Abstract
The electrochemical performances of Li4Ti5O12 (LTO) and Li4Ti5O12-rutile TiO2 (LTO–RTO) composite electrodes at low temperatures were evaluated. The electrochemical performance of both electrodes decreased at low temperatures; regardless, the LTO–RTO electrode performed better than the LTO electrode. First, high viscosity and low ion conductivity of liquid electrolytes at low temperatures significantly reduce electrochemical performance. Second, cycling at low temperatures changes the crystal structure of LTO–based electrodes, impeding lithium ion diffusion and even causing the diffusion path to change from easy to difficult. However, changes in the crystal structure of the LTO–RTO electrode were not sufficient to change this path; thus, diffusion continued along the 8a-16c-8a pathway. Finally, from the perspective of dynamics, aggravation of a side reaction, increase in charge transfer resistance and polarization, and decrease in lithium ion diffusion at low temperatures reduce the electrochemical performance of LTO–based anode materials. However, the activation energy based on lithium ion diffusion is lower in the LTO–RTO electrode than the LTO electrode. The results confirmed that the electrochemical performance of the LTO–RTO electrode was better than that of the LTO electrode at low temperatures.
Introduction
Fossil fuel has started to diminish, and air pollution has escalated in severity; thus, clean and renewable energy sources are urgently needed. Li-ion batteries, which exhibit high energy density and non-memory effect and support quick charging, can efficiently replace fossil fuel as energy source for electric vehicles, plug-in hybrid electric vehicles, and energy storage devices1–3. Li4Ti5O12 (LTO) is indisputably a high-potential anode material because of its distinct features, including the following: “zero-strain” structure during charge/discharge, which ensures a long cycling life4,5; high charge/discharge platform, which can avoid the deposition of lithium metal on the electrode surface and the formation of lithium dendrites during charge/discharge at high rates or low temperatures, thereby strengthening safety6; and 2-phase electrochemical reaction mechanism. The last feature indicates that the voltage distinctly changes when a phase completely transforms to another, signaling the end of charging/discharging and thus ensuring safety6,7. Low ionic and electron conductivities have long been acknowledged to significantly influence performance; as such, these factors have been largely studied. The discharge specific capacity of the LTO electrode has exceeded 140 mAh g−1 at 20 C (1 C = 175 mA g−1) by optimization methods, such as decreasing particle size, controlling morphology, doping ions, coating, compositing, and so on8–12. However, using the LTO anode material in Li-ion batteries is challenged with a non-negligible problem in its practical application, which is poor electrochemical performance at low temperatures13. The low-temperature electrochemical performance of the LTO electrode was first studied by Allen et al.14. Since then, researches have been conducted on the influence of synthetic methods, carbon coating, La-doping, unique microstructure, modified conductive agent super-p, and new types of electrolytes for low-temperature electrochemical performance9,15–19. References9,15–19 indicated that the appropriate particle size, large specific surface area, fewer contact points between particles, and high electrode conductivity could effectively enhance the low-temperature electrochemical performance to a certain extent. However, the mechanisms for the electrochemical performance of the LTO electrode decreasing at low temperatures were rarely explored. Thus, in the current study, the low-temperature electrochemical performance of LTO and LTO–RTO anode materials was investigated, and its mechanisms were explored by analyzing the influence of electrolytes, crystal structure variation of the anode material, lithium ion diffusion path, lithium ion diffusion efficiency, and activation energy, among others. Moreover, the improved methods were presented by reducing the melting point of the liquid electrolyte or by coating an RTO layer on the surface of the LTO nanosheet.
Results and Discussion
Characteristics of the crystal structure of as-prepared anode materials
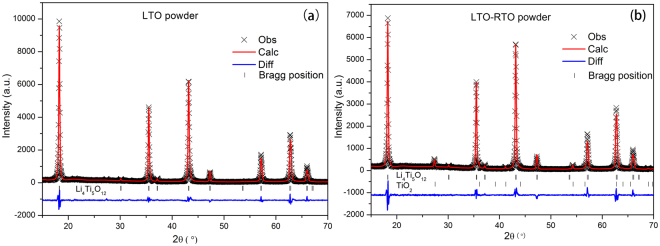
Figure 1 shows the XRD patterns of the LTO powder and the LTO–RTO powder. The patterns suggest that all diffraction peaks of LTO are in accordance with a cubic spinel structure with the Fd-3 m space group (JCPDS: 49–0207); however, some weak diffraction peaks in the pattern of LTO–RTO were detected at 27.5°, 36.1°, 54.3°, which correspond to rutile-TiO2 (JCPDS: 21–1276). These findings indicate that the composite consisted of Li4Ti5O12 and rutile-TiO2.
Figure 1.
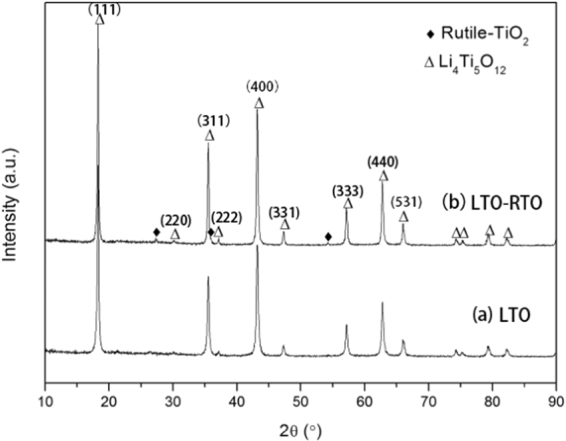
XRD patterns of as-prepared LTO powder (a) and LTO–RTO powder (b).
To elucidate the crystalline structures of LTO and LTO–RTO, as well as ascertain the rutile-TiO2 content in the LTO–RTO crystals, Rietveld refinement was conducted. Profile (Rp), weighted profile residuals (Rwp), and goodness of fit (χ2) are used to estimate refinement results. The result is generally reasonable when Rp, Rwp, and χ2 are less than 6%, 10%, and 3. The results of Rietveld refinement are presented in Fig. 2 and Table 1 based on a model of Li1 atoms occupying the 8a (1/8, 1/8, 1/8) position, Li2/Ti atoms occupying the 16d (1/2, 1/2, 1/2) position, and O atoms occupying the 32e (x, x, x). The results showed that the RTO content in the LTO–RTO powder was 3.257 wt%. Several significant differences in structure were observed between LTO and LTO–RTO. Compared with LTO–RTO, LTO had larger lattice parameter (a), lattice volume (V), oxygen coordinate (x), Li1-O bond distance, and Li1-Li1 bond distance but smaller Li2/Ti-O bond distance. These differences indicated the presence of larger tetrahedrons and smaller octahedrons in LTO and the opposite in LTO–RTO20. The number of free octahedral voids determined the Li-insertion capacity at the potential range of 1–2.5 V21, and the smaller value of x suggested decreasing structural distortion22. Thus, LTO–RTO electrodes are predicted to exhibit a better electrochemical performance compared with the LTO electrode when Li ion diffusion is from 8a-16c along the <011> direction.
Figure 2.
Rietveld refinement patterns of the LTO powder (a) and the LTO–RTO powder (b).
Table 1.
Structural parameters of the LTO and LTO–RTO crystals.
| Sample | a, nm | V, nm3 | x | Li1-O, nm | Li2/Ti-O, nm | Li1-Li1 | wt (RTO)% | Rp, Rwp, χ2 |
|---|---|---|---|---|---|---|---|---|
| LTO | 8.361737(2) | 584.641 | 0.261786 | 1.98107(2) | 1.99675(2) | 3.621 | / | 6.47%, 8.33%, 2.449 |
| LTO–RTO | 8.356956(2) | 583.639 | 0.258768 | 1.93625(2) | 2.01863(3) | 3.619 | 3.257 | 8.39%, 10.63%, 2.724 |
Low-temperature electrochemical performance
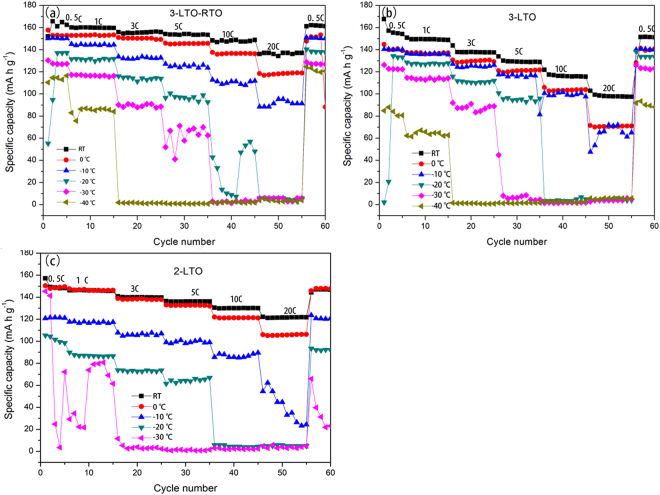
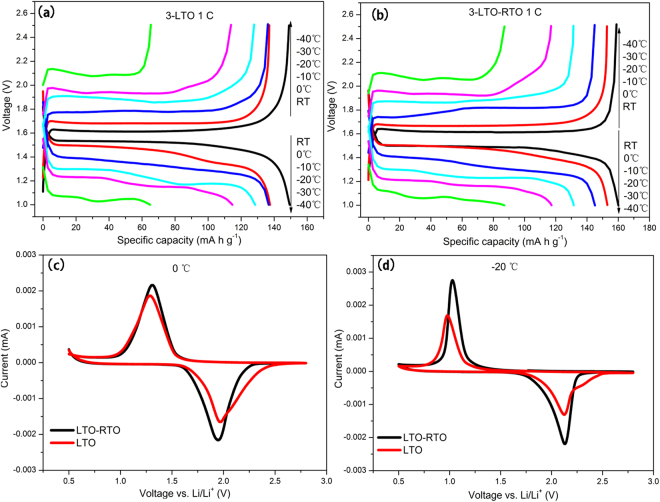
Figure 3 shows the electrochemical performance of the LTO and LTO–RTO electrodes between room temperature (RT) and −40 °C. The LTO electrode and the LTO–RTO electrode cycled in 1 M LiPF6 ethylene carbonate (EC) + ethyl methyl carbonate (EMC) + dimethyl carbonate (DMC) ternary electrolyte (volume ratio, 1:1:1) are identified as 3-LTO, 3-LTO-RTO, respectively. Similarly, the LTO electrode cycled in 1 M LiPF6 EC + DMC binary electrolyte (volume ratio, 1:1) is identified as 2-LTO. The electrochemical performance of cells lowered with a decrease in temperature regardless of the electrode materials and the electrolytes of cells. Compared with the electrochemical performance of 3-LTO-RTO and 3-LTO at different temperatures, the LTO–RTO electrode exhibits the better electrochemical performance at any testing temperature. Under room-temperature conditions, the discharge specific capacity of the LTO electrode are 155 and 165 mAh g−1 at 0.5 C, respectively, whereas those of the LTO–RTO electrode are 149 and 162 mAh g−1 at 1 C, respectively. At −40 °C, the discharge specific capacity of the LTO electrode are 86 and 65 mAh g−1 at 0.5 and 1 C, respectively, whereas those of the LTO–RTO electrode are 115 and 86 mAh g−1 at 0.5 and 1 C, respectively. At −40 °C, 55.48% and 43.62% of the discharge capacity of the LTO electrodes are maintained at 0.5 C, whereas 69.70% and 51.58% of the discharge capacity of the LTO–RTO electrodes are maintained at 1 C. At −40 °C, the superior low-temperature capacity of the LTO–RTO electrode should be related to the intrinsic characteristics of the electrode material.
Figure 3.
The electrochemical performances of (a) 3-LTO-RTO, (b) 3-LTO, and (c) 2-LTO at different temperatures.
Mechanisms on the reduction of the electrochemical performances of the LTO–based electrodes at low temperature and the reasons of the LTO–RTO electrodes exhibit higher specific capacities compared with the LTO electrodes are explored.
Effect of electrolyte
The electrochemical performances of the LTO and LTO–RTO electrodes in the ternary electrolyte apparently declined when cycled at −40 °C. The LTO electrodes in the binary electrolyte almost failed to charge and discharge below −30 °C. Meanwhile, the electrochemical performance of the LTO–based electrodes in the ternary electrolyte was superior to that of the LTO electrodes in the binary electrolyte below 0 °C. Data on the electrochemical performance of LTO in different electrolytes are presented in Figure S1. These results indicate that the sharp decrease in low-temperature electrochemical performance of LTO–based electrodes is most likely related to the electrolyte. And the ternary electrolyte can be more suitably used at low temperatures. The viscosity and ionic conductivity of the liquid electrolytes are widely regarded as key factors influencing the electrochemical performance of batteries23,24. However, viscosity increases, whereas ionic conductivity decreases when the electrodes are cycled at low temperatures24. The melting points of EC, DMC, and EMC are 37 °C, 3 °C, and −55 °C, respectively. The proportion of EC in the binary electrolyte is 50 vol.%, which is greater than that in the ternary electrolyte (about 33 vol.%). Thus, the electrolyte using EC and DMC as the solvent is easier to crystallize and exhibits higher viscosity and lower ionic conductivity, compared with the electrolyte using EC, DMC, and EMC as the solvent at low temperatures. The freezing point of the ternary electrolyte is ~−50 °C and that of the binary electrolyte is ~−30 °C. The conductivity of the ternary electrolyte is 4~10 × 10−3 S cm−1 and ~2 × 10−3 S cm−1 at 20 °C and −20 °C, respectively. And the conductivity of the binary electrolyte is 8.8 × 10−3 S cm−1 and 0.58 × 10−3 S cm−1 at 20 °C and −20 °C, respectively25,26. Thus, lithium ion diffusion in the electrolyte and charge transfer between the electrode/electrolyte interfaces become more difficult. This analysis indicates that the good electrochemical performance in LTO–based materials in ternary electrolyte is attributed to lower viscosity and higher ionic conductivity at low temperatures. Therefore, reducing the melting point of liquid electrolytes can potentially improve performance at low temperatures. On the basis of the results of the electrolyte, only the ternary electrolyte was adapted to proceed with the subsequent investigations.
Variation in crystal structure during cycling at low temperatures
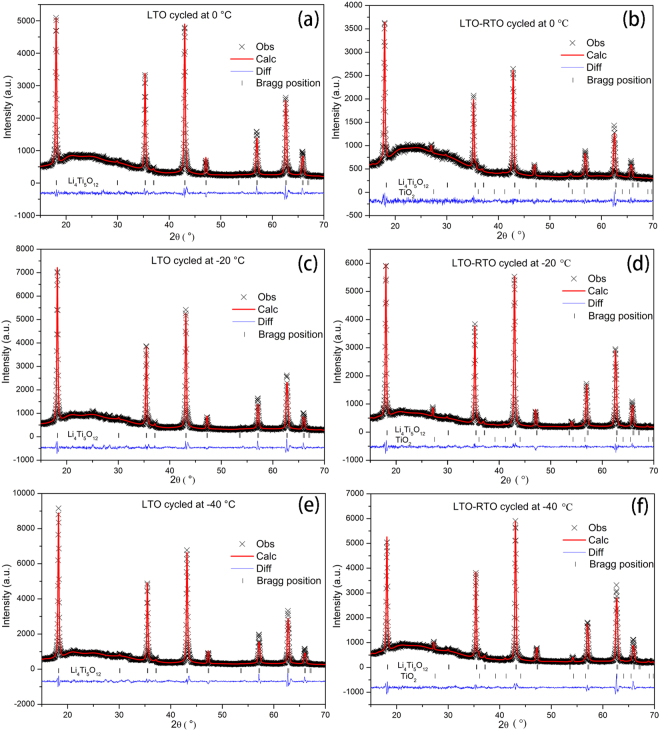
Figure 4 shows the Rietveld refinement patterns of the cycled LTO and LTO–RTO electrodes after charge and discharge at 0 °C, −20 °C, and −40 °C, respectively. The values of Rwp, Rp, and χ2 for all samples are almost less than 10%, 6%, and 3, respectively, which verifies the reliability of the Rietveld refinement results. The structural parameters obtained from Rietveld refinement are listed in Table 2.
Figure 4.
Rietveld refinement patterns of the cycled LTO powder at 0 °C (a), −20 °C (c), and −40 °C (e) as well as the cycled LTO–RTO powder at 0 °C, (b), −20 °C, (d), and −40 °C (f).
Table 2.
Structural parameters of LTO powder and LTO–RTO powder cycled 15 times at 0 °C, −20 °C, and −40 °C.
| Sample | a, nm | V, nm3 | x | Li1-O, nm | Li2/Ti-O, nm | Li1-Li1, nm | Rp, Rwp, χ2 |
|---|---|---|---|---|---|---|---|
| LTO~0 °C | 8.350098 | 582.203 | 0.258519 | 1.93106(2) | 2.01890(3) | 3.616 | 4.58%, 6.09%, 1.752 |
| LTO~−20 °C | 8.352905 | 582.791 | 0.259828 | 1.95064(2) | 2.00949(3) | 3.617 | 4.98%, 6.50%, 2.281 |
| LTO~−40 °C | 8.351963 | 582.594 | 0.258546 | 1.93188(2) | 2.01914(3) | 3.617 | 5.53%, 7.61%, 3.052 |
| LTO–RTO~0 °C | 8.346919 | 581.539 | 0.260629 | 1.96083(3) | 2.00194(4) | 3.614 | 3.62%, 4.66%, 1.281 |
| LTO–RTO~−20 °C | 8.349110 | 581.997 | 0.258189 | 1.92606(1) | 2.02122(2) | 3.615 | 3.82%, 4.60&, 1.524 |
| LTO–RTO~−40 °C | 8.349153 | 582.006 | 0.260355 | 1.95739(2) | 2.00457(3) | 3.615 | 5.36%, 7.26%, 2.131 |
All parameters of the crystal structure change irregularly, regardless of whether such parameters refer to the LTO or LTO–RTO; However, the parameters of the lattice and the Li1-Li1 bond length related to lithium ion diffusion decrease, compared with those of the un–cycled powder (Table 1). The studies by Laumann et al. and Dolotko et al.20,27 indicate that the changes in the crystal structure of Li4Ti5O12 without charge/discharge are regular whether at high or low temperatures. Consequently, the structure is affected not only by temperature but also by lithium ion insertion/extraction during charge/discharge. Dolotko et al.20 suggested that the lithium ion diffusion path is more probable along 8a-32e-32e-8a rather than 8a-16c-8a by valence bond theory at low temperatures. Therefore, it is speculated that the lithium ion diffusion path of Li4Ti5O12–based materials could change at low temperatures. The diffusion path of lithium ions could significantly affect low-temperature electrochemical performance, but this has yet to be verified by further experimentation. Ideally, lithium ion intercalated/deintercalated processes are fully reversible, and the structure returns to the original state after charging. However, the truth deviates from the ideal, particularly charging/discharging under the condition of high rates or low temperatures. Lithium ions cannot fully intercalate while discharging nor deintercalate while charging, Consequently, lithium ions accumulate continually in octahedral 16c positions and tetrahedral 8a positions (Lithium ions in Li4Ti5O12 are believed to be along the [011] orientation diffusing from 8a-16c-8a during the charge/discharge process28,29.). The XRD peak intensities of (311) and (400) are related to the number of lithium ions at 8a (tetrahedron) and 16c (octahedron) sites, respectively21. Therefore, a simple method can be used to investigate the diffusion path of lithium ions. The XRD peak intensity ratios of (311)/(111) and (400)/(111) (referred to as I(311)/I(111) and I(400)/I(111), respectively) gained from the measured XRD patterns (Fig. 4) are listed in Table 3. For the cycled LTO–RTO electrode, the values of I(311)/I(111) and I(400)/I (111) increased when the tested temperatures decreased from 0 °C to −40 °C. However, for the cycled LTO electrode, the opposite result was obtained. The former conformed to the regulations, which lithium ions accumulated continually in octahedral 16c and tetrahedral 8a positions, and the diffusion path remained unchanged along with 8a-16c-8a. However, the latter indicated that the diffusion path of lithium ions in the LTO electrode had been changed. Combined with the results obtained by Dolotko et al.20, the diffusion path of lithium ions may be along 8a-32e-32e-8a. Given the low-temperature electrochemical performance of the LTO and LTO–RTO electrodes (Fig. 3), the conclusion is that diffusion of lithium ions is more difficult and requires more energy in the LTO electrode than in the LTO–RTO electrode. Therefore, on the basis of the crystal structure, the change in diffusion path in the LTO electrodes resulting from the change in structural parameters during cycling at low temperatures could reduce the electrochemical performance of the LTO electrodes. By contrast, for the LTO–RTO electrode, the main diffusion path of lithium ions remains along 8a-16c-8a, according to results listed in Table 3. Thus, the variation in crystal structure is not sufficient to contribute to the reduction in electrochemical performance at low temperatures.
Table 3.
Intensity ratios of (400)/(111) and (311)/I(111) for the cycled LTO and LTO–RTO electrodes at 0 °C, −20 °C, and −40 °C.
| Sample | I(311)/I(111) | I(400)/I(111) |
|---|---|---|
| LTO~0 °C | 0.67 | 1.112 |
| LTO~−20 °C | 0.55 | 0.882 |
| LTO~−40 °C | 0.57 | 0.860 |
| LTO-RTO~0 °C | 0.52 | 0.839 |
| LTO-RTO~−20 °C | 0.68 | 1.111 |
| LTO-RTO~−40 °C | 0.83 | 1.388 |
Dynamical features of LTO–based anode materials cycled at low temperatures
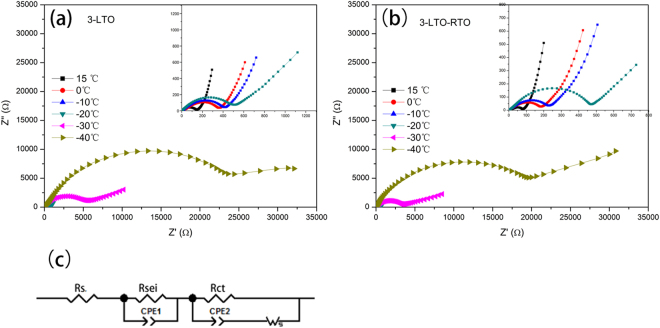
The electrochemical impedance spectra (EIS) of LTO–RTO composites and LTO electrodes cycled 3 times at 0.5 C was measured at different temperatures. The results are presented in Fig. 5. As shown, all Nyquist plots consist of the real-axis intercept at a high frequency, a minimal depressed semicircle and a large depressed semicircle from a high-frequency range to a medium-frequency range, and a slope line about 45° at a low-frequency range, which corresponded to the ohmic resistance (Rs), charge transfer resistance (Rct), and the Warburg impedance (Ws), successively, except for the first depressed semicircle. The first depressed semicircle is usually associated with the resistance of the solid electrolyte interface (SEI) film (Rsei)30. SEI film was previously thought to be impossible to generate in the potential range of 1–2.5 V in Li4Ti5O12-based anode materials. However, the SEI film has been recently proved in Li4Ti5O12-based electrodes in the potential range of 1–2.5 V31,32. To further ascertain the existence of the SEI film under this condition, the SEM and FTIR spectra of 3-LTO before and after cycling were obtained. The results are presented in Figure S2. SEI film clearly exists on the electrode surface, as determined from the SEM images. In Figure S2(d), characteristic peaks of Li2CO3 (1488, 1434, and 865 cm−1) and ROCO2Li (1627 cm−1) are found in all electrodes. This finding presents a direct evidence for the existence of the SEI film. Moreover, the intensity of these characteristic peaks decreased after cycling. Li2CO3 and ROCO2Li generated in the electrode without cycling may be attributable to the existence of trace amounts of HF and H2O in the electrolyte, which mainly causes the formation of an SEI film31. As the cycle proceeds, the SEI film may be dissolved and then formed repeatedly under the action of Ti3+ catalysis, which can increase the SEI film resistance31. This condition may be more serious with a decrease in temperature.
Figure 5.
Electrochemical impedance spectroscopy (EIS) of 3-LTO composite (a), 3-LTO-RTO (b) and the equivalent circuit diagram (c).
On the basis of the aforementioned analysis, the corresponding equivalent circuit diagram is chosen and shown in Fig. 5(c). The impedance parameters are listed in Table 4. With a reduction in the temperature, Rs, Rsei and Rct increase gradually. Rs is mainly related to electrolyte resistance, whereas Rsei reflects film resistance. An increase in Rsei indicates that a side reaction is aggravated. The value for Rsei of the LTO electrodes is larger than that of the LTO–RTO electrodes at the same testing temperature, suggesting that the side reaction of LTO–RTO electrodes was suppressed to a certain extent. Above three resistances, the value of Rct is far greater than those of the two others. The charge transfer resistance between the electrode and the electrolyte is weighted through the value of Rct. The increase in Rct indicates that the interface dynamic activity of cells decreases when cycling occurs at low temperatures.
Table 4.
Impedance parameters and DLi of 3-LTO-RTO and 3-LTO.
| Sample | Temperature (°C) | Rs (Ω) | Rsei (Ω) | Rct (Ω) | R (Ω) | DLi (cm2 s−1) |
|---|---|---|---|---|---|---|
| 3-LTO-RTO | 15 | 2.7 | 6.7 | 67.7 | 77.1 | 1.96 × 10−14 |
| 0 | 4.7 | 25.7 | 181.9 | 212.3 | 4.46 × 10−15 | |
| −10 | 5.1 | 50.7 | 234.1 | 289.9 | 3.10 × 10−15 | |
| −20 | 5.2 | 65.0 | 455.0 | 525.2 | 1.65 × 10−15 | |
| −30 | 16.9 | 254.1 | 3779.0 | 4050.0 | 2.01 × 10−17 | |
| −40 | 20.1 | 463.8 | 20600.0 | 21083.9 | 2.81 × 10−18 | |
| 3-LTO | 15 | 5.2 | 7.8 | 142.2 | 155.2 | 1.49 × 10−14 |
| 0 | 5.5 | 26.7 | 353.0 | 385.2 | 3.11 × 10−15 | |
| −10 | 6.5 | 58.3 | 401.0 | 465.8. | 1.98 × 10−15 | |
| −20 | 7.7 | 136.4 | 513.5 | 657.6 | 3.66 × 10−16 | |
| −30 | 21.6 | 394.3 | 5758.0 | 6173.9 | 4.54 × 10−18 | |
| −40 | 23.5 | 514.7 | 268300.0 | 27368.2 | 3.57 × 10−19 |
Meanwhile, the bulk dynamics activity is weighted through the efficiency of lithium ion diffusion (DLi) at different temperatures. The calculated process of DLi and the Z′~ω−1/2 plots (Figure S3) are presented in Supplementary Information. The results of the diffusion coefficient of the LTO and LTO–RTO electrodes are listed in Table 4. As shown, the lithium ion diffusion coefficients of LTO–based anode materials decreased with a decrease in temperature, indicating that the bulk dynamic activity of active materials decreases at low temperatures. Meanwhile, the DLi value of LTO–RTO active materials is greater than that of LTO active materials at the same temperature. Therefore, LTO–RTO presents an advantage over the LTO in this aspect.
To confirm the advantage of the LTO–RTO electrode in terms of lithium ion diffusion, activation energy was calculated based on the lithium ion diffusion coefficient. The detailed calculation and the plotting of DLi~1000/T (Figure S4) are presented in Supplementary Information. The calculated activation energy of 3-LTO-RTO was 41.64 KJ/mol and that for 3-LTO was 60.99 KJ/mol. The activation energy of 3-LTO-RTO was markedly lower than that of 3-LTO at low temperatures, which proved that lithium ion diffusion was easier in the 3-LTO-RTO electrode than in the 3-LTO electrode. This finding confirms the previous deduction that the lithium ion diffusion path in the LTO–RTO electrode remained unchanged along the 8a-16c-8a path; however, the lithium ion diffusion path in the LTO electrode can change, and lithium ions can be diffused toward a difficult path where a greater activation energy is required.
To further investigate the dynamics of lithium ion diffusion in the bulk, the voltage difference between the charge platform and the discharge platform (ΔE) and the voltage difference between the oxidation peak and the reduction peak (ΔV) were measured33,34. Figure 6(a) and (b) show the charge/discharge voltage curves of the LTO and LTO–RTO electrodes at 1 C from room temperature to −40 °C. Figure 6(c) and (d) present the CV curves of the LTO and LTO–RTO electrodes at 0 °C and −20 °C. The ΔE value of both LTO–RTO and LTO increased gradually with a decrease in temperature, indicating that the polarization increased while the dynamics of lithium ion diffusion decreased with a decrease in temperature. The ΔV value of the LTO–RTO electrode was smaller than that of the LTO electrode, suggesting that the LTO–RTO electrodes had better lithium ion diffusion dynamics than the LTO electrodes.
Figure 6.
Charge/discharge curves of the LTO (a) and LTO–RTO (b) electrodes; CV curves of the LTO–RTO and LTO electrodes at 0 °C (c) and −20 °C (d), respectively.
Briefly, by the analyses of Rsei, Rct, DLi, activation energy, ΔE, and ΔV from the perspective of the dynamics, these results indicate that with a decrease in temperature, a side reaction is aggravated; in addition, the rate of charge transfer between electrolytes and electrodes and the lithium ion diffusion coefficient in the bulk decrease when LTO–based anode materials are cycled at low temperatures. These occurrences lead to the degradation of electrochemical performance (Fig. 3). However, the lithium ion diffusion coefficient of LTO–RTO active materials is greater than that of LTO active materials at the same temperature; the LTO–RTO electrodes have better lithium ion diffusion dynamics than the LTO electrodes; and the activation energy of the LTO–RTO electrodes on the basis of the lithium ion diffusion coefficient is less than that of the LTO electrodes. All of these findings help explain why the LTO–RTO electrode exhibits better electrochemical performance than the LTO electrode.
Conclusion
On the basis of the aforementioned findings, the following results are obtained.
With a decrease in temperature, the electrochemical performance of Li4Ti5O12–based anode materials decreased at low temperatures (e.g., 0 °C–−40 °C). Compared with that at room temperature, the capacity retention rates of the LTO electrode at −40 °C were 55.48% at 0.5 C and 43.62% at 1 C. In addition, the LTO–RTO electrode exhibited capacity retention rates of 69.70% and 51.58% under the same condition. The LTO–RTO electrode exhibited a better electrochemical performance than the LTO electrode.
First, the decrease in the low-temperature electrochemical performance of the Li4Ti5O12–based electrodes is influenced by the electrolyte. The viscosity of the electrolyte increases and the ionic conductivity of the electrolyte is reduced when the cell is cycled at low temperatures. Moreover, the electrolyte using a high proportion of EC or DMC as the solvent is easier to crystallize at low temperatures because of the higher melting points of EC (37 °C) and DMC (3 °C). Consequently, the electrochemical performance of the Li4Ti5O12-based electrodes at low temperatures decreases.
Second, the decrease in the low-temperature electrochemical performance of the Li4Ti5O12–based electrodes is related to changes in the crystal structure. When cycling at low temperatures, the crystal structure parameters the of Li4Ti5O12-based electrodes change; the lattice parameters and the Li1-Li1 bond length related to lithium ion diffusion decrease at low temperatures. On the basis of these changes in the crystal structure, for the LTO electrode, the lithium ion diffusion path of 8a-16c-8a changes. The 8a-32e-32e-8a path might have hindered the lithium ion diffusion. However, variations in the crystal structure parameters of the LTO–RTO electrode failed to sufficiently change the lithium ion diffusion path. Thus, the LTO–RTO electrode exhibited a better electrochemical performance compared with the LTO electrode when cycling was performed at low temperatures.
Finally, from the perspective of dynamics, the results for Rsei, Rct, DLi, ΔE, and ΔV indicate that the dynamics of the Li4Ti5O12 anode materials decrease with a decrease in temperature, thereby reducing the electrochemical performance of the Li4Ti5O12–based electrode at low temperatures. However, the active energy based on lithium ion diffusion of the LTO–RTO electrode was lower than that of the LTO electrode. This result also explains why the LTO–RTO electrode exhibited a better electrochemical performance at low temperatures, compared with the LTO electrode.
Methods
Material synthesis
Spinel LTO and LTO–RTO were synthesized using the gel–hydrothermal method with lithium hydroxide monohydrate (LiOH•H2O) as the lithium source and titanium (IV) butoxide (Ti(C4H9O)4) as the titanium source. Firstly, 16.25 g of Ti(C4H9O)4 was dissolved in 34 mL alcohol as A. Secondly, 1.68 g of LiOH•H2O was dissolved in 20 mL of distilled water as B. Then, solution B was dropped slowly into solution A with magnetic stirring. The gained white suspension was transferred into a 100 mL stainless-steel autoclave and then reacted at 180 °C for 36 h. After filtrating and drying at 80 °C for 7 h, precipitate was calcined at 600 °C for 10 h and transferred into final product. By changing the amount of Ti(C4H9O)4 to 15.10 g, the pure LTO was obtained using the same procedure.
Electrode fabrication and coin cell assembly and disassembly
LTO or LTO-RTO compound, acetylene black, polyvinylidene (weight ratio, 80:10:10) were dissolved in n-methyl pyrrolidinone to form a slurry, coated on a copper foil substrate, and dried at 80 °C for 12 h. The prepared copper foil was cut into small discs with an area of 1.58 cm2. CR2025 coin cells were assembled in an Ar-filled glove box (H2O < 0.1 ppm and O2 < 4 ppm) by using prepared discs as the positive electrode, metallic lithium as the counter electrode, a microporous polyethylene film as the separator, and 1 M LiPF6 EC + EMC + DMC as the ternary electrolyte (the volume ratio, 1:1:1) or 1 M LiPF6 EC + EMC as the binary electrolyte (volume ratio, 1:1) as the electrolyte. The cells were disassembled and then washed in the DMC several times.
Structural characterization
The crystalline phases of the samples were characterized by X-ray diffraction (Rigaku DMAX2000) with Cu kα radiation. Data for crystalline phase identification were collected by continuous scanning at 2θ = 10°–90°. Data for phase structure refinement were collected by step scanning at 2θ = 15°–70° with a step of 0.03° and a counting time of 2 s per step. Rietveld refinements were performed using the GSAS program with the EXPGUI interface. During the entire process, background parameters, zero, polar, lattice parameters, atomic fractional coordinates, atomic isotropic displacement parameters, phase fractions, profile parameters, and SH Pref Orient parameters were fitted using the shifted Chebyshev function as the background function and the Pearson VII function as the profile function.
Electrochemical characterization
The galvanostatic charge and discharge test, with a potential range of 1–2.5 V (vs. Li/Li+), was executed by LAND CT2001A from room temperature to −40 °C. Before testing the electrochemical performance, all cells were placed on their corresponding testing temperatures for 4 h. Data were obtained by electrochemical impedance spectroscopy (EIS) using an electrochemical workstation (CHI660E) at 10−2–105 Hz frequency range. Data were collected by cyclic voltammetry (CV) with a scan rate of 5 mV s−1 within the voltage range of 0.5–2.5 V by using an electrochemical workstation (CHI600E).
Morphological and constituent characterization of SEI film
Surface morphology of the LTO electrodes cycled in the ternary electrolyte was characterized by field emission scanning electron microscopy (SEM, Hitachi S-4800). The surface group constituent of the cycled LTO electrodes was characterized in the ternary electrolyte by Fourier transform infrared spectroscopy (FTIR, TENSOR 27) between 200–2000 cm−1.
Electronic supplementary material
Mechanisms of the decrease in low-temperature electrochemical performance of Li4Ti5O12-based anode materials
Author Contributions
J.M. developed the concept. Q.H. designed and performed the experiments and wrote the manuscript. J.M., Q.H., and Z.Y. participated in the data acquisition and analysis. J.M. reviewed and revised the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-15504-4.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Armand M, Tarascon JM. Building better batteries. Nature. 2008;7:451. doi: 10.1038/451652a. [DOI] [PubMed] [Google Scholar]
- 2.Etacheri V, Marom R, Elazari R, Salitra G, Aurbach D. Challenges in the development of advanced Li-ion batteries: a review. Energy Environ. Sci. 2011;4:3243–3262. doi: 10.1039/c1ee01598b. [DOI] [Google Scholar]
- 3.Zaghib K, Dontigny M, Guerfi A, Julien C. Safe and fast-charging Li-ion battery with long shelf life for power applications. J. Power Sources. 2011;196:3949–3954. doi: 10.1016/j.jpowsour.2010.11.093. [DOI] [Google Scholar]
- 4.Ohzuku T, Ueda A, Yamamoto N. Zero-strain insertion material of Li[Li1/3Ti5/3]O4 for rechargeable lithium cells. J. Electrochem. Soc. 1995;142(5):1431–1435. doi: 10.1149/1.2048592. [DOI] [Google Scholar]
- 5.Ariyoshi K, Yamato R, Ohzuku T. Zero-strain insertion mechanism of Li[Li1/3Ti5/3]O4 for advanced lithium-ion (shuttlecock) batteries. Electrochim. Acta. 2005;51:1125–1129. doi: 10.1016/j.electacta.2005.05.053. [DOI] [Google Scholar]
- 6.Jansen AN, et al. Development of a high-power lithium-ion battery. J. Power Sources. 1999;81–82:902–905. doi: 10.1016/S0378-7753(99)00268-2. [DOI] [Google Scholar]
- 7.Wagemaker M, Simon DR, Kelder E, Mulder FM. A Kinetic Two-Phase and Equilibrium Solid Solution in Spinel Li4+xTi5O12. Adv. mater. 2006;18:3169–3173. doi: 10.1002/adma.200601636. [DOI] [Google Scholar]
- 8.Ge H, et al. Nanoparticles–Constructed Spinel Li4Ti5O12 with Extra Surface Lithium Storage Capability towards Advanced Lithium-ion Batteries. Electrochim. Acta. 2016;211:119–125. doi: 10.1016/j.electacta.2016.06.046. [DOI] [Google Scholar]
- 9.Liu J, Wei XF, Liu XW. Two-Dimensional Wavelike Spinel Lithium Titanate for Fast Lithium Storage. Sci Rep-UK. 2015;5:9782. doi: 10.1038/srep09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang P, et al. Improved rate capability and cycling stability of novel terbium-doped lithium titanate for lithium-ion batteries. Electrochim. Acta. 2016;210:935–941. doi: 10.1016/j.electacta.2016.06.017. [DOI] [Google Scholar]
- 11.Ni HF, Song WL, Fan LZ. Double carbon decorated lithium titanate as anode material with high rate performance for lithium-ion batteries. Prog. Nat. Sci-Mater. 2016;26:283–288. doi: 10.1016/j.pnsc.2016.05.005. [DOI] [Google Scholar]
- 12.Wu LB, et al. Strategy for Synthesis of Nanosheets Consisting of Alternating Spinel Li4Ti5O12 and Rutile TiO2 Lamellas for High-Rate Anodes of Lithium-Ion Batteries. ACS Appl. Mater. Interfaces. 2017;9:4649–4657. doi: 10.1021/acsami.6b15021. [DOI] [PubMed] [Google Scholar]
- 13.Zhao B, Ran R, Liu ML, Shao ZP. A comprehensive review of Li4Ti5O12-based electrodes for lithium-ion batteries: The latest advancements and future perspectives. Mater. Sci. Eng. R. 2015;98:1–71. doi: 10.1016/j.mser.2015.10.001. [DOI] [Google Scholar]
- 14.Allen JL, Jow TR, Wolfenstine J. Low temperature performance of nanophase Li4Ti5O12. J. Power Sources. 2006;159:1340–1345. doi: 10.1016/j.jpowsour.2005.12.039. [DOI] [Google Scholar]
- 15.Yuan T, Cai R, Ran R, Zhou Yk, Shao ZP. A mechanism study of synthesis of Li4Ti5O12 from TiO2 anatase. J. Alloy. Compd. 2010;505:367–373. doi: 10.1016/j.jallcom.2010.04.253. [DOI] [Google Scholar]
- 16.Yuan T, Yu X, Cai R, Zhou YK, Shao ZP. Synthesis of pristine and carbon-coated Li4Ti5O12 and their low-temperature electrochemical performance. J. Power Sources. 2010;195:4997–5004. doi: 10.1016/j.jpowsour.2010.02.020. [DOI] [Google Scholar]
- 17.Bai YJ, Gong C, Qi YX, Lun N, Feng J. Excellent long-term cycling stability of La-doped Li4Ti5O12 anode material at high current rates. J. Mater. Chem. 2012;22:19054. doi: 10.1039/c2jm34523d. [DOI] [Google Scholar]
- 18.Marinaro M, et al. Improved low-temperature electrochemical performance of Li4Ti5O12 composite anodes for Li-ion batteries. Electrochim. Acta. 2013;109:207–213. doi: 10.1016/j.electacta.2013.07.093. [DOI] [Google Scholar]
- 19.Yoshima K, Harada Y, Takami N. Thin hybrid electrolyte based on garnet-type lithium-ion conductor Li7La3Zr2O12 for 12 V-class bipolar batteries. J. Power Sources. 2016;302:283–290. doi: 10.1016/j.jpowsour.2015.10.031. [DOI] [Google Scholar]
- 20.Dolotko O, et al. Neutron diffraction study of Li4Ti5O12 at low temperatures. Solid State Sci. 2014;36:101–106. doi: 10.1016/j.solidstatesciences.2014.08.002. [DOI] [Google Scholar]
- 21.Ge H, Li N, Li DY, Dai CS, Wang DL. Study on the theoretical capacity of spinel lithium titanate induced by low-potential intercalation. J. Phys. Chem. C. 2009;113:6324–6326. doi: 10.1021/jp9017184. [DOI] [Google Scholar]
- 22.Song H, et al. An upper limit of Cr-doping level to Retain Zero-strain Characteristics of Li4Ti5O12 Anode Material for Li-ion Batteries. Sci. Rep-UK. 2017;7:43335. doi: 10.1038/srep43335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zaghib K, et al. Electrochemical and thermal characterization of lithium titanate spinel anode in C-LiFePO4//C-Li4Ti5O12 cells at sub-zero temperatures. J. Power Sources. 2014;248:1050–1057. doi: 10.1016/j.jpowsour.2013.09.083. [DOI] [Google Scholar]
- 24.Rodrigues MTF, Lin XR, Gullapalli H, Grinstaff MW, Ajayan PM. Rate limiting activity of charge transfer during lithiation from ionic liquids. J. Power Sources. 2016;330:84–91. doi: 10.1016/j.jpowsour.2016.08.119. [DOI] [Google Scholar]
- 25.Plichta EJ, Behl WK. A low-temperature electrolyte for lithium and lithium-ion batteries. J. Power Sources. 2000;88:192–196. doi: 10.1016/S0378-7753(00)00367-0. [DOI] [Google Scholar]
- 26.Das A, Thakur AK, Kumar K. Exploring low temperature Li+ ion conducting plastic battery electrolyte. Ionics. 2013;19:1811–1823. doi: 10.1007/s11581-013-0898-x. [DOI] [Google Scholar]
- 27.Laumann A, et al. Lithium migration at high temperatures in Li4Ti5O12 studied by neutron diffraction. Chem. Mater. 2011;23:2753–2759. doi: 10.1021/cm103332y. [DOI] [Google Scholar]
- 28.Schmid W, Wilkening M. Discriminating the Mobile Ions from the Immobile Ones in Li4+xTi5O12: 6Li NMR Reveals the Main Li+ Diffusion Pathway and Proposes a Refined Lithiation Mechanism. J. Phys. Chem. C. 2016;120:11372–11381. doi: 10.1021/acs.jpcc.6b02828. [DOI] [Google Scholar]
- 29.Tanaka S, et al. Atomic and electronic structures of Li4Ti5O12/Li7Ti5O12 (001) interfaces by first-principles calculations. J Mater. Sci. 2014;49:4032–4037. doi: 10.1007/s10853-014-8102-x. [DOI] [Google Scholar]
- 30.Lu W, Xiong SZ, Xie K, Pan Y, Zheng C. Identification of solid electrolyte interphase formed on graphite electrode cycled in trifluoroethyl aliphatic carboxylate-based electrolytes for low-temperature lithium-ion batteries. Ionics. 2016;22:2095–2102. doi: 10.1007/s11581-016-1743-9. [DOI] [Google Scholar]
- 31.Liu JL, et al. Gassing behavior of lithium titanate based lithium ion batteries with different types of electrolytes. J. Power Sources. 2015;286:380–387. doi: 10.1016/j.jpowsour.2015.03.172. [DOI] [Google Scholar]
- 32.He YB, et al. Gassing in Li4Ti5O12-based batteries and its remedy. Sci. Rep. 2012;2:913. doi: 10.1038/srep00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu GY, et al. Facile synthesis of nanocrystalline Li4Ti5O12 by microemulsion and its application as anode material for Li-ion batteries. J. Power Sources. 2012;220:84–88. doi: 10.1016/j.jpowsour.2012.07.087. [DOI] [Google Scholar]
- 34.Zhang H, et al. Li2ZrO3-coated Li4Ti5O12 with nanoscale interface for high performance lithium-ion batteries. Appl. Surf. Sci. 2016;368:56–62. doi: 10.1016/j.apsusc.2016.01.244. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mechanisms of the decrease in low-temperature electrochemical performance of Li4Ti5O12-based anode materials