Abstract
Mitochondrial dysfunction is central to the pathogenesis of neurological disorders. Neurons rely on oxidative phosphorylation to meet their energy requirements and thus alterations in mitochondrial function are linked to energy failure and neuronal cell death. Furthermore, dysfunctional mitochondria are reported to increase the steady-state levels of reactive oxygen species derived from the leakage of electrons from the electron transport chain. Research aimed at understanding mitochondrial dysfunction and its role in neurological disorders has been primarily geared towards neurons. In contrast, the role that dysfunctional mitochondria have in glial cells’ function and its implication for neuronal homeostasis and brain function has been largely understudied. Except for oligodendrocytes, astrocytes and microglia do not degenerate upon the impairment of mitochondrial function, as they rely primarily on glycolysis to produce energy and have a higher antioxidant capacity than neurons. However, recent evidence highlights the role of mitochondrial metabolism and signaling in glial cell function. In this work, we review the functional role of mitochondria in glial cells and the evidence regarding its potential role regulating neuronal homeostasis and disease progression.
Keywords: Astrocytes, microglia, oligodendrocytes, mitochondria, glycolysis, free fatty acid oxidation, calcium, redox, inflammation
1. Introduction
Mitochondria are involved in a myriad of other processes relevant for cell function besides energy (ATP) production (Yin et al. 2014), making them more than simply powerhouses of the cell. Mitochondria are a hub for signaling processes that include the maintenance of calcium (Ca2+) homeostasis and the formation of signaling molecules and thus, signaling events (Bonini; Chandel 2015). For example, cell death progression is well known to be triggered by the release of mitochondrial pro-death proteins. Alterations in mitochondrial functions are expected to have important implications for cellular function and disease progression. Correspondingly, numerous pathological conditions have been connected to mitochondrial dysfunction.
Neuronal cell death in brain disorders (neurodegeneration) and injury (neurotoxicity and ischemia) has been linked to a variety of alterations in mitochondrial homeostasis/function including traffic, quality control and turnover, homeostasis (bioenergetics and electron transport) and signaling (metabolism and Ca2+ handling) (Chaturvedi and Flint Beal 2013; Yin et al. 2014). Compared to other cell types, neurons are more dependent on mitochondrial oxidative phosphorylation (OXPHOS) to fulfill their energy demands. Mitochondrial dysfunction with the concomitant energy failure and increased generation of reactive oxygen species (ROS) are considered central to neuronal cell loss in brain disorders because neurons have a limited capacity to upregulate glycolysis or to counteract oxidative damage (Fernandez-Fernandez et al. 2012; Herrero-Mendez et al. 2009). As such, research has been primarily directed at understanding the causes and consequences of mitochondrial dysfunction in neuronal populations affected during neurodegeneration or brain injury (Moran et al. 2012; Yin et al. 2014).
While initially considered as accessory cells to neurons, glial cells are now recognized to be essential for neuronal cell homeostasis, survival and proper brain function and development (Bolanos 2016; Fernandez-Fernandez et al. 2012; Kubik and Philbert 2015). Importantly, genetic modifications or xenobiotics (i.e. pesticides [rotenone or paraquat], metals [lead, arsenic], antibiotics and drugs that target the integrity of mitochondrial DNA) recognized to alter mitochondrial function in neurons are expected to alter mitochondrial function in glial cells as well (Ballinger; Chan; Kubik and Philbert 2015; Meyer et al. 2013). Unfortunately, very few studies have addressed the pathological implications of mitochondrial dysfunction in glial cells and its consequences in neurological disorders. Herein, we review the current evidence demonstrating the importance of mitochondrial homeostasis and signaling in glial function and how their functional deficiency has important implications for brain disorders and injury that lead to or are a consequence of neuronal cell death.
2. Glial cell types and their functional roles
Glial cells can be generally classified as macroglia (astrocytes and oligodendrocytes) or microglia. Macroglia originate from the embryonic ectoderm, while microglia originate from the mesoderm and enter the vertebrate brain during embryogenesis. While initially grouped under the term “glia” (Greek term for glue), it is now clearly established that glial cells regulate a number of physiological processes required for proper neuronal survival and brain function. Refinement and revision of counting techniques have demonstrated that while the overall ratio of neurons to glial varies between different regions in the brain, a ratio of ~1:1 glia to neuron exists in the entire human brain, which is significantly smaller than previous estimates (~10:1). Oligodendrocytes are reported to be the most abundant type of glial cells (45–75%), followed by astrocytes (19–40%), and microglia (10% or less) (von Bartheld et al. 2016).
Oligodendrocytes are responsible for axon myelination at large membrane extensions, providing axons with an “insulating coat” that enhances nerve impulse conduction (Figure 1.4). Oligodendrocytes have several extensions that form several internodal segments of myelin separated by gaps (Ranvier nodes) (Baumann and Pham-Dinh 2001; Snell 2010). Oligodendrocytes are found in both gray and white matter, but are a major fraction of all the cells in white matter.
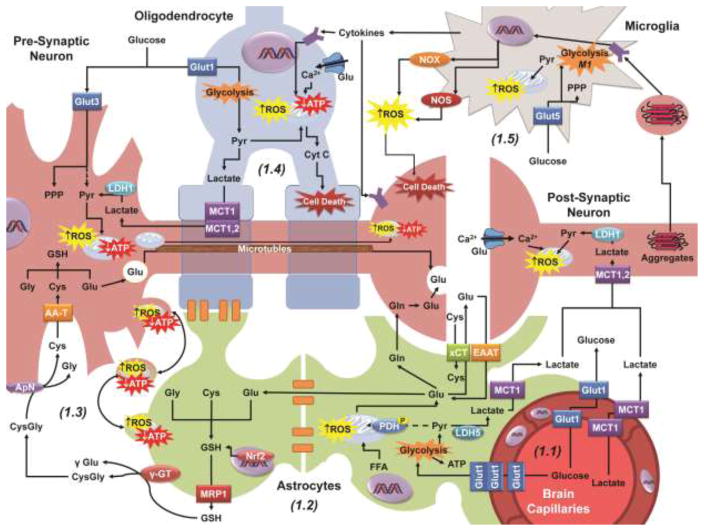
Figure 1.
Neuronal metabolism, redox homeostasis and signaling are supported by neighboring glial cells. 1.1: Glucose and lactate enter the brain through Glut1 (glucose transporter 1) and MCT1 (monocarboxylate transporter 1) transporters in the vascular epithelium. Glucose (Glut3) and lactate (MCT1 or 2) are uptaken from the extracellular space by neuronal calls to fuel the TCA cycle for the generation of ATP and biosynthesis of essential molecules. 1.2: As a component of the blood brain barrier (BBB), astrocytes uptake glucose from the capillary epithelium via Glut1 as well, converting the majority of pyruvate (Pyr) generated into lactate which is exported by MCT1. Astrocytes also uptake the neurotransmitter glutamate (Glu) from the synaptic cleft via EAAT (excitatory amino acid transporters) to be (a) converted into glutamine (Gln), (b) exchanged for extracellular cystine (Cys) by xCT, (c) feed into the TCA cycle, or (d) for GSH synthesis. Astrocytes form extended networks with other glia (oligodendrocytes and astrocytes) via gap junctions, sharing nutrients and molecular components with cells more distal to the capillaries. 1.3: Astrocytes contribute to the redox state of neuronal cells by exporting GSH via MRP1 which is broken down by γGT and ApN into its amino acid components to be uptaken and reassembled as GSH in neuronal cells. Dysfunctional or damaged mitochondrial, likely capable of generating ROS, are transferred from neurons to astrocytes to be degraded by mitophagy. 1.4: Oligodendrocytes wrap neuronal projections (myelin sheaths) improving signal conduction and like astrocytes, have been proposed to shuttle lactate to the neurons. 1.5: Microglia are activated by a variety of factors, including cytokines, oxidized proteins, and protein aggregates. Activated microglia migrate to the site of damage and can induce neuronal or oligodendrocyte cell death through the release of cytokines, and the generation of ROS via NADPH oxidases (NOX) and nitric oxide synthases (NOS). AA-T, amino acid transporters; LDH1 or 5, lactate dehydrogenase isoform 1 or 5.
Astrocytes are small cells with processes that are radially arranged, and have considerable molecular, structural, and functional diversity at the regional level. Astrocyte extensions cover the external surface of brain capillaries (perivascular feet), the synaptic cleft between the pre-synaptic and the post-synaptic terminals, and the bare segments of axons at the Ranvier nodes (Figure 1.2). Astrocytes also form highly organized domains interconnected via gap junctions with other astrocytes and oligodendrocytes (Figure 1.2). Additionally, astrocytes regulate neurotransmitter levels in the synaptic cleft, provide neurons with energetic and antioxidant precursors (Figure 1.2), play an important role in neuro/synaptogenesis and tissue repair, and also regulate blood flow and inflammatory processes by the release of signaling mediators (Sofroniew and Vinters 2010).
Microglial cells are resident macrophages distributed throughout the central nervous system (CNS) (Byrne and Roberts 2009). As innate immune cells, microglia are activated by infection, tissue injury, or xenobiotics. Upon activation, microglia cells retract their cytoplasmic extensions and migrate to the site of injury, where they proliferate and become antigen presenting cells. Microglia phagocytose degenerating cells and act as sources of immunoregulatory and neuromodulatory factors such as cytokines, chemokines and neurotrophic factors. Microglia can be activated by cell-surface receptors for endotoxins, cytokines, chemokines, misfolded proteins, serum factors and ATP (Figure 1.5). While mild activation is a key adaptive immune response, continuous activation or overactivation of microglia is thought to contribute to neurodegeneration (Finsen and Owens 2011; Hanisch 2013; Hanisch and Kettenmann 2007).
3. Mitochondrial dysfunction in glial cells and its effect on neuronal function/survival
3.1. Cell death
Apoptosis is a ubiquitous homeostatic mechanism critical for the turnover of cells throughout the lifespan of multi-cellular organisms. However, dysregulation of apoptosis occurs as either a cause or consequence of distinct pathologies that include neurodegenerative disorders (Fadeel and Orrenius 2005). The signaling pathways that regulate the progression of apoptosis have been extensively characterized and divided in two pathways. Induction of apoptosis via the extrinsic pathway is triggered by the activation of the death receptors leading to the activation of initiator caspases. (Lavrik et al. 2005).
The intrinsic mitochondrial pathway of apoptosis is activated by a wide variety of stimuli that regulate the expression and function of the Bcl-2 (B-cell lymphoma 2) family of (anti or pro) apoptotic proteins. The BH3-only Bcl-2 family members (Bad, Bid, Bim and NOXA) regulate the anti-apoptotic Bcl-2 proteins (Bcl-2, Bcl-xl and Mcl-1) to promote apoptosis. The pro-apoptotic effector proteins Bax and Bak are sufficient and necessary for inducing the permeabilization of the outer mitochondrial membrane and the release of Cyt C (Figure 2.6). However, the activation of BH3-only proteins derepresses the direct inhibition of Bax and Bak by anti-apoptotic Bcl-2 proteins. Released Cyt C leads to the recruitment of Apaf1 and caspase 9 into a platform (apoptosome) that activates caspase 9 and subsequently, executioner caspases 3, 6 and 7. The extrinsic/death receptor pathway can crosstalk to the intrinsic/mitochondrial pathway of apoptosis by an amplification loop induced by caspase dependent cleavage/activation of Bid (Green and Llambi 2015).
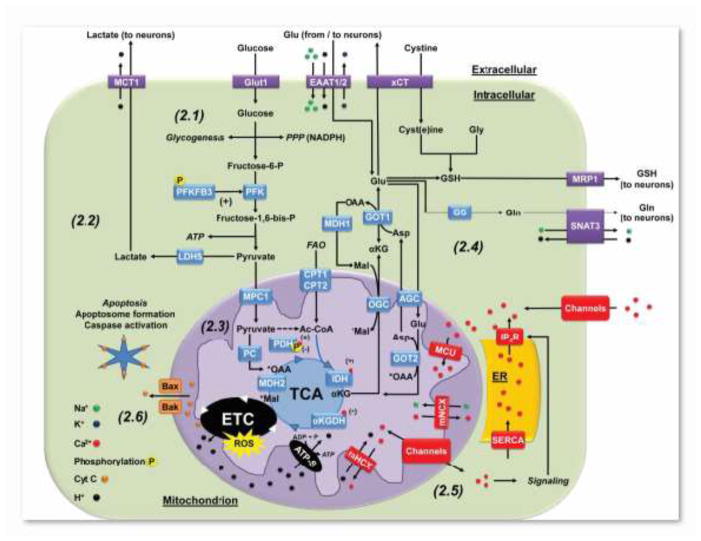
Figure 2.
Mitochondrial metabolism and signaling in astrocytes. 2.1: Glucose in astrocytes is used for glycogenesis, NADPH production through the PPP, or glycolysis. Astrocytes are highly glycolytic due to the expression of high levels of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3 (PFKFB3), whose byproduct fructose-2,6-bisphosphate (F2,6P2), is a positive effector of the glycolytic enzyme 6-phosphofructo-1-kinase (PFK1). In addition, the activity of PFKFB3 is increased by phosphorylation by 5′-AMP-activated protein kinase (AMPK) (Bolanos 2016). 2.2: Astrocytes primarily derive ATP from glycolysis rather than oxidative phosphorylation, where pyruvate is converted to lactate by LDH5 and exported to the extracellular space to be consumed by neurons. 2.3: Astrocytes carboxylate pyruvate to oxaloacetate (OAA) via pyruvate carboxylase (PC) to regenerate TCA cycle intermediates. Phosphorylation of pyruvate dehydrogenase (PDH) restricts the conversion of pyruvate to acetyl-CoA (Ac-CoA). Thus, FAO has been proposed to be the primary contributor of Ac-CoA to the TCA cycle. 2.4: Alpha ketoglutarate (αKG) generated from the TCA cycle can be transported to the cytosol and converted to Glu by glutamic-oxaloacetic transaminase 1 or aspartate (Asp) aminotransferase (GOT1) as part of the malate-Asp shuttle. Glu has three central metabolic pathways in astrocytes. 1) Glu can be converted to Gln by GS and exported to neurons by the sodium-coupled neutral amino acid transporter 3 (SNAT3). 2) Glu is exchanged via xCT for extracellular cystine that is reduced to Cys. Extracellular Glu can be uptaken back by astrocytes by EAAT1/2. Finally, 3) Glu, Gly and Cys are precursors of GSH, which is also exported to neurons via MRP1. 2.5: The ER acts as a store for intracellular calcium, where the sarco/endoplasmic reticulum calcium ion ATPase (SERCA) pumps cytosolic Ca2+ into the ER. Ca2+ signaling is tightly regulated by the activation of IP3R that release Ca2+ from ER stores, as well as by the activation of plasma membrane Ca2+ channels. Mitochondria can buffer Ca2+ by its transport across the inner mitochondrial membrane to the matrix viaMCU), while the export is performed by mNCX and mHCX. Mitochondria can also transport Ca2+ in and out of the mitochondria via the activation of distinct Ca2+ permeable channels. In the matrix, Ca2+ stimulates TCA carbon flux by binding to PDH, IDH, and αKGDH, increasing the activity of the ETC and ATP production. 2.6: Cyt C is held close to the inner mitochondrial membrane by cardiolipin (not shown), acting as a component of ETC. Dissociation of Cyt C from cardiolipin, through oxidative or enzymatic means, coupled with permeabilization of the outer mitochondrial membrane by the formation of Bax/Bak oligomeric channels, allows Cyt C to escape into the cytosol. Cytosolic Cyt C associates with apoptotic protease-activating factor 1 (APAF1), forming the apoptosome and leading to the activation of caspases to initiate apoptosis. AGC, aspartate-glutamate carrier; CPT1 or 2, carnitine palmitoyltransferase isoform 1 or 2; MDH1 or 2, malate dehydrogenase isoform 1 or 2; MPC1, mitochondrial pyruvate carrier 1; OGC, 2-oxoglutarate (α-ketoglutarate) carrier.
While a number of studies have reported the induction of apoptosis in astrocytes and microglia under different experimental conditions, very little evidence exists about the loss or degeneration of these glial cells with respect to human disorders. Conversely, oligodendrocytes are known to degenerate in demyelinating disorders such as multiple sclerosis, and to be affected directly or indirectly by the majority of known disorders in the CNS including ischemia, trauma and neurodegeneration. Glutamate/Ca2+ excitotoxicity, inflammation (cytokines) and oxidative stress are common triggers for oligodendrocyte injury in these pathological situations (Figure 1.4). Oligodendrocytes express ionotropic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainite receptors whose activation induces Ca2+ overflow and apoptotic cell death via the intrinsic mitochondrial pathway via activation of Bax and caspase 3 (Figure 1.4) (Ruiz et al. 2010; Sanchez-Gomez et al. 2011). The high lipid and iron content of oligodendrocytes also makes them susceptible to oxidative damage induced by cytokines (Zhang et al. 2005).
3.2. Bioenergetics and metabolism
Neurons are dependent on high rates of OXPHOS to meet their energy requirements, to maintain and restore ionic gradients, and for the uptake and recycling of neurotransmitters. In contrast, astrocytes are highly glycolytic (Figure 2.1), but a large portion of glucose is converted to lactate and released to the extracellular space. Interestingly, glucose consumption in astrocytes exceeds their energy expenditure, which is explained by the astrocytes-neuron lactate shuttle hypothesis where lactate is shuttled from astrocytes (and oligodendrocytes) as a fuel for OXPHOS in neurons (Figure 1.1 and 2.2) (Belanger et al. 2011; Funfschilling et al. 2012a; Lee et al. 2012; Morrison et al. 2013). What limits OXPHOS in astrocytes? Recent studies have demonstrated that the activity of pyruvate dehydrogenase (PDH), which provides a route of entry for pyruvate into the tricarboxylic acid (TCA or Krebs) cycle, is reduced by its phosphorylation in astrocytes (Figure 1.1 and 2.3) (Halim et al. 2010). Interestingly, astrocytes have the same oxidative capacity as neurons, but are resilient to mitochondrial dysfunction (Di Monte et al. 1992).
Other carbon sources can fuel OXPHOS in astrocytes. Glutamate can be metabolized through the TCA cycle, but astrocytes primarily metabolize it to glutamine by the activity of glutamine synthase (GS) (Figure 2.4). However, when the extracellular concentration of glutamate increases to levels observed during synaptic transmission, the proportion of glutamate metabolized by the TCA cycle increases as well, while its conversion to glutamine decreases concomitantly (McKenna 2013; Nissen et al. 2015; Schousboe et al. 2014). Importantly, glutamate also exerts a stimulatory effect on glycolysis as well (Loaiza et al. 2003; Pellerin and Magistretti 1994).
Acetate is also used as a carbon source by astrocytes, but its physiological significance has not been established (Belanger et al. 2011; Jiang et al. 2013). Astrocytes can oxidize free fatty acids (FFA) and ketone bodies, but neurons and oligodendrocytes can only use ketone bodies as these cell types would be highly vulnerable to ROS formation generated by FFA oxidation due to their high lipid content (Iglesias et al. 2016; Schonfeld and Reiser 2013). Twenty percent of total energy expenditure in the brain is linked to FFA oxidation (FAO), which occurs primarily in astrocytes (Ebert et al. 2003). As mentioned above, astrocytes exhibit high rates of OXPHOS (Lovatt et al. 2007), but a larger proportion of astrocyte PDH is phosphorylated compared to neuronal PDH, inhibiting the conversion of pyruvate to acetyl-CoA (Halim et al. 2010). Thus, FAO might actually be a major source for acetyl-CoA into the TCA cycle (Panov et al. 2014) (Figure 2.3).
Oligodendrocytes have similar rates of glycolysis compared to astrocytes, but release less lactate since a larger proportion of pyruvate derived from glucose is metabolized via PDH into the TCA cycle. Similar to astrocytes, oligodendrocytes can carboxylate pyruvate to oxaloacetate via pyruvate carboxylase (PC) to replenish TCA intermediates (anaplerosis) or recycle pyruvate (Figure 2.3) (Amaral et al. 2016). In astrocytes however, pyruvate carboxylation also serves to compensate for the loss of TCA intermediates due to the generation of glutamate and subsequently glutamine that is then shuttled to neurons (glutamate-glutamine cycle) (Figure 1.2 and 2.4) (Schousboe et al. 2014). Lactate metabolism in oligodendrocytes has been demonstrated to participate in oligodendrocyte differentiation and myelination.(Rinholm et al. 2011). Importantly, mitochondrial respiration/metabolism seems to be primarily involved in oligodendrocyte differentiation, while glycolysis appears to be sufficient to maintain post-myelinated (differentiated) oligodendrocytes (Funfschilling et al. 2012b). Accordingly, demyelination disorders linked to mitochondrial dysfunction seem to be primarily linked to increased oxidative damage and changes in FFA metabolism but not energy failure (Lin et al. 2012; Swalwell et al. 2011; Viader et al. 2013).
3.3. Calcium
Calcium (Ca2+) signaling is tightly coupled to its homeostasis. Ca2+ gradients across membranes and cellular compartments are established by the activity of Ca2+ pumps/transporters. The controlled activation of Ca2+ fluxes allows its release and the subsequent activation of a diverse array of signal transducers including kinases, enzymes and ion channels. Mitochondria are now recognized as important Ca2+ reservoirs or sinks. The regulation of Ca2+ signaling is not a simple process of its release and subsequent compartmentalization. Instead, it involves a highly localized release and controlled diffusion of Ca2+ across intracellular compartments and in most cases, the coordinated action of more than one Ca2+ reservoir and release/uptake system. The spatiotemporal complexity of this process is reflected by the existence of patterns of Ca2+ waves or sparks that are decoded by transducers selectively localized in different cellular compartments. Sequestration of Ca2+ within the mitochondrial matrix is partially driven by the negative environment generated by the extrusion of protons (H+) across the inner mitochondrial membrane by the ETC (Figure 2.3). Translocation of Ca2+ into the matrix is mediated by the mitochondrial Ca2+ uniporter (MCU) in an energy-independent manner (Figure 2.5). Ca2+ release from the mitochondria is mediated by Ca2+ exchangers (the sodium [Na+)]/Ca2+ [mNCX] and mitochondrial proton [H+]/Ca2+ exchangers [mHCX]), or the opening of the mitochondrial permeability transition pore under pathological conditions (Figure 2.5). Importantly, mitochondria act as important buffers for Ca2+ release/influx from the endoplasmic reticulum (ER) and the plasma membrane that contribute to the regulation of Ca2+ signaling (Figure 2.5) (Rizzuto et al. 2012).
Very little is known about the impact of mitochondrial Ca2+ homeostasis on glial signaling. However, as in other cell types, functional mitochondria in astrocytes and oligodendrocytes regulates Ca2+ waves generated by the activation of inositol 1,4,5-triphosphate (IP3) receptors (IP3R) and the release of Ca2+ from the ER (Boitier et al. 1999; Simpson and Russell 1996; Smith et al. 2005). Mitochondrial Ca2+ has also been shown to regulate vesicular glutamate release from astrocytes that modulates synaptic communication and excitability (Reyes and Parpura 2008). Ca2+ accumulation in mitochondria also modulates oxidative phosphorylation and energy production. PDH activity is regulated by a Ca2+-dependent dephosphorylation, while Ca2+ binding also regulates α-ketoglutarate (αKGDH)- and isocitrate (IDH)-dehydrogenase activity, which increases NADH levels, electron flow and ATP synthesis (Figure 2.5) (Rizzuto et al. 2012). Accordingly, Ca2+ release from the ER stimulates mitochondrial-dependent energy production in astrocytes (Wu et al. 2007). Not only do mitochondria regulate Ca2+ accumulation and dynamics, but also its release. A recent report demonstrated that Ca2+ release via mNCX is coupled to store-operated Ca2+ entry (triggered by Ca2+ depletion from ER stores) and regulates astrocytes proliferation and excitotoxic glutamate release (Parnis et al. 2013). In microglia, mitochondrial Ca2+ influx via the mitochondrial transient receptor potential vanilloid 1 channel (TRPV1) depolarizes mitochondria resulting in mtROS production, mitogen activated protein kinase (MAPK) activation, and enhanced migration (Miyake et al. 2015).
3.4. Inflammation
Inflammation is a key contributor to most neurological disorders. In a steady “basal” state, microglia performs continuous surveillance of the CNS, secrete neurotrophic factors, such as insulin-like growth factor 1 (IGF1), brain-derived neurotrophic factor (BDNF), transforming growth factor-β (TGFβ) and nerve growth factor (NGF), and promote synapse pruning for refinement of neuronal circuits during development. Classical activation of microglia (M1) conveys the production of ROS and nitrogen species (RNS) and the release of pro-inflammatory cytokines (tumor necrosis factor [TNF] and interleukin-1β [IL-1β]) to promote brain tissue repair upon injury (removal of cell debris and restoring of tissue integrity) and, upon prolonged activation, neuronal dysfunction as well. Disease-associated factors such as xenobiotics, protein aggregates, and damage (DAMPs) or pathogen-associated molecular patterns (PAMPS) can activate microglia through a variety of surface receptors. These receptors include Toll-like receptors (for lipopolysacharide [LPS], oxidized low-density lipoprotein [LDL] and molecules released by damaged or dead cells including high-mobility group box 1 [HMGB1] and nucleotides), nucleotide-binding oligomerization domain (Nod)-like receptors (for amyloid proteins), advanced glycation end-products receptors or RAGE (that are also activated by HMGB1), and purinergic receptors (for purines and pyrimidines including nucleoside triphosphates, e.g. ATP) (Hu et al. 2014). Pro-inflammatory cytokines released from microglia also “activate” astrocytes, which might produce TNF to potentiate microglia activation as well. As such, co-cultures of microglia and astrocytes produce more neurotoxic factors than either activated cell type alone (Saijo and Glass 2011). Whether astrocytes can be activated in the absence of microglia is still unclear since most studies using primary cultures of astrocytes also contain at least 5% of microglia that significantly contribute to astrocyte activation (Facci et al. 2014; Marinelli et al. 2015). The alternative (M2-like) phenotype of microglia is observed to be induced by transforming growth factor-β (TGFβ), IL-4, IL-6 and IL-10 secreted from glioma cells (Saijo and Glass 2011).
Mitochondrial dysfunction triggers inflammatory responses (West). During inflammation, changes in mitochondrial metabolism contribute to the activation of microglia. The M1 phenotype of microglia was recently reported to be paralleled by a metabolic switch from mitochondrial OXPHOS to glycolysis that enhances carbon flux to the PPP (Figure 1.5) (Gimeno-Bayon et al. 2014; Orihuela et al. 2016; Voloboueva et al. 2013). Interestingly, inhibition of complex I activity activates microglial cells (Shaikh and Nicholson 2009; Ye et al. 2016; Yuan et al. 2013), while impairment of mitochondrial fission reduces the production of pro-inflammatory signals (Park et al. 2013). Induction of the M2-like phenotype results in no observable changes in mitochondrial oxygen consumption or lactate production (Orihuela et al. 2016). However, mitochondrial toxins such as 3-nitropropionic acid and rotenone impair the transition to the M2 phenotype induced by IL-4 (Ferger et al. 2010). These results suggest that mitochondrial dysfunction in microglia can exacerbate the pro-inflammatory M1 phenotype and result in the release of neurotoxic pro-inflammatory cytokines, and enhanced ROS/RNS formation (Tang and Le 2016).
3.5. Redox homeostasis and detoxification of xenobiotics
In general, neurons have limited defense mechanisms against ROS compared to astrocytes. This enhanced resistance to oxidative damage in astrocytes is observed despite the fact that astrocytes have a deficient mitochondrial respiration and increased ROS formation when compared to neurons (Lopez-Fabuel et al. 2016). A comparative study also demonstrated that astrocytes are more resistant to oxidative damage than microglia or oligodendrocytes (Hollensworth et al. 2000). Astrocytes contain higher levels of endogenous antioxidants and antioxidant systems that include NADPH and G6PD (glucose-6-phosphate dehydrogenase). Astrocytes’ resistance to oxidative damage is explained by the activation of the antioxidant response via the nuclear factor erythroid-2-related factor 2 (Nrf2) transcription factor (Garcia-Nogales et al. 2003; Shih et al. 2003). Both neurons and astrocytes can synthesize GSH, but neurons depend on the supply of GSH precursors from astrocytes (Figure 1.3). GSH is released from astrocytes via the ATP-binding cassette transporters subfamily C member 1 transporter (ABCC1, or multidrug-resistance-associated protein 1 [MRP1]) (Hirrlinger and Dringen 2005). Extracellular GSH is then degraded by the γ-glutamyl transpeptidase (γGT) to produce l-cysteine-l-glycine (CysGly), which is cleaved further by the neuronal aminopeptidase N (ApN) into the amino acids glycine and cysteine that are taken up by neurons for de novo GSH synthesis (Figure 1.3) (Aoyama et al. 2008; Belanger et al. 2011). The glutamate-glutamine cycle might also be involved in the regulation of the neuronal redox environment by astrocytes since GSH synthesis also requires glutamate. The importance of astrocytes for neuronal redox homeostasis was evidenced by a recent study demonstrating that conditional depletion of astrocytes promotes neuronal injury by oxidative stress (Schreiner et al. 2015). Astrocytes are also the first line of defense against xenobiotics entering into the brain since their extensions cover the external surface of capillaries as part of the blood brain barrier. Detoxification of electrophiles is dependent the formation of irreversible adducts with GSH that in many cases depends on the activity of glutathione-S-transferases (GST) and their efflux through MRPs (Dringen et al. 2015).
But what is the role of mitochondria in redox homeostasis in astrocytes and neurons? The loss of GSH by its export to neurons or due to the detoxification of electrophiles is expected to prompt astrocytes to replenish GSH precursors. Interestingly, GSH depletion upregulates mitochondrial activity in astrocytes (Vasquez et al. 2001) and we have recently observed that mitochondrial OXPHOS is essential for the detoxification of electrophiles via the GSH/MRP system (manuscript in preparation), but the exact mechanisms that regulate this phenomenon are still unclear.
4. Conclusions and Perspectives
Mitochondrial dysfunction has been widely recognized as central to the pathogenesis of neurological disorders. However, the majority of current research efforts have been focused on understanding the causes and consequences of mitochondrial dysfunction in neuronal cells that rely on OXPHOS to generate energy and are also more sensitive to mitochondrial ROS formation. Less is known about the functional role of mitochondria in glial cells and its implications for neuronal survival and brain function. In this work, we have provided an overview of the role of mitochondria in glial cell function that includes metabolism, redox homeostasis, Ca2+ signaling, inflammation and cell death. The evidence so far clearly demonstrates the importance of mitochondrial health in glial cells and its relevance to neuronal function. Nevertheless, this review also highlights our limited understanding of mitochondria function in glial cells and the need for further investigations in this area that is expanding. For example, recent studies have demonstrated that damaged mitochondria can be transferred from neuronal axons for their turnover in astrocytes (Davis et al. 2014), and conversely, astrocytes have been shown to transfer mitochondria to promote neuronal survival (Hayakawa et al. 2016) (Figure 1.3). Many questions remain to be answered regarding the role of mitochondrial in neurological disorders, but it is time for us to think about mitochondrial health and dysfunction in a more inclusive context outside neuronal cells.
Acknowledgments
This work was supported by the National Institutes of Health Grants P20RR17675 Centers of Biomedical Research Excellence (COBRE), and the Office of Research of the University of Nebraska-Lincoln.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amaral AI, Hadera MG, Tavares JM, Kotter MR, Sonnewald U. Characterization of glucose-related metabolic pathways in differentiated rat oligodendrocyte lineage cells. Glia. 2016;64:21–34. doi: 10.1002/glia.22900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama K, Watabe M, Nakaki T. Regulation of neuronal glutathione synthesis. J Pharmacol Sci. 2008;108:227–238. doi: 10.1254/jphs.08r01cr. [DOI] [PubMed] [Google Scholar]
- Ballinger S, Baumann N, Pham-Dinh D Mitochondrial Toxicity of Tobacco Smoke Air Pollution. This Special Issue. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81:871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- Belanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14:724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Boitier E, Rea R, Duchen MR. Mitochondria exert a negative feedback on the propagation of intracellular Ca2+ waves in rat cortical astrocytes. J Cell Biol. 1999;145:795–808. doi: 10.1083/jcb.145.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolanos JP. Bioenergetics and redox adaptations of astrocytes to neuronal activity. J Neurochem. 2016;139(Suppl 2):115–125. doi: 10.1111/jnc.13486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini M. Oxidative signaling in mitochondrial homeostasis. This Special Issue. [Google Scholar]
- Byrne JH, Roberts JL. From molecules to networks: an introduction to cellular and molecular neuroscience. Academic Press/Elsevier; Amsterdam; Boston: 2009. [Google Scholar]
- Chan SS. Inherited mitochondrial genomic instability and chemical exposures. doi: 10.1016/j.tox.2017.07.014. This Special Issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandel NS. Evolution of Mitochondria as Signaling Organelles. Cell Metab. 2015;22:204–206. doi: 10.1016/j.cmet.2015.05.013. [DOI] [PubMed] [Google Scholar]
- Chaturvedi RK, Flint Beal M. Mitochondrial diseases of the brain. Free Radic Biol Med. 2013;63:1–29. doi: 10.1016/j.freeradbiomed.2013.03.018. [DOI] [PubMed] [Google Scholar]
- Davis CH, Kim KY, Bushong EA, Mills EA, Boassa D, Shih T, Kinebuchi M, Phan S, Zhou Y, Bihlmeyer NA, Nguyen JV, Jin Y, Ellisman MH, Marsh-Armstrong N. Transcellular degradation of axonal mitochondria. Proc Natl Acad Sci U S A. 2014;111:9633–9638. doi: 10.1073/pnas.1404651111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Monte DA, Wu EY, Delanney LE, Irwin I, Langston JW. Toxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in primary cultures of mouse astrocytes. J Pharmacol Exp Ther. 1992;261:44–49. [PubMed] [Google Scholar]
- Dringen R, Brandmann M, Hohnholt MC, Blumrich EM. Glutathione-Dependent Detoxification Processes in Astrocytes. Neurochem Res. 2015;40:2570–2582. doi: 10.1007/s11064-014-1481-1. [DOI] [PubMed] [Google Scholar]
- Ebert D, Haller RG, Walton ME. Energy contribution of octanoate to intact rat brain metabolism measured by 13C nuclear magnetic resonance spectroscopy. J Neurosci. 2003;23:5928–5935. doi: 10.1523/JNEUROSCI.23-13-05928.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facci L, Barbierato M, Marinelli C, Argentini C, Skaper SD, Giusti P. Toll-like receptors 2, -3 and -4 prime microglia but not astrocytes across central nervous system regions for ATP-dependent interleukin-1beta release. Sci Rep. 2014;4:6824. doi: 10.1038/srep06824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadeel B, Orrenius S. Apoptosis: a basic biological phenomenon with wide-ranging implications in human disease. J Intern Med. 2005;258:479–517. doi: 10.1111/j.1365-2796.2005.01570.x. [DOI] [PubMed] [Google Scholar]
- Ferger AI, Campanelli L, Reimer V, Muth KN, Merdian I, Ludolph AC, Witting A. Effects of mitochondrial dysfunction on the immunological properties of microglia. J Neuroinflammation. 2010;7:45. doi: 10.1186/1742-2094-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Fernandez S, Almeida A, Bolanos JP. Antioxidant and bioenergetic coupling between neurons and astrocytes. Biochem J. 2012;443:3–11. doi: 10.1042/BJ20111943. [DOI] [PubMed] [Google Scholar]
- Finsen B, Owens T. Innate immune responses in central nervous system inflammation. FEBS Lett. 2011;585:3806–3812. doi: 10.1016/j.febslet.2011.05.030. [DOI] [PubMed] [Google Scholar]
- Funfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Mobius W, Diaz F, Meijer D, Suter U, Hamprecht B, Sereda MW, Moraes CT, Frahm J, Goebbels S, Nave KA. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012a;485:517–521. doi: 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Mobius W, Diaz F, Meijer D, Suter U, Hamprecht B, Sereda MW, Moraes CT, Frahm J, Goebbels S, Nave KA. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012b;485:517–521. doi: 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Nogales P, Almeida A, Bolanos JP. Peroxynitrite protects neurons against nitric oxide-mediated apoptosis. A key role for glucose-6-phosphate dehydrogenase activity in neuroprotection. J Biol Chem. 2003;278:864–874. doi: 10.1074/jbc.M206835200. [DOI] [PubMed] [Google Scholar]
- Gimeno-Bayon J, Lopez-Lopez A, Rodriguez MJ, Mahy N. Glucose pathways adaptation supports acquisition of activated microglia phenotype. J Neurosci Res. 2014;92:723–731. doi: 10.1002/jnr.23356. [DOI] [PubMed] [Google Scholar]
- Green DR, Llambi F. Cell Death Signaling. Cold Spring Harb Perspect Biol. 2015:7. doi: 10.1101/cshperspect.a006080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halim ND, McFate T, Mohyeldin A, Okagaki P, Korotchkina LG, Patel MS, Jeoung NH, Harris RA, Schell MJ, Verma A. Phosphorylation status of pyruvate dehydrogenase distinguishes metabolic phenotypes of cultured rat brain astrocytes and neurons. Glia. 2010;58:1168–1176. doi: 10.1002/glia.20996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch UK. Proteins in microglial activation--inputs and outputs by subsets. Curr Protein Pept Sci. 2013;14:3–15. doi: 10.2174/1389203711314010003. [DOI] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, Ji X, Lo EH. Transfer of mitochondria from astrocytes to neurons after stroke. Nature. 2016;535:551–555. doi: 10.1038/nature18928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero-Mendez A, Almeida A, Fernandez E, Maestre C, Moncada S, Bolanos JP. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nat Cell Biol. 2009;11:747–752. doi: 10.1038/ncb1881. [DOI] [PubMed] [Google Scholar]
- Hirrlinger J, Dringen R. Multidrug resistance protein 1-mediated export of glutathione and glutathione disulfide from brain astrocytes. Methods Enzymol. 2005;400:395–409. doi: 10.1016/S0076-6879(05)00023-6. [DOI] [PubMed] [Google Scholar]
- Hollensworth SB, Shen C, Sim JE, Spitz DR, Wilson GL, LeDoux SP. Glial cell type-specific responses to menadione-induced oxidative stress. Free Radic Biol Med. 2000;28:1161–1174. doi: 10.1016/s0891-5849(00)00214-8. [DOI] [PubMed] [Google Scholar]
- Hu X, Liou AK, Leak RK, Xu M, An C, Suenaga J, Shi Y, Gao Y, Zheng P, Chen J. Neurobiology of microglial action in CNS injuries: receptor-mediated signaling mechanisms and functional roles. Prog Neurobiol. 2014;119–120:60–84. doi: 10.1016/j.pneurobio.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias J, Morales L, Barreto GE. Metabolic and Inflammatory Adaptation of Reactive Astrocytes: Role of PPARs. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-9833-2. [DOI] [PubMed] [Google Scholar]
- Jiang L, Gulanski BI, De Feyter HM, Weinzimer SA, Pittman B, Guidone E, Koretski J, Harman S, Petrakis IL, Krystal JH, Mason GF. Increased brain uptake and oxidation of acetate in heavy drinkers. J Clin Invest. 2013;123:1605–1614. doi: 10.1172/JCI65153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubik LL, Philbert MA. The role of astrocyte mitochondria in differential regional susceptibility to environmental neurotoxicants: tools for understanding neurodegeneration. Toxicol Sci. 2015;144:7–16. doi: 10.1093/toxsci/kfu254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavrik I, Golks A, Krammer PH. Death receptor signaling. J Cell Sci. 2005;118:265–267. doi: 10.1242/jcs.01610. [DOI] [PubMed] [Google Scholar]
- Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A, Jin L, Zhang PW, Pellerin L, Magistretti PJ, Rothstein JD. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487:443–448. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CS, Sharpley MS, Fan W, Waymire KG, Sadun AA, Carelli V, Ross-Cisneros FN, Baciu P, Sung E, McManus MJ, Pan BX, Gil DW, Macgregor GR, Wallace DC. Mouse mtDNA mutant model of Leber hereditary optic neuropathy. Proc Natl Acad Sci U S A. 2012;109:20065–20070. doi: 10.1073/pnas.1217113109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loaiza A, Porras OH, Barros LF. Glutamate triggers rapid glucose transport stimulation in astrocytes as evidenced by real-time confocal microscopy. J Neurosci. 2003;23:7337–7342. doi: 10.1523/JNEUROSCI.23-19-07337.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Fabuel I, Le Douce J, Logan A, James AM, Bonvento G, Murphy MP, Almeida A, Bolanos JP. Complex I assembly into supercomplexes determines differential mitochondrial ROS production in neurons and astrocytes. Proc Natl Acad Sci U S A. 2016;113:13063–13068. doi: 10.1073/pnas.1613701113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovatt D, Sonnewald U, Waagepetersen HS, Schousboe A, He W, Lin JH, Han X, Takano T, Wang S, Sim FJ, Goldman SA, Nedergaard M. The transcriptome and metabolic gene signature of protoplasmic astrocytes in the adult murine cortex. J Neurosci. 2007;27:12255–12266. doi: 10.1523/JNEUROSCI.3404-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli C, Di Liddo R, Facci L, Bertalot T, Conconi MT, Zusso M, Skaper SD, Giusti P. Ligand engagement of Toll-like receptors regulates their expression in cortical microglia and astrocytes. J Neuroinflammation. 2015;12:244. doi: 10.1186/s12974-015-0458-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna MC. Glutamate pays its own way in astrocytes. Front Endocrinol (Lausanne) 2013;4:191. doi: 10.3389/fendo.2013.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JN, Leung MC, Rooney JP, Sendoel A, Hengartner MO, Kisby GE, Bess AS. Mitochondria as a target of environmental toxicants. Toxicol Sci. 2013;134:1–17. doi: 10.1093/toxsci/kft102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake T, Shirakawa H, Nakagawa T, Kaneko S. Activation of mitochondrial transient receptor potential vanilloid 1 channel contributes to microglial migration. Glia. 2015;63:1870–1882. doi: 10.1002/glia.22854. [DOI] [PubMed] [Google Scholar]
- Moran M, Moreno-Lastres D, Marin-Buera L, Arenas J, Martin MA, Ugalde C. Mitochondrial respiratory chain dysfunction: implications in neurodegeneration. Free Radic Biol Med. 2012;53:595–609. doi: 10.1016/j.freeradbiomed.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Morrison BM, Lee Y, Rothstein JD. Oligodendroglia metabolically support axons and maintain structural integrity. Trends in cell biology. 2013;23:10. doi: 10.1016/j.tcb.2013.07.007. doi:1016/j.tcb.2013.1007.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen JD, Pajecka K, Stridh MH, Skytt DM, Waagepetersen HS. Dysfunctional TCA-Cycle Metabolism in Glutamate Dehydrogenase Deficient Astrocytes. Glia. 2015;63:2313–2326. doi: 10.1002/glia.22895. [DOI] [PubMed] [Google Scholar]
- Orihuela R, McPherson CA, Harry GJ. Microglial M1/M2 polarization and metabolic states. Br J Pharmacol. 2016;173:649–665. doi: 10.1111/bph.13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panov A, Orynbayeva Z, Vavilin V, Lyakhovich V. Fatty acids in energy metabolism of the central nervous system. Biomed Res Int. 2014;2014:472459. doi: 10.1155/2014/472459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Choi H, Min JS, Park SJ, Kim JH, Park HJ, Kim B, Chae JI, Yim M, Lee DS. Mitochondrial dynamics modulate the expression of pro-inflammatory mediators in microglial cells. J Neurochem. 2013;127:221–232. doi: 10.1111/jnc.12361. [DOI] [PubMed] [Google Scholar]
- Parnis J, Montana V, Delgado-Martinez I, Matyash V, Parpura V, Kettenmann H, Sekler I, Nolte C. Mitochondrial exchanger NCLX plays a major role in the intracellular Ca2+ signaling, gliotransmission, and proliferation of astrocytes. J Neurosci. 2013;33:7206–7219. doi: 10.1523/JNEUROSCI.5721-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes RC, Parpura V. Mitochondria modulate Ca2+-dependent glutamate release from rat cortical astrocytes. J Neurosci. 2008;28:9682–9691. doi: 10.1523/JNEUROSCI.3484-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinholm JE, Hamilton NB, Kessaris N, Richardson WD, Bergersen LH, Attwell D. Regulation of oligodendrocyte development and myelination by glucose and lactate. J Neurosci. 2011;31:538–548. doi: 10.1523/JNEUROSCI.3516-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, De Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol. 2012;13:566–578. doi: 10.1038/nrm3412. [DOI] [PubMed] [Google Scholar]
- Ruiz A, Matute C, Alberdi E. Intracellular Ca2+ release through ryanodine receptors contributes to AMPA receptor-mediated mitochondrial dysfunction and ER stress in oligodendrocytes. Cell Death Dis. 2010;1:e54. doi: 10.1038/cddis.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo K, Glass CK. Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol. 2011;11:775–787. doi: 10.1038/nri3086. [DOI] [PubMed] [Google Scholar]
- Sanchez-Gomez MV, Alberdi E, Perez-Navarro E, Alberch J, Matute C. Bax and calpain mediate excitotoxic oligodendrocyte death induced by activation of both AMPA and kainate receptors. J Neurosci. 2011;31:2996–3006. doi: 10.1523/JNEUROSCI.5578-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonfeld P, Reiser G. Why does brain metabolism not favor burning of fatty acids to provide energy? Reflections on disadvantages of the use of free fatty acids as fuel for brain. J Cereb Blood Flow Metab. 2013;33:1493–1499. doi: 10.1038/jcbfm.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schousboe A, Scafidi S, Bak LK, Waagepetersen HS, McKenna MC. Glutamate metabolism in the brain focusing on astrocytes. Adv Neurobiol. 2014;11:13–30. doi: 10.1007/978-3-319-08894-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner B, Romanelli E, Liberski P, Ingold-Heppner B, Sobottka-Brillout B, Hartwig T, Chandrasekar V, Johannssen H, Zeilhofer HU, Aguzzi A, Heppner F, Kerschensteiner M, Becher B. Astrocyte Depletion Impairs Redox Homeostasis and Triggers Neuronal Loss in the Adult CNS. Cell Rep. 2015;12:1377–1384. doi: 10.1016/j.celrep.2015.07.051. [DOI] [PubMed] [Google Scholar]
- Shaikh SB, Nicholson LF. Effects of chronic low dose rotenone treatment on human microglial cells. Mol Neurodegener. 2009;4:55. doi: 10.1186/1750-1326-4-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih AY, Johnson DA, Wong G, Kraft AD, Jiang L, Erb H, Johnson JA, Murphy TH. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J Neurosci. 2003;23:3394–3406. doi: 10.1523/JNEUROSCI.23-08-03394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson PB, Russell JT. Mitochondria support inositol 1,4,5-trisphosphate-mediated Ca2+ waves in cultured oligodendrocytes. J Biol Chem. 1996;271:33493–33501. doi: 10.1074/jbc.271.52.33493. [DOI] [PubMed] [Google Scholar]
- Smith IF, Boyle JP, Kang P, Rome S, Pearson HA, Peers C. Hypoxic regulation of Ca2+ signaling in cultured rat astrocytes. Glia. 2005;49:153–157. doi: 10.1002/glia.20083. [DOI] [PubMed] [Google Scholar]
- Snell RS. Clinical neuroanatomy. Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia: 2010. [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swalwell H, Kirby DM, Blakely EL, Mitchell A, Salemi R, Sugiana C, Compton AG, Tucker EJ, Ke BX, Lamont PJ, Turnbull DM, McFarland R, Taylor RW, Thorburn DR. Respiratory chain complex I deficiency caused by mitochondrial DNA mutations. Eur J Hum Genet. 2011;19:769–775. doi: 10.1038/ejhg.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Le W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol Neurobiol. 2016;53:1181–1194. doi: 10.1007/s12035-014-9070-5. [DOI] [PubMed] [Google Scholar]
- Vasquez OL, Almeida A, Bolanos JP. Depletion of glutathione up-regulates mitochondrial complex I expression in glial cells. J Neurochem. 2001;76:1593–1596. doi: 10.1046/j.1471-4159.2001.00223.x. [DOI] [PubMed] [Google Scholar]
- Viader A, Sasaki Y, Kim S, Strickland A, Workman CS, Yang K, Gross RW, Milbrandt J. Aberrant Schwann cell lipid metabolism linked to mitochondrial deficits leads to axon degeneration and neuropathy. Neuron. 2013;77:886–898. doi: 10.1016/j.neuron.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voloboueva LA, Emery JF, Sun X, Giffard RG. Inflammatory response of microglial BV-2 cells includes a glycolytic shift and is modulated by mitochondrial glucose-regulated protein 75/mortalin. FEBS Lett. 2013;587:756–762. doi: 10.1016/j.febslet.2013.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bartheld CS, Bahney J, Herculano-Houzel S. The search for true numbers of neurons and glial cells in the human brain: A review of 150 years of cell counting. J Comp Neurol. 2016;524:3865–3895. doi: 10.1002/cne.24040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West P. Mitochondrial Dysfunction as a Trigger of Innate Immune Responses and Inflammation. doi: 10.1016/j.tox.2017.07.016. This Special Issue. [DOI] [PubMed] [Google Scholar]
- Wu J, Holstein JD, Upadhyay G, Lin DT, Conway S, Muller E, Lechleiter JD. Purinergic receptor-stimulated IP3-mediated Ca2+ release enhances neuroprotection by increasing astrocyte mitochondrial metabolism during aging. J Neurosci. 2007;27:6510–6520. doi: 10.1523/JNEUROSCI.1256-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Jiang Z, Chen X, Liu M, Li J, Liu N. Electron transport chain inhibitors induce microglia activation through enhancing mitochondrial reactive oxygen species production. Exp Cell Res. 2016;340:315–326. doi: 10.1016/j.yexcr.2015.10.026. [DOI] [PubMed] [Google Scholar]
- Yin F, Boveris A, Cadenas E. Mitochondrial energy metabolism and redox signaling in brain aging and neurodegeneration. Antioxid Redox Signal. 2014;20:353–371. doi: 10.1089/ars.2012.4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan YH, Sun JD, Wu MM, Hu JF, Peng SY, Chen NH. Rotenone could activate microglia through NFkappaB associated pathway. Neurochem Res. 2013;38:1553–1560. doi: 10.1007/s11064-013-1055-7. [DOI] [PubMed] [Google Scholar]
- Zhang X, Haaf M, Todorich B, Grosstephan E, Schieremberg H, Surguladze N, Connor JR. Cytokine toxicity to oligodendrocyte precursors is mediated by iron. Glia. 2005;52:199–208. doi: 10.1002/glia.20235. [DOI] [PubMed] [Google Scholar]