Abstract
Smoldering multiple myeloma (SMM) is an asymptomatic clonal plasma cell disorder that frequently progress to multiple myeloma (MM), a disease at high risk of pneumococcal infections. Moreover, if the polysaccharide vaccine is poorly immunogenic in MM, the 13-valent conjugated vaccine has never been tested in clonal plasma cell disorders. We evaluated its immunogenicity for 7 serotypes in 20 patients ≥ 50 years of age with smoldering multiple myeloma (SMM) pre and post routine-vaccination with PCV13.
Concentrations of IgG specific for 7 serotypes were measured at baseline, 1, 6, and 12 months after vaccination by standardized ELISA and an Opsonophagocytic Assay (OPA). The primary endpoint was the proportion of patients responding to at least 5 of the 7 serotypes by ELISA at one month.
At 1 month post vaccination, 12 patients (60%) were responders by ELISA, among whom 8 were also responders by OPA. At 6 months, 6 (30% of total) of the 12 responders had persistent immunity, and only 2 (10% of total) at 12 months. These results suggested a partial response in this population and a rapid decrease in antibody levels in the first months of vaccination.
Although one injection of the 13-valent pneumococcal conjugate vaccine is immunogenic in some patients with SMM, the response is transient. Repeated injections are likely to be needed for effective and sustained protection.
Keywords: Immunology, Vaccines, Infectious disease
1. Introduction
Smoldering multiple myeloma (SMM) is an asymptomatic clonal plasma cell (PC) disorder defined by the presence of a serum monoclonal protein of ≥30 g/L and/or 10% to 60% clonal bone marrow PCs with no evidence of end-organ damage [1]. SMM is distinguished from monoclonal gammopathy of undermined significance (MGUS) primarily for clinical reasons, because the risk of progression to malignancy in the first 5 years after diagnosis is different: 10% per year in SMM vs 1% per year in MGUS [2].
SMM is biologically heterogeneous; it is a clinically defined entity comprising a subset of patients with biological premalignancy (ie, MGUS) and a subset with biological malignancy (ie, multiple myeloma) who have not yet developed hypercalcemia, renal failure, anemia, or lytic bone lesions and/or other myeloma-defining events [3]. Thus, SMM includes patients who behave like those with MGUS (with a very low rate of progression) and those who develop clinical symptoms and end-organ damage within the first 2 years of diagnosis [4]. It is therefore very important to achieve a good prevention against infectious agents in this subpopulation of patients who can rapidly progress into multiple myeloma a more severe and immune compromised form of the disease particularly at risk for pneumococcal infection. Immunoglobulin levels and the ability to mount a humoral response to infection or vaccination are reduced in patients with MM [5]. Infectious complications, and particularly invasive pneumococcal infections, are frequent during MM course (incidence is 15 times higher than that observed in a control population) and represent a major cause of morbidity and mortality [6, 7].
Two pneumococcal vaccines are available in France: a 23-valent polysaccharide vaccine (Pneumo23®, PPV23) and a 13-valent conjugate polysaccharide vaccine (Prevenar13®, PCV13). Both vaccines induce the synthesis of anti-capsular IgG with both neutralizing and opsonizing capacities [8].
The 23 polysaccharides included in PPV23 are involved in 85–90% of invasive pneumococcal infections in Europe and in the USA [9]. Vaccination with PPV23 is recommended for at-risk adults and children > 2 years of age. Since this unconjugated vaccine does not induce antigen memory, a booster is sometimes proposed after 5 years. However, the recall responses are sometimes impaired by the hyporesponse phenomenon observed for polysaccharide vaccine [10] [11].
The PCV13 vaccine contains purified capsular polysaccharide from 13 serotypes conjugated to a mutant of diphtheria toxoid (CRM197). The coupling of T-cell independent pneumococcal polysaccharide antigens to a carrier protein transforms them into T-cell dependent antigens. The conjugate vaccine is recommanded for children less than 5 year of age and licensed for adults and immunocompromised patients. Many studies in immunocompetent children have shown clinical efficacy in terms of immunization and protection against invasive disease, but also in terms of reducing the percentage of infection and carriage of penicillin-resistant and nonresistant Pneumococcus (PRP) strains [12, 13, 14].
In MM patients, PPV23 is poorly immunogenic, with ∼40% response rate [15, 16]. Furthermore, there is an increased risk of inducing hyporesponsiveness after multiple injections of polysaccharide antigen performed every 5 years.
Most data on immunogenicity of these pneumococcal vaccines, used alone or in a combined schedule, were collected from children, HIV positive individuals, transplant recipients, or sickle cell disease patients [17]. To the best of our knowledge, there are no studies on the immunogenicity of PCV13 among MM patients, and there is no referenced strategy for pneumococcal vaccination in this population. Thus, there is an urgent need to analyze the potential benefit of PCV administered in this at-risk population. Therefore, we conducted a pilot study in 20 patients with smoldering multiple myeloma to determine the immunogenicity of PCV13 and the persistence of the response for one year post vaccination.
2. Material and methods
2.1. Study population
The study protocol was approved by the ethical committee (Protocol 2012/27NICB) and we received inform consent for all patients.
20 patients aged ≥ 50 years with smoldering multiple myeloma (SMM) who had not previously received a pneumococcal vaccine, were enrolled in this study for routine-vaccination against pneumococcus. Patients were defined as SMM if they met the following criteria: 1) Serum paraprotein ≥30 g/L, AND/OR 2) Clonal plasma cells from 10% to 60% on bone marrow sample, AND 3) No myeloma-related organ impairment. Patients were enrolled in 2012 in St Louis Hospital and Cochin Hospital.
2.2. Vaccination
SMM patients were included after having received one dose of PCV13 (Prevenar13®; Pfizer) in during a routine-visit according to recommendations (baseline). Blood samples were obtained at baseline and 1 month, 6 months, and 12 months.
2.3. Serological evaluation
2.3.1. ELISA
IgG antibody concentrations for seven serotypes (4, 6B, 9 V, 14, 18C, 19F and 23F) were determined using a modified enzyme linked immunosorbent [www.vaccine.uab.edu] [18]. We choose to analyse the seven PCV7 serotypes for the availability of these serology in our lab, their reproducibility and robustness.
Briefly, 96-well plates (Corning, Inc., Corning, NY) were coated with a serotype-specific pneumococcal PS antigen (American Type Culture Collection, Manassas, VA) and incubated 5 hours at 37 °C. Reference sera (007sp), QC sera, or patient specimens were pre-absorbed with 5 μg/ml pneumococcal C-polysaccharide (Statens Serum Institut, Copenhagen, Denmark) and 10 μg/ml serotype 22F capsular polysaccharide (American Type Culture Collection) for 30 minutes at room temperature before being serially diluted. After washing plates, serially diluted serum was added and plates were incubated at room temperature for 2 hours. Plates were then washed, and alkaline phosphatase-conjugated goat anti-human immunoglobulin G (IgG; Southern Biotech, Birmingham, AL) was added. After another 2 hour incubation and washing, substrate (p-nitrophenyl phosphate in diethanolamine buffer, pH 9.8) was added to the plates. After a final incubation, the optical density was measured at 405 nm. Anti-pneumococcal antibody levels were determined in each specimen by analysis of linear regression plots compared with the reference serum (007sp) (National Institute for Biological Standards and Control (NIBSC)).
There is in the paediatric population a threshold of protection commonly admitted by 0.35 μg/ml, which serves as a reference for all pneumococcal immunogenicity studies in children. Currently, there are no validated correlates of pneumococcal vaccine protection in adults. Several studies have defined a response criteria for pneumococcal vaccine particularly adapted for evaluation of long-term protection in immunocompromised patients [19, 20]. These criteria include: a two fold increase in serotype specific IgG vaccine concentration and an IgG-concentration ≥ 1μg/ml over the pre-vaccination levels [21].
2.3.2. Opsonophagocytic assay
Whereas ELISA is at that time the gold standard method for the evaluation of pneumococcal vaccine immunogenicity, Opsonophagocytic assay gives more information about how functional the immune response is and results would be more effective and relevant to in vivo immunity when evaluated by OPA, and tend to replace ELISA for vaccine immunogenicity evaluation. Opsonophagocytic activities of antibodies for seven serotypes (4, 6B, 9 V, 14, 18C, 19F and 23F) were measured by a multiplexed opsonophagocytic killing assay (MOPA, [www.vaccine.uab.edu] [22]. All serum samples were incubated at 56 °C for 30 min before being tested. Sera were serially diluted in round-bottom 96-well plates (Corning Inc., Corning, NY). Frozen aliquots of target pneumococci were thawed, washed twice, diluted to a bacterial density of ∼50,000CFU/ml, and added to the plates. After 30 min of incubation at room temperature with shaking at 700 rpm, complement and HL60 cells (ATCC) that had been differentiated to phagocytes were added to each well. Plates were incubated in a tissue culture incubator (37 °C, 5% CO2) with shaking at 700 rpm. After a 45-min incubation, plates were placed on ice for 20 min. Ten μl of each well were spotted onto four different Todd-Hewitt broth-yeast extract agar plates. After application of an overlay agar containing one of four antibiotics to each agar plate and overnight incubation at 37 °C, the number of bacterial colonies in the agar plates was enumerated.
Opsonization titers (OT) were defined as interpolated reciprocal serum dilution that kills 50% of the bacteria in the assay. The assay sensitivity is the lowest dilution of sera tested (limit of detection LOD), which is normally 4 for undiluted sera, and is the same for each serotype. However, to quantify functional antibodies with more precision, the lower limit of quantification was determined for each serotype-specific assay during assay validation.
The LLOQs for the various serotypes were: serotype 4: 24; serotype 6B: 131; serotype 9 V: 38; serotype 14: 85; serotype 18C: 47; serotype 19F: 74 and serotype 23F: 30.
For OPA titers higher than the LLOQ were considered accurate and their values were reported. Titers below the LLOQ were set to a value of 2 (half of LOD) [23].
In our study, immunological response for a serotype was defined as at least an Opsonisation titer ≥ LLOQ and four fold increase from baseline in OPA.
2.4. Statistics analysis
Primary endpoint was the proportion of patients developing an immunological response toward at least 70% of serotypes at one month in ELISA. These Patients, developing an immunological response toward at least 70% of serotypes at one month, were defined as responders. Patient characteristics were compared using Mann-Whitney's Ranksum test for quantitative variable and Fisher’s exact test for categorical variable. The percentages of responders are provided together with their 95% confidence interval (95%CI). Quantitative variables were compared using Student’s t-test and categorical variables were compared using the chi-square test. All tests were 2-sided at the level of 0.05. All analyses were performed using Graph Pad Prism 5.0 software (GraphPad, San Diego, CA).
3. Results
3.1. Population data
Twenty patients with SMM were recruited for the study. The median age of the study population was 62 years (range 50–74) with 15 females (75%) and 5 males (25%). Fourteen patients had IgG MM, five had IgA MM, and one had Bence Jones MM. No one received a treatment for their SMM. We compared the age, gender, type and quantity of monoclonal immunoglobulin, quantity of IgG − IgA − IgM and percentage of plasma cells in bone marrow (Table 1). We also did a phenotypic analysis in our patients (CD3, CD4, CD8, CD19, CD20, CD5, CD38, CD27, CD16-CD56)(data not shown). We did not found any significant differences between responders and non-responders group.
Table 1.
Demographics and Disease Status.
| N = 20 | Non-responders | Responders | p-value |
|---|---|---|---|
| Age: median (range) years | 63.3 (56.5–74.6) | 62.6 (56.3–73.1) | 0.7£ |
| Gender: female/male | 5/3 | 10/2 | 0.35# |
| Predominant type of monoclonal Ig: IgG/IgA/Bencejones |
5/2/1 | 9/3/0 | 0.76# |
#referred to Fisher’s exact test, £ referred to Mann-Whitney’s Ranksum test.
3.2. Pneumococcal serotype-specific Ig concentrations and titers
3.2.1. Prior to immunization
There were no significance differences at baseline in antibody levels and titers between the Non-Responders group (NR) (n = 8) and Responders group (R) (n = 12) (Table 2).
Table 2.
Geometric mean (95% CI) Concentrations by ELISA (a) and Titers by OPA (b) of IgG Antibody to Seven Pneumococcal Serotypes.
|
a) | |||||
|---|---|---|---|---|---|
| Serotypes | Baseline | M1 | M6 | M12 | |
| 4 | NR | 0.21 (0.1–0.4) | 0.56$ (0.3–1.2) | 0.35 (0.1–0.8) | 0.37 (0.1–0.9) |
| R | 0.26 (0.1–0.5) | 1.36$ (0.5–3.5) | 0.65 (0.3–1.4) | 0.43 (0.2–0.8) | |
| 6B | NR | 0.68 (0.4–1.2) | 0.95 (0.4–2.1) | 0.82 (0.4–1.9) | 0.71 (0.3–1.6) |
| R | 0.69 (0.5–1.1) | 3.31$,# (1.9–5.6) | 1.15 (0.6–2.2) | 1.08 (0.5–2.2) | |
| 9V | NR | 0.53 (0.3–1.0) | 0.78 (0.4–1.5) | 0.61 (0.2–1.5) | 0.47 (0.2–1.2) |
| R | 0.52 (0.3–0.9) | 2.93$,# (1.4–6.0) | 1.04 (0.5–2.3) | 0.92 (0.4–1.9) | |
| 14 | NR | 1.08 (0.4–2.9) | 3.47 (1.0–12) | 2.71 (0.9–8.1) | 2.05 (0.4–10.0) |
| R | 1.08 (0.6–2.0) | 10.84$ (5.1–23.0) | 5.7$ (2.6–12.6) | 3.75$ (1.3–10.5) | |
| 18C | NR | 0.53 (0.3–1.0) | 1.46$ (0.4–1.5) | 0.76 (0.3–1.8) | 0.68 (0.2–2.0) |
| R | 0.57 (0.3–1.0) | 4.59$,# 2.5–8.4) | 1.36$ (0.7–2.5) | 1.29 (0.9–1.8) | |
| 19F | NR | 1.13 (0.6–2.0) | 2.51$ (1.4–4.5) | 1.27 (0.7–2.4) | 1.45 (0.7–3.0) |
| R | 1.15 (0.6–2.3) | 5.39$,# (2.9–9.9) | 3.06$,# (1.5–6.1) | 1.56 (0.7–3.3) | |
| 23F | NR | 0.51 (0.2–1.1) | 1.3 (0.4–4.0) | 0.96 (0.3–3.0) | 0.85 (0.2–3.2) |
| R | 0.62 (0.2–1.7) | 3.92$ (1.2–12.8) | 1.18 (0.4–3.2) | 0.98 (0.4–2.6) | |
| b) | |||||
| Serotypes | Baseline | M1 | M6 | M12 | |
| 4 | NR | 2 (2–2) | 13.4 (4.4–40.5) | 6.4 (2.5–16.2) | 4.2 (1.8–9.8) |
| R | 2 (2–2) | 19.9$ (3.7–107.1) | 29.5$,# (6.4–136.0) | 10.4$ (1.2–86.9) | |
| 6B | NR | 4.4 (1.4–14.2) | 7.5 (1.6–35.4) | 4.8 (1.3–18.4) | 3.2 (1.1–8.7) |
| R | 2 (2–2) | 259.7$,# (43.0–1568) | 44.1$ (5.1–382.3) | 25.5$ (2.4–271.7) | |
| 9V | NR | 3.7 (1.5–9.6) | 4.8 (1.7–13.2) | 5.6 (1.7–18.6) | 4.3 (1.4–13.7) |
| R | 3.2 (1.0-10.1) | 133.9$,# (50.0–358.4) | 21.5 (3.8–122.5) | 5.4 (1.1–26.2) | |
| 14 | NR | 4.2 (1.4–12.3) | 43.9$ (7.1–269.7) | 32.9$ (6.6–165.4) | 4.9 (1.3–19.0) |
| R | 3.8 (0.8-18.0) | 529.7$,# (62.7–4474) | 268.7$ (42.3–1707) | 60.7$ (5.0-736.9) | |
| 18C | NR | 5.3 (1.7–16.2) | 45.6$ (10.2–203.8) | 14.6 (3.1–69.3) | 6.0 (1.7–21.6) |
| R | 3.5 (0.9–12.7) | 618.6$,# (180.2-2124) | 182.9$(87.3-383.6) | 67.1$ (9.0–496.8) | |
| 19F | NR | 3.0 (1.2–7.0) | 5.9 (1.7–20.8) | 4.2 (1.4–12.7) | 3.2 (1.1–8.9) |
| R | 3.4 (0.9–12.5) | 113.6$,# (11.6–1112) | 14.2 (1.4–145.3) | 19.9 (1.4–290.7) | |
| 23F | NR | 2.4 (1.6–3.8) | 8.7 (2.1–32.9) | 4.3 (1.4–13.4) | 2.8 (1.3–5.8) |
| R | 4.2 (1.2–14.8) | 207.6$,# (33.8-1273) | 35.3 (4.6–270.0) | 24.8 (4–156) | |
Note: Responders at one month were defined as at least an IgG- two fold increased from baseline and ≥ 1 μg/ml by ELISA and as at least a four-fold increased from baseline and ≥ lower limits of quantification (LLOQ) by OPA, for more than 5 serotypes. Data are in geometric means: antibody concentrations are in μg/ml (95% confidence limits). R: Reponders group, NR: Non-reponders group, $ p < 0.05 vs. baseline, # p < 0.05 vs. NR group.
By ELISA, only serotypes 14 (NR: 1.08 μg/ml (0.4–2.9)/R: 1.08 (0.6–2.0) μg/ml) and 19F (NR: 1.13 μg/ml (0.6–2.0)/R: 1.15 μg/ml (0.6–2.3) μg/ml) had antibodies levels above the 1 μg/ml threshold in pre-vaccination samples. Nine patients (45%) had antibody concentrations ≥ 1 μg/ml for these two serotypes. The lowest antibody level was for serotype 4, with a GMC of 0.21 μg/ml (0.1–0.4) for Non Responders group and 0.26 μg/ml (0.1–0.5) for Responders group. Two patients (one in both groups) (10%) had antibody concentrations ≥ 1 μg/ml for this serotype. Six patients (30%) had as antibody concentrations ≥ 1 μg/ml in ELISA for at least 3 serotypes (data not shown).
By OPA none of the serotypes had GMTs above the LLOQ at baseline. The highest antibody titer was for serotype 18C, with a GMT of 5.3 (1.7–16.2) for Non Responders group. As in ELISA, serotype 4 had the lowest titer (NR: 2 (2–2)/R: 2 (2–2)).
3.2.2. Impact of vaccination
At 1 month post vaccination, 12 patients (60%) were responders by ELISA (Fig. 1a). In the Non-Responder group, the GMC increased significantly (> 2-fold) from baseline for 3 serotypes: serotypes 4 (0.21 μg/ml to 0.56 μg/ml, p = 0.026), 18C (0.53 μg/ml to 1.46 μg/ml, p = 0.037), and 19F (1.13 μg/ml to 2.51 μg/ml, p = 0.021). GMC increased significantly in the Responders group for all serotypes from baseline (Table 2). GMCs in the Responders group were significantly higher than those in the Non-Responders group for serotypes 6B (3.31 μg/ml vs 0.95 μg/ml; p = 0.011), 9 V (2.93 μg/ml vs 0.78 μg/ml; p = 0.009), 18C (4.59 μg/ml vs 1.46 μg/ml; p = 0.005) and 19F (5.39 μg/ml vs 2.51 μg/ml; p = 0.035).
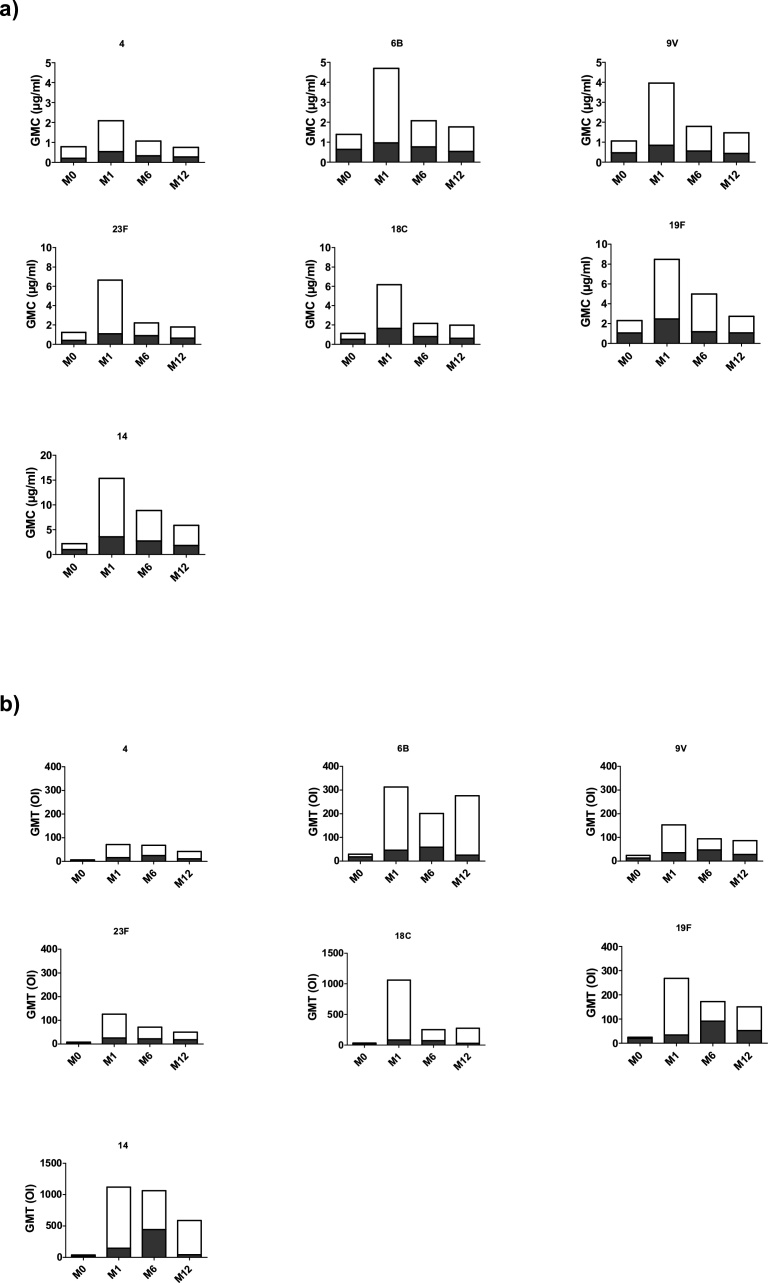
Fig. 1.
Geometric mean of concentration in μg/ml (GMC) by ELISA(a) and titers (GMT) by OPA (b) of IgG Antibody to Seven Pneumococcal Serotypes between Responders and Non Responders. Comparison between the GMC by ELISA (a) and GMT by OPA (b) of the Responders (in white) and Non Responders (in grey).
Serotype 4 was the least immunogenic serotype with a GMC total (define as GMC of R and NR together) of 0.87 μg/ml. Only 8 patients (40%) had antibodies levels ≥ 1 μg/ml for this serotype. Serotype 14 had the highest GMC total with 6.13 μg/ml. 18 patients (95%) had antibodies levels ≥ 1 μg/ml for this serotype.
The functional activity of the anti-polysaccharide antibody was assessed by the Opsonophagocytic Assay (OPA) (Fig. 1b). In our study, 8 patients (40%) had functional antibodies against Streptococcus pneumoniae at one month after immunization and were considered responders. For the Non-Responders group, none of the seven serotypes had GMTs above the LLOQ. In the Responders group, antibody titers from all serotypes increased significantly (> 4 fold) from baseline. Except for serotype 4, antibody titers for all serotypes in the Responders group were significantly higher than those of Non-Responders group.
These results show that despite the overall low response rate (40%), PCV13 induces the production of functional antibodies.
3.2.3. Persistence of the response after immunization
The proportion of responders at 6 months is shown is Fig. 2. Seven (35%) patients had persistent immunity by ELISA and only six (30%) by OPA.
Fig. 2.
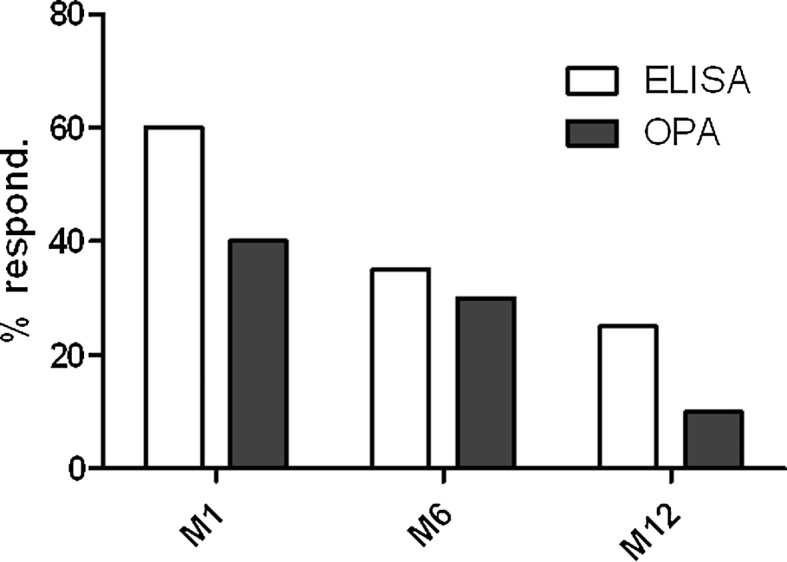
Percentages of responders for pneumococcal vaccine at 1, 6 and 12 months after vaccine. For ELISA, responders patients for each serotype at a specific time point were defined as at least a twofold increase in IgG anti-PS concentration from baseline and a concentration of IgG anti-PS ≥ 1 μg/ml for each serotype. Patients were assigned as responder when more than 5 serotypes upon the 7 serotypes tested fit with those criteria. For OPA, responders patients for each serotype at a specific time point were defined as at least a fourfold increase in Ig anti-PS titers from baseline and a titer of Ig anti-PS ≥ LLOQ for each serotype. Patients were assigned as responder when more than 5 serotypes upon the 7 serotypes tested fit with those criteria.
By ELISA, we observed a decrease in GMC for all serotypes in both groups compared to those in the one month post immunization samples (Table 2). No serotypes were significantly higher than baseline for Non-Responders group. GMCs in this group were<xps:span class="ceCheck"> ≥ 1 μg/ml for only serotypes 14 [2.71 μg/ml (0.9–8.1)] and 19F [1.27 μg/ml (0.7–2.4)]. The decrease in GMCs from 1 month to 6 months after immunization was significant in the Responders group for serotypes 6B (p = 0.009) and 18C (p < 0.001). Despite this decrease in GMC, all serotypes except serotype 4 [0.65 μg/ml (0.3–1.4)] had a GMC ≥ 1 μg/ml.
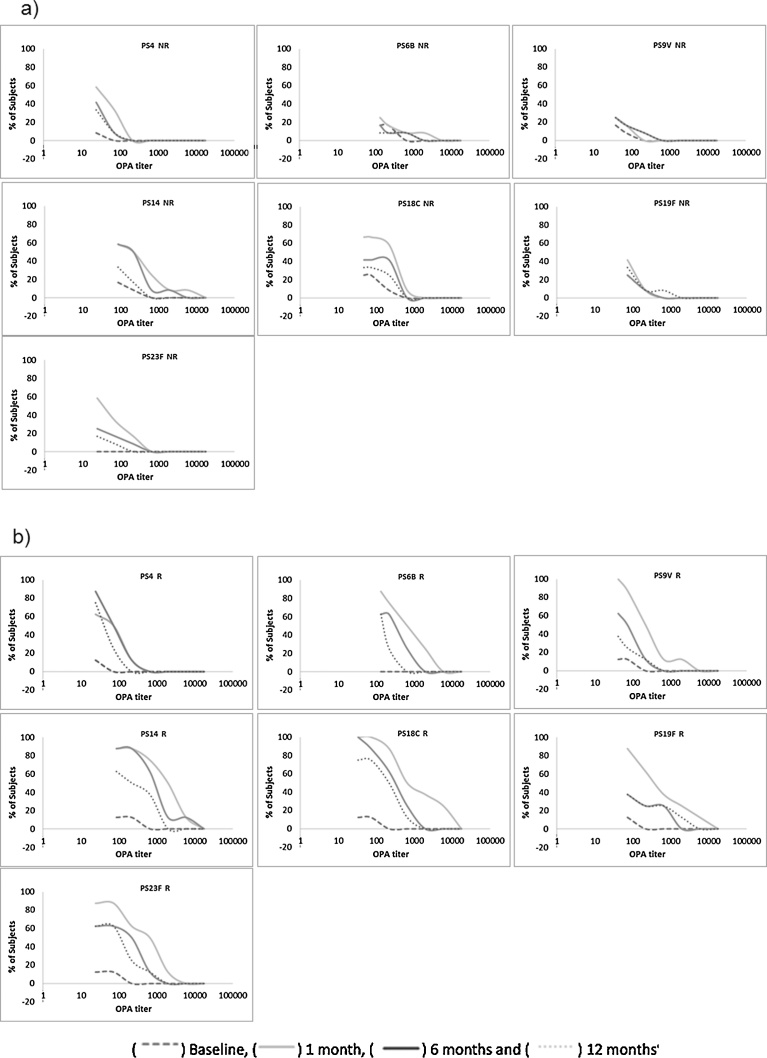
A similar decrease was observed in OPA in both groups (Fig. 3). For the Responders group, all serotypes except 9 V, 19F and 23F were significantly higher than the baseline. But only serotype 4 (p = 0.0247) was significantly higher than the Non-Responders group.
Fig. 3.
Reverse Cumulative Distribution Curves for OPA titers. Anti-pneumococcal OPA titers are depicted on the x-axis using a log scale. The cumulative percentage of Subjects with given OPA titers is in the y-axis for patients considered as Non Responders (NR) in a) and as Responders (R) in b).
Only 5 patients (25%) were considered to be responders by ELISA at 12 months after immunization (Fig. 2). Only serotype 14 had a GMC significantly higher than baseline (p = 0.022) after one year. For the six remaining serotypes, a small continued decrease was observed and GMC reached baseline levels. There were no significant differences between Non-Responders and Responders groups.
By OPA, we found only 2 patients (10%) to be responders at 12 months after immunization. Serotype18C was the only serotype with GMTs above the LLOQ with all others below this threshold one year after immunization.
4. Discussion
Here, we evaluated in a pilot study the immunogenicity of PCV13 using both OPA and ELISA methods in 20 patients with smoldering multiple myeloma (SMM) and the persistence of a protective immunity for one year after vaccination. Although the conjugated vaccine is immunogenic at one month, the persistence of antibody is weak with a severe decrease at 6 month after immunization with antibody concentrations close to basal levels one year post vaccine.
To our knowledge, this study is the first to evaluate the immunogenicity of pneumococcal vaccination in SMM patients. Pneumococcal vaccines have been evaluated in MGUS and in MM patients [24, 25]. However, SMM patients differ from MM patients as they have no evidence of end-organ damage and/or other myeloma-defining events. SMM patients also differ from MGUS because the risk of progression to malignancy in the first 5 years after diagnosis is 10% per year in SMM vs. 1% per year in MGUS. It is therefore very important to achieve a good protection against infectious agents in this subpopulation of patients who can rapidly progress into multiple myeloma a more severe and immune compromised form of the disease particularly at risk for pneumococcal infection [7].
There have been multiple studies exploring vaccination of MM patients with the polysaccharide vaccine. For example, Hinge et al. investigated the serological response to Pneumovax® in sixty MM patients [16]. They found that 33% of the patients responded to the vaccine, and that this response was statistically associated with the severity of the disease and the response to chemotherapy. Robertson et al. found, in a random group of MM patients, a 40% response one month post vaccination [26]. Schmid et al. found that 43% of patients with MM or Waldenström at different stages of the diseases responded to the vaccine [25]. Taken together, these studies demonstrated that PPV23 is poorly immunogenic in MM patients with response rates ranging from 33% to 43%.
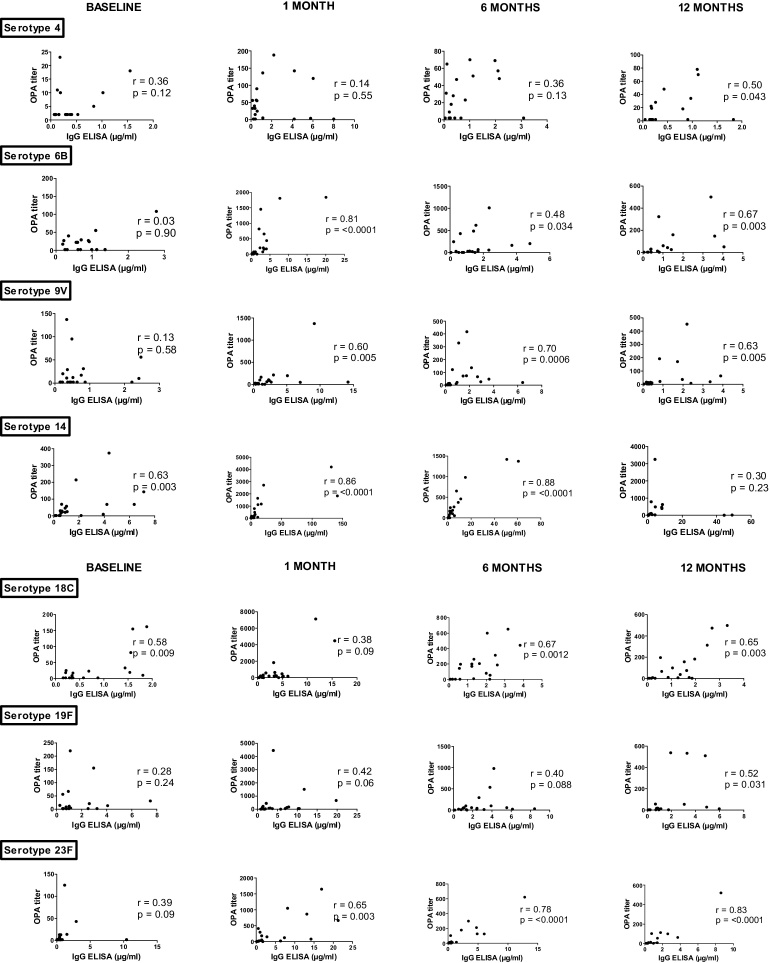
So far, ELISA was the only technique used to quantify antibody levels in these studies. Methods able to evaluate the opsonophagocytic capabilities of anti-PS antibodies have been developed based on the idea that the primary mechanism for protection against invasive pneumococcal disease is mediated by opsonophagocytic antibodies. Therefore, the in-vitro determination of opsonophagocytic activity of antibodies to pneumococcal capsular polysaccharide may represent a better surrogate marker for in-vivo protection [27]. Poor correlations between ELISA and OPA have been found in particular populations such as elderly adults and immunodeficient patients who produced antibodies with low opsonophagocytic capabilities [27]. These nonfunctional antibodies may be able to bind to polysaccharide coated on a plate and thus be positive on ELISA, but are not able to opsonize whole bacteria [28]. Interestingly, a recent paper found a good correlation between ELISA and OPA after pneumococcal vaccine in MGUS but not in MM patients [24]. In our hands as in MGUS patients, we found a good correlation between ELISA and OPA in SMM patients (supplementary data), except for baseline with only 2 serotypes (14 and 18C) on the seven tested which had good correlations between the two tests (Fig. 4).
Fig. 4.
Correlation between anti-pneumococcal Ig concentration (ELISA) and opsonophagocytic antibody titers (OPA) per serotypes and per visite. Values on the x and y axes differ between the different pneumococcal serotypes. r and p values were calculated by the Spearman rank sum test.
One injection of PCV13 in SMM patients induces the production of functional antibodies in only 40% of recipients one month after immunization and results in poor long-term protection, with only 10% of recipients positive one year after immunization.
Due to the heterogeneity of patients enrolled in the various clinical trials performed so far to evaluate pneumococcal vaccine efficacy, the small size of our population (20 patients) and the different techniques used to evaluate the immunogenicity of the anti-PS response (ELISA and OPA), caution should be used when comparing our results with those of previous studies. However, the percentage of responders, evaluated by ELISA in our SMM population, seems to be quite similar with those reported in MM patients vaccinated with either PPV or PCV.
The poor immunogenicity of one dose of pneumococcal conjugated vaccine has been reported in immunocompromised patients with an other hematological malignancy. Sinisialo et al. evaluated the immunogenicity of one dose of the 7-valent conjugate vaccine in patients with chronic lymphocytic leukemia [29]. They found that 40% of the patients responded to at least 6 serotypes of the vaccine, and that the timing for vaccination during the course of the disease has an impact on this response. Highest antibody responses were found in patients vaccinated in an early stage of disease (i.e. before initiation of chemotherapy and the development of hypogammaglobulinaemia).
Some authors suggest that 2 or 3 doses of PCV (or higher single doses) could increase the immunogenicity of the vaccine in immunocompromised patients. Indeed, in a recent study in elderly (patients >70 years) [30], vaccination with a double dose of PCV (1 ml) was more immunogenic than vaccination with a single dose. Such strategy could be useful to achieve good protection against pneumococcal infection with PCV vaccine in SMM. In addition, administration of PPV23 at least two month after PCV has been recommended in some immunocompromised patients. The conjugate and non-conjugate vaccines activate the T-dependent and T-independent anti-pneumococcal responses respectively and enlarge the serotype coverage to 23 pneumococcal serotypes. Such strategy could also be of help to increase vaccine immunogenicity and durability of the response.
Declarations
Author contribution statement
Mathilde Bahuaud: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Helene Bodilis: Conceived and designed the experiments; Analyzed and interpreted the data.
Marion Malphettes, Anais Maugard Landre, Didier Bouscary, Odile Launay, Jean-Paul Fermand: Conceived and designed the experiments.
Caroline Matondo: Performed the experiments.
Frédéric Batteux: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Pfizer Inc. (RFIZ2012).
Competing interest statement
The authors declare the following conflict of interests: Odile Launay; participated in industry-sponsored clinical trials that included Pneumococcal vaccines
Additional information
No additional information is available for this paper.
Acknowledgements
The author thanks Robert Burton for his critical reading of the manuscript.
References
- 1.Rajkumar S.V., Dimopoulos M., Palumbo A., Blade J., Merlini G., Mateos M.V., Kumar S., Hillengass J., Kastritis E., Richardson P., Landgren O., Paiva B., Dispenzieri A., Weiss B., LeLeu X., Zweegman S., Lonial S., Rosinol L., Zamagni E., Jagannath S., Sezer O., Kristinsson S.Y., Caers J., Usmani S.Z., Lahuerta J.J., Johnsen H.E., Beksac M., Cavo M., Goldschmidt H., Terpos E., Kyle R.A., Anderson K.C., Durie B.G., Miguel J.F. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–48. doi: 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
- 2.Kyle R.A., Remstein E.D., Therneau T.M., Dispenzieri A., Kurtin P.J., Hodnefield J.M., Larson D.R., Plevak M.F., Jelinek D.F., Fonseca R., Melton L.J., 3rd, Rajkumar S.V. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N. Engl. J. Med. 2007;356(25):2582–2590. doi: 10.1056/NEJMoa070389. [DOI] [PubMed] [Google Scholar]
- 3.Rajkumar S.V. Multiple myeloma: 2012 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2012;87(1):78–88. doi: 10.1002/ajh.22237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landgren O., Waxman A. Multiple myeloma precursor disease. JAMA. 2010;304(21):2397–2404. doi: 10.1001/jama.2010.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalambokis G., Christou L., Tsianos E. Multiple myeloma presenting with an acute bacterial infection. Int. J. Lab Hematol. 2009;31(4):375–383. doi: 10.1111/j.1751-553X.2009.01154.x. [DOI] [PubMed] [Google Scholar]
- 6.Perry R., Hebbel R., Oken M. Influence of treatment and response status on infection risk in multiple myeloma. Am. J. Med. 1981;71(6):935–940. doi: 10.1016/0002-9343(81)90303-x. [DOI] [PubMed] [Google Scholar]
- 7.Twoney J. Infections complicating multiple myeloma and chronic lymphocytic leukemia. Arch. Intern. Med. 1973;132(4):562–565. [PubMed] [Google Scholar]
- 8.Felmann C., Anderson R. Review: current and new generation pneumococcal vaccines. J. Infect. 2014;69(4):309–325. doi: 10.1016/j.jinf.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 9.WHO . Weekly Epidemiological Report; 2012. Pneumococcal Vaccines WHO positionpaper 2012.ttp://www.who.int/wer [Google Scholar]
- 10.Clutterbuck E.A., Lazarus R., Yu L.M., Bowman J., Bateman E.A., Diggle L., Angus B., Peto T.E., Beverley P.C., Mant D., Pollard A.J. Pneumococcal conjugate and plain polysaccharide vaccines have divergent effects on antigen-specific B cells. J. Infect. Dis. 2012;205(9):1408–1416. doi: 10.1093/infdis/jis212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Brien K.L., Hochman M., Goldblatt D. Combined schedules of pneumococcal conjugate and polysaccharide vaccines: is hyporesponsiveness an issue? Lancet Infect. Dis. 2007;7(9):597–606. doi: 10.1016/S1473-3099(07)70210-4. [DOI] [PubMed] [Google Scholar]
- 12.Rose M., Gruendler M., Schubert R., Kitz R., Schulze J., Zielen S. Safety and immunogenicity of sequential pneumococcal immunization in preschool asthmatics. Vaccine. 2009;27(38):5259–5264. doi: 10.1016/j.vaccine.2009.06.054. [DOI] [PubMed] [Google Scholar]
- 13.Navarro D., Escribano A., Cebrián L., Gimeno C., García-Maset L., García-de-Lomas J., Spanish Pneumococcal Infection Study Network Type-specific antibodies to pneumococcal capsular polysaccharide acquired either naturally or after vaccination with Prevenar in Children with underlying chronic or recurrent lunig diseases. Clin. Vaccine Immunol. 2006;13(6):665–670. doi: 10.1128/CVI.00079-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rose M., Hey C., Kujumdshiev S., Gall V., Schubertand R., Zielen S. Immunogenicity of pneumococcal vaccination of patients with cochlear implants. J. Infec. Dis. 2003;190(3):551–557. doi: 10.1086/422395. [DOI] [PubMed] [Google Scholar]
- 15.Lazarus H.M., Lederman M., Lubin A., Herzig R.H., Schiffman G., Jones P., Wine A., Rodman H.M. Pneumococcal vaccination: the response of patients with multiple myeloma. Am. J. Med. 1980;69(3):419–439. doi: 10.1016/0002-9343(80)90014-5. [DOI] [PubMed] [Google Scholar]
- 16.Hinge M., Hingels H., Slotved H., Mølle I. Serologic response to a 23-valent pneumococcal vaccine administered prior to autologous stem cell transplantation in patients with multiple myeloma. APMIS. 2012;120(11):935–940. doi: 10.1111/j.1600-0463.2012.02922.x. [DOI] [PubMed] [Google Scholar]
- 17.Fletcher M., Balmer P., Bonnet E., Dartois N. PCVs in individuals at increased risk of pneumococcal disease: a litterature review. Expert Rev. Vaccines. 2015;14(7):975–1030. doi: 10.1586/14760584.2015.1037743. [DOI] [PubMed] [Google Scholar]
- 18.Wernette C.M., Frasch C.E., Madore D., Carlone G., Goldblatt D., Plikaytis B., Benjamin W., Quataert S.A., Hildreth S., Sikkema D.J., Käyhty H., Jonsdottir I., Nahm M.H. Enzyme-Linked ImmunoSorbent Assay for quantification of Human Antibodies to Pneumococcal Polysaccharides. Clin. Diagn. Lab Immunol. 2003;10(4):514–519. doi: 10.1128/CDLI.10.4.514-519.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lombardi F., Belmonti S., Fabbiani M., Morandi M., Rossetti B., Tordini G., Cauda R., De Luca A., Di Giambenedetto S., Montagnani F. Immunogenicity and safety of the 13-Valent Pneumococcal Conjugate Vaccine versus the 23-Valent Polysaccharide vaccine in Unvaccinated HIV-Infected Adults: A pilot Prospective Controlled Study. PLoS One. 2016;11(6) doi: 10.1371/journal.pone.0156523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Roux A., Schmidt N., Rose M., Zielen S., Pletz M., Lode H. Immunogenity of the pneumococcal polysaccharide vaccine in COPD patients. The effect of systemic steroids. Respir. Med. 2004;98(12):1187–1194. doi: 10.1016/j.rmed.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 21.Sadlier C., O'Dea S., Bennett K., Dunne J., Conlon N., Bergin C. Immunological efficacy of pneumococcal vaccine strategies in HIV-infected adults: a randomized clinical trial. Sci. Rep. 2016;6:32076. doi: 10.1038/srep32076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burton R.L., Nahm M.H. Development and validation of a fourfold muliplexed opsonization assay (MOPA4) for pneumococcal antibodies. Clin. Vaccine Immunol. 2006;13(9):1004–1009. doi: 10.1128/CVI.00112-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juergens C., Patterson S., Trammel J., Greenberg D., Givon-Lavi N., Cooper D., Gurtman A., Gruber W.C., Scott D.A., Dagan R. Post Hoc analysis of a randomized double-blind trial of the correlation of functional and binding antibody responses elicited by the 13-valent and 7-valent pneumococcal conjugate vaccines and association with nasopharyngeal colonization. Clin. Vaccine Immunol. 2014;21(9):1277–1281. doi: 10.1128/CVI.00172-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karlsson J., Roalfe L., Hogevik H., Zancolli M., Andréasson B., Goldblatt D., Wennerås C. Poor correlation between pneumococcal IgG and IgM titers and opsonophagocytic activity in vaccinated patients with multiple myeloma and waldenstrom's macroglobulinemia. Clin. Vaccine Immunol. 2016;23(4):379–385. doi: 10.1128/CVI.00654-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmid G.P., Smith R.P., Baltch A.L., Hall C.A., Schiffman G. Antibody response to pneumococcal vaccine in patients with multiple myeloma. J. Infect. Dis. 1981;143(4):590–597. doi: 10.1093/infdis/143.4.590. [DOI] [PubMed] [Google Scholar]
- 26.Robertson J.D., Nagesh K., Jowitt S.N., Dougal M., Anderson H., Mutton K., Zambon M., Scarffe J.H. Immunogenicity of vaccination against infleunza: Streptococcus pneumoniae and Haemophilus influenzae type B in patients with multiple myeloma. Br. J. Cancer. 2000;82(7):1261–1265. doi: 10.1054/bjoc.1999.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song J.Y., Moseley M.A., Burton R.L., Nahm M.H. Pneumococcal vaccine and opsonic pneumococcal antibody. J. Infect. Chemother. 2013;19(3):412–425. doi: 10.1007/s10156-013-0601-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park S., Nahm M.H. Older Adults have a low Capacity to opsonize Pneumococci due to low IgM Antibody response to Pneumococcal Vaccinations. Infect. Immun. 2017;79(1):314–320. doi: 10.1128/IAI.00768-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sinisalo M., Vilpo J., Itälä M., Väkeväinen M., Taurio J., Aittoniemi J. Antiboby response to 7-valent conjugated pneumococcal vaccine in patients with chronic lymphocytic leukaemia. Vaccine. 2007;26(1):82–87. doi: 10.1016/j.vaccine.2007.10.053. [DOI] [PubMed] [Google Scholar]
- 30.Lode H., Schmoele-Thoma B., Gruber W., Ahlers N., Fernsten P., Baker S., Razmpour A., Siber G., Hackell J., Lockhart S., Burkhardt O., Welte T., de Roux A. Dose-ranging study of a single injection of pneumococcal conjugate vaccine (1 ×, 2 ×, or 4 ×) in healthy subjects aged 70 years or older. Vaccine. 2011;29(31):4940–4946. doi: 10.1016/j.vaccine.2011.04.132. [DOI] [PubMed] [Google Scholar]