Abstract
Background
The effects of prenatal substance exposure on neurobehavioral outcomes are inherently confounded by the effects of the postnatal environment, making it difficult to disentangle their influence. The goal of this study was to examine the contributing effects of prenatal substance use and parenting style (operationalized as contingent responding during the play episodes of the Still-face paradigm [SFP]) on infant affect.
Methods
A prospective cohort design was utilized with repeated assessment of substance use during pregnancy and the administration of the SFP, which measures infant response to a social stressor, at approximately 6 months of age. Subjects included 91 dyads classified into four groups: 1) Control (n=34); 2) Medication assisted therapy for opioid dependence (MAT; n=19); 3) Alcohol (n=15); 4) Alcohol+MAT (n=23). Mean % of positive infant affect and mean % of maternal responsiveness (watching, attention seeking, and contingent responding) was compared among the five SFP episodes across the four study groups by MANOVA. Mixed effects modelling was used to estimate the contributing effects of the study groups and maternal responsiveness on infant affect.
Results
Maternal contingent responding was associated with increase (β̂ =0.84; p<0.0001) and attention seeking with decrease (β̂ =−0.78; p<0.0001) in infant positive affect. The combined effect of prenatal exposures and covariates explained 15.8% of the variability in infant positive affect, while the model including contingent responding and covariates explained 67.1% of the variability.
Conclusions
Higher maternal responsiveness was a much stronger predictor of infant behavior than prenatal exposures, providing the basis for future intervention studies focusing on specific parenting strategies.
Keywords: infant affect, stress reactivity, maternal contingent responsiveness, alcohol, opioids
INTRODUCTION
Substance use disorders are a complex and prevalent public health problem which affects all segments of the population, including vulnerable groups such as pregnant women. It is estimated that 5.4% of pregnant women use illegal drugs and 10.8 % use alcohol (1). The effects of maternal drug exposure on neurocognitive outcomes in prenatally-exposed infants are complex and vary by the substance of abuse, timing of exposure, patterns of administration, and frequency of use (2). The devastating effects of prenatal alcohol exposure (PAE), collectively known as Fetal Alcohol Spectrum Disorders (FASD), are well described (3). Ongoing attention problems (4), poor memory and executive functioning skills (5), and deficits in verbal comprehension, working memory, and full-scale IQ (6) are associated with PAE and often result in learning disabilities in affected children (7). Neurodevelopmental outcomes commonly associated with PAE might be mediated, at least in part, by hypothalamic-pituitary-adrenal (HPA) dysregulation (8) leading to increased stress reactivity and poor stress regulation in PAE children (9). Alternatively, HPA dysregulation and increased stress reactivity in children affected by PAE might be the basis for some of the PAE-induced behavioral deficits (10) and increased vulnerability to secondary psychopathologies, such as mental health problems, inappropriate sexual behavior, and learning disabilities (11).
Opioid use disorder has reached epidemic proportion in the United States (12) with an accompanying four-fold increase in antepartum maternal opiate use from 2000 to 2009. As a result, neonatal abstinence syndrome (NAS) now affects 5.8 per 1,000 hospital births (13). Emerging evidence suggests that consequences of NAS beyond the neonatal period might include impaired cognitive and language development (14), increased risk of developing attention deficit/hyperactivity disorder, diagnoses of disruptive behaviors (15), and parent-reported behavior problems (16). A recent study reported less “positivity” (lower scores for smiling and laughter) among opioid-exposed infants and lower responsiveness to soothing (17), suggesting that prenatal opioid exposure might affect infant emotional reactivity and maternal-infant interaction. Finally, polysubstance use may present the greatest risk to the developing fetus (18), while little is known about the effect of polysubstance use on stress reactivity and regulation in young children.
The long-term effects of prenatal drug and/or alcohol exposure on neurocognitive and behavioral outcomes are inherently confounded by the effects of the postnatal environment, making it difficult to disentangle the influence of prenatal and postnatal factors. The ‘nature vs. nurture’ concept is not new, but continues to present a challenge for the interpretation of perinatal epidemiology and substance use research. The importance of postnatal factors was demonstrated in a study of children exposed to heroin during pregnancy, which found that children adopted at a young age had intellectual skills similar to non-exposed controls; however, heroin-exposed children who remained with their biological parents had lower intellectual test scores compared to non-exposed controls (19). In another study, toddlers prenatally exposed to polysubstance use were found to have adverse outcomes, such as lower self-regulation and increased externalizing problems at 3 years of age, when their mothers were observed using harsh behaviors during a video-taped free play paradigm (20). Heightened infant reactivity and distress, mother-infant attachment disorders, and decreased sensitivity and responsiveness towards the child have been associated in research with parenting practices among mothers with various substance use disorders (21–26). Substance-using mothers of children with prenatal exposures have been shown to perform poorer than non-using caregivers of children with prenatal exposures on caregiver-child interaction assessments, highlighting the particular vulnerability of the maternal-child relationship when maternal substance use is present (27). Despite these reports suggesting that the quality of maternal-infant interaction might be an important factor that may impact child psychopathology, we are not aware of any studies directly assessing the role of prenatal substance use versus supportive parenting style on infant emotional regulation.
Emotional regulation has been conceptualized as the ability to control the response to an environmental stressor and the ability to recover from the distressful situation (28). Difficulty with emotional regulation has been associated with later externalizing behavioral problems (29, 30) and impairments in executive functioning (31). Emotional regulation in infancy is often measured by infant positive and negative affect in relation to a social stressor (32). The Still-face paradigm (SFP) is an established experimental procedure that measures an infant’s reaction to the introduction of a disruptive event, specifically to their mother’s ‘still-face’ - a social stressor which indicates a mismatch or non-responsiveness in the communication between mother and infant (33). The SFP effect is robust and results in a pattern of reduced positive affect and increased negative affect during the ‘still-face episodes’ and increased positivity during the reunion or play episodes (34). Meta-analyses also reveal that higher maternal responsiveness or sensitivity predicts higher infant affect, and the maternal-infant interaction during the SFP are predictive of secure attachment at 1 year of age (34). Our earlier study found that PAE infants had increased cortisol reactivity, elevated heart rate, and increased negative affect during the stressor episodes of the SFP (35). Infants with moderate to heavy PAE have been found to have greater physiologic stress reactivity between 6 and 15 months as measured by salivary cortisol during the SFP, compared to infants with mild or no PAE exposure (36).
The specific aims of this prospective cohort study were to examine: 1) the differences in maternal behavior styles during the SFP between women who used alcohol, opioids, both substances in combination, or abstained from alcohol and illicit drugs during pregnancy; 2) the contributing effects of prenatal substance use and parenting style (operationalized as maternal contingent responding) on infant positive affect during maternal-infant play episodes of the SFP. We hypothesized that substance using women will be less likely to engage in contingent responding behavior style, and that maternal contingent responding would be an equally important predictor of infant affect as prenatal exposure to substances of abuse.
METHODS
Study design and population
Data were derived from two consecutive prospective cohort studies conducted at the University of New Mexico (UNM) with the same study population. The UNM Human Research Review Committee approved both studies and patients gave written informed consent. The Biomarkers, Infant Neurodevelopment, and Growth (BINGO) study (PI: Bakhireva, supported by NIAAA 1R03AA020170 and 1P20 AA017608 grants) was conducted at UNM in 2011–2012 and served as a pilot study to the larger, ongoing Ethanol, Neurodevelopment, Infant and Child Health (ENRICH) cohort study (MPIs: Bakhireva, Stephen; supported by NIAAA R01 AA021771), which began in 2013. Participants were recruited from UNM-affiliated prenatal care clinics. Both studies included three visits: 1) prenatal, during one of the first prenatal care appointments; 2) early postpartum, during the hospital stay after labor and delivery; and 3) neurodevelopmental and SFP assessment of children at ~ 6 months of age. The following eligibility criteria were applied to all participants: 1) at least 18 years old; 2) singleton pregnancy; 3) currently residing and planning to stay in the Albuquerque metropolitan area to complete all study visits; 4) ability to give informed consent in English; and 5) no fetal diagnosis of a major structural anomaly.
Pregnant women in both cohort studies were recruited into one of four mutually exclusive study groups, as follows: participants 1) without perinatal substance exposures (Control); 2) with opioid use disorder who prenatally received medication assisted therapy (MAT; either methadone or buprenorphine) and did not use alcohol in pregnancy; 3) with alcohol use during pregnancy (Alcohol); and 4) with MAT and alcohol use during pregnancy (Alcohol+MAT). While the focus of both cohorts was to ascertain the effects of prenatal alcohol exposure on infant outcomes, MAT and Alcohol+MAT groups were included, in addition to unexposed controls, to better match pre- and post-natal environmental factors across groups. Participants classified into the control group needed to 1) be a lifetime abstainer of illicit drugs and tobacco products (reported use of ≤100 cigarettes in lifetime); and ≤2) abstain from alcohol use since the last menstrual period (LMP) and be no more than a light alcohol user ( 2 standard drinks/week on average) before the LMP. Participants classified into the alcohol-exposed groups (Alcohol, Alcohol+MAT) had to 1) self-report at least moderate levels of drinking (37) in the periconceptional period (≥3 drinks per week or ≥2 binge drinking episodes [‘binge’ defined as ≥4 drinks per occasion] during the month surrounding the LMP) using the Timeline Follow-Back assessment method; and 2) continue drinking during pregnancy, as confirmed by self-report or positive ethanol biomarker. The self-reported cutoffs for risky alcohol use employed in this study and our conjunctive use of ethanol biomarkers in pregnancy are rigorous and well-supported by the literature (37–41). The final sample size for this analysis was 91 maternal-infant pairs who had completed the three study visits as of January 2017.
Assessment of substance use and covariates at study visits 1 and 2
Quantity and frequency of alcohol and substance use were captured using repeated prospective timeline follow-back (TLFB) interviews (42), as previously described (43–45). Self-reported data on alcohol use was verified by a state-of-the-art battery of six ethanol biomarkers at delivery, including phosphatidylethanol (PEth) in newborn dried blood spots (PEth-DBS), and five in maternal specimens: urine ethylglucuronide (uEtG), urine ethylsulfate (uEtS), whole blood PEth, gamma-glutamyltranspeptidase (GGT), and carbohydrate-deficient transferrin (%dCDT). Analytical methods for all biomarkers have been described elsewhere (44, 45). This panel of biomarkers, with detection windows varying from a few days to approximately 2 months (see review by Bakhireva and Savage, 2011 (46)), was chosen to capture different alcohol consumption patterns (e.g., chronic moderate use vs, recent binge episode). Subjects who reported abstinence from alcohol during pregnancy but tested positive for one of the ethanol biomarkers were disqualified from the study and disenrolled.
Self-reported use of MAT and recreational drugs was confirmed by a review of electronic medical records (EMR) containing the results of urine drug screens conducted at the clinic, and with study-specific urine drug tests conducted at enrollment and at admission for labor and delivery.
Participants’ demographic (age, education level, race, ethnicity, marital status), medical, and reproductive characteristics were obtained during the baseline interview. The second interview occurred during the hospital stay after delivery and captured any changes in drug and alcohol use later in pregnancy. Information regarding pregnancy and newborn outcomes (i.e., gestational age at delivery, infant sex and weight, maternal/newborn complications) was abstracted from patient EMR.
The SFP and maternal responsiveness style at the study visit 3
Infants born in the cohort were brought in for a neurodevelopmental assessment at approximately 6 months of age (allowed range: 4–8 months). For infants born preterm (before 37 weeks gestation), the age at assessment was adjusted for prematurity. Developmental specialist and eesearch assistants, blinded to subject exposure status, described the SFP to maternal participants at the start of the study visit and then conducted the assessment while the infant was awake and content. SFP assessments took 15 minutes to complete, including camera setup, positioning the baby, and giving instructions. As previously described in contrast to the original version, which included three episodes, the modified SFP consists of five episodes of 120 seconds each (9). These are 1) a baseline play interval to assess typical mother-infant interaction; 2) the ‘still-face’ episode, in which the mother does not make eye contact or respond to her infant while maintaining a neutral expression; 3) a reunion or play episode, in which the mother resumes typical interaction; 4) a second ‘still-face’ episode; and 5) a second reunion or play episode.
Offline video coding was performed by reliable coders trained by Dr. Lowe who has extensive experience with the SFP (35, 47, 48).Infant affect was coded second-by-second during all five episodes as follows: 3 (rhythmic crying for ≥3 seconds), 2 (shorter cry in duration, a protest or yell), 1 (mild fuss/frown), 0 (baby is neutral), +1 (corners of the mouth straight, soft coo), +2 (corners of the mouth go up, cheeks raised, chuckle or small giggle), +3 (laugh for ≥2 s). To systematically differentiate between fussing and crying, duration of ≥2 seconds was used to code crying. The percentage of time the infant displayed positive affect over the duration of each 120-second episode was calculated in a similar fashion to previous emotional regulation studies (47, 49), where infant positive affect was defined as a score greater than 0 (neutral).
Maternal responsiveness to child behavior was coded using a second-by-second system developed by (50) and described elsewhere (9, 47, 49). Briefly, maternal responsiveness was coded during the play episodes (episodes 1, 3, and 5) of the SFP using the following ordinal scale: 1) Watching (the mother is neutral as she watches her infant’s behavior); 2) Attention Seeking (the mother is attempting to gain her infant’s attention by using various strategies, such as calling the infant’s name or clapping her hands); 3) Contingent Responsiveness (the mother mimics her infant’s behavior in an exaggerated fashion as the infant responds; the mother and infant then take turns initiating these behaviors and responding to one another). The percentage of time the mother is engaged in each type of responsive behavior over the 120 seconds during the reunion/play episodes was calculated and used for analyses. Due to the small percent time participants were engaged in “watching” behavior, subsequent analyses focused only on contingent responsiveness and attention seeking. To estimate inter-rater reliability, every 7th tape was coded by a second rater and inter-class correlation between the two raters was calculated. The inter-rater reliability for coding of infant affect ranged from 0.76 to 0.91 across episodes. Inter-class correlation coefficients between 2 coders for maternal responsiveness ranged from 0.94 to 0.99. In addition to administration of the SFP, a brief demographic form captured current family gross annual income. At the infant 6-month visit, Beck Depression Inventory (BDI) was also administered.
Power calculations and data analyses
A priori power calculations were based on an earlier study examining correlation (r) between maternal responsiveness and infant affect (48), which indicated that 19 patients per group would achieve 80% power to detect a difference of −0.46 between r=0.30 under the H0 (maternal watching and positive affect) and r=0.76 under H1 (maternal CR and positive affect). We note that this number increases to 23 subjects per group when adjusting for up to seven covariates.
Socio-demographic and medical characteristics, as well as quantity and frequency of substance use were compared among the study groups by analysis of variance (ANOVA) or Fisher's exact tests for continuous and categorical variables, respectively. Since the ANOVA F-test is robust to the assumption of normality (51), that assumption was relaxed. Further, in cases where the equal-variances assumption was violated, Welch's modified F-test (52) was used to assess significant differences in the means. Mean percentage of time during each episode the mother engaged in a particular type of responsive behavior was compared among the study groups by ANOVA.
Mean percentage of positive infant affect was compared among the five SFP episodes across the four study groups according to the Wilks' Lambda test statistic within the multivariate analysis of variance (MANOVA), as illustrated previously (53). Similarly, the percentage of each type of maternal responsiveness over the 120 seconds was compared among episodes 1, 3, and 5 (“still-face” episodes 2 and 4 were not included due to the lack of any maternal interaction per SFP protocol). In bivariate analysis, the effects of maternal contingent responding and attention seeking behaviors on infant positive affect were examined using mixed effects models. Both MANOVA and mixed effects models are advanced techniques for analysis of repeated measures, which allow for examination of the outcome (infant affect) over all episodes while adjusting for the covariance structure. Specifically, we used the unstructured (UN) covariance structure based on the restricted likelihood ratio test (G2) as described elsewhere (54, 55). Mixed effects modelling provides additional flexibility in model fitting for clustered repeated measures compared to traditional regression analysis with the adjustment for baseline infant affect (56). In addition to mixed effect modelling which evaluated maternal responsiveness and infant affect in the same context, we conducted lagged analysis to examine the effect of maternal responsiveness during the earlier episodes of the SFP (episodes 1 and 3) on infant affect at the end of the SFP (episode 5).
Multiple mixed effects models were used to repeat this analysis after adjusting for the study group, SFP episode, infant age at assessment, infant sex, household income, maternal BDI score, maternal education level, and marital status (single parent vs. two-parent household). To explain the variability in infant positive affect by a set of independent variables within the settings of linear mixed effect models, we followed the steps of Xu (57) using R2 = 1-RSS/RSS0, where RSS is the sum of squared residuals from the model, and RSS0 is the sum of squared residuals from an intercept-only model. All analyses were conducted in SAS 9.3 (Gary, NC).
RESULTS
The study population included a large proportion of ethnic minorities (include 60.4% Hispanic/Latina, 4.4% African American, 4.4% American Indian) and spanned both low and high educational categories (28.6% had less than a high school education, 56.0% completed high school to some college, 15.4% had a college degree or above). Most patients (60.4%) were recruited during the second trimester of pregnancy (mean gestational age: 24.1±8.1 weeks). As shown in Table 1, the study groups did not differ with respect to maternal age, gestational age at delivery, age at SFP assessment, health insurance, or infant sex (all with p’s>0.05). Some differences among the study groups were observed for infant birth weight, gestational age at enrollment (both MAT groups were recruited earlier in gestation), race and ethnicity (MAT-only group had a higher proportion of Hispanic/Latina), marital status (lower proportion of married/cohabitating women in the MAT groups), education (higher proportion of subjects with college or professional degrees among controls), gravidity (higher proportion of primigravida among controls), BDI scores (higher proportion of subjects with BDI>13 among both MAT groups), and maternal income (lower among both MAT groups).
Table 1.
Demographic and Medical Characteristics of Participants (N= 91)
| Controls (n=34) | MAT (n=19) | Alcohol (n=15) | Alcohol+MAT (n=23) | pa | |
|---|---|---|---|---|---|
|
| |||||
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| Age* (years) | 27.1 ±5.7 | 28.4 ±5.2 | 30.3 ± 6.9 | 26.7 ±5.5 | 0.230 |
| Gestational age at enrollment (weeks) | 25.5 ± 8.0 | 20.9 ±6.7 | 28.2±6.9 | 22.1±8.7 | 0.022 |
| Gestational age at delivery (weeks) | 39.2±1.2 | 38.6 ±1.9 | 37.7 ±3.0 | 38.7 ±2.0 | 0.233w |
| Infant birth weight (grams) | 3286 ±398 | 2981 ±612 | 2856 ± 727 | 2961 ± 567 | 0.029w |
| Age at SFP assessment(months)* | 6.9±1.1 | 6.5±1.0 | 6.5±1.3 | 7.1±1.1 | 0.308 |
| n (%) | n (%) | n (%) | n (%) | ||
| Ethnicity: | 0.027 | ||||
| Hispanic/Latina | 20 (58.8) | 16 (84.2) | 5 (33.3) | 14 (60.9) | |
| Non-Hispanic/Latina | 14 (41.2) | 3 (15.8) | 10 (66.7) | 9 (39.1) | |
| Race: | <0.001 | ||||
| White | 33 (97.1) | 17 (89.5) | 8 (53.3) | 18 (78.3) | |
| African American | 0 (0.0) | 0 (0.0) | 4 (26.7) | 1 (4.4) | |
| American Indian | 0 (0.0) | 0 (0.0) | 3 (20.0) | 1 (4.4) | |
| Other | 1 (2.9) | 2 (10.5) | 0 (0.0) | 3 (13.0) | |
| Marital status: | 0.003 | ||||
| Single/separated/divorced | 9 (26.5) | 13 (68.4) | 7 (46.7) | 16 (69.6) | |
| Married/cohabitating | 25 (73.5) | 6 (31.6) | 8 (53.3) | 7 (30.4) | |
| Education Level: | 0.006 | ||||
| Less than high school | 5 (14.7) | 10 (52.6) | 3 (20.0) | 8 (34.8) | |
| High school to some college | 18 (52.9) | 9 (47.4) | 10 (66.7) | 14 (60.9) | |
| College/professional degree | 11 (32.35) | 0 (0.0) | 2 (13.3) | 1 (4.4) | |
| Health Insurance: | 1.000 | ||||
| No Insurance | 1 (2.9) | 0 (0.0) | 0 (0.0) | 1 (4.4) | |
| Any other insurance | 33 (97.1) | 19 (100) | 15 (100) | 22 (95.7) | |
| Primigravida | 0.016 | ||||
| Yes | 15 (44.12) | 3 (15.8) | 4 (26.7) | 2 (8.7) | |
| No | 19 (55.9) | 16 (84.2) | 11 (73.3) | 21 (91.3) | |
| Infant’s gender: | 0.540 | ||||
| Boy | 16 (47.1) | 12 (63.2) | 6 (40.0) | 10 (43.5) | |
| Girl | 18 (52.9) | 7 (36.8) | 9 (60.0) | 13 (56.5) | |
| BDI Score | 0.010 | ||||
| ≤13 | 33 (97.1) | 15 (79.0) | 15 (100) | 17 (73.9) | |
| >13 | 1 (2.94) | 4 (21.1) | 0 (0.0) | 6 (26.1) | |
| Income | <0.001 | ||||
| < $20,000 | 7 (20.6) | 13 (72.2) | 5 (33.3) | 16 (69.6) | |
| $20,000–$40,000 | 8 (23.5) | 5 (27.8) | 7 (46.7) | 5 (21.7) | |
| >$40,000 | 19 (55.8) | 0 (0.00) | 3 (20.0) | 2 (8.7) | |
For the continuous variables, p-values corresponds to an ANOVA F-test or Welch's modified F-test. For the categorical variables, p-values correspond to the Fisher's exact test.
Age at assessment was adjusted for prematurity
Welch's modified F-test was used to assess significant differences in the means instead of the ANOVA F-test
On average, controls reported no more than minimal alcohol use in the periconceptional period and no use during pregnancy, tested negative for all ethanol biomarkers, and had negative urine drug tests in accordance with the study group eligibility criteria (Table 2). Subjects in the Alcohol group reported use of 1.55 ±2.13 AA/day in the periconceptional period and 0.12±0.41 during pregnancy (equivalent to approximately 22 and 2 standard drinks per week, respectively). Subjects in the Alcohol+MAT group reported use of 0.84±1.70 AA/day in the periconceptional period and 0.005±0.02 during pregnancy (equivalent to approximately 12 and 0.1 standard drinks per week, respectively). In the Alcohol and Alcohol+MAT groups, the mean maximum number of drinks consumed in 24 hours during pregnancy was 10.7±13.5 and 8.8±11.0, respectively. More than half of the alcohol-exposed participants (60.0% in the Alcohol and 60.9% in the Alcohol+MAT groups) tested positive for at least one ethanol biomarker. The most prevalent positive ethanol biomarker among both exposed groups was newborn PEth-DBS (data not shown). More than one-half of participants in both MAT groups had co-exposure with other opioids (heroin and/or opioid analgesics). Tobacco use was highly prevalent in the MAT groups (> 65%), while 21.4% of participants in the Alcohol group reported tobacco use in pregnancy. Co-exposure with marijuana was also prevalent in the exposed groups and ranged from 31.6% in the MAT group to 40.0% in the Alcohol group. Polysubstance use, including alcohol, tobacco, and illicit drugs, was prevalent with 1.80±1.37 substances in the Alcohol group, 2.37±1.21 in the MAT group, and 3.13±1.32 in the Alcohol+MAT group.
Table 2.
Alcohol and Substance Use Patterns by Study Group (N= 91)
| Controls (n= 34) | MAT (n= 19) | Alcohol (n= 15) | Alcohol+MAT (n= 23) | pa | |
|---|---|---|---|---|---|
| Alcohol Use: | |||||
| 12 months prior to enrollment: | |||||
| AUDIT past 12 months: (Mean ± SD) | 0.65 ±0.77 | 0.58 ±0.90 | 12.7 ±9.9 | 10.1 ±7.5 | <0.001w |
| AUDIT≥8: n (%b) | 0 (0.0) | 0 (0.0) | 9 (60.0) | 12 (52.2) | <0.001 |
| Periconceptional period:c | |||||
| AA/day: (Mean ± SD) | 0.00±0.01 | 0.00±0.00 | 1.55 ±2.13 | 0.84 ±1.70 | <0.001 |
| During pregnancy beyond periconceptional period: | |||||
| Maximum number of drinks in 24 hours | 0.00±0.00 | 0.13±0.55 | 10.7 ±13.5 | 8.8 ±11.0 | <0.001 |
| AA/day average across pregnancy | 0.00±0.00 | 0.00±0.00 | 0.12 ±0.41 | 0.005 ±0.02 | 0.103 |
| Positive for ≥1 ethanol biomarker at study visit 1 or visit 2: n (%) | 0 (0.0) | 0 (0.0) | 9 (60.0) | 14 (60.9) | <0.001 |
| Substance Use During Pregnancy: | |||||
| MAT (methadone, buprenorphine): n (%) | 0 (0.0) | 19 (100) | 0 (0.0) | 23 (100) | <0.001 |
| Other opioids: n (%) | 0 (0.00) | 12 (63.2) | 3 (20.0) | 12(52.2) | <0.001 |
| Marijuana: n (%) | 0 (0.00) | 6 (31.6) | 6 (40.0) | 8 (34.8) | <0.001 |
| Cocaine: n (%) | 0 (0.0) | 1 (5.3) | 2 (13.3) | 3 (13.0) | 0.068 |
| Methamphetamine: n (%) | 0 (0.0) | 3 (15.8) | 0 (0.0) | 2 (8.7) | 0.036 |
| Tobacco: n (%) | 0 (0.0) | 14 (73.7) | 3 (21.4) | 15 (65.2) | <0.001 |
MAT, opioid maintenance therapy.
AA, absolute ounces of alcohol (1 standard drink = approximately 0.5 AA)
For the continuous variables, p-values corresponds to an ANOVA F-test or Welch's modified F-test. For the categorical variables, p-values correspond to the Fisher's exact test.
All percentages are column percentage with each of the study groups as the denominator
One month around LMP
Welch's modified F-test was used to assess significant differences in the means instead of the ANOVA F-test
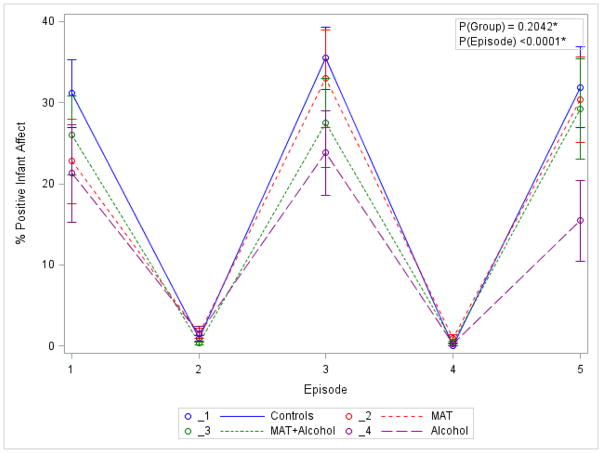
A classic SFP pattern was observed with respect to variability of infant affect between SFP episodes (Figure 1). That is, mean positive affect was much higher during the reunion/play episodes 1, 3, and 5, and was low during the ‘still-face’ episodes 2 and 4 (Wilks’ Lambda=0.33, p<0.0001). No overall difference among the four study groups was observed for this repeated measures pattern in the MANOVA model (Wilks’ lambda=0.78, p=0.204). However, a post-hoc cross-sectional analysis examining the source of variation at episode 5 demonstrated a trend for a lower positive infant affect in the Alcohol group compared to the Control group at the borderline significance level (p=0.053).
Figure 1. Variability of Infant Positive Affect during the SFP.
*p-values for group and episode correspond to the two MANOVA models examining group effect over episodes and mean difference over episodes respectively (in the latter we use a MANOVA model with only an intercept).
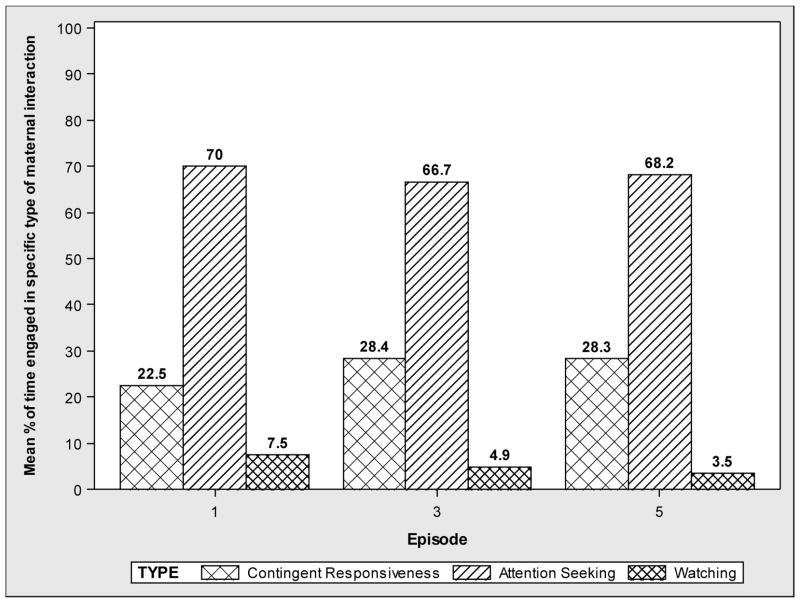
With respect to maternal behaviors, attention seeking was by far the most prevalent behavior type in each episode (66.7–70%), and ‘watching’ was the least prevalent (3.5–7.5%; Figure 2). The most supportive maternal behavior, contingent responding, was demonstrated by less than one-third (22.5–28.4%) of maternal participants. The prevalence of the contingent responding and attention seeking maternal behaviors did not vary by study group at any episode (all p’s>0.05; data not shown). There were no significant interactions between the study group and contingent responding (p=0.786), study group and attention seeking (p=0.875), infant sex and attention seeking (p=0.473), or infant sex and contingent responding (p=0.146) with respect to infant affect, while adjusting for infant age and sex, maternal education, marital status, income, BDI score, and SFP episode.
Figure 2.
Prevalence of Different Types of Maternal Interaction during the SFP Play Episodes
Results of univariate mixed effects model examining the effect of the study group demonstrated that alcohol exposure was associated with a reduction in infant positive affect across repeated measures of the SFP (β=−12.1; p=0.04). Results become non-significant, however, after adjustment for the type of maternal interaction and covariates (p’s < 0.05, Table 3). In the same multivariable mixed effects model, maternal contingent responding was positively associated with infant positive affect (β̂ =0.84; p<0.001) after adjusting for the study group (prenatal exposures), SFP episode, infant age, infant gender, BDI, income, maternal marital status, and maternal education (Table 3). The effect of infant age, infant sex, maternal education, maternal marital status, BDI, income, SFP episode, and study group were non-significant (all p-values >0.05). Similarly, maternal attention seeking was significantly associated with infant affect; however, the direction of association changed. That is, attention seeking was associated with reduction in infant positive affect after controlling for other factors (β̂ =−0.78; p<0.001). In the lagged analyses, contingent responsiveness at episodes 1 and 3 was positively associated with infant affect at episode 5 (β̂ =0.36 and β̂ =0.46, respectively; both p’s<0.01). Similarly, attention seeking at episodes 1 and 3 was negatively associated with infant affect at episode 5 (β̂ =−0.33 and β̂ = 0.51, respectively; both p’s<0.01).
Table 3.
Predictors of Positive Infant Affect: Results of Multivariable Mixed Effect Modelling
| Model A | Model B | |||
|---|---|---|---|---|
|
|
||||
| Predictors | β̂ | p | β̂ | p |
| Study group: | 0.501 | 0.502 | ||
| MAT | −0.20 | 0.965 | 0.198 | 0.970 |
| Alcohol+MAT | −4.17 | 0.310 | −2.07 | 0.656 |
| Alcohol | −4.83 | 0.243 | −6.55 | 0.163 |
| Controls | Reference | Reference | ||
| SFP episode: | 0.090 | 0.407 | ||
| 5 | −3.55 | 0.052 | −0.12 | 0.954 |
| 3 | −0.06 | 0.974 | 2.07 | 0.306 |
| 1 | Reference | Reference | ||
| Maternal interaction* (1 unit increase) | ||||
| Contingent responsiveness for Model A | 0.84 | <.001 | −0.78 | <0.001 |
| Attention seeking for Model B | ||||
| Infant age | 0.39 | 0.760 | −0.20 | 0.889 |
| Infant gender: | ||||
| Boy | −3.16 | 0.268 | −2.99 | 0.352 |
| Girl | Reference | Reference | ||
| Maternal education: | 0.264 | 0.144 | ||
| College or higher | −8.72 | 0.122 | −12.1 | 0.060 |
| HS/Some college/vocational | −1.27 | 0.702 | −1.93 | 0.605 |
| Less than HS | Reference | Reference | ||
| Maternal marital status: | ||||
| Married or living with spouse | −1.01 | 0.742 | −0.56 | 0.873 |
| Single/separated/divorced/widowed | Reference | Reference | ||
| Beck Depression Inventory score: | ||||
| > 13 | −5.87 | 0.198 | −14.4 | 0.006 |
| ≤13 | Reference | Reference | ||
| Income: | 0.537 | 0.640 | ||
| < $20,000 | 4.73 | 0.303 | 4.59 | 0.377 |
| $20,000–$40,000 | 2.92 | 0.404 | 2.71 | 0.493 |
| >$40,000 | Reference | Reference | ||
MAT, medication assisted therapy for opioid dependence; SFP, still-face paradigm; HS, high school
Contingent responsiveness and attention seeking, as predictors, could not be included in one model due to multicollinearity (both models were conducted with the entire sample size)
The model including the combined effect of the study group and covariates (SFP episode, infant age, infant sex, income, BDI, maternal marital status, and maternal education) explained 15.8 % of the variability in infant positive affect, while the model containing maternal contingent responding and covariates explained 67.1% of the variability in infant positive affect (Table 4). The combined effect of the model containing attention seeking and covariates explained 59.4% of the variability in positive affect. Cumulatively, these results indicate that infant positive affect was maximized after the SFP episode if the mother interacted in a responsive or sensitive manner. Adding study group to the model, which included SFP episode, infant age, infant sex, maternal education, marital status, income, and BDI score as predictors of infant positive affect, increased the coefficient of determination (R2) by only 6.7%, while adding maternal contingent responding to the same model increased R2 by 58.0% (Table 4).
Table 4.
Determinants of Explained Variability in Infant Positive Affect
| Modela | Primary exposure and Independent variables | RSS | R2 |
|---|---|---|---|
| 0 | Intercept only | 154,519 | NA |
| 1 | SFP episode, infant age, infant gender, and maternal education, marital status, income, and BDI score | 140,393 | 0.091 |
| 2 | Study group, SFP episode, infant age, infant gender, and maternal education , marital status, income, and BDI score | 130,038 | 0.158 |
| 3 | Contingent interaction, SFP episode, infant age, infant gender, and maternal education, marital status, income, and BDI score | 50,875 | 0.671 |
| 4 | Attention seeking, SFP episode, infant age, infant gender, and maternal education, marital status, income, and BDI score | 62,700 | 0.594 |
Infant positive affect is the outcome of interest in all models RSS, Residual sum of squares
R2, percent of the total variance explained by the model
DISCUSSION
The results of this study support and expand our previous finding in a different population (47), that a supportive parenting style, which includes acknowledgement of infant affect (such as when they were happy or sad) and uses playful games to re-engage the infant (such as peek-a-boo) had a much stronger effect on infant emotional regulation than prenatal exposures to alcohol and/or opioids. With respect to exposure to substances of abuse, there were no significant effects of prenatal substance exposure on infant affect, although there was a trend (p=0.053) for a lower infant affect in the Alcohol group compared to Controls in the last reunion/play episode, when the child is more likely to accumulate “carry-over effect” from prior SFP episodes. The lack of influence of prenatal substance exposures is further supported by the result indicating that the model containing maternal contingent responding accounted for 67% of the variance, in contrast to 16% for the model testing the group effect. This powerful observation has implications for future intervention studies focusing on specific parenting strategies for affected patients.
We also observed that maternal interaction style did not vary among the study groups. These results are consistent with a study by Tronick and colleagues (2005), which found no differences in maternal interaction style or infant self-regulation between mothers who used opioids prenatally and those who did not (58), although infant affect was not included as a measure.
A variety of maternal characteristics, such as maternal sensitivity and maternal self-esteem, have been found to predict infant emotional reactivity (59). Prior studies also indicate that sensitive maternal responding results in less negativity among infants in the general population (60, 61). In a study of mothers with diagnosed depression, infants showed more negative affect during the SFP at 6 months and also had more externalizing problems at 18 months of age (62). The longitudinal effect of maternal contingent responding has also been shown to increase positive affect in full-term infants at 4 and 9 months of age in contrast to maternal attention seeking behavior, which decreased infant positivity (48). Attention seeking behavior is that which occurred without regard to the infant’s response, and was often out of synchrony with the infant and therefore upsetting or annoying to the infant. In contrast, a mother who used contingent responding behavior was more sensitive to their infant’s response and needs. Mesman (2009) reported a number of studies using the Still Face Paradigm, which found that increased maternal sensitivity predicted more infant positive affect. Maternal sensitivity was observed when mothers used contingent responding behaviors, which are defined by the mother’s responding to her infant’s behavior. These studies further support the current findings and emphasize the importance of looking at mother-infant interactions to better understand emotional reactivity in infants. It is possible that some of the emotional dysregulation found in infants prenatally exposed to drugs and alcohol could be mediated by improved interactions between the mother-infant dyad. Animal studies provide further support for the influence of environmental factors that may alter mother-infant bonding in humans (63). Rat pups bond with rat dams with rapid attachment learning based in the olfactory and somatosensory systems to first imprint the maternal odor and then maternal touch (63). Non-alcohol exposed rat dams nurse cross-fostered PAE pups differently than control pups, indicating that maternal behavior in response to PAE is also altered (64). These early social deficits carry-over, such that PAE female rats display poorer maternal behavior (e.g., reduced pup retrieval) with their own pups (65, 66).
Parent-infant relationships consist of interactions that are mutually regulated (67), and which are important for infants’ development of self-regulatory skills (33). Gunning found that higher levels of maternal sensitivity (defined as being accepting, responsive, non-demanding, and attuned to the infant) were associated with more regulated infant behaviors during the SFP (68). Early parent-infant sensitivity at 3, 5 and 7 months resulted in infants who were more positive in their affect during the SFP, and was also predictive of later parent-infant attachment at 12–14 months of age (69). A growing body of literature has demonstrated that the effects of prenatal substance exposures may be ameliorated, to some degree, by early intervention programs that engage parents in positive interactions (70) and teach cognitive control through social interaction and play (71, 72). A ‘dynamic interactive model’ was proposed by Kodituwakku and Kodituwakku (73), which incorporates intervention in the areas of pharmacology, nutrition, social interaction, and behavioral control.
The current results are framed in the context of the known limitations to this study. The SFP has been used extensively in the literature, but is only a proxy for a stressful situation; one can only infer that the measure of affect indicates the infant was upset and therefore stressed. Early in life there are many situations that cause an infant to become upset, and the ability to self-calm or be soothed by a parent can be indicative of the infant’s ability to regulate their emotions. We acknowledge that the limited effect of prenatal exposure on infant affect could be due to the following reasons: 1) small sample size in the Alcohol group, which potentially resulted in the results being of borderline statistical significance; 2) light-to-moderate levels of alcohol consumption in this sample, especially beyond the periconceptional period; 3) prenatal substance exposure being a more distant measure as compared to maternal behavior which is measured in the same dyadic context during the SFP. Furthermore, assessing infant affect and maternal interaction style within the same paradigm limits the generalizability of the results; future studies would benefit from evaluating the effect of parental style earlier in life on more distant infant behavioral outcomes. Though there were demographic differences between the groups, these were controlled for in the multivariable analyses. Co-exposures with other substances, especially tobacco and marijuana, were prevalent among the three exposed groups; however, since Controls had no co-exposure to these substances by definition we could not adjust for them in multivariable analyses. Finally while we controlled for the key socio-demographic (marital status, maternal education, family income), medical (depressive symptoms), and infant (age at assessment, sex) factors, we recognize that there are multiple other pre- and postnatal factors which can affect infant stress reactivity and maternal behavior.
An important strength of our study was the prospective cohort design. We began following the subjects during pregnancy, which allowed us to capture substance use in greater detail beginning in the periconceptional period and throughout pregnancy. Repeated, prospective, self-reported measures of substance use were augmented by a state-of-the art battery of six ethanol biomarkers, study-specific urine drug tests, and a comprehensive review of EMR for clinically-indicated urine drug screen information.
In conclusion, we found that infants of mothers who used contingent responsiveness demonstrated more positive affect during play episodes of the SFP. Additionally, infants displayed less positivity when their mothers used attention seeking behaviors. Maternal behavior did not vary among the exposed and unexposed subjects; however, maternal behavior had a much greater influence on infant affect compared to prenatal exposures. Our findings are relevant to infants exposed to drugs and alcohol, as they are often described as dysregulated, easily over-stimulated, and irritable. These results are important because they suggest that modifiable postnatal factors play a role in infant positivity, which may help mediate effects of prenatal substance exposures.
Future directions may include developing strategies for teaching parents who have infants prenatally exposed to alcohol and other substances how to respond in a sensitive manner that is responsive to their infant’s emotion. Parents can be taught that certain behaviors, such as attention seeking, can be less pleasing or possibly annoying to their infant, while simple games, such as ‘peek-a-boo,’ can be fun and engaging. This could potentially help improve infants’ positivity, which is relevant as children prenatally exposed to alcohol have been found to have problems with ‘negative affectivity’ and irritability (74). Future work should also explore mediation analysis in the context of the SFP, focusing on the complexity of controlling for postnatal environment and maternal interaction as mediators. Future studies should examine the effects of the timing of exposure and different patterns of substance use on infant stress reactivity. Finally, longitudinal studies should also explore if the improvement of infant-mother interactions lead to decreased behavioral problems and/or improved social functioning across childhood in this vulnerable population.
Highlights.
This paper examined the contributing effects of prenatal substance use (alcohol, opioids, and their combination) and parenting style (operationalized as contingent responding during the play episodes of the Still-face paradigm [SFP]) on infant affect in a prospective cohort of 91 maternal-infant dyads.
The combined effect of prenatal exposures and covariates explained 15.8% of the variability in infant positive affect, while the model including contingent responding and covariates explained 67.1% of the variability.
Maternal behavior did not vary among the exposed and unexposed subjects; however, maternal behavior explained more variability in infant affect than prenatal exposures, providing the basis for future intervention studies focusing on specific parenting strategies.
Acknowledgments
We are thankful to Crystal Aragon, M.A.; Natalia Moss, PhDc,; Joy Van Meter, B.A.; Steven Bishop, M.S.; Sonnie Williams, B.S.; Laura Garrison, M.A., and Yuridia Leyva, M.S for recruitment of study subjects, data collection, coding and/or reviewing the SFP videos, and for assistance with the data management and analysis. We are also in debt to Dr. William Rayburn for assisting with the research design and providing access to the study population, and to Sandra Cano, M.A. for coordinating research activities.
Financial disclosure: This work has been supported by research grants from NIAAA/NIH (1R01AA021771, 1R03AA020170). For statistical support, we acknowledge the University of New Mexico Clinical & Translational Science Center (UL1TR001449) and the Mountain West Clinical Translational Research Infrastructure Network (5 U54 GM104944) from the National Institute of General Medical Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Behnke M, Smith VC, Levy S, Ammerman SD, Gonzalez PK, Ryan SA, et al. Prenatal substance abuse: short-and long-term effects on the exposed fetus. Pediatrics. 2013;131(3):e1009–e24. doi: 10.1542/peds.2012-3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson BL, Levitt P, Stanwood GD. Prenatal exposure to drugs: effects on brain development and implications for policy and education. Nat Rev Neurosci. 2009;10(4):303–12. doi: 10.1038/nrn2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riley E, Infante MA, Warren K. Fetal Alcohol Spectrum Disorders: An Overview. Neuropsychol Rev. 2011;21(2):73–80. doi: 10.1007/s11065-011-9166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Streissguth AP, Bookstein FL, Sampson PD, Barr HM. Attention: Prenatal alcohol and continuities of vigilance and attentional problems from 4 through 14 years. Development and Psychopathology. 1995;7(03):419–46. [Google Scholar]
- 5.Kodituwakku PW, Kalberg W, May PA. The effects of prenatal alcohol exposure on executive functioning. Alcohol research & health : the journal of the National Institute on Alcohol Abuse and Alcoholism. 2001;25(3):192–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Min MO, Singer LT, Minnes S, Wu M, Bearer CF. Association of Fatty Acid Ethyl Esters in Meconium and Cognitive Development during Childhood and Adolescence. J Pediatr. 2015;166(4):1042–7. doi: 10.1016/j.jpeds.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coles CD, Kable JA, Taddeo E. Math performance and behavior problems in children affected by prenatal alcohol exposure: intervention and follow-up. Journal of developmental and behavioral pediatrics : JDBP. 2009;30(1):7–15. doi: 10.1097/DBP.0b013e3181966780. [DOI] [PubMed] [Google Scholar]
- 8.Raineki C, Chew L, Mok P, Ellis L, Weinberg J. Short- and long-term effects of stress during adolescence on emotionality and HPA function of animals exposed to alcohol prenatally. Psychoneuroendocrinology. 2016;74:13–23. doi: 10.1016/j.psyneuen.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haley DW, Handmaker NS, Lowe J. Infant stress reactivity and prenatal alcohol exposure. Alcoholism, clinical and experimental research. 2006;30(12):2055–64. doi: 10.1111/j.1530-0277.2006.00251.x. [DOI] [PubMed] [Google Scholar]
- 10.Wieczorek L, Fish EW, O'Leary-Moore SK, Parnell SE, Sulik KK. Hypothalamic-pituitary-adrenal axis and behavioral dysfunction following early binge-like prenatal alcohol exposure in mice. Alcohol. 2015;49(3):207–17. doi: 10.1016/j.alcohol.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore EM, Riley EP. What Happens When Children with Fetal Alcohol Spectrum Disorders Become Adults? Curr Dev Disord Rep. 2015;2(3):219–27. doi: 10.1007/s40474-015-0053-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tetrault JM, Butner JL. Non-Medical Prescription Opioid Use and Prescription Opioid Use Disorder: A Review. The Yale journal of biology and medicine. 2015;88(3):227–33. [PMC free article] [PubMed] [Google Scholar]
- 13.Patrick SW, Davis MM, Lehman CU, Cooper WO. Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012. Journal of perinatology : official journal of the California Perinatal Association. 2015;35(8):667. doi: 10.1038/jp.2015.63. [DOI] [PubMed] [Google Scholar]
- 14.Beckwith AM, Burke SA. Identification of early developmental deficits in infants with prenatal heroin, methadone, and other opioid exposure. Clinical pediatrics. 2015;54(4):328–35. doi: 10.1177/0009922814549545. [DOI] [PubMed] [Google Scholar]
- 15.Ornoy A. The impact of intrauterine exposure versus postnatal environment in neurodevelopmental toxicity: long-term neurobehavioral studies in children at risk for developmental disorders. Toxicology letters. 2003;140–141:171–81. doi: 10.1016/s0378-4274(02)00505-2. [DOI] [PubMed] [Google Scholar]
- 16.de Cubas MM, Field T. Children of methadone-dependent women: developmental outcomes. The American journal of orthopsychiatry. 1993;63(2):266–76. doi: 10.1037/h0079429. [DOI] [PubMed] [Google Scholar]
- 17.Locke RL, Lagasse LL, Seifer R, Lester BM, Shankaran S, Bada HS, et al. Effects of prenatal substance exposure on infant temperament vary by context. Dev Psychopathol. 2016;28(2):309–26. doi: 10.1017/S0954579415000504. [DOI] [PubMed] [Google Scholar]
- 18.Chen W-JA, Maier SE. Combination drug use and risk for fetal harm. Alcohol research & health : the journal of the National Institute on Alcohol Abuse and Alcoholism. 2011;34(1):27. [PMC free article] [PubMed] [Google Scholar]
- 19.Ornoy A, Michailevskaya V, Lukashov I, Bar-Hamburger R, Harel S. The developmental outcome of children born to heroin-dependent mothers, raised at home or adopted. Child abuse & neglect. 1996;20(5):385–96. doi: 10.1016/0145-2134(96)00014-2. [DOI] [PubMed] [Google Scholar]
- 20.Eiden RD, Coles CD, Schuetze P, Colder CR. Externalizing behavior problems among polydrug cocaine-exposed children: Indirect pathways via maternal harshness and self-regulation in early childhood. Psychology of addictive behaviors : journal of the Society of Psychologists in Addictive Behaviors. 2014;28(1):139–53. doi: 10.1037/a0032632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eiden RD, Schuetze P, Coles CD. Maternal cocaine use and mother-infant interactions: Direct and moderated associations. Neurotoxicology and teratology. 2011;33(1):120–8. doi: 10.1016/j.ntt.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Locke TF, Newcomb M. Child maltreatment, parent alcohol and drug-related problems, polydrug problems, and parenting practices: a test of gender differences and four theoretical perspectives. Journal of family psychology : JFP : journal of the Division of Family Psychology of the American Psychological Association (Division 43) 2004;18(1):120–34. doi: 10.1037/0893-3200.18.1.120. [DOI] [PubMed] [Google Scholar]
- 23.Parolin M, Simonelli A. Attachment Theory and Maternal Drug Addiction: The Contribution to Parenting Interventions. Frontiers in psychiatry. 2016;7:152. doi: 10.3389/fpsyt.2016.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suchman NE, DeCoste CL, McMahon TJ, Dalton R, Mayes LC, Borelli J. Mothering From the Inside Out: Results of a second randomized clinical trial testing a mentalization-based intervention for mothers in addiction treatment. Dev Psychopathol. 2017;29(2):617–36. doi: 10.1017/S0954579417000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rutherford HJ, Williams SK, Moy S, Mayes LC, Johns JM. Disruption of maternal parenting circuitry by addictive process: rewiring of reward and stress systems. Frontiers in psychiatry. 2011;2:37. doi: 10.3389/fpsyt.2011.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gottwald SR, Thurman SK. The effects of prenatal cocaine exposure on mother--infant interaction and infant arousal in the newborn period. Topics in Early Childhood Special Education. 1994;14(2):217–31. [Google Scholar]
- 27.Krauss RB, Thurman SK, Brodsky N, Betancourt L, Giannetta J, Hurt H. Caregiver interaction behavior with prenatally cocaine-exposed and nonexposed preschoolers. Journal of Early Intervention. 2000;23(1):62–73. [Google Scholar]
- 28.Bagner DM, Coxe S, Hungerford GM, Garcia D, Barroso NE, Hernandez J, et al. Behavioral Parent Training in Infancy: A Window of Opportunity for High-Risk Families. J Abnorm Child Psychol. 2016;44(5):901–12. doi: 10.1007/s10802-015-0089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eisenberg N, Cumberland A, Spinrad TL, Fabes RA, Shepard SA, Reiser M, et al. The relations of regulation and emotionality to children's externalizing and internalizing problem behavior. Child Dev. 2001;72(4):1112–34. doi: 10.1111/1467-8624.00337. [DOI] [PubMed] [Google Scholar]
- 30.Clark CA, Woodward LJ, Horwood LJ, Moor S. Development of emotional and behavioral regulation in children born extremely preterm and very preterm: biological and social influences. Child Dev. 2008;79(5):1444–62. doi: 10.1111/j.1467-8624.2008.01198.x. [DOI] [PubMed] [Google Scholar]
- 31.Ursache A, Blair C, Stifter C, Voegtline K. Emotional reactivity and regulation in infancy interact to predict executive functioning in early childhood. Developmental psychology. 2013;49(1):127–37. doi: 10.1037/a0027728. [DOI] [PubMed] [Google Scholar]
- 32.Tronick E, Als H, Adamson L, Wise S, Brazelton TB. The infant's response to entrapment between contradictory messages in face-to-face interaction. Journal of the American Academy of Child Psychiatry. 1978;17(1):1–13. doi: 10.1016/s0002-7138(09)62273-1. [DOI] [PubMed] [Google Scholar]
- 33.Tronick E, Beeghly M. Infants' meaning-making and the development of mental health problems. American Psychologist. 2011;66(2):107. doi: 10.1037/a0021631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mesman J, van IJzendoorn MH, Bakermans-Kranenburg MJ. The many faces of the still-face paradigm: a review and meta-analysis. Developmental Review. 2009;29(2):120–62. [Google Scholar]
- 35.Haley DW, Handmaker NS, Lowe J. Infant stress reactivity and prenatal alcohol exposure. Alcoholism, clinical and experimental research. 2006;30(12):2055–64. doi: 10.1111/j.1530-0277.2006.00251.x. [DOI] [PubMed] [Google Scholar]
- 36.Jirikowic T, Chen M, Nash J, Gendler B, Carmichael Olson H. Regulatory Behaviors and Stress Reactivity among Infants at High Risk for Fetal Alcohol Spectrum Disorders: An Exploratory Study. Journal of Mental Health Research in Intellectual Disabilities. 2016;9(3):171–88. [Google Scholar]
- 37.Flak AL, Su S, Bertrand J, Denny CH, Kesmodel US, Cogswell ME. The association of mild, moderate, and binge prenatal alcohol exposure and child neuropsychological outcomes: a meta-analysis. Alcoholism, clinical and experimental research. 2014;38(1):214–26. doi: 10.1111/acer.12214. [DOI] [PubMed] [Google Scholar]
- 38.Meyer-Leu Y, Lemola S, Daeppen JB, Deriaz O, Gerber S. Association of moderate alcohol use and binge drinking during pregnancy with neonatal health. Alcoholism, clinical and experimental research. 2011;35(9):1669–77. doi: 10.1111/j.1530-0277.2011.01513.x. [DOI] [PubMed] [Google Scholar]
- 39.Carter RC, Jacobson JL, Molteno CD, Jiang H, Meintjes EM, Jacobson SW, et al. Effects of heavy prenatal alcohol exposure and iron deficiency anemia on child growth and body composition through age 9 years. Alcoholism, clinical and experimental research. 2012;36(11):1973–82. doi: 10.1111/j.1530-0277.2012.01810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ware AL, Glass L, Crocker N, Deweese BN, Coles CD, Kable JA, et al. Effects of prenatal alcohol exposure and attention-deficit/hyperactivity disorder on adaptive functioning. Alcoholism, clinical and experimental research. 2014;38(5):1439–47. doi: 10.1111/acer.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoyme HE, Kalberg WO, Elliott AJ, Blankenship J, Buckley D, Marais AS, et al. Updated Clinical Guidelines for Diagnosing Fetal Alcohol Spectrum Disorders. Pediatrics. 2016;138(2) doi: 10.1542/peds.2015-4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sobell L, Sobell M. Timeline Follow-Back. In: Litten R, Allen J, editors. Measuring Alcohol Consumption. Humana Press; 1992. pp. 41–72. [Google Scholar]
- 43.Bakhireva LN, Lowe JR, Gutierrez HL, Stephen JM. Ethanol, Neurodevelopment, Infant and Child Health (ENRICH) prospective cohort: Study design considerations. Advances in pediatric research. 2015;2(2015) doi: 10.12715/apr.2015.2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gutierrez HL, Hund L, Shrestha S, Rayburn WF, Leeman L, Savage DD, et al. Ethylglucuronide in maternal hair as a biomarker of prenatal alcohol exposure. Alcohol. 2015;49(6):617–23. doi: 10.1016/j.alcohol.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bakhireva LN, Leeman L, Savich RD, Cano S, Gutierrez H, Savage DD, et al. The validity of phosphatidylethanol in dried blood spots of newborns for the identification of prenatal alcohol exposure. Alcoholism, clinical and experimental research. 2014;38(4):1078–85. doi: 10.1111/acer.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bakhireva LN, Savage DD. Focus on: biomarkers of fetal alcohol exposure and fetal alcohol effects. Alcohol research & health : the journal of the National Institute on Alcohol Abuse and Alcoholism. 2011;34(1):56–63. [PMC free article] [PubMed] [Google Scholar]
- 47.Lowe J, Handmaker N, Aragón C. Impact of mother interactive style on infant affect among babies exposed to alcohol in utero. Inf Mental Hlth J. 2006;27(4):371–82. doi: 10.1002/imhj.20098. [DOI] [PubMed] [Google Scholar]
- 48.Lowe JR, MacLean PC, Duncan AF, Aragón C, Schrader RM, Caprihan A, et al. Association of maternal interaction with emotional regulation in 4-and 9-month infants during the Still Face Paradigm. Infant Behav Dev. 2012;35(2):295–302. doi: 10.1016/j.infbeh.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Erickson SJ, Lowe JR. The role of maternal responsiveness in predicting infant affect during the still face paradigm with infants born very low birth weight. Inf Mental Hlth J. 2008;29(2):114–32. doi: 10.1002/imhj.20172. [DOI] [PubMed] [Google Scholar]
- 50.Haley DW, Stansbury K. Infant stress and parent responsiveness: regulation of physiology and behavior during still-face and reunion. Child development. 2003;74(5):1534–46. doi: 10.1111/1467-8624.00621. [DOI] [PubMed] [Google Scholar]
- 51.Devore JL, Berk KN. Modern mathematical statistics with applications. Cengage Learning; 2007. [Google Scholar]
- 52.Welch B. On the comparison of several mean values: an alternative approach. Biometrika. 1951;38(3/4):330–6. [Google Scholar]
- 53.Qeadan F On MANOVA using STATA, SAS & R. A short course in biostatistics for the Mountain West Clinical Translational Research Infrastructure Network. University of New Mexico Health Sciences Center; Albuquerque, New Mexico: 2015. [Google Scholar]
- 54.Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. John Wiley & Sons; 2012. [Google Scholar]
- 55.Qeadan F. Longitudinal Data Analysis by Example. 2016 Available from: http://hsc.unm.edu/research/ctsc/Docs/longitudinal.pdf.
- 56.Cnaan A, Laird NM, Slasor P. Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat Med. 1997;16(20):2349–80. doi: 10.1002/(sici)1097-0258(19971030)16:20<2349::aid-sim667>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 57.Xu R. Measuring explained variation in linear mixed effects models. Statistics in medicine. 2003;22(22):3527–41. doi: 10.1002/sim.1572. [DOI] [PubMed] [Google Scholar]
- 58.Tronick EZ, Messinger DS, Weinberg MK, Lester BM, Lagasse L, Seifer R, et al. Cocaine exposure is associated with subtle compromises of infants' and mothers' social-emotional behavior and dyadic features of their interaction in the face-to-face still-face paradigm. Developmental psychology. 2005;41(5):711–22. doi: 10.1037/0012-1649.41.5.711. [DOI] [PubMed] [Google Scholar]
- 59.Mastergeorge AM, Paschall K, Loeb SR, Dixon A. The Still-Face Paradigm and bidirectionality: Associations with maternal sensitivity, self-esteem and infant emotional reactivity. Infant Behav Dev. 2014;37(3):387–97. doi: 10.1016/j.infbeh.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 60.Rosenblum KL, McDonough S, Muzik M, Miller A, Sameroff A. Maternal representations of the infant: Associations with infant response to the still face. Child development. 2002;73(4):999–1015. doi: 10.1111/1467-8624.00453. [DOI] [PubMed] [Google Scholar]
- 61.Tarabulsy GM, Provost MA, Deslandes J, St-Laurent D, Moss E, Lemelin J-P, et al. Individual differences in infant still-face response at 6 months. Infant Behav Dev. 2003;26(3):421–38. [Google Scholar]
- 62.Moore GA, Cohn JF, Campbell SB. Infant affective responses to mother's still face at 6 months differentially predict externalizing and internalizing behaviors at 18 months. Developmental psychology. 2001;37(5):706. [PubMed] [Google Scholar]
- 63.Landers MS, Sullivan RM. The development and neurobiology of infant attachment and fear. Developmental neuroscience. 2012;34(2–3):101–14. doi: 10.1159/000336732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marino MD, Cronise K, Lugo JN, Jr, Kelly SJ. Ultrasonic vocalizations and maternal-infant interactions in a rat model of fetal alcohol syndrome. Developmental psychobiology. 2002;41(4):341–51. doi: 10.1002/dev.10077. [DOI] [PubMed] [Google Scholar]
- 65.Barron S, Riley EP. Pup-induced maternal behavior in adult and juvenile rats exposed to alcohol prenatally. Alcoholism, clinical and experimental research. 1985;9(4):360–5. doi: 10.1111/j.1530-0277.1985.tb05560.x. [DOI] [PubMed] [Google Scholar]
- 66.Wilson JH, Kelly SJ, Wilson MA. Early postnatal alcohol exposure in rats: maternal behavior and estradiol levels. Physiology & behavior. 1996;59(2):287–93. doi: 10.1016/0031-9384(95)02094-2. [DOI] [PubMed] [Google Scholar]
- 67.Beeghly M, Tronick E. Early resilience in the context of parent–infant relationships: A social developmental perspective. Current problems in pediatric and adolescent health care. 2011;41(7):197–201. doi: 10.1016/j.cppeds.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gunning M, Halligan SL, Murray L. Contributions of maternal and infant factors to infant responding to the Still Face paradigm: A longitudinal study. Infant Behav Dev. 2013;36(3):319–28. doi: 10.1016/j.infbeh.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 69.Braungart-Rieker JM, Zentall S, Lickenbrock DM, Ekas NV, Oshio T, Planalp E. Attachment in the making: Mother and father sensitivity and infants’ responses during the Still-Face Paradigm. Journal of experimental child psychology. 2014;125:63–84. doi: 10.1016/j.jecp.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bertrand J. Interventions for children with fetal alcohol spectrum disorders (FASDs): overview of findings for five innovative research projects. Research in developmental disabilities. 2009;30(5):986–1006. doi: 10.1016/j.ridd.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 71.Diamond A, Barnett WS, Thomas J, Munro S. Preschool program improves cognitive control. Science (New York, NY) 2007;318(5855):1387–8. doi: 10.1126/science.1151148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bodrova E, Leong DJ. Play and early literacy: A Vygotskian approach. Play and literacy in early childhood: Research from multiple perspectives. 2007:185–200. [Google Scholar]
- 73.Kodituwakku PW, Kodituwakku EL. From research to practice: an integrative framework for the development of interventions for children with fetal alcohol spectrum disorders. Neuropsychol Rev. 2011;21(2):204–23. doi: 10.1007/s11065-011-9170-1. [DOI] [PubMed] [Google Scholar]
- 74.Kable JA, O'Connor MJ, Olson HC, Paley B, Mattson SN, Anderson SM, et al. Neurobehavioral Disorder Associated with Prenatal Alcohol Exposure (ND-PAE): Proposed DSM-5 Diagnosis. Child psychiatry and human development. 2015 doi: 10.1007/s10578-015-0566-7. [DOI] [PubMed] [Google Scholar]