Abstract
Enzyme replacement therapy (ERT) is a newly approved disease-modifying treatment for hypophosphatasia (HPP), a rare metabolic bone disorder. With an orphan drug and ultra-rare disease, sharing information about responders and non-responders is particularly important, as any one centre's familiarity with its use will be limited. Nearly all published data in infants and very young children with life-threatening HPP are from three small clinical trials that have reported generally positive outcomes. We describe in detail a patient with perinatal HPP for whom treatment with ERT was not successful. Lessons learned from this case can inform clinical decision-making and provide topics for the research agenda. We also discuss practical and ethical challenges related to treatment of an ultra-rare disease with an expensive new medication in a publicly funded healthcare system.
Keywords: Hypophosphatasia, Alkaline phosphatase, ALPL, Skeletal dysplasia, Enzyme replacement therapy, Asfotase alfa, Orphan drug
1. Introduction
Hypophosphatasia (HPP; MIM#241500) is a rare metabolic disorder characterized by hypophosphatasemia and consequent defective skeletal mineralization [1]. The ALPL gene (MIM#171760) encodes the tissue-nonspecific isozyme of alkaline phosphatase (TNSALP), and mutations in ALPL are currently the only known cause of HPP [1], [2]. The phenotypic spectrum of HPP is divided into six clinical categories, with perinatal HPP being the most severe and odontohypophosphatasia the least debilitating overall [1], [3]. Genotype-phenotype correlations exist but are imperfect [4], [5], [6]. Historically, perinatal HPP was considered a lethal condition, with most dying soon after birth of restrictive lung disease [7], [8].
Until recently, management of all forms of HPP was entirely symptomatic. There are now promising results with a disease-modifying treatment: enzyme replacement therapy (ERT) with recombinant human bone-targeted TNSALP (asfotase alfa, Strensiq®; Alexion Pharmaceuticals, Inc.) [1], [9]. Regulatory bodies in Canada, the European Union, the United States, and Japan approved asfotase alfa for treatment of paediatric-onset HPP in 2015. To date, most available data regarding perinatal HPP are from three open-label phase II studies [10], [11], [12]. The initial clinical trial publication described treatment outcomes of ten children with perinatal or infantile disease, where all but one required breathing support at baseline [10]. Treatment with a one-time dose of 2 mg/kg IV, followed by 1–3 mg/kg/dose SC three times a week, resulted in radiographic evidence of skeletal healing in nine of ten participants. The sole case fatality in the study period was attributed to sepsis. After 48 weeks, six of nine were breathing ambient air without ventilator support while only one remained on full mechanical ventilation. In a follow-up publication that included additional individuals, 16 of 21 with perinatal or infantile HPP who required ventilator assistance but were treated with asfotase alfa survived for over 1 year [11]. Twelve of these 16 participants were successfully extubated. Similarly positive results have since been reported by a Japanese group, which conducted an open-label single-arm prospective study that included 6 individuals with perinatal HPP (and 13 individuals overall) [12].
To date, factors that influence treatment response and outcome are unknown, and when and how to declare treatment non-response is unclear. We report a patient with perinatal HPP for whom treatment with asfotase alfa was not successful. Based on this experience, we identify lessons learned that may help to inform the initiation and monitoring of treatment of affected neonates.
2. Case report
The infant was born to healthy non-consanguineous parents of Palestinian descent. An older sister is healthy, and the family history was non-contributory. The early pregnancy was uncomplicated. A level II fetal ultrasound at approximately 18–20 weeks gestation was reportedly normal, and throughout the pregnancy fetal movements were unremarkable. Another fetal ultrasound was performed at 40 weeks 2 days gestation for an unclear indication, and showed polyhydramnios and short femurs (65 mm; corresponding to 33 weeks 2 days gestation). Labour was induced at a hospital with a level II neonatal intensive care unit (NICU), and a baby girl was born at 40 weeks 3 days gestation by vaginal delivery. Birth weight (3310 g), length (49 cm), and head circumference (34 cm) were all near the 50th centile. There was a nuchal cord. Apgar scores were 7 and 8 at one and 5 min, respectively. She had significant subcostal retractions and poor air entry bilaterally. She required continuous positive airway pressure (CPAP) and fraction of inspired oxygen (FiO2) up to 0.8 in the immediate neonatal period. The NICU team arrived at 10 min of life. A skeletal dysplasia was suspected on the basis of the chest x-ray and an abnormal physical examination, with depressible skull bones, a large anterior fontanelle, a bell-shaped chest, and the appearance of rhizomelia. Over her first two days of life, she was stable on CPAP with positive end-expiratory pressure (PEEP) 5–8 cm H2O and FiO2 0.3–0.4. Her work of breathing decreased and her rate of breathing improved. Serial arterial gases revealed a pH within the normal range and pCO2 < 50 mmHg. For example, on day 2 the result was pH 7.36, pCO2 47 mmHg, pO2 70 mmHg, and bicarbonate 29 mmol/L (on FiO2 0.42). On day 3, she had increasing oxygen requirements slowly over a period of hours. Her work of breathing worsened, with severe subcostal retractions and tachypnea up to 100–110 breaths per minute. A chest x-ray reportedly showed new severe confluent airspace opacities and ground glass infiltrates. Her arterial blood gas result was pH 7.07, pCO2 103 mmHg, pO2 68 mmHg, and bicarbonate 30 mmol/L (on FiO2 0.98). She was intubated and ventilated for hypoxic hypercarbic respiratory failure, and repeat imaging revealed improved aeration but a persistent ground-glass appearance. She was transferred to our level IV NICU with pressure control tidal volumes between 15 and 20 mL, PEEP 6 cmH2O, and FiO2 0.55. The blood gas result at that time was pH 7.44, pCO2 43 mmHg, pO2 63 mmHg, and bicarbonate 29 mmol/L.
Initial laboratory investigations at our hospital were notable for an undetectable serum ALP level, with normal serum calcium and phosphate. Urine phosphoethanolamine was markedly elevated at 551 (range 1–63 mmol/mol cre). Plasma pyridoxal-5-phosphate (PLP) was elevated at 2660 (range 20–96 nmol/L). Plasma pyrophosphate (PPi) was not measured, as the assay is not commercially available. Skeletal x-rays on day 4 showed significantly diminished skull ossification, thin poorly mineralized ribs, and flaring and rachitic changes at the ends of the long bone metaphyses (Fig. 1 and Fig S1). No fractures were identified. There were no major findings on brain MRI, echocardiogram, or abdominal ultrasound done in the first week of life. She was diagnosed clinically with perinatal HPP. Later, targeted sequencing of ALPL and parental testing confirmed compound heterozygous pathogenic variants in the proband: c.[1171C>T];[1348C>T] / p.[(Arg391Cys)];[(Arg450Cys)] (NM_000478.4). Our patient's genotype is not previously reported. Her specific heterozygous variants were experimentally associated with 10.3% and 4.0% of wild-type enzyme activity, respectively, predicting a severe phenotype [4].
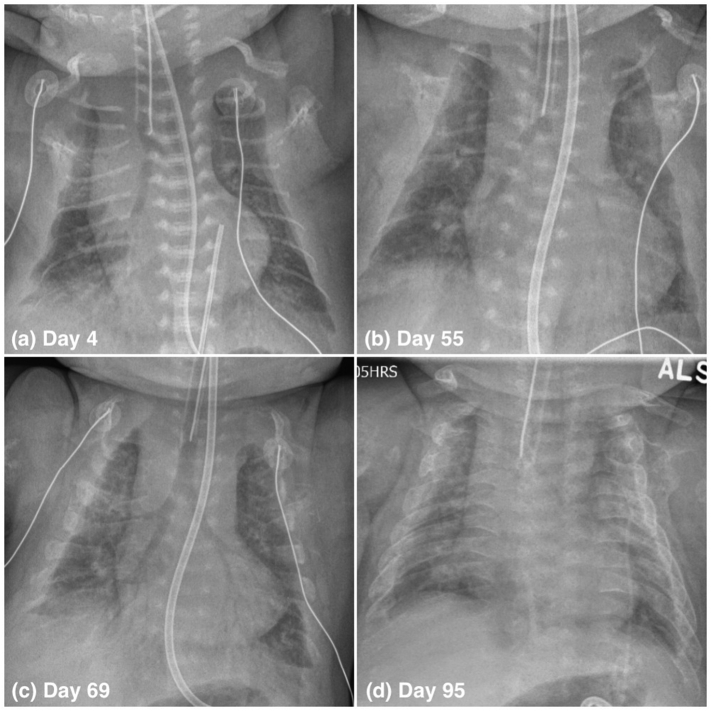
Fig. 1.
Chest x-rays of an infant with perinatal HPP treated with ERT, on days 4 (a), 55 (b), 69 (c), and 95 (d) of life. Improvement in ossification of the ribs first occurred between days 55 and 69. See text for details.
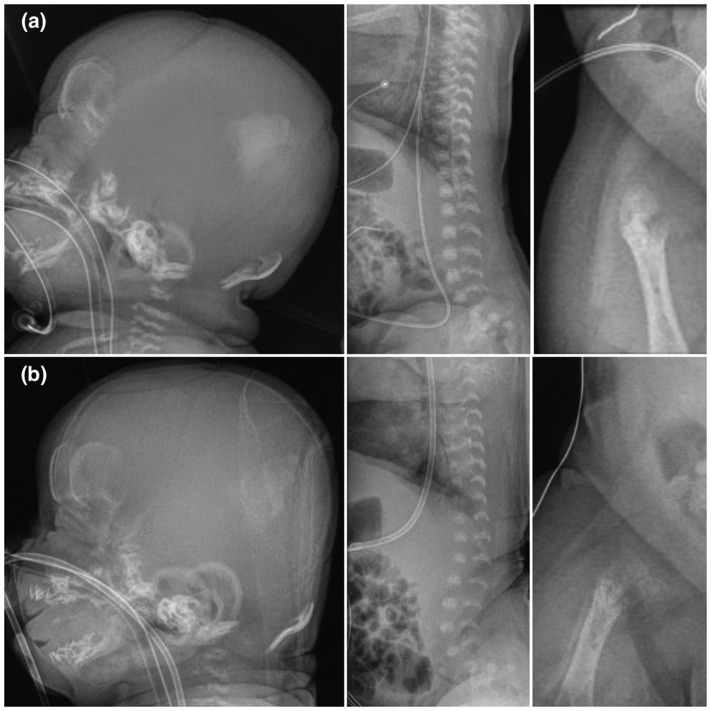
Fig. S1.
Selected additional x-rays of an infant with perinatal HPP treated with ERT. (a) On day 4, there was extremely poor ossification, including of the skull bones and vertebral bodies, and metaphyseal widening and irregularities in the long bones. (b) By day 59, there had been a mild interval increase in the degree of mineralization of the parietal and frontal bones, and there was evidence of periosteal reaction involving the long bones. Otherwise, there was a generalized reduction in bone mineralization affecting the entire skeleton with worsening osteopenia, irregularity, and fragmentation involving the metaphyses of the long bones. See text and Fig. 1 for additional information.
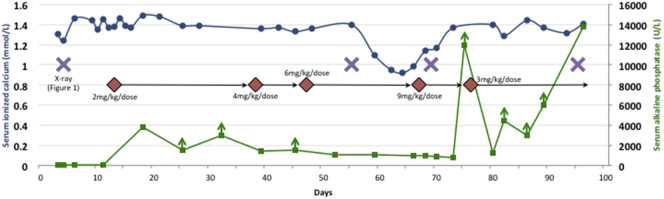
Expert consultation was sought from author C.R-G. The infant was considered a suitable candidate for ERT based on her initial chest x-rays, which showed a degree of pulmonary hypoplasia consistent with some previous treated neonates, and the minimal pressures required for ventilation. However, concerns were raised about cost (including of prolonged NICU care) and resource allocation. Uncertainties surrounding treatment with asfotase alfa were ultimately outweighed by the certainty of the outcome without treatment. The goals of ERT were to prolong life, help to wean from the ventilator, allow for good quality of life, and improve bone quality. A family meeting was held on day 8, and the parents elected to proceed with treatment. Asfotase alfa was initiated at 2 mg/kg/dose SC three times a week on day 13. The dose was sequentially increased to a supraphysiologic level in the absence of signs of clinical improvement, to a maximum of 9 mg/kg/dose on day 67 (Fig. 2). This decision was informed by the facts that the initial lower doses were well tolerated, that there remained no alternative therapies, and that author C.R-G. had prior positive clinical experiences increasing the dose of ERT. Throughout the treatment course, she was weaned from a maximum PEEP of 10 cmH2O to 7 cmH2O, but she did not tolerate further attempts at weaning. There were no significant episodes of aspiration pneumonia, severe atelectasis, or infection. Spontaneous breathing trials on days 55 and 56 were unsuccessful, with immediate respiratory distress. A chest x-ray on day 55 (Fig. 1) and additional skeletal x-rays on day 59 (Fig. S1) showed a generalized reduction in bone mineralization affecting almost the entire skeleton, with worsening osteopenia. She required immediate re-intubation after an unplanned extubation on day 69. The resulting chest x-ray showed some improvement in rib ossification compared with two weeks prior (Fig. 1). The ERT was decreased to 3 mg/kg/dose on day 75 because of very high ALP levels (> 12,000 U/L) consistent with drug absorption (Fig. 2).
Fig. 2.
Line graph of serum ionized calcium (left horizontal axis) and serum ALP (right horizontal axis) over time. ALP was measured on the Abbott Architect® system using an enzyme activity assay. An ALP level < 20 U/L was undetectable. Levels reported as greater than a value are denoted with vertical arrows, and were not reported as exact values because there was insufficient sample quantity for further dilution. Changes in the dosing of asfotase alfa and selected x-rays are also indicated. See text and Fig. 1 for details.
Irritability was a significant component of her presentation and was managed with opiates. She was more distressed, with an increased oxygen requirement, during nursing interventions. Her disease course was otherwise notable for an absence of seizures. For a period of time, expressed breast milk was supplemented with a calcium-restricted formula because of hypercalcaemia. Later, calcium supplementation was started on day 59 because of hypocalcaemia (Fig. 2) suggestive of “hungry bones”. This has been seen previously in reported patients with infantile HPP early in the course of treatment, when skeletal mineralization starts to occur in response to asfotase alfa [10], [12]. There was no evidence of calcium deposition on serial renal imaging and ocular examinations. There was also no evidence of increased intracranial pressure, a potential complication of craniosynostosis, on ocular exam. Feeding intolerance resulted in an uncomplicated gastrostomy tube insertion on day 90. There were no definite drug-related serious adverse events. We did not test for anti-asfotase alfa antibodies.
On day 95, the chest x-ray showed further interval increase in bone formation and ossification, but a persistently small chest size with accompanying suspected pulmonary hypoplasia (Fig. 1). Her ventilator settings and blood gas results had remained grossly unchanged for months. For example, on day 93 she was found to have pH 7.39, pCO2 40 mmHg, pO2 66 mmHg, and bicarbonate 23 mmol/L, in the setting of PEEP 7 cmH2O, peak inspiratory pressure 21 cmH2O, mean airway pressure 13 cmH2O, tidal volume 4.7 mL/kg, and FiO2 0.21. She was no longer receiving opiates for pain or other sedating medications. Pulmonary function could not be further assessed in the setting of intubation. There was no evidence of pulmonary hypertension on repeat echocardiogram, with an inability to estimate right ventricular systolic pressure due to inadequate tricuspid regurgitation jet, a round septum throughout the cardiac cycle, normal pulmonary vascular resistance (right ventricular ejection time (RVET)/pulmonary artery acceleration time (PAAT) ratio of 2.5), and normal right ventricular systolic function. She remained unable to be placed in the sitting position without associated respiratory distress. The attending physicians were concerned about the potential for progressive damage to her alveoli secondary to prolonged mechanical ventilation, and the likelihood of long-term – if not life-long - ventilator dependence. The parents were opposed to long-term ventilation. Her peripheral limb movements were limited, and she was not observed to move her hands against gravity. The prognosis with respect to gross and fine motor outcome was therefore also guarded.
Multidisciplinary meetings with a bioethicist explored the complex ethical issues associated with this case. In particular, the NICU team wrestled with determining what would constitute benefit, what would be a reasonable amount of time to allow for the realization of this benefit, and whether the potential benefits would outweigh the ongoing harms. Potential conflicts of interest of physicians who were involved with prior clinical trials, and who should determine what is in the best interests of the baby, were extensively discussed. The parents consulted with a religious leader in their community. Lack of a sustainable funding model for asfotase alfa did not play a role in decision-making. Ultimately, the team recommended a plan to stop ERT, extubate, and provide supportive care. She died on day 100. Post-mortem examination was declined.
3. Discussion
3.1. Analysis of an unsuccessful course of treatment
The natural history of untreated perinatal HPP is one of progressive skeletal demineralization. Such demineralization, and associated clinical deterioration, was seen early in the treatment courses of critically ill patients with life-threatening HPP enrolled in the clinical trials [10], [11]. With ongoing respiratory support and dose adjustment, ultimately their respiratory statuses improved and the vast majority continued on to successful extubation. Five years or more follow-up of such children continues to show excellent clinical outcomes with very good safety profiles (C.R-G., personal communication). The clinical and radiologic deterioration initially seen in our patient was not unexpected, and the hypocalcaemia (Fig. 2) was initially felt to be a good prognostic sign. Importantly, an improvement in mineralization was seen only after increasing the ERT dose much higher than what was in the protocols in the trials [10], [11]. The failure of growth of the lungs and chest wall, with no improvement in lung function and an inability to spontaneously breathe after three months in spite of good rib mineralization and low ventilatory settings, is what ultimately led to the decision to stop ERT. It is possible, but was felt to be very unlikely, that continued treatment with ERT would have altered the outcome for this infant.
The cause of poor chest wall growth is not readily apparent. One possibility is failure of postnatal alveolar development, as has been described in bronchopulmonary dysplasia (albeit associated with a supplemental oxygen requirement) [13]. Abnormal lung mechanics in the setting of lung hypoplasia is also a consideration (see below). This poor clinical course has been previously seen in an unrelated child with molecularly confirmed perinatal HPP (homozygous for p.(Gly334Asp) in ALPL) treated aggressively from birth until 13 months of age with escalating ventilatory support and asfotase alfa (C.R-G., personal communication). Marked decreased alveolarization consistent with severe pulmonary hypoplasia was noted on postmortem lung biopsy. Genetic variability at the ALPL locus and elsewhere in the genome may play a role in treatment response, as it does in modulating baseline disease severity [5], [6], in both direct and indirect ways. For example, genetic factors may contribute to arrest of alveolar septation [14] or influence the metabolism of ALPL substrates like inorganic pyrophosphates [6]. An International HPP Registry was recently established, which will act as a genotype-phenotype registry and include data regarding treatment response and outcome [15]. Future research studies may be able to use this Registry to search for genome-wide modifiers of treatment response.
Careful medical management remains paramount in the era of asfotase alfa. In spite of the excellent early results of treatment of life-threatening HPP with ERT, there will continue to be infants for whom treatment will not be effective even with assisted ventilation, most likely due to irreversible lung hypoplasia. It can be difficult to isolate the effectiveness of the ERT from that of the remainder of the care plan. We cannot rule out the possibility that anti-asfotase alfa antibodies contributed to initial treatment non-response, but this was not found to be a major determinant of treatment outcomes in the clinical trials [10], [11], [12]. Our paediatric pulmonology team was involved in this patient's care. However, because of the absence of prenatal detection, the birth at a peripheral hospital and subsequent need for transfer, and the initial diagnostic uncertainty, the early respiratory management was not informed by what is known about the lung pathology in HPP. In retrospect, the deterioration on day 3 of life may have been secondary to the relatively high PEEP. Throughout the admission we had difficulty assessing lung volumes and function in our intubated patient, and alternative techniques are deserving of further study in this population [16]. We recommend future care providers ensure early coordination of respiratory management and make special efforts to obtain objective lung parameters [16], given the strong likelihood of pulmonary hypoplasia in perinatal HPP.
Given the many uncertainties in the clinical course of perinatal HPP, we initially sought a framework to assess response to ERT. In the absence of one, we created a preliminary treatment and monitoring guide for use in clinical practice (Box 1). A first draft of a feasible monitoring schedule was created out of necessity several weeks into the management of our patient, based on discussions between the care teams and the expert consultant (C.R-G.). It was later refined post-mortem after reviewing the clinical course in its entirety, hospital-wide rounds featuring a panel including a neonatologist, clinical geneticist, and bioethicist, and review of the literature. We used clinical (e.g., response to handling, ventilator settings), biochemical (e.g., ALP level), and radiologic (e.g., mineralization of ribs on chest x-ray) parameters as markers of treatment response. Direct assays of TNSALP may be preferable to measuring serum ALP but in our case this could not be done in the hospital's laboratory. The proposed tool is intended to be generalizable to other tertiary care paediatric centres where asfotase alfa may be used, and to serve as a practical framework to promote inter-collegial and inter-professional communication. We anticipate future progress in the field that will help to refine our suggestions. Systematic data collection may also enhance our understanding of the course under treatment.
Box 1.
Proposed asfotase alfa treatment initiation and monitoring guide for hospitalized patients with a confirmed diagnosis of life-threatening HPP.
|
3.2. Using orphan drugs in clinical practice
There are practical and ethical challenges related to using an expensive new medication for an ultra-rare disorder in a publicly funded healthcare system. Treatment decisions take place within a larger clinical and societal context [17], and are often complicated by a relative lack of robust clinical data [18]. An initial consideration is the clinical status of the baby with perinatal HPP after delivery, with careful evaluation with respect to likelihood of good response to asfotase alfa. As shown in our case, specific variables include lung volume on the admission x-ray and ventilator settings. The ALPL genotype and other genetic testing results are not currently used in decision-making but may become important in the future. These patient factors, the family's perceptions of risks and benefits, and access to funding to support the high cost of treatment, all play important roles in the initiation of treatment. Positive data are emerging on outcomes after treatment [10], [11], [12], [19], with 7 years of follow-up in some cases, but questions about quality of life and long-term sustainability cannot yet be answered. It is important to establish at the outset hard endpoints and measures of treatment efficacy. Given the early published success with ERT, a trial of such treatment is likely indicated in most infants with perinatal and life-threatening HPP. The principles of a long-term treatment plan, formulated by the multidisciplinary team in collaboration with the family, require better articulation and refinement. We recommend engaging in multidisciplinary discussions with a bioethicist early on to consider the complex ethical questions and uncertainties, and regular engagement with parents as part of a shared decision-making process (Box 1).
Approval of an orphan drug does not guarantee accessibility. Asfotase alfa was not on our hospital's drug formulary, and there was no prior experience using this treatment. Data on efficacy for orphan drugs may be limited or underwhelming, but bolstered by arguments related to a “rule of rescue” and equity of access. Fortunately, our institution has a decision-making framework for the approval of costly off-formulary medications. Having a policy already in place was helpful in distancing frontline workers from this difficult decision, without discounting their perspectives. We also discovered early on that the drug insurance plan of the parents might not cover asfotase alfa. The question was raised whether the hospital would have a moral obligation to provide the drug on discharge if effective. Asfotase alfa offers the promise of turning life-threatening HPP into a treatable disease, but given the costs formal guidelines are needed to assist clinicians with decisions about initiation and withdrawal of ERT. For example, in Canada national committees of metabolic experts have provided oversight of all ERT for Gaucher, infantile Pompe, and Fabry disease. We would support a similar coordinated strategy for paediatric-onset HPP. Different considerations may apply to other countries and practice settings, and will influence the medical and extra-medical factors that inform treatment decisions with orphan drugs.
4. Conclusions
Asfotase alfa is a revolutionary treatment for HPP, and thoughtful application will help to maximize therapeutic benefit. Yet, as with other new and orphan drugs, sharing information on non-responders is essential when any one centre's experience will be limited and the initial literature may be skewed towards reports of positive outcomes. Lessons learned may be generalizable to novel pharmacotherapy for other rare diseases.
The following are the supplementary data related to this article.
Conflicts of interest
Author C.R-G. sits on the Scientific Advisory Board of Alexion Pharmaceuticals and patient advocacy groups, is a principal investigator of industry-sponsored clinical trials (Alexion Pharmaceuticals; Shire; Vitaflo; Actelion), and has received honoraria for invited symposia and travel from Alexion Pharmaceuticals. P.K. has received honoraria for invited symposia from Alexion Pharmaceuticals. The other authors have no conflicts of interest to declare.
Funding sources
Not applicable.
Acknowledgments
Acknowledgements
The authors thank the patient's family for their participation and feedback on this manuscript, and the many healthcare professionals involved in her care.
References
- 1.Whyte M.P. Hypophosphatasia - aetiology, nosology, pathogenesis, diagnosis and treatment. Nat. Rev. Endocrinol. 2016;12:233–246. doi: 10.1038/nrendo.2016.14. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg C.R., Taylor C.L., Haworth J.C. A homoallelic Gly317-->Asp mutation in ALPL causes the perinatal (lethal) form of hypophosphatasia in Canadian mennonites. Genomics. 1993;17:215–217. doi: 10.1006/geno.1993.1305. [DOI] [PubMed] [Google Scholar]
- 3.Whyte M.P., Zhang F., Wenkert D. Hypophosphatasia: validation and expansion of the clinical nosology for children from 25 years experience with 173 pediatric patients. Bone. 2015;75:229–239. doi: 10.1016/j.bone.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 4.Zurutuza L., Muller F., Gibrat J.F. Correlations of genotype and phenotype in hypophosphatasia. Hum. Mol. Genet. 1999;8:1039–1046. doi: 10.1093/hmg/8.6.1039. [DOI] [PubMed] [Google Scholar]
- 5.Wenkert D., McAlister W.H., Coburn S.P. Hypophosphatasia: nonlethal disease despite skeletal presentation in utero (17 new cases and literature review) J. Bone Miner. Res. 2011;26:2389–2398. doi: 10.1002/jbmr.454. [DOI] [PubMed] [Google Scholar]
- 6.Mornet E. Molecular genetics of hypophosphatasia and phenotype-genotype correlations. In: Fonta C., Dordrecht Négyessy L., editors. Neuronal Tissue-Nonspecific Alkaline Phosphatase (TNAP) Springer; 2015. pp. 25–43. [Google Scholar]
- 7.Taketani T., Onigata K., Kobayashi H. Clinical and genetic aspects of hypophosphatasia in Japanese patients. Arch. Dis. Child. 2014;99:211–215. doi: 10.1136/archdischild-2013-305037. [DOI] [PubMed] [Google Scholar]
- 8.Leung E.C., Mhanni A.A., Reed M. Outcome of perinatal hypophosphatasia in Manitoba Mennonites: a retrospective cohort analysis. JIMD Rep. 2013;11:73–78. doi: 10.1007/8904_2013_224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott L.J. Asfotase alfa in perinatal/infantile-onset and juvenile-onset hypophosphatasia: a guide to its use in the USA. BioDrugs. 2016;30:41–48. doi: 10.1007/s40259-016-0161-x. [DOI] [PubMed] [Google Scholar]
- 10.Whyte M.P., Greenberg C.R., Salman N.J. Enzyme-replacement therapy in life-threatening hypophosphatasia. N. Engl. J. Med. 2012;366:904–913. doi: 10.1056/NEJMoa1106173. [DOI] [PubMed] [Google Scholar]
- 11.Whyte M.P., Rockman-Greenberg C., Ozono K. Asfotase alfa treatment improves survival for perinatal and infantile hypophosphatasia. J. Clin. Endocrinol. Metab. 2016;101:334–342. doi: 10.1210/jc.2015-3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitaoka T., Tajima T., Nagasaki K. Safety and efficacy of treatment with asfotase alfa in patients with hypophosphatasia: results from a Japanese clinical trial. Clin. Endocrinol. 2017;87:10–19. doi: 10.1111/cen.13343. [DOI] [PubMed] [Google Scholar]
- 13.Bourbon J., Boucherat O., Chailley-Heu B., Delacourt C. Control mechanisms of lung alveolar development and their disorders in bronchopulmonary dysplasia. Pediatr. Res. 2005;57:38R–46R. doi: 10.1203/01.PDR.0000159630.35883.BE. [DOI] [PubMed] [Google Scholar]
- 14.KH Yu, Li J., Snyder M., Shaw G.M., O'Brodovich H.M. The genetic predisposition to bronchopulmonary dysplasia. Curr. Opin. Pediatr. 2016;28:318–323. doi: 10.1097/MOP.0000000000000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kishnani P., Langman C., Linglart A. A longitudinal, prospective, long-term registry of patients with hypophosphatasia. Bone Abstr. 2015;4:154. [Google Scholar]
- 16.Rodriguez E., Bober M.B., Davey L. Respiratory mechanics in an infant with perinatal lethal hypophosphatasia treated with human recombinant enzyme replacement therapy. Pediatr. Pulmonol. 2012;47:917–922. doi: 10.1002/ppul.22527. [DOI] [PubMed] [Google Scholar]
- 17.Zlotnik Shaul R., Vitale D. Can we afford it?: ethical consideration of expensive drug treatment for neonates and infants. Clin. Pharmacol. Ther. 2009;86:587–589. doi: 10.1038/clpt.2009.211. [DOI] [PubMed] [Google Scholar]
- 18.Janoudi G., Amegatse W., McIntosh B., Sehgal C., Richter T. Health technology assessment of drugs for rare diseases: insights, trends, and reasons for negative recommendations from the CADTH common drug review. Orphanet J. Rare Dis. 2016;11:164. doi: 10.1186/s13023-016-0539-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okazaki Y., Kitajima H., Mochizuki N. Lethal hypophosphatasia successfully treated with enzyme replacement from day 1 after birth. Eur. J. Pediatr. 2016;175:433–437. doi: 10.1007/s00431-015-2641-2. [DOI] [PubMed] [Google Scholar]