Abstract
Background and Aims
To evaluate the feasibility and early safety of catheter-directed irreversible electroporation (IRE) of the normal common bile duct (CBD) in swine.
Methods
IRE (2000 V, 90 pulses, 100 μs pulse) was performed in the CBD of 6 Yorkshire pigs using a catheter electrode under endoscopic guidance. Ductal patency was assessed with immediate retrograde cholangiography and contrast-enhanced CT imaging at 1 or 7 days after treatment. Animals were killed at either 1 (n=4, 2 ablations/animal) or 7 days (n=2, 1 ablation/animal) after treatment. The biliary tract was extracted en bloc and length of the ablation along CBD mucosa was measured. The depth of ablation was quantified using H&E stained cross-sections of the treated CBD wall. Single-sample hypothesis testing was performed to verify whether depth of ablation in the CBD was representative outcome of IRE treatment.
Results
IRE of the CBD did not result in perforation or obstruction of the organ at 1 or 7 days after treatment. The length of ablation along the CBD mucosa was 17.27 ± 5.55 mm on Day 1 samples, and transmural ablation of the CBD wall was representative outcome of the treatment (7/8 samples, p<0.05). Day 1 samples demonstrated loss of epithelium, transmural necrosis, with preservation of lumen integrity. Day 7 samples demonstrated re-epithelialization, with diffuse transmural fibrosis of the CBD wall. These findings were absent from sham tissue samples.
Conclusions
Intraluminal catheter-directed IRE is feasible and safe for the full thickness ablation of the normal porcine CBD without impacting lumen patency up to 1 week after treatment.
Introduction
Endoscopic or percutaneous focal ablation has been piloted for relieving stent in-growth and debulking of biliary tract tumors (1–2), with the goal of increasing the duration of ductal patency. However, limited efficacy and the potential risk of treatment related adverse events have impeded broader clinical use (3–5).
Background
Percutaneous Irreversible Electroporation (IRE) of peri-biliary tumors in patients has been reported to be safe (6–8), and likewise, IRE of the normal swine bile duct did not result in perforation or stricture formation (9). These features make IRE an attractive candidate for endoscopic ablation of tumors in the bile duct. However, the lack of suitable ablation catheters and experience with the technique has impeded clinical translation. The purpose of this study was to evaluate the feasibility, reproducibility and early safety of endoscopic ablation of the normal porcine common bile duct (CBD) with IRE using a catheter mounted electrode.
Materials and Methods
Prototype Catheter
An electrode (20 mm length, 7F) was fabricated in-house and mounted on a modified 240cm balloon dilation catheter (CRE Balloon Dilation Catheter, Boston Scientific, USA) to serve as a monopolar device (Figure 1) that can be used with a grounding pad return electrode for IRE of the swine CBD.
Figure 1.
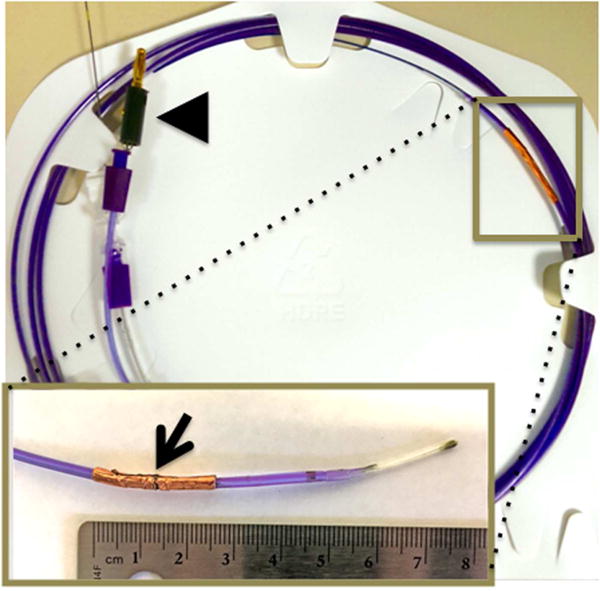
Catheter developed for endoscope guided IRE of the common bile duct. Catheter mounted electrode developed for endoscopic intraluminal IRE. Inset shows the electrode at the distal tip of the catheter (arrowhead). Arrowhead depicts the proximal tip with the connector used to connect the catheter to generator.
Treatment
Six female Yorkshire swine (30–50 kg) were used under an Institutional Animal Care and Use Committee (IACUC) approved protocol for this study. Animals were sedated and placed under general anesthesia using techniques previously described by Srimathveeravalli et al (10). The ablation catheter was placed in the CBD under endoscopic (TJF-160 VF Duodenoscope, Olympus North America, USA) and fluoroscopic guidance. Pulses from a commercial generator (Nanoknife, AngioDynamics Inc, USA) were delivered between the electrode mounted on the catheter and a grounding pad (ESRS grounding pad; Bovie Medical, USA) attached to the flank of the animal. All treatments were performed with a single set of experimental parameters (2000 V, 90 pulses, 100 μs pulse). The CBD distal to the treatment site was catheterized but not exposed to pulse delivery, and was considered internal sham control. Cholangiography with contrast was performed immediately after treatment to assess duct patency. Animals received either one (n=2) or two (n=4) ablations, and were killed at 7 and 1 day after treatment respectively. Contrast enhanced CT imaging was performed in all animals before killing.
Histologic Analysis
The CBD and surrounding liver was removed en bloc and the duct was dissected longitudinally to expose the mucosal surface. High resolution photographs of the exposed mucosal surface were used to measure the length and width of the ablation. The entire treatment region along with the adjacent normal tissue was formalin-fixed, and slides were stained with hematoxylin and eosin (H&E). The histology slides from animals killed 1 day after IRE were examined to identify layers of CBD wall demonstrating treatment effects and the extent of ablation in each layer was scored using a schema previously described by Srimathveeravalli et al(10). Histology samples from 7 days after treatment were evaluated for the integrity of the lumen wall, patency of the lumen and patterns of post-ablation healing.
Statistics
Single sample hypothesis testing (SPSS v22, IBM, USA) was performed on the histology scores assigned to Day 1 samples to assess whether depth of ablation was representative of treatment applied.
Results
Procedure Outcomes
IRE was successfully performed in all 6 animals without any interruption. Immediate postprocedure retrograde cholangiography showed patency of the CBD without extravasation of the contrast or ductal dilation (Figure 2). Follow-up CT imaging on Day 7 showed no obvious signs of inflammation, dilation or perforation of the CBD (Supplemental Figure 1).
Figure 2.
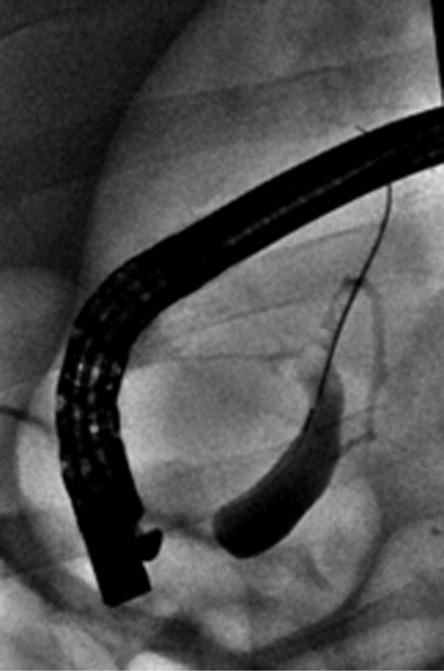
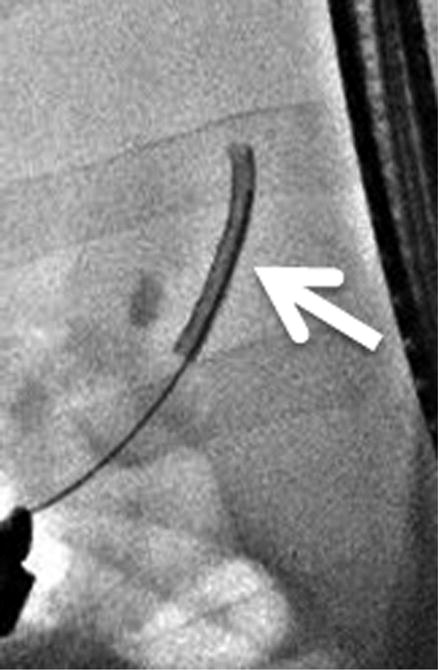
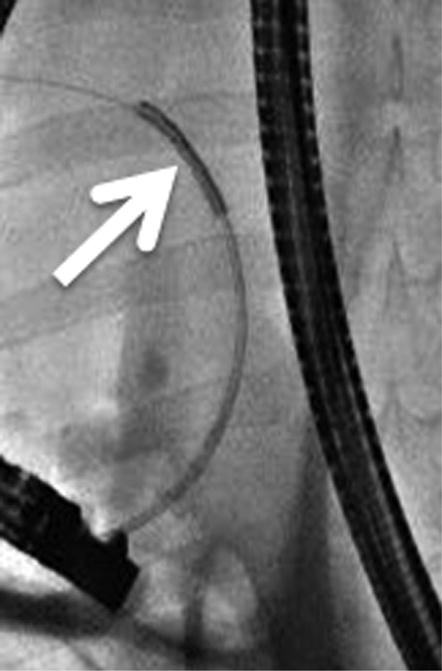
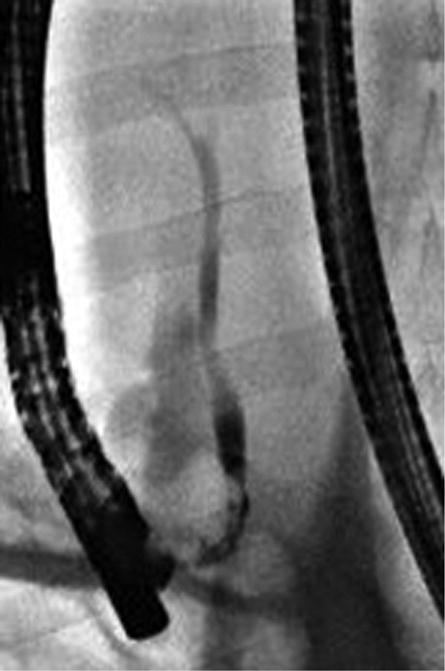
Intra-procedural cholangiography. A, Cholangiography was performed after cannulation of the guidewire into common bile duct. B, C, The IRE catheter was inserted into the common bile duct through the inner lumen of the endoscope. Arrows indicate the location of the electrode at the proximal and distal sites of treatment. D, The IRE catheter was removed after completion of pulse delivery. Retrograde cholangiography shows the immediate post-treatment patency of the bile duct and no evidence of extravasation of contrast material.
Gross Pathology
Clearly demarcated focal lesions were observed on the mucosal surface of the CBD (Figure 3), and the mean length of the lesions (17.27 ± 5.55 mm) was slightly smaller than the length of the electrode mounted on the catheter. There were no observable changes in the vasculature surrounding the duct.
Figure 3.
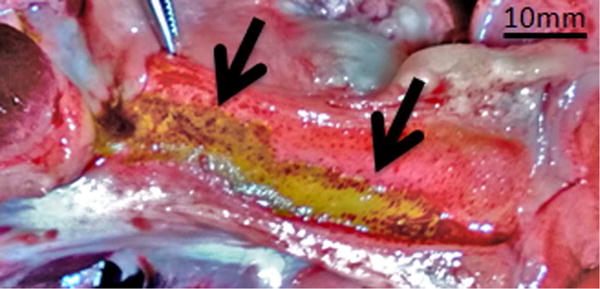
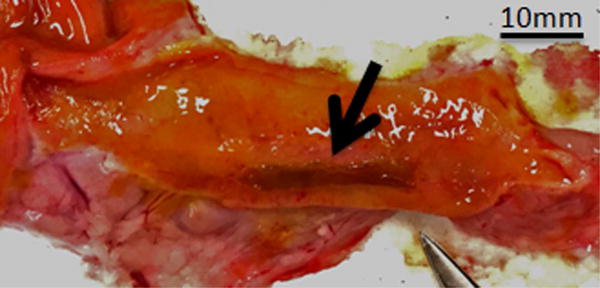
Gross appearance of the bile duct mucosa after treatment with IRE. A, Specimen from animal killed 1 day after treatment. Two distinct locations of treatment can be observed (arrows). Mucosa of the treated regions has a yellow tinge and is surrounded with brown/reddish region. B, Specimen from animal killed 7 days after treatment. Mucosa at the site of treatment appears dark brown (arrow) when compared with surrounding normal bile duct.
Histopathology
Day 1 samples revealed epithelial sloughing and complete transmural cell necrosis (Table 1). There was abundant presence of inflammatory cells, and edema of the ductal wall (Figure 4A and B). Collagen matrix within the treatment regions demonstrated edema related increase in spacing between fibers, but the extracellular matrix appeared intact. Tissue samples that underwent sham treatment had the histologic appearance of normal bile duct wall. Single sample hypothesis testing verified that transmural ablation was the typical outcome (7/8 samples, p<0.05) after IRE of the CBD. Day 7 samples showed mild thickening of the bile duct wall, with diffuse fibrosis, and the presence of small number of inflammatory cells (Figure 4C and D). Regeneration of the epithelium was observed within the treatment region, with occasional regions of mucosal ulceration. Lumen diameter in treated regions appeared no different when compared to adjacent untreated normal duct.
Table 1.
Details of animal assignment, length of the ablation along the mucosa and the depth of ablation penetration within the bile duct wall for all treatments.
| Animal no. | Killed | Ablation No. | Gross measurement Length*Width (mm) | Pathology findings
|
|||
|---|---|---|---|---|---|---|---|
| Depth of necrosis in ductal wall
|
|||||||
| Mucosa | Transmural | Periductal tissue | Score | ||||
| 1 | 24 | 1 | 23.60*5.75 | Complete | Complete | Complete | 4 |
| hours | 2 | 16.59*6.46 | Complete | Complete | Complete | 4 | |
|
| |||||||
| 2 | 24 | 1 | 20.50*5.18 | Complete | Complete | Complete | 4 |
| hours | 2 | 13.20*3.72 | Complete | Complete | Complete | 4 | |
|
| |||||||
| 3 | 24 | 1 | 21.23*6.83 | Complete | Complete | Partial | 3 |
| hours | 2 | 21.38*6.79 | Partial | Partial | None | 1 | |
|
| |||||||
| 4 | 24 | 1 | 11.30*3.06 | Complete | Complete | Partial | 3 |
| hours | 2 | 7.23*4.57 | Complete | Complete | Partial | 3 | |
|
| |||||||
| 5 | 7 days | 1 | 20.44*2.78 | Complete | Complete | Partial | NA |
|
| |||||||
| 6 | 7 days | 1 | Not obvious | Complete | Complete | Partial | NA |
Figure 4.
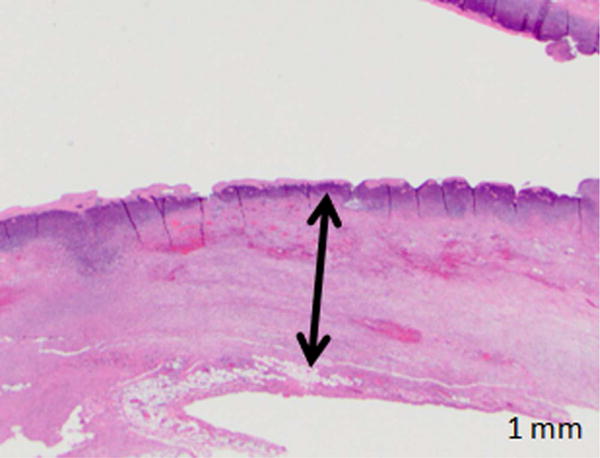
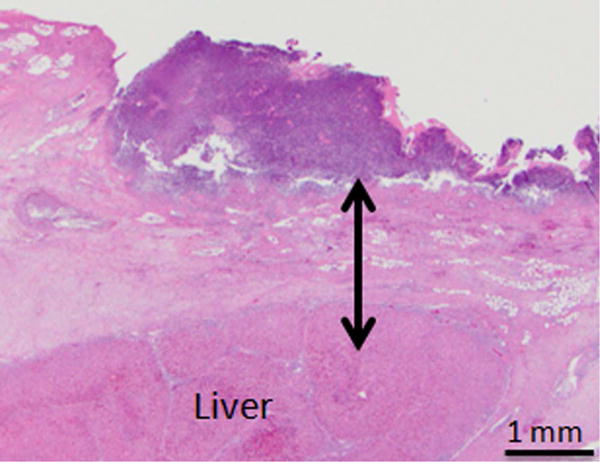
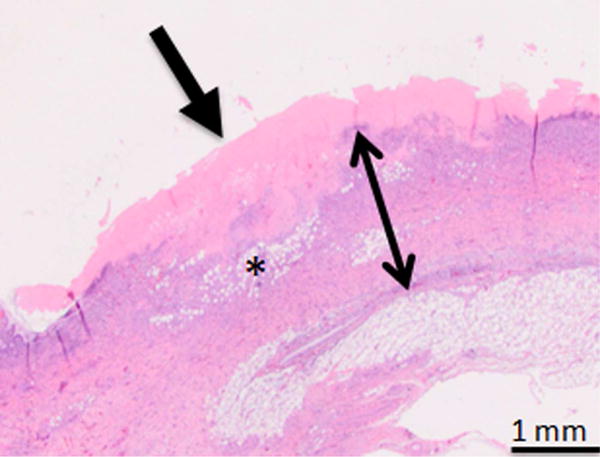
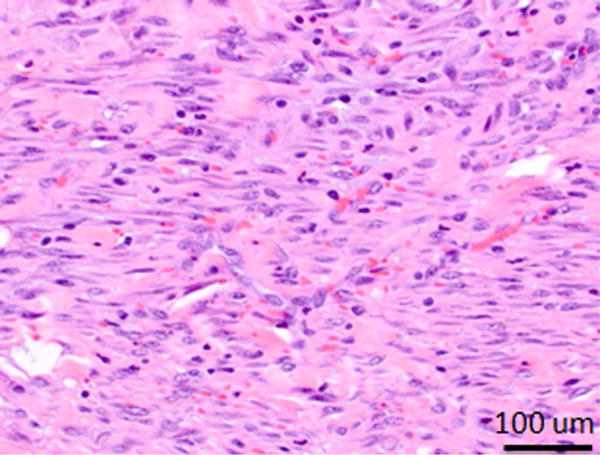
Photomicrographs from H&E stained of treated samples 1 and 7 days after treatment. A, B, Bile duct specimens acquired 1 day after endoscopy-guided IRE (A: Animal No.1 B: Animal No.2, 10× magnification). Line with arrows demarcates the extent of ablation along the bile duct wall. Bile duct epithelium was missing in all samples and was assumed to be sloughed off due to treatment. Full-thickness necrosis of the bile duct wall was observed. C, H&E staining of bile duct specimen 7 days after endoscopy-guided IRE (Animal No.5, 10× magnification). Line with arrows demarcates the extents of treatment effects in the bile duct wall. There is a focally extensive area of ulceration, characterized by necrosis and loss of the mucosa (arrow). This region was associated with superficial neutrophilic exudate covered by eosinophilic necrotic debris. The ful- thickness of the bile duct wall under the ulcerated area demonstrated edema (asterisk), and fibrosis. D, High magnification (100×) image of the region exhibiting signs of fibrosis in the treated bile duct wall.
Discussion
Radiofrequency ablation (RFA) before biliary stenting has been shown to increase the duration of patency and is also used to control tumor invasion into the stent (1,2). Despite its demonstrable benefits, deeper tissue penetration of RFA is associated with risk of perforation and cholangitis (3–5). These risks primarily arise because of the thermal mechanism of RFA and laser-ablation, whereby deeper penetration for better outcomes is difficult to achieve while maintaining a good safety profile. In comparison (Table 2), sub-millisecond long electric pulses are used to alter the transmembrane potential of cells during IRE, which induces pore formation in the cell membrane and leads to cell death from loss of homeostasis. Relatively little energy is delivered during IRE, and tissue heating during treatment is negligible. These features of IRE aid sparing of the extracellular matrix from ablation effects, allowing penetrative ablation of the biliary tract with reduced risk of adverse events. IRE ablations were also reproducible in depth and size, barring minor variations which may be related to the contact geometry of the electrode with CBD wall.
Table 2.
Comparison of IRE with other ablation modalities that have been evaluated for endoscopic biliary ablation.
| IRE | RFA | Laser | PDT | |
|---|---|---|---|---|
| Energy | Electricity (DC) | Electricity (AC) | Photonic | Photonic |
| Ablation Mechanism | Loss of cell homeostasis | Thermal damage | Thermal damage | Reactive oxygen species |
| ECM | No Effect | Denaturation | Vaporization | Unknown |
| Energy Deposited | + | ++ | +++ | ++ |
| Ablation Depth | +++ | +++ | + | ++ |
Choi et al. reported ductal narrowing without loss of patency arises within 7 days after percutaneous IRE of the bile duct (9), with no further changes in patency during an 8-week follow-up period. In comparison, endoscopy-guided intraluminal IRE did not affect ductal patency in both animals surviving 1 week after treatment. It may be concluded that the potential for stricture formation will remain low based on the histologic appearance of tissue healing, and results from prior studies (9). These results can also be compared with RFA of the bile duct where stricture formation typically occurred within 7 days of treatment (4,5).
There are several limitations to this study. Large animal models of biliary tumors do not exist, limiting the ability to assess the effect of intraluminal IRE on tumors. Pilot trials evaluating endoscopic IRE of biliary tumors in patients with long-term follow-up will be necessary to establish the efficacy of this technique, and to validate our preclinical findings. The prototype catheter will require refinements to fit through the working channel of scopes used for spyglass cholangioscopy, and future patient use.
In summary, this study demonstrates the preclinical safety of intraluminal IRE of the CBD, paving the way for evaluation of efficacy and durability of this technique in future patient study. This new approach to biliary ablation may be most relevant for tumor debulking before stenting, and for treating tumor in-growth in stents without affecting the patency of normal bile duct.
Supplementary Material
Supplementary figure 1.
CT images (A, B) 24 hours and (C, D) 7days after endoscopy-guided IRE (A, C: original image, B, D: 2.5x magnification of inset). On both 24 hours and 7 days after treatment, intact common bile ducts can be seen (arrows in C and D) without any evidence of perforation, dilation or stenosis.
Acknowledgments
Funding Sources: The authors acknowledge the support of NIH Cancer Center Support Grant (P30 CA008748) for core laboratory services that were used for the presented work.
Acronyms
- IRE
Irreversible Electroporation
- CBD
Common Bile Duct
- IACUC
Institutional Animal Care and Use Committee
- CT
Computed Aided Tomography
- H&E
Hematoxylin and Eosin
- RFA
Radiofrequency Ablation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steel AW, Postgate AJ, Khorsandi S, et al. Endoscopically applied radiofrequency ablation appears to be safe in the treatment of malignant biliary obstruction. Gastrointest Endosc. 2011 Jan;73(1):149–53. doi: 10.1016/j.gie.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 2.Strand DS, Cosgrove ND, Patrie JT, et al. ERCP-directed radiofrequency ablation and photodynamic therapy are associated with comparable survival in the treatment of unresectable cholangiocarcinoma. Gastrointest Endosc. 2014 Nov;80(5):794–804. doi: 10.1016/j.gie.2014.02.1030. [DOI] [PubMed] [Google Scholar]
- 3.Zhou C, Wei B, Gao K, et al. Biliary tract perforation following percutaneous endobiliary radiofrequency ablation: A report of two cases. Oncol Lett. 2016 Jun;11(6):3813–3816. doi: 10.3892/ol.2016.4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shin JU, Lee KH, Kim SA, et al. Intraductal thermal injury using a heat probe and radiofrequency ablation electrode in a swine model of biliary stenosis. Clin Res Hepatol Gastroenterol. 2013 Apr;37(2):159–65. doi: 10.1016/j.clinre.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Park JS, Jeong S, Kim JM, et al. Development of a Swine Benign Biliary Stricture Model Using Endoscopic Biliary Radiofrequency Ablation. J Korean Med Sci. 2016 Sep;31(9):1438–44. doi: 10.3346/jkms.2016.31.9.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dollinger M, Zeman F, Niessen C, et al. Bile Duct Injury after Irreversible Electroporation of Hepatic Malignancies: Evaluation of MR Imaging Findings and Laboratory Values. J Vasc Interv Radiol. 2016 Jan;27(1):96–103. doi: 10.1016/j.jvir.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Silk MT, Wimmer T, Lee KS, et al. Percutaneous ablation of peribiliary tumors with irreversible electroporation. J Vasc Interv Radiol. 2014 Jan;25(1):112–8. doi: 10.1016/j.jvir.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 8.DeOliveira ML, Glenck M, et al. Irreversible electroporation in HPB cancer treatment - A new ablation modality. HPB. 2016 Apr;18(2):e745. [Google Scholar]
- 9.Choi JW, Lu DS, Osuagwu F, et al. Assessment of chronological effects of irreversible electroporation on hilar bile ducts in a porcine model. Cardiovasc Intervent Radiol. 2014 Feb;37(1):224–30. doi: 10.1007/s00270-013-0731-y. [DOI] [PubMed] [Google Scholar]
- 10.Srimathveeravalli G, Silk M, Wimmer T, et al. Feasibility of catheter-directed intraluminal irreversible electroporation of porcine ureter and acute outcomes in response to increasing energy delivery. J Vasc Interv Radiol. 2015 Jul;26(7):1059–66. doi: 10.1016/j.jvir.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1.
CT images (A, B) 24 hours and (C, D) 7days after endoscopy-guided IRE (A, C: original image, B, D: 2.5x magnification of inset). On both 24 hours and 7 days after treatment, intact common bile ducts can be seen (arrows in C and D) without any evidence of perforation, dilation or stenosis.


