Abstract
Ethnopharmacological relevance
Artemisia annua has been used for >2,000 yrs to treat fever and is more recently known for producing the important antimalarial drug, artemisinin.
Aim of the study
Artemisinin combination therapies (ACTs) are effective for treating malaria, but are often unavailable to those in need. Dried leaves of A. annua (DLA) have recently been studied as a cost effective alternative to traditional ACTs. DLA was shown to dramatically increase oral bioavailability compared to pure artemisinin, so more investigation into the mechanisms causing this increased bioavailability is needed.
Materials and Methods
In this study, we used a simulated digestion system coupled with Caco-2 cell permeability assays to investigate the intestinal permeability of DLA compared to pure artemisinin. We also determined the effects of different phytochemicals (7 flavonoids, 3 monoterpenes, 2 phenolic acids, scopoletin and inulin) and the cytochrome P450 isoform CYP3A4 on artemisinin intestinal permeability.
Results
Artemisinin permeability delivered as digested DLA significantly increased by 37% (Papp = 8.03×10−5 cm s−1) compared to pure artemisinin (Papp = 5.03×10−5 cm s−1). However, none of the phytochemicals tested or CYP3A4 had any significant effect on the intestinal permeability of artemisinin. We also showed that essential oil derived from A. annua negatively affected the intestinal permeability of artemisinin, but only after simulated digestion. Finally, we showed that A. annua essential oil reduced the transepithelial electrical resistance of Caco-2 monolayers, but only in the presence of bile. Although also reduced by essential oils, artemisinin Papp subsequently recovered in the presence of plant matrix.
Conclusions
These results shed light on the mechanisms by which DLA enhances the oral bioavailability of artemisinin.
Keywords: antiprotozoal, drug transport, essential oils, flavonoids, malaria, terpenes
Graphical abstract
A. annua phytochemicals increase artemisinin permeability through Caco-2 cells.
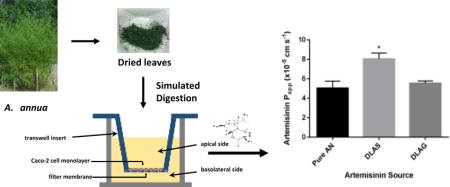
1.0 Introduction
Malaria, a disease caused by parasites of the Plasmodium genus, remains a major global health problem across the developing world. There are over 3 billion people at risk of contracting malaria, about half the world’s population, and each year there are over 400,000 deaths due to the disease (WHO, 2016). Although the vast majority of malaria infections can be treated effectively with artemisinin combination therapies (ACTs), there remains a large population, mostly in rural Africa, that does not have access or the financial resources to receive treatment. As a result, in 2015 about 90% of deaths due to malaria occurred in Sub-Saharan Africa. Of these deaths, about 70% were children under the age of 5 (WHO, 2016).
Artemisia annua L., the plant that naturally produces artemisinin (AN) in its glandular trichomes, has been used traditionally in China to treat malaria dating back as far as the second century BCE (Hsu, 2006). Recently, consumption of the dried leaves of A. annua (DLA) has been studied as a potential low-cost treatment option for people living in rural malaria endemic regions. In mouse studies, DLA was shown to be 5 times more effective than pure artemisinin at clearing Plasmodium parasites from the blood (Elfawal et al., 2012) and three times better at slowing the development of resistant parasites (Elfawal et al., 2015). In a small human trial in Kenya, patients treated twice daily for 6 days with tablets made from DLA achieved >90% parasite clearance at 28 days with <10% recrudescence, a result comparable with many ACTs (ICIPE, 2005). More recently, DLA successfully treated 18 patients who had ACT and i.v. artesunate resistant malaria (Daddy et al., 2017). Furthermore, when oral delivery of DLA was compared to oral delivery of pure artemisinin in mice, there was about 45 times more artemisinin found in the serum of mice given DLA (Weathers et al., 2011). The mechanism causing this striking increase in drug bioavailability is yet to be fully determined. Our group recently showed through simulated digestion experiments that digestion of DLA results in about 4 times higher solubility of artemisinin in the resulting digestate and this is largely from essential oils found in the plant material (Desrosiers and Weathers, 2016). Artemisinin has very low aqueous solubility, so its increased solubility from DLA partially explains the 45-fold increase in serum concentration, however, there are likely other mechanisms in play.
One potential mechanism for the increased artemisinin bioavailability afforded by DLA is modulation of the intestinal permeability of artemisinin. Several phytochemicals found in A. annua have either increased the rate of transport of other drugs or inhibited key enzymes that mediate the first-pass metabolism of artemisinin. For example, De Magalhães et al. showed that tea infusions made from various A. annua cultivars inhibited CYP3A4 (Melillo de Magalhães et al., 2012), an enzyme present in the intestine involved in the metabolism of artemisinin (Svensson and Ashton, 1999). Quercetin, a flavonoid found in A. annua, also increased the absorption of doxorubicin in rats (Choi et al., 2011), while tamarixetin, another flavonoid, increased absorption of fluvastatin in rats (Wang et al., 2014). Further, other studies showed that flavonoids found in A. annua, such as quercetin and rutin, inhibited CYP3A4 as well as other cytochrome P450 enzymes that mediate the metabolism of artemisinin (Wang et al., 2014).
In this study, we used the Caco-2 cell model of the intestinal epithelium to measure the intestinal permeability of artemisinin when delivered as pure drug or as DLA simulated digestate. We showed that delivery as digested DLA increased the rate of artemisinin transport across the intestinal epithelium. We also tested a wide variety of phytochemicals found in A. annua for their effects on artemisinin permeability and tested the role of CYP3A4 in this process by upregulating its expression and activity in Caco-2 cells.
2.0 Materials and Methods
2.1 Plant Material
We used two Artemisia annua L. cultivars in these studies. The first, SAM (DLAS) (voucher MASS 317314), is a high artemisinin and flavonoid producing cultivar, about 1.4% and 0.3% (w/w) respectively, and was propagated clonally by rooted cuttings. DLAS was field grown in Stow, MA and harvested at the floral budding stage, dried and processed as detailed in (Weathers et al., 2014). The second cultivar used, GLS (DLAG) (vouchers OR State Univ 171772 and 170353), is a mutant cultivar lacking glandular trichomes and producing no artemisinin, 25% of the flavonoids of DLAS, and negligible levels of essential oils as measured by our lab (Table S1) and others (Tellez et al., 1999). DLAG was grown in the lab under glass-filtered sunlight, harvested during the vegetative stage, dried and processed as DLAS.
2.1 Chemicals and Reagents
All chemicals and reagents used were at least research grade from Sigma Aldrich (St. Louis, MO, USA) unless otherwise stated. A. annua essential oils were purchased from Bella Mira (Mannford, OK, USA) or Jiangxi Jinyuan Natural Perfume Company (Ji’an, Jiangxi, China). Rutin, eupatorin, casticin, and isovitexin were purchased from ChromaDex (Irvine, CA, USA).
2.3 Caco-2 Cell Culture
The Caco-2 cell line was purchased from the American Type Culture Collection (ATCC: HTB-37) and cultured in Dulbecco’s Modified Eagle Medium (DMEM) (Life Technologies, Carlsbad, CA, USA) containing 4.5 g/L D-glucose, 110 mg/L sodium pyruvate, 20% Fetal Bovine Serum (FBS) (Rocky Mountain Biologicals, Missoula, MT, USA), 1X GlutaMAX (Life Technologies, Carlsbad, CA, USA), and 1X penicillin-streptomycin (Life Technologies, Carlsbad, CA, USA) in a humidified incubator at 37° C and 5% CO2. Cells were harvested using TrypLE (Life Technologies, Carlsbad, CA, USA), resuspended in culture medium, and seeded at a density of 2.6×105 cells/cm2 on 12 well polyethylene terephthalate transwell ThinCerts hanging well inserts (0.4 μm pore size, 1.13 cm2 culture area) (Greiner Bio-One, Kremsmünster, Austria). Culture medium was changed every other day for 21–28 days and 24 hours before performing permeability experiments. All cells used for permeability experiments were between passages 32 and 52.
2.4 Transepithelial Electrical Resistance and Lucifer Yellow Assays
To ensure monolayer integrity throughout the experimental period, transepithelial electrical resistance (TEER) was measured before and after experiments using the EVOM2 epithelial voltmeter (World Precision Instruments, Sarasota, FL, USA). TEER values vary throughout the literature, so we set a TEER cutoff based on Lucifer yellow rejection. Lucifer yellow is a fluorescent dye that is only transported paracellularly and is thus used as a marker of Caco-2 tight junction integrity. To determine Lucifer yellow rejection rate, we performed a Lucifer yellow permeability assay using Caco-2 cells cultured in hanging wells. First, the TEER of Caco-2 monolayers was recorded and then 0.5 mL 100 μM Lucifer yellow in Hank’s balanced salt solution (HBSS) was added to the apical side of the hanging wells. Hanging wells were then inserted into 12 well plates prefilled with 1.5 mL HBSS in each well and then stirred on a nutator (TCS Scientific, New Hope, PA, USA) at 24 RPM in a humidified, 37° C incubator for 1 hour. Afterwards samples were taken from the apical and basolateral sides of the hanging well and read on a fluorescent plate reader. The Lucifer yellow rejection value was calculated using the equation: LY % rejection = 100*(1−RFUbasolateral/RFUapical) where RFU is the relative fluorescent units recorded by the plate reader. It was determined that TEER values below 290 Ω*cm2 had Lucifer yellow rejection values below 95% and as a result, wells with a TEER value below 290 Ω*cm2 were not used for permeability assays.
2.5 Simulated Digestion
Simulated digestion was performed using the method described in Weathers et al. 2014 (Weathers et al., 2014). Either 0.36 g DLA or 2 mg pure artemisinin was digested in a 50 mL conical tube. Digestions were run through oral, gastric, and intestinal stages of digestion and then filtered through Whatman #1 chromatography paper (0.16 mm thickness, porosity <10 μm) to separate liquid and solid fractions. Only the liquid fraction of the digestate was used for permeability experiments.
2.6 CYP3A4 Upregulation
Under normal culture conditions, the human cytochrome P450 isoform CYP3A4 is not expressed in Caco-2 cells. To better mimic the in vivo conditions, CYP3A4 expression was induced by adding 0.5 μM 1α,25-dihydroxyvitamin D3 (VD3) to the culture media as described by Schmiedlin-Ren et al. 2001 (Schmiedlin-Ren et al., 2001). We performed RNA isolation and qPCR for CYP3A4 on cells cultured in this VD3 media to confirm the upregulation of CYP3A4 transcription. RNA isolation was performed using the SV Total RNA Isolation System (Promega, Madison, WI, USA) according to the manufacturer’s instructions. Using qScript cDNA SuperMix (Quanta Biosciences, Beverly, MA, USA), cDNA was prepared from total RNA according to the manufacturer’s instructions. Using PerfeCta SYBR Green FastMix, low ROX (Quanta Biosciences, Beverly, MA, USA), qPCR was performed according to the manufacturer’s instructions with an Applied Biosystems 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). The following primers were used for qPCR: CYP3A4F (5′-CCTTACATATACACACCCTTTGGAAG-3′), CYP3A4R (5′-GGTTGAAGAAGTCCTCCTAAGCT-3′) (Nowakowski et al. 2002), ActinF (5′-AGAGCTACGAGCTGCCTGAC-3′), ActinR (5′-GGATGCCACAGGACTCCA-3′). CYP3A4 upregulation was also verified at the level of enzymatic activity with a luciferin-based P450-Glo assay specific for CYP3A4 (Promega, Madison, WI, USA) following the manufacturer’s instructions. Caco-2 cells were cultured in monolayers on 12 well plates for 21–28 days in either standard or VD3 containing media prior to P450-Glo assays. Cells were then incubated for 2 hours with culture media containing 3 μM Luciferin-IPA, a CYP3A4-specific substrate that is converted to a luminogenic substrate by CYP3A4. After the 2 hour incubation, the medium was removed and combined with Luciferin Detection Reagent and luminescence measured on a PerkinElmer Victor3 plate reader (PerkinElmer, Waltham, MA, USA).
2.7 Permeability Experiments
Artemisinin permeability experiments were performed in 12 well plates with transwell inserts having a developed cell monolayer separating the wells into an apical and basolateral chamber. Culture medium was first decanted and cells on transwell inserts were washed 3 times in HBSS pre-warmed to 37° C. Inserts were then placed in a new 12 well plate pre-filled with 1.5 mL warm HBSS in each basolateral chamber and 0.5 mL warm HBSS was added to the apical chamber. The plate was placed in a humidified 37° C incubator on a nutator mixer and mixed at 24 RPM for 20 minutes to wash off excess media. During the 20 minute incubation, donor solutions were prepared and warmed to 37° C. Donor solutions were either the liquid digestate fraction from a simulated digestion or pure artemisinin with or without a pure test compound dissolved in 2.5% dimethyl sulfoxide (DMSO) in HBSS. The solubility of artemisinin in donor solutions was checked by filtering through a 0.45 μm nylon syringe filter. Filtered and unfiltered donor solutions had no significant difference in artemisinin content; artemisinin was fully soluble at 50 ug/mL in water, DLAG, and DLAS donor solutions. After incubation, the TEER of each well was recorded, HBSS was decanted from the apical chambers, 0.5 mL donor solution was added to the apical chamber and the plate was placed on the nutator in the incubator. Samples of the donor solution were taken to determine the artemisinin content at time 0. Experiments were performed for 60 minutes. At each 15 minute interval, the transwell inserts were removed and placed into a new 12 well plate pre-filled with pre-warmed HBSS and placed immediately back into the incubator on the nutator. Samples were then taken from the basolateral chamber for extraction and analysis. After 60 minutes, the TEER of each well was recorded to validate integrity of the monolayer and a sample was taken from the apical chamber of each transwell insert for extraction and analysis.
2.8 Artemisinin and Papp Analysis
Artemisinin from samples was extracted by adding a 1:1 volume of methylene chloride, briefly vortexing, and sonicating in a sonication water bath for 30 minutes. The organic layer was then removed and dried under a mild stream of nitrogen gas, frozen, and stored at −20° C before analysis. Artemisinin analysis was by gas-chromatography mass-spectrometry (GC-MS) using the method detailed in Towler and Weathers 2015 (Towler and Weathers, 2015). Apparent permeability (Papp) was calculated using the formula: where ΔQ/Δt is the amount of drug transported per time, A is the surface area of the transwell insert, and Co is the original concentration of drug in the donor chamber at time 0. This equation requires that sink conditions be maintained, thus transwell inserts were placed in new receiver chambers with pre-warmed HBSS at each 15 minute time point. Percent recovery of artemisinin was calculated by dividing the total artemisinin recovered in each well by the original amount of artemisinin in the donor solution and multiplying by 100. The percent recoveries of artemisinin for each experiment are shown in Table S2.
2.9 Statistical Analysis
Statistical analysis was performed using GraphPad Prism 6. Each experiment was the average of ≥3 wells and any experiment where a significant difference was observed between the control and experimental wells was subsequently repeated. One-way ANOVA and students T-tests were used where appropriate to determine statistical significance (p<0.05).
3.0 Theory
In animal studies artemisinin is significantly more bioavailable from per os delivery of DLA than from its pure form. Using the Caco-2 permeability assay and simulated digestion methods, we tested digestates of DLA and individual A. annua phytochemicals for their ability to enhance transport of artemisinin across intestinal cells. This approach improved our understanding of DLA enhancement of artemisinin bioavailability.
4.0 Results and Discussion
4.1 Digestate of A. annua Increases Intestinal Permeability of Artemisinin
To determine if digested DLA increased the intestinal permeability of artemisinin, we performed a Caco-2 permeability assay using simulated digestates of DLAS compared to a pure artemisinin control. When delivered as DLAS digestate, the intestinal permeability of artemisinin was significantly greater by 37% than artemisinin delivered as pure drug (p = 0.013) (Figure 1). This result suggested that one or more phytochemicals in A. annua may increase the intestinal absorption of artemisinin. Next, we performed a similar experiment using the glandless (DLAG) cultivar of A. annua that produces no artemisinin, very few flavonoids, and negligible levels of essential oils. We combined pure artemisinin with a digestate of DLAG and compared to a pure artemisinin control in a Caco-2 assay. Interestingly, there was no change in intestinal permeability when artemisinin was combined with digestate from DLAG (Figure 1). These results suggested that one or more phytochemicals produced by DLAS, but not in DLAG, were responsible for the increased intestinal permeability afforded by the DLAS digestate. In addition to testing the Papp of artemisinin in the apical-to-basolateral direction, we also tested the Papp of artemisinin in the basolateral-to-apical direction. The Papp of artemisinin in the basolateral-to-apical direction was not significantly different from the Papp of artemisinin in the apical-to-basolateral direction suggesting active efflux does not play a role in artemisinin absorption. Unfortunately, permeability of artemisinin delivered as digestate from the basolateral-to-apical direction could not be determined because digestates significantly weakened the integrity of the tight junctions in the Caco-2 monolayers as determined by a sharp decrease in TEER.
Figure 1.
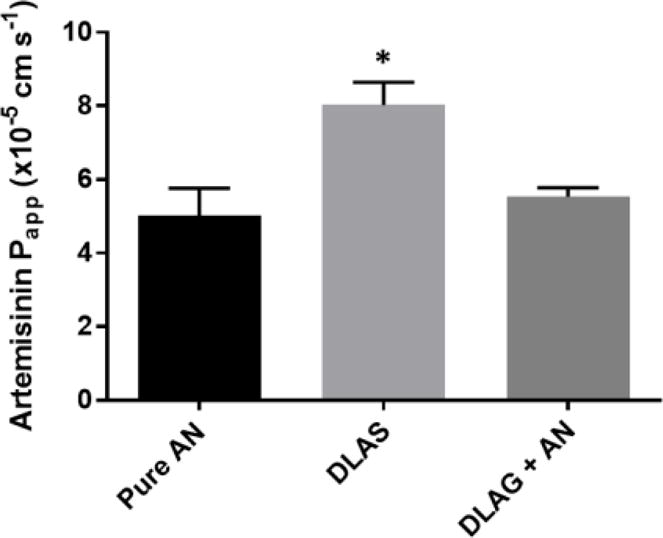
Papp of artemisinin in Caco-2 permeability assays when delivered as pure AN drug, DLAS, or DLAG+AN. n ≥3; *, p <0.05 compared to pure drug control.
4.2 Flavonoids and Other Phytochemicals Do Not Alter Artemisinin Papp
Flavonoids, a group of phytochemicals responsible for many of the pigments in plants, significantly enhanced the intestinal uptake of several drugs. For example oral administration of genistein increased the bioavailability of paclitaxel in rats (Li and Choi, 2007). Furthermore, several flavonoids also increased intestinal absorption of ochratoxin A in Caco-2 cells at physiologically relevant concentrations (Sergent et al., 2005). A. annua is known for having high flavonoid content, so we hypothesized that the flavonoids present in DLAS may have enhanced the intestinal permeability of artemisinin. To test this hypothesis, we performed Caco-2 assays using pure artemisinin in combination with various pure flavonoids having different structural variations and known to be present in A. annua and compared their Papp to the Papp of pure artemisinin. As shown in Table 1 below, none of the tested flavonoids significantly altered the Papp of artemisinin.
Table 1.
Flavonoid effects on Papp of AN
| Flavonoid | Papp (×10−5)(cm s−1) (±SD) |
|---|---|
| Pure AN | 5.59 ± 0.94 |
| +Quercetin | 6.93 ± 1.21 |
| +Rutin | 7.18 ± 1.00 |
| +Eupatorin | 5.57 ± 0.27 |
| +Kaempferol | 5.39 ± 0.28 |
| +Casticin | 7.09 ± 0.92 |
| +Isovitexin | 6.45 ± 0.89 |
| +Apigenin | 5.98 ± 0.56 |
AN, artemisinin.
Other phytochemicals known to increase bioavailability of other xenobiotics or that were considered to also affect intestinal absorption of artemisinin were also tested. Although inulin, a polysaccharide produced by A. annua, increased intestinal absorption of magnesium, calcium, and other minerals (Scholz-Ahrens and Schrezenmeir, 2007) it did not increase the intestinal permeability of artemisinin (Table 2). Recently, a combination of three phytochemicals found in A. annua, artemisinic acid (AA), arteannuin B (AB), and the coumarin scopoletin, increased oral bioavailability of artemisinin in mice (Zhang et al., 2016). This combination, however, did not increase the Papp of artemisinin in our Caco-2 assays (Table 2). Finally, chlorogenic acid and rosmarinic acid, two phenolic acids often found in A. annua (Zang et al., 2014) did not alter the Papp of artemisinin (Table 2). Together these results suggested that flavonoids were not responsible for the increased Papp of artemisinin afforded by DLAS. Furthermore, the results showed that neither inulin, phenolic acids, nor the combination of AA, AB and scopoletin altered artemisinin intestinal absorption. While these compounds do not alter artemisinin intestinal absorption, they may have affected artemisinin bioavailability via some combination of compounds or some other mechanism yet to be investigated, such as inhibition of artemisinin metabolism in the liver.
Table 2.
Effects of other phytochemicals of interest on AN Papp
| Phytochemical | Papp (×10−5)(cm s−1) (±SD) |
|---|---|
| Pure AN | 5.94 ± 0.69 |
| +Chlorogenic Acid | 6.17 ± 0.45 |
| +Rosmarinic Acid | 5.98 ± 0.75 |
| +Inulin | 6.56 ± 0.44 |
| +AA+AB+Scopoletin | 5.62 ± 1.22 |
AA, artemisinic acid; AB, arteanuin B; AN, artemisinin.
4.3 Intestinal CYP3A4 Does Not Play a Role in Increasing DLA Delivered Artemisinin
In humans, artemisinin is primarily metabolized in the liver by the cytochrome P450 isoform, CYP2B6. However, CYP3A4 also contributes in a minor role and is expressed in the liver as well as the small intestine. There are many phytochemicals found in A. annua, such as quercetin, luteolin, apigenin, and kaempferol, that have been shown to inhibit CYP450 enzymes (Basheer and Kerem, 2015), so we hypothesized that inhibition of intestinal CYP3A4 by phytochemicals produced by A. annua would allow more artemisinin to pass through the intestine resulting in higher bioavailability. While CYP3A4 is expressed in the human intestine, it is not expressed in Caco-2 cells under normal culture conditions unless induced by VD3. Thus, to test our hypothesis, we first induced expression of CYP3A4 by including VD3 in the cell culture medium. CYP3A4 expression increased ~175 fold with VD3 treatment as determined by qPCR (Table S3). A luciferin-based CYP3A4 activity assay confirmed that CYP3A4 activity increased 183% in VD3 treated cells compared to untreated cells (p = 0.012). Next we performed Caco-2 permeability assays using pure artemisinin, digested DLAS, or pure artemisinin combined with quercetin, a known CYP3A4 inhibitor found in DLAS, on cells treated with VD3 media. As expected, the Papp of DLAS +VD3 was significantly higher than the Papp of pure artemisinin +VD3 (p = 0.02) (Table 3). However, addition of VD3 to media did not alter the Papp of artemisinin, regardless of whether it was delivered as pure drug, DLAS, or in combination with quercetin. These results suggested that intestinal CYP3A4 does not play a major role in determining the bioavailability of artemisinin; this needs in vivo verification. Furthermore, the mechanism by which DLAS increased intestinal permeability of artemisinin seems independent of CYP3A4.
Table 3.
Papp of artemisinin delivered as pure drug, DLA, or as pure drug + quercetin in media ± VD3
| Donor Solution | Papp (×10−5)(cm s−1) (±SD) |
|---|---|
| Pure AN −VD3 | 6.17 ±1.13 |
| Pure AN +VD3 | 6.27 ± 1.66 |
| DLAS Digestate −VD3 | 8.03 ± 1.23* |
| DLAS Digestate +VD3 | 9.40 ± 1.05* |
| Quercetin −VD3 | 6.93 ± 1.21 |
| Quercetin +VD3 | 6.38 ± 0.89 |
AN, artemisinin; DLAS, dried leaf Artemisia annua; VD3, 1α,25-dihydroxyvitamin D3;
p≤0.05 compare to pure AN controls.
4.4 Digested A. annua Essential Oils Alter Papp and TEER of Caco-2 Cells
Recently our group showed that essential oil (EO) from A. annua increased the digestive solubility of artemisinin (Desrosiers and Weathers, 2016). Since EO increased artemisinin solubility and contains >100 phytochemicals (Ćavar et al., 2012), we hypothesized that this fraction of A. annua would increase the intestinal permeability of artemisinin in Caco-2 assays. A. annua cultivars vary significantly in the amount and chemical profiles of EO, so we used EOs from two different sources (USA and China) and their relative contents are shown in Table S4. The range of total EOs in A. annua also varies between 0.3% and 4.0% (v/w) (Bilia et al., 2014). Thus, we performed Caco-2 permeability assays with digested EO from two sources at two different concentrations corresponding to these known ranges of EO produced by A. annua cultivars. Surprisingly, the digestate of EO from both sources and at both concentrations caused a decrease in the Papp of artemisinin as well as a sharp decrease in the TEER suggesting a loss of the integrity of tight junctions (Table 4).
Table 4.
Effects of 2 different digested A. annua essential oils at different concentrations on artemisinin Papp
| Donor Solution | Papp (×10−5)(cm s−1) (±SD) | Loss of TEER |
|---|---|---|
| Pure AN | 5.22 ± 0.52 | − |
| AN+US EO Digestate (4%) | 3.39 ± 0.41* | + |
| AN+US EO Digestate (0.3%) | 3.86 ± 0.53* | + |
| AN+Chinese EO Digestate (4%) | 3.71 ± 0.66* | + |
| AN+Chinese EO Digestate (0.3%) | 3.76 ± 0.60* | + |
| AN+DLAG+US EO Digestate (4%) | 4.99 ± 0.57 | + |
| AN+DLAG+US EO Digestate (0.3%) | 5.06 ± 0.43 | + |
AN, artemisinin; EO, essential oil; TEER, transepithelial electrical resistance; US, United States;
p<0.05 compare to pure AN controls.
To confirm the drop in TEER was due to weakening of the tight junctions and not an artifact produced by the TEER measuring equipment, we performed a Lucifer yellow assay on the cells after the permeability experiment. Lucifer yellow is a fluorescent dye that only passes paracellularly and thus is only transported when tight junctions lose their integrity (Rastogi et al., 2013). After treatment with US and Chinese EO digestates, Caco-2 cells had Lucifer yellow rejection rates of 83.7% and 84.0%, respectively, compared with 97.6% for pure artemisinin. To determine which phytochemical in the EO caused the decreased Papp and TEER we tested three major components of A. annua EO, camphor (at 3 concentrations), eucalyptol, and caryophyllene in Caco-2 permeability assays. As shown in Table 5 below, none of the tested compounds altered the TEER or Papp of artemisinin. Interestingly, when digestate made with EO combined with artemisinin and DLAG plant material was used in Caco-2 assays, there was no observed decrease in Papp however, the TEER still dropped sharply suggesting that the A. annua plant matrix may counteract the decreased intestinal permeability, but not the reduced tight junction integrity caused by EO, as indicated by the TEER loss.
Table 5.
Effects of undigested pure compounds found in A. annua essential oil on artemisinin Papp and TEER
| EO Phytochemical | Papp (×10−5)(cm s−1) (±SD) | Loss of TEER? |
|---|---|---|
| Pure AN | 5.45 ± 0.86 | − |
| Camphor 1:1 | 5.84 ± 0.92 | − |
| Camphor 1:2 | 6.20 ± 1.22 | − |
| Camphor 1:10 | 4.74 ± 0.87 | − |
| Eucalyptol | 5.72 ± 0.34 | − |
| Caryophyllene | 6.13 ± 0.56 | − |
AN, artemisinin; EO, essential oil; TEER, transepithelial electrical resistance
Bile is an emulsifier that is an important component of lipid digestion and facilitates the formation of micelles from ingested lipids (Maldonado-Valderrama et al., 2011). To determine the role of digestion and bile in the digestate liquid on altering the Papp and TEER, we performed Caco-2 permeability assays with undigested EO and with bile extract added to EO without performing the simulated digestion process. Without digestion, neither of the tested EO’s altered the artemisinin Papp. On the other hand, addition of bile extract to both EO sources resulted in a drop in the TEER after the experiment (Table 6).
Table 6.
Effects of undigested essential oils ± bile on artemisinin Papp and TEER
| Compound | Bile added? | Papp (×10−5)(cm s−1) (±SD) | Loss of TEER? |
|---|---|---|---|
| Pure AN | − | 4.82 ± 0.60 | − |
| Pure AN | + | 4.12 ± 0.38 | − |
| AN+US EO (4%) | − | 4.85 ± 0.35 | − |
| AN+Chinese EO (4%) | − | 4.17 ± 0.10 | − |
| AN+US EO (4%) | + | 4.81 ± 0.51 | + |
| AN+Chinese EO (4%) | + | 5.67 ± 0.64 | + |
AN, artemisinin; EO, essential oil; TEER, transepithelial electrical resistance; US, United States.
Taken together, these results suggested that EO from A. annua modulated the tight junctions of enterocytes, but only after digestion. Surprisingly, this decrease in tight junction integrity correlated with a decrease in Papp. It is possible that in EO digestates, some of the free artemisinin becomes sequestered in micelles formed by the bile emulsifier interacting with the EO. In this scenario, the free fraction of artemisinin is decreased reducing the rate of diffusion and thus the Papp. Although the tight junctions between enterocytes are more tenuous, there is a weaker driving force for the diffusion of lipophilic artemisinin across the membrane. A similar phenomenon was observed in several cases where surfactants were added to increase solubility of highly lipophilic drugs (Dahan and Miller, 2012). Additionally, it is known that lipophilic drugs incorporate into lipid micelles in digestive fluid, especially when oil is present (Pullakhandam and Failla, 2007). The drugs in solution are in an equilibrium state between micellarized and free drug states. While the free drug is capable of permeating the intestinal membrane, drug in the micellarized state is not and thus the overall permeability decreases with increased micelle concentration. This was demonstrated by Yano et al. who showed that the Papp of six different lipophilic drugs decreased with increasing micelle concentration in a Caco-2 system (Yano et al., 2010).
5.0 Conclusions
Understanding the mechanisms by which DLAS increases the bioavailability of artemisinin in vivo is important for the development of DLAS as a malaria therapeutic. While we previously showed that DLAS increased the solubility of artemisinin, solubility is only one factor that governs the overall bioavailability of a drug. Here, using Caco-2 cell permeability assays we showed that DLAS digestate also increased intestinal permeability of artemisinin when compared to pure drug. This increase in permeability helps to, but does not fully explain the >40 times increase in artemisinin bioavailability afforded by DLAS in murine studies.
There are several other known factors that drive bioavailability of artemisinin. For example, we previously showed artemisinin was significantly more soluble when delivered as DLAS (Desrosiers and Weathers, 2016). Drug bioavailability is also largely dictated by the liver. Recently it was shown that chrysoplenetin, a polymethoxylated flavonoid found in A. annua, delivered orally to rats in conjunction with artemisinin increased the oral bioavailability of artemisinin and inhibited several CYP450 enzymes in the rat liver (Wei et al., 2015). It is therefore possible that phytochemicals found in A. annua inhibit the first pass metabolism of artemisinin by liver CYP450 enzymes resulting in higher levels of the drug in the blood. The liver is our next study target for explaining the enhanced bioavailability of artemisinin from DLA.
This study also determined that intestinal CYP3A4 is an unlikely contributor to the bioavailability of artemisinin and showed that flavonoids and other phytochemicals of varying structure are not sufficient alone to alter artemisinin intestinal permeability. Although there were some deleterious effects of digested A. annua EO on tight junctions and a drop in artemisinin Papp, the plant matrix appeared to counteract the Papp decline. While the observed increases in solubility and intestinal permeability help explain the increased bioavailability of artemisinin from DLAS, there may still be other mechanisms at play. These mechanisms should be investigated to better understand the factors that govern artemisinin oral bioavailability when delivered via DLA.
Supplementary Material
Acknowledgments
The authors thank Dr. Melissa Towler, Dr. Andy Butler, Daryl Johnson, Dr. Sarah Hernandez, and Prof. Jill Rulfs of Worcester Polytechnic Institute for technical assistance. We are also grateful for Award Number NIH-R15AT008277-01 from the National Center for Complementary and Integrative Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Complementary and Integrative Health or the National Institutes of Health.
Abbreviations
- AA
artemisinic acid
- AB
arteannuin B
- ACT
artemisinin combination therapy
- AN
artemisinin
- DLA
dry leaf Artemisia
- DLAS
DLA A. annua cultivar with ~1.4% artemisinin
- DLAG
DLA glandless
- GLS
glandless A. annua cultivar that contains no artemisinin
- TEER
trans-epithelial electrical resistance
- VD3
1α,25-dihydroxyvitamin D3
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
M. Desrosiers – conceived and designed study, data acquisition and interpretation, drafted article
P. Weathers - conceived and designed study, data interpretation, edited article
Both authors approve of the final submitted version of the article.
CLASSIFICATION: Gastro-intestinal system
References
- Basheer L, Kerem Z. Interactions between CYP3A4 and dietary polyphenols. Oxidative medicine and cellular longevity 2015. 2015 doi: 10.1155/2015/854015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilia AR, Santomauro F, Sacco C, Bergonzi MC, Donato R. Essential oil of Artemisia annua L.: an extraordinary component with numerous antimicrobial properties. Evidence-Based Complementary and Alternative Medicine 2014. 2014 doi: 10.1155/2014/159819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JS, Piao YJ, Kang KW. Effects of quercetin on the bioavailability of doxorubicin in rats: Role of CYP3A4 and P-gp inhibition by quercetin. Archives of Pharmacal Research. 2011;34(4):607–613. doi: 10.1007/s12272-011-0411-x. [DOI] [PubMed] [Google Scholar]
- Daddy NB, Kalisya LM, Bagire PG, Watt RL, Towler MJ, Weathers PJ. Artemisia annua dried leaf tablets treated malaria resistant to ACT and iv artesunate: case reports. Phytomedicine. 2017 doi: 10.1016/j.phymed.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan A, Miller JM. The Solubility–Permeability Interplay and Its Implications in Formulation Design and Development for Poorly Soluble Drugs. The AAPS Journal. 2012;14(2):244–251. doi: 10.1208/s12248-012-9337-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers MR, Weathers PJ. Effect of leaf digestion and artemisinin solubility for use in oral consumption of dried Artemisia annua leaves to treat malaria. Journal of Ethnopharmacology. 2016;190:313–318. doi: 10.1016/j.jep.2016.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfawal MA, Towler MJ, Reich NG, Golenbock D, Weathers PJ, Rich SM. Dried whole plant Artemisia annua as an antimalarial therapy. PLoS ONE. 2012;7:e52746. doi: 10.1371/journal.pone.0052746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfawal MA, Towler MJ, Reich NG, Weathers PJ, Rich SM. Dried whole-plant Artemisia annua slows evolution of malaria drug resistance and overcomes resistance to artemisinin. Proceedings of the National Academy of Sciences. 2015;112(3):821–826. doi: 10.1073/pnas.1413127112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu E. The history of qing hao in the Chinese materia medica. Transactions of The Royal Society of Tropical Medicine and Hygiene. 2006;100(6):505–508. doi: 10.1016/j.trstmh.2005.09.020. [DOI] [PubMed] [Google Scholar]
- ICIPE. Whole-leaf Artemisia annua-based antimalarial drug: report on proof-of-concepts studies, Nairobi, Kenya. Nairobi, Kenya: 2005. [Google Scholar]
- Li X, Choi JS. Effect of genistein on the pharmacokinetics of paclitaxel administered orally or intravenously in rats. International Journal of Pharmaceutics. 2007;337(1–2):188–193. doi: 10.1016/j.ijpharm.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Maldonado-Valderrama J, Wilde P, Macierzanka A, Mackie A. The role of bile salts in digestion. Advances in Colloid and Interface Science. 2011;165(1):36–46. doi: 10.1016/j.cis.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Melillo de Magalhães P, Dupont I, Hendrickx A, Joly A, Raas T, Dessy S, Sergent T, Schneider YJ. Anti-inflammatory effect and modulation of cytochrome P450 activities by Artemisia annua tea infusions in human intestinal Caco-2 cells. Food Chemistry. 2012;134(2):864–871. doi: 10.1016/j.foodchem.2012.02.195. [DOI] [PubMed] [Google Scholar]
- Pullakhandam R, Failla ML. Micellarization and intestinal cell uptake of β-carotene and lutein from drumstick (Moringa oleifera) leaves. Journal of medicinal food. 2007;10(2):252–257. doi: 10.1089/jmf.2006.250. [DOI] [PubMed] [Google Scholar]
- Rastogi H, Pinjari J, Honrao P, Praband S, Somani R. The impact of permeability enhancers on assessment for monolayer of colon adenocarcinoma cell line (CACO-2) used in in vitro permeability assay. Journal of Drug Delivery and Therapeutics. 2013;3(3):20–29. [Google Scholar]
- Schmiedlin-Ren P, Thummel KE, Fisher JM, Paine MF, Watkins PB. Induction of CYP3A4 by 1α,25-Dihydroxyvitamin D3 Is Human Cell Line-Specific and Is Unlikely to Involve Pregnane X Receptor. Drug Metabolism and Disposition. 2001;29(11):1446–1453. [PubMed] [Google Scholar]
- Scholz-Ahrens KE, Schrezenmeir J. Inulin and Oligofructose and Mineral Metabolism: The Evidence from Animal Trials. The Journal of Nutrition. 2007;137(11):2513S–2523S. doi: 10.1093/jn/137.11.2513S. [DOI] [PubMed] [Google Scholar]
- Sergent T, Garsou S, Schaut A, Saeger SD, Pussemier L, Peteghem CV, Larondelle Y, Schneider YJ. Differential modulation of ochratoxin A absorption across Caco-2 cells by dietary polyphenols, used at realistic intestinal concentrations. Toxicology Letters. 2005;159(1):60–70. doi: 10.1016/j.toxlet.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Svensson USH, Ashton M. Identification of the human cytochrome P450 enzymes involved in the in vitro metabolism of artemisinin. British Journal of Clinical Pharmacology. 1999;48(4):528–535. doi: 10.1046/j.1365-2125.1999.00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez MR, Canel C, Rimando AM, Duke SO. Differential accumulation of isoprenoids in glanded and glandless Artemisia annua L. Phytochemistry. 1999;52(6):1035–1040. [Google Scholar]
- Towler MJ, Weathers PJ. Variations in key artemisinic and other metabolites throughout plant development in Artemisia annua L. for potential therapeutic use. Industrial Crops and Products. 2015;67:185–191. doi: 10.1016/j.indcrop.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HJ, Pao LH, Hsiong CH, Shih TY, Lee MS, Hu OY-P. Dietary Flavonoids Modulate CYP2C to Improve Drug Oral Bioavailability and Their Qualitative/Quantitative Structure–Activity Relationship. The AAPS Journal. 2014;16(2):258–268. doi: 10.1208/s12248-013-9549-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers PJ, Arsenault PR, Covello PS, McMickle A, Teoh KH, Reed DW. Artemisinin production in Artemisia annua: studies in planta and results of a novel delivery method for treating malaria and other neglected diseases. Phytochemistry Reviews. 2011;10(2):173–183. doi: 10.1007/s11101-010-9166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers PJ, Jordan NJ, Lasin P, Towler MJ. Simulated digestion of dried leaves of Artemisia annua consumed as a treatment (pACT) for malaria. Journal of Ethnopharmacology. 2014;151(2):858–863. doi: 10.1016/j.jep.2013.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S, Ji H, Yang B, Ma L, Bei Z, Li X, Dang H, Yang X, Liu C, Wu X, Chen J. Impact of chrysosplenetin on the pharmacokinetics and anti-malarial efficacy of artemisinin against Plasmodium berghei as well as in vitro CYP450 enzymatic activities in rat liver microsome. Malaria Journal. 2015;14(1):432. doi: 10.1186/s12936-015-0929-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Malaria Fact Sheet. 2016 http://www.who.int/mediacentre/factsheets/fs094/en/
- Yano K, Masaoka Y, Kataoka M, Sakuma S, Yamashita S. Mechanisms of Membrane Transport of Poorly Soluble Drugs: Role of Micelles in Oral Absorption Processes. Journal of Pharmaceutical Sciences. 2010;99(3):1336–1345. doi: 10.1002/jps.21919. [DOI] [PubMed] [Google Scholar]
- Zang M, Zhu F, Li X, Yang A, Xing J. Auto-induction of phase I and phase II metabolism of artemisinin in healthy Chinese subjects after oral administration of a new artemisinin-piperaquine fixed combination. Malaria Journal. 2014;13(1):214. doi: 10.1186/1475-2875-13-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Gong MX, Qiu F, Li J, Wang MY. Effects of arteannuin B, arteannuic acid and scopoletin on pharmacokinetics of artemisinin in mice. Asian Pacific Journal of Tropical Medicine. 2016;9(7):677–681. doi: 10.1016/j.apjtm.2016.05.004. [DOI] [PubMed] [Google Scholar]
- Ćavar S, Maksimović M, Vidic D, Parić A. Chemical composition and antioxidant and antimicrobial activity of essential oil of Artemisia annua L. from Bosnia. Industrial Crops and Products. 2012;37(1):479–485. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


