Abstract
Objective
Cochlear Implants (CIs) have a limited number of independent stimulation channels due to the highly conductive nature of the fluid-filled cochlea. Attempts to develop highly focused stimulation to improve speech perception in CI users includes the use of simultaneous stimulation via multiple current sources. Focused multipolar (FMP) stimulation is an example of this approach and has been shown to reduce interaction between stimulating channels. However, compared with conventional biphasic current pulses generated from a single current source, FMP is a complex stimulus that includes extended periods of stimulation before charge recovery is achieved, raising questions on whether chronic stimulation with this strategy is safe. The present study evaluated the long-term safety of intracochlear stimulation using FMP in a preclinical animal model of profound deafness.
Approach
Six cats were bilaterally implanted with scala tympani electrode arrays two months after deafening, and received continuous unilateral FMP stimulation at levels that evoked a behavioural response for periods of up to 182 days. Electrode impedance, electrically-evoked compound action potentials (ECAPs) and auditory brainstem responses (EABRs) were monitored periodically over the course of the stimulation program from both the stimulated and contralateral control cochleae. On completion of the stimulation program cochleae were examined histologically and the electrode arrays were evaluated for evidence of platinum (Pt) corrosion.
Main results
There was no significant difference in electrode impedance between control and chronically stimulated electrodes following long-term FMP stimulation. Moreover, there was no significant difference between ECAP and EABR thresholds evoked from control or stimulated cochleae at either the onset of stimulation or at completion of the stimulation program. Chronic FMP stimulation had no effect on spiral ganglion neuron (SGN) survival when compared with unstimulated control cochleae. Long-term implantation typically evoked a mild foreign body reaction proximal to the electrode array; however stimulated cochleae exhibited a small but statistically significant increase in the tissue response. Finally, there was no evidence of Pt corrosion following long-term FMP stimulation; stimulated electrodes exhibited the same surface features as the unstimulated control electrodes.
Significance
Chronic intracochlear FMP stimulation at levels used in the present study did not adversely affect electrically-evoked neural thresholds or SGN survival but evoked a small, benign increase in inflammatory response compared to control ears. Moreover chronic FMP stimulation does not affect the surface of Pt electrodes at suprathreshold stimulus levels. These findings support the safe clinical application of an FMP stimulation strategy.
2. Introduction
Cochlear Implants (CIs) have a limited number of independent stimulation channels due to the highly conductive nature of the fluid-filled cochlea (Boex et al., 2003, Bierer, 2007, Black et al., 1981). A single neural population can be activated by stimuli from several stimulation channels, leading to these stimuli being perceptually indistinguishable or confused. Furthermore, attempts to stimulate several cochlear electrodes simultaneously can produce unpredictable interactions resulting in unpleasant auditory percepts (Hartmann and Klinke, 1990, Shannon, 1983, Stickney et al., 2006). As a result, contemporary CI’s typically stimulate one monopolar electrode at a time in a sequential manner via a single current source (McDermott et al., 1992, Wilson et al., 1991). Although the use of sequential stimulation overcomes a number of the adverse effects of these interactions, such stimulation provides a relatively crude representation of the dynamic spatiotemporal properties of human speech. Therefore, while CI subjects typically receive significant benefit in speech understanding in quiet, it comes as no surprise that performance drops severely in noise and the rich aural texture of music or tonal languages are not conveyed effectively (Sucher and McDermott, 2007, Fu et al., 1998).
Spatially restricting the spread of intracochlear electrical stimulation using current focusing techniques aims to maximize the number of channels that activate independent neural populations. Spatially restricted activation allows stimulation of multiple sites along the cochlea without producing the adverse effects associated with interactions. It is anticipated that this approach will produce a more natural sound perception for CI subjects by preserving the fine temporal structure of speech across several independent channels.
Two broad approaches have been used in an attempt to develop focused stimulation channels in CIs. First, a single current source has been used in combination with electrode geometries designed to reduce the extent of the current field compared with monopolar stimulation, including bipolar; tripolar and common ground electrode configurations (Bierer, 2007, Litvak et al., 2007, Miyoshi et al., 1999, Jolly et al., 1996, Clark et al., 1983, Kral et al., 1998). The second approach requires the simultaneous use of multiple independently controllable current sources to drive the vector addition of several current fields to actively restrict the field of excitation (Townshend and White, 1987, Rodenhiser and Spelman, 1995, van den Honert and Kelsall, 2007).
Focused multipolar (FMP) stimulation, first described by (van den Honert and Kelsall, 2007), is an example of this second approach. Current-focusing using FMP stimulation has been shown to be effective at restricting the field of excitation in modelling (Frijns et al., 2011), acute preclinical studies (George et al., 2015a, George et al., 2014) and initial clinical trials (Long et al., 2014, Marozeau et al., 2015). Although shown to be a promising stimulation strategy, there has been no evaluation of the underlying long-term safety issues associated with this approach.
As a result of producing a more restricted field of excitation, FMP stimulation enables the use of more complex stimuli than that from conventional sequential biphasic stimulation generated from a single current source. The FMP current waveform can: (i) be continuous and without discrete gaps in time when shorting of electrodes would normally occur; (ii) deliver a different distribution of applied charge (although the maximum charge is still limited in the same way as conventional strategies); and (iii) have significantly longer (up to several milliseconds) time before reversal of the applied charge, which occurs at the completion of each biphasic current pulse during conventional stimulation.
Charge recovery is used in all neural stimulators to ensure levels of direct current (DC) are kept below specified safe maximum (CEN, 2010). This is achieved via electrode shorting and/or capacitive coupling (Patrick et al., 1990). Electrodes can be shorted together at a time when no current is to be delivered; ensuring that any net charge delivered to the electrodes during the previous stimulation pulse is recovered. Alternatively, capacitive coupling recovers any delivered net charge by slowly allowing it to dissipate from an electrode via a bleed resistor. Because FMP channels can be applied asynchronously the current through any given electrode varies continuously. Shorting as a means of charge recovery therefore can only occur with FMP stimulation if periodic times are allowed when all electrodes have nominally delivered no net charge. At such times shorting can occur and can be used to augment the charge recovery obtained through capacitors and bleed resistors. Such a combination of capacitive coupling and periodic shorting was used for charge recovery in the experiments described here.
The aim of the present study was to use chronic in vivo electrical stimulation using a system based on a clinical stimulator to evaluate whether asynchronous FMP stimulation using Platinum (Pt) electrodes can be performed safely in an animal model of profound sensorineural hearing loss. The stimulus used was designed to represent a ‘worst case’ scenario in terms of charge recovery time and degree of focusing using FMP stimulation.
3. Materials and methods
This study was performed using a total of 6 cats. All procedures were conducted with approval from the Bionics Institute Animal Research and Ethics Committee, and were performed in accordance with the Australian Code of Practice for the Care and Use of Animals for scientific purposes and followed the principals of the US National Institutes of Health guidelines regarding the care and use of animals for experimental procedures.
Deafening protocol
Animals were deafened as neonates using daily subcutaneous injection of neomycin (neomycin trisulphate; 60mg/kg; subcutaneously [s.c.]) (Leake et al., 1999, Fallon et al., 2009). After 3 weeks of treatment each animal was anaesthetised with 3–4% isoflurane and 500 ml/min oxygen, and maintained at 1–2% isoflurane. Hearing status was examined by monitoring auditory brainstem responses (ABRs). ABRs were recorded differentially using subcutaneous stainless steel electrodes (vertex positive; neck negative and thorax ground) following procedures described previously (Coco et al., 2007). Briefly, 100 stimuli were presented at a rate of 25 per second and responses amplified by 103, filtered, averaged and displayed. If there was evidence of hearing in response to a 100 dB peak-equivalent Sound Pressure Level (p.e. SPL) acoustic click, the neomycin injections were continued until a complete loss of hearing occurred.
Cochlear implant surgery
Animals were bilaterally implanted at 8 weeks of age using aseptic surgical techniques. Each animal was pre-medicated (xylazine, 1mg/kg; s.c.; atropine sulphate, 0.05 ml/kg s.c.) and anaesthesia was maintained at a surgical level using a closed circuit anaesthetic machine (1.5–2.5% isoflurane and 1.5 l/min oxygen). The bulla cavity was opened, flushed with amoxicillin (10mg/ml) and the round window membrane incised. The electrode array (details below) was inserted into the scala tympani of the cochlea and the round window sealed with crushed muscle to prevent perilymph leakage. The leadwire was fixed at the bulla and on the skull, and then passed subcutaneously to exit the body through an incision over the scapula. During surgery the animal’s respiratory rate, expired CO2 and body temperature were monitored and maintained within normal levels (respiration rate 10–20; expired CO2 3–5%; body temperature 37 + 1 °C). Analgesic (Carprofen 4mg/kg s.c.), systemic antibiotic (clavulox 1 ml/20kg; s.c.) and compound sodium lactate fluids (5 ml/kg/hour; s.c.) were administered during or shortly after surgery.
Scala tympani electrode array
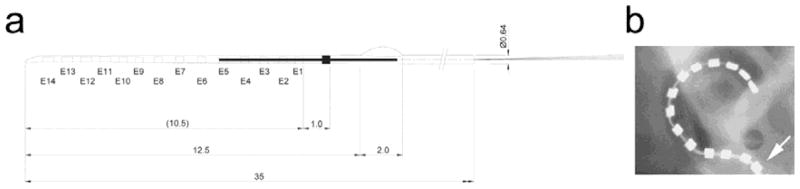
The Hybrid-L 14 electrode array (HL14; Cochlear Ltd., Sydney, Australia) was used in this study. This array consists of 14 half-band platinum (Pt) electrodes (Fig. 1) with electrode 1 the most basal and electrode 14 the most apical. Electrode 14 was located approximately 10.5 mm from the round window, an electrode insertion depth of approximately 50% of the length of the cat scala tympani (Shepherd et al., 2011). Each Pt electrode was connected via a leadwire to a wearable stimulator (details below) located in a backpack (Fallon et al., 2009, Wise et al., 2011). One cochlea of each animal was chronically stimulated while the contralateral cochleae served as a deafened, implanted chronically unstimulated control. Finally, in addition to the intracochlear electrode array, each implant also contained two 1 mm diameter extracochlear Pt ball electrodes located in the temporalis muscle and served as a reference for evoked potential and electrode impedance recordings.
Figure 1.
(a) Schematic diagram of the Hybrid L-14 electrode array used in this study. The electrode array contains 14 Pt electrodes on a silicone carrier. The array tapered from 0.35 mm wide at electrode 14 to 0.5 mm at electrode 1. All dimensions are in mm. (b) Plane x-ray of a left cat cochlea implanted with a Hybrid L electrode array. This array can be inserted into the upper basal turn allowing 14 electrodes to be located within the cochlea. Electrode E1 (arrow) is located at the round window.
Electrode impedance
In order to check the status of the intracochlear electrodes, the impedance of each electrode was measured against the extracochlear return electrode each week throughout the chronic stimulation period using Custom Sound EP 2.0 software (Cochlear Ltd, Sydney, Australia). This impedance monitoring software controls the amplitude of the stimulus current (100 current level in this study (CL; where current in μA = 17.5 × (100(CL/255))) and records the peak voltage developed between the cochlear electrode and an extracochlear reference electrode. In the present study the current pulse was 25 μs/phase.
Electrically-evoked potentials
Electrically-evoked auditory nerve compound action potentials (ECAPs) were recorded in awake animals on a weekly basis using Neural Response Telemetry (NRT) and Custom Sound EP 2.0 software. ECAP recordings were obtained from both the chronically stimulated and control electrode arrays using 25μs/phase biphasic pulses at 80 pulses per second in a monopolar electrode configuration. Recordings were made at 10 CL (21 μA) intensity intervals from below threshold to a maximum of 250 CL (1,600 μA) or the highest level that did not produce an aversive response. ECAP thresholds and growth functions were examined longitudinally for evidence of changes in neural excitability during the course of chronic stimulation and compared statistically with responses recorded from the contralateral implanted/deafened control cochlea (Irving et al., 2014). ECAP threshold was defined as the lowest current amplitude required to elicit a clear ECAP with an amplitude of at least 20 μV.
Electrically-evoked auditory brainstem recordings (EABRs) were recorded monthly with animals anaesthetised using isoflurane as described above. Biphasic current pulses of 25 μs duration were generated, and delivered to the electrode of interest using a monopolar electrode configuration. EABRs were recorded differentially in the same way as ABRs (Coco et al., 2007). Threshold was determined visually to be the lowest stimulus intensity required to elicit a clear EABR with amplitude of at least 0.2 μV.
Stimulator design and the chronic stimulation program
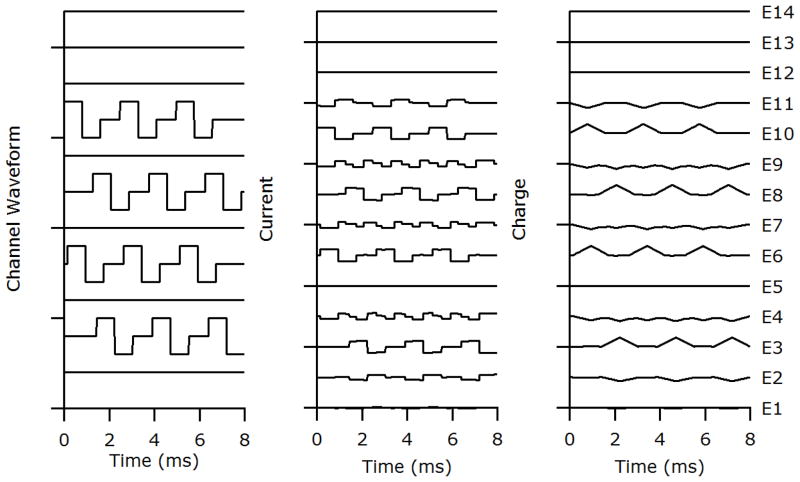
The battery powered stimulator used in this study contained 33 independent linear current sources, two for each intracochlear and extracochlear electrode and one global current source. FMP stimulation, used in the present study, produced quite complex current waveforms when viewed on an individual electrode (Fig. 2; middle panel). Stimulation was organised into channels where each channel stimulated a single, highly focused, sector of auditory neurons. Each channel achieved focusing by delivering current to a number of electrodes simultaneously. The relative magnitudes of the currents in individual electrodes are known as the channel’s ‘weight vectors’. Because stimulation is highly focused a number of channels can be delivered simultaneously with minimal channel interactions (George et al., 2015a).
Figure 2.
The stimulus used was designed to represent a ‘worst case’ scenario in terms of charge recovery time and degree of focusing using FMP stimulation. The left panel represents the stimulus waveforms to be delivered to specified channels and is comprised of 4 biphasic pulse trains on non-adjacent FMP channels. Note that these waveforms represent the positions of focused channels that will be delivered to the neural elements, not the current delivered to each electrode. The middle panel represents the currents to be delivered to specified electrodes. The currents are calculated based on the sum of all the channel waveforms (left panel) transformed into appropriate electrode currents as described in the text. They are designed to achieve maximal focusing of each channel. The right panel represents the net charge delivered to each electrode. Unlike the very rapid charge recovery associated with a conventional biphasic current pulse delivered sequentially, complete charge recovery using FMP stimulation was not ensured until the end of the 8 ms stimulus frame when all electrodes were shorted for 100 μs. The 8 ms stimulus was then repeated in a continuous loop.
In this experiment four channels of stimulation were delivered to the stimulated cochlea simultaneously (Fig. 2; left panel). Each channel delivered biphasic pulses of 800 μs per phase duration with an 800 μs inter pulse gap, repeated indefinitely. The timing of the pulses in each channel was staggered so that the pulses overlapped but did not start or stop at the same time (Fig. 2, left panel). The resulting current and charge delivered to each electrode are shown in the centre and right panels of Fig 2 respectively. This stimulation sequence was chosen to maximise the time for certain electrodes to fully recover delivered charge. An 8 ms burst of pre-recorded stimulation was presented to the animals in a repeating loop.
For each FMP channel, the weight vector was constructed based on the strategy adapted from (van den Honert and Kelsall, 2007). Briefly, a trans-impedance matrix was measured for each animal using proprietary software. The trans-impedance matrix was then inverted and transformed with the voltage matrix defining the desired location of stimulus and degree of focusing for all channels. The result of the transform provided a set of weight vectors (i.e. relative currents) for all channels (George et al., 2015b).
Stimulus levels for each animal were initially set at 40 μA peak current and increased to a long-term maximum level tolerated by each animal (Table 1). The level of stimulation was confirmed to be suprathreshold for each animal using simple behavioural observation and included increased attention and head orientation to the stimulated side (Fallon et al., 2009). The stimulus levels used in the present study, measured as charge/phase (Table 1), were similar to the maximum stimulus levels we have previously used to evaluate the safety associated with stimulation using biphasic current pulse generated by a single current source delivered to bipolar electrodes (Shepherd et al., 1983) and nearly an order of magnitude greater than the typical levels we have used in similar studies using monopolar cochlear electrodes (Xu et al., 1997).
Table 1.
Description of subjects in study
| Animal ID | Hearing status | Duration of deafness (days) | Duration of stimulation (days) | Stimulated side | Stimulation Level (μA)1 (peak) | Charge/phase (nC/phase) |
|---|---|---|---|---|---|---|
| FMP1 | Profoundly deaf | 248 | 168 | Right | 140 | 115 |
| FMP2 | Profoundly deaf | 262 | 182 | Right | 180 | 144 |
| FMP3 | Profoundly deaf | 246 | 167 | Right | 180 | 144 |
| FMP4 | Profoundly deaf | 267 | 182 | Left | 180 | 144 |
| FMP5 | Profoundly deaf | 250 | 159 | Right | 180 | 144 |
| FMP6 | Profoundly deaf | 275 | 173 | Right | 180 | 144 |
Notes: Current on peak (highest weighted) electrode of the FMP channel. Current was gradually increased to this level within the first few weeks of the chronic stimulation program. In some cases the stimulus was increased beyond the level indicated in the Table but subsequently reduced because of an aversive response.
Given the continuous nature of the stimulus it was not possible to obtain an objective measure of evoked potential threshold using the FMP stimulus. All electrodes were capacitively coupled using a 0.1 μF series capacitor and had bleed resistors to ensure charge recovery. A 100 μs period of shorting occurred at the end of each 8 ms burst to recover any charge not fully recovered by the bleed resistors. Shorting was achieved by connecting all electrodes to a common line using programmable switches within the stimulator integrated circuit. The timing of current delivery and the shorting period is described in Figure 2.
One month following cochlear implant surgery chronic stimulation commenced (24 hours/day except for 1–2 hours weekly to change batteries) for a period that varied from 159–182 days (Table 1). Stimulation levels were confirmed to be suprathreshold and within the animal’s comfort range during the daily monitoring.
Histology
At the end of the experimental period, all animals were euthanized with sodium pentobarbitone and systemically perfused with heparinised normal saline at 37°C followed by 4% paraformaldehyde at 4°C. The electrode arrays were removed for examination under a scanning electron microscope (SEM; see below) and the cochleae postfixed for 1 h and washed three times in 0.1 M phosphate buffered saline. The cochleae were decalcified in 10% (wt/vol) ethylene diamine tetra-acetic acid for two weeks, cryoprotected with 15%, then 30% sucrose overnight, and snap frozen in Tissue-Tek compound. Cochlear tissue was cryosectioned in the mid-modiolar plane at a thickness of 12 μm and a representative series of the entire cochlea were stained with hematoxylin and eosin.
The extent of auditory nerve survival, measured as spiral ganglion neuron (SGN) density, foreign body response and new-bone formation were quantified and compared statistically with data from the contralateral control cochlea. SGNs were quantified in mid-modiolar sections using a Zeiss Axioplan microscope by a single observer blinded to the experimental cohorts. SGNs were identified within Rosenthal’s canal and counted within the lower basal, upper basal, lower middle, upper middle, and apical cochlear regions. Only SGNs exhibiting a clear nucleus and nucleoli were counted (Wise et al., 2011). The area of Rosenthal’s canal was measured using Image J software and the density of the SGNs was determined. SGN density data for each cochlear region were averaged from 5 sections that were spaced 76-μm apart, ensuring that no SGN was counted more than once.
The tissue response of the cochlea in response to cochlear implantation and chronic electrical stimulation was determined by measuring the percentage of the area of the scala tympani occupied by the foreign body response in both the chronically stimulated and implanted unstimulated control ears. Tissue response was quantified in hematoxylin and eosin-stained sections at three cochlear locations adjacent to the electrode array using a Zeiss Axioplan microscope, which was then analysed using Image J (Wise et al., 2011). The extent of the tissue response associated with implantation and chronic FMP stimulation was compared statistically with the implanted, unstimulated control cochlea.
Scanning Electron Microscopy
Following explantation, both the control and stimulated electrode arrays from each animal were examined for evidence of Pt corrosion using a FEI QUANTA 200 SEM. All 14 Pt electrodes on each electrode array were examined and photographed at low (X 600) and medium (X 2000) magnification. A region of each electrode surface was then randomly selected and photographed at higher magnification (× 4000 and ×10,000). The surface condition of each Pt electrode was evaluated by an investigator blinded to the experimental groups. Surface features, including mechanical damage, pitting corrosion, intergranular corrosion and surface deposits, were recorded. The severity of Pt corrosion was graded from 0 (no corrosion); 1 (no evidence of corrosion but electrode at least partially coated with organic material); 2 (localized minor corrosion); 3 (localized moderate corrosion); 4 (widespread corrosion); 5 (severe and extensive corrosion). This grading system is a slightly modified version of one we have previously developed (Shepherd et al., 1985, Shepherd and Clark, 1991).
Statistical analysis
Evoked potential and electrode impedance data were examined statistically by comparing the stimulated versus the unstimulated control cochleae using a 2 way analysis of variance (ANOVA; electrode and side). Statistical comparisons of SGN density and extent of tissue response were made by comparing the stimulated cochlea with the unstimulated control cochlea via repeated measures (RM) ANOVA using p<0.05 level of significance. Post hoc comparisons were made using the Holm Sidak method. Finally, statistical comparison of Pt electrode surfaces examined using SEM was performed using Kruskal-Wallis 1 way ANOVA (stimulated and control electrodes).
4. Results
Experiments to determine the safety of chronic intracochlear FMP stimulation included the evaluation of changes in electrode impedance, electrically-evoked neural thresholds, SGN survival, the extent of tissue response and the status of the Pt electrode surface when compared with the contralateral implanted, unstimulated control side. At the time of implant surgery all animals were profoundly deaf as evidenced by a lack of an ABR in response to an acoustic click at 100 dB SPL.
Electrode impedance
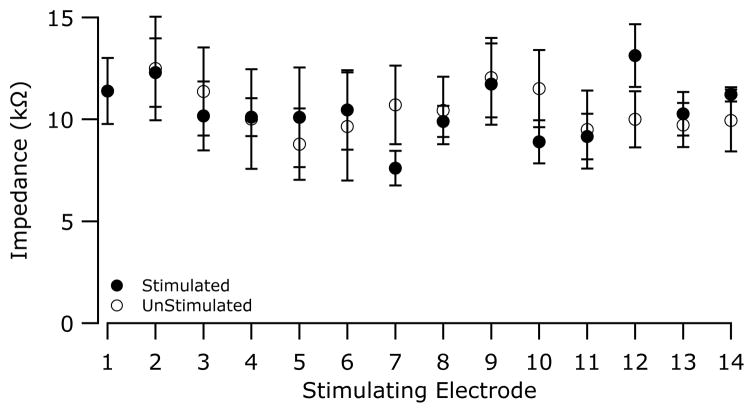
Figure 3 illustrates the mean electrode impedance of all stimulated electrodes and their contralateral unstimulated control electrodes at completion of the chronic stimulation program. While the stimulated electrodes were activated continuously for periods of between 159–182 days (Table 1), there was no statistically significant difference between the two cohorts (2-Way ANOVA, electrode (p = 0.57), side (p = 0.61), electrode × side (p= 0.898), n =6).
Figure 3.
Electrode impedance (mean + SEM) of both the chronically stimulated and control electrodes recorded at the completion of each animal’s stimulation program. The impedance of the chronically stimulated electrodes showed no significant difference to that of the controls (2-Way ANOVA, electrode (p > 0.55), side (p > 0.60), electrode × side (p = 0.898), n =6).
Electrically-evoked responses
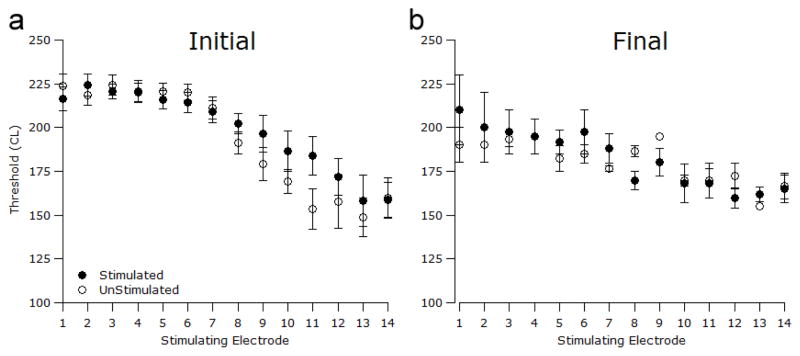
Representative ECAP waveforms recorded from a chronically stimulated cochlea at the outset and on completion of 182 days of FMP stimulation is illustrated in Fig. 4. These responses maintained typical ECAP morphology and exhibited little change in threshold or waveform morphology over the duration of the stimulation regime. Figure 5 illustrates the combined ECAP thresholds for all animals in this study recorded at the outset of stimulation and on completion of each animal’s stimulation program (Table 1). Both stimulated and control electrode arrays exhibited lower thresholds for more apically located electrodes, a common finding (Fallon et al., 2009), reflecting the tapered nature of the scala tympani. Importantly, there was no statistically significant difference in ECAP threshold between chronically stimulated and control cochleae at either time point (2-Way ANOVAs, electrode (p’s < 0.001), side (p’s > 0.1), n=6). Furthermore, there was no significant interaction between electrode and side (p’s > 0.1) indicating the chronically stimulated electrodes did not exhibit an increase in threshold.
Figure 4.
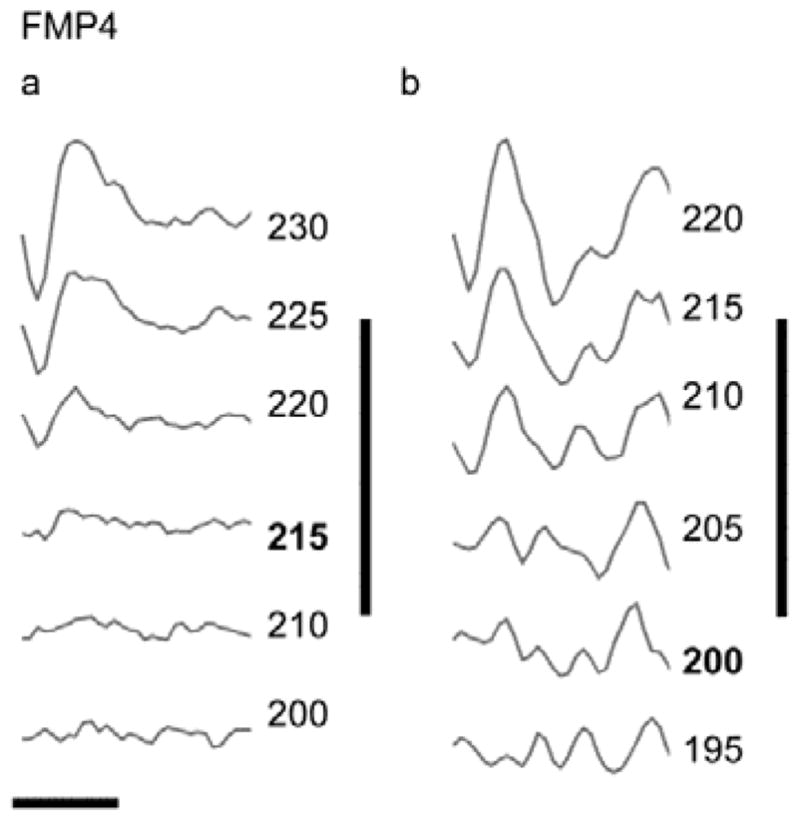
Representative ECAP waveforms recorded from the left (stimulated) cochlea of FMP4 at the (a) initial and (b) final recording following 182 days of electrical stimulation. Both set of responses were evoked by stimulation from electrode 6. Stimulus level is given in CL (bold = threshold). In this example there is a slight decrease (15 CLs) in threshold over the course of the stimulation program. Vertical bar = 150 μV; horizontal bar = 1 ms.
Figure 5.
Mean ECAP thresholds (+ SEM) for all electrodes recorded at (a) the outset (Initial) and (b) on completion (Final) of the chronic stimulation program. There was no significant difference between the sides at either time point (2-Way ANOVAs (Electrode (p < 0.001), Side (p > 0.1), n=6)).
Similar trends were observed in the EABR thresholds (data not shown). Again, there was no significant difference between EABR thresholds evoked from stimulated and control cochleae at either onset of stimulation or the final set of EABRs recorded at completion of the stimulation program (2-Way ANOVAs (Electrode (p < 0.001), Side (p > 0.1), n=6)).
Cochlear pathology
General cochlear pathology
Representative photomicrographs of both the stimulated and unstimulated control cochlea from two cases are illustrated in Fig. 6. Each panel includes the upper basal (UB), upper middle (UM) and apical (A) turns. These micrographs illustrate a number of important points associated with this study. First, all cochleae exhibited widespread loss of hair cells and the organ of Corti, consistent with a severe-profound hearing loss. Second, there was an extensive loss of SGNs particularly in the basal and middle turns of all animals in this cohort consistent with the long-term duration of deafness used in this study (246–275 days; Table 1). Finally, if present, any tissue response was restricted to the basal turn proximal to the electrode array.
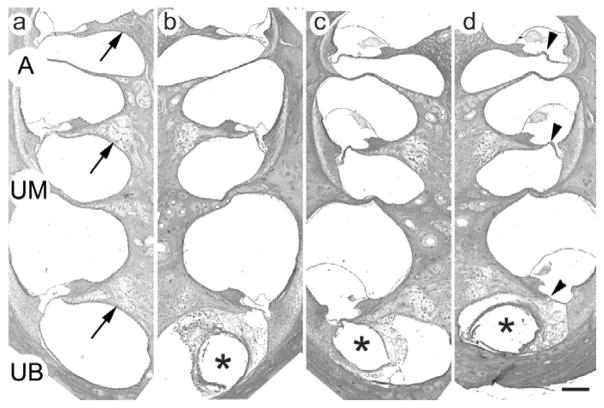
Figure 6.
Representative photomicrographs illustrating the upper basal (UB), upper middle (UM) and apical (A) turns of (a) the control and (b) the stimulated cochlea of animal FMP4. The cochleae in this example were implanted for a period of 210 days and the left cochlea (b) stimulated for 182 days. Tissue response to the chronically implanted electrode array is evident in the basal turn of the stimulated cochlea (* illustrates the location of the electrode array). There was no tissue response in the control cochlea (a). (c) and (d) are photomicrographs from the stimulated and control cochlea respectively of animal FMP6. The cochleae in this example were implanted for a period of 201 days and the right cochlea (c) stimulated for 173 days. Tissue response to the chronically implanted electrode array is evident in the basal turn of both the stimulated and control cochleae (* in (c) and (d)). All animals in this cohort exhibited widespread hair cell and organ of Corti loss (arrowhead; for clarity illustrated in (d) only). Extensive loss of SGNs (arrow; for clarity illustrated in (a) only) occurred in the basal and middle turns of both cohorts; near normal SGN survival was apparent in the apical turn. The Hybrid L14 electrode arrays were inserted into the upper basal turn, reflecting the tissue response in this region of all stimulated and some control cochleae. There was no evidence of a tissue response distal to the electrode array. Scale bar = 200 μm.
SGN survival
Figure 7 illustrates the SGNs within Rosenthal’s canal for the upper basal (UB), upper middle (UM) and apical (A) turns from each cochlea illustrated in Fig. 6. The extensive loss of SGNs in the upper basal and middle turns demonstrates the typical “u” shaped profile of SGN loss in animals deafened with this technique (Hardie and Shepherd, 1999). Given the highly focussed nature of FMP stimulation (George et al., 2015a), excitation of SGNs would be restricted to the basal turn proximal to the electrode array. There was no difference in SGN survival in the basal turn of these cochleae (Fig. 7b & c) compared to the same region in the contralateral control cochleae (Fig. 7a & d respectively), and this was confirmed quantitatively by measuring SGN density (Fig. 8). In contrast, both cohorts exhibited a highly significant effect of cochlear region with lowest SGN densities consistently observed in the upper basal and middle turns of both stimulated and control cochleae.
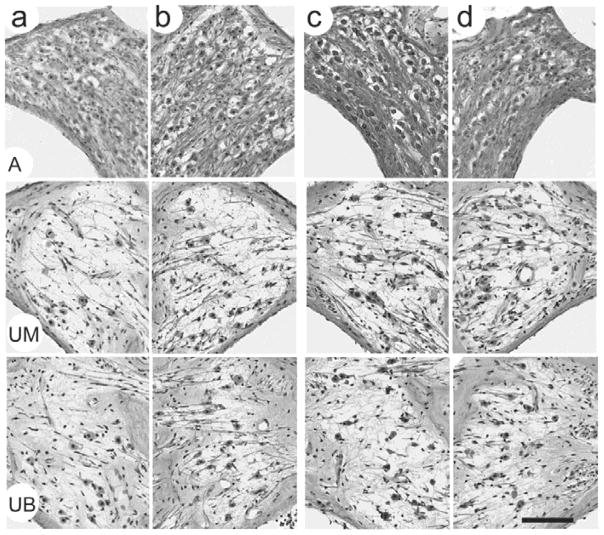
Figure 7.
Representative photomicrographs illustrating the SGNs in Rosenthal’s canal in the apical (A), upper middle (UM) and upper basal (UB) turns of the four cochleae illustrated in Figure 6. (a) control and (b) stimulated cochlea of FMP4; (c) stimulated and (d) control cochlea of FMP6. The extensive loss of SGNs observed in both the basal and middle turns of all cochleae is a feature of both the deafening technique and the extended duration of deafness associated with the present study. Chronic FMP stimulation was restricted to the basal turn; importantly we observed no difference in the SGN population in this cochlear region when comparing stimulated versus control cochleae. Scale bar = 100 μm
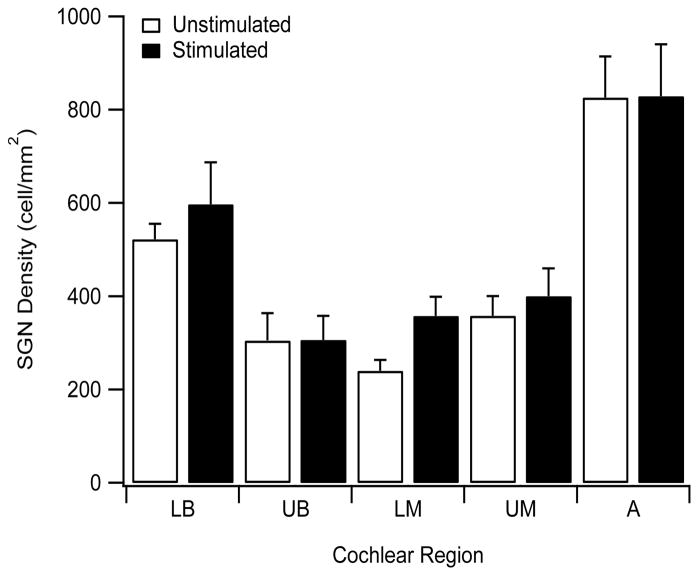
Figure 8.
Mean SGN density for the five cochlear regions examined for both stimulated and unstimulated control cochleae. 2-Way RM ANOVA demonstrated a highly statistically significant effect of cochlear region (p < 0.001; n=6) typical of the “U” shaped distribution of SGN survival associated with this deafening technique. There was no significant difference in SGN density between stimulated and control cochleae (p > 0.1). LB, lower basal; UB, upper basal; LM, lower middle; UM, upper middle; A, apical.
Tissue response
Figure 9 illustrates the typical tissue response adjacent to an electrode array. A thin, mature fibrous tissue capsule surrounded the electrode array with a variable amount of loose areolar tissue occupying an additional region of the scala tympani. In this, and some other cochleae, a number of chronic inflammatory cells were also evident.
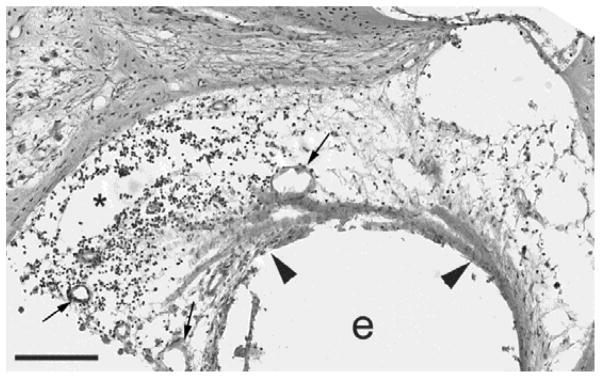
Figure 9.
Photomicrograph of the upper basal turn of stimulated cochlea FMP4 illustrating the typical tissue response to chronic cochlear implantation and electrical stimulation using the FMP strategy; control cochleae typically exhibited a reduced tissue response. In this example the tissue response consisted of a thin mature fibrous tissue capsule (arrowheads) surrounding the electrode array (e), with some loose areolar tissue and some chronic inflammatory cells (*; monocytes and macrophages) scattered within the scala tympani outside the electrode-tissue capsule. Evidence of angiogenesis associated with the tissue response was apparent (arrow). Scale bar = 100 μm.
Three control cochleae showed no evidence of an inflammatory response to the electrode array (e.g. Fig. 6a). Seven of the 12 cochleae examined (five stimulated, two controls) exhibited a minimal-mild chronic inflammatory response consisting of a thin, mature fibrous tissue capsule with evidence of angiogenesis, monocytes and macrophages and occasional foreign body giant cells, and a benign histiocytic response. The stimulated and control cochleae from animal FMP2 exhibited a small number of acute inflammatory cells (neutrophils) in addition to a chronic inflammatory response. Finally, there was no evidence of new bone formation in any cochlea in this study.
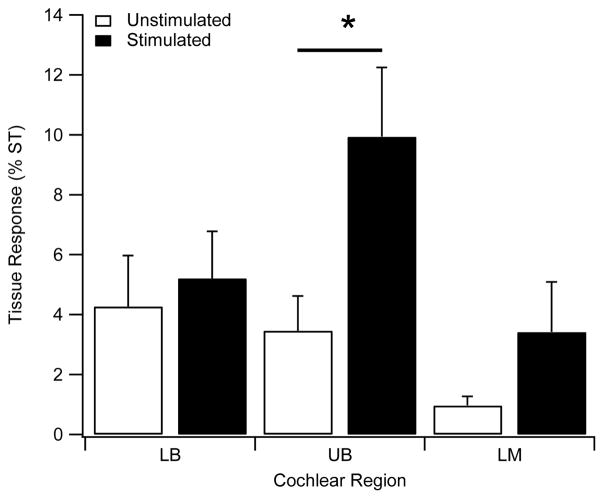
We quantified the tissue response in each cochlea by measuring the area of tissue occupying the scala tympani. This was restricted to an average of <10% of the scala tympani area across all animals in the study. Although not extensive, the stimulated cochleae exhibited an increased tissue response compared with the controls. This difference was statistically significant (Fig. 10; 2-Way RM ANOVA Region × Side p < 0.05; n=6).
Figure 10.
The extent of tissue response in the scala tympani expressed as a percentage of the scala tympani cross sectional area for both chronically implanted control and stimulated cochleae (n=6). Chronic electrical stimulation evoked a small but significant increase in fibrous tissue in a region of the cochlea associated with the electrode array 2-Way RM ANOVA (Cochlear region and Treatment (Stimulated Vs Unstimulated) with a significant main effect of treatment Stimulated > Unstimulated (p=0.034). Post-hoc UB Stimulated > UB Unstimulated (p=0.004; Holm Sidak). Significant main effect of region with UB significantly different to LB and LM in the Stimulated cohort (p=0.009; Holm Sidak). There were no interactions. LB = lower basal; UB = upper basal; LM = lower middle. (* p < 0.05).
Electrode corrosion
The electrode array from one cochlea was not recovered following completion of the chronic stimulation program. Therefore the following analysis is based on the examination of 11 arrays (six control; five stimulated).
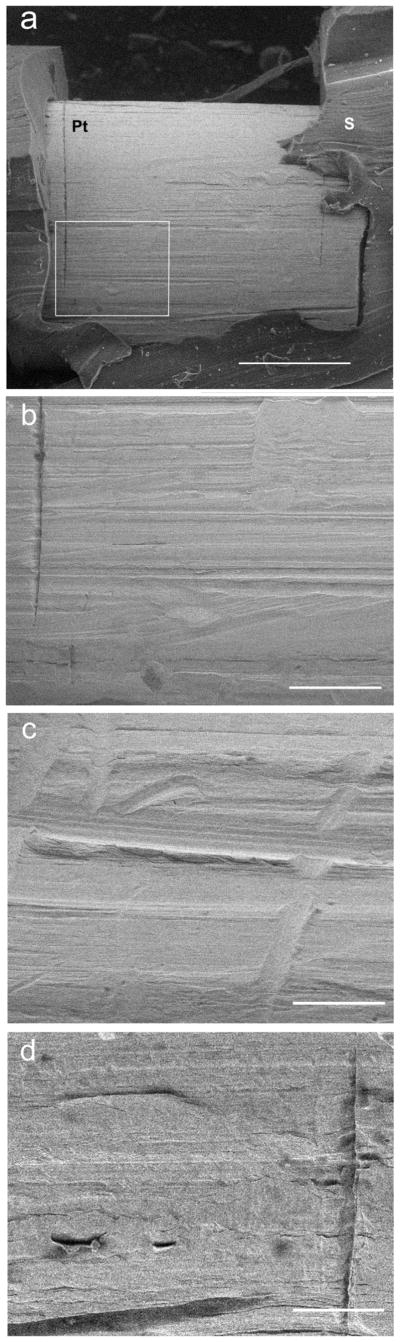
Scanning electron microscopy of the electrode surface provided a high magnification topographical examination of the Pt surface for evidence of corrosion. Representative micrographs from both control and chronically stimulated electrodes are illustrated in Figure 11. Although subject to chronic electrical stimulation using the FMP strategy, there was no evidence of corrosion on any Pt electrode. The most common surface features included sharp score marks associated with manufacturing and occasional organic debris (Fig. 11).
Figure 11.
Representative SEM micrographs from control and stimulated electrodes used in this study. (a) Low power micrograph of E9 from control electrode array of FMP4. This electrode was implanted for 182 days but not electrically stimulated. Although there is abundant score marks associated with the manufacture of the array there was no evidence of Pt corrosion. Pt, platinum electrode; S, silicone. (b) Higher-power image from (a) (box) showing the sharp score marks associated with the manufacturing process without evidence of Pt corrosion. This electrode was graded 0. (c) Micrograph of E8 from the chronically stimulated electrode array of FMP4 following 182 days of stimulation. Electrodes E6–E10 were the most activated electrodes on the array using the FMP stimulation strategy. While sharp score marks associated with manufacture were present there was no evidence of Pt corrosion. This electrode was graded 0. (d) SEM micrograph of E6 from chronically stimulated electrode array of FMP1 following 167 days of stimulation. In addition to the sharp score marks associated with manufacture, the Pt surface showed evidence of some cellular debris consistent with the tissue response observed in this cochlea. There was no evidence of corrosion. This electrode was graded 1. (a) scale bar = 100 μm; (b–d); scale bar = 25 μm.
Electrodes E6–E10 were the most extensively stimulated electrodes on the stimulated array; electrodes E1–E5 and E11–E14 received much less stimulation. The control electrode arrays were not subject to chronic stimulation but received minimal stimulation during the periodic ECAP and EABR recordings. We compared the SEM surface grading of these three electrode cohorts and observed no significant difference between electrodes E6–E10 and the remaining electrodes on the stimulated array and all electrodes on the control array (p=0.896; H=0.219 with 2 degrees of freedom, Kruskal-Wallis 1 Way ANOVA on Ranks).
5. Discussion
This study examined the functional and histological response of deafened cochleae following long-term intracochlear electrical stimulation using FMP stimulation. The stimulus levels used were above levels of auditory perception and the duration of the stimulation program was extensive (continuous stimulation for periods of up to 182 days). To our knowledge this is the first study of this kind to evaluate the preclinical safety of a current focusing strategy that utilises simultaneous stimulation from multiple independent current sources. The results demonstrated that extended periods of FMP stimulation did not result in statistically significant changes to: (i) the thresholds of electrically-evoked auditory responses; (ii) SGN survival; or (iii) the surface features of the Pt electrodes. The only significant stimulus induced change observed in the present study was a small but statistically significant increase in the extent of a mild foreign body response adjacent to the scala tympani electrode array compared with chronically implanted controls. Taken together, these findings support the application of FMP stimulation clinically as a strategy to improve the clinical performance of CI users, particularly in a noisy environment.
It is important to note that the complex FMP stimulus used in the present study employed stimulus levels of 115–180 nC/phase (Table 1). These levels are close to or above the stimulus levels we have previously used in chronic studies evaluating the safety of charge balanced current pulses presented sequentially in a bipolar (100–180 nC/phase, (Shepherd et al., 1983); 20–62.5 nC/phase, (Xu et al., 1997)) or monopolar (11–17.5 nC/phase, (Xu et al., 1997)) electrode configuration.
Safe electrical stimulation is achieved by injecting charge into the biological environment without damaging the surrounding tissue. This is accomplished using both capacitive mechanisms and a series of electrochemical reactions that convert the charge carriers from electrons (in the electrode) to ions (in the electrolyte). To ensure safe stimulation these reactions must be limited to reversible Faradaic reactions; these electrochemical reaction products remain localised to the electrode tissue interface and can therefore be readily reversed by a rapid charge recovery process (Fallon and Carter, 2016, Cogan, 2008, Cogan et al., 2016). Sequential stimulation ensures that charge recovery occurs soon after completion of the second phase of a biphasic current pulse. Long durations between the stimulus and the subsequent charge recovery allows the diffusion of the electrochemical reaction products away from to the electrode, reducing the potential for reversing the reaction and resulting in the formation of toxic electrochemical species being permanently released into the biological environment (Fallon and Carter, 2016, Cogan, 2008, Cogan et al., 2016, Shepherd et al., 2014).
The SEM results in the present study showed no statistical difference in the condition of the surface of FMP stimulated Pt electrodes compared with unstimulated control electrodes. This result is consistent with our previous observations where we reported no evidence of corrosion following chronic stimulation using biphasic current pulses presented sequentially in both experimental animals (Shepherd et al., 1985) and following long-term clinical use (Shepherd and Clark, 1991). This result, combined with the lack of any significant acute pathological response within the stimulated cochleae, provides evidence that the chronic FMP stimulus did not produce adverse electrochemical products, including Pt dissolution, within the cochlear environment.
While SEM analysis of electrodes provide a relatively gross indication of the extent of Pt dissolution, this analysis is far more sensitive than optical inspection of electrodes and becomes an important analysis technique particularly following long-term implantation or stimulation at high charge densities. A recent clinical report, for example, demonstrated evidence of Pt corrosion from the surface of cochlear implant electrodes after typically more than a decade of use. The authors then used energy dispersive spectroscopy to identify particulate Pt in the relatively extensive tissue capsule surrounding the electrode array (Nadol et al., 2014).
Prolonged durations of stimulus induced neuronal activity can also induce injury by placing the neural population under severe metabolic stress. Neurons can exhibit a significant reduction in excitability (McCreery et al., 1992, Tykocinski et al., 1995), reflecting an inability of the target neural population to maintain homeostasis following excessive stimulation (Agnew et al., 1993, McCreery, 2004). For these reasons, careful evaluation of the safety of new stimulation strategies using stimulators and animal models that closely conform to the proposed clinical application is particularly important. Significantly, we observed no evidence of damage related to metabolic stress despite the extended duration and continuous nature of the focused stimulation strategy used in the present study.
A full electrode array/leadwire assembly was implanted bilaterally in each animal in the present study. This provided the ability to record electrically-evoked potentials from both chronically stimulated and control cochleae longitudinally over the duration of the study. The electrically-evoked recordings (ECAP and EABRs) in the present study showed that the chronically stimulated auditory nerve remained physiologically viable for the duration of the stimulus program. Moreover, these longitudinally recorded responses exhibited relatively stable thresholds and exhibited no significant increase in threshold when compared with control cochleae, implying that there was no significant reduction in neural function as a result of chronic FMP stimulation.
Finally, we reported a small but statistically significant increase in the foreign body reaction associated with chronic FMP stimulation. This finding is not unique to FMP stimulation as we have previously reported that electrically stimulated cochleae are slightly more predisposed to evoking a foreign body response following stimulation using conventional biphasic current pulses delivered from a single current source compared with implanted control cochleae (Shepherd et al., 1994, Xu et al., 1997). Foreign body reactions are also commonly observed in clinical material. (Seyyedi and Nadol, 2014), for example, reported that 96% of the post mortem cochlear implant cases they examined showed evidence of a foreign body response.
6. Conclusion
The functional, histopathological and SEM results in the present study have indicated that chronic intracochlear electrical stimulation using FMP stimulation does not adversely affect the SGNs or the cochlea in general, or result in any measurable Pt electrode corrosion. Moreover FMP stimulation does not adversely affect the condition or function of the Pt electrode. This study provides an important basis for the safe application of improved speech processing strategies based on FMP stimulation. Focused stimulation strategies such as FMP also have application in other neural prostheses where the target neural population is highly localized and excitation of non-target neurones could cause adverse side effects.
Acknowledgments
We thank Dr Zach Smith, Dr Chris Long, Dr Chris van den Honert, Shaun Kumar, Rob Bennett, Harish Krishnamoorthi and Dr Joerg Pesch from Cochlear Ltd., Ceara McGowan, Brianna Flynn, Ella Trang, Amy Morley, Nicole Critch, Helen Feng, and Vanessa Maxim from the Bionics Institute, Dr Fenella Long and Dr Sue Peirce from St Vincent’s and the Victorian Eye and Ear Hospitals, Dr Richard Williams, Department of Pathology, St Vincent’s Hospital and Roger Curtain from Bio21, University of Melbourne. We gratefully acknowledge the supply of stimulators and electrode arrays from Cochlear Ltd and funding through an Australian Research Council (LP130100220), National Health and Medical Research Council (APP 1081478) and the National Institutes of Health (R01DC015031). We also note that the Institute receives contract research funding from Cochlear Ltd. for research outside this study. Finally, the Bionics Institute acknowledges the support it receives from the Victorian Government through its Operational Infrastructure Support Program.
References
- AGNEW WF, MCCREERY DB, YUEN TG, BULLARA LA. MK-801 protects against neuronal injury induced by electrical stimulation. Neuroscience. 1993;52:45–53. doi: 10.1016/0306-4522(93)90180-n. [DOI] [PubMed] [Google Scholar]
- BIERER JA. Threshold and channel interaction in cochlear implant users: evaluation of the tripolar electrode configuration. The Journal of the Acoustical Society of America. 2007;121:1642–53. doi: 10.1121/1.2436712. [DOI] [PubMed] [Google Scholar]
- BLACK RC, CLARK GM, PATRICK JF. Current distribution measurements within the human cochlea. IEEE transactions on bio-medical engineering. 1981;28:721–5. doi: 10.1109/TBME.1981.324668. [DOI] [PubMed] [Google Scholar]
- BOEX C, DE BALTHASAR C, KOS MI, PELIZZONE M. Electrical field interactions in different cochlear implant systems. The Journal of the Acoustical Society of America. 2003;114:2049–57. doi: 10.1121/1.1610451. [DOI] [PubMed] [Google Scholar]
- CEN. EN4550502-2-3. European Committee for Standardization; 2010. Particular requirements for cochlear and auditory brainstem implant systems. [Google Scholar]
- CLARK GA, CROSBY PA, DOWELL R, KUZMA JA, MONEY DK, PATRICK J, SELIGMAN P, TONG YC. The preliminary clinical trial of a multichannel cochlear implant hearing prosthesis. J Acoust Soc Am. 1983;74:1911–1914. [Google Scholar]
- COCO A, EPP SB, FALLON JB, XU J, MILLARD RE, SHEPHERD RK. Does cochlear implantation and electrical stimulation affect residual hair cells and spiral ganglion neurons? Hear Res. 2007;225:60–70. doi: 10.1016/j.heares.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COGAN SF. Neural stimulation and recording electrodes. Annu Rev Biomed Eng. 2008;10:275–309. doi: 10.1146/annurev.bioeng.10.061807.160518. [DOI] [PubMed] [Google Scholar]
- COGAN SF, GARRETT DJ, GREEN RA. Electrochemical principles of safe charge injection. In: SHEPHERD RK, editor. Neurobionics: The biomedical engineering of neural prostheses. Hoboken: John Wiley & Sons Inc; 2016. [Google Scholar]
- FALLON JB, CARTER PM. Principles of recording from and electricalstimulation of neural tissue. In: SHEPHERD RK, editor. Neurobionics: The Biomedical Engineering of Neural Prostheses. New York: John Wiley & Sons; 2016. [Google Scholar]
- FALLON JB, IRVINE DR, SHEPHERD RK. Cochlear implant use following neonatal deafness influences the cochleotopic organization of the primary auditory cortex in cats. J Comp Neurol. 2009;512:101–14. doi: 10.1002/cne.21886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIJNS JH, DEKKER DM, BRIAIRE JJ. Neural excitation patterns induced by phased-array stimulation in the implanted human cochlea. Acta oto-laryngologica. 2011;131:362–70. doi: 10.3109/00016489.2010.541939. [DOI] [PubMed] [Google Scholar]
- FU QJ, SHANNON RV, WANG X. Effects of noise and spectral resolution on vowel and consonant recognition: acoustic and electric hearing. J Acoust Soc Am. 1998;104:3586–96. doi: 10.1121/1.423941. [DOI] [PubMed] [Google Scholar]
- GEORGE SS, SHIVDASANI MN, WISE AK, SHEPHERD RK, FALLON JB. Electrophysiological channel interactions using focused multipolar stimulation for cochlear implants. Journal of neural engineering. 2015a;12:066005. doi: 10.1088/1741-2560/12/6/066005. [DOI] [PubMed] [Google Scholar]
- GEORGE SS, WISE AK, FALLON JB, SHEPHERD RK. Evaluation of focused multipolar stimulation for cochlear implants in long-term deafened cats. Journal of neural engineering. 2015b;12:036003. doi: 10.1088/1741-2560/12/3/036003. [DOI] [PubMed] [Google Scholar]
- GEORGE SS, WISE AK, SHIVDASANI MN, SHEPHERD RK, FALLON JB. Evaluation of focused multipolar stimulation for cochlear implants in acutely deafened cats. Journal of neural engineering. 2014;11:065003. doi: 10.1088/1741-2560/11/6/065003. [DOI] [PubMed] [Google Scholar]
- HARDIE NA, SHEPHERD RK. Sensorineural hearing loss during development: morphological and physiological response of the cochlea and auditory brainstem. Hear Res. 1999;128:147–65. doi: 10.1016/s0378-5955(98)00209-3. [DOI] [PubMed] [Google Scholar]
- HARTMANN R, KLINKE R. Impulse patterns of auditory nerve fibres to extra- and intracochlear electrical stimulation. Acta Otolaryngol Suppl. 1990;469:128–34. [PubMed] [Google Scholar]
- IRVING S, WISE AK, MILLARD RE, SHEPHERD RK, FALLON JB. A partial hearing animal model for chronic electro-acoustic stimulation. Journal of neural engineering. 2014;11:046008. doi: 10.1088/1741-2560/11/4/046008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOLLY CN, SPELMAN FA, CLOPTON BM. Quadrupolar stimulation for Cochlear prostheses: modeling and experimental data. IEEE Trans Biomed Eng. 1996;43:857–65. doi: 10.1109/10.508549. [DOI] [PubMed] [Google Scholar]
- KRAL A, HARTMANN R, MORTAZAVI D, KLINKE R. Spatial resolution of cochlear implants: the electrical field and excitation of auditory afferents. Hear Res. 1998;121:11–28. doi: 10.1016/s0378-5955(98)00061-6. [DOI] [PubMed] [Google Scholar]
- LEAKE PA, HRADEK GT, SNYDER RL. Chronic electrical stimulation by a cochlear implant promotes survival of spiral ganglion neurons after neonatal deafness. J Comp Neurol. 1999;412:543–62. doi: 10.1002/(sici)1096-9861(19991004)412:4<543::aid-cne1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- LITVAK LM, SPAHR AJ, EMADI G. Loudness growth observed under partially tripolar stimulation: model and data from cochlear implant listeners. The Journal of the Acoustical Society of America. 2007;122:967–81. doi: 10.1121/1.2749414. [DOI] [PubMed] [Google Scholar]
- LONG CJ, HOLDEN TA, MCCLELLAND GH, PARKINSON WS, SHELTON C, KELSALL DC, SMITH ZM. Examining the electro-neural interface of cochlear implant users using psychophysics, CT scans, and speech understanding. Journal of the Association for Research in Otolaryngology : JARO. 2014;15:293–304. doi: 10.1007/s10162-013-0437-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAROZEAU J, MCDERMOTT HJ, SWANSON BA, MCKAY CM. Perceptual interactions between electrodes using focused and monopolar cochlear stimulation. Journal of the Association for Research in Otolaryngology : JARO. 2015;16:401–12. doi: 10.1007/s10162-015-0511-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCREERY D. Tissue reaction to electrodes: The problem of safe and effective stimulation of neural tissue. In: HORCH KW, DHILLON GS, editors. Neural Prosthesis: Theory and Practice. World Scientific Publishing; River Edge, NJ: 2004. [Google Scholar]
- MCCREERY DB, AGNEW WF, YUEN TG, BULLARA LA. Damage in peripheral nerve from continuous electrical stimulation: comparison of two stimulus waveforms. Med Biol Eng Comput. 1992;30:109–14. doi: 10.1007/BF02446202. [DOI] [PubMed] [Google Scholar]
- MCDERMOTT HJ, MCKAY CM, VANDALI AE. A new portable sound processor for the University of Melbourne/Nucleus Limited multielectrode cochlear implant. Journal of the Acoustical Society of America. 1992;91:3367–3371. doi: 10.1121/1.402826. [DOI] [PubMed] [Google Scholar]
- MIYOSHI S, SHIMIZU S, MATSUSHIMA J, IFUKUBE T. Proposal of a new method for narrowing and moving the stimulated region of cochlear implants: animal experiment and numerical analysis. IEEE Trans Biomed Eng. 1999;46:451–60. doi: 10.1109/10.752942. [DOI] [PubMed] [Google Scholar]
- NADOL JB, JR, O’MALLEY JT, BURGESS BJ, GALLER D. Cellular immunologic responses to cochlear implantation in the human. Hearing research. 2014;318:11–7. doi: 10.1016/j.heares.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATRICK JF, SEIIGMAN PM, MONEY DK, KUZMA JA. Engineering. In: CLARK GM, TONG YC, PATRICK JF, editors. Cochlear Prostheses. Edinburgh: Churchill Livingston; 1990. [Google Scholar]
- RODENHISER KL, SPELMAN FA. A method for determining the driving currents for focused stimulation in the cochlea. IEEE Trans Biomed Eng. 1995;42:337–42. doi: 10.1109/10.376127. [DOI] [PubMed] [Google Scholar]
- SEYYEDI M, NADOL JB., JR Intracochlear inflammatory response to cochlear implant electrodes in humans. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2014;35:1545–51. doi: 10.1097/MAO.0000000000000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHANNON RV. Multichannel electrical stimulation of the auditory nerve in man. II. Channel interaction. Hear Res. 1983;12:1–16. doi: 10.1016/0378-5955(83)90115-6. [DOI] [PubMed] [Google Scholar]
- SHEPHERD R, VERHOEVEN K, XU J, RISI F, FALLON J, WISE A. An improved cochlear implant electrode array for use in experimental studies. Hear Res. 2011;277:20–7. doi: 10.1016/j.heares.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEPHERD RK, CLARK GM. Scanning electron microscopy of platinum scala tympani electrodes following chronic stimulation in patients. Biomaterials. 1991;12:417–23. doi: 10.1016/0142-9612(91)90011-x. [DOI] [PubMed] [Google Scholar]
- SHEPHERD RK, CLARK GM, BLACK RC. Chronic electrical stimulation of the auditory nerve in cats. Physiological and histopathological results. Acta Oto-Laryngologica Supplement. 1983;399:19–31. doi: 10.3109/00016488309105589. [DOI] [PubMed] [Google Scholar]
- SHEPHERD RK, FALLON JB, MCDERMOTT H. Medical Bionics. In: BRAHME A, editor. Comprehensive Biomedical Physics. Amsterdam: Elsevier; 2014. [Google Scholar]
- SHEPHERD RK, MATSUSHIMA J, MARTIN RL, CLARK GM. Cochlear pathology following chronic electrical stimulation of the auditory nerve: II Deafened kittens. Hearing Research. 1994;81:150–166. doi: 10.1016/0378-5955(94)90162-7. [DOI] [PubMed] [Google Scholar]
- SHEPHERD RK, MURRAY MT, HOUGHTON ME, CLARK GM. Scanning electron microscopy of chronically stimulated platinum intracochlear electrodes. Biomaterials. 1985;6:237–42. doi: 10.1016/0142-9612(85)90019-5. [DOI] [PubMed] [Google Scholar]
- STICKNEY GS, LOIZOU PC, MISHRA LN, ASSMANN PF, SHANNON RV, OPIE JM. Effects of electrode design and configuration on channel interactions. Hear Res. 2006;211:33–45. doi: 10.1016/j.heares.2005.08.008. [DOI] [PubMed] [Google Scholar]
- SUCHER CM, MCDERMOTT HJ. Pitch ranking of complex tones by normally hearing subjects and cochlear implant users. Hear Res. 2007;230:80–7. doi: 10.1016/j.heares.2007.05.002. [DOI] [PubMed] [Google Scholar]
- TOWNSHEND B, WHITE RL. Reduction of electrical interaction in auditory prostheses. IEEE Trans Biomed Eng. 1987;34:891–7. doi: 10.1109/tbme.1987.326102. [DOI] [PubMed] [Google Scholar]
- TYKOCINSKI M, SHEPHERD RK, CLARK GM. Reduction in excitability of the auditory nerve following electrical stimulation at high stimulus rates. Hear Res. 1995;88:124–42. doi: 10.1016/0378-5955(95)00108-g. [DOI] [PubMed] [Google Scholar]
- VAN DEN HONERT C, KELSALL DC. Focused intracochlear electric stimulation with phased array channels. J Acoust Soc Am. 2007;121:3703–16. doi: 10.1121/1.2722047. [DOI] [PubMed] [Google Scholar]
- WILSON BS, FINLEY CC, LAWSON DT, WOLFORD RD, EDDINGTON DK, RABINOWITZ WM. Better speech recognition with cochlear implants. Nature. 1991;352:236–8. doi: 10.1038/352236a0. [DOI] [PubMed] [Google Scholar]
- WISE AK, FALLON JB, NEIL AJ, PETTINGILL LN, GEANEY MS, SKINNER SJ, SHEPHERD RK. Combining cell-based therapies and neural prostheses to promote neural survival. Neurotherapeutics. 2011;8:774–87. doi: 10.1007/s13311-011-0070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XU J, SHEPHERD RK, MILLARD RE, CLARK GM. Chronic electrical stimulation of the auditory nerve at high stimulus rates: a physiological and histopathological study. Hearing Research. 1997;105:1–29. doi: 10.1016/s0378-5955(96)00193-1. [DOI] [PubMed] [Google Scholar]