Abstract
Introduction
Block scheduling during residency is an innovative model separating inpatient and ambulatory rotations. We hypothesized this format may have a positive impact on resident sleep and wellness as compared to a traditional format.
Methods
We performed a single-center, cross-sectional, observational study of residents rotating in the medical intensive care unit (MICU). Residents were observed for 4 weeks at a time: Internal Medicine (IM) residents for 3 MICU weeks followed by one ambulatory week, and non-IM residents for 4 weeks in the MICU. We monitored daily total sleep time (TST) utilizing actigraphy, and wellness measures with weekly Epworth Sleepiness Scale (ESS) and Perceived Stress Scale (PSS) questionnaires.
Results
64 of 110 (58%) eligible residents participated, 49 (45%) were included in the final analysis. Mean daily TST for the entire cohort was 6.53h (± 0.78h). Residents slept significantly longer during the ambulatory block compared to the MICU block (mean TST 6.97h ±1.00h vs 6.43h ± .78h; p < .0005). Sleep duration during night call was significantly shorter than day shift (mean TST 6.07h ±1.16h vs 6.50h ± .73h; p = <.0005). 390 of 490 (80%) of ESS and PSS questionnaires were completed, scores significantly declined while in the MICU. IM residents had significant improvement in TST, ESS and PSS scores (p < .05) at the end of the ambulatory week. Non-IM residents, who remained in the MICU for a fourth week, continued a trend of decline in perceived wellness.
Conclusion
Despite duty hour restrictions, residents are getting inadequate sleep. As MICU days accumulate, measures of resident wellness decline. Residents in a block schedule experienced improvement in all measured parameters during the ambulatory week, while residents in a traditional schedule continued a downward trend. Block scheduling may have the previously unrecognized benefit of repaying sleep debt, correcting circadian misalignment and improving wellness.
INTRODUCTION
The hazards of long work hours and sleep deprivation in residency training programs have been consistently demonstrated. Fatigued residents have reduced working memory capacity(1), impaired reaction time and vigilance(2, 3), and increased medical errors(1, 4). In addition to the threat to patient safety, potential harm to residents exists as well. Residents working prolonged hours are at increased risk of motor vehicle accidents(5, 6) burnout(7), and depression(8). Reducing these hazards, primarily through the elimination of sleep deprivation, has been a goal of the Accreditation Council of Graduate Medical Education’s (ACGME) evolving duty hour restrictions. In 2003 the ACGME instituted the maximum 80-hour work week and 30 hours of consecutive duty. In 2011 regulations were updated to include a maximum of 16-hour consecutive duty for first year residents. The ACGME does not provide specific guidance on sleep duration goals for trainees however seven hours per night is the minimum recommended duration according to a recent consensus statement from American Academy of Sleep Medicine and Sleep Research Society(9) and represents a good minimum standard for trainees. In response to duty hour guidelines, residency curricula have had to adapt to the decreased total time in the hospital. Traditionally Internal Medicine (IM) residents attend their ambulatory clinics while on inpatient rotations which can be difficult to fit into a shorter workday. Additionally, the increased need for night call shifts due to 16 hour shifts for first year residents have introduced challenges to scheduling required ambulatory clinic time. Block (also called “X+Y” or “X:Y”) scheduling is a curricular adaptation that responds to these needs including a separation of competing demands in two different environments and is being implemented by a growing number of IM residency programs in the United States. In this model residents have complete separation of inpatient and ambulatory rotations, spending “X” number of consecutive weeks on an inpatient rotation, followed by “Y” number of consecutive weeks on an ambulatory rotation. The goal is to improve ambulatory training by minimizing conflicts between inpatient and outpatient care and allow immersion in the ambulatory experience(10, 11). As large scale changes to graduate medical education structure are made it is important to continually assess their impact. While data supports the notion that sleep deprivation and resident fatigue are dangerous, studies demonstrating improved sleep duration and risk reduction with duty hour restrictions are limited and inconsistent(4, 12–14). In part, this reflects that the full breadth of sleep disruptions impacting trainees has yet to be considered. Beyond duty hour restrictions, the schedule itself presents a challenge. Humans have endogenous circadian rhythms that can become misaligned when the timing of the sleep cycle is disturbed(15). Such misalignment has long been demonstrated to contribute to deficits in alertness and performance in shift workers(16). The early morning shifts and night call of an inpatient rotation put trainees at risk for disrupted and diminished sleep and its consequences. The effect of block scheduling on resident sleep has not been studied, but one potential benefit is the improvement in the various aspects of sleep that the more forgiving schedule of the ambulatory week(s) provide. We hypothesized that this novel format would positively impact trainees sleep and perceptions of wellness compared to a more traditional schedule.
METHODS
Design Overview
This was an observational study conducted at a single academic medical center, observing residents’ objective sleep time, subjective sleepiness and stress levels during the MICU and ambulatory rotations.
Study Setting
Oregon Health & Science University (OHSU) is a large, urban academic medical center in the United States with an IM residency program of 98 core internal medicine residents. The MICU is a 16-bed closed unit, meaning the MICU team has primary responsibility for all patients admitted to the MICU. The MICU team consists of an attending, fellow and eight rotating residents. There are four interns from internal medicine, emergency medicine, anesthesia and neurology and four senior internal medicine residents.
Participants
After approval by the institutional review board (IRB) (approval number IRB00009878), all residents rotating through the MICU during the study period were invited to participate. Residents received orientation to the protocol at conferences prior to study commencement, and an email invitation two weeks prior to starting their rotation. Those who chose to participate were met on their first MICU day where informed consent, questionnaires and actigraphy watches were provided. Those who opted out most commonly did so due to concerns over the size of the watch or concerns about privacy. If a resident opted out they were not observed in the study in any way.
Schedules
The OHSU IM curriculum uses block scheduling in a 3+1 format. The 3-week inpatient rotation, in this case MICU, is followed by a 1-week ambulatory rotation [FIGURE 1]. The residents from non-IM programs do not use this scheduling format and continue to rotate for a fourth week in the MICU. The MICU uses a night call system and there are no 24-hour call shifts for either interns or upper level residents. Both IM and non-IM residents work night call which is typically 4 to 5 consecutive nights per rotation followed by a post call day and off day. Night call blocks are scheduled at any point during the 3-week inpatient rotation. The day shift is 6am to 6pm and the night shift is 6pm to 6am, with overlap for sign-out, shifts average 12.5–13 h/day. All residents have one day off in seven. The ambulatory block rotation is one week long, beginning with a full weekend off, followed by eight half days of clinics, 2 half days of didactics and scheduled hours from 8am to 5pm. This schedule format did not change during the study.
Figure 1.
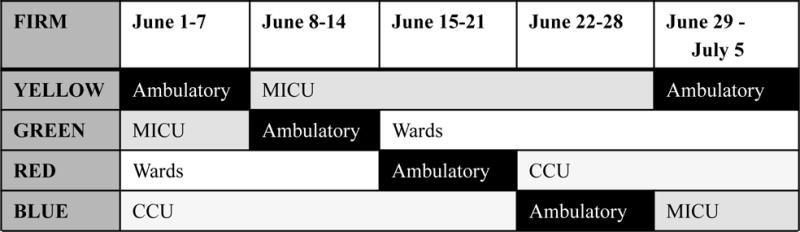
Example Internal Medicine Residency 3:1 (Inpatient:Ambulatory) block schedule. Residency divided into 4 “Firms” named by color. Each firm rotates through ambulatory and inpatient weeks together. This represents only a 5 week sample, all firms were included in the study
Measurements
All subjects wore continuous wrist actigraphy (ActiGraph Corp, Pensacola, FL) throughout their four week study period. Actigraphy is a device worn on the wrist which measures the wearer’s total sleep time (TST), sleep latency, sleep efficiency, light exposure, steps, and activity level. These measurements are made through detection of movement and light, interpreted through specialized software algorithms. The Sadeh algorithm was used for calculating TST in our study(17). Actigraphy is well validated for TST where it performs close to polysomnography(18), and we chose to focus on this measurement. Actigraphy infers sleep from lack of movement so overestimation of TST in subjects who are awake but lie motionless can occur(19). Subjects did not self-report or keep sleep diaries, recognizing this method can be additive, we did not want to overburden with questionnaires and felt that actigraphy plus the ESS was sufficient to capture the desired data. Subjects were asked to fill out two questionnaires, Epworth Sleepiness Scale (ESS © MW Johns 1990–1997. Used under License) and the Perceived Stress Scale (PSS) (questionnaires included in appendices with permission). Questionnaires were filled out at the start of the MICU rotation to establish a baseline and then weekly through the end of the individual four-week observation period. The ESS is an eight item questionnaire that is a commonly used in sleep medicine to evaluate subjective sleepiness(20–22). Scores range from 0–24 and a score of 10 or higher indicates pathologic sleepiness. The PSS is a 10-item questionnaire that evaluates how unpredictable, uncontrollable, and overloaded respondents find their lives(23). Scores range from 0–40 and higher scores have been associated with greater vulnerability to stressful life-event-elicited depressive symptoms(23). Normative data showed an average PSS score of 15.5 for men and 16.1 for women(24). The questionnaires were distributed electronically via RedCap, a secure, web-based, research database(25).
Statistical Analysis
A paired sample t-test was used to assess differences in TST during MICU and ambulatory blocks. A longitudinal mixed effects model was used to assess trends in subjects’ sleepiness and stress over the four weeks of observation, controlling for correlation of repeated measures within an individual. Time was treated as the categorical variable.
RESULTS
During the ten-month study period, 64 of the potential 110 residents (58%) rotating through the MICU consented to participate. Fifteen subjects (23%) were excluded for inadequate adherence with actigraphy, defined as wearing the watch for less than 75% of the study period. Subjects excluded for incomplete watch data, also had their questionnaire results excluded. 49 participants (45%) were included in the final analysis of the study, 40 were IM residents, and 9 non-IM residents [FIGURE 2]. 390 of 490 (80%) questionnaires were completed.
Figure 2.
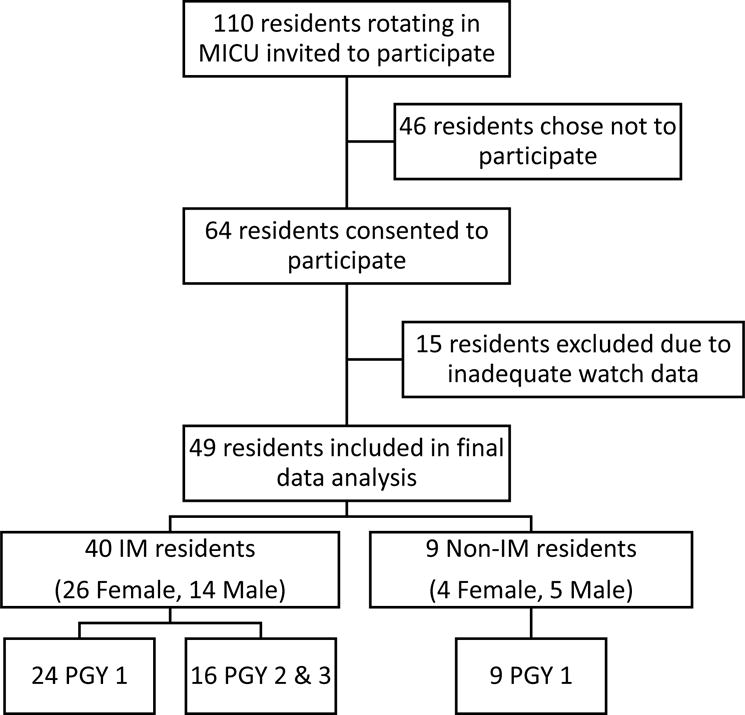
Characteristics of Participants – of the 110 residents invited to participate, 64 residents volunteered. 15 residents that participated were excluded due to inadequate watch data. Of the 49 resident included in the final analysis the majority (40) were Internal Medicine (IM). The largest level of training represented was 1st year residents (PGY-1), there were more females (30) than males (19) that participated.
The mean daily TST for the entire cohort was 6.53h (± 0.78h) overall, and 6.43h (± 0.78h) while in the MICU. 41 of 49 (84%) participants had a TST less than seven hours per day. Residents slept significantly longer when on day shifts compared to night call (mean TST 6.50h ± .73h vs 6.07h ±1.16h; p = 0.0003). There was no significant difference in sleep duration based on level of training, specialty, or gender. Among the IM residents operating in the block schedule, a statistically significant improvement in TST of 0.54 hours was observed in the ambulatory block vs the MICU block (mean, 6.97h ± 1.00h vs 6.43h ± 0.84h; p < .0005) [FIGURE 3]. The number of residents sleeping more than seven hours also improved from 10% to 45% during the ambulatory block. No week-to-week difference in TST was observed for the non-IM residents, remaining in the MICU for the entire 4-week observation period in the traditional fashion.
Figure 3. Box plot of Total Sleep Time by group.
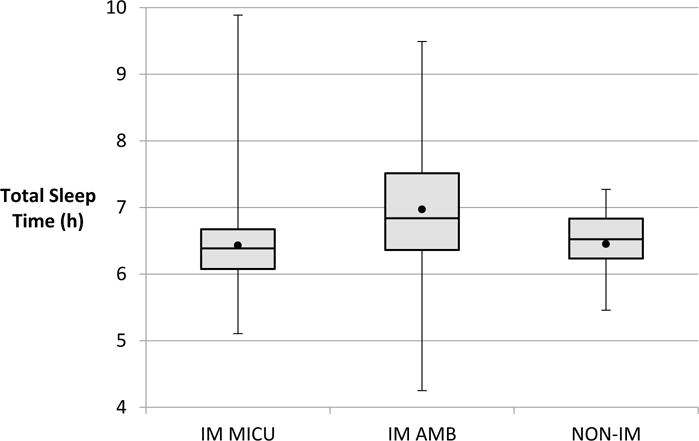
IM MICU = Internal Medicine residents during their medical intensive care unit rotation. IM AMB = Internal Medicine residents during their ambulatory rotation. NON-IM = Non-Internal Medicine residents during their MICU rotation. ● = mean total sleep time.
In regards to wellness measures, the mean PSS score was 17.1 (±5.5) and the mean ESS score was 8.6 (± 3.6) for the entire study. Similar to TST, we did not observe a significant difference in these measures based on level of training, specialty or gender. Contrary to our observed differences in TST in day vs night call, there was no significant difference in ESS (9.8 ± 4.5 vs 9.8 ± 4.9; p = 0.95) and PSS (16.8 ± 5.5 vs 16.6 ± 5.7; p = 0.79) scores. As residents accumulated time in the MICU their ESS scores increased significantly and by week 3 they had crossed the threshold of pathologic sleepiness (baseline mean, 6.5 ± 4.3 vs week 3 mean 10.5 ± 4.7; p <0.05). PSS scores had also significantly increased from baseline by week 3 (mean, 16.1 ± 5.8 vs 18.7 ± 5.5; p<0.05). Among the block schedule residents, sleepiness and stress levels peaked at the end of the MICU block and significantly improved after the ambulatory block (ESS mean, 10.69 vs 7.19; p<0.05) (PSS mean, 19.35 vs 16.6; p< 0.05). By the end of the ambulatory block sleepiness and stress levels were not significantly different from baseline (ESS mean, 6.59 ± 4.5 vs 7.19 ± 4.5; p = 0.62) (PSS mean 16.38 ± 6.2 vs 16.61 ± 6.1; p = 0.37). For those residents who remained in the ICU according to a traditional schedule, stress and sleepiness scores continued to trend upward in the fourth week [FIGURE 4].
Figure 4.
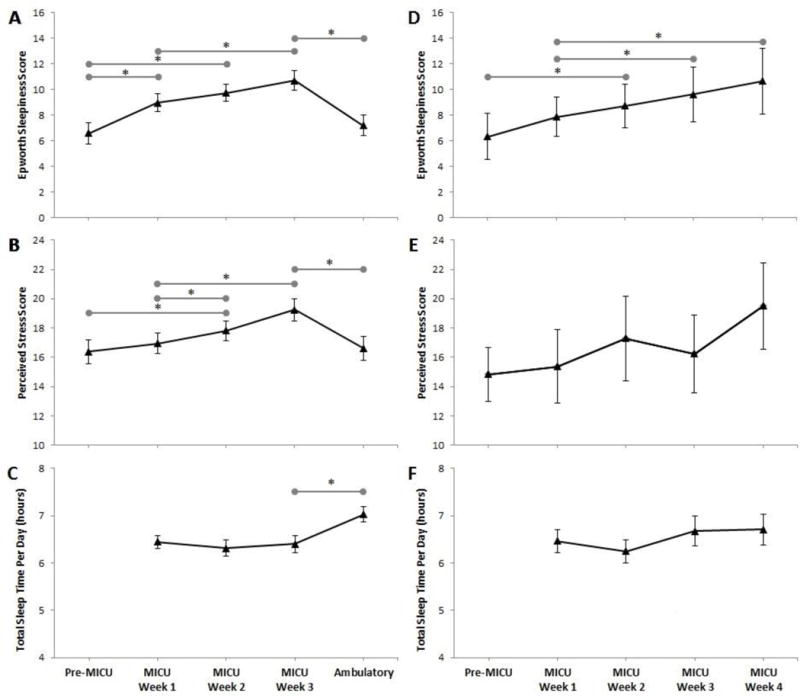
Trend of mean Epworth Sleepiness Scale, Perceived Stress Scale and Total Sleep Time by week. Internal Medicine (IM) cohort (A, B, C), n=40. Non-Internal Medicine cohort (D, E, F), n=9. Error Bars = standard error of the mean reported. Grey horizontal bars = statistically significant difference between spanned weeks (*p < 0.05).
DISCUSSION
We set out to evaluate resident sleep patterns in a residency program operating with a block schedule format in the post-2011 ACGME duty hour era. Our cohort’s mean TST of 6.53h, an average that notably includes off-days, suggests that despite increased opportunity to sleep, residents remain sleep deprived. In our study the overwhelming majority (84%) of residents slept less than seven hours, with the worst sleep observed during night call (6.07h). Normative data on sleep duration is limited primarily to survey data, but by comparison only 37.9% of an age matched cohort reported sleeping less than seven hours in a 2014 survey(26). Regularly sleeping less than seven hours is associated with adverse health outcomes and impaired performance(9). While there are arguments to be made that less sleep may be sufficient in some contexts (27), the rebound observed during the ambulatory block suggests 6.4 hours does not represent our cohort’s habitual sleep duration but rather an acute decrease. Previous studies have demonstrated that duty hour restrictions may not be sufficient to improve resident sleep(12, 14, 28). Desai et al, performed a randomized control trial comparing 2003 and 2011 compliant models and found no difference in mean duration of sleep in the two groups. Nonetheless the numbers we observed were unexpectedly low. When compared to prior studies of sleep duration in residents, including pre 80-hour rule cohorts, our results were amongst the lowest published [TABLE 1].
Table 1.
Studies of Resident Sleep Hours
| Study | Population Studied | ACGME Rules | Measurment Method | Total Sleep Time (h) |
|---|---|---|---|---|
| Parthasarathy 2007(13) |
IM residents in ICU IM residents in ICU |
Pre-2003 2003 Compliant |
Actigraphy | 6.7 7.1 |
| Landrigan 2008(28) |
Pediatric residents, any rotation Pediatric residents, any rotation |
Pre-2003 2003 Compliant |
Sleep Log | 7.4 7.5 |
| Gohar(1) 2009 |
IM residents – Call Month IM residents – Noncall Month |
Pre-2003 2003 Compliant |
Actigraphy | 6.4 7.3 |
| Anderson 2012(34) |
IM residents - ICU Q3 30 hr call IM residents – Ambulatory |
2003 Compliant | Actigraphy | 6.3 7.8 |
| Desai 2013(12) |
IM residents – Inpatient rotation q4 30 hour call IM residents – Inpatient rotation q5 30 hour call IM residents – Night call model |
2003 Compliant 2003 Compliant 2011 Compliant |
Actigraphy | 7.6 7.7 8.2 |
| Our Results | IM residents – MICU rotation IM residents – Ambulatory rotation Non-IM residents – MICU rotation |
2011 Compliant 2011 Compliant 2011 Compliant |
Actigraphy | 6.4 7.0 6.5 |
ACGME, Accreditation Council for Graduate Medical Education; IM, Internal Medicine; q4, every 4 days; q5, every 5 days; h, hour
The reasons for the short sleep duration were not investigated in this study but likely reflect the myriad factors, beyond duty hours, that influence sleep patterns such as inability to achieve quality sleep, night call associated sleep disturbance (i.e., circadian misalignment) and use of time for non-sleep activities. It is not difficult to conceive the struggle of maintaining seven hours of sleep in a 13 hour per day work schedule. The remaining four hours of non-sleep time are quickly used traveling to work, spending time with family, exercising, and doing other activities that maintain work-life balance. Furthermore, the ACGME rules principally address the problem of acute sleep deprivation, leaving chronic sleep deprivation a hazardous reality in residency. Van Dongen showed that subjects randomized to 6 hours of sleep or less per night suffered neurocognitive deficits comparable to 1–2 days without sleep. These effects were seen within a week and there was a dose-response effect as sleep debt accumulated (29). Similarly, our study showed a progressive increase in stress and sleepiness as residents accumulated time in the MICU. There was not a corresponding trend of decreasing sleep duration, rather a stably insufficient amount of sleep, suggesting that accumulation of sleep debt contributed to the observed decline in wellness. The progressive decline also suggests that a single day off out of 6 provides inadequate recovery time. This is supported by work done by Belkany, showing that in contrast to acute sleep deprivation, the performance degradation seen in chronic sleep deprivation requires more time to recover to baseline(30). Our data suggest the ambulatory block, which begins with a full weekend off prior to start of the rotation, has no night call and less required hours, provides the necessary time for repaying sleep debt and improving overall wellness. IM residents experienced a significant improvement in sleep duration during the ambulatory block and upon its completion sleepiness and stress levels had improved to pre-MICU baselines. Conversely, non-IM residents, who remained in the MICU for a fourth week, providing a pseudo-control group, continued to have up trending PSS and ESS scores. It is likely that the decline in alertness across the MICU block also owed something to the effects of circadian misalignment. As touched upon above, circadian misalignment refers to the mismatch between the endogenous circadian rhythm in alertness and the self-selected or imposed timing of sleep and wakefulness(15). The improvement in alertness seen during the ambulatory block may similarly be at least somewhat related to realignment between the hypothalamic circadian pacemaker and the sleep/wake schedule. While it is unsurprising that resident sleep and wellness scores improve when on a lower stress and time intensive rotation, this is a previously unrecognized and significant benefit of the block scheduling format. Sleep deprivation affects a number of areas in a resident’s life including learning, motivation, communication and performing tasks (31). In addition, Min et al found that sleep quality has an impact on self-reported wellness(32). Program Directors should be cognizant of the potential for persistent sleep problems which could affect resident wellness even if a block schedule is not possible for a residency program. Awareness of the exact nature of schedules including duration of night rotations, days off and subsequent transitions are vital in improving resident wellness. Frequent conversations regarding sleep and the importance of wellness should be emphasized by programs and changes in scheduling should be attempted to minimize impact on sleep.
The shortcomings of our study include the relatively small number of residents, from a single academic medical center, limiting generalizability. The traditional schedule “control” group was particularly small (9 residents) and from varied, non-IM specialties, which introduces the possibility of inherent differences between the two groups accounting for observed differences, rather than the schedule. Our analysis also assumes that sleep duration was largely influencing our measures of wellness, but we recognize that the reverse may also be true; that the observed increases in stress and sleepiness are a consequence of the stressful MICU environment, and that sleep duration and schedule were not as influential as concluded. Studying various inpatient rotations would help to determine the role different environments and patient populations play. Finally, our conclusion that the ambulatory week accounts for the observed recuperation would have been bolstered by studying non-IM residents during their first week of a new rotation. It is possible that simply changing rotations accounts for this improvement.
CONCLUSION
Despite duty hour restrictions, residents are getting inadequate sleep. As days of inadequate sleep accumulate, measures of resident wellness progressively decline. We found that a block scheduling format, an innovation that has already been shown to increase resident satisfaction, reduce fragmentation of care, and enhance learning opportunities(11, 33) has the added benefit of allowing residents to pay back some sleep debt, correct circadian misalignment and improve sleepiness and stress back to baseline levels. As the ACGME and residency programs contemplate new ways to ensure resident well-being and patient safety, block scheduling is an example of a how a change outside the scope of duty hour restrictions has potential to enhance the rules already in place.
Supplementary Material
Acknowledgments
Grant Support/Source of Funding:
This publication was made possible with support from the Oregon Clinical and Translational Research Institute (OCTRI), grant number UL1 RR024140 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Abbreviation List
- ACGME
Accreditation Council of Graduate Medical Education
- ESS
Epworth Sleepiness Scale
- IM
Internal Medicine
- MICU
medical intensive care unit
- OHSU
Oregon Health & Science University
- PSS
Perceived Stress Scale
- TST
total sleep time
Footnotes
DR. JAMES BORDLEY (Orcid ID : 0000-0003-4139-1274)
Authors contributions:
Substantial contributions to the conception of the work or acquisition of data: JB, AA, MA, RK, AP, BK, JE, AS, SD, AK
Drafting the work or revising it critically for important intellectual content: JB, SD, AK
Final approval of the version to be published: JB, AA, MA, RK, AP, BK, JE, AS, SD, AK
Agreement to be accountable for all aspects of the work: JB, AA, MA, RK, AP, BK, JE, AS, SD, AK
Conflicts of/competing interests:
The authors declare no conflict of interest.
Prior Abstract/Poster Presentation:
Bordley J, Desai S, Khan A, et al. Trends in Sleepiness and Perceived Stress in Medical Trainees During Intensive Care Unit Rotation Followed by Outpatient Clinics in a Block Schedule. American Thoracic Society International Conference 2015 Denver, CO
Contributor Information
James Bordley V, Fellow, Department of Medicine, Division of Pulmonary & Critical Care, Oregon Health & Science University, Portland, OR.
Algene G. Agustin, Research Assistant, Department of Medicine, Division of Pulmonary & Critical Care, Oregon Health & Science University, Portland, OR.
Mohamed A. Ahmed, Research Assistant, Department of Medicine, Division of Pulmonary & Critical Care, Oregon Health & Science University, Portland, OR.
Raeesa Khalid, Research Assistant, Department of Medicine, Division of Pulmonary & Critical Care, Oregon Health & Science University, Portland, OR.
Anthony M. Paluso, Research Assistant, Department of Medicine, Division of Pulmonary & Critical Care, Oregon Health & Science University, Portland, OR
Bethany S. Kobza, Research Assistant, Department of Medicine, Division of Pulmonary & Critical Care, Oregon Health & Science University, Portland, OR
Aaron W. Spaugy, Fellow, Department of Medicine, Division of Pulmonary & Critical Care, Oregon Health & Science University, Portland, OR.
Jonathan Emens, Assistant Professor, Department of Psychiatry, Oregon Health & Science University and Portland VAMC, Portland, OR.
Sima S. Desai, Associate Professor, Department of Medicine, Division of Hospital Medicine, Oregon Health & Science University, Portland, OR.
Akram Khan, Associate Professor, Department of Medicine, Division of Pulmonary & Critical Care, Oregon Health & Science University, Portland, OR.
References
- 1.Gohar A, Adams A, Gertner E, Sackett-Lundeen L, Heitz R, Engle R, et al. Working memory capacity is decreased in sleep-deprived internal medicine residents. J Clin Sleep Med. 2009;5(3):191–7. [PMC free article] [PubMed] [Google Scholar]
- 2.Gander P, Millar M, Webster C, Merry A. Sleep loss and performance of anaesthesia trainees and specialists. Chronobiology international. 2008;25(6):1077–91. doi: 10.1080/07420520802551428. [DOI] [PubMed] [Google Scholar]
- 3.Saxena AD, George CF. Sleep and motor performance in on-call internal medicine residents. Sleep. 2005;28(11):1386–91. doi: 10.1093/sleep/28.11.1386. [DOI] [PubMed] [Google Scholar]
- 4.Landrigan CP, Rothschild JM, Cronin JW, Kaushal R, Burdick E, Katz JT, et al. Effect of reducing interns’ work hours on serious medical errors in intensive care units. The New England journal of medicine. 2004;351(18):1838–48. doi: 10.1056/NEJMoa041406. [DOI] [PubMed] [Google Scholar]
- 5.Barger LK, Cade BE, Ayas NT, Cronin JW, Rosner B, Speizer FE, et al. Extended work shifts and the risk of motor vehicle crashes among interns. The New England journal of medicine. 2005;352(2):125–34. doi: 10.1056/NEJMoa041401. [DOI] [PubMed] [Google Scholar]
- 6.Ware JC, Risser MR, Manser T, Karlson KH., Jr Medical resident driving simulator performance following a night on call. Behavioral sleep medicine. 2006;4(1):1–12. doi: 10.1207/s15402010bsm0401_1. [DOI] [PubMed] [Google Scholar]
- 7.Barrack RL, Miller LS, Sotile WM, Sotile MO, Rubash HE. Effect of duty hour standards on burnout among orthopaedic surgery residents. Clinical orthopaedics and related research. 2006;449:134–7. doi: 10.1097/01.blo.0000224030.78108.58. [DOI] [PubMed] [Google Scholar]
- 8.Rosen IM, Gimotty PA, Shea JA, Bellini LM. Evolution of sleep quantity, sleep deprivation, mood disturbances, empathy, and burnout among interns. Academic medicine: journal of the Association of American Medical Colleges. 2006;81(1):82–5. doi: 10.1097/00001888-200601000-00020. [DOI] [PubMed] [Google Scholar]
- 9.Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, Buysse D, et al. Recommended Amount of Sleep for a Healthy Adult: A Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. J Clin Sleep Med. 2015;11(6):591–2. doi: 10.5664/jcsm.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrison JW, Ramaiya A, Cronkright P. Restoring Emphasis on Ambulatory Internal Medicine Training-The 3ratio1 Model. J Grad Med Educ. 2014;6(4):742–5. doi: 10.4300/JGME-D-13-00461.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mariotti JL, Shalaby M, Fitzgibbons JP. The 4:1 schedule: a novel template for internal medicine residencies. J Grad Med Educ. 2010;2(4):541–7. doi: 10.4300/JGME-D-10-00044.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desai SV, Feldman L, Brown L, Dezube R, Yeh HC, Punjabi N, et al. Effect of the 2011 vs 2003 duty hour regulation-compliant models on sleep duration, trainee education, and continuity of patient care among internal medicine house staff: a randomized trial. JAMA Intern Med. 2013;173(8):649–55. doi: 10.1001/jamainternmed.2013.2973. [DOI] [PubMed] [Google Scholar]
- 13.Parthasarathy S, Hettiger K, Budhiraja R, Sullivan B. Sleep and well-being of ICU housestaff. Chest. 2007;131(6):1685–93. doi: 10.1378/chest.06-1398. [DOI] [PubMed] [Google Scholar]
- 14.Sen S, Kranzler HR, Didwania AK, Schwartz AC, Amarnath S, Kolars JC, et al. Effects of the 2011 duty hour reforms on interns and their patients: a prospective longitudinal cohort study. JAMA Intern Med. 2013;173(8):657–62. doi: 10.1001/jamainternmed.2013.351. discussion 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dijk DJ, Lockley SW. Integration of human sleep-wake regulation and circadian rhythmicity. Journal of applied physiology (Bethesda, Md: 1985) 2002;92(2):852–62. doi: 10.1152/japplphysiol.00924.2001. [DOI] [PubMed] [Google Scholar]
- 16.Drake C, Wright K. Shift Work, Shift-Work Disorder, and Jet Lag. In: Kryger M, Roth T, Dement W, editors. Principles and Practice of Sleep Medicine. 5th. St. Louis: Elsevier Saunders; 2011. pp. 784–98. [Google Scholar]
- 17.Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep. 1994;17(3):201–7. doi: 10.1093/sleep/17.3.201. [DOI] [PubMed] [Google Scholar]
- 18.Marino M, Li Y, Rueschman MN, Winkelman JW, Ellenbogen JM, Solet JM, et al. Measuring sleep: accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep. 2013;36(11):1747–55. doi: 10.5665/sleep.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin JL, Hakim AD. Wrist actigraphy. Chest. 2011;139(6):1514–27. doi: 10.1378/chest.10-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 21.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15(4):376–81. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 22.Johns MW. Sleepiness in different situations measured by the Epworth Sleepiness Scale. Sleep. 1994;17(8):703–10. doi: 10.1093/sleep/17.8.703. [DOI] [PubMed] [Google Scholar]
- 23.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–96. [PubMed] [Google Scholar]
- 24.Cohen S, Janicki-Deverts D. Who’s Stressed? Distributions of Psychological Stress in the United States in Probability Samples from 1983, 2006, and 20091. Journal of Applied Social Psychology. 2012;42(6):1320–34. [Google Scholar]
- 25.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Wheaton AG, Chapman DP, Cunningham TJ, Lu H, Croft JB. Prevalence of Healthy Sleep Duration among Adults–States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65(6):137–41. doi: 10.15585/mmwr.mm6506a1. [DOI] [PubMed] [Google Scholar]
- 27.Yetish G, Kaplan H, Gurven M, Wood B, Pontzer H, Manger PR, et al. Natural sleep and its seasonal variations in three pre-industrial societies. Curr Biol. 2015;25(21):2862–8. doi: 10.1016/j.cub.2015.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landrigan CP, Fahrenkopf AM, Lewin D, Sharek PJ, Barger LK, Eisner M, et al. Effects of the accreditation council for graduate medical education duty hour limits on sleep, work hours, and safety. Pediatrics. 2008;122(2):250–8. doi: 10.1542/peds.2007-2306. [DOI] [PubMed] [Google Scholar]
- 29.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26(2):117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 30.Belenky G, Wesensten NJ, Thorne DR, Thomas ML, Sing HC, Redmond DP, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res. 2003;12(1):1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- 31.Papp KK, Stoller EP, Sage P, Aikens JE, Owens J, Avidan A, et al. The effects of sleep loss and fatigue on resident-physicians: a multi-institutional, mixed-method study. Academic medicine: journal of the Association of American Medical Colleges. 2004;79(5):394–406. doi: 10.1097/00001888-200405000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Min AA, Sbarra DA, Keim SM. Sleep disturbances predict prospective declines in resident physicians’ psychological well-being. Medical education online. 2015;20(1):28530. doi: 10.3402/meo.v20.28530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaudhry SI, Balwan S, Friedman KA, Sunday S, Chaudhry B, Dimisa D, et al. Moving forward in GME reform: a 4 + 1 model of resident ambulatory training. J Gen Intern Med. 2013;28(8):1100–4. doi: 10.1007/s11606-013-2387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson C, Sullivan JP, Flynn-Evans EE, Cade BE, Czeisler CA, Lockley SW. Deterioration of neurobehavioral performance in resident physicians during repeated exposure to extended duration work shifts. Sleep. 2012;35(8):1137–46. doi: 10.5665/sleep.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


