– | – | 62.5 (125) |
0.030 | nd | na | |
|
Salmonella typhimurium ATCC 14028 |
7.81 (7.81) {1} |
7.81 (7.81) {1} |
125 (500) {4} |
62.5 (125) {2} |
0.98 (7.81) {8} |
500 (>1000) {>2} |
– | – |
62.5 (250) {4} |
– | – | 31.25 (62.5) |
0.061 | nd | na | |
|
Escherichia coli ATCC 25922 |
7.81 (7.81) {1} |
3.91 (7.81) {2} |
125 (250) {2} |
62.5 (125) {2} |
0.98 (1.95) {2} |
1000 (>1000) {>1} |
– | – |
31.25 (31.25) {1} |
– | – | 7.81 (15.62) |
0.004 | nd | na | |
|
Pseudomonas aeruginosa ATCC 9027 |
500 (1000) {2} |
– |
250 (500) {2} |
62.5 (125) {2} |
– | – | – | – | – | – | – | – | 0.488 | nd | na | |
| Fungi |
Candida albicans ATTC 10231 |
125 (250) {2} |
500 (>1000) |
125 (500) {4} |
62.5 (125) {2} |
– | 500 (>1000) {>2} |
125 (>1000) {>8} |
250 (>1000) {>4} |
31.25 (62.5) {2} |
125 (250) {2} |
1000 (>1000) {>1} |
na | na | na | 0.98 |
|
Candida parapsilosis ATTC 22019 |
250 (500) {2} |
1000 (>1000) {>1} |
125 (1000) {8} |
62.5 (250) {4} |
– | 1000 (>1000) {>1} |
500 (>1000) {>2} |
500 (>1000) {>2} |
125 (1000) {8} |
1000 (>1000) {>1} |
1000 (>1000) {>1} |
na | na | na | 1.95 | |
na - not applicable; nd – not determined; ‘-’ – no activity; MIC – Minimal Inhibitory Concentration; MBC – Minimal Bactericidal Concentration; MFC - Minimal Fungicidal Concentration; Compounds with bactericidal effect (MBC/MIC ≤ 4) or fungicidal effect (MFC/MIC ≤ 4) are marked in bold.
Table 1B.
The activity data expressed as MIC (MBC or MFC) [µg/ml] and {MBC/MIC or MFC/MIC ratio} against the reference strains of microorganisms. The standard chemotherapeutics agents: nitrofurantoin (NIT), ciprofloxacin (CIP), cefuroxime (CFX) and fluconazole (FLU) were used as positive control.
| Species | MIC (MBC or MFC) [µg/ml] and {MBC/MIC or MFC/MIC ratio} of the tested compounds |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 39 | 40 | 41 | 42 | 43 | 44 | 45 | 46 | 47 | 48 | NIT | CIP | CFX | FLU | ||
| Gram-positive bacteria |
Staphylococcus aureus ATCC 25923 |
7.81 (31.25) {4} |
3.91 (7.81) {2} |
3.91 (7.81) {2} |
3.91 (3.91) {1} |
3.91 (3.91) {1} |
500 (>1000) {>2} |
0.98 (1.95) {2} |
3.91 (7.81) {2} |
7.81 (15.62) {2} |
3.91 (3.91) {1} |
15.62 (15.62) |
0.488 | 0.49 | na |
|
Staphylococcus aureus ATCC 6538 |
7.81 (31.25) {4} |
0.488 (0.98) {2} |
3.91 (3.91) {1} |
3.91 (3.91) {1} |
3.91 (3.91) {1} |
500 (>1000) {>2} |
0.48 (1.95) {4} |
3.91 (7.81) {2} |
7.81 (15.62) {2} |
3.91 (3.91) {1} |
15.62 (15.62) |
0.244 | 0.98 | na | |
|
Staphylococcus aureus ATCC 43300 |
3.91 (15.62) {4} |
3.91 (3.91) {1} |
0.98 (3.91) {4} |
1.95 (1.95) {1} |
1.95 (1.95) {1} |
62.5 (125) {2} |
0.48 (0.98) {2} |
0.98 (1.95) {2} |
0.98 (1.95) {2} |
1.95 (3.91) {2} |
7.81 (15.62) |
0.244 | nd | na | |
|
Staphylococcus epidermidis ATCC 12228 |
0.031 (0.122) {4} |
0.015 (0.061) {4} |
0.24 (0.48) {2} |
0.48 (0.48) {1} |
0.24 (0.24) {1} |
3.91 (62.5) {16} |
0.12 (0.24) {2} |
0.98 (0.98) {1} |
0.98 (1.95) {2} |
0.98 (1.95) {2} |
3.91 (7.81) |
0.122 | 0.24 | na | |
|
Micrococcus luteus ATCC 10240 |
– | – | – | 250 (>1000) {>4} |
125 (>1000) {>8} |
500 (>1000) {>2} |
125 (>1000) {>8} |
125 (>1000) {>8} |
500 (>1000) {>2} |
31.25 (62.5) {2} |
62.5 (62.5) |
0.976 | 0.98 | na | |
|
Bacillus subtilis ATCC 6633 |
7.81 (15.62) {2} |
0.244 (0.244) {1} |
0.98 (1.95) {2} |
1.95 (1.95) {1} |
0.98 (0.98) {1} |
62.5 (1000) {16} |
0.12 (0.48) {4} |
0.98 (0.98) {1} |
0.98 (0.98) {1} |
1.95 (3.91) {2} |
3.91 (3.91) |
0.031 | 15.63 | na | |
|
Bacillus cereus ATCC 10876 |
3.91 (31.25) {8} |
0.98 (7.81) {8} |
1.95 (1.95) {1} |
0.98 (0.98) {1} |
0.98 (0.98) {1} |
31.25 (>1000) {>32} |
0.98 (1.95) {2} |
3.91 (7.81) {2} |
1.95 (1.95) {1} |
7.81 (7.81) {1} |
7.81 (15.62) |
0.061 | 31.25 | na | |
| Gram-negative bacteria |
Bordetella bronchiseptica ATCC 4617 |
1000 (>1000) {>1} |
– | – | – | – | – | – | – | – | 15.62 (125) {8} |
125 (>1000) |
0.976 | nd | na |
|
Klebsiella pneumoniae ATCC 13883 |
– | – | – | – | – | – | – | 1000 (>1000) {>1} |
– |
62.5 (62.5) {1} |
15.62 (31.25) |
0.122 | nd | na | |
|
Proteus mirabilis ATCC 12453 |
– | – | – | – | – | – | – | 1000 (>1000) {>1} |
– |
31.25 (62.5) {2} |
62.5 (125) |
0.030 | nd | na | |
|
Salmonella typhimurium ATCC 14028 |
– | – | – | – | – | – | – | 125 (>1000) {>8} |
– |
31.25 (31.25) {1} |
31.25 (62.5) |
0.061 | nd | na | |
|
Escherichia coli ATCC 25922 |
1000 (>1000) {>1} |
– |
250 (250) {1} |
– | 1000 (>1000) {>1} |
– | – | 15.62 (>1000) {>64} |
– |
31.25 (125) {4} |
7.81 (15.62) |
0.004 | nd | na | |
|
Pseudomonas aeruginosa ATCC 9027 |
– | – | – | – | – | – | – | – | – | – | – | 0.488 | nd | na | |
| Fungi |
Candida albicans ATTC 10231 |
– | – | – | – | – | – | – | – | – | 250 (>1000) {>4} |
na | na | na | 0.98 |
|
Candida parapsilosis ATTC 22019 |
1000 (>1000) {>1} |
1000 (>1000) {>1} |
– | – | – | – | – | – | – | – | na | na | na | 1.95 | |
na - not applicable; nd – not determined; ‘-’ – no activity; MIC – Minimal Inhibitory Concentration; MBC – Minimal Bactericidal Concentration; MFC - Minimal Fungicidal Concentration; Compounds with bactericidal effect (MBC/MIC ≤ 4) or fungicidal effect (MFC/MIC ≤ 4) are marked in bold.
Staphylococcus epidermidis ATTC 12228 was the most sensitive to all compounds, while Micrococcus luteus ATTC 10240 was the least susceptible. The minimum inhibitory concentrations (MIC) of nitrofurazone analogues (with the exception of inactive 39, 40 and 41), which inhibited the growth of micrococci and killed them (MBC) ranged from 31.25 µg/ml to 1000 µg/ml and from 62.5 µg/ml to >1000 µg/ml, respectively.
The bioactivity of compounds 30, 31 and 44 against Gram-positive bacteria was lower (MIC = 3.91–500 µg/ml and MBC = 7.81 to >1000 µg/ml).
Some of the compounds showed activity towards Gram-negative bacteria. The all reference rods from Enterobacteriaceae family were susceptible to compounds 28–33, 36 and 48 at concentrations from 0.98 µg/ml (S. typhimurium ATTC 14028 and E. coli ATTC 25922 against 32) to 500 µg/ml (P. mirabilis ATCC 12453 against 30). The other compounds indicated mainly mild bioactivity or had no effect towards these bacteria. Among the studied compounds, derivatives 28, 30, and 31 exhibited also some activity against P. aeruginosa ATTC 9027 (MIC = 62.5–500 µg/ml and MBC = 125–1000 µg/ml). The same substances (28, 30, and 31) showed simultaneously the widest spectrum of antimicrobial activity against all tested reference Gram-positive and Gram-negative bacteria and fungi.
The activity of compounds 28, 29, 32–43, and 45–48 was from two to two thousand times higher, depending on the compounds and bacterial strains, in comparison with the activity of nitrofurantoin (Table S1 in Supplementary Materials). It is worth to mention especially compounds 32 and 38, which showed almost two thousand times higher activity than nitrofurantoin against Bacillus subtilis ATCC 6633 and Staphylococcus epidermidis ATCC 12228, respectively.
Compounds 32, 33, 36, 38, 39 and 40 showed from 2 to 61 times better activity than ciprofloxacin on the basis of MIC values. Especially, compound 38 can be considered as the best analogue because its MIC value was 61 times lower than the MIC of ciprofloxacin against Staphylococcus epidermidis ATCC 12228 (Table S2 in Supplementary Materials).
The antibacterial activity of all tested compounds (28–48) against Gram-positive bacteria was also in some cases higher, depending on the compounds and bacterial strains, than the activity of cefuroxime (Table S3 in Supplementary Materials). Especially it is worth to mention compound 32 whose MIC value against Bacillus subtilis ATCC 6633 was almost 8000 times lower than the MIC of cefuroxime.
Moreover 30, 31 and 36 possessed good fungicidal or fungistatic bioactivity against yeasts belonging to Candida spp. ATTC with MIC = 31.25–125 µg/ml and MFC = 125–1000 µg/ml and it was higher than that of fluconazole used as the reference substance. The remaining newly synthesized compounds were less active or inactive towards reference fungi (Table 1A, Table 1B).
4. Conclusions
In our research we synthesized and evaluated a series of 21 nitrofurazone analogues for in vitro antimicrobial activity. All synthesized compounds have been identified by means of 1H NMR and 13C NMR spectroscopy, and subjected to in vitro antimicrobial assays. Our antimicrobial screening results revealed that several synthesized compounds possessed very high bactericidal activity, mainly against Gram-positive bacteria. It is worth to stress that in many cases the activity of obtained derivatives was far better than the activity of commonly used chemotherapeutic agents.
Confilct of interest
The authors declare no conflict of interest.
Acknowledgements
This project was partially supported by a research grant for young scientists granted to Dr Łukasz Popiołek (MNmb25).
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jsps.2017.05.006.
Appendix A. Supplementary material
References
- Backes G.L., Neumann D.M., Jursic B.S. Synthesis and antifungal activity of substituted salicylaldehyde hydrazones, hydrazides and sulfohydrazides. Bioorg. Med. Chem. 2014;22:4629–4636. doi: 10.1016/j.bmc.2014.07.022. [DOI] [PubMed] [Google Scholar]
- Bala S., Uppal G., Kajal A., Kamboj S., Sharma V. Hydrazones as promising lead with diversity in bioactivity-therapeutic potential in present scenario. Int. J. Pharm. Sci. Rev. Res. 2013;18(1):65–74. [Google Scholar]
- Clinical and Laboratory Standards Institute, 2012. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. M27-S4. Wayne, PA, USA.
- Coates A., Hu Y., Bax R., Page C. The future challenges facing the development of new antimicrobial drugs. Nat. Rev. Drug. Discov. 2002;1:895–910. doi: 10.1038/nrd940. [DOI] [PubMed] [Google Scholar]
- Cukurovali A., Yilmaz E. Synthesis, characterization, investigation of biological activity and theoretical studies of hydrazone compounds containing choloroacetyl group. J. Mol. Struct. 2014;1075:566–578. [Google Scholar]
- Çıkla P., Özsavcı D., Bingöl-Özakpınar Ö., Şener A., Çevik Ö., Özbaş-Turan S., Akbuğa J., Şahin F., Küçükgüzel Ş.G. Synthesis, cytotoxicity and pro-apoptosis of etodolac hydrazide derivatives as anticancer agents. Arch. Pharm. Chem. Life Sci. 2013;346:367–379. doi: 10.1002/ardp.201200449. [DOI] [PubMed] [Google Scholar]
- Deep A., Jain S., Sharma P.C.H., Verma R., Kumar M., Dora Ch.P. Design and biological evaluation of biphenyl-4-carboxylic acid hydrazide-hydrazone for antimicrobial activity. Acta Pol. Pharm. 2010;67(3):255–259. [PubMed] [Google Scholar]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. EUCAST discussion document E. Dis 5.1. Clin. Microbiol. Infect. 2003;9:1–7. [Google Scholar]
- He H., Wang X., Shi L., Yin W., Yang Z., He H., Liang Y. Synthesis, antitumor activity and mechanism of action of novel 1,3-thiazole derivatives containing hydrazide–hydrazone and carboxamide moiety. Bioorg. Med. Chem. Lett. 2016;26:3263–3270. doi: 10.1016/j.bmcl.2016.05.059. [DOI] [PubMed] [Google Scholar]
- Koçyiğit-Kaymakçıoğlu B., Oruç E., Unsalan S., Kandemirli F., Shvets N., Rollas S., Anatholy D. Synthesis and characterization of novel hydrazide–hydrazones and the study of their structure–antituberculosis activity. Eur. J. Med. Chem. 2006;41:1253–1261. doi: 10.1016/j.ejmech.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Koçyiğit-Kaymakçıoğlu B., Oruç-Emre E., Unsalan S., Rollas S. Antituberculosis activity of hydrazones derived from 4-fluorobenzoic acid hydrazide. Med. Chem. Res. 2009;18:277–286. [Google Scholar]
- Kumar D., Kapoor A., Thangadurai A., Kumar P., Narasimhan B. Synthesis, antimicrobial evaluation and QSAR studies of 3-ethoxy-4-hydroxybenzylidene/4-nitrobenzylidene hydrazides. Chin. Chem. Lett. 2011;22:1293–1296. [Google Scholar]
- Kumar D., Kumar N.M., Ghosh S., Shah K. Novel bis(indolyl)hydrazide–hydrazones as potent cytotoxic agents. Bioorg. Med. Che. Lett. 2012;22:212–215. doi: 10.1016/j.bmcl.2011.11.031. [DOI] [PubMed] [Google Scholar]
- Küçükgüzel Ş.G., Oruç E.E., Rollas S., Şahin F., Özbek A. Synthesis, characterization and biological activity of novel 4-thiazolidinones, 1,3,4-oxadiazoles and some related compounds. Eur. J. Med. Chem. 2002;37:197–206. doi: 10.1016/s0223-5234(01)01326-5. [DOI] [PubMed] [Google Scholar]
- Küçükgüzel Ş.G., Mazi A., Şahin F., Öztürk S., Stables J.P. Synthesis and biological activities of diflunisal hydrazide-hydrazones. Eur. J. Med. Chem. 2003;38:1005–1013. doi: 10.1016/j.ejmech.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Küçükgüzel Ş.G., Koç D., Çıkla P., Özsavcı D., Bingöl-Özakpınar Ö., Mega-Tiber P., Orun O., Erzincan P., Erdem S.S., Şahin F. Synthesis of tolmetin hydrazide-hydrazones and discovery of a potent inducer in colon cancer cells. Arch. Pharm. Chem. Life Sci. 2015;348(10):730–742. doi: 10.1002/ardp.201500178. [DOI] [PubMed] [Google Scholar]
- Loncle C., Brunel J.M., Vidal N., Dherbomez M., Letourneux Y. Synthesis and antifungal activity of cholesterol-hydrazone derivatives. Eur. J. Med. Chem. 2004;39:1067–1071. doi: 10.1016/j.ejmech.2004.07.005. [DOI] [PubMed] [Google Scholar]
- McCalla D.R., Reuvers A., Kaiser Ch. Mode of action of nitrofurazone. J. Bacteriol. 1970:1126–1134. doi: 10.1128/jb.104.3.1126-1134.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moellering R.C., Jr Discovering new antimicrobial agents. Int. J. Antimicrob. Agents. 2011;37:2–9. doi: 10.1016/j.ijantimicag.2010.08.018. [DOI] [PubMed] [Google Scholar]
- Mohareb R.M., El-Sharkawy K.A., Hussein M.M., El-Sehrawi H.M. Synthesis of hydrazide-hydrazone derivatives and their evaluation of antidepressant, sedative and analgesic agents. J. Pharm. Sci. Res. 2010;2(4):185–196. [Google Scholar]
- Moldovan C.M., Oniga O., Pârvu A., Tiperciuc B., Verite P., Pîrnău A., Crişan O., Bojiţă M., Pop R. Synthesis and anti-inflammatory evaluation of some new acyl-hydrazones bearing 2-aryl-thiazole. Eur. J. Med. Chem. 2011;46:526–534. doi: 10.1016/j.ejmech.2010.11.032. [DOI] [PubMed] [Google Scholar]
- Morjan R.Y., Mkadmh A.M., Beadham I., Elmanama A.A., Mattar M.R., Raftery J., Pritchard R.G., Awadallah A.M., Gardiner J.M. Antibacterial activities of novel nicotinic acid hydrazides and their conversion into N-acetyl-1,3,4-oxadiazoles. Bioorg. Med. Chem. Lett. 2014;24:5796–5800. doi: 10.1016/j.bmcl.2014.10.029. [DOI] [PubMed] [Google Scholar]
- Mukherjee D.D., Kumar N.M., Tantak M.P., Das A., Ganguli A., Datta S., Kumar D., Chakrabarti G. Development of novel bis(indolyl)-hydrazide−hydrazone derivatives as potent microtubule-targeting cytotoxic agents against A549 lung cancer cells. Biochemistry. 2016;55:3020–3035. doi: 10.1021/acs.biochem.5b01127. [DOI] [PubMed] [Google Scholar]
- Nasr T., Bondock S., Youns M. Anticancer activity of new coumarin substituted hydrazide-hydrazone derivatives. Eur. J. Med. Chem. 2014;76:539–548. doi: 10.1016/j.ejmech.2014.02.026. [DOI] [PubMed] [Google Scholar]
- Özkay Y., Tunalı Y., Karaca H., Ișıkdağ I. Antimicrobial activity and a SAR study of some novel benzimidazole derivatives bearing hydrazone moiety. Eur. J. Med. Chem. 2010;45:3293–3298. doi: 10.1016/j.ejmech.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Pavan F.R., da S. Maia P.I., Leite S.R.A., Deflon V.M., Batista A.A., Sato D.N., Franzblau S.G., Leite C.Q.F. Thiosemicarbazones, semicarbazones, dithiocarbazates and hydrazide/hydrazones: anti – mycobacterium tuberculosis activity and cytotoxicity. Eur. J. Med. Chem. 2010;45:1898–1905. doi: 10.1016/j.ejmech.2010.01.028. [DOI] [PubMed] [Google Scholar]
- Pieczonka A.M., Strzelczyk A., Sadowska B., Mlostoń G., Stączek P. Synthesis and evaluation of antimicrobial activity of hydrazones derived from 3-oxido-1H-imidazole-4-carbohydrazides. Eur. J. Med. Chem. 2013;64:389–395. doi: 10.1016/j.ejmech.2013.04.023. [DOI] [PubMed] [Google Scholar]
- Popiołek Ł., Biernasiuk A. Hydrazide-hydrazones of 3-methoxybenzoic acid and 4-tert-butylbenzoic acid with promising antibacterial activity against Bacillus spp. J. Enz. Inh. Med. Chem. 2016;31(S1):62–69. doi: 10.3109/14756366.2016.1170012. [DOI] [PubMed] [Google Scholar]
- Popiołek Ł., Biernasiuk A. Design, synthesis and in vitro antimicrobial activity of hydrazide-hydrazones of 2-substituted acetic acid. Chem. Biol. Drug Des. 2016;88:873–883. doi: 10.1111/cbdd.12820. [DOI] [PubMed] [Google Scholar]
- Popiołek Ł., Kosikowska U., Wujec M., Malm A. Synthesis and antimicrobial evaluation of new Schiff base hydrazones bearing 1,2,4-triazole moiety. Phosphorus, Sulfur Silicon Relat. Elem. 2014;189(11):1611–1623. [Google Scholar]
- Popiołek Ł., Biernasiuk A., Malm A. Synthesis and in vitro antimicrobial activity of nalidixic acid hydrazones. J. Het. Chem. 2016;53:1589–1594. [Google Scholar]
- Popiołek Ł., Stefańska J., Kiełczykowska M., Musik I., Biernasiuk A., Malm A., Wujec M. Synthesis, dissociation constants, and antimicrobial activity of novel 2,3-disubstituted-1,3- thiazolidin-4-one derivatives. J. Het. Chem. 2016;53:393–402. [Google Scholar]
- Rambabu N., Dubey P.K., Ram B., Balram B. Synthesis, characterization and antimicrobial activity of some novel hydrazone derivatives of anacardic acid. Der Pharm. Chem. 2015;7(4):90–97. [Google Scholar]
- Rasras A.J.M., Al-Tel T.H., Al-Aboudi A.F., Al-Qawasmeh R.A. Synthesis and antimicrobial activity of cholic acid hydrazone analogues. Eur. J. Med. Chem. 2010;45:2307–2313. doi: 10.1016/j.ejmech.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Rollas S., Kűçűkgűzel Ş.G. Biological activities of hydrazone derivatives. Molecules. 2007;12:1910–1939. doi: 10.3390/12081910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkauskas K., Mickevičius V., Kantminienė K., Stasevych M., Komarovska-Porokhnyavets O., Musyanovych R., Novikov V. Synthesis and antimicrobial activity of 1,3-disubstituted pyrrolidinones with hydrazone and naphthoquinone moieties. Chemija. 2013;4(1):74–80. [Google Scholar]
- Satyanarayana G.V., Rao V.L., Chary M.T., Ram B., Balram B., Chinmaiyee V. Synthesis and antimicrobial activity of novel hydrazone derivatives of 4-(4-chlorophenyl)cyclohexanecarboxylic acid. J. Appl. Chem. 2014;3(3):1232–1238. [Google Scholar]
- Şenkardes S., Kaushik-Basu N., Durmaz İ., Manvar D., Basu A., Atalay R., Küçükgüzel G.Ş. Synthesis of novel diflunisal hydrazide-hydrazones as anti-hepatitis C virus agents and hepatocellular carcinoma inhibitors. Eur. J. Med. Chem. 2016;108:301–308. doi: 10.1016/j.ejmech.2015.10.041. [DOI] [PubMed] [Google Scholar]
- Velezheva V., Brennan P., Ivanov P., Kornienko A., Lyubimov S., Kazarian K., Nikonenko B., Majorov K., Apt A. Synthesis and antituberculosis activity of indole–pyridine derived hydrazides, hydrazide–hydrazones, and thiosemicarbazones. Bioorg. Med. Chem. Lett. 2016;26:978–985. doi: 10.1016/j.bmcl.2015.12.049. [DOI] [PubMed] [Google Scholar]
- Wardakhan W.W., El-Sayed N.N.E., Mohareb R.M. Synthesis and anti-tumor evaluation of novel hydrazide and hydrazide-hydrazone derivatives. Acta Pharm. 2013;63:45–57. doi: 10.2478/acph-2013-0004. [DOI] [PubMed] [Google Scholar]
- Wiegand I., Hilpert K., Hancock R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008;3(2):163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.