Abstract
Angiogenesis is crucial for tissue growth and repair in mammals, and is chiefly regulated by Vascular Endothelial Growth Factor (VEGF) signaling. We evaluated the effect of chemical inhibition of VEGF receptor signaling in animals with superior regenerative ability, axolotl salamanders, to determine the impact on vascularization and regenerative outgrowth. Following tail amputation, treated animals (100nM PTK787) and controls were examined microscopically and measured over the month-long period of regeneration. Treatment with VEGFR inhibitor decreased regenerative angiogenesis; drug-treated animals had lower vascular densities in the regenerating tail than untreated animals. This decrease in neovascularization, however, was not associated with a decrease in regenerative outgrowth or with morphological abnormalities in the regrown tail. Avascular but otherwise anatomically normal regenerative outgrowth over 1mm beyond the amputation plane was observed. The results suggest that in this highly regenerative species, significant early tissue regeneration is possible in the absence of a well-developed vasculature. This research sets the groundwork for establishing a system for the chemical manipulation of angiogenesis within the highly regenerative axolotl model, contributing to a better understanding of the role of the microvasculature within strongly proliferative yet well-regulated environments.
Keywords: angiogenesis, vascular endothelial growth factor, regeneration, morphology, Ambystoma
INTRODUCTION
The vasculature provides many critical components to growing tissues including nutrients, oxygen, and growth factors. In mammals, only extremely limited tissue growth is possible without a vascular network (Jain et al., 2005) and the repair of many tissues depends on the formation of new vessels (Li et al., 2005). This dependence on the vasculature is less clear, however, in epimorphic regeneration, a form a regeneration in which a population of undifferentiated proliferating cells, the blastema, gives rise to the replacement structures (Morgan, 1901). The axolotl (Ambystoma mexicanum), with its capacity for near-perfect, repeatable, epimorphic regeneration throughout its lifespan is the pre-eminent vertebrate model system for regeneration, yet relatively little is known concerning the role of the vasculature in the regenerative process, or why vascularization is relatively poor in the rapidly growing and proliferating early blastema (Mescher, 1996).
The regenerating microvasculature in salamanders develops via angiogenesis (Rageh et al., 2002), the formation of new blood vessels from pre-existing vessels. Angiogenesis is stimulated by a variety of growth factors that are produced in response to injury, chief among them Vascular Endothelial Growth Factor (VEGF). The VEGF signaling pathway has been extensively investigated in mammalian model systems. In addition to its role as a pro-angiogenic factor, VEGF promotes the production of scar tissue in mammals (Johnson and Wilgus, 2014). There are few studies of the role of VEGF signaling in animals with scarless wound healing, such as salamanders. Bayliss et al. (2006) demonstrated that inhibition of VEGFR diminished the vasculature in the regenerating zebrafish tail fin and that limited regenerative regrowth was possible even in avascular tissue. Among the amphibians, VEGF signaling has been most widely studied in Xenopus (e.g., Ciau-Uitz et al., 2013, Ny et al., 2013). There are few studies in salamanders, but the VEGF receptor (VEGFR) is known to be expressed in Cynops newts (Kanao and Miyachi, 2006) and VEGF, in concert with other growth factors, can affect regeneration across segment defects in the axolotl fibula (Chen et al., 2015).
We sought to determine the effect of disrupting VEGFR signaling on regenerative angiogenesis and regenerative growth in Ambystoma mexicanum. With their ability to regenerate endochondral bone, axolotls are vertebrate model with an even greater regenerative capacity than zebrafish. As molecular tools for axolotls are as yet more limited than those for many other model systems (Whited and Tabin, 2009), we employed a chemical genetic approach to modify receptor signaling. A small molecule protein kinase inhibitor originally investigated as a possible anti-angiogenic therapy for tumor treatment, PTK787 acts as a potent, high affinity inhibitor of the vertebrate VEGF receptors. The role of VEGFR in angiogenesis is broadly conserved across chordates. Although PTK787 is most widely studied in mouse models, the drug also blocks vascular regeneration in tunicates (Tiozzo et al., 2008) and zebrafish (Chan et al., 2002; Bayliss et al., 2006; Chimote et al., 2014; Tal et al., 2014; Hlushchuk et al., 2016). It also inhibits VEGFR signaling in frogs (Whittles et al., 2002) and in the chicken chorioallantoic membrane (Hlushchuk et al., 2007). Our objective was to determine if the regenerating vasculature of axolotls is similarly sensitive to the inhibition of VEGFR by PTK787 and what consequences this may have on the spatial and temporal development of the neovasculature and epimorphic regeneration.
Here we show that regenerative angiogenesis in Ambystoma mexicanum is diminished by chemical inhibition of VEGF receptors. This decrease in regenerative vascular density did not significantly affect rate of regenerative outgrowth or overall morphology of the regenerated appendage. Washout of the inhibitor showed reversibility of the effect of the drug on the microvasculature, with vascular densities approaching those of the control animals after several weeks. These studies demonstrate the feasibility of using small molecule inhibitors as a tool to manipulate angiogenesis in the axolotl model of regeneration.
MATERIALS AND METHODS
Animal maintenance
Captive bred larval and juvenile wildtype and albino strains of Ambystoma mexicanum were obtained from the Ambystoma Genetic Stock Center (Lexington, KY). The animals were allowed to acclimate for at least one week prior to any experiments. Axolotls were maintained in an 18°C room on a 12:12 light cycle. Animals were individually housed in plastic tanks containing 40% Holtfreter’s solution, changed daily. Salamanders were fed daily ad libitum with salmon pellets (Rangen, Buhl, ID) supplemented with live black worms and brine shrimp. Animal procedures were carried out in accordance with an approved Towson University IACUC protocol. Repeated measurements of the same animals over multiple time points were recorded for the duration of the experiments, reducing the number of animals needed for the study.
Amputation
Wildtype larval (mean snout vent length [SVL]=23.2mm±4.1), albino larval (17.3mm±1.9), wildtype juvenile (53.8mm±14.2), and albino juvenile (68.1mm±0.5) salamanders were anaesthetized prior to amputation by immersion in 0.01% benzocaine (Ark Pharm, Libertyville, IL) dissolved in 40% Holtfreter’s solution. Once the animal was unresponsive, an ethanol-cleaned scalpel was used to remove the distal 25% of the tail length (tail length measured from cloaca to tail tip). In six wildtype larvae, the forelimb was amputated. All animals survived anesthesia and amputation. A total of 60 tails were amputated over the course of the experiments. Immediately following amputation, size-matched animals were placed in individual drug treatment tanks or control tanks.
Chemical Exposure
Animals were exposed to the drug via their tank water. The kinase inhibitor PTK787/ZK222584 [N-(4-chlorophenyl)-4-(pyridin-4-ylmethyl)phthalazin- 1-amine, vatalanib, (Cayman Chemical, Ann Arbor, MI)] was first dissolved in dimethyl sulfoxide (DMSO) to produce a stock solution of 5mM that was then aliquoted and stored at −20°C. For the chemical exposures, the stock solution was diluted in 40% Holtfreter’s solution to produce 100nM or 500nM concentrations. Since PTK787 had not previously been used in salamanders, these two initial drug concentrations were based on the EC50 and effective dosages of the drug in zebrafish for the partial and complete inhibition of angiogenesis (Bayliss et al., 2006; Chimote et al., 2014). Vehicle control tanks contained the equivalent volume of DMSO dissolved in Holtfreter’s solution. Because our pilot experiment in wildtype larvae showed no significant dose-dependent effect of the 100nM versus 500nM treatments, all subsequent experiments were all performed using the lower 100nM dose. This is well below the LC50 for zebrafish embryos, [1μM, (Chimote et al., 2014)]. PTK787 is one of the most selective and best characterized of the kinase inhibitors (Fabian et al., 2005). At the nanomolar concentrations used in these studies, the drug acts as a specific inhibitor of VEGFR receptor kinases; only at higher doses is it active against kinases of other receptor families, such as PDGFR-B and c-kit (Wood et al., 2000).
Drug solutions were changed daily for the duration of the experiment. The animals’ behavior, including activity level and feeding, was observed for evidence of drug toxicity. Axolotls were maintained in exposure solutions for several weeks until the control animals had regenerated 100% of the tail length that had been amputated. This was followed by a three week washout period to assess reversibility of the drug’s effects.
Microscopy and Measurement
Animals were anesthetized at day four post-amputation and then weekly to immobilize them for microscopy and measurement of regenerative tail outgrowth and then returned to their treatment tanks. Images were digitally captured under a Bausch and Lomb stereoscopic microscope. Vessels filled with circulating blood were directly visualized in live animals without additional staining and images digitally recorded for subsequent analysis of vascular density. Post-acquisition processing of images was limited to cropping and alteration of brightness and contrast applied to the entire image.
Measurements including SVL, tail length, tail width at the amputation plane, and length of the regenerative outgrowth were made using calipers. Mean regenerative outgrowth length (the length of the tissue that has regenerated beyond the amputation plane) and percent regrowth (the regenerative outgrowth length divided by the length of the amputated portion removed) were calculated for each time point. These values were plotted for the control and treatment groups, with a 95% confidence interval used for error bars. Significance levels for t-tests was set at p>0.05.
Image Analysis for Vascular Density
Using ImageJ software (Schneider et al., 2012), vascular density within the regenerating portion of the tail (i.e., the percentage of the regenerated area occupied by blood vessels) was quantified by thresholding images to remove background and select only for vessels within the region of interest.
Histology
A subset of animals was used for further histological analysis. A methylene blue penetration assay [adapted from methods developed for zebrafish re-epithelialization in wound healing, (Richardson et al., 2013)] was used to compare re-epithelialization immediately following amputation in treated and control animals. Additional animals were euthanized by benzocaine overdose at 14 days post-amputation and 21 days post-amputation and then fixed in 4% paraformaldehyde prior to alcian blue and alizarin red whole mount staining to assess cartilage and bone formation within the regenerating tail [methods adapted from (Hanken and Wassersug, 1981)]. In some cases, harvested, fixed tissue was paraffin embedded, thick sectioned (~30μm) using a microtome, and stained with toluidine blue/fast green prior to imaging using a Nikon compound microscope.
RESULTS
Regenerative angiogenesis in control animals
Following amputation, bleeding was minimal and clotting could often be observed at the site of injury. At three and four days post-amputation, we were not able to detect new vessels. At seven days post-amputation, however, a small amount of new vasculature originating from the vessels of the tail stump at the level of the amputation plane and extending into the regenerating portion of the tail was sometimes visible. A more extensive microvasculature, continuous with the circulation of the tail stump, had invaded the regenerating area by day 14 but generally had not reached the region of the wound epithelium. As regeneration proceeded, a vascular plexus formed proximo-distally. Circulating red blood cells were visible within the regenerated vasculature, indicating that these new vessels were open and perfused with blood. Representative images of the regenerating tail in two albino axolotls are shown in Figure 1. Vascular density values for control animals were similar for the wildtype and albino strains, but quantification in the wildtype was complicated in some cases due to pigmentation obscuring some of the microvessels, so data for the albino strain are presented here.
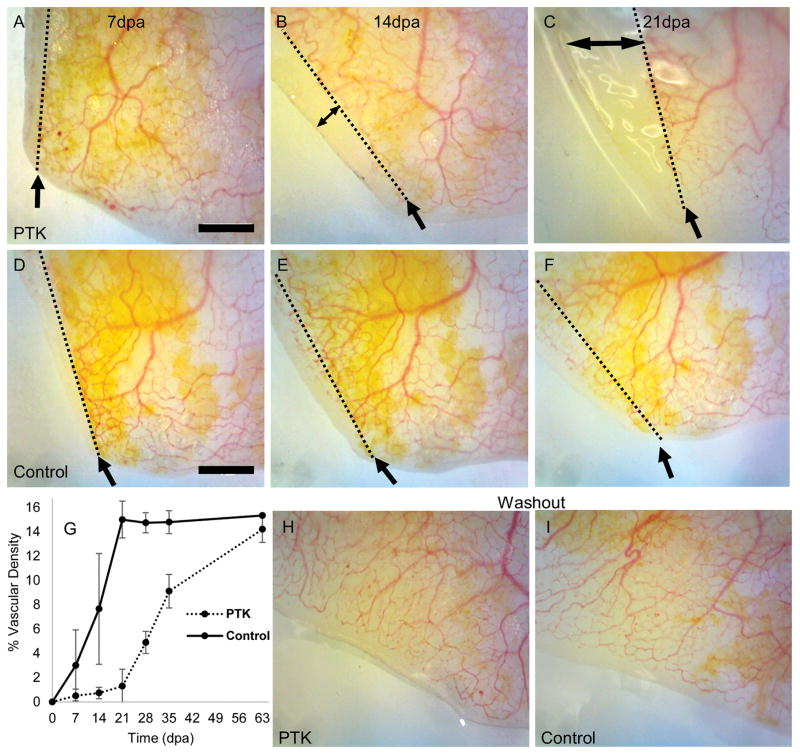
Fig. 1.
Treatment with the VEGFR inhibitor PTK787 decreased vascular density in the regenerating tail. Representative images of the regenerating tail tip of drug-treated (A–C) and control (D–F) juvenile albino axolotls from 7–21 days post amputation (dpa). The direction of regeneration is towards the left, and intact tail is towards the right, in all figures. Single headed arrows point to the level of the amputation plane, shown as a dotted line. Double-headed arrow indicates regenerative outgrowth without detectable vessels in the drug-treated animal. Very little vasculature is visible within the regenerating area in the animal receiving the VEGFR inhibitor compared to the control but otherwise, regenerative outgrowth appears normal. Graph (G) shows mean percent vascular density within the regenerating region of the tail (n=5 animals/group). Error bars represent 95% confidence intervals. Washout of the drug began at day 21. Vascular densities of the regenerating area in the drug-treated juvenile albino axolotls approached those of the control animals after several weeks of washout of the drug. H, I) vasculature in the regenerated region of the tail two weeks after drug washout in same two individuals shown in A–F. Scale bars=1mm.
Inhibition of VEGFR decreased regenerative angiogenesis
Treatment with the VEGFR inhibitor PTK787 decreased the vascular density within the regenerating tail (Fig. 1G). During drug treatment, vascular density was significantly lower (p<0.05) at all time points except 7 days post-amputation, at which there was very little vasculature in either the control or the treated animals. Regenerated tissue over 1 mm in length that lacked grossly visible vasculature was observed in drug-treated animals (Fig. 1C).
The effect of VEGFR inhibition was reversible
Following drug washout, vascular density increased, approaching that of the controls after several weeks, indicating that the effect of PTK787 on angiogenesis was reversible (Fig 1G–I). The regenerated vasculature of drug-treated animals had normal morphology. Observation of the circulation of blood within the regenerated vascular bed confirmed that the regenerated vessels were patent and functional.
Inhibition of VEGFR had little effect on regenerative outgrowth length
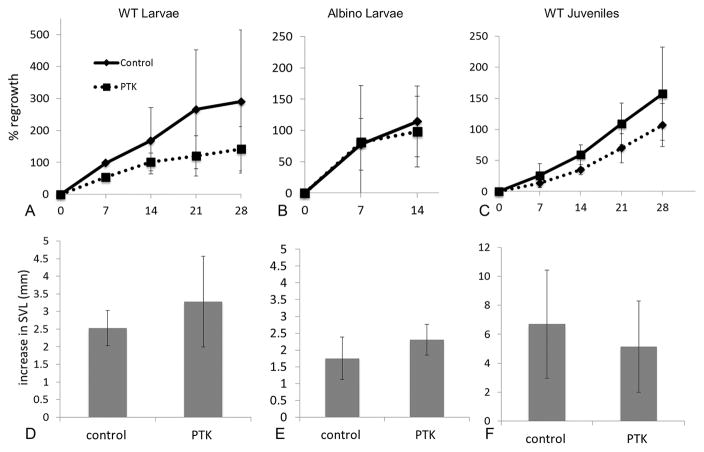
The decrease in vascular density associated with PTK787 treatment was generally not accompanied by a significant decrease in length of the regenerated portion of the tail or a decrease in percentage of amputated tail that had regrown at each time point (Fig. 2A–C). This was true for both strains (wildtype and albino) and ages (larval and juvenile) examined, with the exception of the wildtype larvae at 7 days post-amputation, in which regenerative outgrowth was significantly lower in drug-treated animals. Normalizing tail outgrowth length by dividing by SVL or tail width did not alter these findings. Control wildtype larvae regenerated more quickly (i.e., regrew 100% of the amputated length of the tail sooner) than wildtype juveniles (Fig. 2). Rapid regeneration resulted in the percentage tail regrowth exceeding 100% as the tail fully regenerated and then continued to grow beyond its original, pre-amputation size. Albino larvae displayed a large variation in their tail regrowth rate, particularly within the first week. All wildtype larvae were fully regenerated at an earlier time point than that required for all albino larvae to be fully regenerated.
Fig. 2.
Treatment with VEGFR inhibitor did not alter tail regenerative outgrowth (A–C) or increase in overall body growth (D–F) in the stages (larvae and juvenile) and strains (wildtype and albino) examined. Percent regrowth is the mean percentage of the tail length that was removed that has regrown. A value of 100% regrowth represents full replacement of the amputated tail length, i.e. the tail has regenerated to its original, pre-amputation size. Percentages beyond 100% indicate additional growth beyond replacement length as these immature animals continued to grow. Values for tail regrowth are slightly lower in the drug-treated animals than controls but this did not rise to the level of significance (p<0.05) except as noted in the text. Increase in SVL is the mean increase in snout vent length over three weeks of drug treatment.
No evidence of systemic toxicity of the drug
The mean increase in SVL over the course of drug treatment (Fig. 2D–F) did not differ significantly between PTK787 and control groups for any of the ages or strains studied, suggesting that the drug did not exert a systemic effect on body growth. No changes in behavior (lack of appetite, disequilibrium, lethargy, unusual locomotion, tail or gill curling) were observed in PTK787-treated individuals, consistent with there being no significant toxicity of PTK787 in the nanomolar range.
Inhibition of VEGFR did not alter the gross morphology of the regenerated tail
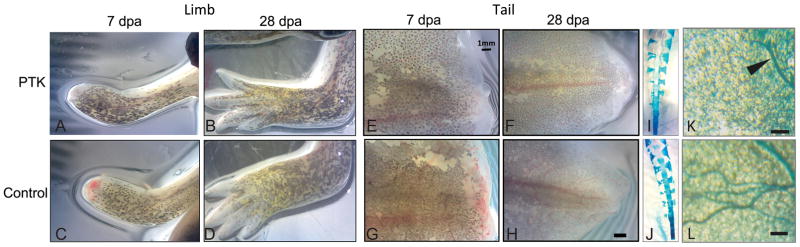
Apart from the decrease in neovascularization, tail regeneration in drug-treated animals appeared normal. The amputation plane showed a similar time course (~3 hours) for exclusion of the majority of the methylene blue dye, suggesting a similar time frame for the initial stages of re-epithelialization in the drug-treated and control groups. No morphological abnormalities in the regenerating tail associated with PTK787 treatment were visible grossly or in whole-mount cleared and stained specimens labelled for markers of cartilage and bone. PTK787 treatment similarly did not alter regeneration of the forelimb at the macroscopic level (Fig. 3). Similar to the differences in vascularity observed in vivo, histology of thick paraffin sections indicated a more extensive vascular network in the regenerated tail tissue of control animals compared to those treated with VEGFR inhibitor (Fig. 3K, L).
Fig. 3.
Treatment with VEGFR inhibitor did not alter the overall gross morphology of the regenerated appendage. Both amputated limb (A–D) and tail (E–L) regenerated normally following PTK787 treatment, with the new appendages closely resembling those prior to amputation. Alcian blue stained tail specimens (I, J) did not reveal any osteological abnormalities associated with drug treatment. Histology of thick paraffin sections indicates that the vascular network of tails treated with VEGFR inhibitor (K) is less well developed than that of controls (L). Scale bar=100 μm. Arrowhead points to a vessel.
DISCUSSION
This study was the first to chemically manipulate angiogenesis in the axolotl model of regeneration. Our findings demonstrate that regenerative angiogenesis in Ambystoma is sensitive to VEGFR signaling, supporting the hypothesis of Rageh et al. (2005) that VEGF plays a role in vascular formation during salamander regeneration. The diminished angiogenesis associated with inhibition of VEGFR, however, does not impede regenerative growth or result in gross morphological abnormalities, indicating that the lower vascular densities were well tolerated within the regenerating tail. That tail regrowth was not significantly impacted by diminished vasculature may indicate that oxygen delivery via water at the wound epithelium and the intact skin is sufficient during this early period of regeneration (Peadon and Singer, 1966). Consistent with PTK787 being a reversible inhibitor of VEGFR, washout of the drug was associated with restoration of vascular densities to control levels after several weeks.
Despite the widespread use of the axolotl as a model of regeneration, the degree to which regrowing appendages are dependent on a blood supply across the stages of regeneration has not been fully characterized. Tassava and Huang (2005) showed that experimentally induced ischemia (via severing or ligature of major tail vessels) during the first week of regeneration caused spinal cord breakdown and inhibited tail regeneration in adult newts (Notophthalamus viridescens). Ischemia that was induced ten days after amputation, however, delayed but did not inhibit regeneration (Tassava and Huang, 2005). This suggests a time-sensitive need for the pre-existing vasculature of the amputation stump for regeneration but does not address the necessity of angiogenesis/neovascularization.
Studies of neovascularization in the regenerating limbs of Notophthalmus viridescens (Peadon and Singer, 1966; Iten and Bryant, 1973), Triturus cristatus, and A. mexicanum (Smith and Wolpert, 1975) indicate that the early blastema lacks a functional vascular network. More recently, Rageh et al. (2002), using immunohistochemical labeling, visualized blood vessels at seven days post-amputation in newt (N. viridescens) forelimb. Whited et al. (2013), using viral vectors to label progenitor cells, found vascular cells at seven but not at three days post-amputation in regenerating axolotl limbs. In our axolotl tail regeneration studies, neovascularization was not observed in vivo at 4 days post-amputation but small outgrowths of vasculature were sometimes observable at seven days post-amputation and were well developed by 14 days post-amputation in control animals. The microvascular architecture, consisting of small vessels sprouting from the pre-existing vessels of the stump and the eventual formation of a more extensive vascular network, was consistent with that described by Rageh et al. (2002) for the regenerating limb. The current study focused on whole mount imaging in vivo as this provides a physiologically-relevant overview of the three dimensional architecture of the blood-perfused vascular network. More sensitive techniques using fixed tissue, for example immunohistochemistry for vascular endothelial cell markers and higher resolution imaging, would be required in future studies to detect any newly formed but unperfused vasculature and as well as any smaller vessels not visible in whole mount imaging. More detailed histological characterization of the neovasculature in the presence of absence of VEGFR inhibition is merited. For example, in light of the complex association between VEGF signaling, pericytes, and the capacity for vascular remodeling (Benjamin et al., 1998), one future approach could assess the effect of VEGFR inhibition on the degree of pericyte coverage on the regenerated vasculature.
As is the case in zebrafish (Bayliss et al., 2006), in axolotls, limited regeneration was still possible despite the diminished angiogenesis resulting from inhibition of VEGFR. In Ambystoma, considerable (>1mm) tissue regeneration was apparent even in the absence of a discernable vasculature. This is greater than that observed during normal newt regeneration [~400μm, (Iten and Bryant, 1973)] and similar to the amount of avascular fin regeneration observed in zebrafish receiving PTK787 (Bayliss et al., 2006). Most cells in mammals are located within 200μm of a capillary (Lovett et al., 2009; Jain et al., 2005) and most tumors cannot grow beyond that size without a vascular supply (Folkman & Kalluri, 2003). Thus the capacity for avascular tissue growth is greater in the ectotherms studied than in mammals, which may have more stringent angiogenic limits to most tissue growth.
Chemical inhibition of VEGFR signaling in axolotls provides a tool to investigate the role of angiogenesis in this highly regenerative vertebrate model. Treatment with PTK787 was well tolerated. There was no overt toxicity as assessed by growth in SVL or off-target morphological abnormalities over the study period. Although younger axolotls regenerated more quickly than older individuals, we did not observe age-dependent effects of the drug, indicating that PTK787 can be used to alter angiogenesis at a variety of ontogenetic stages. Similarly, although the wildtype strain regenerated more quickly than the albino strain, both strains responded similarly to the drug, showing decreased vascularity in the regenerated tail compared to control animals.
Given that simple tissue repair is typically characterized by angiogenesis, the extent to which early epimorphic regeneration can proceed in the absence of a well-developed neovasculature demands explanation. Different phases of regeneration likely have different requirements for vessel formation. That there is relatively little functional neovasculature in the early regenerate compared to later stages suggests that the initial stages of regeneration are less dependent on vascular delivery, and perhaps more dependent on factors from the local microenvironment. During the transient, less vascular, early phase of regeneration in salamanders, regeneration is nerve-dependent and some trophic factors required for subsequent cell proliferation and blastema formation may be supplied locally, for example by growing axons, rather than by capillaries (Mescher 1996; Mescher and Kiffmeyer, 1992; Mescher et al. 1997). It has been hypothesized (Mescher 1996) that angiogenesis may be delayed in early regeneration because essential regenerative processes, such as patterning interactions, might involve changes incompatible with neovascularization. Indeed, in mammals, a less vascular microenvironment favors early regeneration; VEGF and precocious angiogenesis are potent inhibitors of digit regeneration in mice (Yu et al. 2012, 2014). The production of antiangiogenic factors that delay neovascularization in the wound bed may have been essential in the evolution of mammalian digit regeneration (Yu et al., 2014). Early avascularity may create a regeneration-permissive microenvironment more conducive towards the intercellular signaling and regenerative response (Yu et al., 2014). Future studies assessing the effect, for example, of VEGFR activation during early regeneration may help address if delayed angiogenesis is similarly essential for axolotl regeneration.
We have shown that, as with zebrafish (Bayliss et al., 2006), VEGFR signaling can be chemically controlled in axolotls; altering the period of drug administration will allow temporal control over the manipulation of regenerative angiogenesis, facilitating investigations of the role of blood vessel formation during different stages of regeneration. The current study sets the stage for gaining a better understanding of a receptor signaling pathway controlling angiogenesis in Ambystoma, and for investigating the dependence of tissue regrowth on angiogenesis in this highly regenerative model.
Acknowledgments
The authors thank K. Fofana and R. Montoro for technical assistance. This research was supported in part by a Fisher College of Science and Math Undergraduate Research Grant. The Ambystoma Genetic Stock Center is supported by NIH grant P40-OD019794.
LITERATURE CITED
- Bayliss PE, Bellavance KL, Whitehead GG, Abrams JM, Aegerter S, Robbins HS, Cowan DB, Keating MT, O’Reilly T, Wood JM, Roberts TM, Chan J. Chemical modulation of receptor signaling inhibits regenerative angiogenesis in adult zebrafish. Nat Chem Biol. 2006;2:265–273. doi: 10.1038/nchembio778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998;125:1591–8. doi: 10.1242/dev.125.9.1591. [DOI] [PubMed] [Google Scholar]
- Chan J, Bayliss PE, Wood JM, Roberts TM. Dissection of angiogenic signaling in zebrafish using a chemical genetic approach. Cancer Cell. 2002;1:257–267. doi: 10.1016/s1535-6108(02)00042-9. [DOI] [PubMed] [Google Scholar]
- Chen X, Song F, Jhamb D, Li J, Bottino MC, Palakal MJ, Stocum DL. The axolotl fibula as a model for the induction of regeneration across large segment defects in long bones of the extremities. PLoS One. 2015;10:e0130819. doi: 10.1371/journal.pone.0130819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimote G, Sreenivasan J, Pawar N, Subramanian J, Sivaramakrishnan H, Sharma S. Comparison of effects of anti-angiogenic agents in the zebrafish efficacy-toxicity model for translational anti-angiogenic drug discovery. Drug Des Devel Ther. 2014;8:1107–1123. doi: 10.2147/DDDT.S55621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciau-Uitz A, Pinheiro P, Kirmizitas A, Zuo J, Patient R. VEGFA-dependent and -independent pathways synergise to drive Scl expression and initiate programming of the blood stem cell lineage in Xenopus. Development. 2013;140:2632–2642. doi: 10.1242/dev.090829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian MA, Biggs WH, 3rd, Treiber DK, Atteridge CE, Azimioara MD, Benedetti MG, Carter TA, Ciceri P, Edeen PT, Floyd M, Ford JM, Galvin M, Gerlach JL, Grotzfeld RM, Herrgard S, Insko DE, Insko MA, Lai AG, Lélias JM, Mehta SA, Milanov ZV, Velasco AM, Wodicka LM, Patel HK, Zarrinkar PP, Lockhart DJ. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol. 2005;23:329–336. doi: 10.1038/nbt1068. [DOI] [PubMed] [Google Scholar]
- Folkman J, Kalluri R. In: Cancer Medicine. 6. Kufe DW, Pollock RE, Weichselbaum RR, Bast RC Jr, Holland JF, Frei E III, editors. Chapter 11. B.C. Decker; Hamilton, ON, Canada: 2003. pp. 161–194. [Google Scholar]
- Godwin JW, Pinto AR, Rosenthal NA. Macrophages are required for adult salamander limb regeneration. Proc Natl Acad Sci U S A. 2013;110:9415–9420. doi: 10.1073/pnas.1300290110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanken J, Wassersug RJ. The visible skeleton. Funct Photog. 1981;16:22–26. [Google Scholar]
- Hlushchuk R, Baum O, Gruber G, Wood J, Djonov V. The synergistic action of a VEGF-receptor tyrosine-kinase inhibitor and a sensitizing PDGF-receptor blocker depends upon the stage of vascular maturation. Microcirculation. 2007;14:813–825. doi: 10.1080/10739680701370021. [DOI] [PubMed] [Google Scholar]
- Hlushchuk R, Brönnimann D, Correa Shokiche C, Schaad L, Triet R, Jazwinska A, Tschanz SA, Djonov V. Zebrafish caudal fin angiogenesis assay-advanced quantitative assessment including 3-way correlative microscopy. PLoS One. 2016;11:e0149281. doi: 10.1371/journal.pone.0149281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iten LE, Bryant SV. Forelimb regeneration from different levels of amputation in the newt, Notophthalmus viridescens: Length, rate, and stages. Wilhelm Roux’ Arch fur Entwicklungsmechanik der Org. 1973;173:263–282. doi: 10.1007/BF00575834. [DOI] [PubMed] [Google Scholar]
- Jain RK, Au P, Tam J, Duda DG, Fukumura D. Engineering vascularized tissue. Nat Biotechnol. 2005;23:821–823. doi: 10.1038/nbt0705-821. [DOI] [PubMed] [Google Scholar]
- Johnson KE, Wilgus TA. Vascular Endothelial Growth Factor and angiogenesis in the regulation of cutaneous wound repair. Adv Wound Care. 2014;3(10):647–661. doi: 10.1089/wound.2013.0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanao T, Miyachi Y. Lymphangiogenesis promotes lens destruction and subsequent lens regeneration in the newt eyeball, and both processes can be accelerated by transplantation of dendritic cells. Dev Biol. 2006;290:118–124. doi: 10.1016/j.ydbio.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Li WW, Talcott KE, Zhai AW, Kruger EA, Li VW. The role of therapeutic angiogenesis in tissue repair and regeneration. Adv Skin Wound Care. 2005;18(9):491–500. doi: 10.1097/00129334-200511000-00013. [DOI] [PubMed] [Google Scholar]
- Lovett M, Lee K, Edwards A, Kaplan DL. Vascularization strategies for tissue engineering. Tissue Eng Part B Rev. 2009;15:353–70. doi: 10.1089/ten.teb.2009.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher AL. The cellular basis of limb regeneration in urodeles. Int J Dev Biol. 1996;40:785–795. [PubMed] [Google Scholar]
- Mescher AL, Kiffmeyer WR. Axonal release of transferrin in peripheral nerves of axolotls during regeneration. In: Taban CH, Boilly B, editors. Keys for Regeneration. Karger; Basel: 1992. pp. 100–109. [PubMed] [Google Scholar]
- Mescher AL, Connell E, Hsu C, Patel C, Overton B. Transferrin is necessary and sufficient for the neural effect on growth in amphibian limb regeneration blastemas. Dev Growth Differ. 1997;39(6):677–84. doi: 10.1046/j.1440-169x.1997.t01-5-00003.x. [DOI] [PubMed] [Google Scholar]
- Morgan TH. Regeneration. New York: Macmillan Co; 1901. [Google Scholar]
- Ny A, Vandevelde W, Hohensinner P, Beerens M, Geudens I, Diez-Juan A, Brepoels K, Plaisance S, Krieg PA, Langenberg T, Vinckier S, Luttun A, Carmeliet P, Dewerchin M. A transgenic Xenopus laevis reporter model to study lymphangiogenesis. Biol Open. 2013;2:882–890. doi: 10.1242/bio.20134739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peadon AM, Singer M. The blood vessels of the regenerating limb of the adult newt, Triturus. J Morphol. 1966;118:79–89. doi: 10.1002/jmor.1051180106. [DOI] [PubMed] [Google Scholar]
- Rageh MAE, Mendenhall L, Moussad EEA, Abbey SE, Mescher AL, Tassava RA. Vasculature in pre-blastema and nerve-dependent blastema stages of regenerating forelimbs of the adult newt, Notophthalmus viridescens. J Exp Zool. 2002;292:255–266. doi: 10.1002/jez.10015. [DOI] [PubMed] [Google Scholar]
- Richardson R, Slanchev K, Kraus C, Knyphausen P, Eming S, Hammerschmidt M. Adult zebrafish as a model system for cutaneous wound-healing research. J Invest Dermatol. 2013;133:1655–1665. doi: 10.1038/jid.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AR, Wolpert L. Nerves and angiogenesis in amphibian limb regeneration. Nature. 1975;257:224–225. doi: 10.1038/257224a0. [DOI] [PubMed] [Google Scholar]
- Tal TL, McCollum CW, Harris PS, Olin J, Kleinstreuer N, Wood CE, Hans C, Shah S, Merchant FA, Bondesson M, Knudsen TB, Padilla S, Hemmer MJ. Immediate and long-term consequences of vascular toxicity during zebrafish development. Reprod Toxicol. 2014;48:51–61. doi: 10.1016/j.reprotox.2014.05.014. [DOI] [PubMed] [Google Scholar]
- Tassava RA, Huang Y. Tail regeneration and ependymal outgrowth in the adult newt, Notophthalmus viridescens, are adversely affected by experimentally produced ischemia. J Exp Zool A Comp Exp Biol. 2005;303:1031–1039. doi: 10.1002/jez.a.242. [DOI] [PubMed] [Google Scholar]
- Tiozzo S, Voskoboynik A, Brown FD, De Tomaso AW. A conserved role of the VEGF pathway in angiogenesis of an ectodermally-derived vasculature. Dev Biol. 2008;315:243–55. doi: 10.1016/j.ydbio.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whited JL, Tabin CJ. Limb regeneration revisited. J Biol. 2009;8:5. doi: 10.1186/jbiol105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whited JL, Tsai SL, Beier KT, White JN, Piekarski N, Hanken J, Cepko CL, Tabin CJ. Pseudotyped retroviruses for infecting axolotl in vivo and in vitro. Development. 2013;140:1137–1146. doi: 10.1242/dev.087734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittles CE, Pocock TM, Wedge SR, Kendrew J, Hennequin LF, Harper SJ, Bates DO. ZM323881, a novel inhibitor of vascular endothelial growth factor-receptor-2 tyrosine kinase activity. Microcirculation. 2002;9:513–522. doi: 10.1038/sj.mn.7800164. [DOI] [PubMed] [Google Scholar]
- Wood JM, Bold G, Buchdunger E, Cozens R, Ferrari S, Frei J, Hofmann F, Mestan J, Mett H, O’Reilly T, Persohn E, Rösel J, Schnell C, Stover D, Theuer A, Towbin H, Wenger F, Woods-Cook K, Menrad A, Siemeister G, Schirner M, Thierauch KH, Schneider MR, Drevs J, Martiny-Baron G, Totzke F. PTK787/ZK 222584, a novel and potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, impairs vascular endothelial growth factor-induced responses and tumor growth after oral administration. Cancer Res. 2000;60:2178–2189. [PubMed] [Google Scholar]
- Yu L, Yan M, Simkin J, Ketcham PD, Leininger E, Han M, Muneoka K. Angiogenesis is inhibitory for mammalian digit regeneration. Regeneration. 2014;1:33–46. doi: 10.1002/reg2.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Han M, Yan M, Lee J, Muneoka K. BMP2 induces segment-specific skeletal regeneration from digit and limb amputations by establishing a new endochondral ossification center. Dev Biol 2012. 2014;372(2):263–73. doi: 10.1016/j.ydbio.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]