Abstract
Background
Prior studies have yielded inconsistent evidence regarding the association between formaldehyde exposure and amyotrophic lateral sclerosis (ALS).
Methods
We conducted a population case-control study in the Danish National Registries on the relationship between occupationally-derived formaldehyde exposure and ALS. Occupational history was obtained from a comprehensive and prospectively recorded pension database of all paid work in Denmark since 1964, and was linked to a job-exposure matrix to derive individual exposure estimates. Each case was matched to 4 age- and sex-matched population controls alive on the date of the case diagnosis via risk set sampling, and odds ratios (OR) and confidence intervals (CI) were calculated via conditional logistic regression, adjusting for potential confounders.
Results
There were 3,650 incident cases of amyotrophic lateral sclerosis in the Danish National Patient Register from 1982 to 2009. Among controls, 25% were ever employed in jobs with a positive prevalence of formaldehyde exposure. Exposure to formaldehyde was associated with a 1.3-fold increased rate of ALS (95% CI: 1.2–1.4).
Conclusion
This study suggests that formaldehyde exposure, or employment in formaldehyde-exposed occupations, is related to the risk of ALS.
Introduction
Amyotrophic lateral sclerosis (ALS) is a rapidly progressing disease of the upper and lower motor neurons, with median survival estimated at 2–3 years [1]. While no non-genetic causes have been definitely identified, male sex [1], cigarette smoking [2], physical activity [3], physical trauma [4,5], and certain chemicals [6] have all been unevenly implicated as potential risk factors. Associations have also been found with certain occupations, including veterinarians, medical workers, athletes, power-plant operators and military personnel [7]. However these studies, particularly those prior to the mid-2000s, were generally hampered by a lack of objective exposure and outcome ascertainment, clinic-based sampling of cases, family- or convenience-sampling of controls, and/or few exposed cases [7].
Formaldehyde, an organic water-soluble compound with many industrial applications and widespread use, was implicated in ALS risk in a large U.S. cohort [8]. The Cancer Prevention Study-II (CPS-II) queried over one million individuals about a host of chemical exposures, and found an elevated rate of ALS among those who reported an exposure to formaldehyde (RR=1.34; 95% CI 0.93, 1.92). When they restricted the analysis to those who reported their duration of formaldehyde exposure, they found a strongly significant trend with increasing years of exposure (p<0.001). A small case-control study in New England used self-reported occupations and found a non-significant three-fold increase in the most highly exposed, based on 5 highly-exposed cases [9]. A separate study of over 11,000 garment workers found no increase in ALS as compared to the U.S. population, but the study comprised only 8 ALS cases [10]. A more recent study in the U.S. National Longitudinal Mortality Study found an elevated hazard ratio in the most highly exposed (HR=4.44; 95% CI: 1.16–16.85), but this finding was based on only 2 ALS deaths in the highest exposure category, which was comprised entirely of funeral directors [11].
The objective of this study was to assess the risk of ALS in relation to occupational formaldehyde exposure using national registry data in the Danish population. We hypothesized that ALS patients would have higher cumulative levels of formaldehyde exposure.
Methods
Participant Selection
We obtained cases from the Danish National Patient Register, using primary discharge diagnoses of 348.0 (ICD-8) or G12.2 (ICD-10). ICD-8 codes were used in Denmark through 1993, with ICD-10 thereafter. All hospital admissions nationwide are captured by the National Hospital Register, beginning on January 1, 1977 [12]. In a validation sub-study of 173 ALS cases identified this way, we obtained medical records and confirmed the ALS diagnosis in 160 (92.5%) [13]. We limited our case definition to first diagnoses on or after January 1, 1982, a five-year washout period to reduce the inclusion of prevalent cases. Case ascertainment was performed through December 31, 2009, and the index date was the first recorded hospitalization with ALS recorded as the primary discharge diagnosis.
We obtained controls from the Central Person Registry, which covers all residents in Denmark since 1968 [14]. We selected 4 controls for each case, individually matched on sex, age in 1-year windows, and who were free of an ALS diagnosis in the Hospital Register as of the index date (risk-set sampling). All inhabitants of Denmark are assigned a unique 10-digit Central Person number, which includes information on date of birth and sex, and can be used to link between multiple databases, including the Central Person Registry, Danish National Patient Register, and the Danish Pension Fund (see below).
Exposure Ascertainment
We obtained occupational histories from the Danish Pension Fund databases, which maintains employment history on all wage earners aged 16–66, beginning in 1964. In the pension database each employment is recorded with start and end dates, the Central Person number of the employee, an 8-digit tax-number for the company and a 5-digit industry code assigned by Statistics Denmark. The industry code is based on the company’s main activities as classified by Statistics Denmark, which employs an extended version of the 4-digit International Standard Industrial Classification codes.[15]
We employed a previously constructed job-exposure matrix (JEM), the NOCCA-DANJEM. Briefly, this JEM was modified from a Finnish JEM by occupational hygienists and occupational epidemiologists from the five Nordic countries [16]. The Danish version was modified by a Danish occupational epidemiologist (J.H.); thus, the final NOCCA-DANJEM that we employed in this study incorporates industrial measurements of formaldehyde in the original Finnish construction, measurements from Denmark, as well as expert knowledge regarding the translation from Finnish occupations to Danish occupations. The JEM includes industry-specific exposure estimates over four periods: 1945–1959, 1960–1974, 1975–1984, and 1985 thereafter. Because our occupational records did not begin until 1964, we did not use the first period. Thus, the use of the JEM takes two inputs – year and industry – and outputs the estimated prevalence of exposure in a given job along with the estimated mean level of formaldehyde exposure among the exposed in that job. We refer to the output of this process as an “expected” exposure level (ppm for formaldehyde), which is lower than actual ppm experienced on the job when exposed because the group level effectively averages over all people in a given job by multiplying by the exposure prevalence. This specific JEM has not been directly validated, however a cruder version of this JEM did report a significant association between occupational formaldehyde and sino-nasal cancer [17].
Our primary exposure of interest was cumulative expected exposure to formaldehyde (ppm) between 1964 and up to three years prior to the year of interest. For each employment at each time, the expected exposure was calculated as the prevalence of exposure multiplied by the mean level of exposure among the exposed [16], and then summed across jobs and time to obtain cumulative expected exposure. The three-year lag is employed to allow for underlying ALS prior to diagnosis; it is believed that the time from first symptoms to diagnosis is from 9–12 months, although the time from true disease inception to diagnosis is unknown [7]. We also assessed a five-year lag to allow for a longer pre-diagnostic period.
Covariate Ascertainment
In addition to the matching variables of age, sex, and calendar date, we abstracted information on highest socioeconomic status (SES) attained, marital status and history, and residence from the Central Person Registry on the index date. SES was classified into five groups based on an individual’s and, if applicable, his or her spouse’s job titles: academics and managers (1), high salaried (2), low salaried (3), skilled workers (4) and unskilled worker (5).
We also extracted, for each individual, the following covariates through the fourth year prior to the index date: binary flag for having worked that year, total number of years worked prior to that year, whether they were admitted to the hospital in that year, the number of times they had been admitted to the hospital prior to that year, the total number of hospital diagnoses prior to that year, and all hospital discharge diagnoses. We used discharge diagnoses to calculate the Charlson Comorbidity Index, which assigns weights to a handful of conditions to estimate an individual’s underlying health status [18].
We also assessed the relationship between employment as a mortician and ALS rate because morticians are highly exposed to formaldehyde, and a prior study found suggestive evidence of an association [11].
Statistical Methods
All models are conditional logistic regressions with strata defined by the 1:4 matched case-control sets. Under our incidence density sampling approach the resulting odds ratios are valid estimates of rate ratios, and we refer to them as such. Unless otherwise specified, we adjusted for age, sex and calendar date (the original matching variables), SES, marital status, and residence in all analyses. In secondary analyses we further adjusted for the total number of hospital diagnoses an individual had in his or her record up through the fourth year before the index date, whether or not an individual was employed during the fourth year prior to the index date, and an individual’s Charlson Comorbidity Index on the fourth year prior to the index date.
We categorized the cumulative formaldehyde exposure into both a dichotomous ever-exposed group (lagged three years) and into quantiles of exposure, determined by the distribution of exposure among exposed cases. We fit the continuous measure of cumulative exposure using a penalized spline, with degrees of freedom chosen via the Akaike’s Information Criterion (AIC) (pspline function in R).
Because the sensitivity and specificity of ALS diagnoses is considerably lower among individuals with only an outpatient diagnosis [19], we performed a sensitivity analysis in which we excluded all cases that had only an outpatient diagnosis (n=468).
All statistical analyses were conducted using the R Statistical Software, version 3.0.3 (Foundation for Statistical Computing, Vienna, Austria) and SAS, version 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
There were 3,650 newly diagnosed cases of ALS in Denmark between January 1, 1982 and December 31, 2009. Table 1 shows descriptive statistics as of the index date for cases and the 14,600 age-, sex-, and calendar year-matched controls. Cases had a mean age of 65 years and a median age of 67 at diagnosis. Cases tended to reside slightly more often in larger cities, and had slightly higher SES than controls. Cases were more likely to be married as of the index date.
Table 1.
Descriptive statistics of ALS cases and controls, Denmark January 1 1982 – December 31 2009.* Characteristics describe the cases and controls on the case (index) date.
| Cases | Controls | |
|---|---|---|
| Male sex, n (%) | 1954 (54) | 7816 (54) |
| Year of birth, mean (sd) | 1932 (14) | 1932 (14) |
| Age, mean (sd) | 65 (12) | 65 (12) |
| Residence | ||
| Copenhagen | 525 (14) | 1756 (12) |
| Copenhagen suburbs | 787 (22) | 3164 (22) |
| Aarhus/Odense | 405 (11) | 1603 (11) |
| Provincial towns | 1350 (37) | 5785 (40) |
| Rural areas | 525 (14) | 2170 (15) |
| Greenland | 6 (0.16) | 66 (0.45) |
| Unknown | 52 (1.4) | 56 (0.38) |
| SES | ||
| 1 – High | 390 (11) | 1417 (10) |
| 2 | 426 (12) | 1594 (11) |
| 3 | 710 (20) | 2764 (19) |
| 4 | 944 (26) | 3897 (27) |
| 5 – Low | 678 (19) | 2872 (20) |
| Unknown | 502 (14) | 2056 (14) |
| Marriage Status | ||
| Married | 2222 (61) | 7107 (49) |
| Unmarried | 274 (7.5) | 1203 (8.2) |
| Divorced | 359 (10) | 1519 (10) |
| Widower | 795 (22) | 4771 (33) |
N = 3,650 cases and 14,600 controls.
Out of 3,650 cases, 1,068 (29%) had ever been employed in occupations with a positive prevalence of formaldehyde exposure, while only 25% of controls had been employed in such jobs. Among controls, individuals who had worked in occupations with formaldehyde exposures were more likely to be male, were younger, had lower SES, and were less likely to be widowed than individuals who had never worked in exposed jobs (Table 2).
Table 2.
Descriptive statistics by exposure level, among the controls, Denmark January 1 1982 – December 31 2009.* Characteristics describe individuals on the index date.
| Ever-Exposed | Never-Exposed | |
|---|---|---|
| Male sex, n (%) | 2002 (55) | 5814 (53) |
| Year of birth, mean (sd) | 1938 (14) | 1930 (14) |
| Age, mean (sd) | 61 (12) | 67 (12) |
| Residence | ||
| Copenhagen | 391 (11) | 1365 (12) |
| Copenhagen suburbs | 719 (20) | 2445 (22) |
| Aarhus/Odense | 405 (11) | 1198 (11) |
| Provincial towns | 1565 (43) | 4220 (39) |
| Rural areas | 581 (16) | 1589 (15) |
| Greenland | 3 (0.08) | 63 (0.58) |
| Unknown | 2 (0.05) | 54 (0.49) |
| SES | ||
| 1 – High | 328 (8.9) | 1089 (9.9) |
| 2 | 410 (11) | 1184 (11) |
| 3 | 598 (16) | 2166 (20) |
| 4 | 1069 (29) | 2828 (26) |
| 5 – Low | 913 (25) | 1959 (18) |
| Unknown | 348 (9) | 1708 (16) |
| Marriage Status | ||
| Married | 1907 (52) | 5200 (48) |
| Unmarried | 369 (10) | 834 (7.6) |
| Divorced | 550 (15) | 969 (8.9) |
| Widower | 840 (23) | 3931 (36) |
N = 3,666 ever-exposed and 10,934 never-exposed.
The estimated prevalence of exposed workers in jobs with any exposure ranged from 5% (textile) to 90% (plywood and fiberboard). Exposure intensities, which represent the one-year average concentration of the agent in the vicinity of exposed workers, ranged from 0.01ppm (electronics) to 1.5ppm (wood finishers, plywood and fiberboard). Exposure intensity decreased over the three time-periods in the JEM for most occupations, while exposure prevalence typically remained constant. As a result, the “expected” (prevalence × intensity) exposure decreased over time for nearly all occupations.
Among the exposed, the mean cumulative exposure among controls was 0.26 expected ppm, while among cases it was 0.25 expected ppm. The respective median cumulative exposure levels were 0.07 expected ppm for controls, and 0.08 expected ppm for cases.
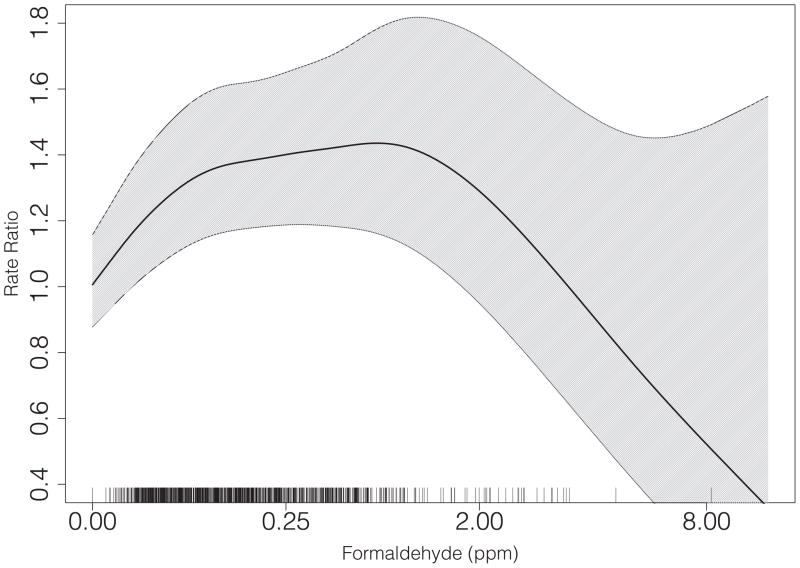
Table 3 displays the results for the association between cumulative formaldehyde exposure, lagged three years, and the rate of ALS incidence. Employment in an exposed job was associated with a 1.3-fold increased rate of ALS diagnosis (95% CI: 1.2–1.4). There was little variation of the association across quartiles of exposure. At higher levels the association declined although confidence intervals grew rapidly at the highest exposures (Figure 1). The relative rate of ALS in those above the 95th percentile of exposure (0.92 expected ppm) as compared to the unexposed was 1.0 (95% CI: 0.8–1.4).
Table 3.
Adjusted* rate ratios and 95% confidence intervals (CI) for association between formaldehyde exposure (expected ppm), lagged three years, with ALS Jan 1 1982–Dec 31 2009.
| Formaldehyde Exposure** | Controls N (%) |
Cases N (%) |
RR* (95% CI) |
|---|---|---|---|
| No Exposure | 10934 (75) | 2582 (71) | 1.0 (ref) |
| Ever Exposure | 3666 (25) | 1068 (29) | 1.3 (1.2–1.4) |
| 1st Quartile | 935 (6.4) | 262 (7.2) | 1.3 (1.1–1.5) |
| 2nd Quartile | 976 (6.7) | 272 (7.5) | 1.2 (1.1–1.4) |
| 3rd Quartile | 873 (6.0) | 268 (7.3) | 1.4 (1.2–1.6) |
| 4th Quartile | 882 (6.0) | 266 (7.3) | 1.3 (1.1–1.5) |
All models adjusted for matching factors (age, sex and calendar date), residence, marital status, and SES.
Quartiles of exposure determined from cases with non-zero exposure, with cutoffs: 0.013, 0.08, and 0.28 ppm.
Figure 1.
Association between expected cumulative formaldehyde exposure (ppm), lagged three years, and the rate of ALS. Curve is fitted via penalized spline, with degrees of freedom chosen via AIC. The rug plot indicates case exposure levels. Note: the x-axis is plotted on a cube-root scale.
Further adjustment for hospitalization history, Charlson Comordity Index, and employment status on the fourth year prior to the index date slightly attenuated the point estimate for ever-exposure (RR = 1.2; 95% CI: 1.1–1.4). There was no evidence of heterogeneity of the association by sex (p=0.97), despite substantial numbers of exposed cases of both sexes (580 men, 488 women). In a sensitivity analysis in which we excluded all cases with only an outpatient diagnosis of ALS (n=468), we found no differences from the main analysis. We also excluded SES from our model on the supposition that it was partially controlling for smoking; we found no difference in the point estimate for dichotomous formaldehyde exposure, nor any heterogeneity when stratifying by SES. Analyses with a 5-year lag for exposure were also no different from the main analysis.
Twenty-three individuals had a history of employment as morticians prior to the index date, with only two developing ALS. The resulting association was thus extremely imprecise, though there was no evidence of a harmful relationship (RR = 0.4; 95% CI: 0.1–1.6).
Discussion
This is the largest study to date, to our knowledge, to assess the relationship between formaldehyde exposure and ALS incidence in a prospectively-followed and nationally-representative cohort, with objective recording of both occupational history and medical diagnoses. We observed an elevated rate of ALS among those employed in jobs with likely exposure to formaldehyde. There was no substantial difference in the strength of the association across quartiles of exposure, but we observed some evidence of a decrease in the association at the highest levels.
Formaldehyde is thought to have direct neurological effects, and can cross the blood-brain barrier freely [20]. In particular, formaldehyde is associated with increased oxidative stress via dysregulation of superoxide dismutase (SOD) [21], as well as with increased mitochondrial membrane permeability [22], both of which are also observed in ALS pathogenesis [23,24]. Notably, a primary source of non-occupational formaldehyde exposure is cigarette smoke [25], one of the potential risk factors for ALS.[2,26] Some studies have found associations between military service and ALS risk [27–29]. While precise exposure estimates are unknown, there is some evidence that military occupations, particularly military deployment, entail exposure to formaldehyde above background levels [30]. A recent study of ALS among US veterans (the GENEVA study) reported an elevated probable exposure to formaldehyde during non-combat military jobs held by cohort members, based on industrial hygiene experts [31].
Both a strength and a limitation of this study is the use of a JEM to estimate individuals’ exposure levels. This approach allowed us to calculate quantitative estimates of formaldehyde based on an individual’s occupational history, rather than simply considering industries or industry-groupings as exposures. No prior study has used JEMs to estimate a cumulative formaldehyde exposure; instead, most studies have relied on either amount of time spent in exposed jobs (e.g. garment workers), or self-reported job history. However, because of variation of tasks within occupations and industries, JEM-based exposure assignments will necessarily still introduce exposure misclassification. Namely, this method will most likely overestimate the proportion of workers classified as “ever exposed” to formaldehyde. However, because occupational history was prospectively recorded in the Danish Pension Fund database, we expect the misclassification to be non-differential with respect to the outcome, and thus to bias our results towards the null. In addition, while the specific JEM we utilized was developed based on industrial measurements of formaldehyde, it has not been specifically validated for estimating formaldehyde exposure. However, an earlier version was used in a study that found an association between formaldehyde exposure and sino-nasal cancer [17].
We cannot rule out confounding by unobserved factors as an explanation for our findings. In particular, we lacked information on smoking habits. However, smoking is not established as a definitive risk factor for ALS, and there is some evidence that it is only a risk factor among women [2]. We did not observe any heterogeneity of our main findings by sex. Furthermore, in prior studies our SES variable has been found to be correlated with smoking in Denmark, thus adjustment for SES would have partially controlled for smoking.[32] We saw no change in the point estimate when removing SES from the model, despite a moderate correlation between SES and exposure to formaldehyde. This may argue against confounding by smoking in our data.
We observed some evidence of an attenuation, and even reversal, of the association at higher exposure levels. This pattern of association is common in occupational epidemiology [33]. It may be explained by the healthy worker survivor effect (a manifestation of time-varying confounding). This bias results from a continual process of selecting out of the exposed occupations those susceptible to the effects of formaldehyde; those who are “available” to accumulate the highest levels are therefore the healthiest or least susceptible. This bias often yields the pattern we observed, wherein the most highly-exposed show attenuated, or even reversed, associations with the outcome [33]. However, it should be noted that adjustment for work status in the fourth year prior to the index date did little to change either the point estimates or the overall shape of the relationship. Lagging is also one way to potentially diminish the healthy worker survivor effect, under the assumption that the effect of underlying disease on future exposure is fully captured within the lag window, which in this study was 3 or 5 years.[34]
In conclusion, we observed a positive association between formaldehyde exposure and ALS. This is the largest study to date to investigate this relationship, and our findings support earlier suggestions of a link between formaldehyde and ALS risk. These findings, taken in conjunction with the fact that formaldehyde acts on pathways implicated in ALS pathogenesis, suggest that formaldehyde should be investigated further as a potential causative risk factor.
Key Messages.
Prior studies have found conflicting evidence concerning the link between formaldehyde exposure and ALS
We observed a 1.3-fold elevation in the rate of ALS in individuals who had been employed in jobs with any potential exposure to formaldehyde
Acknowledgments
Funding:
This work was supported by the National Institute of Environmental Health Sciences [grant number 5R01ES019188-02 to MW]. RMS was supported by the Taplin Fellowship, a National Research Service Award [grant number T32 ES 07069], and an NIH postdoctoral research fellowship [grand number T32 NS 048005].
The funding source had no role in the design or analysis of the study or in the decision to submit the manuscript for publication.
References
- 1.Mitchell JD, Borasio GD. Amyotrophic lateral sclerosis. Lancet. 2007;369:2031–41. doi: 10.1016/S0140-6736(07)60944-1. [DOI] [PubMed] [Google Scholar]
- 2.Alonso A, Logroscino G, Hernan MA. Smoking and the risk of amyotrophic lateral sclerosis: a systematic review and meta-analysis. Journal of Neurology, Neurosurgery & Psychiatry. 2010;81:1249–52. doi: 10.1136/jnnp.2009.180232. [DOI] [PubMed] [Google Scholar]
- 3.Harwood CA, McDermott CJ, Shaw PJ. Physical activity as an exogenous risk factor in motor neuron disease (MND): a review of the evidence. Amyotroph Lateral Scler. 2009;10:191–204. doi: 10.1080/17482960802549739. [DOI] [PubMed] [Google Scholar]
- 4.Pupillo E, Messina P, Logroscino G, Zoccolella S, Chiò A, Calvo A, et al. Trauma and amyotrophic lateral sclerosis: a case-control study from a population-based registry. Eur J Neurol. 2012;19:1509–17. doi: 10.1111/j.1468-1331.2012.03723.x. [DOI] [PubMed] [Google Scholar]
- 5.Peters TL, Fang F, Weibull CE, Sandler DP, Kamel F, Ye W. Severe head injury and amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14:267–72. doi: 10.3109/21678421.2012.754043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sutedja NA, Veldink JH, Fischer K, Kromhout H, Heederik D, Huisman MHB, et al. Exposure to chemicals and metals and risk of amyotrophic lateral sclerosis: a systematic review. Amyotroph Lateral Scler. 2009;10:302–9. doi: 10.3109/17482960802455416. [DOI] [PubMed] [Google Scholar]
- 7.Al-Chalabi A, Hardiman O. The epidemiology of ALS: a conspiracy of genes, environment and time. Nature Reviews Neurology. 2013;9:617–28. doi: 10.1038/nrneurol.2013.203. [DOI] [PubMed] [Google Scholar]
- 8.Weisskopf MG, Morozova N, O’Reilly EJ, McCullough ML, Calle EE, Thun MJ, et al. Prospective study of chemical exposures and amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatr. 2009;80:558–61. doi: 10.1136/jnnp.2008.156976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang F, Quinlan P, Ye W, Barber MK, Umbach DM, Sandler DP, et al. Workplace exposures and the risk of amyotrophic lateral sclerosis. Environ Health Perspect. 2009;117:1387–92. doi: 10.1289/ehp.0900580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinkerton LE, Hein MJ, Meyers A, Kamel F. Assessment of ALS mortality in a cohort of formaldehyde-exposed garment workers. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14:353–5. doi: 10.3109/21678421.2013.778284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts AL, Johnson NJ, Cudkowicz ME, Eum K-D, Weisskopf MG. Job-related formaldehyde exposure and ALS mortality in the USA. J Neurol Neurosurg Psychiatr. 2015 doi: 10.1136/jnnp-2015-310750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scandinavian Journal of Public Health. 2011;39:30–3. doi: 10.1177/1403494811401482. [DOI] [PubMed] [Google Scholar]
- 13.Kioumourtzoglou M-A, Seals RM, Himmerslev L, Gredal O, Hansen J, Weisskopf MG. Comparison of diagnoses of amyotrophic lateral sclerosis by use of death certificates and hospital discharge data in the Danish population. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16:224–9. doi: 10.3109/21678421.2014.988161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pedersen CB, Gøtzsche H, Møller JO, Mortensen PB. The Danish Civil Registration System. A cohort of eight million persons. Dan Med Bull. 2006;53:441–9. [PubMed] [Google Scholar]
- 15.Hansen J, Lassen CF. The Supplementary Pension Fund Register. Scand J Public Health. 2011;39:99–102. doi: 10.1177/1403494810394716. [DOI] [PubMed] [Google Scholar]
- 16.Kauppinen T, Heikkilä P, Plato N, Woldbaek T, Lenvik K, Hansen J, et al. Construction of job-exposure matrices for the Nordic Occupational Cancer Study (NOCCA) Acta Oncol. 2009;48:791–800. doi: 10.1080/02841860902718747. [DOI] [PubMed] [Google Scholar]
- 17.Hansen J, Olsen JH. Formaldehyde and cancer morbidity among male employees in Denmark. Cancer Causes Control. 1995;6:354–60. doi: 10.1007/BF00051411. [DOI] [PubMed] [Google Scholar]
- 18.D’Hoore W, Bouckaert A, Tilquin C. Practical considerations on the use of the Charlson comorbidity index with administrative data bases. J Clin Epidemiol. 1996;49:1429–33. doi: 10.1016/s0895-4356(96)00271-5. [DOI] [PubMed] [Google Scholar]
- 19.Kioumourtzoglou M, Seals R, Himmerslev L, Gredal O, Hansen J, Weisskopf M. Comparison of Diagnoses of Amyotrophic Lateral Sclerosis by Use of Death Certificates and Hospital Discharge Data in the Danish Population. doi: 10.3109/21678421.2014.988161. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shcherbakova LN, Tel’pukhov VI, Trenin SO, Bashilov IA, Lapkina TI. Permeability of the blood-brain barrier to intra-arterial formaldehyde. Biull Eksp Biol Med. 1986;102:573–5. [PubMed] [Google Scholar]
- 21.Gurel A, Coskun O, Armutcu F, Kanter M, Ozen OA. Vitamin E against oxidative damage caused by formaldehyde in frontal cortex and hippocampus: biochemical and histological studies. J Chem Neuroanat. 2005;29:173–8. doi: 10.1016/j.jchemneu.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Strubelt O, Younes M, Pentz R, Kühnel W. Mechanistic study on formaldehyde-induced hepatotoxicity. J Toxicol Environ Health. 1989;27:351–66. doi: 10.1080/15287398909531306. [DOI] [PubMed] [Google Scholar]
- 23.Andersen PM. Amyotrophic lateral sclerosis associated with mutations in the CuZn superoxide dismutase gene. Curr Neurol Neurosci Rep. 2006;6:37–46. doi: 10.1007/s11910-996-0008-9. [DOI] [PubMed] [Google Scholar]
- 24.Norenberg MD, Rao KVR. The mitochondrial permeability transition in neurologic disease. Neurochem Int. 2007;50:983–97. doi: 10.1016/j.neuint.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaller KH, Triebig G, Beyer B. Formaldehyde determination in tobacco smoke--studies under experimental and actual conditions. Zentralbl Hyg Umweltmed. 1989;189:103–10. [PubMed] [Google Scholar]
- 26.Wang H, O’Reilly ÉJ, Weisskopf MG, Logroscino G, McCullough ML, Thun MJ, et al. Smoking and risk of amyotrophic lateral sclerosis: a pooled analysis of 5 prospective cohorts. Arch Neurol. 2011;68:207–13. doi: 10.1001/archneurol.2010.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weisskopf MG, O’Reilly EJ, McCullough ML, Calle EE, Thun MJ, Cudkowicz M, et al. Prospective study of military service and mortality from ALS. Neurology. 2005;64:32–7. doi: 10.1212/01.WNL.0000148649.17706.D9. [DOI] [PubMed] [Google Scholar]
- 28.Seals RM, Kioumourtzoglou M-A, Gredal O, Hansen J, Weisskopf MG. ALS and the Military: A Population-Based Study in the Danish Registries. Epidemiology. 2015:1. doi: 10.1097/EDE.0000000000000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weisskopf MG, Cudkowicz ME, Johnson N. Military Service and Amyotrophic Lateral Sclerosis in a Population-based Cohort. Epidemiology. 2015;26:831–8. doi: 10.1097/EDE.0000000000000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Formaldehyde – Deployment Occupational and Environmental Health Concerns [Internet] U.S. Army Public Health Command; Available from: http://phc.amedd.army.mil/PHC%20Resource%20Library/Formaldehyde%20FS%2055-012-1011.pdf. [Google Scholar]
- 31.Bello A, Woskie SR, Gore R, Sandler DP, Schmidt S, Kamel F. Retrospective Assessment of Occupational Exposures for the GENEVA Study of ALS among Military Veterans. Annals of Work Exposures and Health. 2017;61:299–310. doi: 10.1093/annweh/wxw028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osler M. Smoking habits in Denmark from 1953 to 1991: a comparative analysis of results from three nationwide health surveys among adult Danes in 1953–1954, 1986–1987 and 1990–1991. Int J Epidemiol. 1992;21:862–71. doi: 10.1093/ije/21.5.862. [DOI] [PubMed] [Google Scholar]
- 33.Stayner L, Steenland K, Dosemeci M, Hertz-Picciotto I. Attenuation of exposure-response curves in occupational cohort studies at high exposure levels. Scand J Work Environ Health. 2003;29:317–24. doi: 10.5271/sjweh.737. [DOI] [PubMed] [Google Scholar]
- 34.Arrighi HM, Hertz-Picciotto I. The evolving concept of the healthy worker survivor effect. Epidemiology. 1994;5:189–96. doi: 10.1097/00001648-199403000-00009. [DOI] [PubMed] [Google Scholar]