Abstract
Background
Obesity, a cause of subclinical inflammation, is associated with increased risk of high grade prostate cancer (PC) and poor outcomes. Whether inflammation occurs in periprostatic white adipose tissue (WAT), and contributes to the negative impact of obesity on PC aggressiveness, is unknown.
Methods
In a single-center, cross-sectional design, men with newly diagnosed PC undergoing radical prostatectomy were eligible for study participation. The primary objective was to examine the prevalence of periprostatic WAT inflammation defined by the presence of crown-like structures (CLS-P) as detected by CD68 immunohistochemistry. Secondary objectives were to explore the clinical and systemic correlates of periprostatic WAT inflammation. Tumor characteristics and host factors including BMI, adipocyte diameter, and circulating levels of lipids, adipokines, and other metabolic factors were measured. Wilcoxon rank-sum, Chi-square, or Fisher’s exact tests, and generalized linear regression were used to examine the association between WAT inflammation and tumor and host characteristics.
Results
Periprostatic fat was collected from 169 men (median age 62 years; median BMI 28.3). Periprostatic WAT inflammation was identified in 49.7% of patients and associated with higher BMI (P=0.02), larger adipocyte size (P=0.004), and Gleason grade groups IV/V tumors (P=0.02). The relationship between WAT inflammation and high Gleason grade remained significant after adjusting for BMI (P=0.04). WAT inflammation correlated with higher circulating levels of insulin, triglycerides, and leptin/adiponectin ratio, and lower high density lipoprotein cholesterol, compared to those without WAT inflammation (P’s <0.05).
Conclusions
Periprostatic WAT inflammation is common in this cohort of men with PC and is associated with high grade PC.
Keywords: Body mass index, metabolic dysregulation, obesity, periprostatic white adipose tissue inflammation, prostate cancer
Introduction
Obesity is characterized by a chronic subclinical inflammatory state and is associated with an increased incidence of multiple malignancies including high grade, lethal prostate cancer.1 Obesity is also associated with poor outcomes after a prostate cancer diagnosis including elevated risk of biochemical recurrence, distant metastases, as well as death from cancer or any cause.2,3 Defining the mechanisms by which obesity contributes to prostate cancer development and progression is an active area of investigation - inflammation represents a potential biologic link.
The prostate is contained within a capsule-like structure surrounded by visceral fat. This periprostatic fat pad is biologically active and functionally impacts malignant prostate cells. Extension of tumor cells into the extra-capsular space results in direct cell-to-cell interactions with periprostatic adipocytes and adipocyte-derived factors that may further accelerate tumor progression.4–6 Chemokines derived from tumor cells can direct the migration of adipose stromal cells to the tumor, promoting vascularization, growth and metastatic spread.7 Obesity is associated with altered expression of periprostatic fat genes involved in cell growth and immune surveillance.4 Additionally, increased periprostatic fat area, volume and density are associated with higher stage and grade prostate cancer.8–10 Collectively, these findings suggest an important contributory role of periprostatic adipose tissue to the development and progression of prostate cancer.
Obesity promotes a pathologic process in adipose tissue that involves adipocyte hypertrophy, hypoxia, and eventual cell death.11 This cycle increases chemokine production from adipocytes and immune cell recruitment resulting in white adipose tissue (WAT) inflammation.12 A pathological hallmark of WAT inflammation is the crown-like structure (CLS), comprised of a dead or dying adipocyte surrounded by macrophages.13 These macrophages phagocytize dead adipocytes and become foam cells.14 Recently, we and others have shown that WAT inflammation, detected by the presence of CLS, is an independent predictor of poor prognosis in patients with early-stage breast cancer or tongue cancer.15–17 Despite the proximity between WAT and the prostate, nothing is known about either the existence or clinical importance of WAT inflammation in patients with prostate cancer. Accordingly, the primary objective of this study was to examine the prevalence of periprostatic WAT inflammation, as characterized by CLS, in men with localized prostate cancer. Secondary objectives were to explore the clinical and systemic correlates of periprostatic WAT inflammation and to assess whether these relationships remained significant after adjustment for BMI.
Materials and Methods
Patients and Setting
Using a single-center, cross-sectional design, consecutive men with newly diagnosed prostate adenocarcinoma undergoing radical prostatectomy (RP) at Memorial Sloan Kettering Cancer Center (MSK; NY, USA) were eligible for study participation. Our standardized procedure for tissue procurement and processing was provided to the surgeons and the pathology team prior to initiation of this study. WAT surrounding the prostate was removed en bloc with the prostate and then processed in our tissue procurement core. The institutional review boards of MSK and Weill Cornell Medical College approved the study protocol. Each study participant provided written informed consent.
Tumor Clinicopathology and Clinical Characteristics
Clinicopathologic data were systematically extracted from medical records and independent data review was conducted by two physicians (AG and NI). Height (in meters) and weight (kg) were recorded at the time of surgery and used to calculate BMI as kg/m2. BMI was categorized according to the standard World Health Organization definition as underweight or normal (<25.0 kg/m2), overweight (25.0–29.9 kg/m2), or obese (>30 kg/m2). Preoperative prostate specific antigen (PSA) levels were measured as standard clinical practice and recorded. Gleason scores were categorized by Gleason Grade Groups as follows: (Gleason score ≤ 6 - group I; 3+4=7 - group II; 4+3=7 - group III; 4+4=8 - group IV; and 9–10 - group V).18 Surgical margin status (positive vs. negative) was also recorded.
Detection and Assessment of Periprostatic WAT Inflammation
Formalin-fixed paraffin-embedded (FFPE) blocks of periprostatic fat were prepared immediately following RP. Periprostatic WAT inflammation was defined by the presence of CLS in periprostatic fat (CLS-P). For each patient, a total of 5 periprostatic WAT sections were obtained from FFPE blocks consistent with previous human studies.15,19,20 All sections were immunostained for CD68, a macrophage marker (mouse monoclonal KP1 antibody; Dako; dilution 1:4,000, Carpenteria, California, USA), as previously described.15,19,20 The anti-CD68 stained sections were examined by the study pathologist (D.G.) using light microscopy to detect the presence or absence of CLS-P and the number of CLS-P per slide was recorded.15,19,20 The study pathologist was blinded to the clinical status of the participants. Digital photographs of each slide were generated and WAT area was measured with Image J Software (NIH, Bethesda, MD). The severity of WAT inflammation was quantified as number of CLS-P per square centimeter of WAT (CLS-P/cm2). To assess the proliferative state of macrophages within CLS-P, immunohistochemistry for Ki67 was carried out using mouse monoclonal antibody clone MIB-1 (Dako; catalog #M7240; dilution 1: 100).
Adipocyte Diameter Measurement
Measurement of adipocyte diameters has been described previously.19 Briefly, periprostatic fat H&E sections were photographed at 20× using an Olympus BX50 microscope and MicroFire digital camera (Optronics). From the digitized images, mean diameters were calculated by measuring 30 or more individual adipocytes for each patient using the linear dimensional tool in the Canvas 11 Software (ACD Systems International, Inc., Victoria, British Columbia, Canada).
Systemic (Host) Correlates
A 30 mL fasting blood sample was obtained on the morning of RP. Blood was separated into serum and plasma by centrifugation within 3 hours of collection and stored at −80°C until analysis. Plasma levels of glucose (BioAssay Systems, Hayward, CA), estradiol (Calbiotech, Spring Valley, CA) and insulin (Mercodia, Uppsala, Sweden), leptin, testosterone, sex hormone-binding globulin (SHBG), adiponectin, high sensitivity CRP (hsCRP), and IL-6 (R&D Systems, Minneapolis, MN) were measured by enzyme-linked immunosorbent assay (ELISA). Samples were run in duplicate. Serum levels of total, HDL, and LDL cholesterol, triglycerides, and preoperative PSA were determined in the clinical chemistry laboratory at MSK.
Statistical Analyses
The sample size of 156 patients was determined to ensure at least 80% power to detect a difference of 0.25 in proportion of patients with CLS-P between patients with higher Gleason grade grouping tumors (IV or V) and patients with lower Gleason Grade grouping tumors (I, II, or III), assuming similar proportion of subjects with CLS-P in the two Gleason grade groupings, at a significance level of 0.05 using the two-sided Fisher’s exact test. The calculation also assumed that at least 52 men would have higher grade tumors (IV or V). Demographic and disease characteristics are reported by periprostatic WAT inflammation status – CLS-P positive (CLS-P+) vs. CLS-P negative (CLS-P-) and compared using Chi-square or Fisher’s exact test for categorical variables and Wilcoxon rank-sum test for continuous variables. The association between adipocyte diameter and BMI was examined using Spearman’s method. Differences in the level of a blood biomarker between CLS-P+ and CLS-P− subjects were examined using Wilcoxon rank-sum test. In addition, we examined the association between CLS-P and tumor pathology parameters of interest, including Gleason grade and positive surgical margins, adipocyte diameter and systemic correlates adjusted for BMI using the multivariable generalized linear regression analyses. Specifically, multivariable logistic regression was used when the dependent variable was binary and multivariable linear regression was used when the dependent variable was continuous. Appropriate transformation was applied to continuous variables where appropriate to ensure the underlying model assumptions were met. Potential heteroscedasticity of continuous variables were examined graphically and no heteroscedasticity was observed in our data. All statistical tests were two tailed and P<0.05 was considered statistically significant. P-values were not adjusted for multiple comparisons. Statistical analyses were conducted using R 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
From November 2011 to August 2015, a total of 169 men (median age 62 years, range: 39 –77) were enrolled. Participant characteristics are described in Table 1. Periprostatic WAT inflammation, defined by the presence of CLS-P (Fig. 1A), was present in 84 of 169 (49.7%) men. Notably, some macrophages within CLS-P were Ki67 positive indicating a proliferative state (Fig. 1B).
Table 1.
Clinicopathologic features stratified by WAT inflammation
| Variables | All patients (n=169) |
CLS-P − (n=85) |
CLS-P + (n=84) |
P* |
|---|---|---|---|---|
| Age | ||||
| Median (IQR) | 62 (57, 67) | 63 (58,68) | 61 (55,65) | 0.01 |
| BMI | ||||
| Median (IQR) | 28.3 (26.1, 31.9) | 27.8 (25.8,30.4) | 29.3 (26.5,33.5) | 0.02 |
| Race, n (%) | ||||
| AA | 6 (4%) | 1 (1%) | 5 (7%) | |
| Asian | 6 (4%) | 2 (3%) | 4 (5%) | |
| White | 139 (92%) | 74 (96%) | 65 (88%) | 0.12 |
| Unreported | 18 (11%) | 8 (9%) | 10 (12%) | 0.63 |
| Gleason Grade Group, n (%) | ||||
| I, II, III | 135(80%) | 74 (87%) | 61 (73%) | |
| IV, V | 34(20%) | 11 (13%) | 23 (27%) | 0.02 |
| T Stage, n (%) | ||||
| pT2+ | 4 (2%) | 1 (1%) | 3 (4%) | |
| pT2a | 11 (7%) | 7 (8%) | 4 (5%) | |
| pT2b | 61 (36%) | 31 (36%) | 30 (36%) | |
| pT3a | 65 (38%) | 37 (44%) | 28 (33%) | |
| pT3b | 20 (12%) | 8 (9%) | 12 (14%) | |
| pT4 | 8 (5%) | 1 (1%) | 7 (8%) | 0.13 |
| LN Metastasis, n (%) | ||||
| Negative | 123 (76%) | 66 (80%) | 57 (71%) | |
| Positive | 39 (24%) | 16 (20%) | 23 (29%) | 0.20 |
| Unsampled | 7 (4%) | 3 (4%) | 4 (5%) | 0.72 |
| Seminal Vesicle Invasion, n (%) | ||||
| Negative | 144 (85%) | 76 (89%) | 68 (81%) | |
| Positive | 25 (15%) | 9 (11%) | 16 (19%) | 0.14 |
| Extracapsular Extension, n (%) | ||||
| Negative | 60 (36%) | 29 (34%) | 31 (37%) | |
| Positive | 109 (64%) | 56 (66%) | 53 (63%) | 0.75 |
| Surgical Margins, n (%) | ||||
| Negative | 132(78%) | 72 (85%) | 60 (71%) | |
| Positive | 37(22%) | 13 (15%) | 24 (29%) | 0.04 |
| HTN, n (%) | ||||
| No | 91 (53.85%) | 46 (54.12%) | 45 (53.57%) | |
| Yes | 78 (46.15%) | 39 (45.88%) | 39 (46.43%) | 1.00 |
| DM, n (%) | ||||
| No | 148 (87.57%) | 76 (89.41%) | 72 (85.71%) | |
| Yes | 21 (12.43%) | 9 (10.59%) | 12 (14.29%) | 0.49 |
| Hyperlipidemia, n (%) | ||||
| No | 77 (45.56%) | 41 (48.24%) | 36 (42.86%) | |
| Yes | 92 (54.44%) | 44 (51.76%) | 48 (57.14%) | 0.54 |
| NSAIDs, n (%) | ||||
| No | 103 (60.95%) | 55 (64.71%) | 48 (57.14%) | |
| Yes | 66 (39.05%) | 30 (35.29%) | 36 (42.86%) | 0.35 |
| Statins, n (%) | ||||
| No | 96 (56.8%) | 46 (54.12%) | 50 (59.52%) | |
| Yes | 73 (43.2%) | 39 (45.88%) | 34 (40.48%) | 0.54 |
| Steroids, n (%) | ||||
| No | 166 (98.22%) | 83 (97.65%) | 83 (98.81%) | |
| Yes | 3 (1.78%) | 2 (2.35%) | 1 (1.19%) | 1.00 |
Abbreviation: WAT, white adipose tissue; IQR, interquartile range; BMI, body mass index; AA, African-American; LN, lymph node; T, tumor
P-values for associations with age and BMI were based on Wilcoxon rank sum test. All the others were based on Fisher’s exact test.
Figure 1. Periprostatic WAT inflammation and BMI.
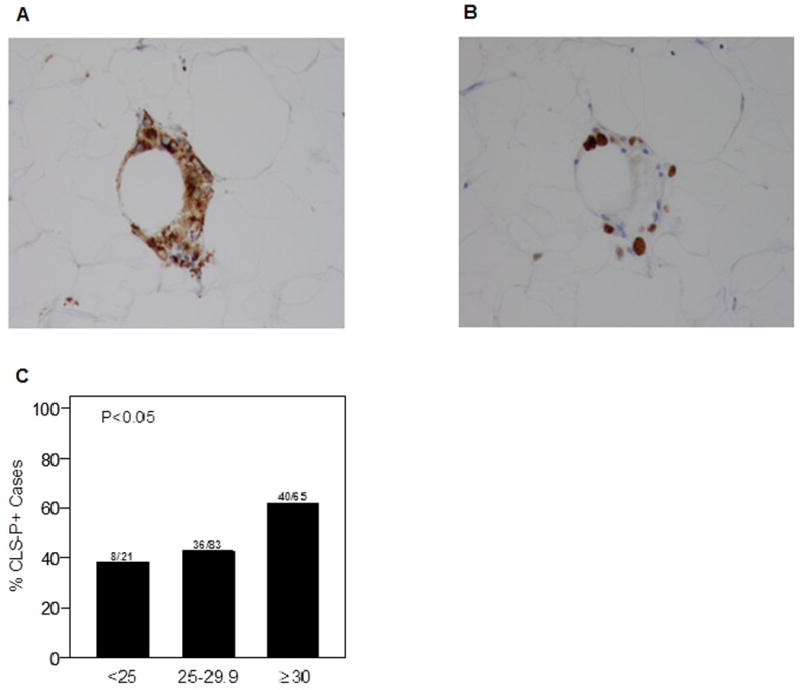
A. CD68-stained slide showing CLS-P (200×). B. Ki-67 stained slide demonstrating the proliferative state of a proportion of CLS-P associated macrophages (200×). C. Prevalence of CLS-P is higher in overweight and obese patients (P<0.05).
Tumor Pathology and Clinical Correlates of Periprostatic WAT Inflammation
Median BMIs were 29.3 (interquartile range: 26.5, 33.5) and 27.8 (interquartile range: 25.8, 30.4) in men with and without periprostatic WAT inflammation, respectively (P=0.02). Overall, 40 of the 65 men classified as obese (61.5%) and 36 of the 83 classified as overweight (43.4%) had periprostatic WAT inflammation; whereas 8 of the 21 men with normal BMI (38.1%) had periprostatic WAT inflammation (Fig. 1C).
Higher Gleason grade tumors were associated with periprostatic WAT inflammation, as evidenced by the diagnosis of higher grade tumors (Gleason grade grouping IV or V) in 23 of 84 men with CLS-P compared to 11 of 85 of those without CLS-P (P=0.02; Fig. 2A). We examined the correlation between CLS-P (presence/absence) and BMI, both as a continuous and as a categorical variable (Table 1, Figure 2). In contrast, we did not observe a statistically significant association between BMI and the diagnosis of higher Gleason grade tumors (P=0.13; Fig. 2B). In the multivariable analysis, the association between periprostatic WAT inflammation and higher Gleason grade grouping (IV or V) remained significant (P=0.04) after adjusting for BMI. Positive surgical margins were seen in a higher proportion of patients with periprostatic WAT inflammation than those without WAT inflammation (P=0.04; Fig. 2C). Elevated BMI did not associate with surgical margin status (P=0.29; Fig. 2D). However, after adjusting for BMI, the association between periprostatic WAT inflammation and positive surgical margins was not significant (P=0.06). Levels of PSA were lower in men with elevated BMI (P=0.01). In contrast, PSA levels were not significantly different between patients with and without periprostatic WAT inflammation (P=0.14).
Figure 2. Periprostatic WAT inflammation, tumor grade, and surgical margins.
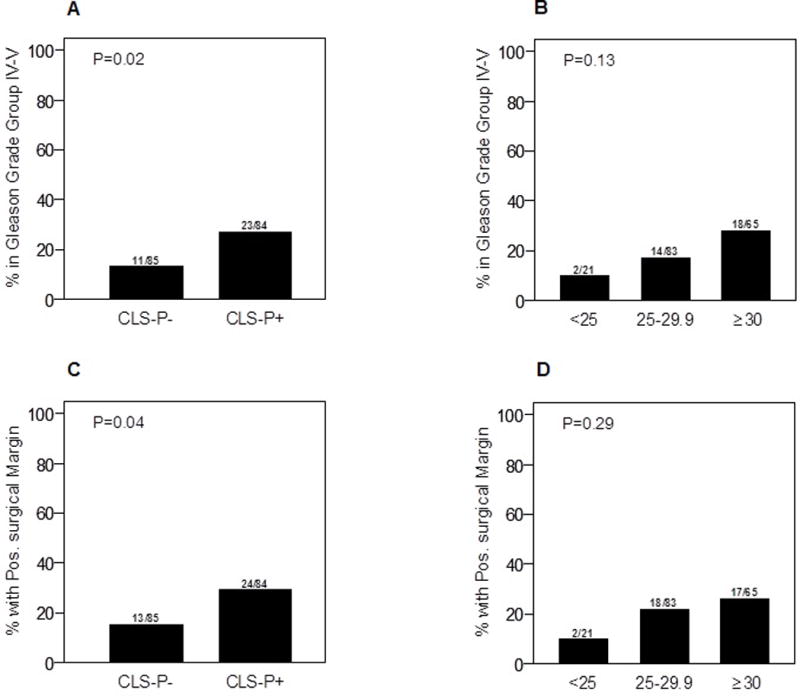
A. Periprostatic WAT inflammation was associated with higher Gleason grade grouping (IV and V; P=0.02). B. BMI is not significantly associated with higher Gleason grade grouping (P=0.13). C. Periprostatic WAT inflammation is associated with positive surgical margins (P=0.04). D. BMI is not associated with surgical margin status (P=0.29).
Adipocyte Diameter and Periprostatic WAT Inflammation
Periprostatic adipocyte diameter correlated with BMI (Spearman’s ρ=0.17; P=0.03; Fig. 3A). Median adipocyte diameter was 102.7 (range: 95.4, 110.4) microns in patients with periprostatic WAT inflammation compared to 98.1 (range: 90.4, 105.8) microns in patients without WAT inflammation (P=0.004; Fig. 3B). The association between larger adipocyte diameter and periprostatic WAT inflammation remained significant after adjustment for BMI (P=0.009).
Figure 3. Adipocyte diameter, BMI, and periprostatic WAT inflammation.
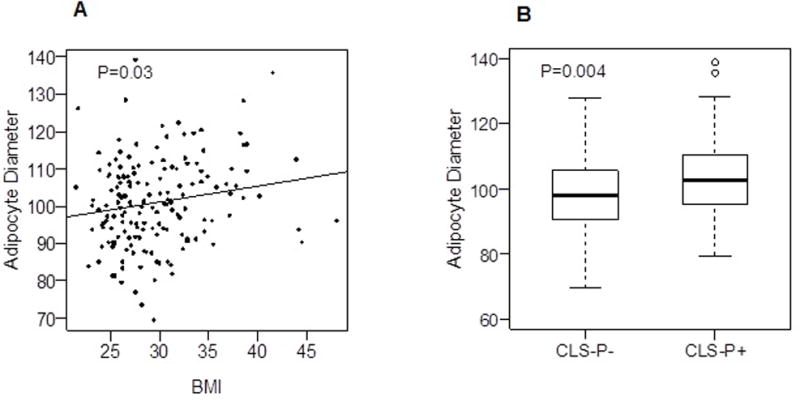
A. A positive correlation was observed between adipocyte diameter and BMI (ρ = 0.17, P = 0.03). B. Patients with periprostatic WAT inflammation have larger mean adipocyte diameter in periprostatic fat (P = 0.004).
Systemic Host Factors and Periprostatic WAT Inflammation
The differences in host factors on the basis of WAT inflammation are shown in Table 2. Periprostatic WAT inflammation was associated with elevated leptin/adiponectin ratio, insulin, and insulin resistance (HOMA2-IR; all P’s<0.05); adiponectin levels were lower in patients with periprostatic WAT inflammation (P=0.01). The association between periprostatic WAT inflammation and insulin resistance remained significant after adjusting for BMI (P=0.04). Triglyceride levels were higher, whereas HDL cholesterol was lower in patients with periprostatic WAT inflammation. The severity of WAT inflammation, quantified as CLS-P per square centimeter of WAT (CLS/cm2), was associated with reduced levels of adiponectin and HDL cholesterol, and elevated triglycerides and insulin (all P’s<0.05). Higher levels of triglycerides and other circulating markers, including insulin, leptin, and hsCRP, were observed in men with high Gleason grade tumors (P’s<0.05).
Table 2.
Measured blood variables stratified by WAT inflammation (n=154)
| Variables Median (range) |
CLS-P − (n=75) |
CLS-P + (n=79) |
P |
|---|---|---|---|
| Preoperative PSA* | 5.79 (0.05,22.47) | 5.90 (0.11,40.70) | 0.14 |
| Testosterone | 5.81 (0.67,50.37) | 5.98 (1.13,40.29) | 0.81 |
| Estradiol | 30.15 (8.42,155.80) | 30.58 (5.66,90.19) | 0.46 |
| SHBG | 43.92 (9.09,96.51) | 42.68 (16.90,127.05) | 0.20 |
| hsCRP (ng/mL) | 1.09 (0.19,12.69) | 2.06 (0.12,22.47) | 0.06 |
| Leptin (pg/mL) | 5.04 (0.56,34.58) | 7.54 (0.61,41.88) | 0.11 |
| Adiponectin (μg/mL) | 7.58 (1.62,25.06) | 5.64 (1.46,21.34) | 0.01 |
| Leptin/Adiponectin ratio | 0.82 (0.02,19.01) | 1.08 (0.04,11.91) | 0.03 |
| Glucose (mg/dL) | 98.74 (73.22,165.94) | 101.69 (40.21,282.30) | 0.18 |
| Insulin (mU/L) | 6.01 (1.02,25.26) | 6.86 (2.33,41.03) | 0.02 |
| HOMA2-IR | 0.68 (0.11,3.17) | 0.81 (0.26,4.60) | 0.01 |
| Total Cholesterol | 178 (123,270) | 187 (106,282) | 0.83 |
| LDL Cholesterol | 110 (44,183) | 110 (49,178) | 0.99 |
| HDL Cholesterol | 50 (31,92) | 46 (26,90) | 0.03 |
| Triglycerides | 100 (48,363) | 121 (44,457) | 0.02 |
PSA was measured preoperatively and was available for 163 of the 169 men (CLS-P − n= 81, CLS-P + n=82)
Abbreviation: WAT, white adipose tissue; PSA, prostate-specific antigen; SHBG, sex hormone-binding globulin; hsCRP, high sensitivity C-reactive protein; HOMA2-IR, homeostasis model assessment 2 – insulin resistance; LDL, low-density lipoprotein; HDL, high-density lipoprotein
Discussion
Periprostatic WAT inflammation, manifested as CLS-P, was present in ~50% of men undergoing RP. The presence of periprostatic WAT inflammation was more common among overweight and obese men but was also detected in ~40% men with BMI <25. Importantly, periprostatic WAT inflammation was associated with more aggressive disease at diagnosis and systemic (host) metabolic dysregulation, even after adjustment for BMI. Taken together, these findings demonstrate that periprostatic WAT inflammation is 1) present in a subset of men with prostate cancer, 2) associated with changes in systemic factors known to promote prostate cancer, and 3) remains a correlate of high grade prostate cancer after adjusting for BMI.
We and others have previously reported that the presence of WAT inflammation is associated with worse prognosis in patients with breast or tongue cancer.15–17 Consistent with our findings in periprostatic fat, WAT inflammation was present in 40%–50% of patients with breast or tongue cancer.17,19,20 Furthermore, as we observed in the setting of breast or tongue cancer, a proportion of normal BMI individuals had periprostatic WAT inflammation while some men with elevated BMI did not have inflammation. Importantly, periprostatic WAT inflammation was associated with higher-grade prostate tumors, while elevated BMI did not correlate with high-grade prostate cancer in this cohort. Thus, periprostatic WAT inflammation was a more sensitive indicator of high grade prostate cancer than BMI in this study.
Here we showed that periprostatic adipocyte hypertrophy was associated with the presence of WAT inflammation, consistent with our findings in other fat depots.19–21 The presence of periprostatic WAT inflammation is likely to be a consequence of hypoxia and possibly endoplasmic reticulum stress. Adipose tissue hypoxia occurs as the fat pad outgrows its supportive vasculature. Enlarged adipocytes may be more sensitive to cell death under hypoxic conditions. Additionally, endoplasmic reticulum stress in hypertrophic adipocytes leads to apoptosis, which can, in turn, elicit an inflammatory response including monocyte recruitment, differentiation and the formation of CLS.22 The accumulation of macrophages within WAT reflects both recruitment and local proliferation.23 In this study, we demonstrate that macrophage proliferation occurs within CLS in periprostatic fat. Additional studies are warranted to determine the mechanisms underlying macrophage proliferation within CLS and to establish whether targeting macrophage recruitment and/or proliferation would be clinically beneficial in patients with prostate cancer.
Periprostatic WAT inflammation is associated with systemic changes that may also contribute to the development of high grade prostate cancer. Specifically, periprostatic inflammation was associated with insulin resistance, dyslipidemia, and altered adipokine levels. Deregulated insulin signaling may play an important role in the development of prostate cancer. Hyperinsulinemia promotes proliferation and inhibits apoptosis of prostate cancer cells24,25 and is associated with de novo steroidogenesis, which can promote prostate neoplasia via activation of the androgen receptor.26 In addition to insulin resistance, lipid derangements are associated with increased risk of prostate cancer recurrence.27 In our study, periprostatic WAT inflammation was associated with higher levels of circulating triglycerides and lower HDL cholesterol. Finally, alterations in adipokines can impact prostate cancer risk. For example, elevated adiponectin levels are associated with a lower risk of developing high grade prostate cancer.28 Here we found that men without periprostatic WAT inflammation have higher adiponectin levels. Thus, our findings collectively suggest that periprostatic WAT inflammation is associated with systemic metabolic alterations that are known to increase the risk of prostate cancer development and progression.
Our study was strengthened by standardized tissue, plasma, and serum collection and the well-established methodology for detection of CLS via morphologic identification by a histopathologist. Nonetheless, this is a single center cross-sectional study with findings that warrant further investigation in independent populations. Additionally, some studies have demonstrated a correlation between elevated BMI and high grade prostate cancer29 while other studies have not supported this observation.30 In our cohort, we did not observe an association between BMI and high grade prostate cancer. In contrast, periprostatic WAT inflammation associated with high grade tumors. Collectively, these findings underscore the limitations of BMI in characterizing the obesity-cancer relationship.
In conclusion, periprostatic WAT inflammation was frequently present in the men in this cohort and was associated with higher grade prostate cancers and systemic metabolic dysfunction. Our findings demonstrate a potential pathophysiologic state linking metabolic dysfunction to high grade prostate cancer that includes inflammation in the periprostatic fat locally and its correlates in the blood systemically. Many of these alterations, such as insulin resistance and dyslipidemia, are associated with the development and progression of prostate cancer.
These findings support further investigation of WAT inflammation as a potential marker of cancer risk and progression and as a novel target for mechanistically-based behavioral, dietary, and/or pharmacologic intervention strategies to prevent prostate cancer and improve outcomes. These efforts would be augmented by the development of non-invasive strategies that can accurately assess periprostatic WAT inflammation to aid in patient selection and monitor the efficacy of potential anti-inflammatory therapies.
Acknowledgments
We wish to thank all the patients who generously volunteered to participate in this study.
Funding support: This work was supported by the Prostate Cancer Foundation (Movember-Challenge Award), Patricia and William Kleh, and Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748). LWJ is supported by grants from the National Cancer Institute and Aktiv Against Cancer.
Footnotes
Conflict of interest disclosures: None
References
- 1.Discacciati A, Orsini N, Wolk A. Body mass index and incidence of localized and advanced prostate cancer–a dose-response meta-analysis of prospective studies. Ann Oncol. 2012;23(7):1665–1671. doi: 10.1093/annonc/mdr603. [DOI] [PubMed] [Google Scholar]
- 2.Cao Y, Ma J. Body mass index, prostate cancer-specific mortality, and biochemical recurrence: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2011;4(4):486–501. doi: 10.1158/1940-6207.CAPR-10-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang X, Zhou G, Sun B, Zhao G, Liu D, Sun J, et al. Impact of obesity upon prostate cancer-associated mortality: A meta-analysis of 17 cohort studies. Oncology letters. 2015;9(3):1307–1312. doi: 10.3892/ol.2014.2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ribeiro R, Monteiro C, Catalan V, Hu P, Cunha V, Rodriguez A, et al. Obesity and prostate cancer: gene expression signature of human periprostatic adipose tissue. BMC medicine. 2012;10:108. doi: 10.1186/1741-7015-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finley DS, Calvert VS, Inokuchi J, Lau A, Narula N, Petricoin EF, et al. Periprostatic adipose tissue as a modulator of prostate cancer aggressiveness. The Journal of urology. 2009;182(4):1621–1627. doi: 10.1016/j.juro.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 6.Laurent V, Guerard A, Mazerolles C, Le Gonidec S, Toulet A, Nieto L, et al. Periprostatic adipocytes act as a driving force for prostate cancer progression in obesity. Nature communications. 2016;7:10230. doi: 10.1038/ncomms10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang T, Tseng C, Zhang Y, Sirin O, Corn PG, Li-Ning-Tapia EM, et al. CXCL1 mediates obesity-associated adipose stromal cell trafficking and function in the tumour microenvironment. Nature communications. 2016;7:11674. doi: 10.1038/ncomms11674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Roermund JG, Hinnen KA, Tolman CJ, Bol GH, Witjes JA, Bosch JL, et al. Periprostatic fat correlates with tumour aggressiveness in prostate cancer patients. BJU international. 2011;107(11):1775–1779. doi: 10.1111/j.1464-410X.2010.09811.x. [DOI] [PubMed] [Google Scholar]
- 9.Woo S, Cho JY, Kim SY, Kim SH. Periprostatic fat thickness on MRI: correlation with Gleason score in prostate cancer. AJR American journal of roentgenology. 2015;204(1):W43–47. doi: 10.2214/AJR.14.12689. [DOI] [PubMed] [Google Scholar]
- 10.Allott EH, Howard LE, Song HJ, Sourbeer KN, Koontz BF, Salama JK, et al. Racial differences in adipose tissue distribution and risk of aggressive prostate cancer among men undergoing radiotherapy. Cancer Epidemiol Biomarkers Prev. 2014;23(11):2404–2412. doi: 10.1158/1055-9965.EPI-14-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 12.Iyengar NM, Hudis CA, Dannenberg AJ. Obesity and cancer: local and systemic mechanisms. Annual review of medicine. 2015;66:297–309. doi: 10.1146/annurev-med-050913-022228. [DOI] [PubMed] [Google Scholar]
- 13.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. Journal of lipid research. 2005;46(11):2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Shapiro H, Pecht T, Shaco-Levy R, Harman-Boehm I, Kirshtein B, Kuperman Y, et al. Adipose tissue foam cells are present in human obesity. J Clin Endocrinol Metab. 2013;98(3):1173–1181. doi: 10.1210/jc.2012-2745. [DOI] [PubMed] [Google Scholar]
- 15.Iyengar NM, Zhou XK, Gucalp A, Morris PG, Howe LR, Giri DD, et al. Systemic Correlates of White Adipose Tissue Inflammation in Early-Stage Breast Cancer. Clin Cancer Res. 2016;22(9):2283–2289. doi: 10.1158/1078-0432.CCR-15-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koru-Sengul T, Santander AM, Miao F, Sanchez LG, Jorda M, Gluck S, et al. Breast cancers from black women exhibit higher numbers of immunosuppressive macrophages with proliferative activity and of crown-like structures associated with lower survival compared to non-black Latinas and Caucasians. Breast Cancer Res Treat. 2016;158(1):113–126. doi: 10.1007/s10549-016-3847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iyengar NM, Ghossein RA, Morris LG, Zhou XK, Kochhar A, Morris PG, et al. White adipose tissue inflammation and cancer-specific survival in patients with squamous cell carcinoma of the oral tongue. Cancer. 2016 doi: 10.1002/cncr.30251. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Epstein JI, Zelefsky MJ, Sjoberg DD, Nelson JB, Egevad L, Magi-Galluzzi C, et al. A Contemporary Prostate Cancer Grading System: A Validated Alternative to the Gleason Score. Eur Urol. 2016;69(3):428–435. doi: 10.1016/j.eururo.2015.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris PG, Hudis CA, Giri D, Morrow M, Falcone DJ, Zhou XK, et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res (Phila) 2011;4(7):1021–1029. doi: 10.1158/1940-6207.CAPR-11-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iyengar NM, Morris PG, Zhou XK, Gucalp A, Giri D, Harbus MD, et al. Menopause is a determinant of breast adipose inflammation. Cancer Prev Res (Phila) 2015;8(5):349–358. doi: 10.1158/1940-6207.CAPR-14-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subbaramaiah K, Howe LR, Bhardwaj P, Du B, Gravaghi C, Yantiss RK, et al. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res (Phila) 2011;4(3):329–346. doi: 10.1158/1940-6207.CAPR-10-0381. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Kawasaki N, Asada R, Saito A, Kanemoto S, Imaizumi K. Obesity-induced endoplasmic reticulum stress causes chronic inflammation in adipose tissue. Scientific reports. 2012;2:799. doi: 10.1038/srep00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amano SU, Cohen JL, Vangala P, Tencerova M, Nicoloro SM, Yawe JC, et al. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell metabolism. 2014;19(1):162–171. doi: 10.1016/j.cmet.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hammarsten J, Hogstedt B. Hyperinsulinaemia: a prospective risk factor for lethal clinical prostate cancer. Eur J Cancer. 2005;41(18):2887–2895. doi: 10.1016/j.ejca.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Cox ME, Gleave ME, Zakikhani M, Bell RH, Piura E, Vickers E, et al. Insulin receptor expression by human prostate cancers. The Prostate. 2009;69(1):33–40. doi: 10.1002/pros.20852. [DOI] [PubMed] [Google Scholar]
- 26.Lubik AA, Gunter JH, Hendy SC, Locke JA, Adomat HH, Thompson V, et al. Insulin increases de novo steroidogenesis in prostate cancer cells. Cancer Res. 2011;71(17):5754–5764. doi: 10.1158/0008-5472.CAN-10-2470. [DOI] [PubMed] [Google Scholar]
- 27.Allott EH, Howard LE, Cooperberg MR, Kane CJ, Aronson WJ, Terris MK, et al. Serum lipid profile and risk of prostate cancer recurrence: Results from the SEARCH database. Cancer Epidemiol Biomarkers Prev. 2014;23(11):2349–2356. doi: 10.1158/1055-9965.EPI-14-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H, Stampfer MJ, Mucci L, Rifai N, Qiu W, Kurth T, et al. A 25-year prospective study of plasma adiponectin and leptin concentrations and prostate cancer risk and survival. Clinical chemistry. 2010;56(1):34–43. doi: 10.1373/clinchem.2009.133272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez C, Freedland SJ, Deka A, Jacobs EJ, McCullough ML, Patel AV, et al. Body mass index, weight change, and risk of prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev. 2007;16(1):63–69. doi: 10.1158/1055-9965.EPI-06-0754. [DOI] [PubMed] [Google Scholar]
- 30.Giovannucci E, Rimm EB, Liu Y, Leitzmann M, Wu K, Stampfer MJ, et al. Body mass index and risk of prostate cancer in U.S. health professionals. J Natl Cancer Inst. 2003;95(16):1240–1244. doi: 10.1093/jnci/djg009. [DOI] [PubMed] [Google Scholar]


