Abstract
BACKGROUND
Warfarin is effective in preventing stroke and systemic embolism in atrial fibrillation (AF) but has several limitations. The new oral anticoagulants (NOACs) address many of these limitations but their impact on prescribing practices in older adults with AF is unknown.
DESIGN
Retrospective observational cohort study
SETTING
Academic medical center in St. Louis, MO, USA
PARTICIPANTS
Patients age ≥75 with AF admitted from October 2010 through September 2015 (N=6568, 50% female, 15% non-white).
MEASUREMENTS
NOACs and warfarin prescribed at discharge were obtained from hospital discharge summaries. Linear regression was used to examine quarterly trends in use of these agents. Multivariable logistic regression was used to assess independent predictors of anticoagulant use.
RESULTS
NOAC use increased over time (correlation coefficient [r]=0.87, p<0.001), warfarin use did not change (r=−0.16, p=0.500), and overall anticoagulant use (NOACs and warfarin) increased (r=0.68, p=0.001). NOAC use increased over time in all age groups (ages 75–79, 80–84, 85–89) except age ≥90, but the rate of NOAC uptake was attenuated by increasing age. There was no consistent relationship between age and warfarin or overall anticoagulant use, except patients age ≥90 had consistently lower use. Overall, <45% of patients were prescribed an anticoagulant. In multivariable analysis, younger age, white race, female gender, higher hemoglobin, higher creatinine clearance, being on a medical service, hypertension, stroke/TIA, no history of intracranial hemorrhage and modified HAS-BLED score <3 increased the likelihood of receiving NOACs.
CONCLUSION
Prescription of anticoagulants for AF increased in older adults primarily due to an increase in the use of NOACs. Nonetheless, less than 45% of patients were prescribed an anticoagulant. Additional research is needed to optimize prescribing practices for older adults with AF.
Keywords: oral anticoagulants, new oral anticoagulants, atrial fibrillation, CHA2DS2-VASc score, race, creatinine clearance, dementia, warfarin
INTRODUCTION
Warfarin is an effective therapy for preventing stroke and systemic embolism in patients with atrial fibrillation (AF), and prior to 2010 it was the only oral anticoagulant available in the U.S. for this purpose. Warfarin has many advantages, including low cost, relative ease of monitoring, and availability of reversal agents, but it also has several disadvantages, including slow onset of action, narrow therapeutic index requiring close monitoring, numerous food and drug interactions, and relatively high risk for major bleeding complications.1, 2 In part for these reasons, warfarin is underutilized in a substantial proportion of patients with AF, and increasing age is associated with a progressive decline in warfarin use.3, 4
Introduction of several new oral anticoagulants (NOACs) has addressed some of these disadvantages. As a group, NOACs have a rapid onset of action with more predictable pharmacokinetics and no need for routine monitoring 2, 5. In addition, the NOACs are at least as effective as warfarin for prevention of ischemic stroke and are associated with lower risk of intracranial hemorrhage,6 an advantage of particular relevance to older adults. For these reasons, NOACs are now recommended as alternative first-line therapy for prevention of stroke and systemic emboli in nonvalvular AF.7 Conversely, limitations to use of NOACs include higher cost, lack of readily accessible monitoring tests and reversal agents, and contraindications in patients with severe kidney or liver disease.2
Although several studies have examined trends in NOAC use in the general population,8–11 to our knowledge no study has assessed NOAC use in older adults. The purpose of this study, therefore, was to examine changes in anticoagulation prescribing practices for older adults with AF since the introduction of NOACs in 2010, with particular attention to the associations of age, sex, race, comorbidities, and socioeconomic status with overall utilization of anticoagulation and trends in uptake of NOACs. We hypothesized that there would be a net increase in anticoagulant use over time driven primarily by increased use of NOACs, and that uptake in NOAC use would be affected by age, kidney function, dementia diagnosis and income but not by sex or race.
METHODS
Study Population and Inclusion Criteria
This was a retrospective observational study. We included patients ≥75 years old with AF admitted to Barnes-Jewish Hospital (a large tertiary academic medical center in St. Louis, MO) from October 2010 through September 2015 and discharged alive. Based on age alone, all patients had a CHA2DS2-VASc (congestive heart failure, hypertension, age ≥75, diabetes, stoke/TIA, vascular disease (peripheral vascular disease or coronary artery disease), age 65–74, sex category (female sex)) score12 of at least 2 and were therefore candidates for anticoagulation. A total of 7012 unique patients met the inclusion criteria; 444 patients with missing data on discharge medications were excluded, yielding 6568 patients for the final analysis. For 2029 patients with multiple admissions, only the last available discharge record with medication data was retained for analysis. Patients were grouped into calendar year quarters based on discharge date. The study was approved by the institutional review board at the Washington University in St. Louis.
Data Collection
Patients fulfilling the inclusion criteria were identified using a searchable electronic medical record database, the Clinical Investigation Data Exploration Repository (CIDER), maintained by the Washington University in St. Louis Center for Biomedical Informatics. Data were collected on patient demographics (age, sex and race), height, weight, selected laboratory (hemoglobin and creatinine) and service type (medical and surgical). Medical services included general medicine, neurology and psychiatry. Surgical services included general surgery, cardiothoracic surgery, gynecology, neurosurgery, ophthalmology, orthopedic surgery, otolaryngology, plastic surgery, trauma surgery and urology. Medical comorbidities were derived from ICD-9 codes and included heart failure, hypertension, diabetes, stroke, transient ischemic attack (TIA), cerebrovascular disease, peripheral vascular disease, coronary artery disease, dementia, liver disease, bleeding, gastrointestinal bleeding, intracranial bleeding, lung/thoracic bleeding, soft tissue hematoma, alcohol abuse and history of fall. Residential zip codes were used as a surrogate for economic status. Discharge medications including NOACs (dabigatran, rivaroxaban, apixaban), warfarin, aspirin, clopidogrel, prasugrel and ticagrelor were obtained from hospital discharge summaries.
Other Variables
Creatinine clearance was calculated using the Cockcroft-Gault equation.13 Body mass index (BMI) was calculated as (height in meters)/(weight in kilograms)2. A modified HAS-BLED score14 was calculated that included hypertension, kidney disease, liver disease, stroke, bleeding history, elderly (age ≥65 years), drugs (aspirin, clopidogrel, prasugrel or ticagrelor) and alcohol abuse but excluded labile international normalized ratio (INR) and non-steroidal anti-inflammatory drugs (NSAIDs) because data for these were not available. Zip codes were used to obtain median income estimates from the United States Census Bureau website (http://factfinder.census.gov/faces/nav/jsf/pages/searchresults.xhtml?refresh=t) and classified in tertiles ($12,589–40,413, $40,460–55,876, and $55,938–205,169).
Statistical Methods
Patient characteristics were summarized by age category (75–79, 80–84, 85–89, ≥90 years) using the mean ± standard deviation or frequency count (percent) for continuous and categorical variables, respectively. Continuous variables were compared using one-way analysis of variance. The chi-square or Fisher’s exact test was used to compare categorical variables. Non-normal and ordinal variables were summarized by the median (1st quartile, 3rd quartile) and compared using the Kruskal-Wallis test.
The linear trend in medication prescribed over time was examined using linear regression with quarter of the year as the independent variable and percent medication as the dependent variable. For this analysis, quarter was treated as a continuous variable with values ranging from 1 to 20 (first quarter October-December 2010; last available quarter July-September 2015). The Pearson correlation coefficient was created to describe strength of linear trend. To compare trends across patient subgroups, separate models were created that included the interaction between time and subgroup. The linear model assumptions of normal and homogeneous errors were evaluated by examination of residual and normal probability plots. No significant deviations were noted.
A multivariable logistic regression model was built to examine independent predictors of NOAC and anticoagulant use. Variables were chosen a priori based on prior literature and clinical experience, including factors affecting risk of stroke, bleeding or likelihood of prescribing anticoagulation (age, race, gender, BMI, hemoglobin and creatinine clearance) or based on results from univariate analysis (p<0.1, see supplementary Tables S1 and S2) and components of the CHA2DS2-VASc score. To adjust for time, a quarterly time variable was included and treated as a continuous variable. When conducting the multivariable analysis, missing data were imputed. Multiple data sets (5) were created, each using a sequential imputation algorithm (see supplementary materials S1).
All statistical analyses were conducted in SAS v9.4 (SAS Institute Inc., Cary, NC). A two tailed p<0.05 was considered statistically significant.
RESULTS
Baseline Characteristics
Baseline characteristics of the cohort stratified by age group are summarized in Table 1. Of 6568 total patients, 36%, 31%, 22% and 11% were aged 75 to79, 80 to 84, 85 to 89 and ≥90 years, respectively. Women comprised 50% of the cohort and 85% of the patients with available race data were white. The percentage of women increased with increasing age (p<0.001). The median income of the cohort was $52,306. The median CHADS2 (congestive heart failure, hypertension, age ≥75, diabetes, stroke/TIA) 15 and CHA2DS2-VASc12 scores were 3 and 4 respectively. The prevalence of comorbidities varied by age group as shown in Table 1. Baseline characteristics of the cohort stratified by anticoagulation use are shown in Supplementary Table S3.
Table 1.
Baseline Characteristics of the Study Cohort Stratified by Age Group
| Characteristic | Overall (N=6568) | Age 75–79 (N=2345) | Age 80–84 (N=2024) | Age 85–89 (N=1445) | Age ≥90 (N=754) | P-value |
|---|---|---|---|---|---|---|
| Women, n (%) | 3303 (50%) | 1040 (44%) | 989 (49%) | 795 (55%) | 479 (64%) | <0.001 |
| White, n (%) | 5298 (85%) | 1953 (86%) | 1627 (85%) | 1175 (86%) | 543 (78%) | <0.001 |
| Median income, $ | 52306 ± 21239 | 51393 ± 20310 | 52375 ± 20898 | 53720 ± 21869 | 52202 ± 23456 | 0.015 |
| Body mass index, n (%) | <0.001 | |||||
| < 25 | 2487 (38%) | 685 (29%) | 769 (38%) | 633 (44%) | 400 (53%) | |
| 25 to < 30 | 2484 (38%) | 922 (39%) | 772 (38%) | 529 (37%) | 261 (35%) | |
| ≥30 | 1597 (24%) | 738 (31%) | 483 (24%) | 283 (20%) | 93 (12%) | |
| Hemoglobin, g/dl | 10.7 ± 1.8 | 10.7 ± 1.9 | 10.8 ± 1.8 | 10.7 ± 1.8 | 10.8 ± 1.8 | 0.24 |
| Creatinine clearance, n (%) | <0.001 | |||||
| 0 to <15 | 169 (3%) | 47 (2%) | 44 (2%) | 47 (4%) | 31 (4%) | |
| 15 to <50 | 2454 (41%) | 561 (27%) | 707 (39%) | 679 (51%) | 507 (73%) | |
| ≥ 50 | 3336 (56%) | 1498 (71%) | 1083 (59%) | 598 (45%) | 157 (23%) | |
| Medical Service, n (%) | 4270 (65%) | 1432 (61%) | 1326 (66%) | 961 (67%) | 551 (73%) | <0.001 |
| Heart failure, n (%) | 3065 (47%) | 968 (41%) | 962 (48%) | 714 (49%) | 421 (56%) | <0.001 |
| Hypertension, n (%) | 5269 (80%) | 1856 (79%) | 1615 (80%) | 1180 (82%) | 618 (82%) | 0.15 |
| Diabetes, n (%) | 1990 (30%) | 804 (34%) | 613 (30%) | 424 (29%) | 149 (20%) | <0.001 |
| Stroke/TIA, n (%) | 391 (6%) | 104 (4%) | 130 (6%) | 98 (7%) | 59 (8%) | <0.001 |
| Cerebrovascular disease, n (%) | 91 (1%) | 22 (1%) | 36 (2%) | 26 (2%) | 7 (1%) | 0.032 |
| Peripheral vascular disease, n (%) | 1077 (16%) | 399 (17%) | 316 (16%) | 237 (16%) | 125 (17%) | 0.66 |
| Coronary artery disease, n (%) | 3208 (49%) | 1129 (48%) | 1005 (50%) | 727 (50%) | 347 (46%) | 0.20 |
| Dementia, n (%) | 769 (12%) | 152 (6%) | 217 (11%) | 235 (16%) | 165 (22%) | <0.001 |
| Liver disease, n (%) | 213 (3%) | 100 (4%) | 59 (3%) | 42 (3%) | 12 (2%) | 0.001 |
| Gastrointestinal bleeding, n (%) | 199 (3%) | 72 (3%) | 65 (3%) | 43 (3%) | 19 (3%) | 0.84 |
| Intracranial bleeding, n (%) | 157 (2%) | 40 (2%) | 56 (3%) | 45 (3%) | 16 (2%) | 0.022 |
| History of fall, n (%) | 350 (5%) | 77 (3%) | 88 (4%) | 111 (8%) | 74 (10%) | <0.001 |
| CHADS2 score, median (Q1, Q3)* | 3.0 (2.0, 3.0) | 3.0 (2.0, 3.0) | 3.0 (2.0, 3.0) | 3.0 (2.0, 3.0) | 3.0 (2.0, 3.0) | 0.007 |
| CHA2DS2VASc score, median (Q1, Q3)* | 4.0 (4.0, 5.0) | 4.0 (3.0, 5.0) | 4.0 (4.0, 5.0) | 4.0 (4.0, 5.0) | 4.0 (4.0, 5.0) | <0.001 |
| Modified HAS-BLED score ≥3, n (%) | 4179 (64%) | 1459 (62%) | 1273 (63%) | 938 (65%) | 509 (68%) | 0.038 |
(1st quartile, 3rd quartile). All percentages indicate the proportion of patients with available data. CHADS2: congestive heart failure, hypertension, age ≥75, diabetes, stroke/TIA. CHA2DS2VASc: congestive heart failure, hypertension, age ≥75, diabetes, stoke/TIA, vascular disease (peripheral vascular disease or coronary artery disease), age 65–74, sex category (female sex). NOAC: new oral anticoagulant; TIA: transient ischemic attack. Modified HAS-BLED: hypertension, kidney disease, liver disease, stroke, bleeding history (bleeding or gastrointestinal bleeding or intracranial bleeding or lung/thoracic bleeding or soft tissue hematoma), elderly (age >65), drugs (aspirin or clopidogrel or prasugrel or ticagrelor), alcohol abuse.
Quarterly Trends in Use of Anticoagulation
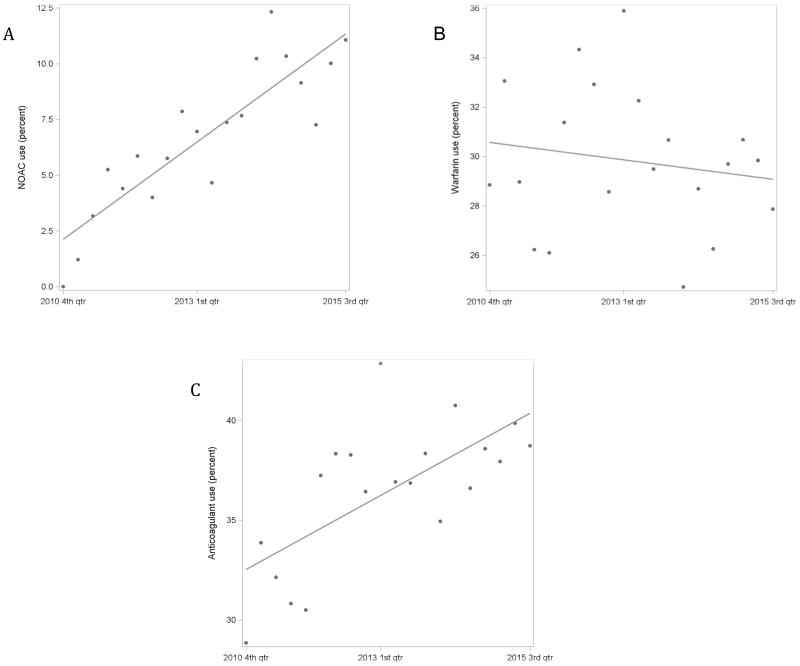
Quarterly trends in NOAC, warfarin and overall anticoagulant use are summarized in Figures 1A, 1B and 1C respectively. NOAC use significantly increased over time (correlation coefficient [r] 0.87, p<0.001). There was no significant change in warfarin use over time (r −0.16, p=0.500). Overall anticoagulant use increased over time (r 0.68, p=0.001), but remained <45% throughout the study period.
Figure 1.
(A) Quarterly trend in NOAC use (correlation coefficient [r] 0.87, p <0.001). (B) Quarterly trend in warfarin use (r −0.16, p=0.500). (C) Quarterly trend in anticoagulant use (r 0.68, p=0.001)).
Quarterly Trends in Use of Anticoagulants by Age Group
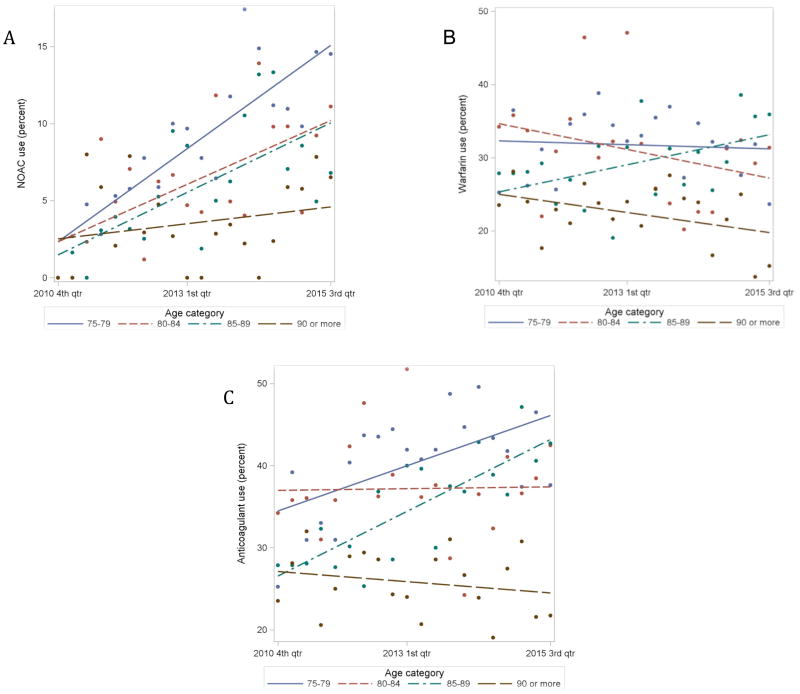
Quarterly trends in NOAC, warfarin and anticoagulant use by age group are summarized in Figures 2A, 2B and 2C respectively. Although uptake of NOACs increased significantly over time for all age groups except age ≥90, the rate of uptake was attenuated by increasing age (p for interaction = 0.007). Use of warfarin increased only in patients aged 85–89 (remaining unchanged in the other age groups), while overall use of anticoagulants increased only in patients aged 75–79 and 85–89 (remaining unchanged in the other age groups). Overall, there was no consistent association between age and prescription of warfarin or any anticoagulant, except that patients ≥ 90 years had consistently lower use. There were significant differences in trends of warfarin and anticoagulant use over time across age groups (p for interaction 0.028 and <0.001, respectively).
Figure 2.
(A) Quarterly trends in NOAC use by age group. 75–79 (r 0.86, p<0.001), 80–84 (r 0.62, p=0.003), 85–89 (r 0.67, p=0.001), ≥90 (r 0.23, p=0.340); p-value for interaction=0.007. (B) Quarterly trends in warfarin use by age group. 75–79 (r −0.07, p=0.750), 80–84(r −0.32, p=0.170), 85–89 (r 0.48, p=0.032), ≥90(r −0.41, p=0.070); p-value for interaction=0.028. (C) Quarterly trends in anticoagulant use by age group. 75–79 (r 0.58, p=0.008), 80–84 (r 0.02, p=0.930), 85–89 (r 0.81, p<0.001) and ≥90
Quarterly Trends in Use of NOACs for Other Subgroups
Quarterly trends by gender, race, renal function, dementia diagnosis and income are summarized in Supplementary Figures S1 through S5. NOAC use increased over time in women and men, whites and non-whites, patients without dementia (no change in patients with dementia), patients in all renal function categories (except stage V chronic kidney disease), and patients in all tertiles of median income. There were no significant differences in rate of NOAC uptake between men and women (p for interaction= 0.93), whites and non-whites (p= 0.51), patients with and without dementia diagnosis (p= 0.05) or across income tertiles (p=0.70). Lower renal function was associated with lower NOAC use over time (p<0.001).
Factors Predictive of NOAC Use
Factors independently predictive of NOAC use are summarized in Table 2. In the multivariable model that included age, gender, race, median income, BMI, hemoglobin, creatinine clearance, service type, heart failure, hypertension, diabetes, stroke/TIA, peripheral vascular disease, coronary artery disease, dementia, gastrointestinal bleeding, intracranial bleeding, modified HAS-BLED score and time, 11 factors independently affected the likelihood of NOAC use. These included age (compared to age group 75–79, age group 80–84 [OR 0.675, p=0.001], 85–89 [OR 0.665, p=0.003] and ≥90 [OR 0.390, p<0.001]), female gender (OR 1.335, p=0.005), white race (OR 1.501, p=0.018), hemoglobin (OR per 1 g/dl increase 1.196, p<0.001), creatinine clearance (≥50 vs <50 mL/min, OR 1.368, p=0.010), medical service (vs surgical service, OR 1.260, p=0.041), hypertension (OR 1.323, p=0.044), stroke/TIA (OR 1.573, p=0.016), history of intracranial bleeding (OR 0.386, p=0.043), HAS-BLED score ≥3 (vs <3 OR 0.744, p=0.015) and time from start of the analysis (OR 1.081 per 1 quarter increase, p<0.001).
Table 2.
Independent Predictors of NOAC Use
| Characteristic | OR* | 95% CI | P-value |
|---|---|---|---|
| Age (vs. 75–79) | |||
| 80–84 | 0.675 | (0.536, 0.852) | 0.001 |
| 85–89 | 0.665 | (0.507, 0.873) | 0.003 |
| ≥ 90 | 0.390 | (0.254, 0.599) | <0.001 |
| Women (vs. men) | 1.335 | (1.090, 1.636) | 0.005 |
| White (vs. non-white) | 1.501 | (1.073, 2.099) | 0.018 |
| Median income (vs. 1st tertile) | |||
| 2nd tertile | 0.957 | (0.851, 1.354) | 0.74 |
| 3rd tertile | 1.257 | (0.737, 1.258) | 0.07 |
| Body mass index (vs. < 25) | |||
| 25 to < 30 | 1.074 | (0.851, 1.354) | 0.55 |
| ≥ 30 | 0.963 | (0.737, 1.258) | 0.78 |
| Hemoglobin (per 1 g/dl increase) | 1.196 | (1.132, 1.263) | <0.001 |
| Creatinine Clearance, ≥ 50 (vs. < 50) | 1.368 | (1.077, 1.736) | 0.010 |
| Medical service (vs. surgical service) | 1.260 | (0.737, 1.258) | 0.041 |
| Heart failure, yes (vs. no) | 1.097 | (1.132, 1.263) | 0.39 |
| Hypertension, yes (vs. no) | 1.323 | (0.851, 1.354) | 0.044 |
| Diabetes, yes (vs. no) | 0.795 | (1.077, 1.736) | 0.05 |
| Stroke/TIA yes (vs. no) | 1.573 | (1.062, 1.101) | 0.016 |
| Peripheral vascular disease, yes (vs. no) | 0.930 | (0.851, 1.354) | 0.61 |
| Coronary artery disease, yes (vs. no) | 0.872 | (0.737, 1.258) | 0.21 |
| Dementia, yes (vs. no) | 0.780 | (1.132, 1.263) | 0.15 |
| Gastrointestinal bleeding, yes (vs. no) | 0.746 | (1.077, 1.736) | 0.43 |
| Intracranial bleeding, yes (vs. no) | 0.386 | (1.062, 1.101) | 0.043 |
| Modified HAS-BLED score, ≥3 yes (vs. <3) | 0.744 | (1.132, 1.263) | 0.015 |
| Time point (per 1 quarter increase) | 1.081 | (1.062, 1.101) | <0.001 |
Adjusted odds ratio of being prescribed a NOAC
NOAC: new oral anticoagulant; TIA: transient ischemic attack. Modified HAS-BLED: hypertension, kidney disease, liver disease, stroke, bleeding history (bleeding or gastrointestinal bleeding or intracranial bleeding or lung/thoracic bleeding or soft tissue hematoma), elderly (age >65), drugs (aspirin or clopidogrel or prasugrel or ticagrelor), alcohol abuse.
Factors Predictive of Anticoagulant Use
Factors independently predictive of anticoagulant use are summarized in Supplementary Table S4. In the multivariable model, 15 factors independently affected the likelihood of anticoagulant use. These included age (compared to age group 75–79, age group ≥90 [OR 0.549, p<0.001]), race (OR for white vs. other 1.495, p<0.001), median income (1st tertile vs. 3rd tertile [OR 1.179, p=0.017]), BMI (compared to BMI <25, BMI 25 to <30 [OR 1.266, p<0.001] and BMI ≥30 [OR 1.406, p<0.001]), hemoglobin (OR per 1 g/dl increase 1.134, p<0.001), medical service (vs. surgical service OR 1.317, p<0.001), heart failure (OR 1.150, p<0.001), cerebrovascular disease (OR 0.522, p=0.016), dementia (OR 0.515, p<0.001), liver disease (OR 0.567, p=0.001), gastrointestinal bleeding (OR 0.635, p=0.009), intracranial bleeding (OR 0.265, p<0.001), history of fall (OR 0.598, p<0.001), modified HAS-BLED score ≥3 (vs <3, OR 0.811, p=0.003) and time from start of the analysis (OR 1.018 per 1 quarter increase, p<0.001).
DISCUSSION
Over a period of 5 years, NOAC use in older adults with AF admitted to a tertiary academic medical center increased progressively, whereas warfarin use remained stable, resulting in an overall increase in prescription of anticoagulants. The overall trend was generally consistent across demographic subgroups defined by age, sex, and race, but the rate of NOAC uptake declined significantly with increasing age.
Prior studies have assessed the effect of the introduction of NOACs on AF treatment patterns in various populations, but no study has assessed treatment patterns specifically in older adults. In one study of newly diagnosed AF patients with a mean age of 69 years evaluated over a similar period of time (2010 to 2015), the authors reported an increase in anticoagulation therapy driven primarily by prescription of NOACs.16 Another study in AF patients hospitalized with TIA or stroke conducted in the first two years after the introduction of NOACs showed no change in anticoagulant use but a trend towards switching from warfarin to NOACs.17 The increase in use of NOACs observed in these studies, including ours, reflects increased familiarity with NOACs and better understanding among physicians of indications for their use. We anticipate that this trend will continue, especially as prices of these drugs decline and with approval of novel reversal agents.
An important finding of our study is that NOAC use increased over time in all age groups except patients ≥90 years, and the rate of new use declined with increasing age. Warfarin and overall anticoagulant use increased only in patients age 85–89, most likely representing a chance finding, and there were no clear trends in usage over time as a function of age, except that patients ≥90 years were less likely to receive treatment.
Although age is a potent risk factor for stroke and systemic embolization in patients with AF, anticoagulation is underutilized in older adults.3, 4 We found that even with the availability of NOACs, overall use of anticoagulation was less than 45% throughout the study period. Factors associated with low utilization rates in our population include non-white race, low income, low BMI, low hemoglobin, being on a surgical service, not having heart failure, cerebrovascular disease, dementia, liver disease, gastrointestinal bleeding, intracranial bleeding, history of fall and a modified HAS-BLED score ≥3. For warfarin, drug and dietary interactions and requirement for close monitoring with frequent blood tests likely also contributed to low utilization rates. Our results suggest that these and other concerns are especially pronounced in patients ≥90 years old. Alternatively, NOACs may have abated these concerns to a greater extent in patients age 75–79 years than in those ≥90 years. Nevertheless, our data indicate that there is considerable opportunity to improve use of anticoagulation in the high-risk group of older adults with AF.
As anticipated, there were no differences in the rate of NOAC uptake between women and men or between whites and non-whites (non-significant p-values for time interaction). However, both gender and race were found to be predictors of NOAC prescription after adjusting for time and other factors. Women were more likely to be prescribed a NOAC compared to men (OR=1.335, p=0.005) and whites were more likely to be prescribed a NOAC compared to non-whites (OR=1.501, p=0.018). In other words, although rate of NOAC uptake did not differ by gender or race, overall use was higher in women and whites than in men and non-whites, respectively, as illustrated in Supplementary Figures S1 and S2.
Also as expected, there was a difference in NOAC use based on renal function, such that patients with higher creatinine clearance were more likely to be prescribed NOACs. In bivariate analysis, NOAC use increased over time in patients without dementia but not in patients with dementia. However, the difference between groups was only marginally significant (p=0.05). After adjusting for time and other factors, dementia diagnosis did not affect the likelihood of being prescribed a NOAC but was associated with lower overall anticoagulant use. Other studies have also shown lower use of anticoagulation in patients with cognitive impairment.18, 19 This may be due to a perception of potentially lower net benefit of anticoagulation in this group, such that the benefit may no longer outweigh an increased risk of bleeding and other side effects. However, this view has been challenged by a recent study showing that discontinuation of warfarin following a diagnosis of dementia is associated with increased risk of stroke and death.20 Of note, having a history of falling was also associated with lower use of anticoagulation overall but not with uptake of NOACs. This may be due to the associated lower risk of intracranial hemorrhage with NOACs.6 NOAC use increased over time in all tertiles of income, but contrary to expectations there was no difference in the rate of uptake across income tertiles after adjusting for time and other variables. Given the higher cost of NOACs, we had expected higher use in patients with higher incomes.
Limitations of study
Our study has several limitations. It is based on data from a single center and may not be representative of other populations. The retrospective design and use of hospital records may be associated with coding errors and other inaccuracies in the data. Specifically, geriatric syndromes such as falls and cognitive impairment are likely undercoded. Other factors not included in our analysis such as education level or marital status may have affected prescription of warfarin and NOACs; in particular, we did not have information on reasons for non-prescription of anticoagulants in individual patients, which may include both physician and patient factors. We used zip code as a surrogate for income, but this may not accurately reflect an individual’s socioeconomic status. However, all patients were potentially eligible for Medicare, which may reduce disparities in ability to pay for medications.
Conclusion
In patients 75 years of age or older admitted to a large academic hospital, the introduction of NOACs was associated with an increase in the use of oral anticoagulation for AF. Nonetheless, despite high risk for stroke based on the CHA2DS2-VASc score, less than 45% of patients in this cohort were discharged on an anticoagulant. Additional research is needed to optimize prescribing practices for older adults with AF, including a better understanding of the reasons for not using anticoagulants in a high proportion of patients.
Supplementary Material
Table S1. Univariate Predictors of NOAC Use
Table S2. Univariate Predictors of Anticoagulant Use
Table S3. Baseline Characteristics of Study Cohort Stratified by Anticoagulant Use
Table S4. Independent Predictors of Anticoagulant Use
Acknowledgments
Funding:
This research was supported by the Mentors in Medicine (MiM) program of the Department of Medicine and Division of Medical Education at the Washington University School of Medicine and a Just-in-Time (JIT) grant (ID# CI044) from the Washington University Institute of Clinical and Translation Sciences grant UL1 TR000448 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
Footnotes
Meetings and Presentations:
This research was presented in part during the Presidential Poster Session at the 2017 Annual Scientific Meeting of the American Geriatrics Society, San Antonio, Texas.
Conflict of interest: The authors have no conflicts of interest.
Author Contributions: R.B.F. contributed to the design, data collection, interpretation of data, drafting and revision of the manuscript. E.N. contributed to the analysis, interpretation of data and revision of the manuscript. M.W.R. contributed to the conception, design, interpretation of data, and revision of the manuscript. M.W.R. is the corresponding author and affirms that he has listed all authors who contributed significantly to this work.
Sponsor’s Role: None.
References
- 1.Landefeld CS, Beyth RJ. Anticoagulant-related bleeding: clinical epidermiology, prediction, and prevention. Am J Med. 1993;95:315–328. doi: 10.1016/0002-9343(93)90285-w. [DOI] [PubMed] [Google Scholar]
- 2.Shafeeq H, Tran TH. New oral anticoagulants for atrial fibrillation: are they worth the risk? PT. 2014;39:54–64. [PMC free article] [PubMed] [Google Scholar]
- 3.Brass LM, Krumholz HM, Scinto JD, et al. Warfarin use following ischemic stroke among Medicare patients with atrial fibrillation. Arch Intern Med. 1998;158:2093–2100. doi: 10.1001/archinte.158.19.2093. [DOI] [PubMed] [Google Scholar]
- 4.Gage BF, Boechler M, Doggette AL, et al. Adverse outcomes and predictors of underuse of antithrombotic therapy in medicare beneficiaries with chronic atrial fibrillation. Stroke. 2000;31:822–827. doi: 10.1161/01.str.31.4.822. [DOI] [PubMed] [Google Scholar]
- 5.Strangier J. Clinical pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor dabigatran etexilate. Clin Pharmacokinet. 2008;47:285–295. doi: 10.2165/00003088-200847050-00001. [DOI] [PubMed] [Google Scholar]
- 6.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–962. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 7.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 8.Kirley K, Qato DM, Kornfield R, et al. National trends in oral anticoagulant use in the United States, 2007 to 2011. Circ Cardiovasc Qual Outcomes. 2012;5:615–621. doi: 10.1161/CIRCOUTCOMES.112.967299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnes GD, Lucas E, Alexander GC, et al. National Trends in Ambulatory Oral Anticoagulant Use. Am J Med. 2015;128:1300–1305. e2. doi: 10.1016/j.amjmed.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desai NR, Krumme AA, Schneeweiss S, et al. Patterns of initiation of oral anticoagulants in patients with atrial fibrillation-quality and cost implications. Am J Med. 2014;127:1075–1082. e1. doi: 10.1016/j.amjmed.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Weitz JI, Semchuk W, Turpie AG, et al. Trends in Prescribing Oral Anticoagulants in Canada, 2008–2014. Clin Ther. 2015;37:2506–2514. e4. doi: 10.1016/j.clinthera.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 13.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 14.Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;135:1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 15.Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 16.Camm AJ, Accetta G, Ambrosio G, et al. Evolving antithrombotic treatment patterns for patients with newly diagnosed atrial fibrillation. Heart. 2017;103:307–314. doi: 10.1136/heartjnl-2016-309832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel PA, Zhao X, Fonarow GC, et al. Novel Oral Anticoagulant Use Among Patients with Atrial Fibrillation Hospitalized with Ischemic Stroke or Transient Ischemic Attack. Circ Cardiovasc Qual Outcomes. 2015;8:383–392. doi: 10.1161/CIRCOUTCOMES.114.000907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bahri O, Roca F, Lechani T, et al. Underuse of oral anticoagulation for individuals with atrial fibrillation in a nursing home setting in France: comparisons of resident characteristics and physician attitude. J Am Geriatr Soc. 2015;63:71–76. doi: 10.1111/jgs.13200. [DOI] [PubMed] [Google Scholar]
- 19.Wilke T, Groth A, Pfannkuche M, et al. Real life anticoagulation treatment of patients with atrial fibrillation in Germany: extent and causes of anticoagulant under-use. J Thromb Thrombolysis. 2015;40:97–107. doi: 10.1007/s11239-014-1136-8. [DOI] [PubMed] [Google Scholar]
- 20.Orkaby AR, Ozonoff A, Reisman JI, et al. Continued use of warfarin in Veterans with atrial fibrillation after dementia diagnosis. J Am Geriatr Soc. 2017;65:249–256. doi: 10.1111/jgs.14573. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Univariate Predictors of NOAC Use
Table S2. Univariate Predictors of Anticoagulant Use
Table S3. Baseline Characteristics of Study Cohort Stratified by Anticoagulant Use
Table S4. Independent Predictors of Anticoagulant Use