Abstract
Purpose
While the estrogen receptor (ER) is the single most widely used biomarker to evaluate breast cancer outcomes, aspects of ER marker biology remain poorly understood. We sought to determine whether quantitative measures of ER, such as protein expression and intensity, were associated with survival, or with survival disparities experienced by Hispanic women.
Methods
A case-cohort study included a 15% random sample of invasive breast cancer cases diagnosed from 1997–2009 in 6 New Mexico counties and all deaths due to breast cancer-related causes. Pathology reports and tissue microarrays served as sources of ER information. Analyses were restricted to women with ≥1% ER immunohistochemical staining. Hazard ratios (HR) and 95% confidence intervals (CI) for breast cancer death were estimated using Cox proportional hazards models.
Results
Included women represented 4336 ER+ breast cancer cases and 448 deaths. Median follow-up was 93 months. ER percent expression was not associated with breast cancer survival after adjustment for standard prognostic factors (p-trend = .76). ER intensity remained a strong and independent risk factor for breast cancer survival in multivariate analyses: Women whose tumors expressed ER at intensity=2 (HR 0.6; 95% CI 0.4–1.0) or 3 (HR 0.5; 95% CI 0.2–0.9) had a reduced risk of breast cancer mortality, compared to ER intensity=1 (p-trend = .02). Neither ER protein expression nor intensity influenced Hispanic survival disparities.
Conclusions
ER percent positive staining is not independently related to breast cancer survival after adjustment for other survival-related factors. ER intensity, in contrast, demonstrates promise for prognostic utility.
Keywords: Breast Neoplasms, Estrogen Receptors, Survival Analysis, Tumor Biomarkers, Case-Cohort Study, Hispanic Americans
Background
The estrogen receptor is an established biomarker with substantial clinical utility. Women whose breast tumors express the estrogen receptor (ER) have been known to have improved prognosis in comparison with those whose are ER negative since at least the 1970s[1,2]. ER positivity also indicates greater likelihood of response to a range of pharmacologic agents involved directly or indirectly in the estrogen-signaling blockade. Such responses may be accompanied by a reduction in breast cancer mortality risk [3] or prolonged disease-free survival [4,5].
However, ER positive status consists of several components which may have a bearing on clinical outcomes, some of which have rarely been examined in clinical practice. One such component is the proportion of cells staining ER positive using immunohistochemistry (IHC). Women with a greater proportion of ER-expressing tumors cells may be more likely to respond to anti-endocrine therapy[6,7] or to experience better prognosis [8,5,4], but not uniformly[9,10]. A second aspect is the intensity of ER staining, a measure usually examined in conjunction with ER percent positivity in relation to clinical outcomes, in the form of either an Allred score [8,6] or an H-score[5,11,12].
ER quantitative measures such as percent positivity and staining intensity are of interest in assessment of breast cancer survival disparities by race and ethnicity. In several studies, African-American women had a greater survival difference, in comparison with white women, for ER+ than ER− disease[13–15], and we have observed a similarly greater disparity in ER+ disease for breast cancer survival in Hispanic relative to non-Hispanic white women in New Mexico. Such differences suggest that aspects of ER biomarker quantity may play a role in mediating survival disparities.
In a population-based case-cohort study, we sought to determine the relationship between ER quantitative measures and breast cancer survival, and to evaluate whether differences in ER biomarker values might contribute to survival differences by Hispanic ethnicity.
Methods
Study Population
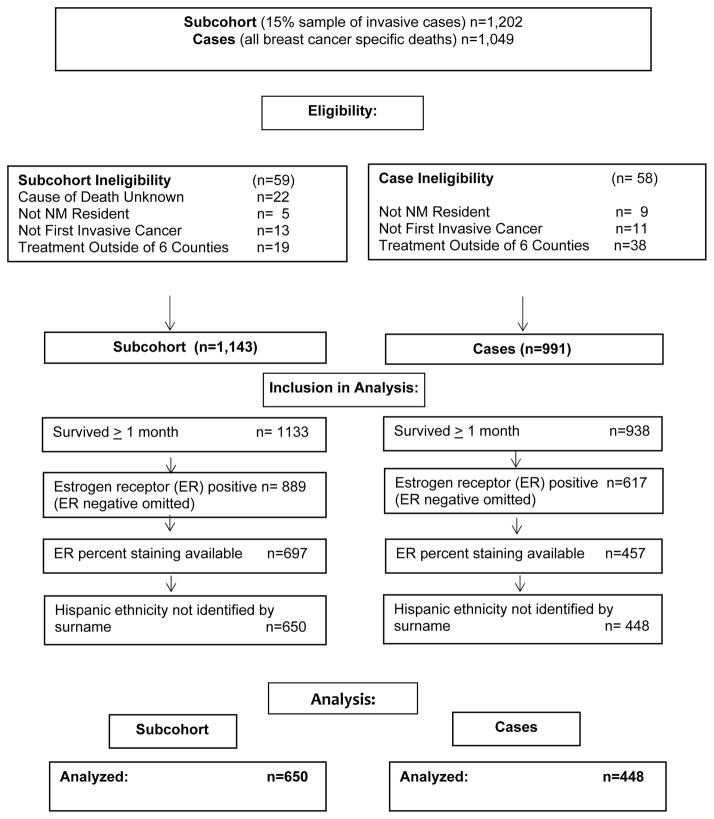
We conducted a population-based case-cohort study of breast cancer survival in 6 counties in the north central region of New Mexico (NM). Eligible breast cancer cases were identified through the NM Tumor Registry (NMTR), a founding participant in the National Cancer Institute (NCI) funded Surveillance Epidemiology End Results (SEER) program. First invasive breast cancer cases diagnosed from 1997–2009 among white female residents of six NM counties (Bernalillo, Sandoval, Santa Fe, Socorro, Torrance, Valencia) were potentially eligible. Included in the study were a 15% random sample of all eligible breast cancer diagnoses (‘subcohort’), as well as all deaths due to breast cancer-related causes (‘cases’) among incident diagnoses (not diagnosed by autopsy or death certificate), selected more than two years post eligibility event to account for lagged reporting (deaths occurring in 2012 were ascertained in 2015). Hispanic ethnicity was categorized according to the North American Association of Central Cancer Registries (NAACR) algorithm for Hispanic origin, with exclusion of women identified only by Hispanic surname. Also excluded were women who were not residents of NM, who had an unknown cause of death, or who received treatment outside the six counties, thus precluding therapy assessment (Figure 1). The study sample size was determined by the goal of identification of mediators of Hispanic survival disparities. For this analysis of estrogen receptor (ER) quantitative measures, only women with tumors known to be ER positive and for whom estrogen receptor percent staining was known are included.
Fig. 1.
Eligibility and Inclusion in Analyses: Population-Based Invasive Breast Cancer Cases Diagnosed from 1997–2009 in six New Mexico counties.
Data Collection
Initial review of medical records was conducted for inpatient and outpatient medical providers recorded by the NM Tumor Registry, and additional care providers were identified through those sources. Medical records were abstracted by certified tumor registrars (CTRs) or a registered health information technologist. Abstractors collected information regarding demographic variables, tumor characteristics, and details regarding treatment, including surgery, chemotherapy, radiation, endocrine, and biological therapy (types, dates of receipt, doses, and agents) through systematic review of paper and electronic records. Information was also obtained regarding the biological markers ER, progesterone receptor (PR) and Her2/neu (ErbB2) if available, including percent of tumor cells staining positive for each marker by immunohistochemistry (IHC), and for Her2, intensity of staining. During most of the time period of the study, ER antibody clone 6F11 (various manufacturers) was the predominant assay used, switching in 2008 to ER clone SP1.
Study pathologists also reviewed tumor and normal tissue from formalin fixed, paraffin embedded (FFPE) biopsy or surgery specimens for construction of tissue microarrays (TMAs). Briefly, two 1.5 mm cores of tumor tissue were selected and embedded in paraffin wax, and 4 um sections were cut and stained for estrogen receptor using ER alpha ID5 monoclonal antibody, using standard methods (DAKO Laboratories, Carpinteria, CA). Assessment by a pathologist (LL) blinded to outcome included nuclear ER percent staining and staining intensity (1 = weak, 2=moderate, or 3=strong) in comparison with positive and negative controls. The average of the two tumor tissue core values was computed for ER percent staining as a continuous variable, but as ER intensity coded in categories did not facilitate averaging (moderate = 2 vs strong=3) the first of the two values was used. Included in this analysis are women for whom either pathology report or TMA review indicated at least 1% of cells staining positive for ER. Only pathology report values were used in the analysis if both were available.
Follow-up and vital status ascertainment
Women were followed until death, loss to follow up, or January 1, 2013. Vital status and cause of death were determined by the NM Tumor Registry, using probabilistic matching to the New Mexico State Vital Statistics Bureau files, and the National Death Index of the National Center for Health Statistics. Vital status was verified by submission of files to the Centers for Medicare and Medicaid Services. As Hispanic women have a lower age-adjusted all-cause mortality rate than non-Hispanic white women, which can mask any elevation in cause-specific mortality rates, only deaths attributed to breast cancer as an underlying cause on the death certificate were included as events in the analysis.
Statistical analysis
ER percent staining was categorized by quintiles in the subcohort, although skewed by the 35% of tumors that scored at exactly 90% staining. ER positivity was also classified to create an Allred score[8]: the proportion score was assigned as: 1–10% staining = 2, 11–33% = 3, 34–66% = 4, and 67–100% = 5, while intensity score was categorized as weak =1, intermediate=2, and strong=3. The proportion and intensity scores were added to obtain the Allred score. Scores of 7 and 8 were combined due to small cell sizes. PR status was considered positive if at least 1% of cells demonstrated evidence of progesterone receptor. Her2 was scored using both percent positive cells and intensity, and according to the guidelines in place during the conduct of the study [16], only tumors expressing Her2 in ≥ 30% of cells at 3+ intensity were considered positive.
Cox proportional hazards models for case-cohort [17] were fit to calculate hazard ratios (HR) and 95% confidence intervals (CI), using an alpha level of .05. Death due to breast cancer-related causes was the end point of interest, and all other events were censored. Time to event was measured in months. Women in the subcohort were weighted by the inverse of the sampling fraction (100%/15% = 6.67). Differences in estrogen receptor percent positive or intensity by Hispanic ethnicity were examined using a test for interaction, by including the main effects (Hispanic ethnicity and estrogen quantitative measure) and their product (Hispanic * estrogen measure) in the statistical model. Pearson correlation coefficients were calculated to determine multicollinearity between variables. Missing covariates were rare or could not be imputed, thus missing indicators were used. The proportional hazards assumption was verified using Schoenfeld residuals [18]. Analyses were adjusted for age (5 year age groups), tumor size (<2 cm, 2–<5 cm, ≥5 cm, skin/chest wall involvement), positive lymph nodes (0, 1–3, 4+) tumor grade (1, 2, 3/4), progesterone receptor (PR) status, Her2/neu status, and Hispanic ethnicity. Adjustment for receipt of chemotherapy, radiotherapy, or endocrine therapy did not alter hazard ratios, and receipt of standard of care was high (> 84%), thus those factors were not included in multivariate models. All analyses were conducted with Statistical Analysis Systems (SAS) software v 9.4 (Cary, N.C.). Institutional review board approval for the study was received from the University of New Mexico Health Sciences Center under a Health Insurance Portability and Accountability Act (HIPAA) waiver of consent for previously collected data. We acknowledge the guidance of the “Reporting recommendations for tumor marker prognostic studies (REMARK)” guidelines in preparation of these results[19].
Results
Included in the study were 650 ER+ women (as defined by ≥ 1% positive staining by immunohistochemistry (IHC)) sampled from the cohort, termed the ‘subcohort’, who represented 4336 total breast cancer cases when weighted by the inverse of the sampling fraction (.15 or 6.67x) in the analysis, and 448 ER+ women who died of breast cancer-related causes. Median follow-up in the subcohort was 93 months. Of women included in the subcohort, almost 20% were less than age 50 years at diagnosis, 21% were Hispanic, and tumor characteristics were generally reflective of the ER+ inclusion criteria, including 91% that were also PR+ (Table 1). Demographic and tumor characteristics of included ER+ women were generally representative of those in the full SEER cohort, with slightly more included women falling into categories of age ≥ 80 years (11.7% vs. 8.8%) and tumor size extension to chest wall/skin (4.8% vs. 2.3%). Mean ER percent positive staining was 79% in the subcohort (median 90%), and did not differ by ethnicity. In initial analyses, the weighted kappa statistic for agreement between the two ER percent categorical measures was .65, while that for ER intensity was .76, on a scale in which 0.61<Kappa≤.80 is considered ‘substantial agreement’.
Table 1.
Characteristics of Estrogen Receptor (ER) Positive Incident Invasive Breast Cancer Cases Diagnosed in Six New Mexico Counties from 1997–2009 (Subcohort – 15% sample of all eligible cases).
| Characteristic | Subcohort (15% sample weight 6.67) N=650 |
Cases (Breast Cancer Deaths) N= 448 |
||||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Age Group | ||||||
| <40 | 20 | 3.1 | 26 | 5.8 | ||
| 40–49 | 105 | 16.1 | 87 | 19.4 | ||
| 50–59 | 168 | 25.8 | 103 | 23.0 | ||
| 60–69 | 157 | 24.2 | 79 | 17.6 | ||
| 70–79 | 124 | 19.1 | 90 | 20.1 | ||
| 80+ | 76 | 11.7 | 63 | 14.1 | ||
| Ethnicity | ||||||
| Hispanic | 138 | 21.2 | 143 | 31.9 | ||
| Non-Hispanic | 512 | 78.8 | 305 | 68.1 | ||
| Year of Diagnosis | ||||||
| 1997–2000 | 188 | 28.9 | 167 | 37.3 | ||
| 2001–2004 | 191 | 29.4 | 165 | 36.8 | ||
| 2005–2009 | 271 | 41.7 | 116 | 25.9 | ||
| Tumor Size | ||||||
| < 2.0 cm | 396 | 61.7 | 132 | 30.1 | ||
| 2–<5 cm | 180 | 28.0 | 173 | 39.4 | ||
| ≥5 cm | 35 | 5.5 | 54 | 12.3 | ||
| Chest wall/Skin | 31 | 4.8 | 80 | 18.2 | ||
| Missing | 8 | 9 | ||||
| Number of Positive Nodes | ||||||
| 0 | 435 | 68.0 | 151 | 35.3 | ||
| 1–3 | 140 | 21.9 | 144 | 33.6 | ||
| 4–9 | 40 | 6.3 | 84 | 19.6 | ||
| 10+ | 24 | 3.8 | 49 | 11.5 | ||
| Missing | 11 | 20 | ||||
| Tumor Grade | ||||||
| Grade I | 206 | 36.1 | 52 | 14.5 | ||
| Grade II | 259 | 45.4 | 162 | 45.1 | ||
| Grade III/IV | 106 | 18.5 | 145 | 40.4 | ||
| Missing | 39 | 39 | ||||
| Progesterone Receptor | ||||||
| Positive | 565 | 91.4 | 361 | 85.3 | ||
| Negative | 53 | 8.6 | 62 | 14.7 | ||
| Missing | 32 | 25 | ||||
| Her2/neu | ||||||
| Positive | 84 | 16.9 | 85 | 25.6 | ||
| Negative | 413 | 83.1 | 247 | 74.4 | ||
| Missing | 72 | 57 | ||||
| Allred Score a | ||||||
| 3 | 4 |
|
5 |

|
||
| 4 | 12 | 4.9 | 9 | 7.3 | ||
| 5 | 12 | 3.8 | 17 | 8.9 | ||
| 6 | 76 | 24.3 | 50 | 26.2 | ||
| 7 | 129 | 41.2 | 67 | 35.1 | ||
| 8 | 81 | 25.8 | 45 | 22.5 | ||
| Missing | 337 | 255 | ||||
Due to small cell sizes, categories 3 and 4 of the Allred score were combined in the analysis.
Scores of 1 or 2 would be considered ER negative and thus are not included.
In univariate analyses, women with tumors that expressed ER protein in 90%, 91–96%, or ≥97% of cells had at least a 40% reduced risk of breast cancer-specific mortality, relative to those with < 60% staining (Table 2). Mortality diminished with increasing category of ER protein staining (p=.001 for trend). In multivariate models, however, the reduction in risk with increasing ER percent positivity was no longer evident, with absence of trend. In additional analyses, ER percent staining was similarly related to mortality among both non-Hispanic white and Hispanic women, with a diminished mortality HR with increasing ER protein in univariate analyses, which again was not apparent after multivariate adjustment. A test for interaction by Hispanic ethnicity also indicated that the relationship did not differ by ethnicity (p>.05). We explored whether adjustment for particular factors led to the change apparent in the multivariate analysis: In Cox proportional hazards models, ER percent positive was no longer related to breast cancer death after adjustment for only age, tumor size, number of positive nodes, and tumor grade, thus adjustment for ER intensity or for PR was not responsible for the loss of significance.
Table 2.
Estrogen Receptor Quantitative Measures in Relation to Breast Cancer Survival among Incident Invasive Breast Cancer Cases Diagnosed in Six New Mexico Counties from 1997–2009 (Subcohort – 15% sample of all eligible cases).
| Characteristic | Subcohort 15% Sample (Weight 6.67) (n=650) | Breast Cancer Deaths (n=448) | Univariate Hazard Ratio | Multivariate Adjusted Hazard Ratioa | ||
|---|---|---|---|---|---|---|
|
| ||||||
| N | % | N | % | (95% CI) | (95% CI) | |
| OVERALL: | ||||||
| ER Percent | ||||||
| 1–59% | 100 | 15.4 | 115 | 25.7 | 1.0(reference) | 1.0 (reference) |
| 60–89% | 113 | 17.4 | 93 | 20.8 | 0.7 (0.5–1.1) | 1.1 (0.6–1.9) |
| 90% | 226 | 34.8 | 135 | 30.1 | 0.5 (0.4–0.7) | 0.9 (0.6–1.5) |
| 91–96% | 118 | 18.1 | 44 | 9.8 | 0.4 (0.2–0.5) | 0.8 (0.4–1.5) |
| 97%+ | 93 | 14.3 | 61 | 13.6 | 0.6 (0.4–0.9) | 1.5 (0.8–2.8) |
| p-value trend | .001 | .76 | ||||
| ER Intensity | ||||||
| 1 | 77 | 24.6 | 68 | 35.3 | 1.0 (reference) | 1.0(reference) |
| 2 | 149 | 47.6 | 79 | 40.9 | 0.6 (0.4–0.9) | 0.6 (0.4–1.0) |
| 3 | 87 | 27.8 | 46 | 23.8 | 0.6 (0.4–1.0) | 0.5 (0.2–0.9) |
| missing | 337 | 255 | ||||
| p-value trend | .04 | .02 | ||||
| NON-HISPANIC: | ||||||
| ER Percent | ||||||
| 1–59% | 74 | 14.5 | 71 | 23.3 | 1.0 (reference) | 1.0 (reference) |
| 60–89% | 87 | 17.0 | 66 | 21.6 | 0.8 (0.5–1.3) | 1.6 (0.8–3.0) |
| 90% | 184 | 35.9 | 93 | 30.5 | 0.5 (0.4–0.8) | 1.0 (0.5–1.8) |
| 91–96% | 100 | 19.5 | 31 | 10.2 | 0.4 (0.2–0.6) | 0.7 (0.3–1.4) |
| 97%+ | 67 | 13.1 | 44 | 14.4 | 0.8 (0.5–1.3) | 1.7 (0.8–3.4) |
| p-value trend | .01 | .93 | ||||
| ER Intensity | ||||||
| 1 | 60 | 23.8 | 47 | 33.3 | 1.0 (reference) | 1.0 (reference) |
| 2 | 118 | 46.8 | 58 | 41.1 | 0.6 (0.4–1.0) | 0.5 (0.3–1.0) |
| 3 | 74 | 29.4 | 36 | 25.5 | 0.6 (0.4–1.1) | 0.5 (0.2–1.0) |
| missing | 260 | 164 | ||||
| p-value trend | .11 | .04 | ||||
| HISPANIC: | ||||||
| ER Percent | ||||||
| 1–59% | 26 | 18.8 | 44 | 30.8 | 1.0 (reference) | 1.0 (reference) |
| 60–89% | 26 | 18.8 | 27 | 18.9 | 0.5 (0.3–1.1) | 1.1 (0.3–4.0) |
| 90% | 42 | 30.4 | 42 | 29.4 | 0.5 (0.3–1.0) | 0.5 (0.2–1.6) |
| 91–96% | 18 | 13.0 | 13 | 9.1 | 0.5 (0.2–1.1) | 1.3 (0.4–4.9) |
| 97%+ | 26 | 18.8 | 17 | 11.9 | 0.4 (0.2–0.8) | 1.1 (0.2–6.4) |
| p-value trend | .01 | .82 | ||||
| ER Intensity | ||||||
| 1 | 17 | 27.9 | 21 | 40.4 | 1.0 (reference) | 1.0 (reference) |
| 2 | 31 | 50.8 | 21 | 40.4 | 0.5 (0.2–1.2) | 0.6 (0.1–3.4) |
| 3 | 13 | 21.3 | 10 | 19.2 | 0.6 (0.2–1.7) | 0.3 (0.1–3.1) |
| Missing | 77 | 91 | ||||
| p-value trend | .24 | .28 | ||||
Multivariate models were adjusted for age, tumor size (<2 cm, 2–<5 cm, ≥5 cm, skin/chest wall involvement), positive lymph nodes (0, 1–3, 4+) tumor grade (1, 2, 3/4), progesterone receptor status, Her2/neu status, and Hispanic ethnicity.
ER percent positive staining was adjusted for ER intensity and vice-versa.
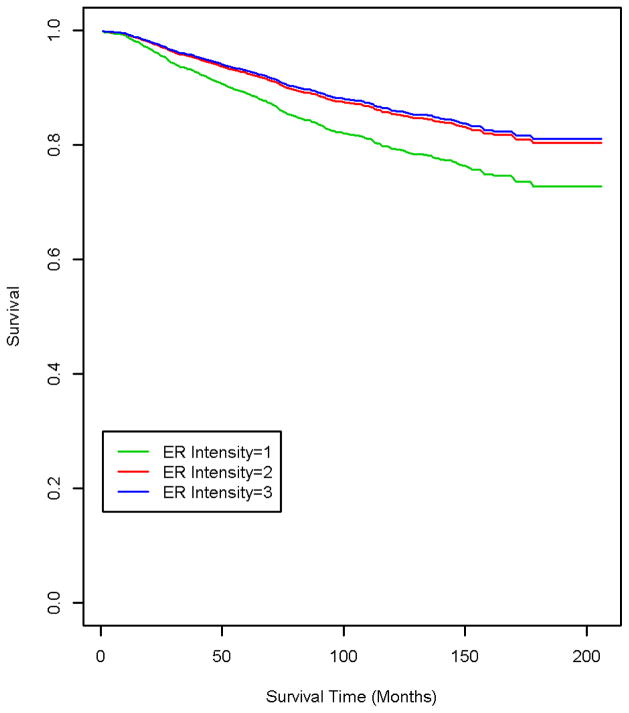
Women whose tumors demonstrated an ER staining intensity of 2 or 3 had a 40% reduction in risk of mortality in univariate analyses, in comparison with those of ER intensity 1 (Table 2). The reduced risk persisted in multivariate models, suggesting that women with ER intensity 3 had half the risk of mortality as those of ER intensity 1 (p-trend= .02) (Figure 2). A test for interaction indicated that the relationship between ER intensity and mortality did not differ by Hispanic ethnicity (p> .05). We verified that the Hispanic HR of 1.9 (95% CI 1.4–2.9) among ER positive women was unaltered when adjusted for ER percent staining or intensity (data not shown).
Fig. 2.
Breast Cancer-Specific Survival by Estrogen Receptor (ER) Staining Intensity among ER Positive Women
We further explored the relationship between ER percent staining, ER intensity, and established prognostic factors in the subcohort (Table 3). Women with increasingly greater ER positive staining had progressively smaller tumor size and lower tumor grade (Chi-square p-trend = .001), consistent with a more favorable prognosis, and increasing ER percent positivity was also associated with PR positivity (p< .0001) and Her2 negative status (p=.0002), but not increasing diagnosis age (p-trend= .08) or number of positive lymph nodes (p=.08). In contrast, increased intensity of ER staining was not associated with any prognostic indicator (tumor size, grade, lymph nodes, PR or HER2 status), except increased ER percent staining (p<.0001) and diagnosis age (p=.007) (data not shown). In the subcohort, the Pearson correlation coefficient between ER percent staining and ER intensity was .27 (p<.0001).
Table 3.
Estrogen Receptor (ER) Quantitative Measures: Association with Common Prognostic Indicators in the Population-based Subcohort Sample.
| Quantitative Measure | Tumor Size (cm) | Positive Nodes | Tumor Grade | |||
|---|---|---|---|---|---|---|
| Mean | Median | Mean | Median | Mean | Median | |
| ER Percent: | ||||||
| 1–59% | 2.5 | 2.1 | 0.9 | 0 | 2.2 | 2.0 |
| 60–89% | 2.3 | 1.6 | 2.2 | 0 | 1.9 | 2.0 |
| 90% | 1.9 | 1.5 | 1.3 | 0 | 1.8 | 2.0 |
| 91–96% | 2.1 | 1.7 | 0.9 | 0 | 1.8 | 2.0 |
| 97%+ | 1.9 | 1.5 | 1.1 | 0 | 1.7 | 2.0 |
| p-trenda | .001 | .08 | .001 | |||
| ER Intensity: | ||||||
| 1 | 2.5 | 1.8 | 1.1 | 0 | 1.9 | 2.0 |
| 2 | 2.0 | 1.5 | 1.7 | 0 | 1.7 | 2.0 |
| 3 | 2.2 | 1.8 | 1.2 | 0 | 1.8 | 2.0 |
| p-trend | .71 | .79 | .41 | |||
Trend test for relationship between ER quantitative measures (left column) and continuous measures of tumor size, positive node count, and grade (Chi-Square or Fisher’s Exact Test)
We sought to determine whether combined measures of ER percent staining and ER intensity, such as the Allred score, contributed to multivariate models, and how that contribution might differ from that of ER intensity alone (Table 4). We could not calculate an H-score because ER percent staining was not collected separately by each ER intensity measure[11]. The Allred score was not statistically significantly related to breast cancer survival in univariate or multivariate analyses, although some categories had to be combined due to small cell sizes. ER intensity remained related to outcome in both models.
Table 4.
Contribution of Estrogen Receptor (ER) Quantitative Measures to Breast Cancer Specific-Mortality Models.
| Prognostic Factor | Univariate Modela Chi-Square | dfc | p-value | Multivariate Modelb Chi-Square | df | p-value |
|---|---|---|---|---|---|---|
| ER intensity | 7.2 | 2 | .03 | 6.24 | 2 | .04d |
| Allred score | 6.6 | 4 | .16 | 5.43 | 4 | .24d |
Univariate model includes the variable of interest + missing value indicator only.
Multivariate model included adjustment for age at diagnosis, tumor size (<2 cm, 2–<5 cm, ≥5 cm, skin/chest wall involvement), positive lymph nodes (0, 1–3, 4+) tumor grade (1, 2, 3/4), progesterone receptor (PR) status, Her2/neu status, and Hispanic ethnicity. Excluded was ER percent positive staining.
DF – degrees of freedom
The contribution of ER intensity alone to the multivariate model was compared to the contribution from a combined ER intensity-ER positivity measure (the Allred score). Both ER intensity and Allred scores were modeled as dummy variables, with Allred categories of 3 and 4 combined due to small cells sizes (Table 1).
ER percent staining and intensity are most often evaluated in relationship to response to endocrine therapy, or survival or recurrence after endocrine therapy. Approximately 80% of ER+ women in the subcohort received endocrine therapy (91% of those guideline-eligible), thus numbers of non-treated women were small. Interaction terms between either ER percent staining or ER intensity with receipt of endocrine therapy in relation to breast cancer-specific survival were non-significant in multivariate models (data not shown).
Discussion
Our data provide an additional perspective on the relationship between the estrogen receptor and breast cancer survival. While the quantity of ER protein staining is strongly related in univariate analysis, our findings indicate that much of that association may be attributable to the strong correlation with other measures of breast cancer prognosis. Our results also suggest that ER intensity is a promising biomarker bearing little relationship to standard indicators of prognosis. However, our findings should be considered in light of the strengths and limitations of the study. Breast cancer tissue samples demonstrate considerable heterogeneity in ER expression from block to block, and even within an individual slide [20]. Some ER percent quantification and all ER intensity measures were conducted on TMAs, where the smaller sample of tumor may be less representative of tumor than the full slide section used in the initial pathologic diagnosis, potentially leading to misclassification. However, ER positive women constituted 82% of our full study (using 1% staining cutpoint). In SEER data collected since implementation of the ASCO/CAP guidelines recommending the 1% threshold [21] (diagnosis years 2011–2013), 84% of invasive tumors in white women are classified as ER positive [22], thus our percent positive appears representative. Absolute proportion of tumor marker staining by IHC can decline with elapsed time in FFPE tissue [23,24], and such was also noted in our study. Adjustment for years elapsed since diagnosis did not alter our results. Using a ≥20% absolute difference between pathology report and TMA as a threshold, 38% of women received a lower ER staining score on TMA and 9% a higher, which did not differ by cohort or case status. Women with missing ER intensity were included in models with a missing value indicator, and we did not employ multiple imputation to estimate intensity values, because they were independent of all other standard prognostic indicators except ER percent positive. Our ER intensity results require confirmation. We could not evaluate whether women with increased ER protein expression or intensity had a greater response to endocrine therapy because approximately 80% of women were treated, thus the study had only a small untreated comparison group. Our study limitations also include lack of uniform treatment, although adjustment for treatment received did not alter hazard ratio estimates. Strengths of the study include the population-based case identification through the NM Tumor Registry, the length of follow-up, and the case-cohort study design, which allowed efficient assessment of outcomes.
The study results should also be interpreted in the context of other findings regarding ER values and breast cancer outcome. In previous investigations, increasing ER percent positive staining (ER fmol/mg in earlier publications) has been significantly associated with response or recurrence following tamoxifen [6] or with breast cancer-specific survival in univariate analyses[7,4], and also with those outcomes after adjustment for nodal status [3], grade[25], tumor size and nodal status[26,27] or localized vs. non-localized disease + histologic grade [12]. In our study, models were adjusted for additional prognostic factors, which may have led to the difference in findings in comparison with most studies. In one follow-up of 670 breast cancer cases for a median 11.4 years, increasing ER positivity also was no longer prognostic after multivariate adjustment[28]. Allred or H-score measures have remained associated with survival-related outcomes in multivariate models in a number of studies, possibly due to inclusion of ER intensity as a component [8,5,12]. The correlation between good prognosis tumor characteristics and increasing ER positivity that we identified also has been noted in a number of investigations [29,8,30,25]. Our finding that women with the highest ER percent positivity (≥ 97%) had a non-significant elevation in breast cancer mortality is also consistent with that seen in several breast cancer studies [31–34].
While IHC staining intensity has previously been considered a somewhat subjective assessment, pathologist agreement regarding strong staining intensity may have gained additional rigor since the advent of HER2 testing, which requires an intensity of 3+ to be considered clinically positive. The lack of ER intensity reads by a second observer, which would facilitate inter-rater reliability assessment, is a limitation of our study. Increasing ER intensity may be indicative of increased binding to ER antigen within a cell, and thus could be considered a surrogate measure of increased ER cellular concentration or density (in contrast to ER percent staining, which indicates only the proportion of cells stained).
Our study differed from others due to restriction to only women with ER positive tumors. Our data suggests that among ER positive women only, increased ER protein staining is indicative of tumor characteristics associated with favorable prognosis, and is not independently associated with survival. In contrast, the results regarding ER intensity, which require confirmation in additional studies, suggest promise for categorizing differential survival in ER+ women.
Acknowledgments
This study was supported by grants R01CA 132877 and 2P30CA11810 from the National Cancer Institute (NCI), and NCI contract HSN26120130010I-Task Order HHSN26100005 to the Surveillance Epidemiology End Results (SEER) program.
Footnotes
Conflicts of Interest
The authors declare that they have no conflict of interest.
References Cited
- 1.Knight WA, Livingston RB, Gregory EJ, McGuire WL. Estrogen receptor as an independent prognostic factor for early recurrence in breast cancer. Cancer research. 1977;37(12):4669–4671. [PubMed] [Google Scholar]
- 2.Walt AJ, Singhakowinta A, Brooks SC, Cortez A. The surgical implications of estrophile protein estimations in carcinoma of the breast. Surgery. 1976;80(4):506–512. [PubMed] [Google Scholar]
- 3.Early Breast Cancer Trialists’ Collaborative G. Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC, Dowsett M, Ingle J, Peto R. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Viale G, Regan MM, Maiorano E, Mastropasqua MG, Dell’Orto P, Rasmussen BB, Raffoul J, Neven P, Orosz Z, Braye S, Ohlschlegel C, Thurlimann B, Gelber RD, Castiglione-Gertsch M, Price KN, Goldhirsch A, Gusterson BA, Coates AS. Prognostic and predictive value of centrally reviewed expression of estrogen and progesterone receptors in a randomized trial comparing letrozole and tamoxifen adjuvant therapy for postmenopausal early breast cancer: BIG 1–98. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(25):3846–3852. doi: 10.1200/JCO.2007.11.9453. [DOI] [PubMed] [Google Scholar]
- 5.Dowsett M, Allred C, Knox J, Quinn E, Salter J, Wale C, Cuzick J, Houghton J, Williams N, Mallon E, Bishop H, Ellis I, Larsimont D, Sasano H, Carder P, Cussac AL, Knox F, Speirs V, Forbes J, Buzdar A. Relationship between quantitative estrogen and progesterone receptor expression and human epidermal growth factor receptor 2 (HER-2) status with recurrence in the Arimidex, Tamoxifen, Alone or in Combination trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(7):1059–1065. doi: 10.1200/JCO.2007.12.9437. [DOI] [PubMed] [Google Scholar]
- 6.Elledge RM, Green S, Pugh R, Allred DC, Clark GM, Hill J, Ravdin P, Martino S, Osborne CK. Estrogen receptor (ER) and progesterone receptor (PgR), by ligand-binding assay compared with ER, PgR and pS2, by immuno-histochemistry in predicting response to tamoxifen in metastatic breast cancer: a Southwest Oncology Group Study. International journal of cancer. 2000;89(2):111–117. [PubMed] [Google Scholar]
- 7.McGuire WL. Steroid receptors in human breast cancer. Cancer research. 1978;38(11 Pt 2):4289–4291. [PubMed] [Google Scholar]
- 8.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1999;17(5):1474–1481. doi: 10.1200/jco.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 9.Ferno M, Stal O, Baldetorp B, Hatschek T, Kallstrom AC, Malmstrom P, Nordenskjold B, Ryden S. Results of two or five years of adjuvant tamoxifen correlated to steroid receptor and S-phase levels. South Sweden Breast Cancer Group, and South-East Sweden Breast Cancer Group. Breast cancer research and treatment. 2000;59(1):69–76. doi: 10.1023/a:1006332423620. [DOI] [PubMed] [Google Scholar]
- 10.Anderson H, Hills M, Zabaglo L, A’Hern R, Leary AF, Haynes BP, Smith IE, Dowsett M. Relationship between estrogen receptor, progesterone receptor, HER-2 and Ki67 expression and efficacy of aromatase inhibitors in advanced breast cancer. Annals of oncology : official journal of the European Society for Medical Oncology. 2011;22(8):1770–1776. doi: 10.1093/annonc/mdq700. [DOI] [PubMed] [Google Scholar]
- 11.Kinsel LB, Szabo E, Greene GL, Konrath J, Leight GS, McCarty KS., Jr Immunocytochemical analysis of estrogen receptors as a predictor of prognosis in breast cancer patients: comparison with quantitative biochemical methods. Cancer research. 1989;49(4):1052–1056. [PubMed] [Google Scholar]
- 12.Ma H, Lu Y, Marchbanks PA, Folger SG, Strom BL, McDonald JA, Simon MS, Weiss LK, Malone KE, Burkman RT, Sullivan-Halley J, Deapen DM, Press MF, Bernstein L. Quantitative measures of estrogen receptor expression in relation to breast cancer-specific mortality risk among white women and black women. Breast cancer research : BCR. 2013;15(5):R90. doi: 10.1186/bcr3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Brien KM, Cole SR, Tse CK, Perou CM, Carey LA, Foulkes WD, Dressler LG, Geradts J, Millikan RC. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16(24):6100–6110. doi: 10.1158/1078-0432.CCR-10-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma H, Lu Y, Malone KE, Marchbanks PA, Deapen DM, Spirtas R, Burkman RT, Strom BL, McDonald JA, Folger SG, Simon MS, Sullivan-Halley J, Press MF, Bernstein L. Mortality risk of black women and white women with invasive breast cancer by hormone receptors, HER2, and p53 status. BMC cancer. 2013;13:225. doi: 10.1186/1471-2407-13-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sparano JA, Wang M, Zhao F, Stearns V, Martino S, Ligibel JA, Perez EA, Saphner T, Wolff AC, Sledge GW, Jr, Wood WC, Davidson NE. Race and hormone receptor-positive breast cancer outcomes in a randomized chemotherapy trial. Journal of the National Cancer Institute. 2012;104(5):406–414. doi: 10.1093/jnci/djr543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF American Society of Clinical Oncology/College of American P. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Archives of pathology & laboratory medicine. 2007;131(1):18–43. doi: 10.1043/1543-2165(2007)131[18:ASOCCO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 17.Barlow WE. Robust variance estimation for the case-cohort design. Biometrics. 1994;50(4):1064–1072. [PubMed] [Google Scholar]
- 18.Xue X, Xie X, Gunter M, Rohan TE, Wassertheil-Smoller S, Ho GY, Cirillo D, Yu H, Strickler HD. Testing the proportional hazards assumption in case-cohort analysis. BMC Med Res Methodol. 2013;13:88. doi: 10.1186/1471-2288-13-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM Statistics Subcommittee of the NCIEWGoCD. Reporting recommendations for tumor marker prognostic studies (REMARK) Journal of the National Cancer Institute. 2005;97(16):1180–1184. doi: 10.1093/jnci/dji237. [DOI] [PubMed] [Google Scholar]
- 20.Chung GG, Zerkowski MP, Ghosh S, Camp RL, Rimm DL. Quantitative analysis of estrogen receptor heterogeneity in breast cancer. Laboratory investigation; a journal of technical methods and pathology. 2007;87(7):662–669. doi: 10.1038/labinvest.3700543. [DOI] [PubMed] [Google Scholar]
- 21.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC American Society of Clinical O, College of American P. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version) Archives of pathology & laboratory medicine. 2010;134(7):e48–72. doi: 10.1043/1543-2165-134.7.e48. [DOI] [PubMed] [Google Scholar]
- 22.National Cancer Institute, Surveillance Research Program, Surveillance Systems Branch. Surveillance, Epidemiology, and End Results (SEER) Program. (Released April 2016, based on the November 2015 submission.) ( www.seer.cancer.gov) Research Data (1973–2013)
- 23.Jacobs TW, Prioleau JE, Stillman IE, Schnitt SJ. Loss of tumor marker-immunostaining intensity on stored paraffin slides of breast cancer. Journal of the National Cancer Institute. 1996;88(15):1054–1059. doi: 10.1093/jnci/88.15.1054. [DOI] [PubMed] [Google Scholar]
- 24.Combs SE, Han G, Mani N, Beruti S, Nerenberg M, Rimm DL. Loss of antigenicity with tissue age in breast cancer. Laboratory investigation; a journal of technical methods and pathology. 2016;96(3):264–269. doi: 10.1038/labinvest.2015.138. [DOI] [PubMed] [Google Scholar]
- 25.Fisher B, Redmond C, Fisher ER, Caplan R. Relative worth of estrogen or progesterone receptor and pathologic characteristics of differentiation as indicators of prognosis in node negative breast cancer patients: findings from National Surgical Adjuvant Breast and Bowel Project Protocol B-06. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1988;6(7):1076–1087. doi: 10.1200/JCO.1988.6.7.1076. [DOI] [PubMed] [Google Scholar]
- 26.Barnes DM, Harris WH, Smith P, Millis RR, Rubens RD. Immunohistochemical determination of oestrogen receptor: comparison of different methods of assessment of staining and correlation with clinical outcome of breast cancer patients. British journal of cancer. 1996;74(9):1445–1451. doi: 10.1038/bjc.1996.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lockwood CA, Ricciardelli C, Raymond WA, Seshadri R, McCaul K, Horsfall DJ. A simple index using video image analysis to predict disease outcome in primary breast cancer. International journal of cancer. 1999;84(3):203–208. doi: 10.1002/(sici)1097-0215(19990621)84:3<203::aid-ijc1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 28.Alexieva-Figusch J, Van Putten WL, Blankenstein MA, Blonk-Van Der Wijst J. The prognostic value and relationships of patient characteristics, estrogen and progestin receptors, and site of relapse in primary breast cancer. Cancer. 1988;61(4):758–768. doi: 10.1002/1097-0142(19880215)61:4<758::aid-cncr2820610421>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 29.McCarty KS, Jr, Miller LS, Cox EB, Konrath J, McCarty KS., Sr Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Archives of pathology & laboratory medicine. 1985;109(8):716–721. [PubMed] [Google Scholar]
- 30.McCarty KS, Jr, Flowers SE, Cox JL, Leight EB, Miller GS, Konrath L, Soper J, Budwit JT, Creasman WTDA. Use of a monoclonal anti-estrogen receptor antibody in the immunohistochemical evaluation of human tumors. Cancer research. 1986;46(8 Suppl):4244s–4248s. [PubMed] [Google Scholar]
- 31.Thorpe SM, Christensen IJ, Rasmussen BB, Rose C. Short recurrence-free survival associated with high oestrogen receptor levels in the natural history of postmenopausal, primary breast cancer. European journal of cancer. 1993;29A(7):971–977. doi: 10.1016/s0959-8049(05)80204-7. [DOI] [PubMed] [Google Scholar]
- 32.Black R, Prescott R, Bers K, Hawkins A, Stewart H, Forrest P. Tumour cellularity, oestrogen receptors and prognosis in breast cancer. Clin Oncol. 1983;9(4):311–318. [PubMed] [Google Scholar]
- 33.Sancho-Garnier H, Delarue JC, Mouriesse H, Contesso G, May-Levin F, Gotteland M, May E. Is the negative prognostic value of high oestrogen receptor (ER) levels in postmenopausal breast cancer patients due to a modified ER gene product? European journal of cancer. 1995;31A(11):1851–1855. doi: 10.1016/0959-8049(95)00387-x. [DOI] [PubMed] [Google Scholar]
- 34.Fowler AM, Solodin N, Preisler-Mashek MT, Zhang P, Lee AV, Alarid ET. Increases in estrogen receptor-alpha concentration in breast cancer cells promote serine 118/104/106-independent AF-1 transactivation and growth in the absence of estrogen. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2004;18(1):81–93. doi: 10.1096/fj.03-0038com. [DOI] [PubMed] [Google Scholar]