Abstract
Detection and toxicity assessment of waterborne contaminants are crucial for protecting human health and the environment. Development of easy-to-implement, rapid and cost-effective tools to measure anthropogenic effects on watersheds are critical for responsible management, particularly in times of increasing development and urbanization. Traditionally, environmental toxicology has focused on limited endpoints, such as lethality and fertility, which are directly affecting population levels. However, more sensitive readings are needed to assess sub-lethal effects. Monitoring of contaminant-induced behavior alterations was proposed before, but is difficult to implement in the wild and performing it in aquatic laboratory models seem more suited. For this purpose, we adapted a photo-dependent swimming response (PDR) that was previously described in zebrafish larva. We first asked if PDR was present in other aquatic animals. We measured PDR in larvae from two freshwater prawn species (Macrobrachium rosenbergii, MR, and Macrobrachium carcinus, MC) and from another fish the fathead minnow (FHM, Pimephales promelas). In all, we found a strong and reproducible species-specific PDR, which is arguing that this behavior is important, therefore an environmental relevant endpoint. Next, we measured PDR in fish larvae after acute exposure to copper, a common waterborne contaminant. FHM larvae were hyperactive at all tested concentrations in contrast to ZF larvae, which exhibited a concentration-dependent hyperactivity. In addition to this well-accepted anxiety-like behavior, we examined two more: photo-stimulated startle response (PSSR) and center avoidance (CA). Both were significantly increased. Therefore, PDR measures after acute exposure to this waterborne contaminant provided as sensitive readout for its detection and toxicity assessment. This approach represents an opportunity to diagnostically examine any substance, even when present in complex mixtures like ambient surface waters. Mechanistic studies of toxicity using the extensive molecular tool kit of ZF could be a direct extension of such approaches.
Keywords: Diagnostic waterborne contaminant screening, zebrafish, fathead minnow, macrobrachium, copper, larval photo-dependent swimming response, photo-stimulated startle response, center avoidance, anxiety-like behavior
1. Introduction
Water resources are continuously and increasingly stressed by human populations. Monitoring of contaminants in drinking water and watersheds represents an essential service of environmental public health (https://www.cdc.gov/nceh/ehs/10-essential-services/index.html), which is crucial for human health and for the environment1–5. Toxicity assessment of anthropogenic compounds has been performed with battery of in-vitro6,7 and in-vivo tests8–12. This helps regulatory agencies to establish toxicity thresholds. However, most thresholds for specific chemicals are based on endpoints, like mortality and fertility, which have an extreme impact on a species survival9,13–15. Increasingly, new endpoints associated with more specific potential adverse outcomes have been examined, such as embryonic spontaneous movements16, larval heartbeat and hatching rates17, shelter seeking18, and molting19 that allow detection of more subtle developmental disruptors. The needs for such tests is growing as new classes of chemicals are identified, often called contaminants of emerging concern (CECs), which have the potential to produce adverse effects even at nanomolar concentrations. Detection of CECs and other contaminants has advanced greatly with current analytical methods4 and more adequate monitoring of aquatic environments20, but read-outs for physiological adverse effects are still lacking. Chronical contaminant exposure probably causes ecological and physiological alterations at concentrations far lower than the presently established thresholds. Thus, it is crucial to develop sensitive assays able to detect more subtle effects of aquatic contaminants.
Alterations of the fauna behavior has been proposed as an alternative to survival monitoring21. Contaminant-induced behavioral changes may serve as a more subtle readout for physiological and ecological fitness22. Within the broad spectrum of behaviors, anxiety-like behaviors are amongst those that can be quantified more readily, and numerous animal assays that were established for biomedical purposes could be adapted for environmental toxicology and diagnostic applications23,24. Increased anxiety-like behaviors are likely to impact survival in the wild by affecting key responses like escaping a predator, avoiding a threat, parenting, or searching for food25. Similarly, social interactions necessary for mating could be compromised26–28. Recently, innovative work showed altered behavior in adult wild perch exposed to psychiatric drugs because of uncontrolled urbanization29. However, these types of studies on animals chronically exposed in their natural habitat are extremely challenging and not easily amenable to diagnostic applications. Reproducing chronical exposure in a laboratory setting comes with the same shortcomings. Therefore, behavioral assays detecting changes after acute exposure would present clear advantages for rapid and cost-effective pollution assessment.
The genetic and developmental laboratory model, zebrafish (ZF, Danio rerio) is widely used for human disease modeling30–34 and biomedical toxicological studies35. Multiple parallel behavior measurements can be performed rapidly, thus enabling high-throughput screening36. Statistical power is increased through adjusted sample size37 and substances can be readily tested at multiple concentrations38, which is indispensable for toxicological studies. Screens for small molecules39, neuro-active drugs40, and mutations affecting locomotor41 and visual systems42 were performed, but not applied to environmental research. Various photo-dependent swimming responses have been previously measured in ZF embryos and larvae43–46. In particular, a highly reproducible photo-dependent swimming response (PDR) was described in 6 day post fertilization (dpf) larvae43,47. To be relevant for aquatic contamination and environmental adverse outcome detection, PDR should be a conserved behavior found in various aquatic species. To be a useful environmental diagnostic tool, PDR should be modulated by waterborne contaminants, especially after acute exposure.
To assess if PDR was a commonly found behavior in larval aquatic species, including in invertebrates, we measured it in two freshwater prawn species. Macrobrachium rosenbergii (MR) is extensively farmed and adults are routinely used in behavior studies48,49, thus they can be assimilated to a laboratory model50,51. Macrobrachium carcinus (MC), also called the big claw river prawn, is mostly found in the wild from Florida to southern Brazil, including most of the Caribbean islands52. Next, we measured PDR in fathead minnow (FHM, Pimephales promelas) which is the model of choice for aquatic toxicology testing53. We found a strong species-specific PDR in all animals tested, arguing that it is strongly conserved behavior thus, a physiological relevant endpoint usable for environmental contaminant toxicity assessment and detection.
To test PDR alteration after acute exposure to a waterborne contaminant, we exposed ZF and FHM larvae to copper (Cu), a common contaminant in watersheds. We found that PDR was significantly altered in both fish species. Animals were more active, FHM larvae at all concentrations tested and ZF larvae in a [Cu]-dependent manner. FHM seems more sensitive but ZF responses were strikingly more reproducible and robust, which would arguably make it the model of choice. Hyperactivity is a well-accepted measure of anxiety-like behavior37, and so are photo-stimulated startle response (PSSR)45,54–56 and center avoidance (CA)57,58, which we also measured. PSSR and CA were also increased, therefore corroborating the observed hyperactivity.
Taken together, we established that PDR was a strong behavior found in two invertebrate and two vertebrate aquatic species. PDR was significantly altered by acute exposure to copper, in both fish species. Highly reproducible [Cu]-dependent hyperactivity in ZF larvae allowed us to further analyze two additional anxiety-like behaviors, PSSR and CA which were both significantly increased. Thus, contaminant-induced PDR-alterations are sensitive and relevant endpoints for diagnostic screening of aquatic contamination. This approach, when coupled with mechanistic studies of toxicity using the extensive molecular tool kit of ZF could be further used for understanding adverse outcomes of a contaminant present in complex mixtures like ambient surface waters.
2. Results
2.1 PDR is a highly reproducible larval behavior in crustacean Macrobrachium rosenbergii (MR) and Macrobrachium carcinus
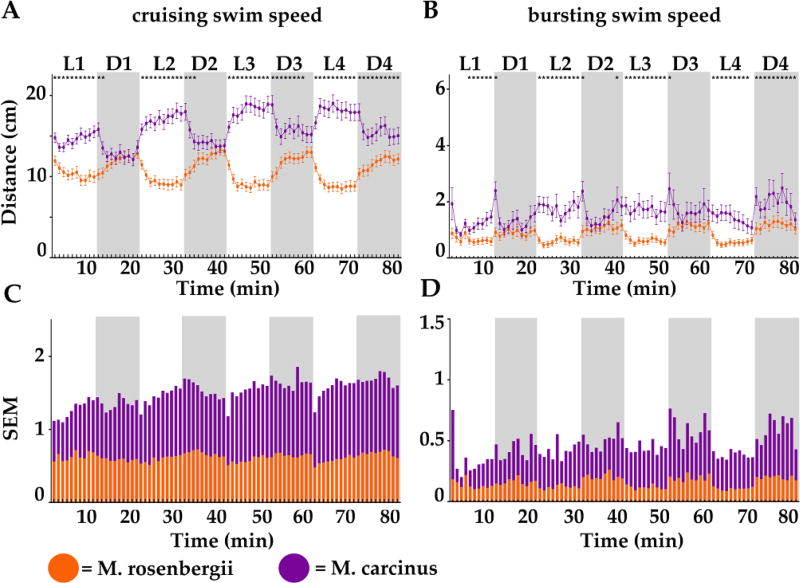
(MC) To validate larval PDR as an appropriate and relevant endpoint for establishing an environmental diagnostic tool, we first asked if this behavior was present in other aquatic species. We tested two invertebrate species Macrobrachium rosenbergii (MR) and Macrobrachium carcinus (MC). We monitored the swimming activity of individual untreated crustacean larvae (stage 1 = 0–2-day post hatching) of MR (Fig.1 orange lines and sur-imposed bars) and of MC (purple lines and bars). We recorded during 4 cycles of alternating light (L1 to L4, white boxes) and dark (D1 to D4, grey boxes) periods of 10 minutes each. We reported the mean travelled distance (in cm) per larva (n = 72) and per minute for 80 consecutive minutes, distinguishing two swim speeds: cruising (Fig.1A and C) and bursting (Fig. 1B and D). Remarkably, both MR and MC showed a clear light dependent behavioral pattern, but inverted when compared to each other.
Figure 1.
Distances travelled by minute by 2-day post-hatching larvae of Macrobrachium rosenbergii (MR, orange) and Macrobrachium carcinus (purple) measured in 4 successive cycles of 10-minute-long alternating light (L, white boxes) and dark (D, grey boxes) periods. A and B. Swimming activity was divided according to swim speed (S): cruising (0.2 cm/s < S < 1.3 cm/s) shown in A and bursting (S > 1.3 cm/s) shown in B. C and D. Standard errors of the mean (SEM) of averaged distances travelled for each recorded time point (also represented as error bars in A and B) were graphed for cruising (in C) and for bursting (in D) for larvae of MR (super-imposed orange bars) or MC (purple bars). Statistical significance (* = p <0.05) of A and B are shown in top panels A and B and values are reported in the supplemental data section.
MR (orange lines) displayed in both swim speeds, high activity in the dark that was greatly lowered in the light. In dark (D1 to D4, dark boxes), MR larvae steadily increased activity to ~12 cm/min in cruising speed (Fig. 1A) and to ~1 cm/min in bursting speed (Fig.1B). Activity was lowered in all light periods (L1 to L4, light boxes) to ~8 cm/min in cruising (Fig. 1A) and ~0.5 cm/min in bursting (Fig. 1B) swim speeds. Remarkably, at all measured time points, there was a highly consistent response from larva to larva as illustrated by the small standard error (SEM, Fig. 1A and B orange error bars), which we also reported separately (Fig. 1C and D, superimposed orange bars), thus highlighting the strong reproducibility of this larval swimming behavior in MR.
MC (purple lines) displayed in cruising swim speed, high activity in the light that was significantly decreased in the dark, but activity was more erratic in bursting swim speed. In light (L1 to L4, light boxes), MC larvae increased activity to ~18 cm/min in cruising speed (Fig. 1A) and to ~2 cm/min in bursting speed (Fig.1B). Activity was lowered in all dark periods (D1 to D4, dark boxes) to ~15 cm/min in cruising (Fig. 1A). Noticeably and unlike MR recordings, the travelled distance was highly variable from larva to larva as indicated by the large SEM (Purple errors bars in Fig. 1A and B). This was even more obvious when reporting SEM of both species for each time point separately (Fig. 1C and D). Values for MC (purple bars) were approximately double the values for MR (sur-imposed orange bars). Interestingly, both species exhibited an opposite PDR when compared to each other suggesting a species-specific response. Finally, the fact that this behavior was found in both tested invertebrates argued that it is a conserved behavior that is found even in distantly related aquatic species and therefore validating it as a good endpoint to be used in an environmental diagnostic tool.
2.2 PDR is a highly reproducible larval behavior in zebrafish (ZF) and fathead minnow (FHM)
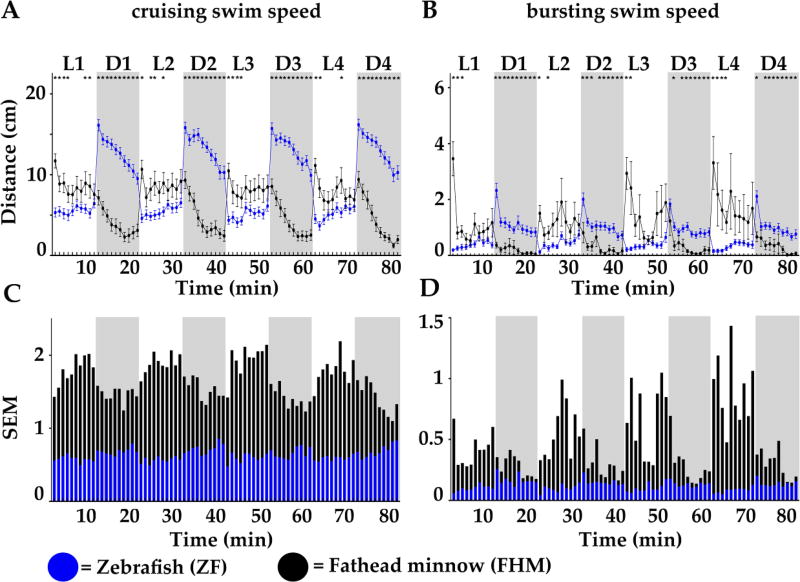
To further validate PDR as a commonly found aquatic behavior, we monitored and compared the swimming activity of individual untreated 6-day post-fertilization (dpf) larva of zebrafish (ZF, Fig. 2 blue lines and bars) and of fathead minnow (FHM, black lines and bars). Mean travel distance/larva (ZF, n = 40, and FHM, n = 40) were reported in two swim speeds: cruising (Fig.2A) and bursting (Fig. 2B). As expected for ZF but also for FHM, we found a strong and highly reproducible PDR, but surprisingly the behavioral pattern was inverted when compared to each other like what we observed in crustaceans’ larvae.
Figure 2.
Distanc2es travelled per minute by 6-day post fertilization (dpf) larvae of zebrafish (ZF, blue) and fathead minnow (FHM, black) in 4 successive cycles of 10 minute long alternating light (L, white boxes) and dark (D, grey boxes) periods. A and B. Swimming activity was divided according to swim speed (S): cruising (0.2 cm/s < S < 2 cm/s) shown in A, and bursting (S > 2.0 cm/s) shown in B. Standard errors of the mean (SEM) of averaged distances travelled for each recorded time point (also represented as error bars in A and B) were graphed for cruising (in C) and for bursting (in D) for ZF (super-imposed blue bars) and FHM (black bars) larvae. Statistical significance (* = p <0.05) of A and B are reported in the supplemental data section.
ZF displayed high activity in the dark that was greatly lowered in the light. During all light periods, cruising activity was drastically reduced to ~5 cm/min. It abruptly rose to 17 cm/min just after the light was turned off and then gradually decreased to 10 cm/min over the rest of each dark period. Likewise, bursting activity in light periods was below 0.5 cm/min, which increased to 2 cm/min when light was turned off and then gradually stabilized to ~1 cm/min during the remaining of the dark periods. Remarkably, at all measured time points the response was highly consistent from larva to larva as illustrated by the small SEM (Blue error bars and Fig. 2C and D blue bars) highlighting the strong reproducibility and the robustness of this swimming behavior in ZF larvae.
FHM displayed high activity in light, which was gradually lowered in dark. During light periods, cruising activity was ~7 cm/min and the bursting activity between 1–3 cm/min. When the light was switched off, a gradual decrease in activity was measured in all dark periods. In cruising swim speed, the travelled distances decreased from ~7 to 1.5 cm/min. In bursting swim speed, the travelled distances quickly dropped below 0.3 cm/min. Importantly, there was a relatively large variation in travelled distances from larva to larva as shown by the large SEM (black errors bars in Fig. 2A and B). SEM in cruising swim speed (Fig. 2C, black bars) varied from ~1.3 to > 2, which was in striking contrast with ZF SEM (superimposed blue bars) which were steadily around~ 0.5 overall. In bursting swim speed (Fig. 2D), differences were even more drastic, especially during light periods, with ZF SEM ~0.3 compared to FHM SEM up to 1.5. This clearly indicated that the swimming behavior was less reproducible in FHM larvae arguing that the ZF model would be more adapted for this type of behavioral assays. Nevertheless, we found a strong species-specific PDR, which again highlighted the physiological importance of this swimming behavior for each species, arguing for the relevance of its usage in an environmental diagnostic tool.
2.3 Acute copper exposure significantly alters PDR in both ZF and FHM larvae
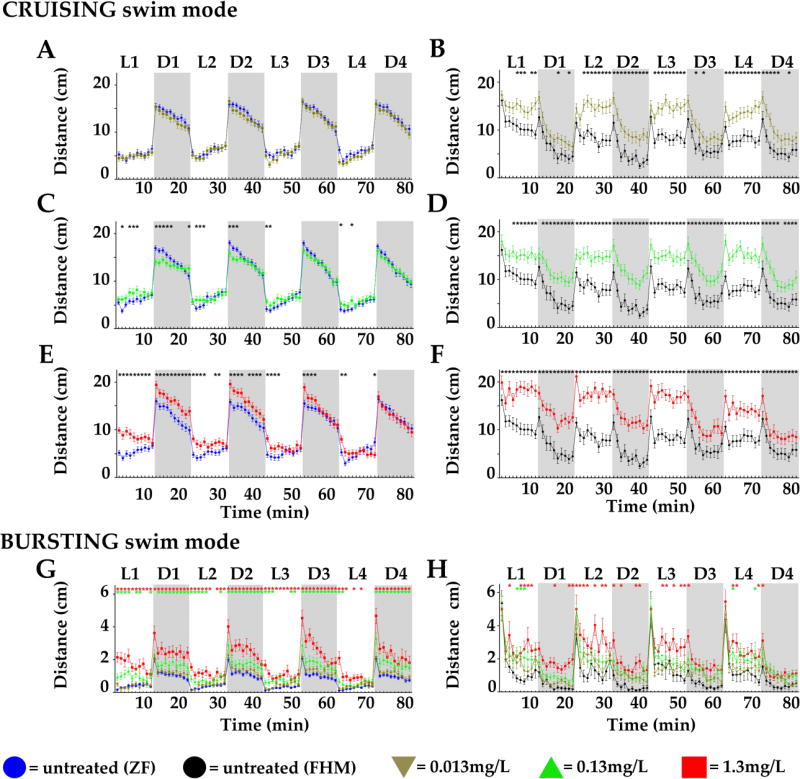
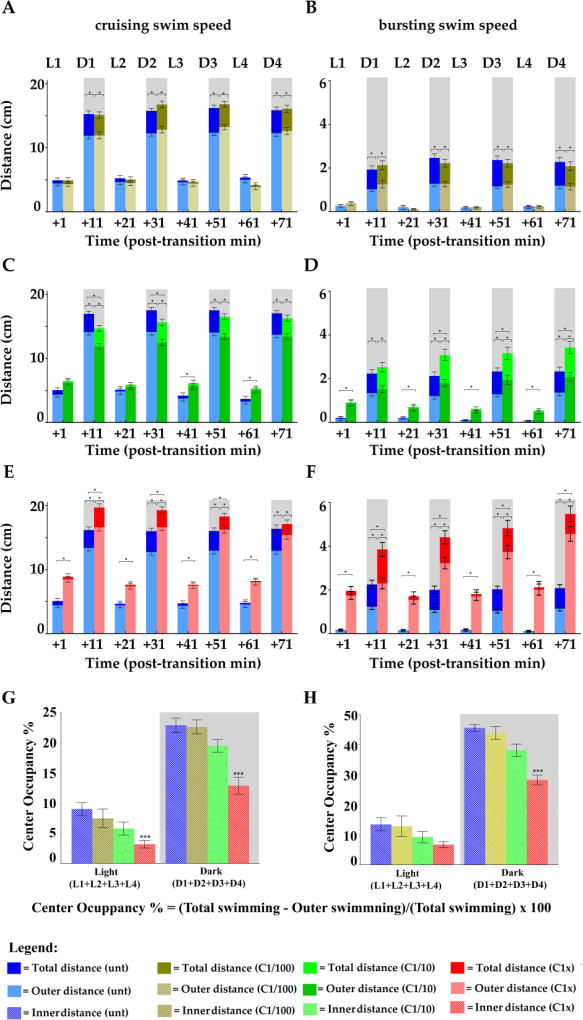
Next, we asked if PDR was affected in a concentration-dependent manner after acute treatment with a contaminant. We chose to work with copper since it is a commonly found metal contaminant in watersheds. We used concentrations of copper sulfate (Cu) ranging from the EPA established maximum contaminant level goal for drinking water (MCLG= 1.3 mg/L, = 1.3 ppm, = 8.23 µM) to a 100-fold dilution that approached water quality criterion values, which are considered environmentally acceptable for copper in freshwater and marine systems. The highest concentration tested was designated C1x (Fig. 3 red lines) and the two additional 10× serial dilutions, were called C1/10 = 0.13 mg/L (green lines) and C1/100 = 0.013 mg/L (khaki lines). Experiments were performed with larvae from ZF (n= 40/concentration, Fig. 3 left panels) and from FHM (n= 40/concentration, Fig. 3 right panels) and two swim speeds analyzed: cruising (Fig.3A, B, C, D, E and F) and bursting (Fig.3G and H).
Figure 3.
Distances travelled per minute by 6-day post fertilization (dpf) larvae of zebrafish (ZF, left panels) or fathead minnow (FHM, right panels) in 4 successive cycles of 10 minute long alternating light (L, white boxes)/dark (D, grey boxes) periods after acute exposure to increasing copper concentrations. A to F. Distances travelled in the cruising swim speed. G and H. Distances travelled in the bursting swim speed. Respective untreated controls for each treatment group are shown for ZF larvae (blue lines) and for FHM larvae (black lines). Distances travelled by larvae after a given copper treatment level are represented as following: C1/100 = 0.013 mg/L = kaki lines and triangles; C1/10 = 0.13 mg/L = green lines and triangles; C1x = 1.3 mg/L = red lines and squares. Error bars represent the mean of the error (SEM) for each mean distance. Statistical significance (* = p <0.05) are reported in the supplemental section.
The distance travelled by ZF larvae Cu-treated with the lowest concentration C1/100 (Fig. 3A and G, khaki lines) was not significantly different at any recorded time point from untreated animals (blue lines). Differences were observed for the C1/10 treated ZF larvae (Fig. 3C and G, green lines, * p < 0.05) at discreet time points, especially just after lights transitions. In the cruising swim speed treated larvae were less active in dark and more active in light. Only at the highest concentration C1x (Fig. 3E red lines, * p < 0.05), treated larvae were hyperactive in light and in dark at most time points in the first two cycles. Noticeably, this effect was then attenuated in the last cycles. In the bursting swim speed (Fig. 3G), we saw a remarkably clear concentration-dependent hyperactivity in most of the time points in light and in dark periods suggesting that bursting would be a useful measure to examine with this contaminant.
The distances travelled by Cu-treated FHM larvae at any concentration, were significantly increased at most time points when compared to untreated (Fig. 3B, D and F, * p < 0.05). However, neither in cruising nor in bursting swim speed (Fig. 3H) was there a clear [Cu]-dependent effect potentially due to the high SEM that we found at all measured time points. Therefore, we concluded that although ZF larvae seemed less sensitive than FHM, it appears as a more reliable model because of the consistency of PDR-contaminant-induced alteration ([Cu]-dependent) and the robustness of the results (small SEM). Consequently, we decided to dissect additional aspects of the PDR in ZF focusing on additional anxiety like-behaviors, such as the photo-stimulated startle response (PSSR) and center avoidance (CA).
2.4 Acute copper exposure of ZF larvae increases the photo-stimulated startle response (PSSR)
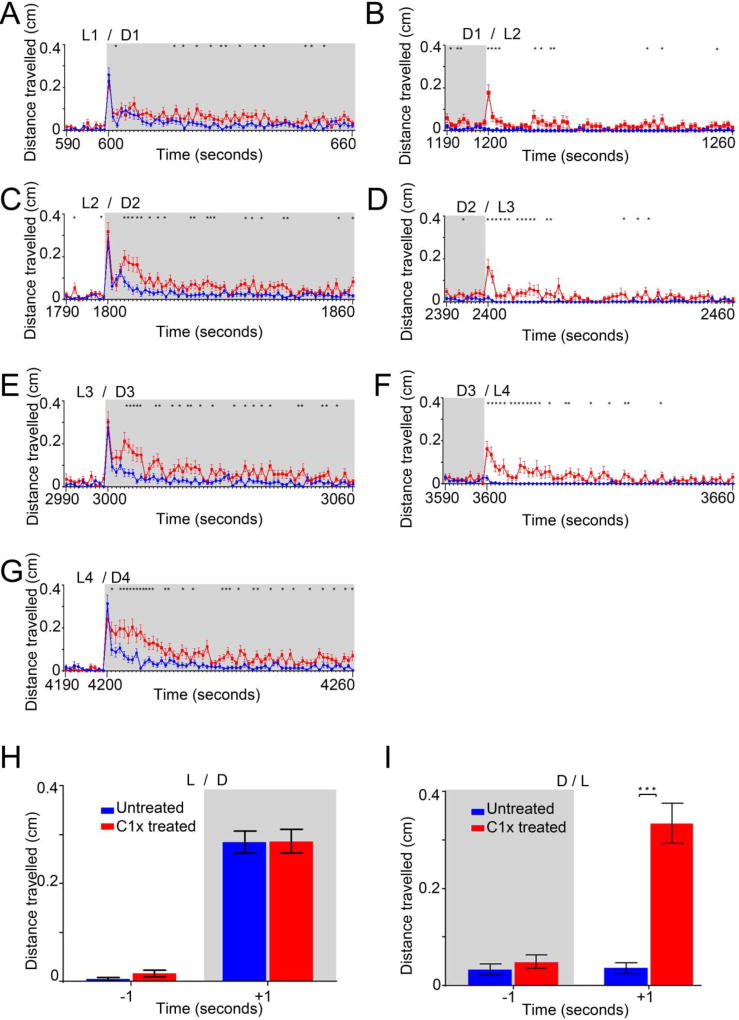
The photo-stimulated startle response (PSSR) is a strong and extremely rapid reflex that is found in most animals after an abrupt change in the surroundings, such as the sudden light transitions (light to dark or dark to light) that we were using in this behavioral assay. PSSR is usually measured in millisecond(s) and though this was below our equipment detection levels, we reasoned that if the PSSR was significantly affected by the contaminant, we would detect it when analyzing travelled distances/second in the fastest swim speed: bursting. So, we analyze and graphed travelled distances in this swim speed, but in cm/second for C1x (Fig.4). We reported swimming activity, 10 seconds prior and 60s after a light transition (Fig. 4A–G), of untreated (blue lines and bars) and copper treated (red lines and bars) larvae.
Figure 4.
Distances per second travelled in the bursting swim speed by 6dpf ZF larvae after an acute copper treatment (C1x = 1.3 mg/L) shown 10 seconds before and 60 seconds after a transition from light to dark (L/D, left panels) and from dark to light (D/L, right panels). A to G. Distances travelled per second during each successive transition (L1/D1, D1/L2, L2/D2, D2/L3, L3/D3, D3/L4, L4/D4) by control untreated animals (blue lines) and by C1x treated animals (red lines). H and I. Distances travelled during the second before (−1) and the second after (+1) all cumulated transitions from light to dark (L/D shown in H) and from light to dark (L/D, shown in I). Statistical significance (* = p <0.05) are reported in the supplemental section.
When the light was turned off (Fig. 4A, C, E, and G), in all cycles we repeatedly detected a high peak of activity during the first second after the L/D transition in all animals, untreated and treated alike, saying that it was Cu-treatment independent. However, as expected, Cu-treated animals travelled significantly more than untreated animals for most of the remaining 59 seconds (*, p < 0.05). More interestingly, when the light was turned on (Fig. 4B, D and F), in all cycles, we detected a strong burst of activity in Cu-treated animals only, suggesting an aberrant or heightened PSSR. Less surprising was the higher activity observed for the remaining 59 seconds in Cu-treated animals.
Next, we averaged all successive single seconds over all cycles, before (−1), and after (+1) a light transition. After transitions from light to dark (L/D, Fig. 4H, +1), we found a strong increase in swimming activity (~0.3 cm/s) in both untreated (blue bars) and Cu-treated animals (red bars). After transitions from dark to light (D/L, Fig. 4I, +1), we found an intense burst of activity (~0.3 cm/s), but only in Cu-treated larvae (***, p <0.0001). These results clearly illustrated a strong and selective effect of copper during L/D transitions that can be assimilated to an aberrant or increased PSSR. Thus, this was clearly demonstrating that the tested waterborne contaminant was significantly and specifically increasing another anxiety-like behavior, which was readily measured in this PDR-alteration assay.
2.5 Acute copper exposure of ZF larvae increases Center avoidance (CA)
The next anxiety-like behavior that we measured was center avoidance (CA) during the first minute after light transitions (Fig.5). To do so, we delimited a virtual inner open space (diameter, d = 0.5 cm) in the center of the well (d = 1 cm) and split the recorded swimming activities accordingly, for both swim speeds. From the total swimming activity that we recorded in the entire well (Fig5, A to F, bars), we subtracted the activity performed in close vicinity to the walls (Outer distance, lighter shade of bars) to calculate the travelled distances in the center. Next, we determined the percentage of center occupancy (CO = swimming activity in the center/total swimming × 100). Consequently, CA would be the direct inverse reading of CO (Fig.5 G and H), as more a larva swam in the center the less it was avoiding it.
Figure 5.
Distances travelled during the first minute after the successive light to dark and dark to light transitions by 6dpf ZF larvae after copper treatments in cruising (left panels) and bursting swim speeds (right panels). Distances travelled exclusively in close vicinity to the walls (=outer distances) are superimposed in a lighter shade on top of each respective total travelled distance in the entire well. Total distances travelled by control untreated animals (dark blue bars) and outer distances (light blue bars) are superimposed for all respective treatments. A and B. Distances travelled by larvae after a copper treatment at C1/100 = 0.013 mg/L (kaki bars). C and D. Distances travelled by larvae after a copper treatment at C1/10 = 0.13 mg/L (green bars). E and F. Distances travelled by larvae after a copper treatment at C1x = 1.3 mg/L (red bars). G and H. Percentage of center occupancy is shown for cruising and bursting swim speeds, respectively. Error bars represent the mean of the error (SEM) for each mean distance. Statistical significance (* = p <0.05) are reported in the supplemental section.
During light periods (Fig.5G and H, white boxes), as expected we found that all larvae spent significantly less time in the center when compared to dark periods (grey boxes). Nevertheless, Cu-treated larvae were increasingly avoiding the center in a [Cu]-dependent manner in both bursting and cruising swim speeds (Fig.5, G and H respectively, white boxes, * p < 0.05). This was even more pronounced during dark periods (grey boxes, * p < 0.05). Therefore, Cu-treated larvae were increasingly avoiding the center in a clear [Cu]-dependent manner, corroborating the two previous measures of anxiety-like behaviors and firmly establishing PDR-alterations as a sensitive read-out for detection and toxicity-assessment of this waterborne contaminant.
3. Discussion
In this work, our goal was to generate new endpoints for a rapid, cost-efficient diagnostic tool for screening aquatic contamination. We hypothesized that sub-lethal effects induced by an acute exposure to a waterborne contaminant in aquatic animals could be detected with the adequate behavioral read-out. To test this hypothesis, we adapted a larval photo-dependent swimming response (PDR) which was previously characterized in ZF larvae for biomedical purposes39,46,59–61. Light/dark responses were described in various terrestrial species like Drosophila62, and Ciona intestinalis63, and are routinely used for anxiety testing in rodent models64. It was described only in a few instances in aquatic species and used for environmental purposes like Daphnia magna65, but never in comparative studies. We showed for the first time that PDR was a reproducible and strong larval behavior that we found in three additional aquatic species, two crustaceans MR and MC and one more fish species, FHM. This underlies the importance of this behavior, possibly for survival, and therefore established its relevance and value as a new environmental endpoint.
We found that PDR was species-specific. MC and FHM were more active during light periods, whereas MR and ZF were more active during dark periods. This species-specificity of PDR might be linked to the circadian rhythm and whether animals are diurnal or nocturnal which would need further exploration. But more relevant to our goal, these results suggested that diagnostic detection of contaminants could be performed even with wildlife fauna. The species-specific PDR would not be an issue as all measurements would be done intra-species, meaning using untreated and treated larvae from the same chosen species. However, more of a concern is the much higher larva-to-larva variability that we found both with MC and FHM larvae. Remarkably, both are the less inbred species which could at least in part explain this variability. More importantly, this will be a real problem to obtain significant results. Sample size would have to be adjusted meaning using more animals, which besides being an ethical issue might not be trivial when getting animal from the wild. Therefore, using a well-established laboratory model like zebrafish would offer clear advantages like, up-scalability and possibility to couple the behavioral assay with molecular and genetic approaches to mechanistically address the contaminant toxicity.
In this study, we performed acute exposure (30 minutes + recording time) to a contaminant, copper, using a range of concentrations that were relevant to human health and aquatic environments. We could discern specific alterations especially with the higher concentration therefore overcoming the difficulty of performing extended exposures. Whether the observed PDR alterations are comparable to behavioral alterations that would result from chronic exposure across different developmental stages, even with lower concentrations, is an open question. Chronic exposure to low concentrations might trigger an adaptive physiological response in the animal that could counteract the subtler deleterious effects overtime. Thus, this approach might generate false positives. To the contrary, not all molecules might generate a detectable effect after acute exposure. We observed PDR alterations after acute exposure to other neurotropic molecules like diphenhydramine (DPH)43 and valproic acid VPA66, but one cannot exclude that some chemicals might go undetected, thus generated false negatives. However, because of the ease of use and low cost of this approach, one could use it in a first-pass screening effort and then complemented it with a variation of the PDR assay using longer exposure times to mimic chronic exposure.
We chose copper, because its deleterious effects have been extensively documented in fish in particular. Previous experiments in larvae showed that exposure to waterborne copper enhanced cortisol production67, induced cellular stress68, provoked hair cell death in the lateral line69, and damaged ciliated cells in the olfactory sensory system70,71. In adult fish, deficits in avoidance memory72, and failure to perceive odorant alarms73 were reported. Furthermore, human post-mortem studies have shown that altered copper levels in human brain correlate with various neurodegenerative diseases74. Taken together, this suggested that copper would most probably alter PDR. Presumably, only few contaminants will affect the nervous system directly or exclusively, and this might be the case for copper too. Other vital organs, like the digestive track for example could be affected. However, when using this diagnostic tool, one does not need to know which organ/tissue is affected, and this is a clear advantage as the test can be performed without any prior knowledge about the nature, the mode of action, or the putative effect of the contaminant(s) at study.
For practical reasons, we did not perform Cu-treatments in crustacean’s larvae. To raise prawn larvae, we had to reproduce a complex and dynamic salinity profile of the water to mimic natural developmental conditions. In the wild, larvae travel upstream in the rivers, going from higher to lower salinity. The early stage larvae that we analyzed required high salinity for survival, rendering exposure to copper sulfate challenging. The bioavailability of the copper will be extremely difficult to determine because of copper oxidation in salt water75,76 and because of sodium-dependent copper uptake across epithelia77. This is obviously adding another layer of complexity and illustrating a short-coming of this assay when using marine wildlife to test a certain class of contaminants.
Our comparative studies of Cu-treatments in fish seemed to highlight a higher sensitivity of FHM larvae. They had strongly altered-PDR even with the lowest tested [Cu] to which ZF seemed largely insensitive. This was suggesting that for FHM, we were using concentrations above their copper sensitivity-threshold. However, PDR-alterations were completed [Cu]-independent, pointing to an absence of cause and effect. Furthermore, FHM swimming behavior was highly variable from larva to larva especially in light and in all swim modes and that was also the case for untreated animals. Possibly, the animals were already at a heighten stress/anxiety level because of other parameters of our experiments (size of the well, intensity of the light, ambient noise…). Alternatively, higher sensitivity might be due to developmental differences78. We age-matched the ZF and FHM larvae and tested 6 dpf larvae for both species, however this might not reflect a real correspondence as development is not necessarily happening at the same rate in two different species. It is also not clear if the FHM higher sensitivity is copper-specific. One comparative study using FHM and ZF with a dozen of different chemicals, reported LC50 which were lower in FHM for some and lower in ZF for others79. Ultimately, using a more sensitive species can appear as advantageous for establishing an environmental diagnostic tool, but it is most certainly strongly offset by a diminished reproducibility and lack of robustness in responses. This was suggesting that, for the specific set-up that we were using, ZF was more appropriate. Ultimately, the fact that the same contaminant can elicit highly different responses in two fish species is illustrative of the importance of performing tests in more than one animal model. Furthermore, it highlights the need to adjust appropriately the experimental conditions (plate size, exposure time, developmental stage, light conditions…) for each tested species. Measuring PDR in optimal conditions for a species will probably be more informative than trying to reproduce the exact same experimental conditions from species to species. This in-turn implies that an optimization phase will be necessary to determine adequate species-specific conditions, rather than using a “one fits all” approach.
Behavioral alterations after sub-lethal exposures are considered to be among the most sensitive indicators of environmental disturbances, far more than the classical endpoints like survival and growth21. Identifying behaviors predictive of adverse outcomes represents an important research need and anxiety-like behaviors seem like a promising avenue to explore. Physiological levels of anxiety promote defensive behaviors alerting an organism about potentially dangerous situations, thus promoting species survival. Dysregulation of anxiety levels in aquatic fauna is already occurring in aquatic ecosystems due to anthropogenic contamination29,80,81. Our study represents an initial effort to rapidly measure anxiety-like behaviors in fish larva. Future studies are needed to understand relationships between PDR measures and ecologically important behaviors like shelter seeking and predator avoidance. Altered exploratory behaviors could be detrimental if animals do not react to stimulus adequately. For example, we showed a strongly altered PSSR in dark to light transitions after acute exposure to MCLG of drinking water for copper. Exposures to such conditions in the wild are not improbable and when coupled to light pollution which is on the rise worldwide, the risk to alter population trajectories seems real. We also found that larvae were increasingly avoiding the center in a clear [CU]-dependent manner. This can disrupt reproduction by altering mating, nest strategies, shelter protection and food seeking behaviors. Therefore, both PSSR and CA behaviors deserve future studies with additional contaminants.
In future studies, this diagnostic tool could be adapted for monitoring adverse effects of isolated or complex mixture of contaminants. Detection of chemicals in water samples from contaminated sites could be performed without any prior knowledge of the nature or concentrations of those contaminants. Furthermore, the use of the zebrafish larvae would allow to rapidly linking altered PDR to damaged organs, tissues, and cells. Classical molecular approaches like whole-mount in situ hybridization (WISH), antibody staining, and use of transgenic lines will help to identify the molecular mechanisms underlying contaminant-induced altered PDR59,66. Eventually, genetically sensitized animals, which are carrying mutations in genes important for establishing and maintaining the neural circuitry implicated in anxiety-like behaviors, like GABA receptors for example, could be used as environmental sentinels. Early detection of aquatic contaminants and better understanding of the mechanisms of toxicity will support protection of human health and the environment.
4. Material and Methods
4.1 Zebrafish care and husbandry
AB × TUB inbred lines designated as TAB5 Zebrafish (Danio rerio) were used82 for all experiments, raised and maintained in the UPR-MSC Satellite Fish Room facility according to standard procedures as recommended83 and following the IACUC authorized protocol (#A880216). All fish were raised and kept at 28°C on 14:10 hour light/dark cycles on a recirculating system (Techniplast®). Water supplied to the system was filtered by reverse osmosis (Siemens) and maintained at a neutral pH (~7.0–7.5) and stable conductivity (1,000 µS/cm) by adding sea salt (Instant Ocean®). Water coming from the filtered system and under the mentioned conditions will be referred to as system water (SW). After each crossing, embryos were collected, rinsed and raised for the first 24-hour post fertilization (hpf) in system water with methylene blue (0.2 %). The following day, fertilized and anatomically normal embryos were raised in SW at 28 °C on 14:10-hour light/dark cycles until 6-day post fertilization (dpf) larvae. Only larvae with an inflated swim bladder devoid of obvious anatomical abnormality and exhibiting upright swimming were used for the behavioral recordings.
4.2 Fathead minnow care and husbandry
Fathead minnow (Pimephales promelas) were raised and maintained according to standard procedures previously reported by our laboratory in Baylor University84. Individuals were housed in a recirculating system supplied with dechlorinated tap water at a constant temperature of 25 °C under a 16:8 light/dark photoperiod. Routine water quality parameters (e.g., alkalinity, hardness, pH, conductivity, ammonia, dissolved oxygen) are routinely measured following standard methods. Copper treatment levels were confirmed using a copper ion selective electrode. Newly fertilized fathead minnow embryos were collected from breeding tiles and were left to mature until 6 dpf with continuous and increasing aeration. After hatching (4dpf), larvae were daily fed with Artemia. Culture and experimental conditions followed IACUC protocols approved at Baylor University.
4.3 M. rosenbergii and M. carcinus care and husbandry
M. rosenbergii were obtained from Caribe Fisheries Inc. in Lajas, PR. M. carcinus were caught in non-urban rivers in Yabucoa and Canovanas, PR, as authorized through permit 2016-IC-145 (R-VS-PVS15-SJ-00560-08092016) of the local Department of Environmental and Natural Resources. Both adult species were maintained in the lab in 5-gallon tanks with continuous filtered and aerated water under a 12:12-hour light/dark cycles. Adults animals from which larvae were to be harvested were fed a high protein (>40%) pelleted purina chow once every two days. Water temperature was maintained at 26–28 °C and the pH adjusted to 7.2–7.5. To perform the larval experiments, both M. rosenbergii and M. carcinus fertilized female prawns were kept individually in 5-gallons tanks with the same conditions until the larvae were hatched. Once hatched, prawn larvae were collected immediately with a mesh and placed in another tank with aerated saline water 10–12 ppt using sea salt (Instant Ocean®). This same water was used throughout the behavioral experiments. Larvae were fed twice a day with newly hatched artemias. The larvae used for the experiments were staged using the presence of sessile eyes and were carefully selected for being free of anatomical abnormalities. All procedures were approved by IACUC (#A3240113).
4.4 Dark/light response behavioral recordings in ZF, FHM, and Macrobrachium larvae
Individual ZF, FHM, and Macrobrachium larvae were placed into a well of a 48-well transparent plate (Greiner bio one, CELLSTAR®). A single larva per well was added into 450 µl of SW. Each well was topped up to 500 µl with 50 µl of SW or 50 µl SW plus the desired amount of copper. Macrobrachium larvae were not exposed to copper concentrations. Plates were placed into the Zebrabox® (Viewpoint, France) for recording. The Zebrabox® is an isolated recording device with a camera and infrared light-emitting base where temperature and light were controlled. Larvae were first habituated to the dark for 30 minutes without recording followed by 80 minutes of recording. The 80-minute recording was divided in 4 × 20 min cycles of alternating light (L) and dark (D) periods of 10 minute each. Three independent experiments were performed for each species
4.5 Swimming activity tracking
Individual ZF, FHM and Macrobrachium larval activity was recorded and measured using the Viewpoint video tracking software (Viewpoint, France). Swim speed thresholds for ZF and FHM were set experimentally and used to define: freezing (< 0.2 cm/s; data not shown) during which larvae displayed minimal activity, cruising swim speed (0.2 > s < 2.0 cm/s) covering most of the commonly measured larval speeds, and bursting swim speed (> 2.0 cm/s), which were short, intermittent and powerful bouts of activity. For the prawn larvae, different speed thresholds were defined: freezing (< 0.2 cm/s), cruising swim speed (0.2 > s < 1.3 cm/s), and bursting swim speed (> 1.3 cm/s). All results were binned into one-minute intervals, therefore resulting in 80 data points. For further analysis, the Viewpoint software was used to recalculate results and time binning.
4.6 Place preference test
We further defined the recording of swimming traveled distance according to the location in the well. We virtually defined an inner perimeter (inner distance= 0.5 cm) within the whole well (total distance = 1.0 cm) for each well using the Viewpoint software. To calculate the outer perimeter (outer distance = 0.5), the inner area was subtracted from the total area.
4.7 Copper treatment and exposure period
All solutions were made fresh before each experiment by diluting copper sulfate (Sigma, # 7758-98-7) in SW. As a reference point, we chose the maximum contamination level goal (MCLG) as set by the United States Environmental Protection Agency (EPA) for copper (C1x = 1.3ppm = 1.3 mg/L = 8.23 µM) in drinking water. A 2-fold magnitude of concentrations were assessed below the C1x that we called C1/100, C1/10. The addition of copper to the well was done 30 minutes prior each recording (the acclimation period is not included in the graphs).
4.8 Statistical analyses
Data from at least 3 independent experiments for each concentration of choice were compiled and analyzed using GraphPad Prism software (v.7). Results were plotted in cruising or bursting swim speeds as mean distance covered, binned into 1 min intervals. We analyzed mean total distances traveled per larva from triplicate experiments and plotted them versus a time axis, displaying the entire time-course of the experiment. Error bars represent the standard error of the mean (SEM). Statistical differences between untreated and treated were calculated using multiple t-tests and were considered significant when p<0.05. We performed Two-way ANOVA in graphs when two or more groups were compared. Detailed tables of results are available in Supplemental data: Tables (S1 to S5b).
Supplementary Material
Highlights.
Our work represents an effort to rapidly measure sensitive and relevant endpoints for diagnostic screening of aquatic contamination.
A photo-dependent swimming response (PDR) showed a reproducible and strong larval quantifiable behavior in four aquatic species: two crustaceans (M. rosenbergii and M. carcinus) and two fish (Zebrafish and Fathead minnow).
The larval PDR is species-specific and dose-dependent.
Contaminant-induced PDR alterations were found at sub-lethal concentrations resulting in anxiety-like behaviors.
We interrogated two additional anxiety-like behaviors that were significantly different suggesting relevance of the PDR assay as a diagnostic screening tool to evaluate the integrity of aquatic fauna.
Acknowledgments
This work was supported by Research Centers for Minority Institutions of National Institutions of Health (RCMI/NIH, Seed Monies; award no. G12RR03051). LC and JC were funded by the National Science Foundation-Center of Research Excellence in Science and Technology (NSF-CREST: Puerto Rican Center of Environmental Neuroscience (PRCEN), award no. HRD-1137725) and received additional support from the Minority Biomedical Research Support Program-Research Initiative for Scientific Enhancement (MBRS-RISE award no. 2 R25 GM061838) at the Medical Science Campus University of Puerto Rico (UPR-MSC). LK and BWB were supported by a grant National Science Foundation (CHE-1339637) and Environmental Protection Agency through the Molecular Design Research Network (modrn.yale.edu). Additional support was provided by the Department of Environmental Science at Baylor University. AA was funded by the MBRS-RISE award no. 2 R25 GM061838 at the MSC-UPR. Infrastructure and instrumentation were available through a Pathway to Independence grant from the National Institutions of Health (NIH-K99/R00-NIH, award no. R00-DC009443) and from a Puerto Rico Science, Research, and Technology Trust grant to MB and from funding from the Department of Anatomy & Neurobiology and the School of Medicine of the UPR-MSC. We want to thank the UPR-MSC´s Puerto Rico Clinical Translational Research Consortium (PRCTRC) for the statistical analysis support (NIMHHD/NIH, award no. 2U54MD007587). We appreciate the effort from Dr. John Bradsher for critical reading of the manuscript. Also, we thank all the graduates (Roberto Rodriguez-Morales and Barbara Manfredi) and undergraduates (Normarie Herrera, Rafael Perez, Nabila Chaudri, Alexis Santana, Victor Román, Joaquín Carrillo, Juan Cantres, and Alejandro Del Valle) students for the invaluable help with the fish room maintenance and fish husbandry.
Abbreviations
- PDR
photo-dependent swimming response
- CEC
chemicals of emerging concern
- ZF
zebrafish
- FHM
fathead minnow
- MC
Macrobrachium carcinus
- MR
Macrobrachium rosenbergii
- PSSR
photo-stimulated startle response
- CA
center avoidance
- MCLG
maximum contaminant level goal
- L
light
- D
dark
- dpf
days post-fertilization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: Luis Colón-Cruz and Martine Behra conceived and designed the ZF experiments; Lauren Kristofco and Bryan W. Brooks designed the FHM experiments. Jonathan Crooke-Rosado and María A. Sosa designed the prawn experiments. Luis Colón-Cruz, Lauren Kristofco, and Jonathan Crooke-Rosado performed the experiments. Luis Colón-Cruz, Jonathan Crooke-Rosado, Aranza Torrado, and Agnes Acevedo analyzed the data and help writing the manuscript. Luis Colón-Cruz and Martine Behra wrote the paper.
Conflicts of Interest: The authors declare no conflict of interest.
References
- 1.Watts G. Something in the water. BMJ. 2011;343:d7236. doi: 10.1136/bmj.d7236. [DOI] [PubMed] [Google Scholar]
- 2.Halder J, Islam N. Water Pollution and its Impact on the Human Health. J. Environ. Hum. 2015;2:36–46. [Google Scholar]
- 3.Torres T, Cunha I, Martins R, Santos M. Screening the Toxicity of Selected Personal Care Products Using Embryo Bioassays: 4-MBC, Propylparaben and Triclocarban. Int. J. Mol. Sci. 2016;17:1762. doi: 10.3390/ijms17101762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gavrilescu M, Demnerová K, Aamand J, Agathos S, Fava F. Emerging pollutants in the environment: Present and future challenges in biomonitoring, ecological risks and bioremediation. N. Biotechnol. 2015;32:147–156. doi: 10.1016/j.nbt.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Brooks BW, Huggett DB, Boxall ABa. Pharmaceuticals and Personal Care Products in the Environment Editorial PHARMACEUTICALS AND PERSONAL CARE PRODUCTS : RESEARCH NEEDS FOR THE NEXT DECADE. Environ. Toxicol. Chem. 2009;28:2469–2472. doi: 10.1897/09-325.1. [DOI] [PubMed] [Google Scholar]
- 6.Dreier DA, Connors KA, Brooks BW. Comparative endpoint sensitivity of in vitro estrogen agonist assays. Regul. Toxicol. Pharmacol. 2015;72:185–193. doi: 10.1016/j.yrtph.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Judson RS, et al. In vitro screening of environmental chemicals for targeted testing prioritization: The toxcast project. Environ. Health Perspect. 2010;118:485–492. doi: 10.1289/ehp.0901392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sipes NS, Padilla S, Knudsen TB. Zebrafish-As an integrative model for twenty-first century toxicity testing. Birth Defects Res. Part C - Embryo Today Rev. 2011;93:256–267. doi: 10.1002/bdrc.20214. [DOI] [PubMed] [Google Scholar]
- 9.Padilla S, et al. Zebrafish developmental screening of the ToxCast??? Phase I chemical library. Reprod. Toxicol. 2012;33:174–187. doi: 10.1016/j.reprotox.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 10.Okamoto A, Yamamuro M, Tatarazako N. Acute toxicity of 50 metals to Daphnia magna. J. Appl. Toxicol. 2015;35:824–830. doi: 10.1002/jat.3078. [DOI] [PubMed] [Google Scholar]
- 11.Ong C, Yung L-YL, Cai Y, Bay B-H, Baeg G-H. Drosophila melanogaster as a model organism to study nanotoxicity. Nanotoxicology. 2014;5390:1–8. doi: 10.3109/17435390.2014.940405. [DOI] [PubMed] [Google Scholar]
- 12.Mesnage R, Defarge N, Rocque LM, De Vendômois JS, Séralini GE. Laboratory rodent diets contain toxic levels of environmental contaminants: Implications for regulatory tests. PLoS One. 2015;10:1–17. doi: 10.1371/journal.pone.0128429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kovrižnych Ja, et al. Acute toxicity of 31 different nanoparticles to zebrafish (Danio rerio) tested in adulthood and in early life stages - comparative study. Interdiscip. Toxicol. 2013;6:67–73. doi: 10.2478/intox-2013-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guilhermino L, Diamantino T, Silva MC, Soares aM. Acute toxicity test with Daphnia magna: an alternative to mammals in the prescreening of chemical toxicity? Ecotoxicol. Environ. Saf. 2000;46:357–362. doi: 10.1006/eesa.2000.1916. [DOI] [PubMed] [Google Scholar]
- 15.Martin TM, Young DM. Prediction of the acute toxicity (96-h LC50) of organic compounds to the fathead minnow (Pimephales promelas) using a group contribution method. Chem. Res. Toxicol. 2001;14:1378–85. doi: 10.1021/tx0155045. [DOI] [PubMed] [Google Scholar]
- 16.Wu L, Jiang Y, Zhang L, Chen L, Zhang H. Toxicity of urban highway runoff in Shanghai to Zebrafish (Danio rerio) embryos and luminous bacteria (Vibrio qinghaiensis.Q67) Environ. Sci. Pollut. Res. 2014;21:2663–2676. doi: 10.1007/s11356-013-2193-9. [DOI] [PubMed] [Google Scholar]
- 17.Huang H, et al. Toxicity, uptake kinetics and behavior assessment in zebrafish embryos following exposure to perfluorooctanesulphonicacid (PFOS) Aquat. Toxicol. 2010;98:139–47. doi: 10.1016/j.aquatox.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valenti TW, et al. Human therapeutic plasma levels of the selective serotonin reuptake inhibitor (SSRI) sertraline decrease serotonin reuptake transporter binding and shelter-seeking behavior in adult male fathead minnows. Environ. Sci. Technol. 2012;46:2427–2435. doi: 10.1021/es204164b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ENMIN ZOU. Impacts of Xenobiotics on Crustacean Molting : The Invisible Endocrine Disruption 1. 2005;38:33–38. doi: 10.1093/icb/45.1.33. [DOI] [PubMed] [Google Scholar]
- 20.Noguera-Oviedo K, Aga DS. Lessons learned from more than two decades of research on emerging contaminants in the environment. J. Hazard. Mater. 2016;316:242–251. doi: 10.1016/j.jhazmat.2016.04.058. [DOI] [PubMed] [Google Scholar]
- 21.Robinson PD. Behavioural toxicity of organic chemical contaminants in fish: application to ecological risk assessments (ERAs) Can. J. Fish. Aquat. Sci. 2009;66:1179–1188. [Google Scholar]
- 22.Gerhardt a. Aquatic Behavioral Ecotoxicology—Prospects and Limitations. Hum. Ecol. Risk Assess. An Int. J. 2007;13:481–491. [Google Scholar]
- 23.Maximino C, et al. Measuring anxiety in zebrafish: A critical review. Behav. Brain Res. 2010;214:157–171. doi: 10.1016/j.bbr.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 24.Clark KJ, Boczek NJ, Ekker SC. Stressing zebrafish for behavioral genetics. Rev. Neurosci. 2011;22:49–62. doi: 10.1515/RNS.2011.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brodin T, et al. Ecological effects of pharmaceuticals in aquatic systems — impacts through behavioural alterations. Philos. Trans. R. Publ. Soc. B. 2014;369:20130580. doi: 10.1098/rstb.2013.0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pottinger TG, Carrick TR. Stress Responsiveness Affects Dominant–Subordinate Relationships in Rainbow Trout. Horm. Behav. 2001;40:419–427. doi: 10.1006/hbeh.2001.1707. [DOI] [PubMed] [Google Scholar]
- 27.Colwill RM, Creton R. Locomotor behaviors in zebrafish (Danio rerio) larvae. Behav. Processes. 2011;86:222–229. doi: 10.1016/j.beproc.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruiz-Gomez M, de L, et al. Behavioral plasticity in rainbow trout (Oncorhynchus mykiss) with divergent coping styles: When doves become hawks. Horm. Behav. 2008;54:534–538. doi: 10.1016/j.yhbeh.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 29.Brodin T, Fick J, Jonsson M, Klaminder J. Dilute concentrations of a psychiatric drug alter behavior of fish from natural populations. Science. 2013;339:814–5. doi: 10.1126/science.1226850. [DOI] [PubMed] [Google Scholar]
- 30.Kalueff AV, Stewart AM, Gerlai R. Zebrafish as an emerging model for studying complex brain disorders. Trends in Pharmacological Sciences. 2014;35:63–75. doi: 10.1016/j.tips.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Homberg JR, et al. Genetic and environmental modulation of neurodevelopmental disorders: Translational insights from labs to beds. Brain Res. Bull. 2016;125:79–91. doi: 10.1016/j.brainresbull.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 32.Brittijn SA, et al. Zebrafish development and regeneration: New tools for biomedical research. Int. J. Dev. Biol. 2009;53:835–850. doi: 10.1387/ijdb.082615sb. [DOI] [PubMed] [Google Scholar]
- 33.McCammon JM, Sive H. Addressing the Genetics of Human Mental Health Disorders in Model Organisms. Annu. Rev. Genomics Hum. Genet. 2015;16 doi: 10.1146/annurev-genom-090314-050048. 150522223126008. [DOI] [PubMed] [Google Scholar]
- 34.Kalueff AV, Stewart AM, Gerlai R. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol. Sci. 2014;35:63–75. doi: 10.1016/j.tips.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalueff AV, et al. Zebrafish neurobehavioral phenomics for aquatic neuropharmacology and toxicology research. Aquat. Toxicol. 2016;170:297–309. doi: 10.1016/j.aquatox.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 36.Reif DM, et al. High-throughput characterization of chemical-associated embryonic behavioral changes predicts teratogenic outcomes. Arch. Toxicol. 2016;90:1459–1470. doi: 10.1007/s00204-015-1554-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng X, et al. Anxiety-related behavioral responses of pentylenetetrazole-treated zebrafish larvae to light-dark transitions. Pharmacol. Biochem. Behav. 2015;145:55–65. doi: 10.1016/j.pbb.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 38.Irons TD, MacPhail RC, Hunter DL, Padilla S. Acute neuroactive drug exposures alter locomotor activity in larval zebrafish. Neurotoxicol. Teratol. 2010;32:84–90. doi: 10.1016/j.ntt.2009.04.066. [DOI] [PubMed] [Google Scholar]
- 39.Rihel J, Schier AF. Behavioral screening for neuroactive drugs in zebrafish. Dev. Neurobiol. 2012;72:373–85. doi: 10.1002/dneu.20910. [DOI] [PubMed] [Google Scholar]
- 40.MacRae CA, Peterson RT. Zebrafish as tools for drug discovery. Nat. Rev. Drug Discov. 2015;14:721–731. doi: 10.1038/nrd4627. [DOI] [PubMed] [Google Scholar]
- 41.Behra M, et al. Acetylcholinesterase is required for neuronal and muscular development in the zebrafish embryo. Nat. Neurosci. 2002;5:111–118. doi: 10.1038/nn788. [DOI] [PubMed] [Google Scholar]
- 42.Neuhauss SCF. Behavioral genetic approaches to visual system development and function in zebrafish. J. Neurobiol. 2003;54:148–160. doi: 10.1002/neu.10165. [DOI] [PubMed] [Google Scholar]
- 43.Kristofco LA, et al. Age matters: Developmental stage of Danio rerio larvae influences photomotor response thresholds to diazinion or diphenhydramine. Aquat. Toxicol. 2016;170:344–354. doi: 10.1016/j.aquatox.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MacPhail RC, et al. Locomotion in larval zebrafish: Influence of time of day, lighting and ethanol. Neurotoxicology. 2009;30:52–58. doi: 10.1016/j.neuro.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 45.Burgess HA, Granato M. Modulation of locomotor activity in larval zebrafish during light adaptation. J. Exp. Biol. 2007;210:2526–39. doi: 10.1242/jeb.003939. [DOI] [PubMed] [Google Scholar]
- 46.Kokel D, Peterson RT. Using the zebrafish photomotor response for psychotropic drug screening. Methods Cell Biol. 2011;105:517–524. doi: 10.1016/B978-0-12-381320-6.00022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Torres-Hernández BA, et al. Reversal of pentylenetetrazole-altered swimming and neural activity-regulated gene expression in zebrafish larvae by valproic acid and valerian extract. Psychopharmacology (Berl) 2016 doi: 10.1007/s00213-016-4304-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vázquez-Acevedo N, et al. GYRKPPFNGSIFamide (Gly-SIFamide) modulates aggression in the freshwater prawn Macrobrachium rosenbergii. Biol. Bull. 2009;217:313–326. doi: 10.1086/BBLv217n3p313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kruangkum T, et al. Spermatophore affects the egg-spawning and egg-carrying behavior in the female giant freshwater prawn, Macrobrachium rosenbergii. Anim. Reprod. Sci. 2015;161:129–137. doi: 10.1016/j.anireprosci.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 50.Muralisankar T, Saravana Bhavan P, Radhakrishnan S, Seenivasan C, Srinivasan V. The effect of copper nanoparticles supplementation on freshwater prawn Macrobrachium rosenbergii post larvae. J. Trace Elem. Med. Biol. 2016;34:39–49. doi: 10.1016/j.jtemb.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 51.Satapornvanit K, Baird DJ, Little DC. Laboratory toxicity test and post-exposure feeding inhibition using the giant freshwater prawn Macrobrachium rosenbergii. Chemosphere. 2009;74:1209–1215. doi: 10.1016/j.chemosphere.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 52.Holthuis LB World, O. F. T. H. E. An Annotated Catalogue of Species. Fao Fish. Synopsis. 1980;1:284. [Google Scholar]
- 53.Ankley GT, Villeneuve DL. The fathead minnow in aquatic toxicology: Past, present and future. Aquat. Toxicol. 2006;78:91–102. doi: 10.1016/j.aquatox.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 54.Ray WJ, et al. Startle response in generalized anxiety disorder. Depress. Anxiety. 2009;26:147–154. doi: 10.1002/da.20479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kimmel CB, Patterson J, Kimmel RO. The development and behavioral characteristics of the startle response in the zebra fish. Dev. Psychobiol. 1974;7:47–60. doi: 10.1002/dev.420070109. [DOI] [PubMed] [Google Scholar]
- 56.Ellis LD, Seibert J, Soanes KH. Distinct models of induced hyperactivity in zebrafish larvae. Brain Res. 2012;1449:46–59. doi: 10.1016/j.brainres.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 57.Richendrfer H, Pelkowski SD, Colwill RM, Creton R. On the edge: Pharmacological evidence for anxiety-related behavior in zebrafish larvae. Behav. Brain Res. 2012;228:99–106. doi: 10.1016/j.bbr.2011.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ahmad F, Richardson MK. Exploratory behaviour in the open field test adapted for larval zebrafish: Impact of environmental complexity. Behav. Processes. 2013;92:88–98. doi: 10.1016/j.beproc.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 59.Baxendale S, et al. Identification of compounds with anti-convulsant properties in a zebrafish model of epileptic seizures. Dis. Model. Mech. 2012;5:773–84. doi: 10.1242/dmm.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gao Y, et al. A High-Throughput Zebrafish Screening Method for Visual Mutants by Light-Induced Locomotor Response. IEEE/ACM Trans. Comput. Biol. Bioinform. 2014;11:693–701. doi: 10.1109/TCBB.2014.2306829. [DOI] [PubMed] [Google Scholar]
- 61.Rihel J, et al. Zebrafish Behavioral Profiling Links Drugs to Biological Targets and Rest/Wake Regulation. Science (80−.) 2010;327:348–351. doi: 10.1126/science.1183090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wheeler DA, Hamblen-Coyle MJ, Dushay MS, Hall JC. Behavior in light-dark cycles of Drosophila mutants that are arrhythmic, blind, or both. J. Biol. Rhythms. 1993;8:67–94. doi: 10.1177/074873049300800106. [DOI] [PubMed] [Google Scholar]
- 63.Zega G, Thorndyke MC, Brown ER. Development of swimming behaviour in the larva of the ascidian Ciona intestinalis. J. Exp. Biol. 2006;209:3405–3412. doi: 10.1242/jeb.02421. [DOI] [PubMed] [Google Scholar]
- 64.Arrant AE, Schramm-Sapyta NL, Kuhn CM. Use of the light/dark test for anxiety in adult and adolescent male rats. Behav. Brain Res. 2013;256:119–127. doi: 10.1016/j.bbr.2013.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Effertz C, von Elert E. Light intensity controls anti-predator defences in Daphnia: the suppression of life-history changes. Proc. Biol. Sci. 2014;281:20133250. doi: 10.1098/rspb.2013.3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Torres-Hernández BA, et al. Reversal of pentylenetetrazole-altered swimming and neural activity-regulated gene expression in zebrafish larvae by valproic acid and valerian extract. Psychopharmacology (Berl) 2016:2533–2547. doi: 10.1007/s00213-016-4304-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Craig PM, Hogstrand C, Wood CM, McClelland GB. Gene expression endpoints following chronic waterborne copper exposure in a genomic model organism, the zebrafish, Danio rerio. Physiol. Genomics. 2009;40:23–33. doi: 10.1152/physiolgenomics.00089.2009. [DOI] [PubMed] [Google Scholar]
- 68.Hernandez PP, et al. Sublethal concentrations of waterborne copper induce cellular stress and cell death in zebrafish embryos and larvae. Biol. Res. 2011;44:7–15. doi: 10.4067/S0716-97602011000100002. [DOI] [PubMed] [Google Scholar]
- 69.Olivari FA, Hernández PP, Allende ML. Acute copper exposure induces oxidative stress and cell death in lateral line hair cells of zebrafish larvae. Brain Res. 2008;1244:1–12. doi: 10.1016/j.brainres.2008.09.050. [DOI] [PubMed] [Google Scholar]
- 70.Dew WA, Wood CM, Pyle GG. Effects of continuous copper exposure and calcium on the olfactory response of fathead minnows. Environ. Sci. Technol. 2012;46:9019–9026. doi: 10.1021/es300670p. [DOI] [PubMed] [Google Scholar]
- 71.Tilton FA, Bammler TK, Gallagher EP. Swimming impairment and acetylcholinesterase inhibition in zebrafish exposed to copper or chlorpyrifos separately, or as mixtures. Comp. Biochem. Physiol. - C Toxicol. Pharmacol. 2011;153:9–16. doi: 10.1016/j.cbpc.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Da Silva Acosta D, et al. Copper at low levels impairs memory of adult zebrafish (Danio rerio) and affects swimming performance of larvae. Comp. Biochem. Physiol. Part - C Toxicol. Pharmacol. 2016;185–186:122–130. doi: 10.1016/j.cbpc.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 73.Dew WA, Azizishirazi A, Pyle GG. Contaminant-specific targeting of olfactory sensory neuron classes: Connecting neuron class impairment with behavioural deficits. Chemosphere. 2014;112:519–525. doi: 10.1016/j.chemosphere.2014.02.047. [DOI] [PubMed] [Google Scholar]
- 74.Davies KM, et al. Localization of copper and copper transporters in the human brain. Metallomics. 2012;5:43–51. doi: 10.1039/c2mt20151h. [DOI] [PubMed] [Google Scholar]
- 75.Lee JA, Marsden ID, Glover CN. The influence of salinity on copper accumulation and its toxic effects in estuarine animals with differing osmoregulatory strategies. Aquat. Toxicol. 2010;99:65–72. doi: 10.1016/j.aquatox.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 76.Smith KS, Balistrieri LS, Todd AS. Applied Geochemistry Using biotic ligand models to predict metal toxicity in mineralized systems. Appl. GEOCHEMISTRY. 2014 doi: 10.1016/j.apgeochem.2014.07.005. [DOI] [Google Scholar]
- 77.Handy RD, Eddy FB, Baines H. Sodium-dependent copper uptake across epithelia: A review of rationale with experimental evidence from gill and intestine. Biochim. Biophys. Acta - Biomembr. 2002;1566:104–115. doi: 10.1016/s0005-2736(02)00590-4. [DOI] [PubMed] [Google Scholar]
- 78.Jeffries MKS, et al. Alternative methods for toxicity assessments in fish: Comparison of the fish embryo toxicity and the larval growth and survival tests in zebrafish and fathead minnows. Environ. Toxicol. Chem. 2014;33:2584–2594. doi: 10.1002/etc.2718. [DOI] [PubMed] [Google Scholar]
- 79.Corrales J, et al. Toward the Design of Less Hazardous Chemicals: Exploring Comparative Oxidative Stress in Two Common Animal Models. 2017 doi: 10.1021/acs.chemrestox.6b00246. [DOI] [PubMed] [Google Scholar]
- 80.Hamilton TJ, Holcombe A, Tresguerres M. CO2-induced ocean acidification increases anxiety in rockfish via alteration of GABAA receptor functioning. Proc. Biol. Sci. 2014;281:20132509. doi: 10.1098/rspb.2013.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hamilton TJ, Kwan GT, Gallup J, Tresguerres M. Acute fluoxetine exposure alters crab anxiety-like behaviour, but not aggressiveness. Sci. Rep. 2016;6:19850. doi: 10.1038/srep19850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.LaFave Matthew C, Varshney Gaurav K, V M, Mullikin James C, B SM. A defined zebrafish line for high-thoughput genetics and genmoics: NHGRI-1. Genetics. 2014 doi: 10.1534/genetics.114.166769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Westerfield M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio) 5. University of Oregon Press; Eugene: 2007. p. 2007. [Google Scholar]
- 84.Corrales J, et al. Chem. Res. Toxicol. ASAP; 2016. Toward the Design of Less Hazardous Chemicals: Exploring Comparative Oxidative Stress in Two Common Animal Models. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.