Introduction
Netherton syndrome (NS), also known as Comèl-Netherton syndrome, was clinically described in 1964 by Wilkinson et al1 and is characterized by the triad of ichthyosis linearis circumflexa, trichorrhexis invaginata, and atopic diathesis.1 The single entities, ichthyosis linearis circumflexa and the “bamboo hair” were previously described by Comèl2 in 1949 and Netherton3 in 1958, respectively.
The incidence of NS is 1 per 200,000 births4; however, this number is probably underestimated. Not only is NS a challenging diagnosis, but it is also easily misdiagnosed as atopic dermatitis.4
The clinical presentation of NS is variable. In most patients, a generalized erythroderma and scaling is observed at birth or soon after and may be associated with potentially fatal complications, such as hypernatremia, altered thermoregulation, failure to thrive, and sepsis. Ichthyosis linearis circumflexa develops gradually and is characterized by a serpiginous or circinate scaling and pruriginous polycyclic erythematous plaques with a double-edged scale, frequently distributed on the trunk and extremities. Moreover, many patients present with eczematous plaques in flexural sites, resembling atopic dermatitis. Hair shaft abnormalities, characteristically trichorrhexis invaginata, are predominantly found in infancy or early childhood. Scalp hair grows slowly and is sparse, thin, and fragile. Lastly, immune imbalance is also a characteristic feature of NS and includes elevation of IgE, eosinophilia, and allergic reactions.5 We present the case of a 19-year-old woman with NS treated with infliximab.
Case report
A 19-year-old white woman was referred to our outpatient dermatology clinic with an undiagnosed skin eruption since early childhood. She was born from normal delivery after an uneventful pregnancy from healthy nonconsanguineous parents and did not present any cutaneous lesions at birth. Her sister has a similar dermatosis, which also appeared during childhood.
Clinical examination found a disseminated cutaneous eruption characterized by polycyclic erythematous plaques with a scaling edge, predominantly involving the thighs, trunk, and folds (including symmetrical antecubital lesions). Skin lesions were migratory and were accompanied by pruritus. She also had sparse, thin, fragile hair.
Blood analyses found elevation of total IgE levels to 686 UI/mL (normal range, 150-300 UI/mL). Light microscopic examination of clipped hair from the scalp found bamboo hair with a ball-and-socket appearance (Fig 1).
Fig 1.
Netherton syndrome. Light microscopic examination of scalp hair: “bamboo hair.”
Two skin biopsies were performed, one on the arm and the other on the thigh. The biopsy of the arm found an unilocular subcorneal spongiform pustule with a psoriasiform pattern, whereas the other found irregular acanthosis of epidermis with parakeratosis foci. Immunostaining of skin biopsy specimens with LEKTI antibodies found a weak and focal expression (Fig 2).
Fig 2.
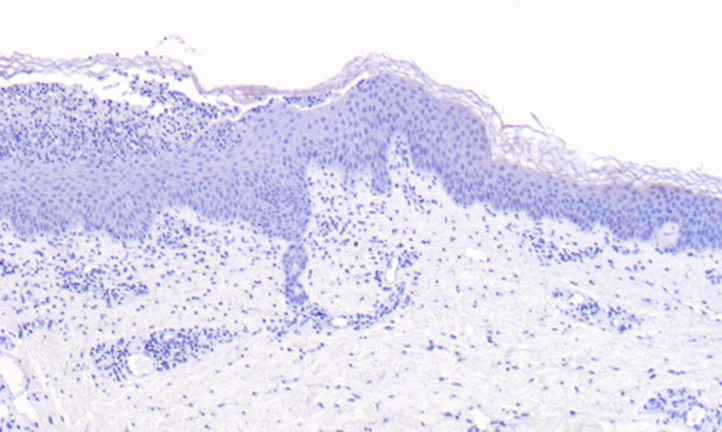
Netherton syndrome. Immunofluorescence staining with polyclonal antibody against LEKTI: very weak and focal expression of LEKTI in the epidermis.
DNA molecular analysis of the serine protease inhibitor Kazal type 5 (SPINK5) gene confirmed the diagnosis of Netherton syndrome, showing heterozygosity for pathogenic mutations in exon 11 and intron 15, c.891C>T and c.1431-12G>A, respectively.
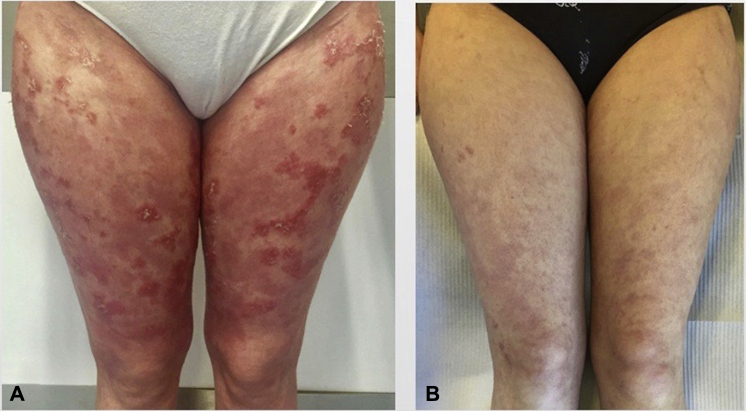
Topical tacrolimus was initiated, but its percutaneous absorption in a patient with a significant skin barrier dysfunction made its continuation challenging. In addition, in the past, administration of oral isotretinoin did not result in clinical improvement of cutaneous lesions. As such, infliximab infusions were administered at a dose of 5 mg/kg in weeks 0, 2, and 6 and continued every other 8 weeks. Clinical improvement was observed after the second infusion, with fewer inflammatory lesions, desquamation, and pruritus. At week 22, the skin was almost cleared of inflammatory lesions (Fig 3), and the patient mentioned stronger and longer hair.
Fig 3.
Netherton syndrome. Clinical response to infliximab. A, At week 0; B, at week 22 (fewer inflammatory lesions and desquamation).
Discussion
NS is an autosomal recessive genodermatosis, caused by mutations in the SPINK5 gene. This gene encodes the multidomain serine protease inhibitor LEKTI,4 which is predominantly expressed in the lamellar granules of epithelium and in thymus. Consequently, immunostaining of skin biopsy specimens with LEKTI antibodies shows absence or reduced distribution of LEKTI protein in the epidermis of affected patients.6
The lack of functional LEKTI leads to uncontrolled proteolytic activity in the stratum corneum and premature corneodesmosome cleavage and is responsible for increased desquamation and loss of skin barrier function.7 Altered epidermal barrier function results in loss of essential anti-inflammatory and antimicrobial mechanisms, which contributes to recurrent bacterial infections.5
Recent studies found that thymic stromal lymphopoietin (TSLP) and tumor necrosis factor (TNF)-α are overexpressed in the epidermis of NS patients.8, 9 These findings might be explained by the unrestricted activity of epidermal proteases, including kallikrein 5 (KLK5), caused by LEKTI deficiency. Consequently, hyperactive KLK5 induces protease-activated receptor 2 (PAR2) signaling by proteolytic cleavage, leading to factor nuclear kappa B activation and TSLP and TNF-α expression.9 Both overexpression of TSLP and thymic expression of the mutated SPINK5 gene, seem to promote an excessive helper T cell (Th) 2 response and elevation of serum IgE levels.10 Therefore, the KLK5–PAR2–TSLP signaling pathway appears to play a key role in the development of atopic manifestations in NS.9
The exact mechanism by which LEKTI deficiency causes hair shaft abnormalities remains unclear. Hair shaft analysis of NS patients finds a reduced number of S-S disulfide bonds in cortical fibers, probably because of increased cleavage of cross-linkage of hair keratin structures, leading to a focal weakness of the hair shaft with consequent invagination of the fully keratinized distal hair shaft into the weaker proximal hair shaft.5 These features correspond to trichorrhexis invaginata, a highly characteristic light microscopic finding of clipped hair.
The histologic features of NS are variable and unspecific. According to the most recent histologic review of NS, the most frequent histologic finding in skin biopsies is psoriasiform hyperplasia. However, other less common findings may be observed, such as compact parakeratosis with large nuclei, subcorneal or intracorneal splitting, the presence of clear cells in the upper epidermis or stratum corneum, dyskeratosis, dermal infiltrate with neutrophils or eosinophils, and dilated blood vessels in the superficial dermis. Considering the histologic diversity, LEKTI staining is mandatory for the definitive diagnosis of NS.11
Treatment of NS represents a great challenge. Several therapeutic options, such as topical corticosteroids, topical calcineurin inhibitors, topical and systemic retinoids, and phototherapy, have been used with variable success and nonnegligible toxicity.
Recent studies suggest that targeting key molecules involved in factor nuclear kappa B activation, such as TNF-α, may reduce skin inflammation in NS.8, 9 To date, only one previous report has been published concerning the successful use of infliximab, a recombinant humanized monoclonal anti–TNF-α antibody, in NS. In that work, Fontao et al.9 found that infusions of infliximab decrease the expression of TSLP, interleukin-6, and interleukin-8 in the skin with a remarkable and prolonged improvement of cutaneous lesions, probably in association with a shift of the Th2 systemic immune response towards a Th1 profile.9 Similarly, in our patient, there was an improvement of the skin lesions and an enhancement of hair growth after infliximab infusions. However, cytokines were not measured.
The administration of drugs targeting specific molecules involved in inflammatory pathways on NS, seems promising in controlling disease activity and minimizing adverse effects. Our case shows the potential use of infliximab in NS. Nonetheless, further studies are necessary to support the efficacy and safety profile of these drugs on NS.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Wilkinson R.D., Curtis G.H., Hawks W.A. Netherton's disease: Trichorrhexis invaginata (bamboo hair), congenital ichthyosiform erythroderma and the atopic diathesis. A histopathologic study. Arch Dermatol. 1964;89:46–54. doi: 10.1001/archderm.1964.01590250052010. [DOI] [PubMed] [Google Scholar]
- 2.Comèl M. Ichthyosis linearis circumflexa. Dermatologia. 1949;98(3):133–136. [PubMed] [Google Scholar]
- 3.Netherton E.W. A unique case of trichorrhexis nodosa; bamboo hairs. AMA Arch Derm. 1958;78(4):483–487. doi: 10.1001/archderm.1958.01560100059009. [DOI] [PubMed] [Google Scholar]
- 4.Sarri C.A., Roussaki-Schulze A., Vasilopoulos Y. Netherton syndrome: a genotype-phenotype review. Mol Diagn Ther. 2017;21(2):137–152. doi: 10.1007/s40291-016-0243-y. [DOI] [PubMed] [Google Scholar]
- 5.Richard G., Ringpfeil F. Ichthyosis, erythroleratodermas and related disorderes. In: Bolognia J.L., Jorizzo J.L., Schaffer J.V., editors. Dermatology. 3rd Ed. Mosby Elsevier Publishing; 2012. pp. 837–870. [Google Scholar]
- 6.Ong C., O'Toole E.A., Ghali L. LEKTI demonstrable by immunohistochemistry of the skin: a potential diagnostic skin test for Netherton syndrome. Br J Dermatol. 2004;151(6):1253–1257. doi: 10.1111/j.1365-2133.2004.06180.x. [DOI] [PubMed] [Google Scholar]
- 7.Fortugno P., Bresciani A., Paolini C. Proteolytic activation cascade of the Netherton syndrome–defective protein, LEKTI, in the epidermis: implications for skin homeostasis. J Invest Dermatol. 2011;131(11):2223–2232. doi: 10.1038/jid.2011.174. [DOI] [PubMed] [Google Scholar]
- 8.Briot A., Deraison C., Lacroix M. Kallikrein 5 induces atopic dermatitis-like lesions through PAR2-mediated thymic stromal lymphopoietin expression in Netherton syndrome. J Exp Med. 2009;206:1135–1147. doi: 10.1084/jem.20082242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fontao L., Laffitte E., Briot A. Infliximab infusions for Netherton syndrome: sustained clinical improvement correlates with a reduction of thymic stromal lymphopoietin levels in the skin. J Invest Dermatol. 2011;131(9):1947–1950. doi: 10.1038/jid.2011.124. [DOI] [PubMed] [Google Scholar]
- 10.Berthold E., Metze D., Kogut M. Diagnostic criteria of Netherton syndrome using noninvasive reflectance confocal microscopy. J Dtsch Dermatol Ges. 2016;14(5):519–521. doi: 10.1111/ddg.12876. [DOI] [PubMed] [Google Scholar]
- 11.Leclerc-Mercier S., Bodemer C., Furio L. Skin biopsy in Netherton syndrome: a histological review of a large series and new findings. Am J Dermatopathol. 2016;38(2):83–91. doi: 10.1097/DAD.0000000000000425. [DOI] [PubMed] [Google Scholar]