Abstract
Objectives
To describe the natural history of frailty transitions in a large cohort of community-dwelling older men and identify predictors associated with progression to or improvement from states of greater frailty.
Design
Prospective cohort study.
Setting
Six U.S. sites.
Participants
5,086 community-dwelling men aged 65 years or older.
Measurements
Frailty was measured at baseline and an average of 4.6 years later. Frailty was defined as ≥ 3 of the following: low lean mass, weakness, self-reported exhaustion, low activity level, and slow walking speed; prefrail as 1-2 components. Separate multivariable logistic regression models were analyzed for progression and improvement in frailty status.
Results
Of the 5,086 men, 8% were frail, 46% were prefrail, and 46% were robust at baseline. Between baseline and follow-up, 35% progressed in frailty status or died, 56% had no change in frailty status, and 15% of prefrail or frail participants improved. However, only 0.5% improved across two levels, from frail to robust. In multivariable models, factors associated with improvement in frailty status included greater leg power, being married, and good or excellent self-reported health, whereas presence of any instrumental activities of daily living (IADL) limitations, low albumin levels or high IL-6 levels, and presence of chronic obstructive pulmonary disease or diabetes mellitus were associated with a decreased likelihood of improvement in frailty status.
Conclusions
Improvement in frailty status was possible in this cohort of community-dwelling older men; however, complete remediation from frail to robust was rare. Several predictors were identified as possible targets for intervention including prevention and improved management of comorbid medical conditions, prevention of IADL disability, physical exercise, and nutritional and social support.
BACKGROUND
Frailty has been proposed as a geriatric syndrome due to multisystem dysregulation that results in decreased physiologic reserve conferring vulnerability to adverse outcomes.1 A validated phenotype of frailty has been shown to predict incident and worsening disability, hospitalization, falls, fractures, and mortality.2, 3 Few studies have examined the natural history and precipitants of frailty transitions over time. Gill and colleagues reported that progression in frailty status was more common than improvement in frailty status among 754 community-dwelling adults age 70 or older over a follow-up period of 4.5 years using the longitudinal Precipitating Events Project cohort.4 Espinoza and colleagues demonstrated comparable trends in frailty status transitions and identified diabetes mellitus (DM) with macrovascular complications, fewer years of education, and a longer follow-up interval as predictors of progression in frailty status.5 In both studies, a small but significant proportion of participants (9 – 14%) improved in frailty status. These results suggest that frailty is a dynamic process, and that prevention or remediation of frailty may be possible. However, a comprehensive evaluation of potential behavioral, clinical, socioeconomic, and physiologic predictors of transitions in frailty status has not previously been conducted in a large cohort of community-dwelling older adults.
Identifying modifiable factors may provide insights into mechanisms of frailty and facilitate development of interventions to delay or reverse its progress. Increased understanding of characteristics associated with improvement in frailty status and knowledge of early risk factors could allow for the identification of older adults most likely to benefit from targeted interventions. The objectives of this study were to 1) determine the patterns and probability of frailty progression and improvement over time, and 2) identify predictors of transitions in frailty status.
METHODS
Participants
Between March 2000 and April 2002, 5994 men who were age ≥ 65 years, able to walk independently, and did not report bilateral hip replacements were recruited to the Osteoporotic Fractures in Men Study (MrOS) study at six US clinical centers (Birmingham, AL; Minneapolis, MN; Palo Alto, CA; Monongahela Valley, near Pittsburgh, PA; Portland, OR and San Diego, CA). The MrOS study rationale, design, and recruitment have been published.6, 7 Between March 2005 and May 2006, an average (± SD) of 4.6 ± 0.4 years after visit 1 (V1), 5229 men participated in a second visit. For these analyses, we excluded 143 men who were missing frailty measurements at either visit and did not die, leaving 5086 men to examine the transition between V1 and visit 2 (V2) (Supplementary Figure S1). Written informed consent was obtained from each participant and the study was approved by the institutional review boards at each institution.
Frailty and Frailty Transitions
Frailty status at baseline has been previously defined using criteria similar to Fried and colleagues2:
Shrinking: lowest quintile of appendicular lean mass (ALM) (adjusted for height and total body fat);
Weakness: lowest quintile of grip strength stratified by body mass index (BMI) (quartiles);
Exhaustion: response of “a little or none” to the question “How much of the time during the past four weeks did you have a lot of energy?” from the Medical Outcomes Study12-item Short Form (SF-12);8
Slowness: lowest quintile of 6-meter walk speed stratified by standing height (median); and
Low physical activity: lowest quintile on the Physical Activity Scale for the Elderly (PASE) score.9
Men meeting none of the above criteria were considered to be robust; those meeting 1 or 2 criteria were considered to be prefrail, and those meeting ≥ 3 criteria were considered to be frail. To jointly analyze the outcomes of frailty status at V2 and mortality between V1 and V2, four levels of frailty status were considered at V2: robust, prefrail, frail, and death. Participants who 1) were not frail at V1 and prefrail or frail at V2, 2) were prefrail at V1 and frail at V2, or 3) died between visits were considered to have progressed in frailty status. Participants who were 1) prefrail at V1 and robust at V2, or 2) frail at V1 and prefrail or robust at V2 were considered to have improved in frailty status.
Measurements
All participants completed a standard self-administered questionnaire that included queries about race and ethnicity, education, marital status, subjective socioeconomic status (SES) compared to the community10, smoking, alcohol, self-reported disease, and self-rated health. The Modified Mini-Mental State Exam (Teng 3MS) was used to assess cognitive function. Instrumental activities of daily living (IADL) and activities of daily living (ADL) were measured using a self-administered questionnaire. Physiologic parameters included leg power, ability to perform chair stands, and fasting serum glucose, creatinine, and albumin. Inflammatory markers, including C-reactive protein (CRP), interleukin-10 (IL-10), and tumor necrosis factor (TNF), were evaluated in a subset of 950 participants. The questionnaires and a description of the measurements of physiologic parameters are further described in the Supplementary Methods S1.
Statistical Analysis
Based on knowledge of the pathogenesis of frailty, we hypothesized that the following variable categories would be associated with frailty transitions: comorbidities, sociodemographics, physical function, lifestyle, and blood markers of inflammation or organ dysfunction.5, 11–14 To assess systemic inflammation we calculated a composite inflammatory burden score, as has been performed previously with this dataset.15 Variables included within each category are listed in the Supplementary Methods S1. Participant characteristics were compared across transitions in frailty status using ANOVA for normally distributed continuous variables, Kruskal-Wallis for non-normal continuous variables, or chi-square tests for categorical variables. Progression outcomes included three transitions (robust to prefrail or frail or death, prefrail to frail or death, and frail to death) and improvement outcomes included two transitions (prefrail to robust and frail to prefrail or robust). All variables significant at the p < 0.05 level for each outcome in age- and site-adjusted models were added to multivariable models by category. We tested for multicollinearity within each category using the variance inflation factor with values greater than three considered positive for multicollinearity. Variables that remained significant in multivariable models at p < 0.05 were subsequently added to final models for each outcome. Inflammatory cytokine variables were entered into separate models given that fewer participants (n = 950) underwent additional testing. Age and site were forced into all models. A posthoc sensitivity analysis was performed using the same multivariable models excluding men who died. All analyses were conducted using SAS version 9.2 (SAS Institute, Inc., Cary, North Carolina).
RESULTS
At V1, men were on average 73.4 (± 5.8) years old with a mean BMI of 27.4 (± 3.8) kg/m2 (Table 1). About 83% were married, 55% were college educated, 44% reported high socioeconomic status compared to the community, and 59% reported past smoking, but only 3% reported currently smoking. Nearly 70% reported one or more medical conditions; the most commonly reported conditions were hypertension (42%), and cancer (29%). Most men (81%) had no IADL limitations. At V1, nearly 8% of the participants met the criteria for frailty, and another 46% met the criteria for prefrailty. Among frail participants, the most prevalent frailty criteria were weakness, slowness, and low activity, while exhaustion was the least prevalent (Supplementary Table S1). Frail men were more likely to report IADL limitations compared to prefrail and robust men (59%, 23% and 9% reporting any IADL limitations, respectively), were more likely to report more than one medical condition (85%, 72%, and 61%, respectively), and were more likely to demonstrate cognitive dysfunction (11%, 5%, and 3%, respectively) (Table 1).
Table 1.
V1 Characteristics of Study Participants
| Characteristics | Frailty Status | ||||
|---|---|---|---|---|---|
| All (N = 5086) | Robust (n = 2322) | Prefrail (n = 2342) | Frail (n = 422) | p | |
| Age (y), mean +/− SD | 73.4 +/− 5.8 | 71.7 +/− 4.9 | 74.3 +/− 5.8 | 78.3 +/− 6.6 | <.001 |
| Body mass index (kg/m2), mean +/− SD | 27.4 +/− 3.8 | 27.3 +/− 3.5 | 27.46 +/− 4.0 | 26.70 +/− 4.4 | 0.04 |
| Non-Hispanic white, n (%) | 4598 (90.4) | 2097 (90.3) | 2135 (91.2) | 366 (86.7) | 0.02 |
| College education, n (%) | 2777 (54.6) | 1335 (57.5) | 1247 (53.3) | 195 (46.2) | <.001 |
| Married, n (%) | 4215 (82.9) | 1998 (86.1) | 1902 (81.2) | 315 (74.6) | <.001 |
| SES status compared to community (top 3 rungs), n (%) | 1028 (44.4) | 949 (40.6) | 139 (33.3) | 2116 (41.8) | <.001 |
| Smoking status, n (%) | |||||
| Never | 1944 (38.2) | 921 (39.7) | 872 (37.2) | 151 (35.8) | 0.08 |
| Past | 2975 (58.5) | 1336 (57.6) | 1388 (59.3) | 251 (59.5) | |
| Current | 166 (3.3) | 64 (2.8) | 82 (3.5) | 20 (4.7) | |
| Hypertension, n (%) | 2115 (41.6) | 863 (37.2) | 1040 (44.4) | 212 (50.2) | <.001 |
| Cancer, n (%) | 1476 (29.0) | 621 (26.7) | 710 (30.3) | 145 (34.4) | <.001 |
| Congestive heart failure n (%) | 265 (5.2) | 77 (3.3) | 127 (5.4) | 61 (14.5) | <.001 |
| Chronic obstructive pulmonary disease, n (%) | 537 (10.6) | 185 (8.0) | 259 (11.1) | 93 (22.0) | <.001 |
| Diabetes mellitus, n (%) | 529 (10.4) | 166 (7.2) | 288 (12.3) | 75 (17.8) | <.001 |
| Osteoporosis (n, %) | 3460 (68.0) | 1409 (60.7) | 1694 (72.3) | 357 (84.6) | <.001 |
| Good/excellent self-reported health, n (%) | 4415 (86.8) | 2190 (94.3) | 1978 (84.5) | 247 (58.7) | <.001 |
| Teng Modified Mini-Mental State < 83, n (%) | 219 (4.3) | 57 (2.5) | 115 (4.9) | 47 (11.2) | <.001 |
| Unable to complete chair stand, n (%) | 124 (2.4) | 12 (0.5) | 45 (2.0) | 67 (16.0) | <.001 |
| Any IADL limitations, n (%) | 983 (19.4) | 206 (8.9) | 529 (22.6) | 248 (59.1) | <.001 |
| Leg power (w), mean +/− SD | 211.3 +/− 62.5 | 234.0 +/− 58.4 | 197.3 +/− 57.3 | 150.1 +/− 52.8 | <.001 |
| Creatinine < 60 unmold/L (n, %) | 55 (1.2) | 17 (0.8) | 28 (1.3) | 10 (2.6) | .009 |
| Albumin < 4 g/dL (n, %) | 381 (8.1) | 132 (6.2) | 183 (8.4) | 66 (17.0) | <.001 |
| TNF-α level in highest quartile (n, %) | 77 (20.5) | 98 (26.0) | 19 (34.6) | 194 (24.0) | 0.03 |
| CRP level in highest quartile (n, %) | 72 (19.2) | 97 (25.7) | 25 (45.5) | 194 (24.0) | <.001 |
| IL-6 level in highest quartile (n, %) | 65 (17.4) | 111 (29.5) | 33 (61.1) | 209 (26.0) | <.001 |
V1 = visit 1; SD = standard deviation; SES = socioeconomic status; IADL = instrumental activities of daily livi
Frailty Transitions
Over an average of 4.6 years, the proportion of frail men increased, while the proportion of robust men decreased (Figure 1 and Supplementary Table S2). Among the 5086 men with frailty measures at both visits, 1791 (35%) progressed in frailty status or died (1223, or 26%, of robust or prefrail men progressed and 568, or 11%, of all men died), 2872 (56%) had no change in frailty status, and 423 (15%) of prefrail or frail participants improved in frailty status.
Figure 1.
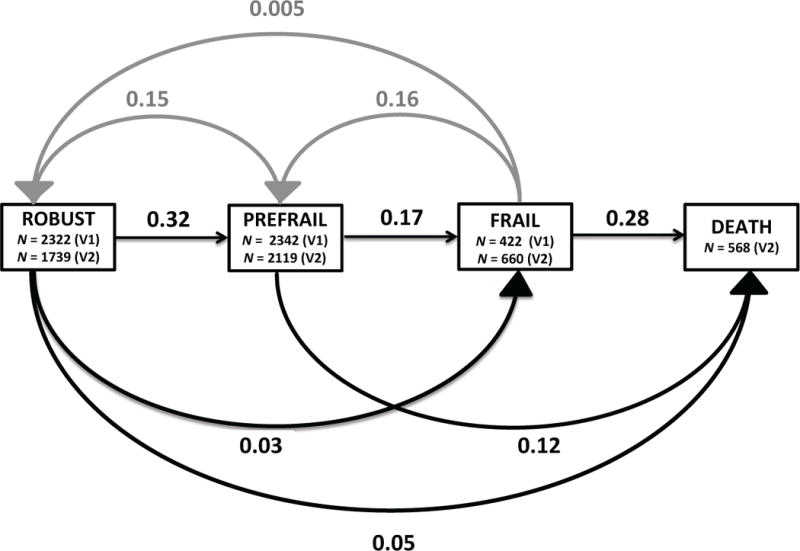
Probability of Transitions between Frailty States. The cumulative probability of transitions between frailty states at Visit 1 and Visit 2 among community-dwelling older men. Black lines represent progression of frailty status, while gray lines represent improvement in frailty status. 95% confidence intervals by transition: frail to robust (.001, 0.02), prefrail to robust (0.14, 0.16), frail to prefrail (0.13, 0.20), robust to prefrail (0.31, 0.34), prefrail to frail (0.16, 0.19), frail to death (0.34, 0.43), robust to frail (0.02, 0.03), robust to death (0.04, 0.06), prefrail to death (0.11, 0.14). V1 = visit 1; V2 = visit 2
The probabilities of each frailty transition are displayed in Figure 1. For all participants, regardless of V1 frailty status, the most likely outcome at V2 was no change in frailty status (0.60 for robust participants, 0.55 for pre-frail participants, and 0.45 for frail participants). The most frequent frailty transitions were from the robust to prefrail state (0.32), and from the frail state to death (0.28). Not all of the transitions were progressive; some participants had improvements in their frailty status. The probability of improving from the frail to prefrail state was 0.16 and the probability of improving from the prefrail to robust state was 0.15. These probabilities were similar to that of transitioning from the prefrail to frail state (0.17). Transitions between one frailty state to the next were more common than transitions across two frailty states. The probability of transitioning from prefrail to death was 0.12, while the probability of improving across two states, from frail to robust, was 0.005 (Figure 1).
Characteristics of participants by frailty status transition are presented in Supplementary Tables S3 and S4. Participants who improved in frailty status were younger, reported higher socioeconomic status, were more likely to be married, had stronger leg power, and reported fewer medical conditions and IADL limitations at V1. Among the 352 participants who improved from prefrail at V1 to robust at V2, the most prevalent improvements in individual frailty criteria were reversal of low physical activity and slowness, with 37% and 28% meeting each of these criteria respectively at V1 but not at V2. Among the 69 participants who improved from frail at V1 to prefrail at V2, the most prevalent improvements in individual frailty criteria were reversal of low physical activity, exhaustion, and slowness, with 45%, 36%, and 34% meeting each of these criteria respectively at V1 but not at V2 (Supplementary Table S5).
Age- and Site-Adjusted Predictors for Improvement and Progression in Frailty State
Many predictors were statistically significant in age- and site-adjusted regression models (Supplementary Table S6). Predictors associated with improvement in frailty status included being married, good or excellent self-reported health, ability to complete chair stands, and greater leg power. Men with two or more comorbidities (and specifically with COPD, DM, or CHF), albumin < 4 g/dL, fasting glucose > 26 mg/dL, CRP or IL-6 levels in the highest quartile, or any IADL limitations were less likely to improve from prefrail or frail states. Predictors associated with progression in frailty status included past or current smoking, one or more comorbidities (specifically hypertension, CHF, COPD, cancer, stroke, and osteoporosis), CRP or TNF-α levels in the highest quartile, albumin < 4 g/dL, fasting glucose > 26 mg/dL, presence of any IADL limitations, and cognitive dysfunction. Men who were non-Hispanic white, college-educated, reported high socioeconomic status, or had greater leg power were less likely to transition to prefrail or frail states.
Multivariable Predictors for Improvement or Progression in Frailty State
We found no evidence of multicollinearity among variables within each category. Results from final multivariable logistic regression models for progression and improvement in frailty status are presented in Tables 2 and 3 respectively. Variables associated with progression in frailty status included a diagnosis of DM, CHF, or cancer, cognitive dysfunction, current smoking, CRP or TNF-α levels in the highest quartile, albumin < 4 g/dL, and any IADL limitations. Greater leg power, college education, and good or excellent self-reported health were associated with a lower likelihood of progression in frailty status. Men with DM were nearly three times as likely to progress in frailty status from the robust state (OR 2.7 95% CI (1.7, 4.3)). A diagnosis of DM, current smoking, any IADL limitations, and greater leg power retained similar strengths of association with progression outcomes in sensitivity analyses excluding men who died.
Table 2.
Age-Adjusted and Multivariate Model Predictors of Progression in Frailty Status from Visit 1 to Visit 2
| Baseline Characteristics2 | Robust to Prefrail, Frail, or Death | Prefrail to Frail or Death | Frail to Death | |||
|---|---|---|---|---|---|---|
| Adjusted1 OR (95% CI) |
Multivariable OR (95% CI) |
Adjusted1 OR (95% CI) |
Multivariable OR (95% CI) |
Adjusted1 OR (95% CI) |
Multivariable OR (95% CI) |
|
| Diabetes mellitus | 2.4 (1.6, 3.4) | 2.7 (1.7, 4.3) | 2.1 (1.6, 2.8) | 1.6 (1.04, 2.5) | 1.8 (1.2, 2.6) | 1.7 (1.01, 3.0) |
| Any IADL limitations | 1.7 (1.3, 2.4) | 1.7 (1.2, 2.3) | 2.7 (2.2, 3.4) | 1.9 (1.4, 2.4) | 2.4 (1.7, 3.5) | 2.4 (1.7, 3.5) |
| Leg power (W)2 | 0.8 (0.7, 0.9) | 0.8 (0.7, 0.9) | 0.6 (0.5, 0.7) | 0.6 (0.5, 0.7) | 0.6 (0.5, 0.8) | 0.7 (0.6, 0.99) |
| Self-reported health (good/excellent) | – | – | 0.4 (0.3, 0.5) | 0.6 (0.5, 0.9) | – | – |
| Teng 3M < 83 | 2.7 (1.5, 4.9) | 2.0 (1.02, 3.9) | – | – | 2.2 (1.4, 3.4) | 2.4 (1.4, 4.1) |
| CRP highest quartile | – | – | 2.1 (1.3, 3.6) | 2.1 (1.3, 3.6) | – | – |
| TNF highest quartile | – | – | – | – | 2.6 (1.1, 6.0) | 2.4 (1.0, 5.8) |
| IL-6 highest quartile | – | – | – | – | 2.6 (1.0, 6.7) | – |
| College education | – | – | 0.7 (0.6, 0.8) | 0.8 (0.6, 0.96) | – | – |
| Cancer | – | – | 1.3 (1.1, 1.6) | 1.4 (1.1, 1.8) | – | – |
| CHF | – | – | 2.1 (1.4, 3.1) | 1.7 (1.1, 2.8) | – | – |
| Current smoking | – | – | 2.6 (1.6, 4.4) | 2.3 (1.2, 4.1) | 2.2 (1.1, 4.3) | 3.8 (1.4, 10.1) |
| Albumin < 4 g/dL | – | – | – | – | 2.1 (1.4, 3.0) | 2.3 (1.4, 3.9) |
Adjusted for age and site
The effect estimates (ORs) for all continuous variables were expressed per standard deviation increment, except age, which was expressed per one-year increment. For continuous variables, odds ratios refer to increments of one standard deviation.
OR = odds ratio; COPD = chronic obstructive pulmonary disease; IADL = instrumental activities of daily living
Table 3.
Age-Adjusted and Multivariable Model Predictors of Improvement in Frailty Status from Visit 1 to Visit 2
| Prefrail to robust | Frail to prefrail or robust | |||
|---|---|---|---|---|
| Adjusted1 OR (95% CI) |
Multivariable OR (95% CI) |
Adjusted1 OR (95% CI) |
Multivariable OR (95% CI) |
|
| Albumin < 4 g/dL | – | – | 0.2 (0.1, 0.7) | 0.2 (0.1, 0.8) |
| IL-6 highest quartile | 0.3 (0.1, 0.7) | 0.3 (0.1, 0.7) | – | – |
| COPD | – | – | 0.3 (0.1, 0.7) | 0.3 (0.1, 0.9) |
| Diabetes Mellitus | – | – | 0.3 (0.1, 0.8) | 0.2 (0.1, 0.8) |
| Any IADL limitations | 0.4, (0.2, 0.5) | 0.5 (0.3, 0.8) | 0.3 (0.2, 0.6) | 0.4 (0.2, 0.8) |
| Leg power (W)2 | 1.6 (1.4, 1.8) | 1.5 (1.2, 1.7) | 2.1 (1.4, 3.1) | 2.0 (1.2, 3.3) |
| Married | 1.6 (1.1, 2.2) | 1.5 (1.02, 2.2) | 4.3 (1.9, 10.5) | 3.6 (1.1, 11.7) |
| Self-reported health (good/excellent) | 2.2 (1.5, 3.2) | 1.6 (1.03, 2.6) | – | – |
Adjusted for age and site
The effect estimates (ORs) for all continuous variables were expressed per standard deviation increment, except age, which was expressed per one-year increment. For continuous variables, odds ratios refer to increments of one standard deviation.
OR = odds ratio; COPD = chronic obstructive pulmonary disease; IADL = instrumental activities of daily living
Greater leg power, being married, and good or excellent self-reported health were associated with improvement in frailty status. In particular, being married was associated with a 3.6 times greater likelihood of improving from the frail state (OR 3.6 95% CI 1.1–11.7). IL-6 level in the highest quartile was associated with a lower likelihood of improvement from the prefrail to robust state, whereas presence of any IADL limitations, DM, COPD, and albumin level < 4 g/dL were associated with a decreased likelihood of improvement from the frail to prefrail state or robust states. With the exception of good or excellent self-reported health which dropped out, all variables retained similar strengths of association with improvement outcomes in sensitivity analyses excluding men who died.
DISCUSSION
Our findings demonstrate that frailty status was dynamic in older community-dwelling MrOS participants over approximately five years. During this time, 35% of the cohort progressed in frailty status or died, 56% had no change in frailty status, and 15% of prefrail or frail participants improved. Improvement in frailty status occurred in a significant proportion of men, indicating that the prefrail and frail states are not irreversible. However, remediation across two states, from frail to robust, was extremely rare. Variables associated with progression differed from factors associated with improvement in frailty status, with some variables (e.g. smoking, CHF, cancer) significantly associated with progression or improvement between one but not all stages along the frailty pathway, suggesting that different factors may play a role in the initiation, progression, and reversal of frailty.
The development and progression of frailty is thought to be associated mechanistically with a systemic inflammatory state and neuroendocrine dysregulation.16, 17 Poor nutrition is also implicated, with diet quality previously shown to be inversely associated with prevalent and future frailty status in the MrOS cohort.17 Predictors of frailty transitions identified in our multivariate models were consistent with our understanding of likely causal pathways. Men with high levels of inflammatory markers were more likely to progress in frailty status and less likely to improve (CRP and IL-6 in the highest quartile, respectively). Low albumin levels were associated with a lower likelihood of improving from the frail state. Factors directly related to functional ability, such as IADL limitations, self-reported health, and leg power, reflect downstream effects of convergent, multi-system physiologic processes, and explained a significant degree of variance in frailty transition outcomes. Finally, our findings suggest that certain patient populations, particularly those with DM or COPD and smokers, may be particularly high-risk for progression in frailty status as well as unlikely to improve, and that men who are married are more likely to improve.
DM and COPD have previously been shown to be associated with frailty.5, 18 Frailty has been identified as a useful prognostic indicator of increased mortality risk in patients with these conditions.19, 20 Our results are novel in that they identify COPD and DM as predictive both of progression in frailty status and decreased likelihood of improvement. The link between DM and sarcopenia is well known; both muscle mass and muscle quality appear to be reduced in older diabetics.21 There is evidence to suggest that DM adversely affects skeletal muscle via direct glucose toxicity, activation of protein degradation pathways due to insulin resistance, reduction in motor neurons, and contributions to the inflammatory-catabolic mileau.21, 22 COPD and frailty have overlapping pathophysiologic features as well; prior studies have demonstrated that the lung inflammatory response in COPD is accompanied by systemic inflammatory changes, oxidative stress, increased basal metabolic rate leading to unexplained weight loss, and loss of skeletal muscle mass.23 Thus, the two conditions may compound one another reciprocally. Beyond general awareness among clinicians that frail older adults with comorbid COPD or DM are a particularly high-risk group, future research should evaluate potential interventions in these patients with the goal of reducing frailty-related adverse outcomes.
The protective effects of close personal relationships, and conversely the negative health outcomes associated with social isolation, are well-known.24, 25 Prior associations have been demonstrated between marital status and morbidity and mortality25, 26, with the protective effect of marriage more pronounced in men than in women.27 Marital interaction studies demonstrate physiologic changes in cardiovascular, neuroendocrine, and immune pathways in response to social interactions between study participants and their spouses28. However, few prior studies have investigated the link between marital status and frailty. In one study, marital status was shown to be associated with the onset of frailty in older adults.29 Our results add to this emerging area of research by demonstrating a four-fold increased likelihood in improvement from the frail to prefrail or robust state in married participants. This finding highlights the importance of considering social support in research efforts to reverse frailty through targeted interventions; future studies may choose to evaluate whether interventions with supervised or group components are more beneficial for older adults with limited social support.
Finally, we found that leg power was a significant predictor for all frailty state transition outcomes. Consistent with these findings, a secondary analysis of results from the InVEST trial, a multi-center randomized clinical trial comparing two rehabilitative exercise programs in community-dwelling adults with mobility limitations, found that leg power was the best rehabilitative impairment target, as changes in leg power were associated with clinically meaningful differences in gait speed and Short Physical Performance Battery scores.31 Of note, long-term complications of DM may result in losses in muscle quality, function and mass, however; gains in leg power may be achieved with targeted training interventions in this group as well.32
Limitations of this study include use of a single-sex and predominantly non-Hispanic white cohort, the possibility of missed transitions over short intervals, the possibility of incidental fluctuations in frailty score over short time periods, inability to capture trends in frailty transitions occurring over longer time periods, and little information regarding acute precipitants that may have contributed to progression in frailty status. Future studies should examine more vulnerable populations of older adults, such as those in assisted living or who are homebound or in a nursing home, with higher levels of disability. In addition, it may be beneficial to perform sub-group analyses of transitions occurring acutely over shorter intervals, as such transitions likely reflect distinct physiologic processes.
In conclusion, our results demonstrate that improvement in frailty status in older community-dwelling adults is possible and associated with social, functional, and clinical factors, although complete recovery from frailty is rare. Future studies should evaluate targeted interventions in both frail and prefrail older adults, as our results suggest that the likelihood of improvement is similar from both baseline states. Effective strategies might specifically address predictors of improvement and progression in frailty status, including preservation of functional ability through interventions that target strength and lower-extremity power, improved management of comorbid medical conditions such as DM and COPD, and through social and nutritional support.
Supplementary Material
Supplementary Methods S1
Supplementary Figure S1. Data Analysis Flowchart
Supplementary Table S1. Prevalence of Frailty Components by Visit
Supplementary Table S2. Visit 2 Frailty Category and Death Status by Visit 1 Frailty Category
Supplementary Table S3. Participant Characteristics by Progression in Frailty Status Between Visit 1 and Visit 2
Supplementary Table S4. Participant Characteristics by Improvement in Frailty Status Between Visit 1 and Visit 2
Supplementary Table S5. Improvement in Individual Frailty Criteria Among Participants who Improved in Overall Frailty Status Between V1 and V2
Supplementary Table S6. Age- and Site-Adjusted Associations for Progression or Improvement in Frailty Status from Visit 1 to Visit 2 Grouped by Category
Acknowledgments
Preparation of Manuscript: L.R.P., T.D. Sponsor’s role: The National Institutes of Health had no role in the designs of the study, collection analysis of the data, or in the preparation of the manuscript.
Funding Sources: NIA and AFAR Medical Student Training in Aging Research (LRP), Columbia University College of Physicians and Surgeons Dean’s Research Fellowship (LRP), K23 AG040168-01A1 (TD), The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, UL1 TR000128, and P60 AR054731.
Footnotes
Conflicts of Interests: The authors declare no personal or financial conflicts of interest.
Author contributions: Study concept and design: S.L.H., P.M., K.E., N.L., E.B., T.D. Acquisition of participants and/or data: S.L.H. Data analysis and interpretation of data: L.R.P., S.L.H., T.D.
References
- 1.Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. Journal of the American Geriatrics Society. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 2.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. The journals of gerontology Series A, Biological sciences and medical sciences. 2001;56:M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 3.Cawthon PM, Marshall LM, Michael Y, et al. Frailty in older men: prevalence, progression, and relationship with mortality. Journal of the American Geriatrics Society. 2007;55:1216–1223. doi: 10.1111/j.1532-5415.2007.01259.x. [DOI] [PubMed] [Google Scholar]
- 4.Gill TM, Gahbauer EA, Allore HG, Han L. Transitions between frailty states among community-living older persons. Archives of internal medicine. 2006;166:418–423. doi: 10.1001/archinte.166.4.418. [DOI] [PubMed] [Google Scholar]
- 5.Espinoza SE, Jung I, Hazuda H. Frailty transitions in the San Antonio Longitudinal Study of Aging. Journal of the American Geriatrics Society. 2012;60:652–660. doi: 10.1111/j.1532-5415.2011.03882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study–a large observational study of the determinants of fracture in older men. Contemporary clinical trials. 2005;26:569–585. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemporary clinical trials. 2005;26:557–568. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Ware J, Kosinski M, Keller SD. How to score the SF-12 Physical and Mental Summary Scores. Third. Lincoln, RI: QualiyMetric, Inc.; 1998. [Google Scholar]
- 9.Washburn RA, Ficker JL. Physical Activity Scale for the Elderly (PASE): the relationship with activity measured by a portable accelerometer. The Journal of sports medicine and physical fitness. 1999;39:336–340. [PubMed] [Google Scholar]
- 10.Adler NE, Epel ES, Castellazzo G, Ickovics JR. Relationship of subjective and objective social status with psychological and physiological functioning: preliminary data in healthy white women. Health psychology: official journal of the Division of Health Psychology, American Psychological Association. 2000;19:586–592. doi: 10.1037//0278-6133.19.6.586. [DOI] [PubMed] [Google Scholar]
- 11.Dodds RM, Sayer AA. Sarcopenia, frailty and mortality: the evidence is growing. Age and ageing. 2016;45:570–571. doi: 10.1093/ageing/afw148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kojima G, Iliffe S, Walters K. Smoking as a predictor of frailty: a systematic review. BMC geriatrics. 2015;15:131. doi: 10.1186/s12877-015-0134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woods NF, LaCroix AZ, Gray SL, et al. Frailty: emergence and consequences in women aged 65 and older in the Women’s Health Initiative Observational Study. Journal of the American Geriatrics Society. 2005;53:1321–1330. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- 14.Rizzoli R, Reginster JY, Arnal JF, et al. Quality of life in sarcopenia and frailty. Calcified tissue international. 2013;93:101–120. doi: 10.1007/s00223-013-9758-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cauley JA, Barbour KE, Harrison SL, et al. Inflammatory Markers and the Risk of Hip and Vertebral Fractures in Men: the Osteoporotic Fractures in Men (MrOS) Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2016;31:2129–2138. doi: 10.1002/jbmr.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X, Mao G, Leng SX. Frailty syndrome: an overview. Clinical interventions in aging. 2014;9:433–441. doi: 10.2147/CIA.S45300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shikany JM, Barrett-Connor E, Ensrud KE, et al. Macronutrients, diet quality, and frailty in older men. The journals of gerontology Series A, Biological sciences and medical sciences. 2014;69:695–701. doi: 10.1093/gerona/glt196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lahousse L, Ziere G, Verlinden VJ, et al. Risk of Frailty in Elderly With COPD: A Population-Based Study. The journals of gerontology Series A, Biological sciences and medical sciences. 2016;71:689–695. doi: 10.1093/gerona/glv154. [DOI] [PubMed] [Google Scholar]
- 19.Cacciatore F, Testa G, Galizia G, et al. Clinical frailty and long-term mortality in elderly subjects with diabetes. Acta diabetologica. 2013;50:251–260. doi: 10.1007/s00592-012-0413-2. [DOI] [PubMed] [Google Scholar]
- 20.Galizia G, Cacciatore F, Testa G, et al. Role of clinical frailty on long-term mortality of elderly subjects with and without chronic obstructive pulmonary disease. Aging clinical and experimental research. 2011;23:118–125. doi: 10.1007/BF03351076. [DOI] [PubMed] [Google Scholar]
- 21.Morley JE, Malmstrom TK, Rodriguez-Manas L, Sinclair AJ. Frailty, sarcopenia and diabetes. Journal of the American Medical Directors Association. 2014;15:853–859. doi: 10.1016/j.jamda.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Jang HC. Sarcopenia, Frailty, and Diabetes in Older Adults. Diabetes & metabolism journal. 2016 doi: 10.4093/dmj.2016.40.3.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agusti AG, Noguera A, Sauleda J, Sala E, Pons J, Busquets X. Systemic effects of chronic obstructive pulmonary disease. The European respiratory journal. 2003;21:347–360. doi: 10.1183/09031936.03.00405703. [DOI] [PubMed] [Google Scholar]
- 24.House JS, Landis KR, Umberson D. Social relationships and health. Science (New York, NY) 1988;241:540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- 25.Kiecolt-Glaser JK, Newton TL. Marriage and health: his and hers. Psychological bulletin. 2001;127:472–503. doi: 10.1037/0033-2909.127.4.472. [DOI] [PubMed] [Google Scholar]
- 26.Johnson NJ, Backlund E, Sorlie PD, Loveless CA. Marital status and mortality: the national longitudinal mortality study. Annals of epidemiology. 2000;10:224–238. doi: 10.1016/s1047-2797(99)00052-6. [DOI] [PubMed] [Google Scholar]
- 27.Umberson D. Gender, marital status and the social control of health behavior. Social science & medicine (1982) 1992;34:907–917. doi: 10.1016/0277-9536(92)90259-s. [DOI] [PubMed] [Google Scholar]
- 28.Uchino BN, Cacioppo JT, Kiecolt-Glaser JK. The relationship between social support and physiological processes: a review with emphasis on underlying mechanisms and implications for health. Psychological bulletin. 1996;119:488–531. doi: 10.1037/0033-2909.119.3.488. [DOI] [PubMed] [Google Scholar]
- 29.Trevisan C, Veronese N, Maggi S, et al. Marital Status and Frailty in Older People: Gender Differences in the Progetto Veneto Anziani Longitudinal Study. Journal of women’s health (2002) 2016 doi: 10.1089/jwh.2015.5592. [DOI] [PubMed] [Google Scholar]
- 30.Bean JF, Kiely DK, Herman S, et al. The relationship between leg power and physical performance in mobility-limited older people. Journal of the American Geriatrics Society. 2002;50:461–467. doi: 10.1046/j.1532-5415.2002.50111.x. [DOI] [PubMed] [Google Scholar]
- 31.Bean JF, Kiely DK, LaRose S, Goldstein R, Frontera WR, Leveille SG. Are changes in leg power responsible for clinically meaningful improvements in mobility in older adults? Journal of the American Geriatrics Society. 2010;58:2363–2368. doi: 10.1111/j.1532-5415.2010.03155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strollo SE, Caserotti P, Ward RE, Glynn NW, Goodpaster BH, Strotmeyer ES. A review of the relationship between leg power and selected chronic disease in older adults. The journal of nutrition, health & aging. 2015;19:240–248. doi: 10.1007/s12603-014-0528-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods S1
Supplementary Figure S1. Data Analysis Flowchart
Supplementary Table S1. Prevalence of Frailty Components by Visit
Supplementary Table S2. Visit 2 Frailty Category and Death Status by Visit 1 Frailty Category
Supplementary Table S3. Participant Characteristics by Progression in Frailty Status Between Visit 1 and Visit 2
Supplementary Table S4. Participant Characteristics by Improvement in Frailty Status Between Visit 1 and Visit 2
Supplementary Table S5. Improvement in Individual Frailty Criteria Among Participants who Improved in Overall Frailty Status Between V1 and V2
Supplementary Table S6. Age- and Site-Adjusted Associations for Progression or Improvement in Frailty Status from Visit 1 to Visit 2 Grouped by Category


