Abstract
Mycobacterium avium subsp. paratuberculosis (MAP) is the etiologic agent of Johne’s disease in ruminants causing chronic diarrhea, malnutrition, and muscular wasting. Neonates and young animals are infected primarily by the fecal–oral route. MAP attaches to, translocates via the intestinal mucosa, and is phagocytosed by macrophages. The ensuing host cellular immune response leads to granulomatous enteritis characterized by a thick and corrugated intestinal wall. We review various tissue culture systems, ileal loops, and mice, goats, and cattle used to study MAP pathogenesis. MAP can be detected in clinical samples by microscopy, culturing, PCR, and an enzyme-linked immunosorbent assay. There are commercial vaccines that reduce clinical disease and shedding, unfortunately, their efficacies are limited and may not engender long-term protective immunity. Moreover, the potential linkage with Crohn’s disease and other human diseases makes MAP a concern as a zoonotic pathogen. Potential therapies with anti-mycobacterial agents are also discussed. The completion of the MAP K-10 genome sequence has greatly improved our understanding of MAP pathogenesis. The analysis of this sequence has identified a wide range of gene functions involved in virulence, lipid metabolism, transcriptional regulation, and main metabolic pathways. We also review the transposons utilized to generate random transposon mutant libraries and the recent advances in the post-genomic era. This includes the generation and characterization of allelic exchange mutants, transcriptomic analysis, transposon mutant banks analysis, new efforts to generate comprehensive mutant libraries, and the application of transposon site hybridization mutagenesis and transposon sequencing for global analysis of the MAP genome. Further analysis of candidate vaccine strains development is also provided with critical discussions on their benefits and shortcomings, and strategies to develop a highly efficacious live-attenuated vaccine capable of differentiating infected from vaccinated animals.
Keywords: Mycobacterium avium subsp. paratuberculosis, Johne’s disease, pathogenesis, transposon mutagenesis, mutant bank
Introduction
Mycobacterium avium subsp. paratuberculosis (MAP) is the etiologic agent of Johne’s disease (JD) in ruminants, a chronic enteritis with significant economic impact and worldwide distribution (1). The potential linkage of MAP to Crohn’s disease (CD) in humans continues to be intensively investigated with dissimilar results (2–4). MAP was first isolated by the German scientists Johne and Frothingham in 1895 (5). It causes disease primarily in ruminants (cattle, sheep, goats, deer, etc.), but there are also reports of infection in non-ruminants, especially in wildlife (6). In the United States, annual losses to the cattle industry have been estimated from $250 million (7) to $1.5 billion (8). A recent analysis of published data using a Bayesian method (9), adjusting for sensitivity and specificity, determined that the true United States dairy herd-level prevalence of MAP was actually 91.1% compared to the 70.4% reported in 2007 (10). In beef cattle, herd-level prevalence of MAP is 7.9% (11). Even though JD was first observed in the United States in the early 1900s, the focus on research and disease control has only increased in the past 20 years. A Voluntary Bovine JD Control Program is in place to control JD on farms and identify herds with a low risk of infection. Currently, one of the most cost-effective and sensitive testing methods for JD is the identification of MAP in herds by testing environmental fecal samples by culturing from high traffic areas (9). Indeed, the use of improved diagnostics coupled with good management practices have shown to decrease JD transmission (12). Unfortunately, wildlife reservoirs may undermine efforts to control JD in domesticated animals until their role in wildlife is fully understood (13).
Taxonomy and Properties
Mycobacterium avium subsp. paratuberculosis is part of the Mycobacterium avium complex in the genus Mycobacterium and family Mycobacteriaceae. The M. avium complex contains two clearly defined species M. avium and M intracellulare. M. avium is classified into four subspecies based on the comprehensive sequence-based analysis of the 16S-23S ribosomal RNA internal transcribed spacer (14, 15): M. avium subsp. avium, M. avium subsp. hominissuis (MAH), MAP, and M. avium subsp. silvaticum. MAP is a facultative intracellular, Gram-positive, acid-fast (Figure 1A) and small (0.5 × 1.5 μm) rod-shaped (Figure 1B) bacterium. The cell wall is thick and waxy and made up of mycolate and peptidoglycan layers held together by arabinogalactan. It is a slow-growing bacteria with a generation time of over 20 h (16). Initial attempts to cultivate MAP in laboratory media were unsuccessful (17) and it was hypothesized that the inability of MAP to grow under in vitro conditions may be due to the lack of some essential growth factor. Later, it was shown that MAP was able to grow in media supplemented with extracts from other mycobacteria (18, 19) and they concluded that MAP lacked the ability to synthesize some essential growth factor that is synthesized by other species. Mycobactin, an iron-binding siderophore isolated from Mycobacterium phlei, was shown to be the growth factor that is essential for the in vitro cultivation of MAP (20, 21). Since that time, mycobactin dependency has been considered to be taxonomic for MAP. More recently, with the genome sequence discussed further below, a molecular understanding of mycobactin dependency has been discovered by a deletion in the mbtA gene within the mycobactin synthesis operon (22, 23).
Figure 1.
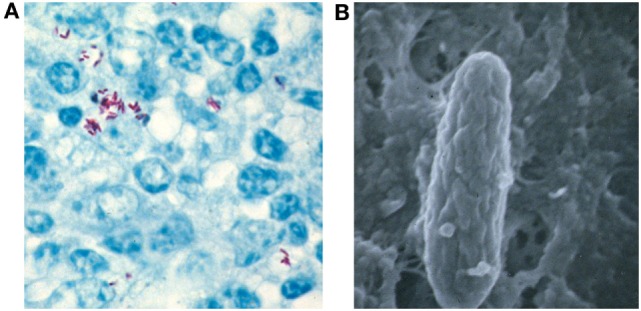
Mycobacterium avium subsp. paratuberculosis (MAP) properties. (A) Acid-fast stain of intestinal epithelium from an experimentally challenged bovine reveals MAP (red rods) inside macrophages. (B) Electron microscopy clearly shows the rod-shaped mycobacteria magnified over 50,000 times.
Pathogenesis
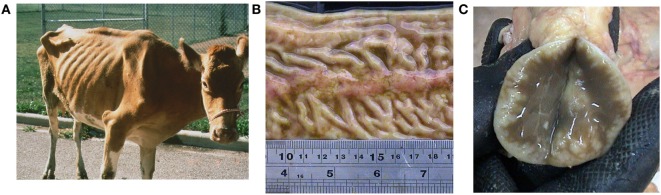
Johne’s disease causes a chronic diarrhea characterized by a malabsorption syndrome that leads to malnutrition and muscular wasting (Figure 2A). Neonates and juvenile animals are infected mainly via the fecal–oral route. Transmission may also occur by the consumption of milk and colostrum from infected cows (24). Calves up to 6 months of age are at higher risk of getting infected, but the risk of infection drops after this age (25). Mouse models have shown that, after ingestion, MAP attachment to and translocation through the intestinal mucosa is mediated by both M-cells and enterocytes (26). Moreover, studies in tissue cultures demonstrated that MAP affects the formation of tight junctions in the intestinal mucosa providing a mechanism for increased permeability (27). There is significant host–pathogen crosstalk during this process as antigens 85 (28), 35 kDa (29), MAP oxidoreductase (30), MAP fibronectin-binding protein (31, 32), and the histone HupB (33) play important roles in MAP epithelial cell adhesion and/or invasion. Previous findings using a co-culture of the bovine mammary epithelial cell line MAC-T (34) and bovine blood-monocyte-derived macrophages (BMDM) suggest that phagosome acidification in MAP-infected epithelial cells leads to interleukin (IL)-1β production, macrophage recruitment, and transepithelial migration (35). Bacilli are subsequently phagocytosed by these sub- and intra-epithelial macrophages (36–38). Once inside phagocytic cells, the ability of MAP to survive and replicate within these phagocytic cells plays a key role in pathogenesis (39, 40). Moreover, use of a culture passage model showed that MAP lipid composition changes in macrophages developing a pro-inflammatory phenotype (41).
Figure 2.
Johne’s disease affected animal caused by Mycobacterium avium subsp. paratuberculosis. (A) Severely debilitated cow with common symptoms of chronic diarrhea, malabsorption, muscular wasting, and malnutrition. The host cellular immune response leads to the typical granulomatous enteritis seen as thickening of the (B) intestinal mucosa with prominent Peyer’s patches, and (C) lymph node showing hyperactive lymphoid tissue (white spots).
The ensuing host cellular immune response leads to the typical granulomatous enteritis pathognomonic of JD (38), characterized by the thick and corrugated appearance of the intestinal wall (Figure 2B) and inflamed lymph nodes (Figure 2C). Tissue macrophages and dendritic cells play a crucial role in the recognition of pathogen-associated molecular patterns in the innate phase via toll-like receptors (42), as well as in antigen processing and the elicitation of cytokine-mediated cellular interactions (43). Control of MAP infections are dependent on a T helper cell, Th1, type response and the subsequent activation of macrophages by interferon-gamma (INF-γ) secreted by Th1 T lymphocytes in the acquired immunity phase (44, 45). The killing mechanism of these activated phagocytic cells involves the generation of nitric oxide by the inducible nitric oxide synthase, whose activity has been already demonstrated in cattle (46). In this context, the overproduction of nitric oxide is particularly high in BMDM isolated from subclinically infected animals (47). However, MAP also affects the function of bovine macrophages as evidenced by unique mRNA expression profiles (48), inhibition of apoptosis and antigen presentation (49), and patterns of cytokine expression that have diagnostic significance (50). Predominantly, MAP drives T helper cells from infected cattle to undergo a Th2 response with enhanced expression of IL-4, IL-5, IL-10, and inhibitors of tissue remodeling factors (51, 52). This humoral response was confirmed in a neonatal calf model (53). Other findings also implicate regulatory T and Th17 cells in the immunopathogenesis of JD in both ruminants and wildlife (49, 54).
Several models have been developed to study MAP pathogenesis. However, MAP elicits responses from the ruminant host immune system that are not manifested in traditional in vitro models. For example, in infected BMDM, MAP bacilli multiply over 4–8 days (44, 55), but infection of the murine J774 macrophage cell line results in a decrease in bacterial loads over time (56, 57). Thus, primary phagocytic cells are preferred to conduct experiments when studying the interaction of MAP with phagocytic cells. A promising systems biology approach based on ileal loops has been developed to follow MAP infection from early to late stages (58). Recently, this model has been applied to compare the host transcriptome profile upon M. avium subsp. avium and MAP infection. Analysis of cellular pathways affected during MAP infection was consistent with intestinal mucosal weakening, the activation of a Th2 response, and phagocytosis inhibition that was not observed with M. avium subsp. avium (59).
Diagnosis and Control
Infected animals shed MAP in the feces before showing any clinical signs, thus acting as a major source of infection to other animals in the herd. Early diagnosis of the infection is very important to prevent the spread of JD. Various diagnostic tests were developed based on the direct and indirect detection of MAP (60). The direct detection of MAP in clinical samples can be accomplished by (i) microscopy, (ii) MAP isolation by culturing, and (iii) the identification of MAP DNA by PCR. Ziehl–Neelsen or acid-fast staining has been used for the examination of clinical samples (Figure 1A). Acid-fast staining is the simplest, fastest, and most economical method of diagnosis, but the specificity and sensitivity are low as it is difficult to differentiate between MAP and other acid-fast bacilli (61). Ziehl–Neelsen staining can be used for the initial screening of MAP, but it has to be confirmed by other specific tests, such as PCR and/or immunoassays. The isolation of MAP by culturing is the “gold standard” for JD diagnosis. The dependence of MAP on mycobactin J for growth in specialized laboratory media can also be used to discriminate MAP from other acid-fast bacilli. Recently, an improved growth medium was described that improves MAP recoverability and sensitivity by 1,000-fold (62). Culture-based diagnosis is very time consuming as MAP grows extremely slow (6–8 weeks for colony formation on solid media). Therefore, a very rapid and sensitive PCR-based testing was used for MAP detection in clinical and environmental samples (63–65). This PCR utilizes primers for a 1.4 kb multicopy insertion element, IS900, which is sequence specific to MAP (60, 66). However, the presence of IS900-like insertion sequences in other mycobacteria was shown to compromise the specificity of this assay leading to false-positive results (64, 67). To overcome false-positive results, multiplex PCR based on the IS900, IS901, IS1245, and dnaJ gene has been developed, but the sensitivity of this test is low due to reagent interference and primer dimer formation (60, 68). In addition, PCR assays with fecal samples are only 70% sensitive and 85% specific (69). Some good progress has been made identifying and using more specific targets for PCR tests (70–72), and this comparative genomic approach has filled a knowledge gap in MAP diagnostics. Some of these targets have even been used in commercial diagnostic kits.
Diagnostic MAP assays created on the indirect detection are based on the host immune response to infection. The delayed-type hypersensitivity skin test has been developed using a Johnin purified protein derivative (73). However, this test is not specific as other environmental mycobacteria can also sensitize the animal and give false-positive results. So delayed-type hypersensitivity skin tests cannot distinguish vaccinated from naturally infected animals. As stated previously, MAP infection leads to a T helper cell response which secrets INF-γ. The measurement of this INF-γ level by an enzyme-linked immunosorbent assay (ELISA) can also be used for the diagnosis of JD using day-old blood sample culture supernatants stimulated with Johnin and co-stimulated with bovine IL-12 and/or human IL-2 (74). Unfortunately, MAP purified protein derivatives are also used as antigens in the INF-γ assay leading to problems with cross-reactivity. The cell wall lipopeptide, L5P, has been studied as an alternative MAP antigen for the assay, but the INF-γ response has been shown to be lower than with Johnin (75). Thus, routinely used methods for MAP diagnosis include the detection of antibodies in serum and milk from infected animals using a commercial ELISA kit: (i) HerdCheck M. paratuberculosis ELISA (IDEXX Laboratories, Inc.), (ii) ParaCheck (CSL/Biocor), (iii) SERELISA ParaTB (Synbiotic Corp.), and (iv) ID Screen® Paratuberculosis Indirect (IDvet Genetics). Compared to PCR diagnosis, an ELISA has a lower sensitivity of 50%, but a much greater specificity of 99.8% (76, 77). More studies are required to identify MAP specific antigens to develop immune-based assays for diagnosis of JD that have a higher level of sensitivity.
Control measures to prevent JD include vaccination (the most cost-effective), testing, and improved herd management based on a producer’s resources, facilities, and operation (78). Unfortunately, though there are JD vaccines that reduce clinical disease and shedding, their efficacies are limited and none afford long-term protective immunity. For example, in the United States, Mycopar® (Boehringer Ingelheim Vetmedica, Inc.) is the only licensed vaccine against JD in cattle. However, this vaccine was derived from M. avium subsp. avium strain 18 (79) and; therefore, does not have an optimal antigenic repertoire. Another bacterin, Silirum® (Zoetis Animal Health) is being tested in Australia and approved for limited use in cattle. This vaccine contains the heat-killed MAP 316F strain. This formulation may possess a better antigenic repertoire, but while using heat-killed bacteria may improve safety, it may also reduce efficacy. Neoparasec® (Rhone-Merieux) contains the live-attenuated MAP strain 316F while Gudair® (Zoetis Animal Health) is heat-killed 316F and both are licensed for use in sheep and goats. However, current vaccines cannot distinguish vaccinated from infected animals, thus compromising JD diagnostic tests (80), and strain 316F was generated in the 1920s by random attenuation procedures (e.g., passages on ox bile) that are only now being investigated (81). In the final analysis, a vaccine of high efficacy is needed to significantly control JD (82).
Testing results on the new generation of human anti-tuberculosis vaccines seem to indicate that live-attenuated vaccines provide better protection than subunit vaccines (83). As JD is caused by a Mycobacterium, it is likely that a similar situation will occur for candidate subunit or bacterin-based vaccines. This was actually the impetus behind the JD Integrated Program—Animal and Plant Health Inspection Service efforts to undertake a standardized vaccine test program. United States and New Zealand researchers contributed 22 blinded live-attenuated vaccine candidates to be evaluated in a three-phase study: BMDM, mouse, and goat models. Though the methodology for animal testing was well developed (84), most of the attenuated transposon mutants tested were first-generation ones and carried the Tn5367 transposase which lead to instability. Moreover, there were unknowns that could not be ascertained before the start of the trial, such as the best immunization route and dosing schedule. Nonetheless, important data and reagents were developed (80). It may still be possible to develop a subunit vaccine capable of controlling infections by eliciting the appropriate type of humoral immunity (85), especially against antigens expressed in the pro-inflammatory phenotype (86).
Zoonotic Potential
Mycobacterium avium subsp. paratuberculosis is among a list of pathogens that have been associated withCD, a human chronic inflammatory bowel disease (2, 87, 88). The cross-reactivity of MAP antigens with those in humans, such as the zinc transporter 8 protein, may underlie the etiology of these diseases (89). MAP is also postulated to be involved in the progression of HIV infection and other immune dysfunction diseases: multiple sclerosis, sarcoidosis, type 1 and 2 diabetes mellitus, Hashimoto’s thyroiditis, and Parkinson’s disease (90–96). Over the years, several studies have investigated the role of MAP in CD with conflicting results (4, 97–100). MAP has been isolated from CD patients by culturing blood, breast milk, and tissue biopsy samples, and samples were shown positive for IS900 by PCR (101–103). Also by this method, MAP was present in the gut of 92% of CD patients and 26% of control individuals (98). Humans are exposed to MAP from various sources that could be contaminated from infected animals, such as drinking water with feces, milk, etc. (104). In the United Kingdom, a study revealed that even after pasteurization, MAP was detected by IS900 PCR in 7% of retail milk for human consumption (105). Nonetheless, this is highly dependent on the pasteurization method since the application of a standard high-temperature short-time pasteurization results in the destruction of MAP (106). However, some bacteria survived the sub-pasteurization heat treatment at lower temperatures used for cheese manufacture. Thus, though humans are widely exposed to MAP, susceptible individuals with a compromised immune system, due to other illness or genetic factors, may be at higher risk for infection and disease. In these individuals, MAP may survive and multiply inside gut macrophages leading to immune dysregulation and inflammation of the intestinal wall resulting in a “leaky gut” (http://www.crohnsmapvaccine.com). Microbes and food materials penetrate the leaky gut causing massive wall inflammation leading to chronic inflammatory bowel disease.
Currently a combination of anti-inflammatory, anti-mycobacterial, and immunosuppressant drugs are used or in trials for CD treatment. Initially, only anti-tuberculosis drugs were used for treatment of MAP infections in humans, but the results were not promising. Later macrolides (e.g., azithromycin and erythromycin), rifabutin, and clofazimine have been used with limited success, but the treatment regimen is very lengthy (2, 66, 107–109). A comprehensive study evaluated culture methods for the determination of MAP in vitro drug susceptibility and concluded that the BACTEC™ MGIT™ system was more rapid and reliable (110). In addition, in vitro studies using drug combinations of anti-microbials and immunomodulators displayed synergistic effects that could be applied to CD treatment (111). Therefore, more studies are needed to identify novel drugs and/or targets for the development of better therapeutics against MAP to shorten and simplify the treatment. Regarding MAP pathogenesis, the European Food Safety Authority has conducted a thorough study and indicated that MAP is eligible to be listed for Union intervention based on Article 5(3) of the Animal Health Law (112). Several species of mammals and birds were listed as susceptible species, including Bovidae, Cervidae, and Leporidae, but there was no consensus on the zoonotic risk of MAP in the context of human diseases, such as CD.
Genomics
The MAP bovine strain K-10 genome has been sequenced, annotated, and re-examined by optical mapping (22, 113, 114). The updated K-10 genome is 4,829,781 bp encoding 4,350 protein-encoding ORFs with a 69.3% GC content. In the genome, about 60% of the ORFs have similar sequences in microbial genetic databases, but only approximately 35% have well predicted or identified functions (115). Nonetheless, about 75% of the MAP genes have counterparts in Mycobacterium tuberculosis with 39 predicted proteins that are unique to MAP. This genome possesses a high redundancy rate due to gene duplications, particularly for those involved in lipid metabolism and redox processes. Gene functions include 150 transcriptional regulators, 16 polyketide synthesis, 14 two-component regulatory systems, 16 serine–threonine protein kinases, 8 mammalian cell entry (mce), 20 insertion elements, and 5 putative prophages. Sequencing also identified and mapped genes involved in glycolysis, pentose phosphate pathway, tricarboxylic acid cycle, and glyoxylate cycle (116). Important differences between the genomes of K-10 and M. tuberculosis human strain H37Rv include the following: (i) no intact Pro-Glu-polymorphic GC-rich sequences (PE-PGRS) genes, (ii) 47 fewer Pro-Pro-Glu (PPE) genes in M. tuberculosis (117), and (iii) the presence of a truncated salicyl-AMP ligase gene (mtbA) which may explain the MAP mycobactin biosynthesis defect discussed earlier (22).
Other MAP strains with complete sequences include the human strain MAP4 (118) and another bovine strain JII-1961 (119). Partial MAP genomic sequences include 10-4404, 2015WD-1, 2015WD-2, ATCC19698, JQ5, S5, S397, JIII-386, and several human isolates (118, 120–122). The MAP ovine genomes of strains S397 and JIII-386 differ by one inversion to the MAH human strain 104, while two inversions are detected between K-10 and MAH, with additional differences involving duplications, deletions, and polymorphisms. Based on these analyses, ovine isolates seem to be an intermediate in the evolution of MAP bovine strains from MAH human strains (115), although this is not definitely established (121). However, the more closely related bovine and human isolates (118, 120) may be the latest strains that diverged in evolution acquiring specific host adaptations in their genomes.
Transposon Mutagenesis
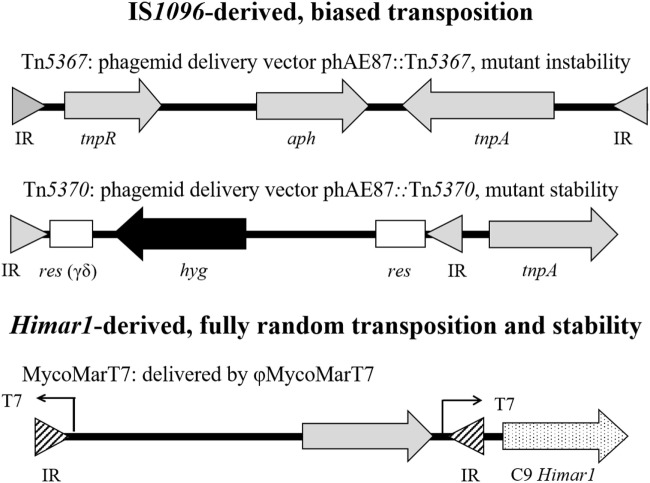
The identification and functional analysis of genes involved in the pathogenesis of MAP requires molecular genetic tools to manipulate its genome. Because the MAP cell wall is very thick and impermeable, it is difficult to introduce plasmid vectors. The shuttle plasmid vector pMV262 carrying a mycobacterial origin of replication (oriM) and an Escherichia coli origin of replication (oriE) have been used to transform MAP (123). However, the transformation efficiency was very low; therefore, a phage-mediated transduction process was developed to introduce DNA into MAP (124). Shuttle phasmids (phagemids) carrying the mycobacteriophage TM4 (125) and an E. coli cosmid have been widely used for the genetic manipulation of MAP. More recently, the generation of MAP mutant libraries using transposon mutagenesis has been the major approach to define virulence determinants and identify genes that are essential and non-essential (126). Two types of transposons have been extensively used in mycobacteria to generate random mutant banks: (i) IS1096-derived and (ii) Himar1-derived transposons (Figure 3).
Figure 3.
Structure of mycobacterial transposons utilized to generate random mutant libraries. Inverted repeat (IR) for Tn5367 or Tn5370 (filled triangle), or Himar1-derived transposon (striped triangle); tnpR, IS1096 resolvase; aph, kanamycin-resistant gene; tnpA, IS1096 transposase; res, resolution site for transposon gamma-delta; hyg, hygromycin resistant gene; and C9 Himar1, Himar1 transposase.
Tn5367 (GenBank Accession KM232614) was the first transposon widely used in mycobacteria including MAP (126–128). This transposon (3,381 bp) carries a kanamycin-resistant drug marker (aph), and the IS1096 transposase (tnpA) and resolvase (tnpR). This transposon has the transposase within the transposed element (126) resulting in a relatively high transposition frequency (ca. 1.0 × 10−5), at least in Mycobacterium smegmatis (129). Tn5367 transposes by a cutting and paste mechanism (130) and may insert in another gene creating either a wild-type or mutated gene, depending on precise or imprecise excisions, in the location of the original insertion. The transposon can be delivered into Mycobacterium by the thermosensitive phasmid derivative of mycobacteriophage TM4 (126). Since the IS1096 transposase is located within the transposed element, the corresponding MAP mutants may not be stable enough to conduct long-term in vivo experiments where bacilli multiply to large numbers. An improved IS1096-derived transposon, Tn5370 (GenBank Accession KM232615), was constructed by removing ORFs dispensable for transposition (e.g., tnpR), therefore, reducing the size to 2,295 bp (128, 130). Moreover, the transposase was engineered into the phasmid outside of the transposable element. Thus, Tn5370, as opposed to Tn5367, generates stable mutants upon transposition. This transposon has the advantage of possessing a hygromycin (hyg) marker within the transposable element outflanked by the γ-δ resolvase. This construction allows for the excision of the drug marker and the generation of unmarked transposon insertion mutants. However, neither Tn5367 nor Tn5370 transposes randomly in mycobacteria (127, 130).
Himar1-derived transposons have also been used to generate random mutant libraries in M. tuberculosis and MAP (131–134). The Himar1 transposon (MycoMarT7) was engineered into the same TM4 phasmid derivative described above. This vector carries the highly active C9 Himar1 transposase outside the inverted repeats, thus yielding stable mutants. In addition, this vector carries the aph drug marker and a T7 promoter that reads outward to facilitate identification of insertion sites by in vitro transcription and cDNA synthesis (131). The major advantage of the Himar1 derivative is the almost fully random recognition sequence (5′-TA-3′) versus the recognition sequence of IS1096-derived transposons (5′-NNPy(A/T)A(A/T)NN-3′) showed experimentally to have a transposition bias in both M. tuberculosis and MAP (127, 130). Most of the MAP transposon mutant libraries were made with Tn5367 (127, 128, 135, 136) and fewer libraries have been constructed using the Himar1-derived transposon MycoMarT7 (132, 133). Our group has constructed a Himar1 library for MAP and used it to demonstrate random genomic insertions (134).
Molecular Genetics in the Post-Genomics Era: Candidate Vaccine Applications
Our understanding of MAP pathogenesis has greatly improved based on the completion of the MAP K-10 genome sequence, and the availability of advanced molecular biology and bioinformatics tools. MAP genomics has many applications, such as molecular detection, determining the molecular evolution of MAP as a pathogen, and identifying virulence determinants, drug targets, attenuation targets for vaccine development, and/or diagnostic antigens. Several strategies were used to mine the MAP genome for virulence determinants or important diagnostic antigens (137–139). One approach relied on identifying MAP genes with known M. tuberculosis homologs known to play important roles in pathogenesis (140, 141). Many of these studies described below have generated deletion mutants to identify the role of these genes in pathogenesis. Usually, phasmids are used to deliver the allelic exchange substrate into MAP to produce the desired gene deletion by homologous recombination. For example, based on the attenuation of M. tuberculosis leuD mutants (140), the corresponding MAP leuD mutants were constructed by allelic exchange, characterized by carbon and nitrogen source utilization, and showed to be attenuated in mice (142, 143). Likewise, a mutant of MAP lipN was constructed and displayed lower levels of colonization in mice compared to the wild-type strain (144). Similarly, lsr2, pknG, and relA deletion mutants were generated (145) and later in vivo mice studies revealed that relA mutants were immunogenic, but had decreased pathogenicity (146). Other studies constructed sigH and sigL deletion mutants, infecting bovine macrophages and mice to demonstrate that this gene plays a role in pathogenesis (147, 148). Recently, sigH and lipN deletion mutants were validated as promising live-attenuated vaccine candidates in goats (149). Nonetheless, these meritorious studies are not comprehensive since genetic islands were identified in the MAP genome demonstrating a gene organization different from the closely related MAH (150). Indeed, differences in gene organization may lead to a context-dependent function (e.g., even homologous genes in MAP and MAH may play different roles in pathogenesis). This effect is further compounded by findings indicating host genome adaptations (115).
In the context of vectored subunit vaccines, success has been achieved in the expression of MAP antigens in Lactobacillus salivarius using a codon optimization strategy (151, 152). Multi-antigen virally vectored vaccines were also engineered and tested in mice and cattle. In this context, the vaccine designated HAV is a fusion of two secreted and two cell surface MAP proteins whose DNA coding sequence was inserted into the human adenovirus 5 and Modified Vaccinia Ankara delivery vectors. Vaccination of naive C57BL/6 mice resulted in a highly immunogenic response with significant levels of IFN-γ and a humoral response against the recombinant antigens (153). The vaccine provided moderate protection against challenge. This vaccine was also tested in cattle with promising results being well tolerated, showing no cross-reactivity against bovine tuberculin, providing some protection against challenge in a calf model and eliciting CD4+, CD8+ IFN-γ producing T-cell populations and, upon challenge, developed early specific Th17 T-cells (154). In addition, a non-recombinant Lacotbacillus casei used as a probiotic immunomodulated and reduced pathology in a mouse MAP infection model (155). Moderate success has been attained with recombinant MAP Hsp70 proteins resulting in a reduction of bacterial shedding upon experimental infection without compromising the diagnosis of bovine tuberculosis (156, 157) and showed some promise as a therapeutic vaccine in cattle (158). Lipoarabinomannan-enriched glycolipid extracts used as potential subunit vaccines against MAP yielded a pro-apoptotic response in bovine macrophages and reduced shedding (159, 160). Studies have also been conducted with purified antigens or antigen cocktails. For example, superoxide dismutase and antigen 85B were shown to elicit T-cell responses consistent with protection in mice (161, 162). These results were further advanced using DNA vector vaccines (145). Various MAP protein cocktails were also tested in mice and cattle, showing reduced tissue burden (163), and INF-γ and humoral responses, respectively (164). Nonetheless, the extensive experience of researchers in the human tuberculosis vaccine field has led to the consensus that live-attenuated vaccines are the best approach for vaccination against mycobacterial diseases (83).
Gene expression in MAP is complex and global regulation has been associated with 19 putative sigma factors with 12 belonging to common mycobacterial gene families. Specific sigma factors may be involved in the expression of survival and virulence genes (165). In addition, MAP virulence determinants are regulated by the LuxR–LuxI quorum sensing system (166). Transcriptomic analysis has been applied extensively and these studies generated large expression databases under conditions of environmental stress (144), iron limitation (167), intracellular survival within BMDM, tissue localization in vivo (168), and ligated jejuno-ileal loops (58, 59). Some differentially regulated genes such as atpC, clpB, dnaJ, dnaK, groEL2, infB, kdpE, mbtC, and pmmA correspond to essential genes in M. tuberculosis (131, 144, 169). Other genes identified as non-essential more likely to be involved in pathogenesis rather than general physiological functions: aceAB, clpX, relA, htpX, lipL, lipN, and lpqP. Indeed M. tuberculosis homologs of lipL, lipN, aceAB, and lpqP were also identified as being required for intracellular survival within macrophages by a genetic approach (170). Another insight from transcriptomic studies revealed significant differences in gene expression with MAP metabolic genes being shut down in tissues, while phagocytic cells upregulated genes involved in iron acquisition and intracellular survival (168). Interestingly, the analysis of databases from different studies does not reveal any significant correlations. Moreover, the fold-changes in gene expression levels differed widely from one study to another. Nonetheless, except for specific and restricted conditions, a common feature is that approximately 20–25% of the genes in the MAP genome change their expression levels based on the condition. Likewise for M. tuberculosis, little correlation was observed among genes that were up- or downregulated in macrophages or infected mice, and those identified by a genetic approach as being required for survival in macrophages and mice (170, 171). In retrospect, these results are not surprising as genetic methods establish gene requirements for a certain condition as an outcome of a cumulative selection process during the infection, independent of their mode of regulation. In this context, transcriptomic approaches may miss a significant number of constitutively or transiently expressed genes involved in pathogenesis. As transcriptomic approaches require discrete rather than continuous samplings, most appropriate time points could be missed.
Transposon mutagenesis is another strategy that has been used to identify MAP virulence genes. In this approach, genes are disrupted using random transposition to generate a mutant library where each mutant is marked with a transposon. Mutants are then screened under different in vitro and in vivo conditions for the functional analysis of genes. Mutants that have a mutation in genes that are essential will not survive. A library of 5,060 Tn5367 transposon mutants was generated to apply a transposon–chromosome junction site sequencing protocol in order to identify the disrupted genes (127). They analyzed 1,150 mutants and identified 970 unique insertion sites. Based on sequencing analysis, they selected 11 mutants for mouse infection experiments and identified some potential virulence genes (gcpE, pstA, kdpC, papA2, impA, umaA1, and fabG2_2). In our study, we generated and screened a MAP transposon library of 13,536 mutants, identifying genes potentially involved in pathogenesis (e.g., MAP1152, MAP1156, MAP1566, and lsr2) (128). Another study used the Himar1 transposon in MAP to isolate 111 mutants attenuated in BMDM from a random screen of 2,290 (132). Unfortunately, all of these studies have limitations: (i) the screening and analysis methods used are laborious and time consuming, especially for slow-growing MAP and (ii) the library used for screening is partial because of transposon bias (134) so it is not possible to perform the functional analysis of the entire MAP genome. To overcome these limitations, it is necessary to generate a comprehensive MAP mutant library and design new and more robust approaches to perform global gene functional analysis.
Transposon site hybridization (TraSH) has been applied for the global analysis of the M. tuberculosis genome (131). In this method, a high-density transposon mutant library is generated and the mutants are pooled. DNA microarray is then applied to map all transposon insertion sites. This approach has some limitations, such as poor resolution, limited dynamic range, and the requirement to develop DNA microarrays. Recently, a new method has been established based on next-generation deep sequencing to analyze the essentiality of genes on a whole-genome basis called transposon sequencing (Tn-Seq) (172). Tn-Seq involves the generation of a comprehensive mutant library using a transposon that inserts randomly without any biases. The mutants are pooled to isolate genomic DNA so the transposon chromosomal junctions can be amplified and sequenced using an Illumina or another suitable platform. This approach has been applied for M. tuberculosis to define essential genes under various conditions (173, 174). More recently, the Tn-Seq method was used to classify MAP essential genes by generating a pool of approximately 100,000 Himar1 transposon mutants (133). However, we have demonstrated that Himar1 transposons recombine with significant loci-dependent biases, making these collections highly underrepresented (134). The application of the Tn-Seq system to a highly saturated Himar1 transposon mutant library will overcome these limitations and provide a global approach to the identification of MAP essential genes and virulence determinants.
Perspective and Overall Summary
Sequencing the MAP K-10 genome, and the availability of advanced molecular biology and bioinformatics tools have allowed the screening and characterization of transposon mutants to identify specific genetic loci involved in pathogenesis, suggesting novel strategies for the control and diagnosis of JD. In addition, several candidate genes for strain attenuation have been mutagenized by allelic exchange and the corresponding mutants were tested with promising results in mice, goats, and cattle. We consider that vaccination with a live-attenuated vaccine is the most cost-effective control measure for MAP infection in livestock. The embodiment of such a vaccine would require the construction of a strain with two deletion mutations and with the capability to differentiate vaccinated from infected animals.
In summary, this article opens with a brief review of JD epidemiology and the staggering economic losses followed by MAP taxonomy and cultivation. The main mechanisms of pathogenesis related to the invasion of the intestinal epithelium and the underlying humoral and cellular responses are discussed. Infection models are concisely summarized thereafter. The article then shifts to the multiple methods of JD diagnosis, including acid-fast stain, culture, PCR, and the use of ELISA to detect the antibody and INF-γ responses. The control of JD is explained in the context of commercially available vaccines and their efficacies. This section also includes results of a recent multi-institutional live-attenuated JD vaccine testing program. MAP zoonotic potential is described based on the diagnostic methods used to detect MAP in human samples along with the various treatment options. Subsequently, we focus on genomics, including the analysis of bovine and ovine genome sequence isolates. This leads to the transposon mutagenesis section where we define the transposons and vectors used and summarize the results obtained with mutant banks. The final post-genomic section discusses the construction of new attenuated mutants by deletion mutagenesis and their vaccine potential. We also discuss antigen and antigen cocktails that have been suggested for subunit vaccine formulations. The manuscript concludes with the discussion of gene expression, global regulators, methods of wide genomic analysis, such as TraSH mutagenesis and Tn-Seq analysis, and our perspective on current and future vaccine efforts.
Author Contributions
GR wrote excerpts of this review as Chapter 1 of his Doctoral Dissertation in Veterinary and Biomedical Science available online at the University of Nebraska-Lincoln (http://digitalcommons.unl.edu/). DZ, JB, JS, YG, MC, and RB contributed and edited the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. Research related to the topics reviewed is supported by the Hatch/Multi State Project (NEB 39-168; RB), the United States Department of Agriculture Agricultural Research Service (JB and JS), the USDA National Institute of Food and Agriculture award number 2013-67015-21239 (RB, JB, JS, and YG).
Abbreviations
MAP, Mycobacterium avium subsp. paratuberculosis; JD, Johne’s disease; MAH, M. avium subsp. hominissuis; BMDM, blood-monocyte-derived macrophages; IL, interleukin; INF-γ, interferon-gamma; ELISA, enzyme-linked immunosorbent assay; CD, Crohn’s disease; TraSH, transposon site hybridization mutagenesis; Tn-Seq, transposon sequencing; DIVA, differentiate vaccinated from infected animals.
References
- 1.Sweeney RW. Transmission of paratuberculosis. Vet Clin North Am Food Anim Pract (1996) 12:305–12. 10.1016/S0749-0720(15)30408-4 [DOI] [PubMed] [Google Scholar]
- 2.Chacon O, Bermudez LE, Barletta RG. Johne’s disease, inflammatory bowel disease, and Mycobacterium paratuberculosis. Annu Rev Microbiol (2004) 58:329–63. 10.1146/annurev.micro.58.030603.123726 [DOI] [PubMed] [Google Scholar]
- 3.Davis WC, Madsen-Bouterse SA. Crohn’s disease and Mycobacterium avium subsp. paratuberculosis: the need for a study is long overdue. Vet Immunol Immunopathol (2012) 145:1–6. 10.1016/j.vetimm.2011.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Momotani E, Romona NM, Yoshihara K, Momotani Y, Hori M, Ozaki H, et al. Molecular pathogenesis of bovine paratuberculosis and human inflammatory bowel diseases. Vet Immunol Immunopathol (2012) 148:55–68. 10.1016/j.vetimm.2012.03.005 [DOI] [PubMed] [Google Scholar]
- 5.Johne HA, Frothingham L. Ein eigenthumlicher fall von tuberculose beim rind (a particular case of tuberculosis in a cow). Deut Z Tiermed Vergl Pathol (1895) 21:438–54. [Google Scholar]
- 6.Motiwala AS, Amonsin A, Strother M, Manning EJ, Kapur V, Sreevatsan S. Molecular epidemiology of Mycobacterium avium subsp. paratuberculosis isolates recovered from wild animal species. J Clin Microbiol (2004) 42:1703–12. 10.1128/JCM.42.4.1703-1712.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ott SL, Wells SJ, Wagner BA. Herd-level economic losses associated with Johne’s disease on US dairy operations. Prev Vet Med (1999) 40:179–92. 10.1016/S0167-5877(99)00037-9 [DOI] [PubMed] [Google Scholar]
- 8.Stabel JR. Johne’s disease: a hidden threat. J Dairy Sci (1998) 81:283–8. 10.3168/jds.S0022-0302(98)75577-8 [DOI] [PubMed] [Google Scholar]
- 9.Lombard JE, Gardner IA, Jafarzadeh SR, Fossler CP, Harris B, Capsel RT, et al. Herd-level prevalence of Mycobacterium avium subsp. paratuberculosis infection in United States dairy herds in 2007. Prev Vet Med (2013) 108:234–8. 10.1016/j.prevetmed.2012.08.006 [DOI] [PubMed] [Google Scholar]
- 10.USDA. Dairy 2007. Part III: reference of dairy cattle health and management practices in the United States. In: USDA , editor. USDA APHIS VS, CEAH, National Animal Health Monitoring System. USDA APHIS VS, CEAH, National Animal Health Monitoring System; (2008). [Google Scholar]
- 11.Lombard JE. Epidemiology and economics of paratuberculosis. Vet Clin North Am Food Anim Pract (2011) 27:525–35, v. 10.1016/j.cvfa.2011.07.012 [DOI] [PubMed] [Google Scholar]
- 12.Espejo LA, Godden S, Hartmann WL, Wells SJ. Reduction in incidence of Johne’s disease associated with implementation of a disease control program in Minnesota demonstration herds. J Dairy Sci (2012) 95:4141–52. 10.3168/jds.2011-4550 [DOI] [PubMed] [Google Scholar]
- 13.Miller RS, Farnsworth ML, Malmberg JL. Diseases at the livestock-wildlife interface: status, challenges, and opportunities in the United States. Prev Vet Med (2013) 110:119–32. 10.1016/j.prevetmed.2012.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frothingham R, Wilson KH. Sequence-based differentiation of strains in the Mycobacterium avium complex. J Bacteriol (1993) 175:2818–25. 10.1128/jb.175.10.2818-2825.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mijs W, De Haas P, Rossau R, Van Der Laan T, Rigouts L, Portaels F, et al. Molecular evidence to support a proposal to reserve the designation Mycobacterium avium subsp. avium for bird-type isolates and ‘M. avium subsp. hominissuis’ for the human/porcine type of M. avium. Int J Syst Evol Microbiol (2002) 52:1505–18. 10.1099/00207713-52-5-1505 [DOI] [PubMed] [Google Scholar]
- 16.Lambrecht RS, Carriere JF, Collins MT. A model for analyzing growth kinetics of a slowly growing Mycobacterium sp. Appl Environ Microbiol (1988) 54:910–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Twort FW, Ingram GLY. Method for isolating and cultivating the Mycobacterium enteritidis chronicae pseudotuberculosis bovis, Johne, and some experiments on the preparation of a diagnostic vaccine for pseudo-tuberculous enteritis of bovines. Proc R Soc Lond B Biol Sci (1912) 84:517–30. 10.1098/rspb.1912.0011 [DOI] [Google Scholar]
- 18.Twort FW, Ingram GLY. Johne’s Disease. London: Bailhiere, Tindall and Cox; (1913). [Google Scholar]
- 19.Twort FW, Ingram GLY. Further experiments on the biology of Johne’s bacillus. Zentr Bakteriol Parasitenk Abt 1 Org (1914) 73:277–83. [Google Scholar]
- 20.Francis J, Macturk HM, Madinaveitia J, Snow GA. Mycobactin, a growth factor for Mycobacterium johnei. I. Isolation from Mycobacterium phlei. Biochem J (1953) 55:596–607. 10.1042/bj0550596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snow GA. Isolation and structure of mycobactin T, a growth factor from Mycobacterium tuberculosis. Biochem J (1965) 97:166–75. 10.1042/bj0970166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li L, Bannantine JP, Zhang Q, Amonsin A, May BJ, Alt D, et al. The complete genome sequence of Mycobacterium avium subspecies paratuberculosis. Proc Natl Acad Sci U S A (2005) 102:12344–9. 10.1073/pnas.0505662102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Kruijf M, Coffey A, O’mahony J. The investigation of the truncated mbtA gene within the mycobactin cluster of Mycobacterium avium subspecies paratuberculosis as a novel diagnostic marker for real-time PCR. J Microbiol Methods (2017) 136:40–8. 10.1016/j.mimet.2017.03.005 [DOI] [PubMed] [Google Scholar]
- 24.Streeter RN, Hoffsis GF, Bech-Nielsen S, Shulaw WP, Rings DM. Isolation of Mycobacterium paratuberculosis from colostrum and milk of subclinically infected cows. Am J Vet Res (1995) 56:1322–4. [PubMed] [Google Scholar]
- 25.Windsor PA, Whittington RJ. Evidence for age susceptibility of cattle to Johne’s disease. Vet J (2010) 184:37–44. 10.1016/j.tvjl.2009.01.007 [DOI] [PubMed] [Google Scholar]
- 26.Bermudez LE, Petrofsky M, Sommer S, Barletta RG. Peyer’s patch-deficient mice demonstrate that Mycobacterium avium subsp. paratuberculosis translocates across the mucosal barrier via both M cells and enterocytes but has inefficient dissemination. Infect Immun (2010) 78:3570–7. 10.1128/IAI.01411-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bannantine JP, Bermudez LE. No holes barred: invasion of the intestinal mucosa by Mycobacterium avium subsp. paratuberculosis. Infect Immun (2013) 81:3960–5. 10.1128/IAI.00575-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuo CJ, Bell H, Hsieh CL, Ptak CP, Chang YF. Novel mycobacteria antigen 85 complex binding motif on fibronectin. J Biol Chem (2012) 287:1892–902. 10.1074/jbc.M111.298687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bannantine JP, Huntley JF, Miltner E, Stabel JR, Bermudez LE. The Mycobacterium avium subsp. paratuberculosis 35 kDa protein plays a role in invasion of bovine epithelial cells. Microbiology (2003) 149:2061–9. 10.1099/mic.0.26323-0 [DOI] [PubMed] [Google Scholar]
- 30.Alonso-Hearn M, Patel D, Danelishvili L, Meunier-Goddik L, Bermudez LE. The Mycobacterium avium subsp. paratuberculosis MAP3464 gene encodes an oxidoreductase involved in invasion of bovine epithelial cells through the activation of host cell Cdc42. Infect Immun (2008) 76:170–8. 10.1128/IAI.01913-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Secott TE, Lin TL, Wu CC. Fibronectin attachment protein homologue mediates fibronectin binding by Mycobacterium avium subsp. paratuberculosis. Infect Immun (2001) 69:2075–82. 10.1128/IAI.69.4.2075-2082.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Secott TE, Lin TL, Wu CC. Fibronectin attachment protein is necessary for efficient attachment and invasion of epithelial cells by Mycobacterium avium subsp. paratuberculosis. Infect Immun (2002) 70:2670–5. 10.1128/IAI.70.5.2670-2675.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lefrancois LH, Pujol C, Bodier CC, Teixeira-Gomez AP, Drobecq H, Rosso ML, et al. Characterization of the Mycobacterium avium subsp. paratuberculosis laminin-binding/histone-like protein (Lbp/Hlp) which reacts with sera from patients with Crohn’s disease. Microbes Infect (2011) 13:585–94. 10.1016/j.micinf.2011.02.002 [DOI] [PubMed] [Google Scholar]
- 34.Patel D, Danelishvili L, Yamazaki Y, Alonso M, Paustian ML, Bannantine JP, et al. The ability of Mycobacterium avium subsp. paratuberculosis to enter bovine epithelial cells is influenced by preexposure to a hyperosmolar environment and intracellular passage in bovine mammary epithelial cells. Infect Immun (2006) 74:2849–55. 10.1128/IAI.74.5.2849-2855.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamont EA, O’grady SM, Davis WC, Eckstein T, Sreevatsan S. Infection with Mycobacterium avium subsp. paratuberculosis results in rapid interleukin-1beta release and macrophage transepithelial migration. Infect Immun (2012) 80:3225–35. 10.1128/IAI.06322-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Momotani E, Whipple DL, Thiermann AB, Cheville NF. Role of M cells and macrophages in the entrance of Mycobacterium paratuberculosis into domes of ileal Peyer’s patches in calves. Vet Pathol (1988) 25:131–7. 10.1177/030098588802500205 [DOI] [PubMed] [Google Scholar]
- 37.Fujimura Y, Owen RL. M cells as portals of infection: clinical and pathophysiological aspects. Infect Agents Dis (1996) 5:144–56. [PubMed] [Google Scholar]
- 38.Lugton I. Mucosa-associated lymphoid tissues as sites for uptake, carriage and excretion of tubercle bacilli and other pathogenic mycobacteria. Immunol Cell Biol (1999) 77:364–72. 10.1046/j.1440-1711.1999.00836.x [DOI] [PubMed] [Google Scholar]
- 39.Kaufmann SH. Immunity to intracellular bacteria. Annu Rev Immunol (1993) 11:129–63. 10.1146/annurev.iy.11.040193.001021 [DOI] [PubMed] [Google Scholar]
- 40.Zhao B, Collins MT, Czuprynski CJ. Effects of gamma interferon and nitric oxide on the interaction of Mycobacterium avium subsp. paratuberculosis with bovine monocytes. Infect Immun (1997) 65:1761–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Everman JL, Eckstein TM, Roussey J, Coussens P, Bannantine JP, Bermudez LE. Characterization of the inflammatory phenotype of Mycobacterium avium subspecies paratuberculosis using a novel cell culture passage model. Microbiology (2015) 161:1420–34. 10.1099/mic.0.000106 [DOI] [PubMed] [Google Scholar]
- 42.El Chamy L, Leclerc V, Caldelari I, Reichhart JM. Sensing of ‘danger signals’ and pathogen-associated molecular patterns defines binary signaling pathways ‘upstream’ of toll. Nat Immunol (2008) 9:1165–70. 10.1038/ni.1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coussens PM, Verman N, Coussens MA, Elftman MD, Mcnulty AM. Cytokine gene expression in peripheral blood mononuclear cells and tissues of cattle infected with Mycobacterium avium subsp. paratuberculosis: evidence for an inherent proinflammatory gene expression pattern. Infect Immun (2004) 72:1409–22. 10.1128/IAI.72.3.1409-1422.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao BY, Czuprynski CJ, Collins MT. Intracellular fate of Mycobacterium avium subspecies paratuberculosis in monocytes from normal and infected, interferon-responsive cows as determined by a radiometric method. Can J Vet Res (1999) 63:56–61. [PMC free article] [PubMed] [Google Scholar]
- 45.Stabel JR. Transitions in immune responses to Mycobacterium paratuberculosis. Vet Microbiol (2000) 77:465–73. 10.1016/S0378-1135(00)00331-X [DOI] [PubMed] [Google Scholar]
- 46.Li RW, Li C, Gasbarre LC. The vitamin D receptor and inducible nitric oxide synthase associated pathways in acquired resistance to Cooperia oncophora infection in cattle. Vet Res (2011) 42:48. 10.1186/1297-9716-42-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khalifeh MS, Al-Majali AM, Stabel JR. Role of nitric oxide production in dairy cows naturally infected with Mycobacterium avium subsp. paratuberculosis. Vet Immunol Immunopathol (2009) 131:97–104. 10.1016/j.vetimm.2009.03.020 [DOI] [PubMed] [Google Scholar]
- 48.Tooker BC, Burton JL, Coussens PM. Survival tactics of M. paratuberculosis in bovine macrophage cells. Vet Immunol Immunopathol (2002) 87:429–37. 10.1016/S0165-2427(02)00065-X [DOI] [PubMed] [Google Scholar]
- 49.Coussens PM, Sipkovsky S, Murphy B, Roussey J, Colvin CJ. Regulatory T cells in cattle and their potential role in bovine paratuberculosis. Comp Immunol Microbiol Infect Dis (2012) 35:233–9. 10.1016/j.cimid.2012.01.004 [DOI] [PubMed] [Google Scholar]
- 50.Weiss DJ, Evanson OA, Mcclenahan DJ, Abrahamsen MS, Walcheck BK. Regulation of expression of major histocompatibility antigens by bovine macrophages infected with Mycobacterium avium subsp. paratuberculosis or Mycobacterium avium subsp. avium. Infect Immun (2001) 69:1002–8. 10.1128/IAI.69.2.1002-1008.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coussens PM, Pudrith CB, Skovgaard K, Ren X, Suchyta SP, Stabel JR, et al. Johne’s disease in cattle is associated with enhanced expression of genes encoding IL-5, GATA-3, tissue inhibitors of matrix metalloproteinases 1 and 2, and factors promoting apoptosis in peripheral blood mononuclear cells. Vet Immunol Immunopathol (2005) 105:221–34. 10.1016/j.vetimm.2005.02.009 [DOI] [PubMed] [Google Scholar]
- 52.Weiss DJ, Evanson OA, Souza CD. Expression of interleukin-10 and suppressor of cytokine signaling-3 associated with susceptibility of cattle to infection with Mycobacterium avium subsp paratuberculosis. Am J Vet Res (2005) 66:1114–20. 10.2460/ajvr.2005.66.1114 [DOI] [PubMed] [Google Scholar]
- 53.Stabel JR, Waters WR, Bannantine JP, Lyashchenko K. Mediation of host immune responses after immunization of neonatal calves with a heat-killed Mycobacterium avium subsp. paratuberculosis vaccine. Clin Vaccine Immunol (2011) 18:2079–89. 10.1128/CVI.05421-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robinson MW, O’brien R, Mackintosh CG, Clark RG, Griffin JF. Immunoregulatory cytokines are associated with protection from immunopathology following Mycobacterium avium subspecies paratuberculosis infection in red deer. Infect Immun (2011) 79:2089–97. 10.1128/IAI.00779-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Woo SR, Heintz JA, Albrecht R, Barletta RG, Czuprynski CJ. Life and death in bovine monocytes: the fate of Mycobacterium avium subsp. paratuberculosis. Microb Pathog (2007) 43:106–13. 10.1016/j.micpath.2007.04.004 [DOI] [PubMed] [Google Scholar]
- 56.Kuehnel MP, Goethe R, Habermann A, Mueller E, Rohde M, Griffiths G, et al. Characterization of the intracellular survival of Mycobacterium avium ssp. paratuberculosis: phagosomal pH and fusogenicity in J774 macrophages compared with other mycobacteria. Cell Microbiol (2001) 3:551–66. 10.1046/j.1462-5822.2001.00139.x [DOI] [PubMed] [Google Scholar]
- 57.Bannantine JP, Stabel JR. Killing of Mycobacterium avium subspecies paratuberculosis within macrophages. BMC Microbiol (2002) 2:2. 10.1186/1471-2180-2-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khare S, Lawhon SD, Drake KL, Nunes JE, Figueiredo JF, Rossetti CA, et al. Systems biology analysis of gene expression during in vivo Mycobacterium avium paratuberculosis enteric colonization reveals role for immune tolerance. PLoS One (2012) 7:e42127. 10.1371/journal.pone.0042127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khare S, Drake KL, Lawhon SD, Nunes JE, Figueiredo JF, Rossetti CA, et al. Systems analysis of early host gene expression provides clues for transient Mycobacterium avium ssp avium vs. persistent Mycobacterium avium ssp paratuberculosis intestinal infections. PLoS One (2016) 11:e0161946. 10.1371/journal.pone.0161946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chaubey KK, Gupta RD, Gupta S, Singh SV, Bhatia AK, Jayaraman S, et al. Trends and advances in the diagnosis and control of paratuberculosis in domestic livestock. Vet Q (2016) 36:203–27. 10.1080/01652176.2016.1196508 [DOI] [PubMed] [Google Scholar]
- 61.Manning EJ, Collins MT. Mycobacterium avium subsp. paratuberculosis: pathogen, pathogenesis and diagnosis. Rev Sci Tech (2001) 20:133–50. 10.20506/rst.20.1.1275 [DOI] [PubMed] [Google Scholar]
- 62.Bull TJ, Munshi T, Mikkelsen H, Hartmann SB, Sorensen MR, Garcia JS, et al. Improved culture medium (TiKa) for Mycobacterium avium subspecies paratuberculosis (MAP) matches qPCR sensitivity and reveals significant proportions of non-viable MAP in lymphoid tissue of vaccinated MAP challenged animals. Front Microbiol (2017) 7:2112. 10.3389/fmicb.2016.02112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vary PH, Andersen PR, Green E, Hermon-Taylor J, Mcfadden JJ. Use of highly specific DNA probes and the polymerase chain reaction to detect Mycobacterium paratuberculosis in Johne’s disease. J Clin Microbiol (1990) 28:933–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cousins DV, Whittington R, Marsh I, Masters A, Evans RJ, Kluver P. Mycobacteria distenct from Mycobacterium avium subsp. paratuberculosis isolated from the faeces of ruminants possess IS900-like sequences detectable IS900 polymerase chain reaction: implications for diagnosis. Mol Cell Probes (1999) 13:431–42. 10.1006/mcpr.1999.0275 [DOI] [PubMed] [Google Scholar]
- 65.Ellingson JL, Stabel JR, Bishai WR, Frothingham R, Miller JM. Evaluation of the accuracy and reproducibility of a practical PCR panel assay for rapid detection and differentiation of Mycobacterium avium subspecies. Mol Cell Probes (2000) 14:153–61. 10.1006/mcpr.2000.0299 [DOI] [PubMed] [Google Scholar]
- 66.Harris NB, Barletta RG. Mycobacterium avium subsp. paratuberculosis in Veterinary Medicine. Clin Microbiol Rev (2001) 14:489–512. 10.1128/CMR.14.3.489-512.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Englund S, Bolske G, Johansson KE. An IS900-like sequence found in a Mycobacterium sp. other than Mycobacterium avium subsp. paratuberculosis. FEMS Microbiol Lett (2002) 209:267–71. 10.1111/j.1574-6968.2002.tb11142.x [DOI] [PubMed] [Google Scholar]
- 68.Rachlin J, Ding C, Cantor C, Kasif S. Computational tradeoffs in multiplex PCR assay design for SNP genotyping. BMC Genomics (2005) 6:102. 10.1186/1471-2164-6-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Clark DL, Jr, Koziczkowski JJ, Radcliff RP, Carlson RA, Ellingson JL. Detection of Mycobacterium avium subspecies paratuberculosis: comparing fecal culture versus serum enzyme-linked immunosorbent assay and direct fecal polymerase chain reaction. J Dairy Sci (2008) 91:2620–7. 10.3168/jds.2007-0902 [DOI] [PubMed] [Google Scholar]
- 70.Bannantine JP, Baechler E, Zhang Q, Li L, Kapur V. Genome scale comparison of Mycobacterium avium subsp. paratuberculosis with Mycobacterium avium subsp. avium reveals potential diagnostic sequences. J Clin Microbiol (2002) 40:1303–10. 10.1128/JCM.40.4.1303-1310.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rajeev S, Zhang Y, Sreevatsan S, Motiwala AS, Byrum B. Evaluation of multiple genomic targets for identification and confirmation of Mycobacterium avium subsp. paratuberculosis isolates using real-time PCR. Vet Microbiol (2005) 105:215–21. 10.1016/j.vetmic.2004.10.018 [DOI] [PubMed] [Google Scholar]
- 72.Stabel JR, Bannantine JP. Development of a nested PCR method targeting a unique multicopy element, ISMap02, for detection of Mycobacterium avium subsp. paratuberculosis in fecal samples. J Clin Microbiol (2005) 43:4744–50. 10.1128/JCM.43.9.4744-4750.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maroudam V, Mohana Subramanian B, Praveen Kumar P, Dhinakar Raj G. Paratuberculosis: diagnostic methods and their constraints. J Vet Sci Technol (2015) 6:259. 10.4172/2157-7579.1000259 [DOI] [Google Scholar]
- 74.Jungersen G, Mikkelsen H, Grell SN. Use of the johnin PPD interferon-gamma assay in control of bovine paratuberculosis. Vet Immunol Immunopathol (2012) 148:48–54. 10.1016/j.vetimm.2011.05.010 [DOI] [PubMed] [Google Scholar]
- 75.Holbert S, Branger M, Souriau A, Lamoureux B, Ganneau C, Richard G, et al. Interferon gamma response to Mycobacterium avium subsp. paratuberculosis specific lipopentapeptide antigen L5P in cattle. Res Vet Sci (2015) 102:118–21. 10.1016/j.rvsc.2015.07.017 [DOI] [PubMed] [Google Scholar]
- 76.Collins MT, Wells SJ, Petrini KR, Collins JE, Schultz RD, Whitlock RH. Evaluation of five antibody detection tests for diagnosis of bovine paratuberculosis. Clin Diagn Lab Immunol (2005) 12:685–92. 10.1128/CDLI.12.6.685-692.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Collins MT. Diagnosis of paratuberculosis. Vet Clin North Am Food Anim Pract (2011) 27:581–91, vi. 10.1016/j.cvfa.2011.07.013 [DOI] [PubMed] [Google Scholar]
- 78.Carter MA. Prevalence and prevention of paratuberculosis in North America. Jpn J Vet Res (2012) 60(Suppl):S9–18. 10.14943/jjvr.60.suppl.s9 [DOI] [PubMed] [Google Scholar]
- 79.Bastida F, Juste RA. Paratuberculosis control: a review with a focus on vaccination. J Immune Based Ther Vaccines (2011) 9:8. 10.1186/1476-8518-9-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hines ME, II, Turnquist SE, Ilha MR, Rajeev S, Jones AL, Whittington L, et al. Evaluation of novel oral vaccine candidates and validation of a caprine model of Johne’s disease. Front Cell Infect Microbiol (2014) 4:26. 10.3389/fcimb.2014.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bull TJ, Schock A, Sharp JM, Greene M, Mckendrick IJ, Sales J, et al. Genomic variations associated with attenuation in Mycobacterium avium subsp. paratuberculosis vaccine strains. BMC Microbiol (2013) 13:11. 10.1186/1471-2180-13-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lu Z, Schukken YH, Smith RL, Grohn YT. Using vaccination to prevent the invasion of Mycobacterium avium subsp. paratuberculosis in dairy herds: a stochastic simulation study. Prev Vet Med (2013) 110:335–45. 10.1016/j.prevetmed.2013.01.006 [DOI] [PubMed] [Google Scholar]
- 83.Walker KB, Brennan MJ, Ho MM, Eskola J, Thiry G, Sadoff J, et al. The second Geneva consensus: recommendations for novel live TB vaccines. Vaccine (2010) 28:2259–70. 10.1016/j.vaccine.2009.12.083 [DOI] [PubMed] [Google Scholar]
- 84.Hines ME, II, Stiver S, Giri D, Whittington L, Watson C, Johnson J, et al. Efficacy of spheroplastic and cell-wall competent vaccines for Mycobacterium avium subsp. paratuberculosis in experimentally-challenged baby goats. Vet Microbiol (2007) 120:261–83. 10.1016/j.vetmic.2006.10.030 [DOI] [PubMed] [Google Scholar]
- 85.Achkar JM, Casadevall A. Antibody-mediated immunity against tuberculosis: implications for vaccine development. Cell Host Microbe (2013) 13:250–62. 10.1016/j.chom.2013.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Everman JL, Bermudez LE. Antibodies against invasive phenotype-specific antigens increase Mycobacterium avium subspecies paratuberculosis translocation across a polarized epithelial cell model and enhance killing by bovine macrophages. Front Cell Infect Microbiol (2015) 5:58. 10.3389/fcimb.2015.00058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chiodini RJ, Van Kruiningen HJ, Thayer WR, Merkal RS, Coutu JA. Possible role of mycobacteria in inflammatory bowel disease. I. An unclassified Mycobacterium species isolated from patients with Crohn’s disease. Dig Dis Sci (1984) 29:1073–9. 10.1007/BF01317078 [DOI] [PubMed] [Google Scholar]
- 88.Di Sabatino A, Paccagnini D, Vidali F, Rosu V, Biancheri P, Cossu A, et al. Detection of Mycobacterium avium subsp. paratuberculosis (MAP)-specific IS900 DNA and antibodies against MAP peptides and lysate in the blood of Crohn’s disease patients. Inflamm Bowel Dis (2011) 17:1254–5. 10.1002/ibd.21461 [DOI] [PubMed] [Google Scholar]
- 89.Niegowska M, Rapini N, Biet F, Piccinini S, Bay S, Lidano R, et al. Seroreactivity against specific L5P antigen from Mycobacterium avium subsp. paratuberculosis in children at risk for T1D. PLoS One (2016) 11:e0157962. 10.1371/journal.pone.0157962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sechi LA, Paccagnini D, Salza S, Pacifico A, Ahmed N, Zanetti S. Mycobacterium avium subspecies paratuberculosis bacteremia in type 1 diabetes mellitus: an infectious trigger? Clin Infect Dis (2008) 46:148–9. 10.1086/524084 [DOI] [PubMed] [Google Scholar]
- 91.Paccagnini D, Sieswerda L, Rosu V, Masala S, Pacifico A, Gazouli M, et al. Linking chronic infection and autoimmune diseases: Mycobacterium avium subspecies paratuberculosis, SLC11A1 polymorphisms and type-1 diabetes mellitus. PLoS One (2009) 4:e7109. 10.1371/journal.pone.0007109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sisto M, Cucci L, D’amore M, Dow TC, Mitolo V, Lisi S. Proposing a relationship between Mycobacterium avium subspecies paratuberculosis infection and Hashimoto’s thyroiditis. Scand J Infect Dis (2010) 42:787–90. 10.3109/00365541003762306 [DOI] [PubMed] [Google Scholar]
- 93.Cossu D, Masala S, Sechi LA. A Sardinian map for multiple sclerosis. Future Microbiol (2013) 8:223–32. 10.2217/fmb.12.135 [DOI] [PubMed] [Google Scholar]
- 94.Waddell LA, Rajic A, Stark KD, Mc ES. The zoonotic potential of Mycobacterium avium ssp. paratuberculosis: a systematic review and meta-analyses of the evidence. Epidemiol Infect (2015) 143:3135–57. 10.1017/S095026881500076X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Arru G, Caggiu E, Paulus K, Sechi GP, Mameli G, Sechi LA. Is there a role for Mycobacterium avium subspecies paratuberculosis in Parkinson’s disease? J Neuroimmunol (2016) 293:86–90. 10.1016/j.jneuroim.2016.02.016 [DOI] [PubMed] [Google Scholar]
- 96.Niegowska M, Delitala A, Pes GM, Delitala G, Sechi LA. Increased seroreactivity to proinsulin and homologous mycobacterial peptides in latent autoimmune diabetes in adults. PLoS One (2017) 12:e0176584. 10.1371/journal.pone.0176584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Graham DY, Markesich DC, Yoshimura HH. Mycobacteria and inflammatory bowel disease. Results of culture. Gastroenterology (1987) 92:436–42. 10.1016/0016-5085(87)90139-9 [DOI] [PubMed] [Google Scholar]
- 98.Bull TJ, Mcminn EJ, Sidi-Boumedine K, Skull A, Durkin D, Neild P, et al. Detection and verification of Mycobacterium avium subsp. paratuberculosis in fresh ileocolonic mucosal biopsy specimens from individuals with and without Crohn’s disease. J Clin Microbiol (2003) 41:2915–23. 10.1128/JCM.41.7.2915-2923.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Scanu AM, Bull TJ, Cannas S, Sanderson JD, Sechi LA, Dettori G, et al. Mycobacterium avium subspecies paratuberculosis infection in cases of irritable bowel syndrome and comparison with Crohn’s disease and Johne’s disease: common neural and immune pathogenicities. J Clin Microbiol (2007) 45:3883–90. 10.1128/JCM.01371-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mortier RA, Barkema HW, Bystrom JM, Illanes O, Orsel K, Wolf R, et al. Evaluation of age-dependent susceptibility in calves infected with two doses of Mycobacterium avium subspecies paratuberculosis using pathology and tissue culture. Vet Res (2013) 44:94. 10.1186/1297-9716-44-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Naser SA, Schwartz D, Shafran I. Isolation of Mycobacterium avium subsp paratuberculosis from breast milk of Crohn’s disease patients. Am J Gastroenterol (2000) 95:1094–5. 10.1111/j.1572-0241.2000.01954.x [DOI] [PubMed] [Google Scholar]
- 102.Naser SA, Ghobrial G, Romero C, Valentine JF. Culture of Mycobacterium avium subspecies paratuberculosis from the blood of patients with Crohn’s disease. Lancet (2004) 364:1039–44. 10.1016/S0140-6736(04)17058-X [DOI] [PubMed] [Google Scholar]
- 103.Kirkwood CD, Wagner J, Boniface K, Vaughan J, Michalski WP, Catto-Smith AG, et al. Mycobacterium avium subspecies paratuberculosis in children with early-onset Crohn’s disease. Inflamm Bowel Dis (2009) 15:1643–55. 10.1002/ibd.20967 [DOI] [PubMed] [Google Scholar]
- 104.McNees AL, Markesich D, Zayyani NR, Graham DY. Mycobacterium paratuberculosis as a cause of Crohn’s disease. Expert Rev Gastroenterol Hepatol (2015) 9:1523–34. 10.1586/17474124.2015.1093931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Millar D, Ford J, Sanderson J, Withey S, Tizard M, Doran T, et al. IS900 PCR to detect Mycobacterium paratuberculosis in retail supplies of whole pasteurized cows’ milk in England and Wales. Appl Environ Microbiol (1996) 62:3446–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stabel JR, Lambertz A. Efficacy of pasteurization conditions for the inactivation of Mycobacterium avium subsp. paratuberculosis in milk. J Food Prot (2004) 67:2719–26. 10.4315/0362-028X-67.12.2719 [DOI] [PubMed] [Google Scholar]
- 107.Selby W, Pavli P, Crotty B, Florin T, Radford-Smith G, Gibson P, et al. Two-year combination antibiotic therapy with clarithromycin, rifabutin, and clofazimine for Crohn’s disease. Gastroenterology (2007) 132:2313–9. 10.1053/j.gastro.2007.03.031 [DOI] [PubMed] [Google Scholar]
- 108.Greenstein RJ, Su L, Juste RA, Brown ST. On the action of cyclosporine A, rapamycin and tacrolimus on M. avium including subspecies paratuberculosis. PLoS One (2008) 3:e2496. 10.1371/journal.pone.0002496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Alcedo KP, Thanigachalam S, Naser SA. RHB-104 triple antibiotics combination in culture is bactericidal and should be effective for treatment of Crohn’s disease associated with Mycobacterium paratuberculosis. Gut Pathog (2016) 8:32. 10.1186/s13099-016-0115-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Krishnan MY, Manning EJ, Collins MT. Comparison of three methods for susceptibility testing of Mycobacterium avium subsp. paratuberculosis to 11 antimicrobial drugs. J Antimicrob Chemother (2009) 64:310–6. 10.1093/jac/dkp184 [DOI] [PubMed] [Google Scholar]
- 111.Krishnan MY, Manning EJ, Collins MT. Effects of interactions of antibacterial drugs with each other and with 6-mercaptopurine on in vitro growth of Mycobacterium avium subspecies paratuberculosis. J Antimicrob Chemother (2009) 64:1018–23. 10.1093/jac/dkp339 [DOI] [PubMed] [Google Scholar]
- 112.More S, Bøtner A, Butterworth A, Calistri P, Depner K, Edwards S, et al. Assessment of listing and categorisation of animal diseases within the framework of the Animal Health Law (Regulation (EU) No 2016/429): paratuberculosis. EFSA J (2017) 15:e04960. 10.2903/j.efsa.2017.4960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wu CW, Schramm TM, Zhou S, Schwartz DC, Talaat AM. Optical mapping of the Mycobacterium avium subspecies paratuberculosis genome. BMC Genomics (2009) 10:25. 10.1186/1471-2164-10-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wynne JW, Seemann T, Bulach DM, Coutts SA, Talaat AM, Michalski WP.Resequencing the Mycobacterium avium subsp. paratuberculosis K10 genome: improved annotation and revised genome sequence. J Bacteriol (2010) 192:6319–20. 10.1128/JB.00972-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bannantine JP, Wu CW, Hsu C, Zhou S, Schwartz DC, Bayles DO, et al. Genome sequencing of ovine isolates of Mycobacterium avium subspecies paratuberculosis offers insights into host association. BMC Genomics (2012) 13:89. 10.1186/1471-2164-13-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Marri PR, Bannantine JP, Golding GB. Comparative genomics of metabolic pathways in Mycobacterium species: gene duplication, gene decay and lateral gene transfer. FEMS Microbiol Rev (2006) 30:906–25. 10.1111/j.1574-6976.2006.00041.x [DOI] [PubMed] [Google Scholar]
- 117.Bannantine JP, Stabel JR, Lamont EA, Briggs RE, Sreevatsan S. Monoclonal antibodies bind A SNP-sensitive epitope that is present uniquely in Mycobacterium avium subspecies paratuberculosis. Front Microbiol (2011) 2:163. 10.3389/fmicb.2011.00163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bannantine JP, Li L, Mwangi M, Cote R, Raygoza Garay JA, Kapur V. Complete genome sequence of Mycobacterium avium subsp. paratuberculosis, isolated from human breast milk. Genome Announc (2014) 2:e01252–13. 10.1128/genomeA.01252-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mobius P, Nordsiek G, Holzer M, Jarek M, Marz M, Kohler H. Complete genome sequence of JII-1961, a bovine Mycobacterium avium subsp. paratuberculosis field isolate from Germany. Genome Announc (2017) 5:e00870–17. 10.1128/genomeA.00870-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wynne JW, Bull TJ, Seemann T, Bulach DM, Wagner J, Kirkwood CD, et al. Exploring the zoonotic potential of Mycobacterium avium subspecies paratuberculosis through comparative genomics. PLoS One (2011) 6:e22171. 10.1371/journal.pone.0022171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mobius P, Holzer M, Felder M, Nordsiek G, Groth M, Kohler H, et al. Comprehensive insights in the Mycobacterium avium subsp. paratuberculosis genome using new WGS data of sheep strain JIII-386 from Germany. Genome Biol Evol (2015) 7:2585–601. 10.1093/gbe/evv154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yue R, Liu C, Barrow P, Liu F, Cui Y, Yang L, et al. The isolation and molecular characterization of Mycobacterium avium subsp. paratuberculosis in Shandong province, China. Gut Pathog (2016) 8:9. 10.1186/s13099-016-0092-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Foley-Thomas EM, Whipple DL, Bermudez LE, Barletta RG. Phage infection, transfection and transformation of Mycobacterium avium complex and Mycobacterium paratuberculosis. Microbiology (1995) 141(Pt 5):1173–81. 10.1099/13500872-141-5-1173 [DOI] [PubMed] [Google Scholar]
- 124.Chacon O, Barletta RG. Chapter 9. Molecular Genetics of Mycobacterium avium subspecies paratuberculosis. In: Behr MA, Collins DM, editors. Paratuberculosis: Organism, Disease, Control. Cambridge, MA: CAB Publishing; (2010). p. 83–93. [Google Scholar]
- 125.Timme TL, Brennan PJ. Induction of bacteriophage from members of the Mycobacterium avium, Mycobacterium intracellulare, Mycobacterium scrofulaceum serocomplex. J Gen Microbiol (1984) 130:2059–66. 10.1099/00221287-130-8-2059 [DOI] [PubMed] [Google Scholar]
- 126.Bardarov S, Kriakov J, Carriere C, Yu S, Vaamonde C, Mcadam RA, et al. Conditionally replicating mycobacteriophages: a system for transposon delivery to Mycobacterium tuberculosis. Proc Natl Acad Sci U S A (1997) 94:10961–6. 10.1073/pnas.94.20.10961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shin SJ, Wu CW, Steinberg H, Talaat AM. Identification of novel virulence determinants in Mycobacterium paratuberculosis by screening a library of insertional mutants. Infect Immun (2006) 74:3825–33. 10.1128/IAI.01742-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rathnaiah G, Lamont EA, Harris NB, Fenton RJ, Zinniel DK, Liu X, et al. Generation and screening of a comprehensive Mycobacterium avium subsp. paratuberculosis transposon mutant bank. Front Cell Infect Microbiol (2014) 4:144. 10.3389/fcimb.2014.00144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cirillo JD, Barletta RG, Bloom BR, Jacobs WR., Jr A novel transposon trap for mycobacteria isolation and characterization of IS1096. J Bacteriol (1991) 173:7772–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.McAdam RA, Quan S, Smith DA, Bardarov S, Betts JC, Cook FC, et al. Characterization of a Mycobacterium tuberculosis H37Rv transposon library reveals insertions in 351 ORFs and mutants with altered virulence. Microbiology (2002) 148:2975–86. 10.1099/00221287-148-10-2975 [DOI] [PubMed] [Google Scholar]
- 131.Sassetti CM, Boyd DH, Rubin EJ. Comprehensive identification of conditionally essential genes in mycobacteria. Proc Natl Acad Sci U S A (2001) 98:12712–7. 10.1073/pnas.231275498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Scandurra GM, Young M, De Lisle GW, Collins DM. A bovine macrophage screening system for identifying attenuated transposon mutants of Mycobacterium avium subsp. paratuberculosis with vaccine potential. J Microbiol Methods (2009) 77:58–62. 10.1016/j.mimet.2009.01.005 [DOI] [PubMed] [Google Scholar]
- 133.Wang J, Pritchard JR, Kreitmann L, Montpetit A, Behr MA. Disruption of Mycobacterium avium subsp. paratuberculosis-specific genes impairs in vivo fitness. BMC Genomics (2014) 15:415. 10.1186/1471-2164-15-415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rathnaiah G, Bannantine JP, Bayles DO, Zinniel DK, Stabel JR, Grohn YT, et al. Analysis of Mycobacterium avium subsp. paratuberculosis mutant libraries reveals loci-dependent transposition biases and strategies to novel mutant discovery. Microbiology (2016) 162:633–41. 10.1099/mic.0.000258 [DOI] [PubMed] [Google Scholar]
- 135.Harris NB, Feng Z, Liu X, Cirillo SLG, Cirillo JD, Barletta RG. Development of a transposon mutagenesis system for Mycobacterium avium subsp. paratuberculosis. FEMS Microbiol Lett (1999) 175:21–26. [DOI] [PubMed] [Google Scholar]
- 136.Cavaignac SM, White SJ, de Lisle GW, Collins DM. Construction and screening of Mycobacterium paratuberculosis insertional mutant libraries. Arch Microbiol (2000) 173:229–31. [DOI] [PubMed] [Google Scholar]
- 137.Bannantine JP, Paustian ML. Identification of diagnostic proteins in Mycobacterium avium subspecies paratuberculosis by a whole genome analysis approach. Methods Mol Biol (2006) 345:185–96. 10.1385/1-59745-143-6 [DOI] [PubMed] [Google Scholar]
- 138.Cho D, Shin SJ, Talaat AM, Collins MT. Cloning, expression, purification and serodiagnostic evaluation of fourteen Mycobacterium paratuberculosis proteins. Protein Expr Purif (2007) 53:411–20. 10.1016/j.pep.2006.12.022 [DOI] [PubMed] [Google Scholar]
- 139.Li L, Munir S, Bannantine JP, Sreevatsan S, Kanjilal S, Kapur V. Rapid expression of Mycobacterium avium subsp. paratuberculosis recombinant proteins for antigen discovery. Clin Vaccine Immunol (2007) 14:102–5. 10.1128/CVI.00138-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sampson SL, Dascher CC, Sambandamurthy VK, Russell RG, Jacobs WR, Jr, Bloom BR, et al. Protection elicited by a double leucine and pantothenate auxotroph of Mycobacterium tuberculosis in guinea pigs. Infect Immun (2004) 72:3031–7. 10.1128/IAI.72.5.3031-3037.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bach H, Sun J, Hmama Z, Av-Gay Y. Mycobacterium avium subsp. paratuberculosis PtpA is an endogenous tyrosine phosphatase secreted during infection. Infect Immun (2006) 74:6540–6. 10.1128/IAI.01106-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chen JW, Faisal SM, Chandra S, Mcdonough SP, Moreira MA, Scaria J, et al. Immunogenicity and protective efficacy of the Mycobacterium avium subsp. paratuberculosis attenuated mutants against challenge in a mouse model. Vaccine (2012) 30:3015–25. 10.1016/j.vaccine.2011.11.029 [DOI] [PubMed] [Google Scholar]
- 143.Chen JW, Scaria J, Chang YF. Phenotypic and transcriptomic response of auxotrophic Mycobacterium avium subsp. paratuberculosis leuD mutant under environmental stress. PLoS One (2012) 7:e37884. 10.1371/journal.pone.0037884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wu CW, Schmoller SK, Shin SJ, Talaat AM. Defining the stressome of Mycobacterium avium subsp. paratuberculosis in vitro and in naturally infected cows. J Bacteriol (2007) 189:7877–86. 10.1128/JB.00780-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Park KT, Dahl JL, Bannantine JP, Barletta RG, Ahn J, Allen AJ, et al. Demonstration of allelic exchange in the slow-growing bacterium Mycobacterium avium subsp. paratuberculosis, and generation of mutants with deletions at the pknG, relA, and lsr2 loci. Appl Environ Microbiol (2008) 74:1687–95. 10.1128/AEM.01208-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Park KT, Allen AJ, Bannantine JP, Seo KS, Hamilton MJ, Abdellrazeq GS, et al. Evaluation of two mutants of Mycobacterium avium subsp. paratuberculosis as candidates for a live attenuated vaccine for Johne’s disease. Vaccine (2011) 29:4709–19. 10.1016/j.vaccine.2011.04.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ghosh P, Wu CW, Talaat AM. Key role for the alternative sigma factor, SigH, in the intracellular life of Mycobacterium avium subsp. paratuberculosis during macrophage stress. Infect Immun (2013) 81:2242–57. 10.1128/IAI.01273-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ghosh P, Steinberg H, Talaat AM. Virulence and immunity orchestrated by the global gene regulator sigL in Mycobacterium avium subsp. paratuberculosis. Infect Immun (2014) 82:3066–75. 10.1128/IAI.00001-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shippy DC, Lemke JJ, Berry A, Nelson K, Hines ME, II, Talaat AM. Superior protection from live-attenuated vaccines directed against Johne’s disease. Clin Vaccine Immunol (2017) 24:e00478–16. 10.1128/CVI.00478-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wu CW, Glasner J, Collins M, Naser S, Talaat AM. Whole-genome plasticity among Mycobacterium avium subspecies: insights from comparative genomic hybridizations. J Bacteriol (2006) 188:711–23. 10.1128/JB.188.2.711-723.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Johnston C, Douarre PE, Soulimane T, Pletzer D, Weingart H, Macsharry J, et al. Codon optimisation to improve expression of a Mycobacterium avium ssp. paratuberculosis-specific membrane-associated antigen by Lactobacillus salivarius. Pathog Dis (2013) 68:27–38. 10.1111/2049-632X.12040 [DOI] [PubMed] [Google Scholar]
- 152.Johnston CD, Bannantine JP, Govender R, Endersen L, Pletzer D, Weingart H, et al. Enhanced expression of codon optimized Mycobacterium avium subsp. paratuberculosis antigens in Lactobacillus salivarius. Front Cell Infect Microbiol (2014) 4:120. 10.3389/fcimb.2014.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bull TJ, Gilbert SC, Sridhar S, Linedale R, Dierkes N, Sidi-Boumedine K, et al. A novel multi-antigen virally vectored vaccine against Mycobacterium avium subspecies paratuberculosis. PLoS One (2007) 2:e1229. 10.1371/journal.pone.0001229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bull TJ, Vrettou C, Linedale R, Mcguinnes C, Strain S, Mcnair J, et al. Immunity, safety and protection of an Adenovirus 5 prime – modified vaccinia virus Ankara boost subunit vaccine against Mycobacterium avium subspecies paratuberculosis infection in calves. Vet Res (2014) 45:112. 10.1186/s13567-014-0112-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cooney MA, Steele JL, Steinberg H, Talaat AM. A murine oral model for Mycobacterium avium subsp. paratuberculosis infection and immunomodulation with Lactobacillus casei ATCC 334. Front Cell Infect Microbiol (2014) 4:11. 10.3389/fcimb.2014.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Koets A, Hoek A, Langelaar M, Overdijk M, Santema W, Franken P, et al. Mycobacterial 70 kD heat-shock protein is an effective subunit vaccine against bovine paratuberculosis. Vaccine (2006) 24:2550–9. 10.1016/j.vaccine.2005.12.019 [DOI] [PubMed] [Google Scholar]
- 157.Santema W, Hensen S, Rutten V, Koets A. Heat shock protein 70 subunit vaccination against bovine paratuberculosis does not interfere with current immunodiagnostic assays for bovine tuberculosis. Vaccine (2009) 27:2312–9. 10.1016/j.vaccine.2009.02.032 [DOI] [PubMed] [Google Scholar]
- 158.Santema W, Rutten V, Segers R, Poot J, Hensen S, Heesterbeek H, et al. Postexposure subunit vaccination against chronic enteric mycobacterial infection in a natural host. Infect Immun (2013) 81:1990–5. 10.1128/IAI.01121-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jolly A, Morsella C, Bass L, Fiorentino MA, Paolicchi FA, Mundo SL. Bovine response to lipoarabinomannan vaccination and challenge with Mycobacterium paratuberculosis. Braz J Microbiol (2013) 44:511–4. 10.1590/S1517-83822013000200029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jolly A, Lompardia S, Hajos SE, Mundo SL. Evidence of a pro-apoptotic effect of specific antibodies in a bovine macrophage model of infection with Mycobacterium avium subsp. paratuberculosis. Vet Immunol Immunopathol (2016) 169:47–53. 10.1016/j.vetimm.2015.12.001 [DOI] [PubMed] [Google Scholar]
- 161.Mullerad J, Hovav AH, Fishman Y, Barletta RG, Bercovier H. Antigenicity of Mycobacterium paratuberculosis superoxide dismutase in mice. FEMS Immunol Med Microbiol (2002) 34:81–8. 10.1111/j.1574-695X.2002.tb00606.x [DOI] [PubMed] [Google Scholar]
- 162.Mullerad J, Michal I, Fishman Y, Hovav AH, Barletta RG, Bercovier H. The immunogenicity of Mycobacterium paratuberculosis 85B antigen. Med Microbiol Immunol (2002) 190:179–87. 10.1007/s00430-001-0104-z [DOI] [PubMed] [Google Scholar]
- 163.Stabel JR, Barnhill A, Bannantine JP, Chang YF, Osman MA. Evaluation of protection in a mouse model after vaccination with Mycobacterium avium subsp. paratuberculois protein cocktails. Vaccine (2012) 31:127–34. 10.1016/j.vaccine.2012.10.090 [DOI] [PubMed] [Google Scholar]
- 164.Thakur A, Aagaard C, Stockmarr A, Andersen P, Jungersen G. Cell-mediated and humoral immune responses after immunization of calves with a recombinant multiantigenic Mycobacterium avium subsp. paratuberculosis subunit vaccine at different ages. Clin Vaccine Immunol (2013) 20:551–8. 10.1128/CVI.05574-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sechi LA, Felis GE, Ahmed N, Paccagnini D, Usai D, Ortu S, et al. Genome and transcriptome scale portrait of sigma factors in Mycobacterium avium subsp. paratuberculosis. Infect Genet Evol (2007) 7:424–32. 10.1016/j.meegid.2007.01.001 [DOI] [PubMed] [Google Scholar]
- 166.Alonso-Hearn M, Eckstein TM, Sommer S, Bermudez LE. A Mycobacterium avium subsp. paratuberculosis LuxR regulates cell envelope and virulence. Innate Immun (2010) 16:235–47. 10.1177/1753425909339811 [DOI] [PubMed] [Google Scholar]
- 167.Janagama HK, Kumar S, Bannantine JP, Kugadas A, Jagtap P, Higgins L, et al. Iron-sparing response of Mycobacterium avium subsp. paratuberculosis is strain dependent. BMC Microbiol (2010) 10:268. 10.1186/1471-2180-10-268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Janagama HK, Lamont EA, George S, Bannantine JP, Xu WW, Tu ZJ, et al. Primary transcriptomes of Mycobacterium avium subsp. paratuberculosis reveal proprietary pathways in tissue and macrophages. BMC Genomics (2010) 11:561. 10.1186/1471-2164-11-561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sassetti CM, Boyd DH, Rubin EJ. Genes required for mycobacterial growth defined by high density mutagenesis. Mol Microbiol (2003) 48:77–84. 10.1046/j.1365-2958.2003.03425.x [DOI] [PubMed] [Google Scholar]
- 170.Rengarajan J, Bloom BR, Rubin EJ. Genome-wide requirements for Mycobacterium tuberculosis adaptation and survival in macrophages. Proc Natl Acad Sci U S A (2005) 102:8327–32. 10.1073/pnas.0503272102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Talaat AM, Lyons R, Howard ST, Johnston SA. The temporal expression profile of Mycobacterium tuberculosis infection in mice. Proc Natl Acad Sci U S A (2004) 101:4602–7. 10.1073/pnas.0306023101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Long JE, Dejesus M, Ward D, Baker RE, Ioerger T, Sassetti CM. Identifying essential genes in Mycobacterium tuberculosis by global phenotypic profiling. Methods Mol Biol (2015) 1279:79–95. 10.1007/978-1-4939-2398-4_6 [DOI] [PubMed] [Google Scholar]
- 173.Griffin JE, Gawronski JD, Dejesus MA, Ioerger TR, Akerley BJ, Sassetti CM. High-resolution phenotypic profiling defines genes essential for mycobacterial growth and cholesterol catabolism. PLoS Pathog (2011) 7:e1002251. 10.1371/journal.ppat.1002251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.DeJesus MA, Ioerger TR. A Hidden Markov Model for identifying essential and growth-defect regions in bacterial genomes from transposon insertion sequencing data. BMC Bioinformatics (2013) 14:303. 10.1186/1471-2105-14-303 [DOI] [PMC free article] [PubMed] [Google Scholar]