Abstract
Background
Increased extracellular glutamate may contribute to L-DOPA induced dyskinesia, a debilitating side effect faced by Parkinson’s disease patients 5–10 years after L-DOPA treatment. Therapeutic strategies targeting post-synaptic glutamate receptors to mitigate dyskinesia may have limited success due to significant side effects. Increasing glutamate uptake may be another approach to attenuate excess glutamatergic neurotransmission to mitigate dyskinesia severity or prolong the time prior to onset. Initiation of a ceftriaxone regimen at time of nigrostriatal lesion, can attenuate tyrosine hydroxylase loss in conjunction with increased glutamate uptake and glutamate transporter GLT-1 expression in a rat 6-hydroxydopamine model. Here, we examined if a ceftriaxone regimen initiated 1 week after nigrostriatal lesion, but prior to L-DOPA, could reduce L-DOPA-induced dyskinesia in an established dyskinesia model.
Methods
Ceftriaxone (200 mg/kg, i.p., once daily, 7 consecutive days) was initiated 7 days post-6-hydroxydopamine lesion (days 7–13) and continued every other week (days 21–27, 35–39) until the end of the study (day 39 post-lesion, 20 days of L-DOPA).
Results
Ceftriaxone significantly reduced abnormal involuntary movements at 5 time points examined during chronic L-DOPA treatment. Partial recovery of motor impairment from nigrostriatal lesion by L-DOPA was unaffected by ceftriaxone. The ceftriaxone-treated L-DOPA group had significantly increased striatal GLT-1 expression and glutamate uptake. Striatal tyrosine hydroxylase loss in this group was not significantly different compared to the L-DOPA alone group.
Conclusions
Initiation of ceftriaxone after nigrostriatal lesion, but prior to and during L-DOPA, may reduce dyskinesia severity without affecting L-DOPA efficacy or reduction of striatal tyrosine hydroxylase loss.
Keywords: Parkinson’s disease, L-DOPA, dyskinesia, glutamate, GLT-1, ceftriaxone, tyrosine hydroxylase
INTRODUCTION
Parkinson’s Disease (PD) is characterized by loss of nigrostriatal dopamine (DA) neurons and tyrosine hydroxylase (TH)1–3 with nigrostriatal terminal fields being more vulnerable early in the disease process3. With progressive degeneration of DA neurons, evidence for concomitant increases in glutamatergic tone have been observed4–11; including decreased striatal glutamate uptake and changes in glutamate transporter GLT-1 function11–13. High concentrations of synaptic glutamate increase Ca2+ influx through the glutamate receptors triggering activation of Ca2+−-dependent enzymes that can lead to excitotoxic cell death 14–20. Increased Ca2+−dependent phosphorylation of TH occurs with TH loss in the rat 6-OHDA PD model21 and is reduced in conjunction with increased glutamate uptake11,22.
PD symptoms can be alleviated by the DA precursor, L-DOPA. However, chronic L-DOPA therapy can lead to L-DOPA induced dyskinesia (LID), affecting 50% of patients by the first 5 years of L-DOPA therapy and 90% of patients after 10 years of therapy23–25. These dyskinesias limit the therapeutic efficacy of L-DOPA and disrupt the quality of life in PD patients. Furthermore, once dyskinesia appears it re-emerges with subsequent exposure to L-DOPA, therefore remaining a significant challenge to mitigate motor impairment.
The development of LID has been attributed to plasticity in pre- and post-synaptic receptor and transporter functions in striatum 26–32, including glutamatergic imbalances 33–40, that may arise from DA neuron loss or L-DOPA treatment40. The only current FDA approved drug for mitigating LID expression is amantadine25,41–43, a NMDA antagonist which, among others, may have limited clinical utility in some cases44 and produce untoward side effects such as psychiatric and memory complications 35,45,46. Recent work has focused upon pharmacological blockade of glutamate receptors directed at alternate subtypes. However, it is not resolved as to whether an antidyskinetic effect, similar or superior to that of amantadine, may be achieved by other glutamate receptor drugs, such as metabotropic glutamate receptor modulators 35,47,48.
Pharmacological-based reduction of striatal or nigral glutamate efflux may reduce LID severity.49–51. GLT-1 is a primary glial glutamate transporter responsible for clearing synaptic glutamate52 and may be involved in neurodegenerative processes11,15–18. Ceftriaxone, a beta-lactam antibiotic, can increase GLT-1 expression with >5 consecutive daily injections11,53–55. When initiated at the time of 6-OHDA, thereby increasing GLT-1 expression during progression of TH loss, ceftriaxone can attenuate TH loss 9 days after 6-OHDA lesion in association with increased uptake of glutamate and reduced Ca2+-dependent phosphorylation of TH 11. Increased GLT-1 expression by ceftriaxone may also reduce DA neuron loss when initiated prior to56 or after 11,57,58 experimental DA lesion. We reasoned that ceftriaxone may reduce LID by augmenting glutamate uptake or reducing the rate of TH loss using an established model of LID59,60. The timing of ceftriaxone administration was modeled in accordance with consideration of the time required to ensure increased GLT-1 expression11,53–55, the longevity of its effect of at least 1 week11, and was initiated at a time post-lesion when TH loss has been shown to be >50%11,21,30,61. The appearance of PD locomotor symptoms are generally considered to be present with >70% loss of striatal TH, but recent evidence suggests symptoms can present with as little as 35% loss in putamen3. The possible clinical translation therefore was to initiate ceftriaxone when significant striatal TH protein loss was expected and end the first 7 days regimen prior to L-DOPA initiation. As such, our paradigm sought to model a preventative strategy to reduce LID severity or time of onset when locomotor symptoms would begin and a PD patient would begin taking L-DOPA. The week-long ceftriaxone regimen continued intermittently thereafter, with a subsequent week-on, week-off regimen, until L-DOPA was given for 20 days, 39 days post-6-OHDA lesion.
METHODS
Animals
Male Sprague Dawley rats (N=38 used across all replicate studies) purchased from Charles River were used in all experiments. All rats were 4–9 months old in the study, and were housed under controlled lighting conditions (12:12 reverse light:dark cycle) with food and water available ad libitum. All animals were used in compliance with OLAW guidelines and an approved protocol by the institutional Animal Care and Use Committee at LSU Health Sciences Center-Shreveport, University of North Texas Health Science Center (UNTHSC), and Binghamton University. The study was conducted in replicate at the 3 institutions, with UNTHSC additionally evaluating amphetamine-induced rotational motor function and Binghamton assessing forepaw adjustment steps in response to L-DOPA with and without ceftriaxone.
Ceftriaxone and L-DOPA treatment
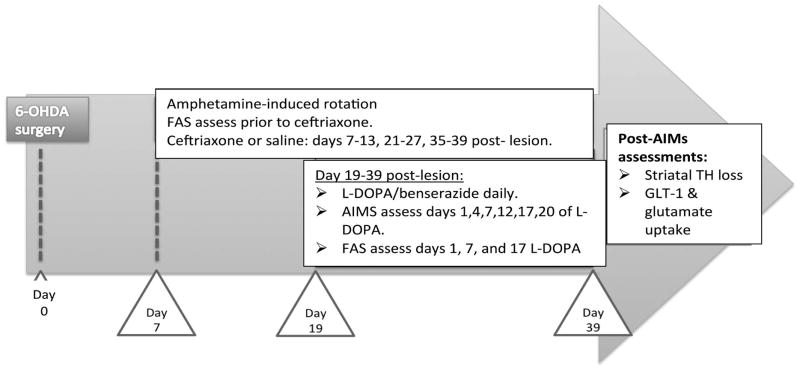
The overall study design is illustrated in Figure 1. On day 7 post-6-OHDA lesion, ceftriaxone (200 mg/kg, i.p.) or saline was administered for 7 consecutive days and every other week (days 7–13, days 21–27 and 35–39). Ceftriaxone (disodium salt hemiheptahydrate) (Hospira, Lake Forest, IL or Toronto Research Chemicals, Inc., Toronto, ON, cat#C245000) was dissolved in sterile saline (0.9%) and prepared daily. The dose of L-DOPA (Sigma, cat # D9628) at one experiment site was 12 mg/kg L-DOPA and 6 mg/kg L-DOPA (methyl ester) (Cayman Chemical, act#16149) was used at the two subsequent studies. In combination with 15 mg/kg benserazide-HCl, i.p., L-DOPA was given for 20 consecutive days, starting on day 19 (day 1 of L-DOPA) until day 39 post-lesion. No significant difference in total ALO was observed between using either dose of L-DOPA in either the L-DOPA alone (experiment site, F(1,5) =1.55, p=0.27; days of L-DOPA, F(5,25) =26.0, p<0.0001) or L-DOPA + ceftriaxone group (experiment site, F(1,11) =1.09, p=0.32; days of L-DOPA, F(5,55) =2.44, p<0.05) and therefore study outcomes were collapsed between study sites.
Figure 1. Treatment paradigm.
Ceftriaxone (Cef; 200mg/kg) or saline (vehicle) is initiated 7 days post lesion for 7 consecutive days every other week until end of study (day 39). 19 days post-lesion, L-DOPA or saline treatment is initiated for 20 consecutive days. During L-DOPA treatment period, 6 behavioral AIMs are performed on days 19, 23, 27, 31, 35 and 39. There were also baseline assessments of FAS on day 7 post-lesion, followed by assessment on day 1, 7, and 17 of L-DOPA treatment. L-DOPA alone group, n=11; L-DOPA + ceftriaxone group, n=18; ceftriaxone alone group, n=9, across 3 study sites. All groups received unilateral 6-OHDA lesion
6-OHDA Lesions
Rats were anesthetized with 40 mg/kg Nembutal intraperitoneal (i.p.) (pentobarbital Lundbeck Inc, Deerfield, IL) with supplement of 9.0, 0.6, and 0.3 mg/kg ketamine, xylazine, and acepromazine, respectively or with 2–3% continuous inhalation isoflurance. Rats were immobilized in a stereotaxic frame to target the medial forebrain bundle ((mfb) coordinates ML +1.5, AP −3.8, DV −8.0) relative to Bregma according to Paxinos and Watson rat brain atlas, 4th ed.62. 6-OHDA (16 μg in 4 μl (4 μg/μl, Tocris, as methylbromide (cat#2547) or Sigma, as ) in 0.02% ascorbic acid (4 mg/ml) was infused unilaterally at 1 μl/min into the medial forebrain bundle. The contralateral mfb was infused with vehicle (0.02% ascorbic acid).. The needle (26 gauge) was left in place for 10 min before removal. Body temperature was maintained at 37° using a heating pad.
Behavioral AIMS ratings
L-DOPA-induced abnormal involuntary movements (AIMs) were rated by two lab members blind to treatment group at 6 time points (days 19, 23, 27, 31, 35, 39 following 6-OHDA lesion, corresponding to days 1, 4, 7, 12, 17, and 20 of daily L-DOPA administration). Twenty minutes after L-DOPA, dyskinetic behaviors were quantified based on frequency during a 1-minute observation period performed every 20 minutes over a period of 140 minutes. Three subtypes of AIMs were evaluated: axial AIMs (dystonic posturing of the upper trunk towards the side contralateral to the lesion), limb AIMs (hyperkinetic or jerky movements of the forelimb contralateral to the lesion), and orolingual AIMs (abnormal jaw movements, facial twitching and tongue protrusion). Each subtype was scored based on frequency from 0 to 4, with 4 occurring continuously during the entire 1-minute observation period and not being interrupted by an experimentor-generated startling sound59,60. Total AIMs scores were calculated by summing the total score from each 20 min observation period. Theoretically, the highest AIMs score achievable in one test day would be 84. This scoring system has been used in a previous study from our lab30, among others 59,60, 63.
Forepaw adjusting steps (FAS)
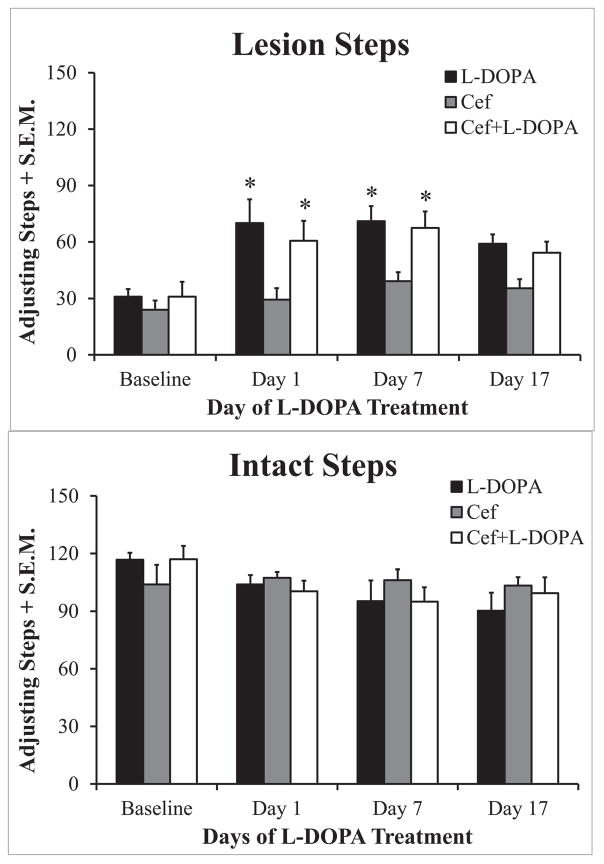
The FAS test measures rat forelimb akinesia, modeling clinical symptoms of PD. Rats with greater than 80% striatal DA depletion perform poorly on the task and L-DOPA treatment partially reverses lesion-induced deficits64,65. For this test one experimenter held both hind legs and one forepaw such that the rat was bearing its weight on the forepaw to be tested. Rats are moved across the table at a speed of 90 cm/10 seconds, during which an additional experimenter recorded the number of adjusting steps taken on the weight-bearing forepaw. Both experimenters were blinded to treatment condition. Rats were dragged for 6 trials per forepaw: 3 backhand (lateral steps away from the torso) and 3 forehand (lateral steps toward the torso) trials, alternating the starting forepaw between animals. Data was represented as the sum of the 3 trials per direction for each forepaw (i.e. left forehand). Fewer steps indicate greater lesion-induced akinesia. This test was used in one of 3 replicate studies performed to verify nigrostriatal lesion evidence and evaluate if ceftriaxone affected L-DOPA-dependent motor recovery. There was no significant difference in FAS at baseline measure taken at day 7 post-lesion, just prior to ceftriaxone initiation in designated groups.
Amphetamine-induced rotation
Amphetamine produces rotations in unilaterally lesioned rats, with high probability of lesion between 50–100 rotations per hour66. We used this test in a replicate study to verify that rats entering 2 treatment groups (L-DOPA or L-DOPA + ceftriaxone) had at least 50 rotations present on day 7 post-lesion. Amphetamine (2 mg/kg, i.p.) was given on day 7 following 6-OHDA and rotations were quantified for 1 hour following injection. There was no significant difference in amphetamine-induced rotation between the L-DOPA alone and L-DOPA + ceftriaxone group (p =0.31, df=9). Rats in the L-DOPA + ceftriaxone group had 137 ±27 rotations (mean ± SEM, n=8).
Preparation of synaptosomes and glutamate uptake
To evaluate the relationship of GLT-1 expression with glutamate uptake, with and without ceftriaxone, striatal glutamate uptake was determined on last day of LID assessment in one of the replicate studies. Synaptosomes were prepared as previously described 11. Striatal tissue was homogenized at 4° C, in 5 mL of 0.32 M sucrose solution then spun at 1000 × g for 10 min. The resulting P1 fraction was stored frozen until processing for TH protein determination. The supernatant was spun further at 16,500 × g for 30 minutes at 4° C, yielding the P2 fraction, aspirated and resuspended in 1 mL of Kreb’s buffer. The P2 fraction was used to determine GLT-1 expression and glutamate uptake. Protein concentration was determined using a BCA assay (Thermo Scientific, Rockford, IL).
Glutamate uptake protocol
The synaptosomal P2 fraction contains glial components67 and ~70% of the levels of glial fibrillary acid protein are recovered in purified glial plasmalemmal vesicles and used to assess glutamate uptake11,22. Uptake was assessed in a 200 μL final volume by reconstituting 30 μg synaptosomal protein in 100 μL in oxygenated Kreb’s buffer at 4 °C. Synaptosomes were heated to 35°C for 5 min, followed by 100 μL of 10 μM 14C(U)-L-glutamic acid (Perkin-Elmer, specific activity 260 mCi/mmol, cat# NEC290E050UC) to give 5 μM final [glutamate]. Uptake time was 90 seconds and terminated with 1 ml of ice-cold Kreb’s buffer. The reuptake time was as close as practically possible to glutamate reuptake time in vivo68,69. Synaptosomes were washed to remove excess labeled-glutamate with equal-osmolarity PBS buffer through a Brandel M24-TI cell harvester. The percent of labeled glutamate recovered was calculated for uptake as pmole/mg protein/min.
Tissue preparation and Western immunoblotting
Synaptosome fractions (P1, P2) were sonicated in 1% sodium dodecyl sulfate solution (pH ~8). Following electrophoresis, proteins were transferred onto nitrocellulose (Bio-Rad Laboratories), stained with Ponceau S, and normalized for protein via relative staining.. These lanes were scanned and quantified by Image J for further normalization to total protein. Membranes were then blocked in PVP buffer (1% polyvinylpyrrolidone and 0.05% Tween 20), and exposed toprimary antibody for 2 hours. Specific primary antibodies were GLT-1 (Santa Cruz, Santa Cruz, CA, cat# 15317) and TH (Millipore, cat # AB152). P2 fraction was used to assess GLT-1 expression and P1 fraction used for TH expression, comparing TH protein expression per protein in lesioned vs contralateral side against a standard curve of TH protein standard 11,21,30,61. TH protein was also determined from processing fresh frozen striatal dissections obtained at the conclusion of 2 replicate studies, as previously described11. No significant difference in TH loss was observed by the lesion in the L-DOPA + ceftriaxone group as a result of the preparation method of tissue for analysis (t=1.57, ns, df=11). Nominal protein loads for linear detection of GLT-1 was 25 μg total protein and 5 – 8 ug for striatal TH. Secondary antibodies (swine anti-rabbit IgG) were used for signal enhancement, followed by 1h incubation with [125I] protein A (PerkinElmer, Waltham, MA) or a chemilluminscence approach using a secondary antibody (Immun-Star #170-5046, goat anti-rabbit) from BioRad and quantified using BioRad imager V3 ChemiDoc Touch.
Statistics
Data were analyzed using Graphed Prism (La Jolla, CA, USA) or Statistica (Tulsa, OK, USA) with correction for multiple comparisons and p-values < 0.05 considered significant. A two-way ANOVA (time × treatment) was used to analyze the sum of axial, limb, orolingual (ALO) AIM scores obtained for each day of dyskinesia evaluation followed by Bonferroni multiple comparison- post hoc tests to determine if significant differences in total ALO were present for each day of evaluation between the L-DOPA alone versus the L-DOPA + ceftriaxone groups. There were few (maximum ALO observed=3) or no AIMs observed in the ceftriaxone alone (no L-DOPA) group in 2 replicate studies (median value of 1; total n=9). This group was used to verify that ceftriaxone itself would not produce AIMS comparable to those in the L-DOPA alone group. Therefore, the statistical evaluations were kept between the L-DOPA alone and L-DOPA + ceftriaxone groups.
FAS data were analyzed with a three-way ANOVA (paw × treatment × time) to determine whether forepaw stepping in the intact or lesioned paw differed depending upon treatment and/or session (day 1, 7 or 17 of L-DOPA treatment). When main effects or interactions were found Bonferroni multiple comparison-Dunn post hoc test was employed to determine if significant differences existed between conditions.
Correlational analysis (Pearson) evaluated GLT-1 expression versus glutamate uptake.. A paired student test determined if differences in glutamate uptake were observed between lesioned and unlesioned striatum for each of the 3 treatment groups. No significant differences between lesioned and unlesioned sides were observed in any group. Thus, the average of uptake between lesioned and unlesioned sides were used to determine differences in uptake existed between the 2 AIMS-relevant groups (unpaired t-test), as little or no AIMS in the CEF alone treatment group was observed.. An unpaired t-test was used to evaluate TH protein loss between the L-DOPA + ceftriaxone and L-DOPA alone groups.
RESULTS
Chronic Ceftriaxone reduces L-DOPA Induced Dyskinesia expression
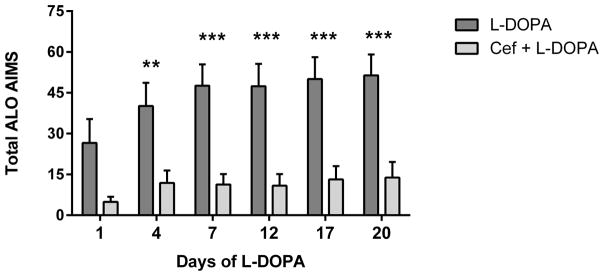
In the 6-OHDA + ceftriaxone, no L-DOPA group, 7 of 9 total rats exhibited a total ALO of 1 or 0. Therefore, any LID produced by ceftriaxone alone in lesioned rats was negligible and statistical comparisons of LID thereafter were between the L-DOPA alone versus the L-DOPA + ceftriaxone group. With the described ceftriaxone regimen (Fig. 1) LID severity, shown as total ALO AIMS, was decreased in all but the first of the 6 days of observation throughout the study compared to L-DOPA alone (Fig 2A). The mean reduction of total ALO in the L-DOPA + ceftriaxone group across all days of observation was 75 ± 4% (mean ± SD).
Figure 2. AIMS expression over course of L-DOPA administration between L-DOPA alone and L-DOPA + ceftriaxone group.
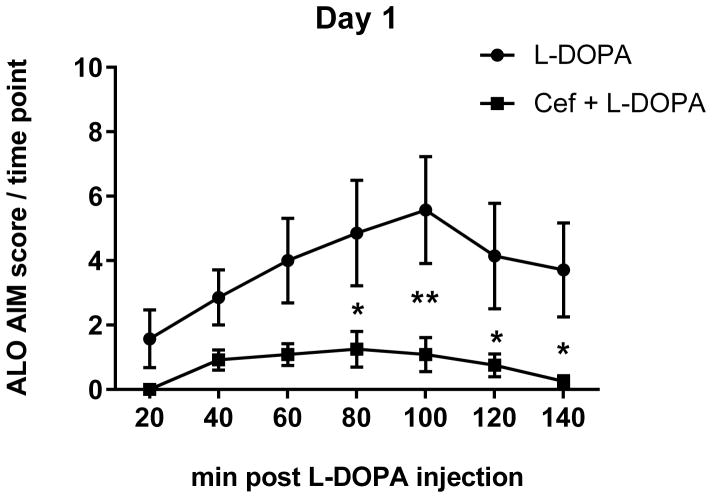
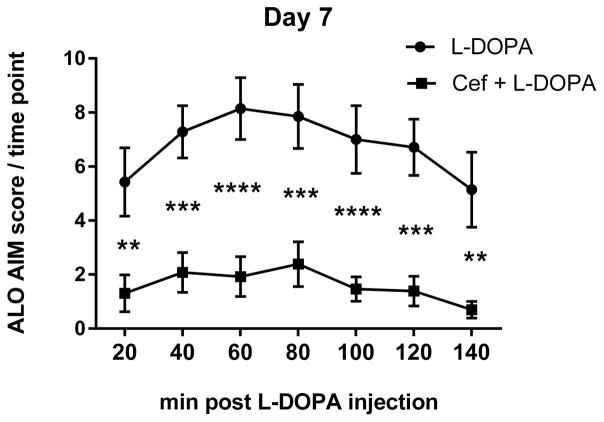
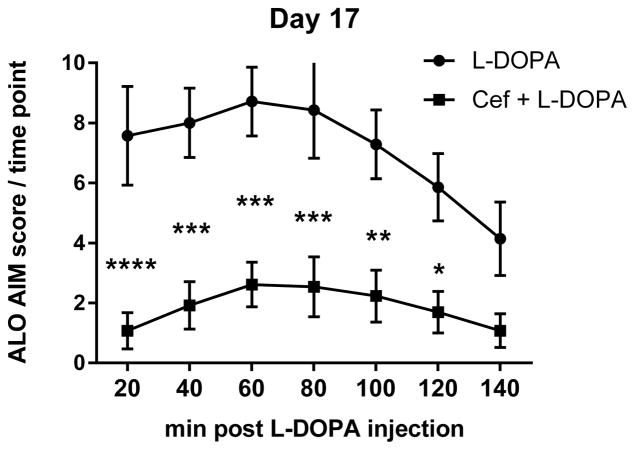
A. The mean cumulative ALO AIMS. The sum of ALO AIMS (as frequency, rating 0–4 for each of the 3 categories of AIMS) observed over the 140 minute observation period following L-DOPA administration between the L-DOPA alone and L-DOPA + ceftriaxone (cef) group (mean ± SEM). Two-way repeated measures ANOVA (matched for individual test subject over time) revealed a significant effect of duration of L-DOPA administration (F(5,90) =13.84, p<0.0001) and treatment (F(1,18 =17.40, p=0.0006). There was also significant interaction between duration of L-DOPA and ceftriaxone treatment (F(5,90)=3.83, p=0.0034) Significant differences in between the two groups were observed on 5 of the 6 observation days ((Day 1, t=2.57,ns; Day 4, t=3.35, **p<0.01; Day 7, t=4.30, ***p<0.001; Day 12, t=4.33, ***p<0.001; Day 17, t=4.37,*** p<0.001; Day 20, t=4.45, ***p<0.001), Bonferroni’s multiple comparison test). B–D. Time course of AIMS onset and severity following L-DOPA administration. ALO AIMS were evaluated on days 1, 4, 7, 12, 17, and 20 of daily L-DOPA administration (corresponding to days 19, 23, 27, 31, 35 and 39 post lesion). Rats treated with 200mg/kg of ceftriaxone pretreatment + L-DOPA displayed significantly less LID compared to L-DOPA alone during the 140 minute observation period for at least four 20 min intervals. Two-way repeated measures ANOVA, followed by Bonferroni post-test showed significant differences between treatments at specific time intervals following L-DOPA. Presented are results from Day 1, 7, and 17 of L-DOPA. B. Day 1. Time interval after L-DOPA, F(6,102)=6.95, p<0.0001; effect of ceftriaxone treatment, F(1,17)=9.66, p=0.006. Significant differences were observed 80–140 min after L-DOPA. C. Day 7. Time interval after L-DOPA, F(6,108)=8.90, p<0.0001; effect of ceftriaxone treatment, F(1,18)=21.96, p=0.0002. Significant differences were observed 20–140 min after L-DOPA. D. Day 17. Time interval after L-DOPA, F(6,108)=10.89, p<0.0001; effect of ceftriaxone treatment, F(1,18)=16.94, p=0.0006. Significant differences were observed 20–120 min after L-DOPA. Statistical outcomes *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 Results not depicted in graphs; Day 4: Time interval after L-DOPA, F(6,108)=9.09, p<0.0001; effect of ceftriaxone treatment, F(1,18)=9.46, p=0.0.007. Significant differences were observed 60–140 min after L-DOPA. Day 12:. Time interval after L-DOPA, F(6,108)=12.08, p<0.0001; effect of ceftriaxone treatment, F(1,18)=18.77, p=0.0004. Significant differences were observed at 20–120 min after L-DOPA Day 20: Time interval after L-DOPA, F(6,108)=8.31, p<0.0001; effect of ceftriaxone treatment, F(1,18)=14.21, p=0.001. Significant differences were observed at 20–120 min after L-DOPA
The reduction of total AIMS in the ceftriaxone group was evident earlier after L-DOPA injection as the regimen of L-DOPA continued, such that AIMS reduction was significant at the first 20 min observation period by day 7 of L-DOPA and continued until 120 min or later after L-DOPA was given (Fig. 2B–D).
Anti-parkinson effects of L-DOPA were not affected by ceftriaxone
The forepaw adjusting steps (FAS) test evaluates nigrostriatal lesion severity by comparing the adjustment steps of the forepaw contralateral to the side of lesion versus the forepaw contralateral to the unlesioned side. As depicted in Figure 4, the number of steps taken with the lesioned forepaw (Fig. 3A) were significantly reduced by 6-OHDA lesion to near 70% less than that of the unlesioned side (Fig. 3B). L-DOPA treatment increased the number of lesioned paw steps taken versus those at baseline both 1 and 7 days 7 post-lesion (Fig. 3A) and this improvement in motor performance was similar in the L-DOPA + ceftriaxone group (Fig. 3A).
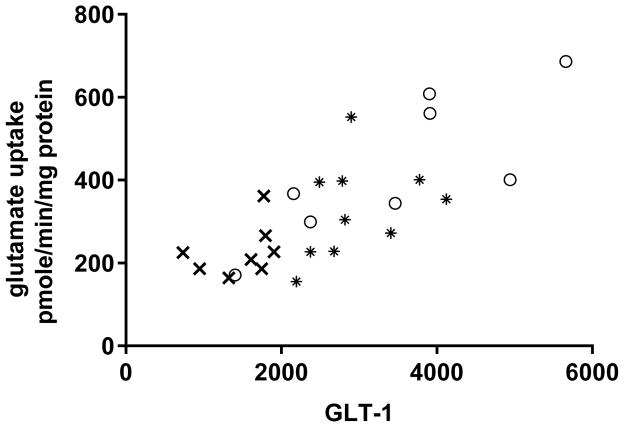
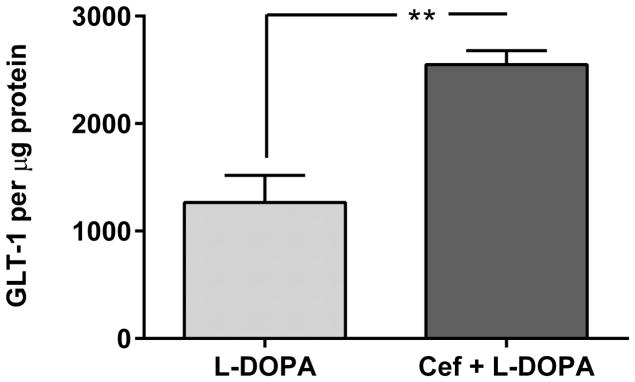
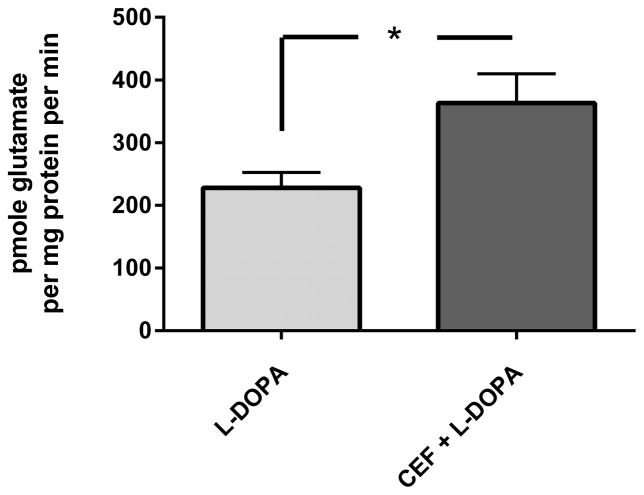
Figure 4. GLT-1 expression and glutamate uptake. A. Striatal GLT-1 protein expression is directly correlated with glutamate uptake.
Combining all treatment groups, a significant positive correlation was observed between striatal GLT-1 protein expression and glutamate uptake (Pearson r= 0.762, p<0.0001,). Note, symbols on the graph ((○) ceftriaxone alone, (*) L-DOPA + ceftriaxone, (x) L-DOPA alone) represent the values obtained from each of the three treatment groups (taken from both lesioned (L) and unlesioned striatum (UL). B. GLT-1 expression in lesioned striatum. The ceftriaxone regimen produced a significant increase in GLT-1 expression, without any difference in relative expression levels observed between the unlesioned striatum (data not shown) and lesioned striatum. In lesioned striatum, GLT-1 expression was significantly increased in the two groups receiving ceftriaxone (L-DOPA + ceftriaxone group vs L-DOPA alone data shown). Student’s unpaired t-test (t=4.81, p<0.01, df=7). C. Glutamate uptake. Striatal glutamate uptake (average uptake values from unlesioned and lesioned striatum of each test subject used for analysis) was greater in the Cef + L-DOPA vs. L-DOPA alone treated rats. Student’s unpaired t-test (t=2.56, *p < 0.05, df=6)
Figure 3. Forepaw adjusting steps.
Effects of 6-OHDA lesion, L-DOPA treatment, and L-DOPA + ceftriaxone (Cef) on forepaw adjusting steps (FAS). Seven days after 6-OHDA lesion surgery, rats (4–5/group) were assessed to establish baseline FAS differences between lesioned (A) and unlesioned (B) forelimbs. Following baseline FAS, animals received daily Cef (CF; 0 or 200 mg/kg, i.p.) in a 7 day on, 7 day off treatment paradigm over 32 days. L-DOPA (0 or 6 mg/kg, i.p.) treatment began 19 days after lesion and continued daily for 19 days. Adjusting steps were recorded 60 min post-L-DOPA treatment and are presented as group mean lesioned forepaw stepping + standard error of the mean (S.E.M). Parametric 3 × 2 × 4 (Treatment × Paw × Day) ANOVAs were used to assess differences. Main effects of Paw (F(1,22) = 138.5, p<0.001) and Time (F(3,66) = 3.6, p<0.02) were found. Importantly a 3-way interaction between factors was also revealed (F(1,22) = 3.6, p<0.004). Bonferroni post-hocs demonstrated significant improvement in stepping with L-DOPA regardless of Cef. treatment on Days 1 and 7 vs. baseline (*p<0.05 vs baseline of the same treatment condition).
GLT-1 protein expression and glutamate uptake
Confirming the proposed role of brain GLT-1, there was a significant positive correlation found between striatal GLT-1 expression and glutamate uptake (Fig. 4A). Importantly, when comparing striatal GLT-1 expression in the unlesioned striatum across treatments, the L-DOPA + ceftriaxone group had significantly increased expression compared to the L-DOPA alone group (t=5.03, p<0.01, df=7, unpaired t-test). This pattern of effects was also seen in the 6-OHDA lesioned striatum (Fig. 4B). No differences in uptake were observed between unlesioned and lesioned striata (data not shown), so the combined average uptake was used to compare uptake between L-DOPA + ceftriaxone and L-DOPA alone groups, given that only these two groups presented ALO AIMS. The L-DOPA + ceftriaxone group showed significant increases in glutamate uptake compared to the L-DOPA alone group (Fig. 4C).
Ceftriaxone treatment and striatal tyrosine hydroxylase expression
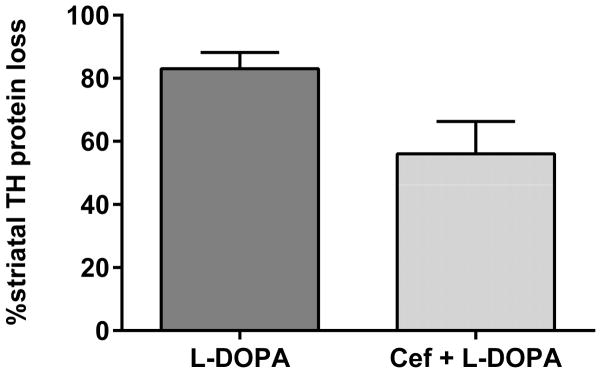
There was no significant difference in striatal TH protein loss between the L-DOPA alone versus the L-DOPA + ceftriaxone group (Fig. 5). Striatal TH loss in the L-DOPA + ceftriaxone group had a 4-fold greater coefficient of variance than the L-DOPA alone group, suggesting the possibility that the severity of TH loss may have correlated with total ALO among test subjects. However, striatal TH loss in the L-DOPA + ceftriaxone group (Pearson r=0.344, p=0.25, 13 pairs) or the L-DOPA alone group (Pearson r=0.348, p=0.44, 8 pairs) did not correlate with the total ALO observed among the test subjects.
Figure 5. Tyrosine hydroxylase protein loss.
Striatal TH loss by 6-OHDA at the end of the study was measured by comparison of the percent of TH protein in the lesioned versus contralateral control striatum in each test subject. There was no significant difference in TH loss between the L-DOPA and Cef + L-DOPA group (t=1.85, p =0.08 (ns), df=18). The variance in TH protein loss was significantly different (coefficient of variance = 17% in L-DOPA alone vs. 65% in Cef + L-DOPA) between the two group (F(12,6)=7.04; p <0.05).
DISCUSSION
Given L-DOPA’s primary role and significant efficacy in the treatment of motor symptoms, but noted side effects, unveiling neurobiological strategies to mitigate LID still remain a critical clinical hurdle70. The aim of this study was to determine if ceftriaxone, previously shown in preclinical studies to increase GLT-1 expression11,53–55, could reduce LID severity or time of onset. The cellular source of increased GLT-1 expression by ceftriaxone is likely astocytes57, although a neuronal GLT-1 component may not be ruled out71. Ceftriaxone also can activate other potentially protective signaling pathways58,72, which were not evaluated in our study. The regimen of ceftriaxone used herein decreased dyskinesia rating scores by ~70%. We also observed increased GLT-1 expression and glutamate uptake, indicating that the increase in GLT-1 expression could reduce extracellular glutamate levels by way of concomitant increases in glutamate uptake. The regimen of ceftriaxone regimen began when TH protein loss would be expected to be at least 50% (as evidenced by confirmation of amphetamine-induced rotations at the start of the ceftriaxone regimen and possibly ~70–80%, as shown in previous studies 11,21,30,61). The possible translation of this study would be that if PD symptoms were detected and L-DOPA was being considered for pharmacotherapy, increasing GLT-1 expression could alleviate the severity or delay the onset of dyskinesia. To add to the translational potential of this study, the ceftriaxone regimen did not appear to alter the beneficial effects of L-DOPA and thus may convey a favorable clinical profile. A logical extension of this work would include interventional ceftriaxone after L-DOPA has begun. In this regard, the results serve as proof of principle that ceftriaxone could also be effective in established LID, since the current ceftriaxone regimen was intermittent.
GLT-1 loss has been observed in 6-OHDA lesion PD and MPTP (1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine) models7,11,12,73. We have previously reported decreased glutamate uptake in association with ~80% loss of TH in the 6-OHDA model11. Therefore, increased GLT-1 and glutamate uptake may reduce LID severity by reducing excess glutamatergic neurotransmission, as seen with observations showing reduced LID in association with reduced glutamate release 49,50. To the converse, others have not reported any difference in striatal glutamate concentrations in the lesioned nigrostriatal pathway 10,74,75, which may be attributed to time-dependent changes in release and uptake and their contributions to extracellular glutamate concentration and when these determinations were made after lesion induction or the lesion site 4,5,40,75. As a result, post-lesion time points and lesion topography are relevant metrics for timing therapeutic strategies. Post-synaptic involvement of glutamate-mediated mechanisms in the long-term effects of L-DOPA therapy have been well-documented. Pathologically-enhanced corticostriatal plasticity may influence LID presentation with glutamate receptors implicated in this process. Increased sensitivity of striatal glutamate AMPA receptors36–38 in dyskinetic rats and AMPA receptor blockade may attenuate the process of priming for LID in animal models of PD. As such, future inquiries to evaluate if modulation of glutamate uptake or release would alter AMPA receptor function or expression in conjunction with differences in LID onset or severity could be informative.
Pre-synaptic alterations with chronic L-DOPA however, have been less examined. The few studies with chronic L-DOPA report increased GLT-1 expression13, 76,77, which could be a compensatory response to alleviate excessive synaptic glutamate accumulation or maintain stores of glutamate for cystine/glutamate antiporter 33, an exchanger responsible for glutathione synthesis. Our data would suggest that such an intrinsic increase in GLT-1 may not be sufficient to delay LID expression, but may constitute a logical target for pharmacological augmentation.. Accordingly, the ability of ceftriaxone and L-DOPA to increase GLT-1 expression more than L-DOPA alone suggests this increase could reach the threshold of the level of GLT-1 expression necessary to prevent or reduce LID.
The expression of other glutamate transporters was not determined in these studies, although we previously reported that ceftriaxone does not affect GLAST expression11. Another consideration with regard to using the contralateral striatum as our control is the issue of clinical translation, given our use of the unilateral 6-OHDA model and known bilateral projections in the basal ganglia78. However, the hemiparkisonian PD model used here was intended for comparison of TH loss against contralateral striatum. As such, our results showed a non-significant trend toward reduced TH loss in the L-DOPA + ceftriaxone group compared with the L-DOPA alone group. This lack of effect by ceftriaxone on striatal TH loss, when beginning 7 days after lesion induction, contrasts our previous observation that ceftriaxone protects against striatal TH protein loss if initiated at the time of 6-OHDA lesion induction11. This difference in striatal TH loss being dependent upon the timing of ceftriaxone initiation indicates that excess extracellular glutamate may contribute to striatal TH loss. However, others have reported a possible restorative effect on nigrostriatal function by ceftriaxone. Ceftriaxone alone (no L-DOPA) reduced AIMs in 6-OHDA lesioned rats long after its cessation and for a time period nearly double (69 days) that in our study79. It is possible that DA function altered by ceftriaxone outside of the striatum could be associated with such locomotor effects80.
In conclusion, the ability of ceftriaxone-induced increased GLT-1 expression to persist under long-term L-DOPA treatment and alleviate LID in a rodent PD model presents evidence that increased glutamate uptake could be a viable therapeutic target for reducing LID development or severity. Moreover, the reduction of dyskinesia was not apparently associated with loss of anti-parkinsonian effects of L-DOPA. This work represents a stepping stone for further evaluation of ceftriaxone as a viable, inexpensive and available and antidyskinetic compound, as it has been proven to be safe as a repurposed drug in other neurodegenerative diseases81.
Acknowledgments
Funding sources: in part by NIH grant AG040261
We thank Victoria Fields, Lauren Leon, Jessica Chumsky, Carolyn Sato, Anne Taylor, and Sharon Bossert for their technical support in this project. This project was funded in part by NIH grant award AG040261 to MFS.
Footnotes
Financial disclosure/Conflict of interest: nothing to disclose
- Research project: Conception, TC, MFS. Organization, TC, MFS, SM. Execution, TC, EK, TM, SM, MAC, MFS.
- Statistical Analysis: TC, MFS, CRB.
- Manuscript: Writing of first draft, TC. Review & Critique, MFS, CRB, TC.
References
- 1.Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: Substantia nigra regional selectivity. Brain. 1991;114:2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- 2.Bezard E, Dovero S, Prunier C, et al. Relationship between the appearance of symptoms and the level of nigrostriatal degeneration in a progressive 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned Macaque model of Parkinson’s disease. J Neurosci. 2001;21:6853–6861. doi: 10.1523/JNEUROSCI.21-17-06853.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kordower JH, Olanow CW, Dodiya HB, et al. Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain. 2013;136:2419–2431. doi: 10.1093/brain/awt192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindefors N, Ungerstedt U. Bilateral regulation of glutamate tissue and extracellular levels in caudate–putamen by midbrain dopamine neurons. Neurosci Lett. 1990;115:248–252. doi: 10.1016/0304-3940(90)90463-j. [DOI] [PubMed] [Google Scholar]
- 5.Meshul CK, Emre N, Nakamura CM, Allen C, Donohue MK, Buckman JF. Time-dependent changes in striatal glutamate synapses following a 6-hydroxydopamine lesion. Neuroscience. 1999;88:1–16. doi: 10.1016/s0306-4522(98)00189-4. [DOI] [PubMed] [Google Scholar]
- 6.Pflibsen L, Stang KA, Sconce MD, Wilson VB, Hood RL, Meshul CK, Mitchell SH. Executive function deficits and glutamatergic protein alterations in a progressive 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. J Neurosci Res. 2015;93:1849–1864. doi: 10.1002/jnr.23638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dervan AG, Meshul CK, Beales M, et al. Astroglial plasticity and glutamate function in a chronic mouse model of Parkinson’s disease. Exp Neurol. 2004;190:145–156. doi: 10.1016/j.expneurol.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Hazell AS, Itzhak Y, Liu H, Norenberg MD. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) decreases glutamate up-take in cultured astrocytes. J Neurochem. 1997;68:2216–2219. doi: 10.1046/j.1471-4159.1997.68052216.x. [DOI] [PubMed] [Google Scholar]
- 9.Izumi Y, Yamamoto N, Matsuo T, et al. Vulnerability to glutamate toxicity of dopaminergic neurons is dependent on endogenous dopamine and MAPK activation. J Neurochem. 2009;110:745–755. doi: 10.1111/j.1471-4159.2009.06178.x. [DOI] [PubMed] [Google Scholar]
- 10.Bianchi L, Galeffi F, Bolam JP, Della Corte L. The effect of 6-hydroxydopamine lesions on the release of amino acids in the direct and indirect pathways of the basal ganglia: a dual microdialysis probe analysis. Eur J Neurosci. 2003;18:856–868. doi: 10.1046/j.1460-9568.2003.02795.x. [DOI] [PubMed] [Google Scholar]
- 11.Chotibut T, Davis RW, Arnold JC, et al. Ceftriaxone increases glutamate uptake and reduces striatal tyrosine hydroxylase loss in 6-OHDA Parkinson’s model. Mol Neurobiol. 2014;49:1282–1292. doi: 10.1007/s12035-013-8598-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung EK, Chen LW, Chan YS, Yung KK. Downregulation of glial glutamate transporters after dopamine denervation in the striatum of 6-hydroxydopamine-lesioned rats. J Comp Neurol. 2008;511:421–437. doi: 10.1002/cne.21852. [DOI] [PubMed] [Google Scholar]
- 13.Massie A, Goursaud S, Schallier A, Vermoesen K, Meshul CK, Hermans E, Michotte Y. (2010) Time-dependent changes in GLT-1 functioning in striatum of hemi-Parkinson rats. Neurochem Int. 2010;57:572–578. doi: 10.1016/j.neuint.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Takahasi K, Foster JB, Lin CG. Glutamate transporter EAAT2: regulation, function, and potential as a therapeutic target for neurological and psychiatric disease. Cell Mol Life Sci. 2015 Jun 2; doi: 10.1007/s00018-015-1937-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rothstein JD, Dykes-Hoberg M, Pardo CA, et al. Knockout of Glutamate Transporters Reveals a Major Role for Astroglial Transport in Excitotoxicity and Clearance of Glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 16.Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med. 1994;330:613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- 17.Wang GJ, Chung HJ, Schnuer J, et al. Dihydrokainate-sensitive neuronal glutamate transport is required for protection of rat cortical neurons in culture against synaptically released glutamate. Eur J Neurosci. 1998;10:2523–2531. doi: 10.1046/j.1460-9568.1998.00256.x. [DOI] [PubMed] [Google Scholar]
- 18.Meldrum B, Garthwaite J. Excitatory amino acid neurotoxicity and neurodegenerative disease. Trends Pharmacol Sci. 1990;11:379–387. doi: 10.1016/0165-6147(90)90184-a. [DOI] [PubMed] [Google Scholar]
- 19.Chase TN, Bibbiani F, Oh JD. Striatal glutamatergic mechanism and extrapyramidal movement disorders. Neurotox Res. 2003;5:139–46. doi: 10.1007/BF03033378. [DOI] [PubMed] [Google Scholar]
- 20.Lipton SA, Nicotera P. Calcium, free radicals and excitotoxins in neuronal apoptosis. Cell Calcium. 1998;23:165–71. doi: 10.1016/s0143-4160(98)90115-4. [DOI] [PubMed] [Google Scholar]
- 21.Salvatore MF. ser31 tyrosine hydroxylase phosphorylation parallels differences in dopamine recovery in nigrostriatal pathway following 6-OHDA lesion. J Neurochem. 2014;129:548–558. doi: 10.1111/jnc.12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salvatore MF, Davis RW, Arnold JC, Chotibut T. Transient striatal GLT-1 blockade increases EAAC1 expression, glutamate reuptake, and decreases tyrosine hydroxylase phosphorylation at ser19. Exp Neur. 2012;234:428–436. doi: 10.1016/j.expneurol.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord. 2001;16:448–458. doi: 10.1002/mds.1090. [DOI] [PubMed] [Google Scholar]
- 24.Grandas F, Galiano ML, Tabernero C. Risk factors for levodopa-induced dyskinesias in Parkinson’s disease. J Neurol. 1999;246:1127–1133. doi: 10.1007/s004150050530. [DOI] [PubMed] [Google Scholar]
- 25.Stocchi F, Tagliati M, Olanow CW. Treatment of levodopa-induced motor complications. Mov Disord. 2008;23(Suppl 3):S599–eS612. doi: 10.1002/mds.22052. [DOI] [PubMed] [Google Scholar]
- 26.Picconi B, Centonze D, Håkansson K, et al. Loss of bidirectional striatal synaptic plasticity in L-DOPA-induced dyskinesia. Nat Neurosci. 2003;6:501–506. doi: 10.1038/nn1040. [DOI] [PubMed] [Google Scholar]
- 27.Santini E, Valjent E, Usiello A, et al. Critical involvement of cAMP/DARPP-32 and extracellular signal-regulated protein kinase signaling in L-DOPA-induced dyskinesia. J Neurosci. 2007;27:6995–7005. doi: 10.1523/JNEUROSCI.0852-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Westin JE, Vercammen L, Strome EM, Konradi C, Cenci MA. Spatiotemporal pattern of striatal ERK1/2 phosphorylation in a rat model of L-DOPA-induced dyskinesia and the role of dopamine D1 receptors. Biol Psychiatry. 2007;62:800–810. doi: 10.1016/j.biopsych.2006.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen W, Plotkin JL, Francardo V, et al. M4 muscarinic receptor signaling ameliorates striatal plasticity deficits in models of L-DOPA-induced dyskinesia. Neuron. 2015;88:762–773. doi: 10.1016/j.neuron.2015.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chotibut T, Fields VF, Salvatore MF. Norepinephrine transporter inhibition with desipramine exacerbates L-DOPA–induced dyskinesia: Role for synaptic dopamine regulation in denervated nigrostriatal terminals. Mol Pharmacol. 2014;86:675–685. doi: 10.1124/mol.114.093302. [DOI] [PubMed] [Google Scholar]
- 31.Cenci MA, Lindgren HS. Advances in understanding L-DOPA-induced dyskinesia. Curr Op Neurobiol. 2007;17:665–671. doi: 10.1016/j.conb.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Cenci MA. Presynaptic mechanisms of L-DOPA-induced dyskinesia: the findings, the debate, and the therapeutic implications. Front Neurology. 2014;5:242. doi: 10.3389/fneur.2014.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robelet S, Melon C, Guillet B, Salin P, Kerkerian-Le Goff L. Chronic L-DOPA treatment increases extracellular glutamate levels and GLT1 expression in the basal ganglia in a rat model of Parkinson’s disease. Eur J Neurosci. 2004;20:1255–1266. doi: 10.1111/j.1460-9568.2004.03591.x. [DOI] [PubMed] [Google Scholar]
- 34.Cenci MA. Glutamatergic pathways as a target for the treatment of dyskinesia in Parkinson’s disease. Biochem Soc Trans. 2014;42:600–604. doi: 10.1042/BST20140006. [DOI] [PubMed] [Google Scholar]
- 35.Rascol O, Perez-Lloret S, Ferreira JJ. New treatments for levodopa-induced motor complications. Mov Disord. 2015;30:1451–1460. doi: 10.1002/mds.26362. [DOI] [PubMed] [Google Scholar]
- 36.Konitsiotis S, Blanchet PJ, Verhagen L, Lamers E, Chase TN. AMPA receptor blockade improves levodopa-induced dyskinesia in MPTP monkeys. Neurology. 2000;54:1589–1595. doi: 10.1212/wnl.54.8.1589. [DOI] [PubMed] [Google Scholar]
- 37.Kobylecki C, Crossman AR, Ravenscroft P. Alternative splicing of AMPA receptor subunits in the 6-OHDA-lesioned rat model of Parkinson’s disease and L-DOPA-induced dyskinesia. Exp Neurol. 2013;247:476–84. doi: 10.1016/j.expneurol.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 38.Kobylecki C, Cenci MA, Crossman AR, Ravenscroft P. Calcium-permeable AMPA receptors are involved in the induction and expression of l-DOPA-induced dyskinesia in Parkinson’s disease. J Neurochem. 2010;114:499–511. doi: 10.1111/j.1471-4159.2010.06776.x. [DOI] [PubMed] [Google Scholar]
- 39.Hadj Tahar A, Gregoire L, Darre A, Belanger N, Meltzer L, Bedard PJ. Effect of a selective glutamate antagonist on L-dopa-induced dyskinesias in drug-naive parkinsonian monkeys. Neurobiol Dis. 2004;15:171–176. doi: 10.1016/j.nbd.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 40.Jonkers N, Sarre S, Ebinger G, Michotte Y. MK801 suppresses the L-DOPA-induced increase of glutamate in striatum of hemi-Parkinson rats. Brain Res. 2002;926:149–155. doi: 10.1016/s0006-8993(01)03147-x. [DOI] [PubMed] [Google Scholar]
- 41.Verhagen Metman L, Del Dotto P, van den Munckhof P, Fang J, Mouradian MM, Chase TN. Amantadine as treatment for dyskinesias and motor fluctuations in Parkinson’s disease. Neurology. 1998;50:1323–1326. doi: 10.1212/wnl.50.5.1323. [DOI] [PubMed] [Google Scholar]
- 42.Wolf E, Seppi K, Katzenschlager R, et al. Long-term antidyskinetic efficacy of amanditine in Parkinson’s disease. Mov Disord. 2010;25:1357–1363. doi: 10.1002/mds.23034. [DOI] [PubMed] [Google Scholar]
- 43.Ory-Magne F, Corvol JC, Azulay JP, et al. Withdrawing amantadine in dsykinetic patients with Parkinson’s disease: the AMANDYSK trial. Neurology. 2014;82:300–307. doi: 10.1212/WNL.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 44.Thomas A, Iacono D, Luciano AL, Armellino K, Di Iorio A, Onofrj M. Duration of Amantadine benefit on dyskinesia of severe Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2004;75:141–143. [PMC free article] [PubMed] [Google Scholar]
- 45.Hallet PJ, Standaert DG. Rationale for and use of NMDA receptor antagonists in Parkinson’s disease. Pharmacol Ther. 2004;102:155–174. doi: 10.1016/j.pharmthera.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 46.Nutt JG, Gunzler SA, Kirchhoff T, et al. Effects of NR2B selective NMDA glutamate Antagonist, CP-101,606, on dyskinesia and Parkinsonism. Mov Disord. 2008;23:1860–1866. doi: 10.1002/mds.22169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iderberg H, Rylander D, Bimpisidis Z, Cenci MA. Modulating mGluR5 and 5-HT1A/1B receptors to treat L-DOPA-induced dyskinesia: Effects of combined treatment and possible mechanisms of action. Exp Neurol. 2013;250:116–124. doi: 10.1016/j.expneurol.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 48.Iderberg H, Maslava N, Thompson AD, et al. Pharmacological stimulation of metabotropic glutamate receptor type 4 in a rat model of Parkinson’s disease and L-DOPA-induced dyskinesia: Comparison between a positive allosteric modulator and an orthosteric agonist. Neuropharmacology. 2015;95:121–129. doi: 10.1016/j.neuropharm.2015.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dupre KB, Ostock CY, Jaunarajs KLE, Botton T, Savage LM, Wolf W, Bishop C. Local modulation of striatal glutamate efflux by serotonin 1A receptor stimulation in dsykinetic, hemiparkinsonian rats. Exp Neurol. 2011;229:288–299. doi: 10.1016/j.expneurol.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paolone G, Brugnoli A, Arcuri L, Mercatelli D, Morari M. Eltoprazine prevents dyskinesias by reducing striatal glutamate and direct pathway neuron activity. Movt Disord. 2015;30:1890–1902. doi: 10.1002/mds.26326. [DOI] [PubMed] [Google Scholar]
- 51.Carta M, Cenci MA. On the effect of eltoprazine in dyskinetic hemiparkinsoniam rats. Mov Disord. 2016;31:149. doi: 10.1002/mds.26519. [DOI] [PubMed] [Google Scholar]
- 52.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 53.Rothstein JD, Patel S, Regan MR, et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 54.Sari Y, Prieto A, Barton SJ, et al. Ceftriaxone-induced up-regulation of cortical and striatal GLT1 in the R6/2 model of Huntington’s disease. J Biomed Sci. 2010;17:62. doi: 10.1186/1423-0127-17-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller BR, Dorner JL, Shou M, et al. Up-regulation of GLT1 expression increases glutamate uptake and attenuates the Huntington’s disease phenotype in the R6/2 mouse. Neuroscience. 2008:329–337. doi: 10.1016/j.neuroscience.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leung TC, Lui CN, Chen LW, et al. Ceftriaxone ameliorates motor deficits and protects dopaminergic neurons in 6-hydroxydopamine-lesioned rats. ACS Chem Neurosci. 2012;3:22–30. doi: 10.1021/cn200072h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hsu CY, Hung CS, Chang HM, Liao WC, Ho SC, Ho YJ. Ceftraixone prevents and reverses behavioral and neuronal deficits in an MPTP-induced animal model of Parkinson’s disease dementia. Neuropharmacol. 2015;91:43–56. doi: 10.1016/j.neuropharm.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 58.Bisht R, Kaur B, Gupta H, Prakash A. Ceftriaxone mediated rescue of nigral oxidative damage and motor deficits in MPTP model of Parkinson’s disease in rats. Neurotox. 2014;44:71–79. doi: 10.1016/j.neuro.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 59.Lundblad M, Andersson M, Winkler C, Kirik D, Wierup N, Cenci MA. Pharmacological validation of behavioural measures of akinesia and dyskinesia in a rat model of Parkinson’s disease. Eur J Neurosci. 2002;15:120–32. doi: 10.1046/j.0953-816x.2001.01843.x. [DOI] [PubMed] [Google Scholar]
- 60.Cenci MA, Lundblad M. Ratings of L-DOPA-Induced Dyskinesia in the Unilateral 6-OHDA Lesion Model of Parkinson’s Disease in Rats and Mice. Curr Prot Neuro. 2007;41:9.25.1–9.25.23. doi: 10.1002/0471142301.ns0925s41. [DOI] [PubMed] [Google Scholar]
- 61.Chotibut T, Apple DM, Jefferis R, Salvatore MF. Dopamine transport loss in 6-OHDA Parkinson’s model is unmet by parallel reduction in dopamine uptake. PLoS ONE. 2012;7:e52322. doi: 10.1371/journal.pone.0052322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2. Academic; Sydney: 1986. [DOI] [PubMed] [Google Scholar]
- 63.Dekundy A, Lundblad M, Danysz W, Cenci MA. Modulation of L-DOPA-induced abnormal involuntary movements by clinically tested compounds: further validation of the rat dyskinesia model. Behav Brain Res. 2007;179:76–89. doi: 10.1016/j.bbr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 64.Conti MM, Meadows SM, Melikhov-Sosin M, Lindenbach D, Hallmark J, Werner DF, Bishop C. Monoamine transporter contributions to L-DOPA effects in hemi-parkinsonian rats. Neuropharmacol. 2016;110:125–134. doi: 10.1016/j.neuropharm.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 65.Meadows SM, Chambers NE, Conti MM, Bossert SC, Tasber C, Sheena E, Varney M, Newman-Tancredi A, Bishop C. Characterizing the differential roles of striatal 5-HT1A auto- and heteroreceptors in the reduction of L-DOPA-induced dyskinesia. Exp Neurol. 2017;292:168–178. doi: 10.1016/j.expneurol.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 66.Hudson JL, Van Horne CG, Stromberg I, et al. Correlation of apomorphine- and amphetamine-induced turning with nigrostriatal dopamine content in unilateral 6-hydroxydopamine lesioned rats. Brain Res. 1993;626:167–174. doi: 10.1016/0006-8993(93)90576-9. [DOI] [PubMed] [Google Scholar]
- 67.Henn FA, Anderson DJ, Rustad DG. Glial contamination of synaptosomal fractions. Brain Res. 1976;101:341–344. doi: 10.1016/0006-8993(76)90274-2. [DOI] [PubMed] [Google Scholar]
- 68.Nickell J, Salvatore MF, Pomerleau F, Apparsundaram S, Gerhardt GA. Reduced plasma membrane surface expression of GLAST mediates decreased glutamate regulation in the aged striatum. Neurobiol Aging. 2007;28:1737–1748. doi: 10.1016/j.neurobiolaging.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 69.Wassum KM, Tolosa VM, Wang J, Walker E, Monbouquette HG, Maidment NT. Silicon wafer-based platinum microelectrode array biosensor for near real-time measurement of glutamate in vivo. Sens Basel Sens. 2008;8:5023–5036. doi: 10.3390/s8085023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pilleri M, Antonini A. Therapeutic strategies to prevent and manage dyskinesias in Parkinson’s disease. Expert Opin Drug Saf. 2015;14:281–294. doi: 10.1517/14740338.2015.988137. [DOI] [PubMed] [Google Scholar]
- 71.Petr GT, Sun Y, Frederick NM, et al. Conditional deletion of the glutamate transporter GLT-1 reveals that astrocytic GLT-1 protects against fatal epilepsy while neuronal GLT-1 contributes significantly to glutamate uptake into synaptosomes. J Neurosci. 2015;35:5187–201. doi: 10.1523/JNEUROSCI.4255-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Y, Zhang X, Qu S. Ceftriaxone protects astrocytes from MPP+ via suppression of NF-κB/JNK/c-Jun signaling. Mol Neurobiol. 2015;52:78–92. doi: 10.1007/s12035-014-8845-z. [DOI] [PubMed] [Google Scholar]
- 73.Holmer HK, Keyghobadi M, Moore C, Meshul CK. l-dopa-induced reversal in striatal glutamate following partial depletion of nigrostriatal dopamine with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neuroscience. 2005;136:333–74. doi: 10.1016/j.neuroscience.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Corsi C, Pinna A, Gianfriddo M, Melani A, Morelli M, Pedata F. Adenosine A2A receptor antagonism increases striatal glutamate outflow in dopamine denervated rats. Eur J Pharmacol. 2003;464:33–38. doi: 10.1016/s0014-2999(03)01352-9. [DOI] [PubMed] [Google Scholar]
- 75.Walker RH, Koch RJ, Sweeney JE, Moore C, Meshul CK. Effects of subthalamic nucleus lesions and stimulation upon glutamate levels in the dopamine-depleted rat striatum. NeuroReport. 2009;20:770–775. doi: 10.1097/WNR.0b013e32832ad556. [DOI] [PubMed] [Google Scholar]
- 76.Lievens JC, Salin P, Nieoullon A, Kerkerian-Le Goff L. Nigrostriatal denervation does not affect glutamate transporter mRNA expression but subsequent levodopa treatment selectively increases GLT1 mRNA and protein expression in the rat striatum. J Neurochem. 2001;79:893–902. doi: 10.1046/j.1471-4159.2001.00644.x. [DOI] [PubMed] [Google Scholar]
- 77.St-Hilaire M, Landry E, Levesque D, Rouillard C. Denervation and repeated L-DOPA induce complex regulatory changes in neurochemical phenotypes of striatal neurons: implication of a dopamine D1-dependent mechanism. Neurobiol Dis. 2005;20:450–460. doi: 10.1016/j.nbd.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 78.Castle M, Aymerich MS, Sanchez-Escobar C, Gonzalo N, Obeso JA, Lanciego JL. Thalamic innervation of the direct and indirect basal ganglia pathways in the rat: ipsi- and contralateral projections. J Comp Neurol. 2005;483:143–153. doi: 10.1002/cne.20421. [DOI] [PubMed] [Google Scholar]
- 79.Kelsey JE, Neville C. The effects of the β-lactam antibiotic, ceftriaxone, on forepaw stepping and L-DOPA-induced dyskinesia in a rodent model of Parkinson’s disease. Psychopharmacology. 2014;231:2405–2415. doi: 10.1007/s00213-013-3400-6. [DOI] [PubMed] [Google Scholar]
- 80.Pruett BS, Salvatore MF. Nigral GFRα1 infusion in aged rats increases locomotor activity, nigral tyrosine hydroxylase, and dopamine content in synchronicity. Mol Neurobiol. 2013;49:548–558. doi: 10.1007/s12035-013-8397-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Berry JD, Shefner JM, Conwit R, et al. Design and Initial Results of a Multi-Phase Randomized Trial of Ceftriaxone in Amyotrophic Lateral Sclerosis. PLoS ONE. 2013;8(4):e61177. doi: 10.1371/journal.pone.0061177. [DOI] [PMC free article] [PubMed] [Google Scholar]