Abstract
Acinetobacter baumannii has been a dreadful problem for ICU physicians for a long time. Bacteremic pneumonia (BP) caused by this organism has a higher mortality compared to other organisms. Between 2012 and 2015, 86 BP and 89 non-bacteremic pneumonia (NBP) patients from five ICUs were enrolled into the study. The 7-day and 14-day mortality rates were higher in BP patients than in NBP patients (P < 0.001). Procalcitonin elevation, high APACHEII score and recent surgery, were independently associated with BP episodes. Acute respiratory distress syndrome, coma, high APACHEII score and procalcitonin elevation, were independently associated with mortality in the BP group. Extensively drug-resistant isolates were detected in 34.9% of BP and 25.8% of NBP isolates. PFGE identified 12 and 9 genotypes in the BP and NBP isolates, respectively, with 6 genotypes shared by both groups. ST195 was the most prevalent type (40%), followed by ST457 (18.9%). The pandemic clonal complex 92 was predominant, accounting for 94.3% of the strains. For all studied periods, mortality remained higher in the BP than the NBP group. Disease severity was the main risk factor for high mortality in the BP group, and other factors related to mortality were infection, and not treatment or microbiology-related.
Introduction
The prevalence of health care associated infections caused by Acinetobacter spp. is increasing among immunocompromised hosts and patients in intensive care units (ICUs)1,2. A large multinational study in Asia (ANSORP) showed that Acinetobacter spp. is the most frequently isolated bacterial species (36.5%) in ventilator associated pneumonia (VAP), and the third frequent species (13.6%) in healthcare associated pneumonia (HAP)3. In China, A. baumannii is the most frequently isolated species in both HAP and VAP patients, accounting for 16.2% and 35.7%, respectively3. Furthermore, A. baumannii has a high resistance rate to carbapenems, with a rate of 58.9% in HAP/VAP as per the ANSORP study, and of 53.7% in bloodstream infection, according to China national data3,4.Therefore infections caused by this organism present a serious clinical challenge for physicians in health-care settings, resulting in much higher crude mortality rate than other bacterial strains from HAP/VAP3.
The relationship between a positive culture of A. baumannii from lower respiratory tract (LRT) sample and the attributed mortality is less clear in the HAP or VAP patients, as the organism may be considered a colonizer or contaminant, and thus may not reflect the true etiologic pathogen of HAP and VAP. It is very difficult to distinguish between A. baumannii colonization and infection even if stringent diagnostic procedures, including quantitative culture for LRT samples, are performed5.
As reported by ANSORP, the “definite pathogen” of HAP or VAP should be the isolates from blood matching those from culture of the LRT specimens, while the “probable pathogen” and “possible pathogen” are defined as an isolate from LRT culture specimens but with a negative blood culture3. In other words, A. baumannii bacteremia is just the result of pulmonary infection without involvement of other infectious foci in nosocomial settings. In order to establish the causal relationship of bacteria causing pneumonia, such as P. aeruginosa and A. baumannii, which have high incidence of colonization in LRT, some authors suggest defining pneumonia caused by a “definite pathogen” as bacteremic pneumonia (BP), and the one caused by a “probable pathogen” or a “possible pathogen” as non-bacteremic pneumonia (NBP)6,7.
Numerous studies have investigated the risk factors and mortality of A. baumannii bacteremia, with findings consistently suggesting that A. baumannii bacteremia has a higher mortality rate than other organisms, ranging from 30% to 60%, especially for imipenem-resistant A. baumannii6,8. However, it remains unclear what the attributed mortality of A. baumannii bloodstream infections is by different sites of infection. Additionally, there is a scarcity of studies on the microbiological characteristics of A. baumannii isolates from bloodstream infections emanating from focal infection.
To date, few studies have comparatively evaluated the clinical features of A. baumannii pulmonary infection with and without bacteremia, and the genetic differences between strains causing focal and bloodstream infections. The objective of this study was to identify independent risk factors associated with mortality due to A. baumannii BP and NBP by comparative analysis of clinical characteristics, final outcomes of bacteremic patients, and microbiological features.
Results
Clinical Characteristics of A. baumannii BP and NBP
A total of 175 patients were enrolled in the study, including 86 BP patients and 89 NBP patients. Male patients accounted for 81.4% (70/86) in the BP group and 83.1% (74/89) in the NBP group. There were no significant differences between the two groups in some risk factors of illness (e.g. underlying diseases with chronic pulmonary disease, cerebrovascular disease). However, patients who suffered with ≥ 3 underlying diseases, were more common in the NBP group (51.7% vs 34.9%, P = 0.025). Compared with the NBP group, more BP patients had higher APACHEII scores, acute respiratory distress syndrome (ARDS) and multiple organ dysfunction syndrome (MODS) (P < 0.05). Multivariate Cox regression analysis showed that APACHEII score (OR = 1.048, 95% CI = 1.021–1.076, P = 0.000), PCT elevation (OR = 1.010, 95%CI = 1.005–1.015, P = 0.000), recent surgery (OR = 1.872, 95% CI = 1.211–2.894, P = 0.005), and central venous catheter (OR = 2.334, 95% CI = 1.054–5.169, P = 0.037), were the risk factors for HAP patients complicated with bloodstream infection. The overall mortality rate of BP patients was higher than that of NBP patients (59.3% vs 40.4%, P = 0.013), especially for 7-day mortality (43.0% vs 13.5%, P < 0.001) and 14-day mortality (48.8% vs 19.1%, P < 0.001) (Table 1). The survival curve for the BP group rapidly declined in the initial five to seven days after the diagnosis, then was on a gradual downward slope from 30 days onwards (Log-rank, X2 = 21.975, P < 0.05) (Fig. 1).
Table 1.
Clinical characteristics of bacteremic and non-bacteremic nosocomial pneumonia patients caused by Acinetobacter baumannii.
| Variable | BP(86) | NBP(89) | Statistic | P value |
|---|---|---|---|---|
| n (%) | n (%) | |||
| age, mean (y) | 69.92 ± 14.65 | 71.63 ± 14.91 | t = −0.765 | 0.445 |
| male gender | 70 (81.4) | 74 (83.1) | χ² = 0.092 | 0.762 |
| APACHEII score in admission of ICU | 23.31 ± 7.28 | 20.06 ± 7.66 | t = 2.88 | 0.004 |
| Co-morbidities at admission | ||||
| coma | 31 (36.0) | 26 (29.2) | 0.930 | 0.335 |
| urinary tract infection | 1 (1.2) | 5 (5.6) | 2.622 | 0.105 |
| wound infection | 3 (3.5) | 0 | 3.159 | 0.076 |
| COPD | 25 (29.1) | 27 (30.3) | 0.034 | 0.854 |
| cerebral vascular disease | 18 (20.9) | 27 (30.3) | 2.026 | 0.155 |
| hypertension | 38 (44.2) | 42 (47.2) | 0.159 | 0.690 |
| diabetes mellitus | 20 (23.3) | 21 (23.6) | 0.003 | 0.958 |
| ARDS | 42 (48.8) | 15 (16.9) | 20.371 | 0.000 |
| malignancy | 14 (16.3) | 23 (25.8) | 2.399 | 0.121 |
| MODS | 16 (18.6) | 13 (14.6) | 0.506 | 0.477 |
| three or more underlying diseases | 30 (34.9) | 46 (51.7) | 5.026 | 0.025 |
| length of ICU prior to culture | 15.34 ± 25.22 | 9.46 ± 13.80 | Z = −0.681 | 0.496 |
| length of MV prior to culture | 13.16 ± 19.51 | 9.22 ± 13.998 | Z = −1.068 | 0.286 |
| C-reactive protein | 68.28 ± 59.65 | 90.13 ± 81.79 | Z = −1.303 | 0.193 |
| PCT in admission of ICU | 18.42 ± 44.60 | 3.05 ± 9.66 | Z = −4.489 | 0.000 |
| Invasive procedures | ||||
| mechanical ventilation | 86 (100) | 89 (100) | — | — |
| recent surgery | 38 (44.2) | 10 (11.2) | 23.856 | 0.000 |
| CRRT | 33 (38.4) | 27 (31.5) | 1.253 | 0.263 |
| central venous catheter | 77 (89.5) | 67 (75.3) | 6.096 | 0.014 |
| foley catheter | 69 (80.2) | 77 (86.5) | 1.249 | 0.264 |
| nasogastric tube | 80 (93.0) | 78 (87.6) | 1.445 | 0.229 |
| Previous antibiotic used (within 1 month) | ||||
| sulbactam+ β-lactams | 38 (44.2) | 27 (30.3) | 3.593 | 0.058 |
| carbapenems | 65 (75.6) | 55 (61.8) | 3.856 | 0.050 |
| fluoroquinolones | 19 (22.1) | 21 (23.6) | 0.056 | 0.813 |
| linezolid | 9 (10.5) | 5 (5.6) | 1.396 | 0.237 |
| glycopeptides | 43 (50.0) | 28 (31.5) | 6.235 | 0.013 |
| cephalosporin | 11 (12.8) | 8 (9.0) | 0.653 | 0.419 |
| amikacin | 3 (3.5) | 1 (1.1) | 1.095 | 0.295 |
| tigecycline | 4 (4.7) | 3 (3.4) | 0.187 | 0.666 |
| used 3 or more antibiotics | 38 (44.2) | 28 (31.5) | 3.015 | 0.082 |
| proportion of XDR strains | 30 (34.9) | 23 (25.8) | 1.693 | 0.193 |
| Outcome | ||||
| duration of ICU | 26.43 ± 33.16 | 33.01 ± 34.83 | Z = −2.690 | 0.007 |
| duration of MV | 25.97 ± 31.69 | 32.16 ± 35.73 | Z = −2.369 | 0.018 |
| Survival ≥30 d | 17 (19.8) | 32 (36.0) | 5.685 | 0.017 |
| in-hospital mortality | 51 (59.3) | 36 (40.4) | 6.218 | 0.013 |
| 7-day mortality | 37 (43.0) | 12 (13.5) | 18.931 | 0.000 |
| 14-day mortality | 42 (48.8) | 17 (19.1) | 17.305 | 0.000 |
| 28-day mortality | 46 (53.5) | 26 (29.2) | 4.778 | 0.029 |
| XDR mortality | 18 (20.9) | 14 (15.7) | 0.791 | 0.374 |
| MDR mortality | 31 (36.0) | 22 (24.7) | 2.658 | 0.103 |
| days of death after culture | 8.92 ± 13.73 | 21.47 ± 23.31 | Z = −3.870 | 0.000 |
Data presented as mean ± standard deviation or n (%); BP, bacteremic pneumonia; NBP, non-bacteremic pneumonia; APACHE, acute physiology and chronic health evaluation; COPD, chronic obstructive pulmonary disease; ARDS, Acute respiratory distress syndrome; MODS, multiple organ dysfunction syndrome; ICU, intensive care units; MV, mechanical ventilation; PCT, procalcitonin; CRRT, continuous renal replacement therapy; XDR, extensively drug-resistant; MDR, multi-drug resistance.
Figure 1.
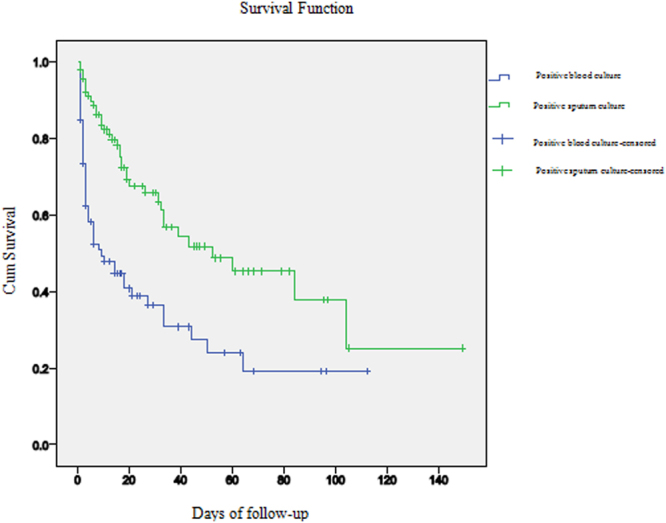
Kaplan–Meier survival curves for patients with NBP and BP.
The clinical characteristics of survivors and non-survivors in the BP group were also studied. The incidence of ARDS (66.7%) and coma (45.1%) in the non-survival group was higher than in the survival group (P < 0.05). Furthermore, the APACHEII score (25.31 ± 7.28) and PCT elevation (26.07 ± 56.32) were higher in the non-survival group than the survival group (P < 0.05). Multivariate Cox regression analysis showed that the high APACHE II score (OR for death = 1.058, 95%CI = 1.022–1.096, P = 0.002) and ARDS (OR for death = 2.229, 95%CI = 1.222–4.065, P = 0.009), coma (OR for death = 2.000, 95%CI = 1.084–3.689, P = 0.027) and PCT elevation (OR for death = 1.007, 95%CI = 1.001 to 1.013, P = 0.025, were independently associated with mortality of the BP group (Table 2).
Table 2.
Clinical characteristics of survivors and non-survivors in the BP group.
| Variable | Survivors (35) | non-survivors (51) | Statistic | P value |
|---|---|---|---|---|
| n (%) | n (%) | |||
| male gender | 25 (71.4) | 45 (88.2) | 3.872 | 0.049 |
| age, mean(y) | 67.29 ± 10.64 | 71.73 ± 16.72 | t = −1.504 | 0.136 |
| length of stay ICU, mean d | 30.51 ± 31.659 | 23.63 ± 34.175 | z = −1.579 | 0.114 |
| length of ICU prior to bacteremia | 17.34 ± 22.643 | 16.22 ± 23.466 | Z = −0.004 | 0.996 |
| mechanical ventilation | 35 (100) | 51 (100) | — | — |
| length of MV, mean d | 32.71 ± 34.335 | 21.33 ± 29.203 | z = −1.958 | 0.050 |
| length of MV prior to bacteremia | 18.23 ± 22.196 | 12.67 ± 22.924 | z = −2.105 | 0.035 |
| Co-morbidities at admission | ||||
| hypoproteinemia | 29 (82.9) | 44 (86.3) | 0.189 | 0.664 |
| ARDS | 8 (22.9) | 34 (66.7) | 15.943 | 0.000 |
| coma | 8 (22.9) | 23 (45.1) | 4.454 | 0.035 |
| wound infection | 1 (2.9) | 2 (3.9) | 0.070 | 0.792 |
| urinary tractin fection | 1 (2.9) | 0 | 1.474 | 0.225 |
| COPD | 9 (25.7) | 16 (31.4) | 0.322 | 0.570 |
| cerebral vascular disease | 5 (14.3) | 13 (25.5) | 1.574 | 0.210 |
| hypertension | 16 (45.7) | 22 (43.1) | 0.056 | 0.813 |
| diabetes mellitus | 8 (22.9) | 12 (23.5) | 0.005 | 0.942 |
| cardiac disorders | 6 (17.1) | 16 (31.4) | 2.208 | 0.137 |
| MODS | 6 (17.1) | 10 (19.6) | 0.083 | 0.773 |
| malignancy | 6 (17.1) | 8 (15.7) | 0.032 | 0.857 |
| 3 or more underlying diseases | 14 (40.0) | 16 (31.4) | 0.680 | 0.410 |
| Invasive procedures | ||||
| CRRT | 13 (37.1) | 20 (39.2) | 0.038 | 0.846 |
| central venous catheter | 32 (91.4) | 45 (88.2) | 0.226 | 0.635 |
| foley catheter | 28 (80.0) | 41 (80.4) | 0.002 | 0.964 |
| nasogastric tube | 34 (97.1) | 46 (90.2) | 1.543 | 0.214 |
| recent surgery | 17 (48.6) | 21 (41.2) | 0.460 | 0.498 |
| Laboratory parameters | ||||
| APACHE II score | 20.40 ± 6.33 | 25.31 ± 7.28 | Z = −3.076 | 0.002 |
| C-reactive protein | 64.34 ± 61.14 | 73.18 ± 55.65 | Z = −1.174 | 0.241 |
| PCT | 7.267 ± 9.65 | 26.07 ± 56.32 | Z = −2.335 | 0.023 |
| proportion of XDR strains | 12 (34.3) | 18 (35.3) | 0.009 | 0.923 |
| Previous antibiotic used (within 1 month) | ||||
| sulbactam+ β-lactams | 16 (45.7) | 22 (43.1) | 0.056 | 0.813 |
| carbapenems | 28 (80.0) | 37 (72.5) | 0.624 | 0.429 |
| tigecycline | 2 (5.7) | 2 (3.9) | 0.150 | 0.698 |
| linezolid | 4 (11.4) | 5 (9.8) | 0.058 | 0.809 |
| fluoroquinolones | 10 (28.6) | 9 (17.6) | 1.439 | 0.230 |
| glycopeptides | 19 (54.3) | 24 (47.1) | 0.434 | 0.510 |
| cephalosporin | 4 (11.4) | 7 (13.7) | 0.098 | 0.754 |
| amikacin | 1 (2.9) | 2 (3.9) | 0.070 | 0.792 |
| used 3or more antibiotics | 17 (48.6) | 21 (41.2) | 0.460 | 0.498 |
| Antimicrobial therapy in the occurrence of BP | ||||
| sulbactam+ β-lactams | 15 (42.9) | 10 (19.6) | 5.441 | 0.020 |
| carbapenems | 25 (71.4) | 44 (86.3) | 2.884 | 0.089 |
| tigecycline | 6 (17.1) | 5 (9.8) | 1.002 | 0.317 |
| aminoglycosides | 2 (5.7) | 1 (2.0) | 0.869 | 0.351 |
| fluoroquinolones | 3 (8.6) | 5 (9.8) | 0.037 | 0.847 |
| Combination antimicrobial therapy | ||||
| tigecycline+sulbactam/β-lactam | 3 (8.6) | 2 (3.9) | 0.820 | 0.365 |
| carbapenems+sulbactam/β-lactams | 9 (25.7) | 8 (15.7) | 1.316 | 0.251 |
| appropriate antimicrobial therapy | 14 (40.0) | 12 (23.5) | 2.670 | 0.102 |
Data presented as mean ± standard deviation or n (%); ICU, intensive care units; MV, mechanical ventilation; ARDS, acute respiratory distress syndrome; COPD, chronic obstructive pulmonary disease ; MODS, multiple organ dysfunction syndrome; CRRT, continuous renal replacement therapy; APACHE, acute physiology and chronic health evaluation; PCT, procalcitonin; XDR, extensively drug-resistant.
Microbiological Studies
Non-repetitive clinical isolates (n = 261) including 172 paired isolates obtained from the BP group (one each from LRT sample and blood culture in each patient) and 89 from NBP group, were identified as ACB complex by the API 20 NE system (bioMérieux Vitek, Marcy l'Etoile, France) . Using the ITS region sequence of the 16S-23S rRNA gene, BP group isolates were discriminated into 3 groups, namely A. baumannii (n = 170), genomic species 3 (n = 1), and genomic species 13TU (n = 1), whilst in the NBP group, isolates were classified as A. baumannii (n = 87), genomic species 3 (n = 1), and genomic species 13TU (n = 1). A. baumannii was the predominant type in both patient groups, accounting for 98.5% of the isolates.
Strains of the BP and NBP groups showed similar antimicrobial resistant profiles. In total, 95.8% of the tested strains were resistant to meropenem, with MIC90 ≥128 µg/ml. The prevalence of extensively drug resistant A. baumannii (XDRAB) was 34.9% in the BP group and 25.8% in the NBP, as well as 34.3% (12/35) in survivors and 35.3% (18/51) in non-survivors in the BP group. The XDRAB isolates had higher susceptibility rates to polymyxin B (96.6%) with MIC90 of 2 µg/ml, and tigecycline (56.6%) with MIC90 of 4 µg/ml (Tables 3 and 4).
Table 3.
Comparison of antimicrobial susceptibilities of Acinetobacter baumannii from BP and NBP groups.
| Antimicrobial agent | BP | NBP | X² | P value | ||
|---|---|---|---|---|---|---|
| R% | MIC50/MIC90 | R% | MIC50/MIC90 | |||
| cefepime | 95.35 | 64/≥128 | 92.13 | 64/≥128 | 0.767 | 0.381 |
| ceftriaxone | 96.51 | ≥128/≥128 | 91.01 | ≥128/≥128 | 2.246 | 0.134 |
| ciprofloxacin | 95.35 | ≥16/≥16 | 95.51 | ≥16/≥16 | 0.002 | 0.960 |
| imipenem | 95.35 | 32/≥128 | 91.01 | 16/≥128 | 1.288 | 0.256 |
| piperacillin/tazobactam | 96.51 | ≥512/4/≥512/4 | 89.89 | 256/4/≥512/4 | 3.005 | 0.083 |
| gentamicin | 89.53 | 32/≥128 | 93.26 | 32/≥128 | 0.774 | 0.379 |
| tobramycin | 87.21 | 32/≥128 | 86.52 | 32/≥128 | 0.018 | 0.892 |
| levofloxacin | 55.81 | 8/≥32 | 56.18 | 8/≥32 | 0.002 | 0.961 |
| tigecycline | 22.09 | 1/4 | 15.73 | 1/4 | 1.157 | 0.282 |
| cefperazone/sulbactam | 87.21 | 64/≥256 | 84.27 | 64/≥256 | 0.309 | 0.579 |
| minocyline | 51.28 | 8/32 | 53.85 | 16/32 | 1.799 | 0.180 |
| amikacin | 65.63 | 128/≥512 | 69.64 | 128/≥512 | 0.152 | 0.697 |
| ceftazidime | 96.51 | ≥128/≥128 | 95.51 | ≥128/≥128 | 0.115 | 0.734 |
| ampicillin/sulbactam | 94.19 | 128/≥512 | 95.51 | 128/≥512 | 0.156 | 0.693 |
| meropenem | 95.35 | ≥32/≥32 | 93.26 | 16/≥32 | 0.355 | 0.551 |
| polymyxin B | 3.49 | 2/2 | 3.37 | 2/2 | 0.002 | 0.966 |
Table 4.
Comparison of antimicrobial susceptibilities of blood isolates in survivors and non-survivors.
| Antimicrobial agent | Survivors | non-survivors | X² | P value | ||
|---|---|---|---|---|---|---|
| R% | MIC50/MIC90 | R% | MIC50/MIC90 | |||
| cefepime | 94.29 | 64/≥128 | 96.08 | 64/≥128 | 0.150 | 0.698 |
| ceftriaxone | 97.14 | ≥128/≥128 | 96.08 | ≥128/≥128 | 0.070 | 0.792 |
| ciprofloxacin | 94.29 | ≥16/≥16 | 96.08 | ≥16/≥16 | 0.150 | 0.698 |
| imipenem | 94.29 | 32/≥128 | 96.08 | 32/≥128 | 0.150 | 0.698 |
| piperacillin/tazobactam | 97.14 | ≥512/4/≥512/4 | 96.08 | ≥512/4/≥512/4 | 0.070 | 0.792 |
| gentamicin | 88.57 | 32/≥128 | 90.20 | 32/≥128 | 0.537 | 0.464 |
| tobramycin | 82.86 | 32/≥128 | 90.20 | 32/≥128 | 1.202 | 0.273 |
| levofloxacin | 57.14 | 8/≥32 | 54.90 | 8/≥32 | 0.042 | 0.837 |
| tigecycline | 20.00 | 1/4 | 23.53 | 1/4 | 0.150 | 0.698 |
| cefperazone/sulbactam | 82.86 | 64/128 | 90.20 | 64/≥256 | 1.002 | 0.317 |
| minocyline | 47.37 | 8/16 | 55.00 | 16/32 | 0.227 | 0.634 |
| amikacin | 64.71 | 128/≥512 | 66.67 | 128/≥512 | 0.014 | 0.907 |
| ceftazidime | 94.29 | 64/≥128 | 98.04 | ≥128/≥128 | 0.869 | 0.351 |
| ampicillin/sulbactam | 91.43 | 128/256 | 96.07 | 128/≥512 | 0.820 | 0.365 |
| meropenem | 94.29 | ≥32/≥32 | 96.08 | ≥32/≥32 | 0.150 | 0.698 |
| polymyxin B | 0 | 2/2 | 5.88 | 2/2 | 2.133 | 0.144 |
PFGE Analysis
The 172 strains from the BP group were classified into 12 genotypes. Seven major types accounted for 62.8% of the isolates, including types A (n = 24), B (n = 20), C (n = 18), D (n = 14), E (n = 12), F (n = 12), and G (n = 8). The remaining 5 types (H, I, J, K, and L) each consisted of 2–4 strains. Isolates from blood and LRT sample of the same patient belonged to the same genotype. Eighty-six strains of the NBP group were classified into 9 genotypes. For the BP group, 69 strains were classified into six clones (A–F), and the remaining 20 strains belonged to 3 types (M, N, and Q).
MLST Analysis
The blood culture isolates of the BP group (n = 86) and all isolates of the NBP group (n = 89) were further typed by MLST. The isolates were assigned to 16 STs, including 2 new STs (STn1 and STn2). The prevalence of ST195, ST457, ST208 and STn1 (1-15-3-2-2-96-3) (a variant of ST457 with one-allele difference) was 40%, 18.9%, 13.1% and 12%, respectively. The five STs had similar distribution in the BP and NBP groups, as well as in the survival and non-survival BP group patients (Table 5). eBURST analysis revealed that eight of 16 STs (ST136, ST195, ST208, ST457, ST369, ST381, STn1, STn2) belonged to the clonal complex 92 (CC92) as shown in Fig. 2, accounting for 94.3% in total.
Table 5.
MLST analysis of strains from bacteremic and non-bacteremic nosocomial pneumonia patients caused by Acinetobacter baumannii.
| STs | BP(86) | NBP(89) | X² | P value | non-survivors(51) | Survivors(35) | X² | P |
|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |||||
| 136 | 5 (5.81) | 5 (5.62) | 0.003 | 0.955 | 2 (3.92) | 3 (8.57) | 0.820 | 0.365 |
| 195 | 36 (41.86) | 34 (38.20) | 0.244 | 0.621 | 22 (43.14) | 14 (40.00) | 0.084 | 0.772 |
| 208 | 7 (8.14) | 16 (17.98) | 3.708 | 0.054 | 4 (7.84) | 3 (8.57) | 0.015 | 0.903 |
| 229 | 1 (1.16) | 1 (1.12) | 0.001 | 0.981 | 0 | 1 (2.86) | 1.474 | 0.225 |
| 369 | 2 (2.33) | 1 (1.12) | 0.375 | 0.54 | 0 | 2 (5.71) | 2.984 | 0.084 |
| 373 | 1 (1.16) | 0 | 1.041 | 0.308 | 1 (1.96) | 0 | 0.694 | 0.405 |
| 381 | 0 | 3 (3.37) | 2.949 | 0.086 | 0 | 0 | — | — |
| 447 | 1 (1.16) | 0 | 1.041 | 0.308 | 0 | 1 (2.86) | 1.474 | 0.225 |
| 457 | 19 (22.09) | 14 (15.73) | 1.157 | 0.282 | 13 (25.49) | 6 (17.14) | 0.84 | 0.359 |
| 605 | 1 (1.16) | 0 | 1.041 | 0.308 | 0 | 1 (2.86) | 1.474 | 0.225 |
| 759 | 0 | 1 (1.12) | 0.972 | 0.324 | 0 | 0 | — | — |
| 782 | 0 | 1 (1.12) | 0.972 | 0.324 | 0 | 0 | — | — |
| 836 | 1 (1.16) | 1 (1.12) | 0.001 | 0.981 | 1 (1.96) | 0 | 0.694 | 0.405 |
| 893 | 0 | 1 (1.12) | 0.972 | 0.324 | 0 | 0 | — | — |
| n1 | 11 (12.79) | 10 (11.24) | 0.1 | 0.752 | 8 (15.69) | 3 (8.57) | 0.942 | 0.332 |
| n2 | 1 (1.16) | 1 (1.12) | 0.001 | 0.981 | 0 | 1 (2.86) | 1.474 | 0.225 |
MLST analysis of strains obtained from survivors and non-survivors among BP group.
Figure 2.
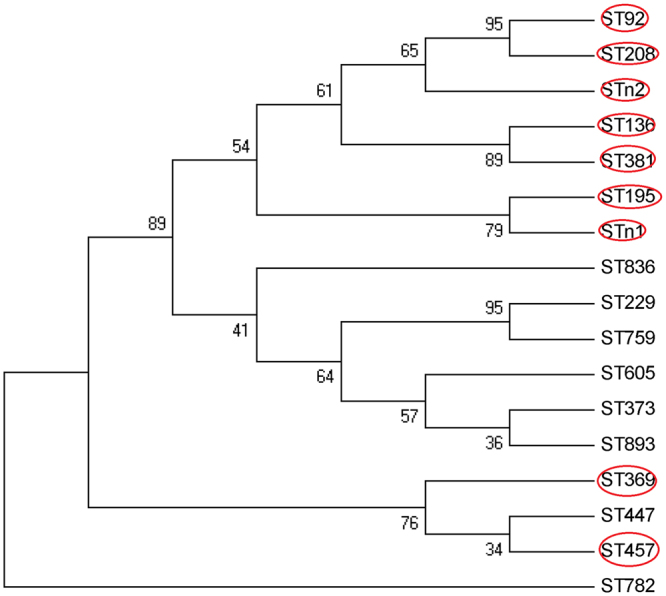
Genetic relatedness of STs detected in 175 strains. Members of CC92 are indicated in red circles.
Discussion
A. baumannii infections can cause high mortality varying from 20% to 70% of nosocomial infections3,9. The difference in the mortality rate depends on several factors including type of microorganism involved, host immunity, severity of illness, and likelihood of receiving antibiotic treatment. Consequently, it is difficult to evaluate infection-related mortality caused by A. baumannii using overall mortality or in-hospital mortality. This study found that BP patients were more prone to death than NBP patients in the shorter duration time period (7 days), with 88.1% (37/42) of BP patient deaths occurring during this period, suggesting that 7-day mortality was a better predictor of patient outcome in A. baumannii BP than 28-day or overall mortality. However, the patients took a turn for the worse when some common risk factors, such as underlying disease, long-term hospitalization, and invasive examination, were taken into consideration. These risk factors also worsened the outcome in 28-day mortality of NBP patients. Mortality remained significantly higher in the BP group for all recorded periods, including 28 days after infection.
Consistent with previous studies, ARDS, coma, high PCT and high APACHEII score, were the risk factors of death in the BP group10–12. However, no association was found between receiving an appropriate antibiotic treatment and the mortality rate. The reasons for this could be: (i) the high prevalence of XDRAB strains in our study collection presented a great challenge for the antimicrobial treatment. According to ESCMID guidelines, the present regimen in the treatment of XDRAB infection, including colistin, tigecycline, and sulbactam, has limited clinical evidence for successful treatment13. Therefore, at the present moment, the recommended antimicrobial therapy for XDRAB is not appropriate as it is not supported by clinical evidence. (ii) due to the restriction on certain doses of tigecycline in China, it is difficult to follow the recommended drug treatment dosage guidelines or expert consensus on XDRAB14. For example, the specified tigecycline daily dosage in China is no more than 100 mg/day, which is an insufficient dose for pneumonia or bacteremia as per guidelines. If the MIC is >1 μg/mL, a higher dosage schedule (75 or 100 mg every 12 hours, after a loading dose of 150 or 200 mg, respectively) is required14. Since 43.6% of the strains in this study had MIC >1 µg/ml, it means that treatment failure may have occurred in <50% of patients using tigecycline at 100 mg/d15. (iii) timely initiation of appropriate therapy is a key point for BP patients due to the severity and rapid development of the disease in this group. However, in this study, positive culture results were usually obtained after 2 or 3 days, which delayed the best treatment time point. Taken together, our results imply that fully assessing the dynamic risk factors of BP, early detection of pathogens, and timely initiation of appropriate antibiotics, may be the key measures to improve the prognosis of BP patients13.
Interestingly, findings from this study suggest that patients who undergo surgery may easily develop BP in comparison to the control group. This may be caused by endotracheal intubation, mechanical ventilation, residual effects of anesthetics, and insufficient coughing due to postoperative wound pain, acquired by surgical patients, resulting in colonizing strains accessing lung tissue. It is necessary to give early passive surveillance and decolonization in management of such patient groups5.
Most studies on A. baumannii are concentrated on clinical features, and there are very few studies on the microbiologic characteristics that promote the progression of the organism from focal to bloodstream infection. Most studies focused on the comparison of carbapenem resistant and carbapenem susceptible isolates, and the association of Acinetobacter genomic species with clinical outcomes13,16. In the present study, the proportion of carbapenem-resistant isolates did not differ significantly between the BP and NBP groups. Likewise, in the BP group, there was no difference in the proportion of individuals in the death and survivor groups. Additionally, 98.5% of the tested strains belonged to A. baumannii, which made it difficult to compare the effect of bacterial phenotype on relative clinical risk between the two groups. Furthermore, there was no significant difference in the ST distribution of strains in the two groups, and 94.3% strains belonged to CC92, the most widely distributed population in the world17. Notably, CC92 is frequently associated with MDR or XDR profile, and is responsible for more treatment failures than other clones when empirical therapy is used. This is especially an issue in China, where other drugs rather than colistin or tigecycline, are used in treatment, and which according to previous studies, explains the increase in indirect mortality in CC92 epidemic areas18,19. Although all patients in the present study were treated with polymyxin, which was used in combination therapy, in 32.4% (n = 30) of the patients, the 30-day mortality rate was still 83.7%. Multivariate analysis revealed that septic shock at diagnosis of XDR-ABC infection was a risk factor for 30-day mortality, which is in agreement to a previous study20. Therefore, early detection of high-risk clones may improve the prognosis of patients.
There are some limitations in this study. Lack of genome sequences of some predominant clones such as ST195 and ST 457 in the BP group is a limitation. Such data could provide extra information to explore the association of genetic characteristics with the occurrence of BP. Notably, ST457 is a predominant type found in almost all patients with bacteremic MDRAB since being described in 2013 in Hong Kong21. To explore the virulence of the emerging clone, we further sequenced the genome of a ST457 strain XH860, and obtained a complete genome (GeneBank accession no. CP014538). Comparison of virulence factors (VFs) between XH860 and the recently published hypervirulent ST10 strain LAC_4 (GeneBank accession no. CP007712) by blasting against VFDB, revealed no difference on the major VFs studied. This suggests that ST457 would also be a hypervirulent clone as ST10. Additionally, some VFs involved in type VI secretion system and quorum sensing were found in XH860 but not in LAC_4. The difference in the distribution of VFs in specific clones needs to be investigated further.
In conclusion, this study found that mortality remained significantly higher in the BP group than the NBP group for all recorded periods, including 28 days of infection, but was especially marked for the 7-day and 14-day mortality. Disease severity was the main risk factor for the high mortality rate in the BP group, and other factors related to mortality in this group were infection related, and not treatment-or microbiology-related.
Methods
Study Design and Inclusion Criteria
This study was carried out from July 2012 to July 2015, and included three tertiary hospitals in South China. All enrolled patients were admitted for a diagnosis of HAP/VAP, and had a positive culture of A. baumannii from lower respiratory tract (LRT), and/or blood culture, according to the American Thoracic Society/Infectious Disease Society of America guidelines22. A. baumannii bacteremic pneumonia (BP) was defined as isolation of A. baumannii from at least one blood culture and one LRT sample culture in the same HAP/VAP patient within 48 hours6. Furthermore, the positive blood culture of A. baumannii was not related to another source of infection, such as catheter-related bacteremia. Non bacteremic pneumonia (NBP) was defined as only the isolation of A. baumannii in LRT sample, and with a negative blood culture from HAP/VAP patients, within 48 hours. Furthermore, organisms other than A. baumannii, detected in both respiratory and blood cultures within this period, were defined as inconsistent microbiology, and such cases were excluded from the study.
Collection of Clinical Data
Clinical data covering demography, risk factors, clinical characteristics, and clinical outcomes, were collected. Data collected included demographic data (gender, age, hospitalization time, ICU stay time etc.); underlying diseases (such as chronic obstructive pulmonary disease, cerebrovascular disease, diabetes, cardiovascular disorders, surgery within 30 days etc.); vital signs (body temperature, heart rate, respiratory rate, blood pressure, oxygenation index); lab examination (white blood cell count, platelet, C-reactive protein, procalcitonin); and other clinical characteristics (APACHE score, invasive operation (such as continuous renal replacement therapy and the central venous catheter, endotracheal intubation or cut channel catheter, gastrointestinal nutrition tube, etc.)), at admission in ICU and within 24 hours of the first positive blood culture or sputum culture. And finally, the use of antibiotics within 30 days before the first positive blood culture or sputum culture of A. baumannii, was recorded. The use of antibiotics for BP and NBP, the proportion of appropriate antibiotic therapy; in-hospital mortality, 7-day, 14-day and 28-day mortality, were documented. The 7-day (14- and 28-day) mortality was defined as death occurring ≤7 days (14 and 28 days) after blood cultures were positive for A. baumannii.
Appropriate antimicrobial therapy was defined as administration of ≥1 antimicrobial agent to which the causative pathogen was susceptible in vitro, within 48 hours after the onset of BP and NBP, and with an approved route and dosage appropriate for end organ function23. Due to the unavailability of polymyxin B and/or colistin in China, antimicrobial agents including carbapenem, sulbactam, or its fixed-dose combinations with ampicillin or cefoperazone, and tigecycline, may be selected for combination therapy at a higher dosage, if A. baumannii isolates show intermediate or minimum inhibitory concentrations (MIC) values closer to the breakpoints of susceptibility. These combination therapies are also considered as appropriate antimicrobial therapy24.
Microbiological Studies
All the LRT samples including sputum, broncho-alveolar lavage, and tracheal aspirates, were collected according to microbiological standard methods of HAP/VAP guidelines3. Non-repetitive clinical isolates were identified as ACB complex by the API 20 NE system (bioMérieux Vitek, Marcy l'Etoile, France) .Since biochemical classification tests cannot distinguish among Acinetobacter calcoaceticus, Acinetobacter baumannii, Acinetobacter genomic species 3 and Acinetobacter genomic species 13TU, all the types were referred to as A. Calcoaceticus-A. baumannii complex (ACB complex) in the routine work. We use the 16S-23S rRNA gene ITS region sequencing to identify ACB complex. Reference strains of Acinetobacter calcoaceticus, Acinetobacter genomic species 3 and Acinetobacter genomic species 13TU, were kindly provided by Fu Y25.
The MIC of each antimicrobial agent was determined by the broth microdilution method, and results were interpreted according to the criteria of the CLSI for broth dilution18. Extensively drug resistant (XDR) was defined as non-susceptibility to at least one agent in all but two or less antimicrobial categories (i.e. bacterial isolates remain susceptible to only one or two categories) according to European expert consensus26.
Genetic Relatedness of Tested Strains
Pulsed-field gel electrophoresis (PFGE) analysis of Apa I-digested genomic DNA was performed to determine the genetic relatedness of A. baumannii isolates using a CHEF-Mapper XA System (Bio-Rad Laboratories, Hercules, CA, USA) as described previously27. The interpreting criteria used was described by Tenover28, combining unweighted pair group method with hierarchic averages (UPGMA). Isolates were assigned the same pulsotype if the value of Dice coefficient of similarity was >80%.
Multilocus sequence typing (MLST) was conducted as previously described29, based on seven housekeeping genes (gltA, gyrB, gdhB, recA, cpn60, gpi, rpoD). The STs were assigned according to A. baumannii MLST database (http://pubmlst.org/abaumannii/). The clonal complexes (CCs) were analyzed by the Based upon Related Sequence Types (BURST) clustering algorithm (eburst.mlst.net).
Statistical Analysis
Statistical analysis was performed using SPSS version 22.0. The dichotomous variables were analyzed by chi-square test or Fisher’s exact test, whereas the continuous variables were analyzed by independent t-test. To construct the survival curve, we used the Kaplan-Meier method, calculating P values by the log-rank test. Multivariate analysis was performed by stepwise logistic regression. Results of in vitro susceptibility were analyzed using WHONET5.6 software.
Notes
Ethics statement
The participating centers either received ethical approval from the Ethics Committees of their respective hospitals (The First Affiliated Hospital of Guangzhou Medical University, Guangzhou General hospital of Guangzhou military and The first People's Hospital of Foshan), or ethical approval was waived due to the observational nature of the study. All subjects signed written informed consent prior to the study. Patient information was anonymized and de-identified prior to analysis. All methods were carried out in accordance with relevant guidelines and regulations.
Acknowledgements
We thank all members of the National surveillance of antimicrobial resistance program for supply of the isolates. This work was supported by a grant from the State Major Infectious Disease Research Program (China Central Government, 2012ZX10004-213) and a grant from the Natural Science Foundation of China (No. 81471989).
Author Contributions
Chao Zhuo designed the study; Yunfang Tan, Xiang Tang, Luxia Wang, Zhenhui Guo steered literature search, data collection and analysis; Yunfang Tan and Kai Zhou drafted the manuscript; Chao Zhuo and Murat Akova reviewed and approved the submission of the manuscript. Kai Zhou and Timothy Kudinha reviewed and revised the manuscript critically. All authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Murat Akova, Email: makova@hacettepe.edu.tr.
Chao Zhuo, Email: chao_sheep@263.net.
References
- 1.Garnacho-Montero J, et al. Optimum treatment strategies for carbapenem-resistant Acinetobacter baumannii bacteremia. Expert Rev Anti Infect Ther. 2015;13:769–777. doi: 10.1586/14787210.2015.1032254. [DOI] [PubMed] [Google Scholar]
- 2.Custovic A, et al. Epidemiological monitoring of nosocomial infections caused by Acinetobacter baumannii. Med Arch. 2014;68:402–406. doi: 10.5455/medarh.2014.68.402-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung DR, et al. High prevalence of multidrug-resistant nonfermenters in hospital-acquired pneumonia in Asia. Am J Respir Crit Care Med. 2011;184:1409–1417. doi: 10.1164/rccm.201102-0349OC. [DOI] [PubMed] [Google Scholar]
- 4.Xu A, et al. National epidemiology of carbapenem-resistant and extensively drug-resistant Gram-negative bacteria isolated from blood samples in China in 2013. Clin Microbiol Infec. 2016;22:S1–S8. doi: 10.1016/j.cmi.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Pogue, J. M., Mann, T., Barber, K. E. & Kayr, K. S. Carbapenem-resistant Acinetobacter baumannii: epidemiology, surveillance and management. Expert Rev Anti-Infe, 383–393 (2013). [DOI] [PubMed]
- 6.Teng S, et al. Comparison of pneumonia- and non-pneumonia-related Acinetobacter baumannii bacteremia: Impact on empiric therapy and antibiotic resistance. Journal of Microbiology, Immunology and Infection. 2015;48:525–530. doi: 10.1016/j.jmii.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Iannini P, Claffey T, Quintiliani R. Bacteremic Pseudomonas pneumonia. JAMA. 1974;230:558–561. doi: 10.1001/jama.230.4.558. [DOI] [PubMed] [Google Scholar]
- 8.Wisplinghoff H, et al. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 9.Lemos EV, et al. Carbapenem resistance and mortality in patients with Acinetobacter baumannii infection: systematic review and meta-analysis. Clin Microbiol Infect. 2014;20:416–423. doi: 10.1111/1469-0691.12363. [DOI] [PubMed] [Google Scholar]
- 10.Jang TN, Lee SH, Huang CH, Lee CL, Chen WY. Risk factors and impact of nosocomial Acinetobacter baumannii bloodstream infections in the adult intensive care unit: a case-control study. J Hosp Infect. 2009;73:143–150. doi: 10.1016/j.jhin.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Punpanich W, Nithitamsakun N, Treeratweeraphong V, Suntarattiwong P. Risk factors for carbapenem non-susceptibility and mortality in Acinetobacter baumannii bacteremia in children. Int J Infect Dis. 2012;16:e811–e815. doi: 10.1016/j.ijid.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Meyer E, et al. Risk factor analysis of blood stream infection and pneumonia in neutropenic patients after peripheral blood stem-cell transplantation. Bone Marrow Transplant. 2007;39:173–178. doi: 10.1038/sj.bmt.1705561. [DOI] [PubMed] [Google Scholar]
- 13.Garnacho-Montero J, et al. Task force on management and prevention of Acinetobacter baumannii infections in the ICU. Intensive Care Med. 2015;41:2057–2075. doi: 10.1007/s00134-015-4079-4. [DOI] [PubMed] [Google Scholar]
- 14.Anthony KB, et al. Clinical and microbiological outcomes of serious infections with multidrug-resistant gram-negative organisms treated with tigecycline. Clin Infect Dis. 2008;46:567–570. doi: 10.1086/526775. [DOI] [PubMed] [Google Scholar]
- 15.Ramirez J, et al. Randomized Phase 2 Trial To Evaluate the Clinical Efficacy of Two High-Dosage Tigecycline Regimens versus Imipenem-Cilastatin for Treatment of Hospital-Acquired Pneumonia. Antimicrob Agents Ch. 2013;57:1756–1762. doi: 10.1128/AAC.01232-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiang MC, et al. Clinical characteristics and outcomes of bacteremia due to different genomic species of Acinetobacter baumannii complex in patients with solid tumors. Infection. 2012;40:19–26. doi: 10.1007/s15010-011-0187-4. [DOI] [PubMed] [Google Scholar]
- 17.Ruan Z, et al. Wide distribution of CC92 carbapenem-resistant and OXA-23-producing Acinetobacter baumannii in multiple provinces of China. Int J Antimicrob Ag. 2013;42:322–328. doi: 10.1016/j.ijantimicag.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 18.Institue, C.A.L.S. Performance standards forantimicrobial susceptibility testing; nineteenth informational supplement.ment. CLSI document M100– S23. Clinical and Laboratory Standards Institute (2013).
- 19.Liu Q, et al. Risk and Prognostic Factors for Multidrug-Resistant Acinetobacter Baumannii Complex Bacteremia: A Retrospective Study in a Tertiary Hospital of West China. PLoS One. 2015;10:e130701. doi: 10.1371/journal.pone.0130701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freire MP, et al. Bloodstream infection caused by extensively drug-resistant Acinetobacter baumannii in cancer patients: high mortality associated with delayed treatment rather than with the degree of neutropenia. Clin Microbiol Infec. 2016;22:352–358. doi: 10.1016/j.cmi.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Cheng VC, et al. Control of hospital endemicity of multiple-drug-resistant Acinetobacter baumannii ST457 with directly observed hand hygiene. Eur J Clin Microbiol Infect Dis. 2015;34:713–718. doi: 10.1007/s10096-014-2281-x. [DOI] [PubMed] [Google Scholar]
- 22.Kalil AC, et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee YT, et al. Impact of appropriate antimicrobial therapy on mortality associated with Acinetobacter baumannii bacteremia: relation to severity of infection. Clin Infect Dis. 2012;55:209–215. doi: 10.1093/cid/cis385. [DOI] [PubMed] [Google Scholar]
- 24.Guan X, et al. Laboratory diagnosis, clinical management and infection control of the infections caused by extensively drug-resistant Gram-negative bacilli: a Chinese consensus statement. Clin Microbiol Infect. 2016;22(Suppl 1):S15–S25. doi: 10.1016/j.cmi.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Fu Y, et al. Wide dissemination of OXA-23-producing carbapenem-resistant Acinetobacter baumannii clonal complex 22 in multiple cities of China. J Antimicrob Chemoth. 2010;65:644–650. doi: 10.1093/jac/dkq027. [DOI] [PubMed] [Google Scholar]
- 26.Magiorakos AP, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infec. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 27.Seifert H, Gerner-Smidt P. Comparison of ribotyping and pulsed-field gel electrophoresis for molecular typing of Acinetobacter isolates. J Clin Microbiol. 1995;33:1402–1407. doi: 10.1128/jcm.33.5.1402-1407.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tenover FC, et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartual SG, et al. Development of a multilocus sequence typing scheme for characterization of clinical isolates of Acinetobacter baumannii. J Clin Microbiol. 2005;43:4382–4390. doi: 10.1128/JCM.43.9.4382-4390.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


