Abstract
Phthalate exposure has been shown to be associated with adverse pregnancy outcomes. However, human studies informing relevant mechanistic pathways are lacking. Experimental studies have reported that matrix metalloproteinases (MMPs), which are responsible for extracellular protein degradation, may be upregulated in response to phthalate exposure. In this exploratory study we measured urinary phthalate metabolite concentrations, plasma MMP levels, and relevant covariates among 134 pregnant women. There were statistically significant or suggestive positive relationships between several phthalates, particularly between metabolites of di-(2-ethylhexyl) phthalate, with MMP-1 and MMP-9 levels. Further research is needed to confirm these results and how they may inform the mechanisms involved between phthalate exposure and adverse pregnancy outcomes.
Keywords: biomarker, enzyme, environment, epidemiology, exposure, pregnancy
Graphical Abstract
INTRODUCTION
Phthalates are a family of chemicals that are widely present in the environment due to their use in industry as well as in personal care and consumer products. High molecular weight phthalates such as di-(2-ethylhexyl) phthalate (DEHP) are widely used as plasticizers with exposure occurring through food packaging, bottled water, children’s toys, medical devices, and other products (Marie et al. 2015). Low molecular weight phthalates such as diethyl phthalate (DEP) or dibutyl phthalate (DBP) are commonly found in lotions, perfumes and deodorants, posing a primary concern for women (Marie et al. 2015).
Matrix metalloproteinases (MMPs) are enzymes that participate in extracellular matrix degradation. MMPs and their inhibitors rely on a delicate balance whose disruption can result in a range of adverse pregnancy outcomes and other health effects (Geng et al. 2016). Several animal and in vitro studies have shown that phthalates induce increased MMPs in a range of cell types. Specifically, DEHP (and its metabolite MEHP) upregulated both MMP-2 and MMP-9, while DBP upregulated MMP-9 (Kim et al. 2015; Scarano et al. 2009; Yao et al. 2012; Zhang et al. 2016; Zhu et al. 2010). Increased circulating levels of MMPs have been reported in human studies of preeclampsia (Eleuterio et al. 2015), gestational hypertension (Ab Hamid et al. 2012), pregnancy loss (Nissi et al. 2013), and preterm birth (Kramer et al. 2010). Increased exposure to phthalates have also been associated with these same adverse health measures in recent epidemiologic studies (Cantonwine et al. 2016; Ferguson et al. 2014; Messerlian et al. 2016; Werner et al. 2015). However, to our knowledge no human studies to date have assessed the relationship between phthalate exposure and circulating MMP levels. The present exploratory study aimed to investigate these relationships to determine the potential for MMPs to help inform mechanism(s) of action between phthalates and adverse pregnancy outcomes.
METHODS
Women who planned to deliver at Brigham and Women’s Hospital in Boston between the years 2006 to 2008 were recruited into a prospective cohort study as described previously (Ferguson et al. 2014). Written informed consent was obtained from the participants and the institutional review boards of Brigham and Women’s Hospital and the University of Michigan approved this study. During the study visit, the women filled out demographic questionnaires and supplied urine and blood samples for biomarker analysis. During their following three study visits, further biological samples were collected. Specimens were stored at −80 degrees Celsius until analysis. For this exploratory study, samples of urine and plasma from 134 women collected at the third study visit (median=26 weeks gestation; range=23–29 weeks) were analyzed for phthalate metabolites and MMPs, respectively.
Nine phthalate metabolites were measured in each urine sample by NSF International using a modified version of the LC-MS/MS method developed at the Centers for Disease Control and Prevention (Lewis et al. 2013). To account for urine dilution, specific gravity (SG) was measured using a handheld refractometer.
Maternal plasma samples were analyzed for matrix metalloproteinases by the University of Michigan Cancer Center Immunology Core. Five MMPs (1, 2, 7, 9 and 10) were measured using EMD Millipore’s MILLIPLEX Multiplex assay kit using the Luminex xMAP platform. Only MMP-1, 2 and 9 were included further in our data analysis since MMP-7 and MMP-10 were not detected in any of the samples. Coefficients of variation for replicate standards at varying concentrations were below 8% for MMP-1, 11% for MMP-2 and 7% for MMP-9.
All statistical analysis was performed using R version 3.3.2. Phthalate metabolite and MMP concentrations were all right-skewed and log-transformed for statistical analysis. All phthalate metabolites were analyzed separately, in addition to a summed value (ΣDEHP) for multiple metabolites of DEHP (MEHP, MEHHP, MEOHP, MECPP) based on molecular weight. Demographic information was collected for covariates of interest, including maternal age, race/ethnicity, education level, health insurance provider and body mass index. Differences in MMP or phthalate levels between categorical covariates were tested using t-tests or one-way ANOVA, and Pearson correlations were calculated for continuous covariates. Next, a crude analysis of the association between phthalates and MMPs was conducted using simple linear regression. Final adjusted models were constructed by including covariates either associated with both phthalates and MMPs or changed effect estimates by 10% or more. The same covariates were included in all models for consistency. To further examine potential non-linear effects, phthalate metabolite concentrations were divided into tertiles and regressed on MMP levels while adjusting for the same covariates.
RESULTS
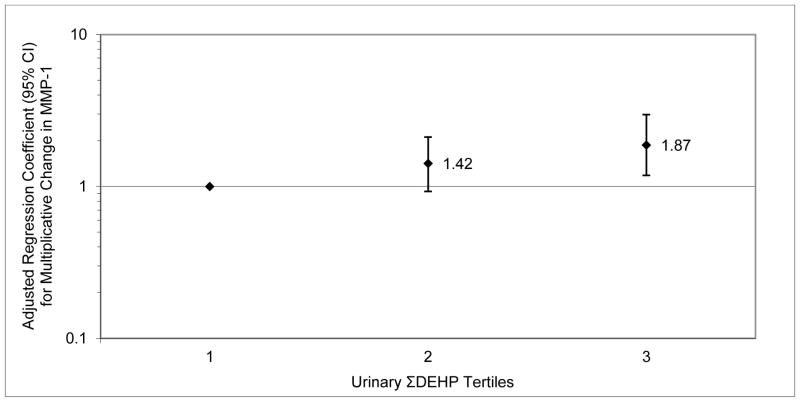
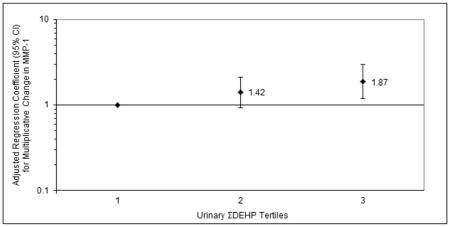
The majority of women in the study were white and college-educated as described previously (Ferguson et al. 2014). There was a suggestive difference in MMP-1 levels by insurance provider (p=0.16). We also found a significant difference between BMI categories in MMP-2 levels (p=0.03) and a suggestive difference in MMP-9 levels (p=0.15) by BMI category. In Table 1, geometric means as well as 25th, 75th, 95th and 100th percentiles of phthalate and MMP levels are presented. Crude results (not presented) were similar to those in the adjusted models (Table 2). We observed a significant positive association between MMP-1 and MECPP as well as suggestive associations for MEHP, MEOHP, ΣDEHP, MBP, MIBP and MCPP. For MMP-2, we found suggestive positive associations with MBZP and MEP. For MMP-9, we observed suggestive associations between MEHHP, MEOHP, MECPP and ΣDEHP. When tertiles of summed DEHP metabolites were regressed on MMP levels, results were consistent with the models using continuous variables and some strong increasing trends were observed. For example, the second and third tertiles of ΣDEHP were associated with increases in MMP-1 of 42% and 87%, respectively, compared to the lowest ΣDEHP tertile (Figure 1).
Table 1.
Distribution of urinary phthalate metabolite and plasma MMP concentrations (N=134)
| Biomarker | Geometric Mean | Percentiles
|
||||
|---|---|---|---|---|---|---|
| 25th | 50th | 75th | 95th | Maximum | ||
|
| ||||||
| Urine (ng/ml) | ||||||
| MEHP | 7.52 | 3.05 | 7.12 | 15.1 | 83.4 | 888 |
| MEHHP | 21.6 | 6.91 | 19.8 | 42.9 | 390 | 2100 |
| MEOHP | 12.9 | 4.25 | 12.1 | 25.9 | 178 | 1200 |
| MECPP | 32.6 | 10.8 | 29.6 | 72.4 | 439 | 4290 |
| ΣDEHP | 26.8 | 10.6 | 24.9 | 56.6 | 387 | 2140 |
| MBZP | 4.75 | 1.93 | 4.36 | 12.4 | 42.7 | 255 |
| MBP | 12.2 | 6.10 | 13.6 | 31.4 | 66.6 | 217 |
| MIBP | 5.60 | 2.31 | 6.62 | 12.3 | 29.8 | 158 |
| MEP | 105 | 31.4 | 80.2 | 308 | 2180 | 5500 |
| MCPP | 1.65 | 0.642 | 1.53 | 3.50 | 14.2 | 116 |
|
| ||||||
| Plasma (ng/ml) | ||||||
| MMP-1 | 0.59 | 0.21 | 0.59 | 0.98 | 2.62 | 7.52 |
| MMP-2 | 50.7 | 36.0 | 54.8 | 75.8 | 123 | 182 |
| MMP-9 | 51.0 | 36.4 | 51.2 | 78.5 | 122 | 211 |
Table 2.
Adjusted percent change (95% Confidence Intervals) in MMP level associated with an interquartile range (IQR) increase in phthalate metabolite concentrations. N=134.
| MMP-1 | MMP-2 | MMP-9 | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| % Δ | 95% CI | p-value | % Δ | 95% CI | p-value | % Δ | 95% CI | p-value | |
|
| |||||||||
| MEHP | 18.10 | −3.92, 44.9 | 0.11 | 1.94 | −11.2, 17.0 | 0.79 | 10.43 | −4.84, 28.3 | 0.19 |
| MEHHP | 15.11 | −8.23, 44.4 | 0.22 | 2.78 | −11.5, 19.6 | 0.71 | 17.45 | −0.16, 38.4 | 0.05 |
| MEOHP | 23.77 | −1.08, 54.6 | 0.06 | 5.57 | −8.97, 22.7 | 0.47 | 14.52 | −2.50, 34.7 | 0.10 |
| MECPP | 25.89 | 0.19, 58.2 | 0.05 | 8.32 | −6.97, 26.4 | 0.30 | 13.60 | −3.92, 34.1 | 0.13 |
| ΣDEHP | 23.50 | −0.33, 52.8 | 0.05 | 6.04 | −8.19, 22.3 | 0.42 | 14.92 | −1.66, 34.3 | 0.08 |
| MBZP | −1.29 | −24.9, 29.5 | 0.92 | 18.22 | −1.11. 41.1 | 0.07 | −2.75 | −20.2, 18.4 | 0.78 |
| MBP | 36.52 | −1.63, 89.5 | 0.06 | 14.38 | −8.02, 42.5 | 0.22 | −0.65 | −21.9, 26.4 | 0.96 |
| MIBP | 28.08 | −6.63, 76.0 | 0.12 | 16.44 | −5.69, 43.8 | 0.16 | −12.96 | −30.9, 9.63 | 0.24 |
| MEP | −1.81 | −21.9, 23.1 | 0.87 | 13.64 | −2.26, 31.8 | 0.10 | −4.46 | −19.0, 12.6 | 0.59 |
| MCPP | 25.09 | −0.84, 58.1 | 0.06 | 9.96 | −5.92, 28.5 | 0.23 | −0.17 | −15.9, 18.5 | 0.98 |
Adjusted for BMI, insurance, specific gravity, age
Figure 1.
Adjusted regression coefficients for multiplicative change MMP-1 level in relation to tertiles of urinary ΣDEHP concentration. P-value for trend = 0.008.
DISCUSSION
The results of this exploratory study suggest that urinary phthalate metabolites are associated with increased circulating MMP levels in pregnant women. We observed the strongest relationships between DEHP metabolites and MMP-1 and MMP-9 levels. We found suggestive positive associations between DBP and DiBP metabolites and MMP-1 as well as between BzBP and DEP metabolites and MMP-2. To our knowledge this is the first human study to test these associations.
Our findings in a human population are supported by several experimental studies that have shown a positive association between phthalate levels and MMP expression in various cell types. In an in vitro study by Kim et al. (2015), endometrial cells treated with DEHP were found to have increased MMP-9 levels. Another in vitro study of breast cancer cells by Zhang et al. (2014) also reported that DEHP was associated with the overexpression of MMP-2 and MMP-9. A third in vitro study by Zhu et al. (2010) reported similar trends in human neuroblastoma cells. Among other phthalates, an in vivo study by Scarano et al. (2009) found that rats treated with DBP had higher MMP-9 activity in the prostate, whereas we found that the DBP metabolites MBP and MCPP were associated with MMP-1, but not associated with MMP-2 or MMP-9. To our knowledge, there are no studies that have tested the effect of phthalates on MMP-1. Future research is needed to obtain a comprehensive understanding of the effects of phthalates on MMP expression and activity.
Despite the present study’s exploratory nature and relatively small sample size, our findings may help inform the existing body of research concerning environmental as they relate to various adverse pregnancy outcomes influenced by inflammatory pathways. MMPs are involved in significant degradation and remodeling of tissues. The role of MMPs prior to parturition are less defined, though MMP-8 levels in amniotic fluid are predictive of intra-amniotic inflammation/infection and early spontaneous delivery (Chaemsaithong et al. 2017; Kim et al. 2016). MMP-1, 2 and 9 have been shown to increase with increasing cervical dilation and play an important role in uterine postpartum involution in animals (Manase et al. 2006; Winkler et al. 1999), while MMP-9 facilitates fetal membrane rupture and placental separation (Tsatas et al. 1999).
Since MMPs are a vital part of the reproductive process, there is a need to assess which chemicals may impact their balance that could play a role in adverse pregnancy outcomes such as preeclampsia and preterm birth (Geng et al. 2016). Phthalates have been found to be associated with adverse pregnancy outcomes, but knowledge on relevant mechanisms is lacking. The present study observed a positive association between metabolites of DEHP, and potentially other phthalates, and MMP levels. However, as an exploratory study our sample size and statistical power were limited and future full-scale studies should address the potential for MMP levels to mediate associations between phthalates or other environmental exposures and adverse pregnancy outcomes.
Highlights.
Exposure to phthalates is common, including among pregnant women, and may be associated with adverse outcomes like preterm birth.
Matrix metalloproteinases (MMP) are enzymes that participate in extracellular matrix degradation and may play a role in a range of adverse pregnancy outcomes.
Experimental studies suggest phthalate exposure may upregulate MMP, but human data are lacking.
In 134 pregnant women we found that increased concentrations of several phthalate metabolites were associated with higher circulating MMP levels in maternal plasma.
Acknowledgments
Funding was provided by the National Institute of Environmental Health Sciences, National Institutes of Health (R01ES018872, P42ES017198, P50ES026049, and U2CES026553).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no competing interests to declare.
References
- Ab Hamid J, Mohtarrudin N, Osman M, Andi Asri AA, Wan Hassan WH, Aziz R. Matrix metalloproteinase-9 and tissue inhibitors of metalloproteinases 1 and 2 as potential biomarkers for gestational hypertension. Singapore Med J. 2012;53:681–683. [PubMed] [Google Scholar]
- Cantonwine DE, Meeker JD, Ferguson KK, Mukherjee B, Hauser R, McElrath TF. Urinary Concentrations of Bisphenol A and Phthalate Metabolites Measured during Pregnancy and Risk of Preeclampsia. Environ Health Perspect. 2016;124:1651–1655. doi: 10.1289/EHP188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaemsaithong P, Romero R, Docheva N, Chaiyasit N, Bhatti G, Pacora P, et al. Comparison of rapid MMP-8 and interleukin-6 point-of-care tests to identify intra-amniotic inflammation/infection and impending preterm delivery in patients with preterm labor and intact membranes. J Matern Fetal Neonatal Med. 2017:1–17. doi: 10.1080/14767058.2017.1281904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eleuterio NM, Palei AC, Rangel Machado JS, Tanus-Santos JE, Cavalli RC, Sandrim VC. Positive correlations between circulating adiponectin and MMP2 in preeclampsia pregnant. Pregnancy Hypertens. 2015;5:205–208. doi: 10.1016/j.preghy.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Meeker JD. Environmental phthalate exposure and preterm birth. JAMA Pediatr. 2014;168:61–67. doi: 10.1001/jamapediatrics.2013.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng J, Huang C, Jiang S. Roles and regulation of the matrix metalloproteinase system in parturition. Mol Reprod Dev. 2016;83:276–286. doi: 10.1002/mrd.22626. [DOI] [PubMed] [Google Scholar]
- Kim SH, Cho S, Ihm HJ, Oh YS, Heo SH, Chun S, et al. Possible Role of Phthalate in the Pathogenesis of Endometriosis: In Vitro, Animal, and Human Data. J Clin Endocrinol Metab. 2015;100:E1502–1511. doi: 10.1210/jc.2015-2478. [DOI] [PubMed] [Google Scholar]
- Kim SM, Romero R, Lee J, Chaemsaithong P, Lee MW, Chaiyasit N, et al. About one-half of early spontaneous preterm deliveries can be identified by a rapid matrix metalloproteinase-8 (MMP-8) bedside test at the time of mid-trimester genetic amniocentesis. J Matern Fetal Neonatal Med. 2016;29:2414–2422. doi: 10.3109/14767058.2015.1094049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MS, Kahn SR, Platt RW, Genest J, Chen MF, Goulet L, et al. Mid-trimester maternal plasma cytokines and CRP as predictors of spontaneous preterm birth. Cytokine. 2010;49:10–14. doi: 10.1016/j.cyto.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Lewis RC, Meeker JD, Peterson KE, Lee JM, Pace GG, Cantoral A, et al. Predictors of urinary bisphenol A and phthalate metabolite concentrations in Mexican children. Chemosphere. 2013;93:2390–2398. doi: 10.1016/j.chemosphere.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manase K, Endo T, Chida M, Nagasawa K, Honnma H, Yamazaki K, et al. Coordinated elevation of membrane type 1-matrix metalloproteinase and matrix metalloproteinase-2 expression in rat uterus during postpartum involution. Reprod Biol Endocrinol. 2006;4:32. doi: 10.1186/1477-7827-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie C, Vendittelli F, Sauvant-Rochat MP. Obstetrical outcomes and biomarkers to assess exposure to phthalates: A review. Environ Int. 2015;83:116–136. doi: 10.1016/j.envint.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Messerlian C, Wylie BJ, Minguez-Alarcon L, Williams PL, Ford JB, Souter IC, et al. Urinary Concentrations of Phthalate Metabolites and Pregnancy Loss Among Women Conceiving with Medically Assisted Reproduction. Epidemiology. 2016;27:879–888. doi: 10.1097/EDE.0000000000000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissi R, Talvensaari-Mattila A, Kotila V, Niinimaki M, Jarvela I, Turpeenniemi-Hujanen T. Circulating matrix metalloproteinase MMP-9 and MMP-2/TIMP-2 complex are associated with spontaneous early pregnancy failure. Reprod Biol Endocrinol. 2013;11:2. doi: 10.1186/1477-7827-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarano WR, Toledo FC, Guerra MT, de Campos SG, Junior LA, Felisbino SL, et al. Long-term effects of developmental exposure to di-n-butyl-phthalate (DBP) on rat prostate: proliferative and inflammatory disorders and a possible role of androgens. Toxicology. 2009;262:215–223. doi: 10.1016/j.tox.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Tsatas D, Baker MS, Rice GE. Differential expression of proteases in human gestational tissues before, during and after spontaneous-onset labour at term. J Reprod Fertil. 1999;116:43–49. doi: 10.1530/jrf.0.1160043. [DOI] [PubMed] [Google Scholar]
- Werner EF, Braun JM, Yolton K, Khoury JC, Lanphear BP. The association between maternal urinary phthalate concentrations and blood pressure in pregnancy: The HOME Study. Environ Health. 2015;14:75. doi: 10.1186/s12940-015-0062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler M, Oberpichler A, Tschesche H, Ruck P, Fischer DC, Rath W. Collagenolysis in the lower uterine segment during parturition at term: correlations with stage of cervical dilatation and duration of labor. Am J Obstet Gynecol. 1999;181:153–158. doi: 10.1016/s0002-9378(99)70452-7. [DOI] [PubMed] [Google Scholar]
- Yao PL, Lin YC, Richburg JH. Mono-(2-ethylhexyl) phthalate (MEHP) promotes invasion and migration of human testicular embryonal carcinoma cells. Biol Reprod. 2012;86:160, 161–110. doi: 10.1095/biolreprod.111.097295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Ma J, Fu Z, Zhang Z, Cao J, Huang L, et al. Promotion of breast cancer cells MDA-MB-231 invasion by di(2-ethylhexyl)phthalate through matrix metalloproteinase-2/-9 overexpression. Environ Sci Pollut Res Int. 2016;23:9742–9749. doi: 10.1007/s11356-016-6158-7. [DOI] [PubMed] [Google Scholar]
- Zhu H, Zheng J, Xiao X, Zheng S, Dong K, Liu J, et al. Environmental endocrine disruptors promote invasion and metastasis of SK-N-SH human neuroblastoma cells. Oncol Rep. 2010;23:129–139. [PubMed] [Google Scholar]