Abstract
Biobanks and national registries represent a powerful tool for genomic discovery, but rely on diagnostic codes that can be unreliable and fail to capture relationships between related diagnoses. We developed an efficient means of conducting genome-wide association studies using combinations of diagnostic codes from electronic health records for 10,845 participants in a biobanking program at two large academic medical centers. Specifically, we applied latent Dirichilet allocation to fit 50 disease topics based on diagnostic codes, then conducted a genome-wide common-variant association for each topic. In sensitivity analysis, these results were contrasted with those obtained from traditional single-diagnosis phenome-wide association analysis, as well as those in which only a subset of diagnostic codes were included per topic. In meta-analysis across three biobank cohorts, we identified 23 disease-associated loci with p < 1e-15, including previously associated autoimmune disease loci. In all cases, observed significant associations were of greater magnitude than single phenome-wide diagnostic codes, and incorporation of less strongly loading diagnostic codes enhanced association. This strategy provides a more efficient means of identifying phenome-wide associations in biobanks with coded clinical data.
INTRODUCTION
In the search for common genetic variations associated with medical disorders, the traditional analytic approach examines single disorders in case-control cohorts ascertained for a specific disorder. With the availability of large-scale biobanks with broad ascertainment, multiple approaches to phenome-wide association – ie, looking across a range of clinical phenotypes to detect genetic association – have been proposed (1). However, relying on individual disorders represented in diagnostic codes may not efficiently capture the underlying architecture of genetic risk. First, the ability of claims codes to accurately capture a given diagnosis varies widely, even when diagnosis-specific classifiers are applied to augment single codes (2,3). As such, approaches that focus on individual diagnostic codes are limited by inaccurate, missing or heterogeneous diagnoses; eg, where individuals with cystic fibrosis might be represented by male infertility, diabetes and chronic rhinosinusitis even in the absence of a diagnostic code for cystic fibrosis (4). Second, under conditions of pleiotropy, where a single variant contributes to risk for multiple disorders, as in some autoimmune and neuropsychiatric disorders, standard phenome-wide approaches do not make efficient use of the correlation structure between diagnoses. Finally, single-code approaches do not capture disease subtypes with different genetic architecture, where these subtypes may be reflected in different patterns of comorbidity, as a recent investigation of diabetes mellitus suggests (5–8).
Here, we describe a method for addressing the problem of mapping genetic space to high-dimensional phenotype space that leverages comorbidity and diagnostic uncertainty to allow efficient genome-wide or single-locus association. This approach facilitates association by capturing diagnostic co-occurrence patterns to reduce dimensionality, thereby decreasing the number of hypotheses being tested, while increasing power by including individuals who may have different manifestations of the same underlying pathology. Specifically, we apply latent Dirichilet allocation (LDA), a means of identifying commonly co-occurring features, to derive a set of 50 disease topics. Then we test those topics for association with common genetic variation and compare this approach to standard methods using single International Classification of Diseases, Ninth Revision (ICD-9)/phenome-wide association studies (PheWAS) codes (9).
MATERIALS AND METHODS
Cohort Derivation and Genotyping
We drew on three cohorts of patients seen in the Brigham and Women’s Hospital network and the Massachusetts General Hospital network, representing the first 15,064 individuals genotyped as part of the Partners HealthCare Biobank initiative (10). These individuals provided informed consent for their electronic health records (EHRs) to be examined in investigations approved by the Partners Institutional Review Board, and provided blood samples for DNA extraction.
DNA was extracted from buffy coat and genotyped using the Illumina Expanded Multi-Ethnic Genotyping Array (MEGA or MEGA-EX) platforms, with common variant arrays incorporating content from the 1000 Genomes Project Phase 3. Single nucleotide polymorphism (SNP) coordinates were remapped based on the TopGenomicSeq provided from Illumina (MEGA_Consortium_v2_15070954_A2.csv); all rsIDs correspond to build 142 of dbSNP. To determine the forward strand of the SNP, we aligned both SNP sequences (alleles A and B) to hg19 using BLAT with default parameters set by the University of California, Santa Cruz Genome Browser (11).
Quality Control and Imputation
Genotyping was done using three versions of the Illumina Multi-Ethnic Global (MEG) array (MEGA n = 4927, MEGA EX n = 5353, MEG n = 4784; mappable variants available for each were 1,411,334, 1,710,339 and 1,747,639, respectively). Each cohort was cleaned, imputed and analyzed separately to avoid batch effects. For each batch, we included subjects with genotyping call rates exceeding 99%; no related individuals based on identity by descent were included (12). From these individuals, any genotyped SNP with a call rate of at least 95%, minor allele frequency of 0.01 or greater and Hardy-Weinberg equilibrium p value < 1 × 10−6 was included. We then imputed using the Michigan Imputation Server implementing Minimac3 (13–15). Imputation used all population subsets from the 1000 Genomes Project Phase 3 v5 as reference panel; haplotype phasing was performed using SHAPEIT (16).
Ancestry
For each cohort, we used principal components analysis of linkage-disequilibrium-pruned genotyped SNPs to characterize population structure, based on EIGENSTRAT, as implemented in PLINK v1.9, and plotted these components with superimposition of HapMap samples to confirm locations of northern European individuals (17–19). Limiting the analysis to these individuals yielded 3,728 + 3,402 + 3,715 = 10,845 analyzable participants.
Topic Identification
For both cohorts, ICD-9 diagnosis codes extracted from each individual’s medical record were grouped into 1,667 PheWAS codes corresponding to clinically meaningful disease categories, with the total number of codes within each PheWAS category preserved (20). Initial model fitting was performed using cohort 1 only, with cohort 2 preserved as an out-of-sample test set. To fit the topic model cohort, the PheWAS code count by subject matrix was frequency-controlled to eliminate PheWAS codes that occurred in < 1% or > 99% of subjects. After frequency control, 508 distinct PheWAS codes were used for the initial unsupervised learning step of model fit.
The PheWAS code count by subject matrix for cohort 1 was used to train an LDA model with 50 topics (9). LDA is a form of unsupervised machine learning typically found in natural language processing (NLP). As topic modeling is drawn from the NLP literature, this preprocessing can be conceptualized as treating each subject’s medical record as a document composed of ICD-9 codes that are lemmatized to PheWAS codes and thereafter analyzed as a term-count document matrix. LDA postulates that the words of a document are a mixture of underlying topics, and documents are composed of each of these topics to varying degrees. The resulting trained LDA model is a distribution of all PheWAS codes over each topic. This distribution can be used to score each collection of PheWAS codes for membership in each of the topics. In the case of illness in a biobank, we use LDA to model biology as a collection of topics or underlying generator processes of observable, but potentially overlapping and incompletely penetrant, pathological states. These states are captured as PheWAS “words.” Having trained the topic model on cohort 1, this model was then used to score each subject in cohort 1 for membership in each of the topics (in-sample) and each subject in cohort 2 for membership in each of the topics (out-of-sample). To perform the PheWAS LDA, we used the Gensim implementation of the LDA algorithm (21,22).
There is no widely accepted method for naming topics, since by definition all PheWAS words arise from all topics at some probability, albeit a vanishingly small probability in many cases. To aid in interpretability, in our discussion of results we name topics subjectively in terms of the preponderance of codes represented toward the top of the list, as interpreted by the two physician authors (THM, RHP); we refer to them below as “topic-name-plus,” as a reminder to the reader that the topic contains more than just a single diagnosis and may contain apparently unrelated terms.
Analysis
Single-locus associations in each cohort were examined individually, and then combined in inverse variance–weighted fixed-effects meta-analyses. In these analyses, only bi-allelic SNPs with minor allele frequencies of at least 1% were retained. Tests for association used linear regression assuming an additive allelic effect, treating each topic as a quantitative trait and adjusting for the first 10 principal components a priori (analyses incorporating 5 or 20 components did not yield meaningfully different results). Association results are presented in terms of independent loci after pruning, using the clump command in PLINK 1.9, with a 250kb window and r2 = 0.2. We present uncorrected p values, but elected to focus on p values less than 1e-15 and loci with at least two associated SNPs (23).
To facilitate comparisons across topics and methods, reported p values are not adjusted for linkage disequilibrium scores. Adjustment for lambda-1000 or linkage disequilibrium score regression intercept did not meaningfully change relative results; lambda values range from 0.990 to 1.017 γ across topics (24).
Secondary analyses examined alternate topic-based phenotypes in which either the most strongly loading diagnostic codes (ie, those with loading > 0.05) or least strongly loading diagnostic codes (ie, those with loading < 0.01) for a given topic were omitted, as a means of understanding the relative contributions of these sets of codes. These analyses utilized the same approach as for the primary analysis of topics. For comparison, we also examined association with the presence or absence of the single most strongly loading diagnostic code in each topic, using logistic regression.
All supplementary materials are available online at http://www.molmed.org.
RESULTS
After exclusions for genotyping quality control, relatedness and ancestry, cohorts 1, 2 and 3 included 2,141/3,728 (57.4%), 1,690/3,402 (49.7%) and 2,089/3,715 (56.23%) female participants, respectively. Mean ages were 57.9 (standard deviation [SD] 16.2), 62.4 (SD 16.0) and 59.4 (SD 16.5).
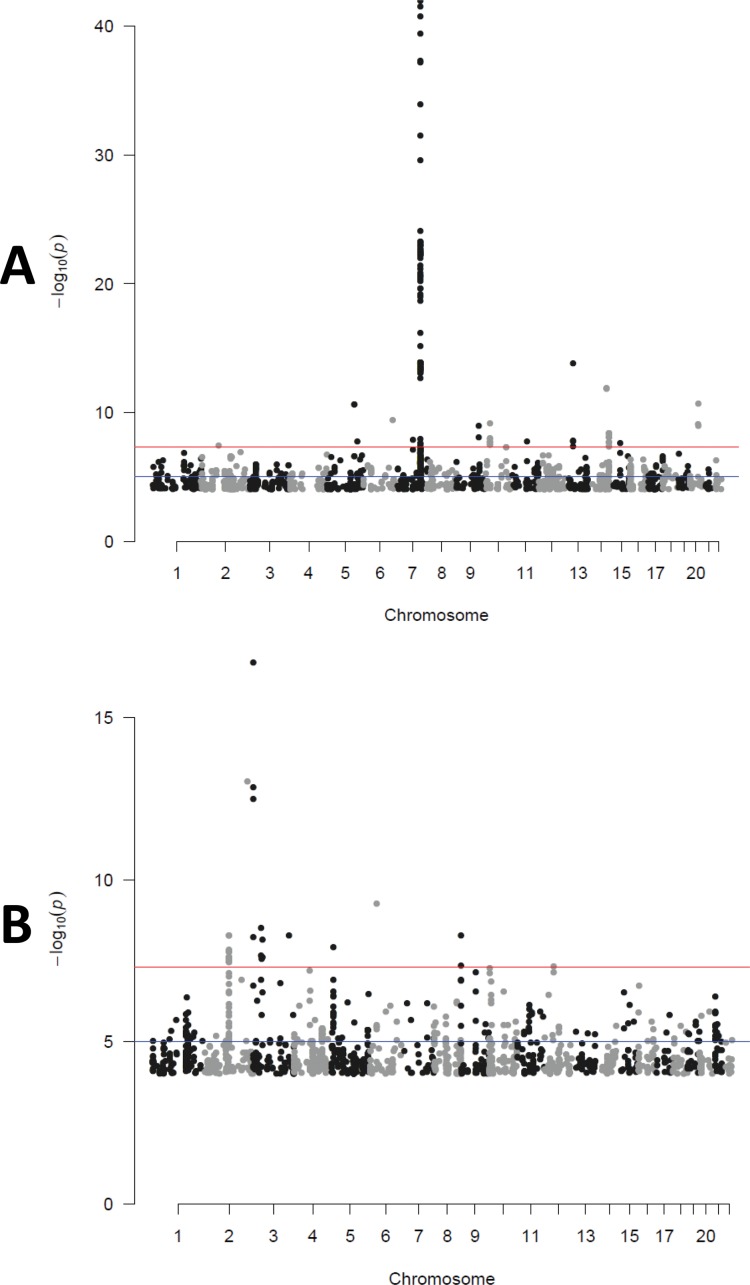
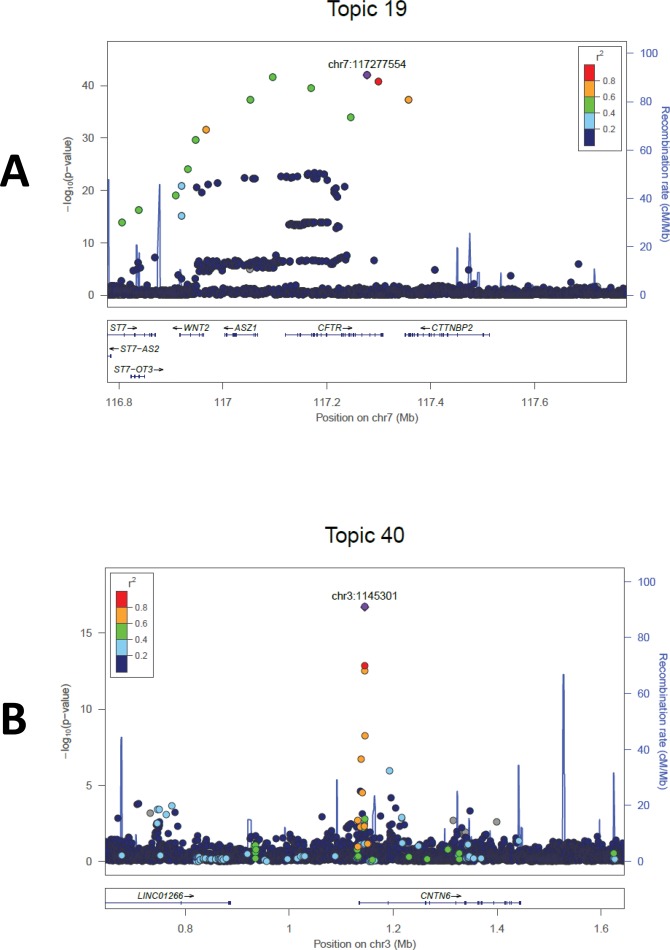
After imputation, a total of 7,781,941 SNPs with minor allele frequency (MAF) of 0.01 or greater were analyzed for each of the 50 topics and meta-analyzed. After genome-wide association analysis for each of the 50 topics, a total of 56 loci spanning 24 topics included at least one SNP with p < 1e-11; 39 of these loci across 22 topics included at least one additional associated SNP at p < 0.01. Table 1 reports the physical position, annotation and association for the most strongly associated SNPs for topics with at least one p < 5e-15 and at least two associated SNPs in a given locus, while Supplementary Table S1 reports the 10 most associated independent SNPs for all 50 topics (for effects by cohort, see Supplementary Table S2). The strongest associations (all p < 1e-15) were observed for pulmonary disease/cystic fibrosis-plus, anemia and fracture-plus, rheumatoid arthritis-plus, pregnancy complications-plus, uterine neoplasm-plus, viral-plus, neoplasm-plus, adrenal and electrolyte disorders-plus, and pituitary and adrenal disorder-plus. Figures 1 and 2 show Manhattan and locus plots for pulmonary disease/cystic fibrosis-plus and neoplasm-plus; for plots for the remainder of these, see Supplementary Materials. Diagnostic codes loading most strongly for each of these topics are listed in Table 2; for the codes loading on all 50 topics, see Supplementary Table S3.
Table 1.
Loci associated with a topic with p < 1e-15
| Topic # | Topic name | CHR | SNP | p value | A1 | A2 | MAF | SNPs in locusA | Locus range | Genes in locusB | GWAS catalogC |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 19 | Pulmonary disease and cystic fibrosis-plus | 7 | 7:117277554 | 1.049E-42 | T | A | 0.023 | 25 | chr7:117041941..117357607 | [ASZ1, CFTR, CTTNBP2] | Cystic fibrosis; esophageal cancer (PMID: 27527254) |
| 19 | Pulmonary disease and cystic fibrosis-plus | 7 | 7:116967838 | 3.005E-32 | A | C | 0.026 | 18 | chr7:116806103..117217725 | [ASZ1, CFTR, ST7, ST7-OT3, WNT2] | Cystic fibrosis; esophageal cancer (PMID: 27527254) |
| 09 | Anemia and fracture-plus | 4 | 4:12459496 | 5.985E-29 | G | C | 0.010 | 10 | chr4:12459496..12585393 | [] | None |
| 24 | Rheumatoid arthritis-plus | 6 | 6:32570417 | 2.14E-28 | C | A | 0.167 | 759 | chr6:32326531..32685550 | [HLA region] | Rheumatoid arthritis |
| 16 | Pregnancy complications-plus | 5 | 5:32198317 | 2.852E-20 | C | T | 0.010 | 4 | chr5:32006737..32198317 | [GOLPH3, PDZD2] | Myocardial infarction (PMID: 26708285) |
| 19 | Pulmonary disease and cystic fibrosis-plus | 7 | 7:117220403 | 2.055E-19 | G | A | 0.108 | 69 | chr7:116971883..117223764 | [ASZ1, CFTR] | Cystic fibrosis; esophageal cancer (PMID: 27527254) |
| 22 | Uterine neoplasm-plus | 14 | 14:62132727 | 3.294E-19 | A | G | 0.018 | 11 | chr14:62109231..62160023 | [FLJ22447, HIF1A-AS1] | None |
| 07 | Viral-plus | 14 | 14:57647875 | 7.58E-19 | G | T | 0.010 | 2 | chr14:57647875..57649586 | [] | None |
| 22 | Uterine neoplasm-plus | 6 | 6:148020784 | 7.105E-18 | G | A | 0.010 | 54 | chr6:147998800..148177349 | [] | None |
| 24 | Rheumatoid arthritis-plus | 6 | 6:32681992 | 9.832E-18 | C | T | 0.202 | 211 | chr6:32433759..32682043 | [HLA region] | Rheumatoid arthritis |
| 40 | Neoplasm-plus | 3 | 3:1145301 | 1.962E-17 | A | G | 0.016 | 10 | chr3:1131967..1145857 | [CNTN6] | Gut microbiome (PMID: 27723756) |
| 22 | Uterine neoplasm-plus | 2 | 2:209079758 | 7.645E-17 | C | G | 0.017 | 4 | chr2:209077907..209084036 | [] | None |
| 01 | Adrenal and electrolyte-plus | 4 | 4:41059660 | 1.813E-16 | A | C | 0.012 | 3 | chr4:40970912..41064140 | [APBB2] | None |
| 22 | Uterine neoplasm-plus | 4 | 4:84272944 | 1.993E-16 | G | T | 0.015 | 2 | chr4:84272944..84273025 | [] | None |
| 25 | Pituitary and adrenal disease-plus | 18 | 18:25741737 | 3.269E-16 | C | A | 0.012 | 5 | chr18:25724075..25753257 | [CDH2] | Resting heart rate (PMID: 27798624) |
| 24 | Rheumatoid arthritis-plus | 6 | 6:32303848 | 3.558E-16 | A | G | 0.204 | 263 | chr6:32114515..32389305 | [HLA region] | Rheumatoid arthritis |
| 07 | Viral-plus | 8 | 8:58987251 | 4.165E-16 | T | C | 0.012 | 5 | chr8:58987251..59037450 | [FAM110B] | None |
Total number of SNPs within r2= 0.2 and 250 kb with p < 0.01
Annotated mRNA in locus
Genes annotated in GWAS catalog
Figure 1.
Manhattan plots for two example topics: (A) pulmonary disease/cystic fibrosis-plus and (B) neoplasm-plus.
Figure 2.
Locus plots for two example topics: (A) pulmonary disease/cystic fibrosis-plus and (B) neoplasm-plus.
Table 2.
Diagnostic codes loading on topics associated with p < 1e-
| topic 01 | topic 07 | topic 09 | topic 16 | topic 19 | topic 22 | topic 24 | topic 25 | topic 40 |
|---|---|---|---|---|---|---|---|---|
| Disorders of adrenal glands | Psoriasis and related disorders | Other anemias | Erythematous conditions | Chronic airway obstruction | Uterine cancer | Rheumatoid arthritis and related inflammatory polyarthropathies | Disorders of the pituitary gland and its hypothalamic control | Secondary malignant neoplasm |
| Fracture of ankle and foot | Human immunodeficiency virus | Fracture of upper limb | Other conditions of the mother complicating pregnancy | Diseases of pancreas | Benign neoplasm of other parts of digestive system | Inflammatory spondylopathies | Benign neoplasm of other endocrine glands | Colorectal cancer |
| Disorders of fluid electrolyte and acid-base balance | Viral infection | Disorders of mineral metabolism | Indications for care or intervention related to labor and delivery (not elsewhere classified) | Cystic fibrosis | Endometrial hyperplasia | Radiotherapy | Cancer of other female genital organs | |
| Symptoms involving skin and other integumentary tissue | Disorders of the autonomic nervous system | Iron deficiency anemias not otherwise specified | Known or suspected fetal abnormality | Pneumonia | Benign neoplasm of colon | Neoplasm of uncertain behavior | Chemotherapy | |
| Pernicious or B12 deficiency anemia | Viral warts and human papillomavirus | Myeloproliferative disease | Hypertension complicating pregnancy | Tobacco use disorder | Cancer of other female genital organs | Disorders of adrenal glands | Radiotherapy | |
| Other disorders of the kidney and ureters | Tobacco use disorder | Gastrointestinal hemorrhage | Disorders of menstruation | Other diseases of lung | Diseases of esophagus | Cancer of other endocrine glands | Cancer of the digestive organs and peritoneum | |
| Urinary tract infection | Hereditary and idiopathic peripheral neuropathy | Malaise and fatigue | Other high-risk pregnancy | Abdominal pain | Nausea and vomiting | Effects of radiation not otherwise specified | Cancer suspected or other | |
| Abdominal pain | Diseases of blood and blood-forming organs | Early or threatened labor; hemorrhage in early pregnancy | Osteoporosis osteopenia and pathological fractures | Postoperative infection | Testicular dysfunction | Pancreatic cancer | ||
| Genitourinary congenital anomalies | Chromosomal anomalies and genetic disorders | Parkinson’s disease | Other disorders of the kidney and ureters | Benign neoplasm of brain and other parts of nervous system | Diseases of blood and blood-forming organs | |||
| Disorders of function of stomach | Pernicious or B12-deficiency anemia | Miscarriage; stillbirth | Neoplasm of unspecified nature of digestive system | Hypothyroidism | Neoplasm of unspecified nature of digestive system | |||
| Musculoskeletal symptoms referable to limbs | Fracture of hand or wrist | Other complications of pregnancy (not elsewhere classified) | Other disorders of intestine | Other headache syndromes | Disorders of fluid electrolyte and acid-base balance | |||
| Hydronephrosis | Pneumonia | Problems associated with amniotic cavity and membranes | ||||||
| Urinary calculus | Immune disorders | Abnormality pelvic soft tissues and organs complicating pregnancy | ||||||
| Anxiety phobic and dissociative disorders | Disorders of fluid electrolyte and acid-base balance | Symptoms affecting skin | ||||||
| Vitamin deficiency | Hemorrhage during pregnancy, childbirth and postpartum | |||||||
| Tobacco use disorder |
We also examined (Table 3) the effect of three alternate phenotypic definitions: examining the topic “tail” only (ie, diagnostic codes with weights < 0.05, the “tail” of the list) or the topic “head” only (ie, diagnostic codes with weights > 0.01, the “head” of the list) and including only the single top-weighted diagnostic code (ie, a standard single diagnosis association). This last comparison allows direct contrast with nominal associations returned by traditional PheWAS, recognizing that here only 50 phenotypes are examined rather than 500 or more.
Table 3.
Sensitivity analysis examining alternate phenotypes omitting the most- or least-strongly loading diagnoses
| Topic # | Topic name | CHR | SNP | P value Full topic (all diagnoses) | P values in sensitivity analysis | ||
|---|---|---|---|---|---|---|---|
| Topic head (diagnoses loading ≥0.01) | Topic tail (diagnoses loading ≤0.05) | Single diagnosis (only top diagnosis) | |||||
| 19 | Pulmonary disease and cystic fibrosis-plus | 7 | 7:117277554 | 1.049E-42 | 1.121E-4 | 7.178E-11 | 0.02882 |
| 19 | Pulmonary disease and cystic fibrosis-plus | 7 | 7:116967838 | 3.005E-32 | 1.686E-3 | 4.765E-3 | 0.0518 |
| 09 | Anemia and fracture-plus | 4 | 4:12459496 | 5.985E-29 | 0.3539 | 6.102E-51 | * |
| 24 | Rheumatoid arthritis-plus | 6 | 6:32570417 | 2.14E-28 | 2.895E-20 | 0.2878 | 7.586E-19 |
| 16 | Pregnancy complications-plus | 5 | 5:32198317 | 2.852E-20 | 0.1513 | 6.545E-44 | * |
| 19 | Pulmonary disease and cystic fibrosis-plus | 7 | 7:117220403 | 2.055E-19 | 4.268E-4 | 1.114E-08 | 0.05133 |
| 22 | Uterine neoplasm-plus | 14 | 14:62132727 | 3.294E-19 | 0.2401 | 0.7799 | 6.231E-3 |
| 07 | Viral-plus | 14 | 14:57647875 | 7.58E-19 | 0.3954 | 0.3554 | * |
| 22 | Uterine neoplasm-plus | 6 | 6:148020784 | 7.105E-18 | 0.5184 | 3.387E-3 | 5.874E-3 |
| 24 | Rheumatoid arthritis-plus | 6 | 6:32681992 | 9.832E-18 | 4.413E-14 | 0.2 | 4.409E-11 |
| 40 | Neoplasm-plus | 3 | 3:1145301 | 1.962E-17 | 9.212E-4 | 3.945E-6 | 0.01377 |
| 22 | Uterine neoplasm-plus | 2 | 2:209079758 | 7.645E-17 | 0.1993 | 0.8465 | * |
| 01 | Adrenal and electrolyte-plus | 4 | 4:41059660 | 1.813E-16 | 0.01646 | 0.1058 | * |
| 22 | Uterine neoplasm-plus | 4 | 4:84272944 | 1.993E-16 | 0.02548 | 0.8802 | * |
| 25 | Pituitary and adrenal disease-plus | 18 | 18:25741737 | 3.269E-16 | 0.06459 | 0.078 | 0.07633 |
| 24 | Rheumatoid arthritis-plus | 6 | 6:32303848 | 3.558E-16 | 5.129E-14 | 0.118 | 3.344E-13 |
| 07 | Viral-plus | 8 | 8:58987251 | 4.165E-16 | 0.01557 | 0.8416 | 0.09888 |
Sparseness of single diagnosis code and low MAF precludes estimate.
DISCUSSION
We applied a topic-modeling approach to identify 50 groups of diagnostic codes in biobank-associated EHR data and then used genome-wide data to examine common-variant associations for each topic. With this novel approach, we identified multiple known loci for autoimmune and pulmonary disease, as well as multiple apparently novel disease loci for pregnancy complications, viral susceptibility, anemia/fracture risk and uterine cancer not previously associated at a genome-wide threshold with disease (based on searching the National Human Genome Research Institute–European Bioinformatics Institute Catalog of published genome-wide association studies) (25). We compared our results to those arising from a standard single-diagnostic-code PheWAS; this approach would not have yielded association at this threshold. Moreover, omitting either the head or the tail of each topic (ie, the most- or least-weighted diagnosis) eliminates the association, suggesting that the observed effect does not arise from a small number of codes.
The identification of robust associations with loci implicated in prior genome-wide association studies demonstrates convergent validity (27,28). We demonstrate that this approach more efficiently detects these known associations (based on magnitude of p value) than single-code association. That is, simply incorporating a single ICD9/PheWAS code yielded weaker evidence of association. These loci and the sensitivity analysis associated with topic pruning (head and tail distributions; Table 1, Figure 1) function as positive controls and illustrations of assay sensitivity.
In nearly all cases, we note that the strongest associations are identified by incorporating all codes loading on a topic, rather than limiting the analysis to only the most strongly loading. Indeed, we observe that omitting such strongly loaded codes does not necessarily reduce the magnitude of association. Interestingly, there is only one example where a single code yielded an association nearly as robust as that observed with the topic, rheumatoid arthritis-plus, which may reflect the distinct genetic architecture of this disorder compared with some others.
In lieu of looking across phenotypes, a recent report describes a method to identify disease subtypes based upon network analysis (29). Our approach is not directly comparable, but may also be valuable in identifying subtypes in the case where a given diagnosis is genetically heterogeneous but the presence of comorbidities helps to define more homogeneous groups. A major advantage of the present approach compared with other unsupervised methods (eg, deep learning) is inspectability: it yields a weighted list of diagnostic codes. This inspectability enhances biological utility, as it allows post hoc clarification of the results, as illustrated by the sensitivity analysis. While we describe its application for genomics, it may be useful for other approaches drawing on coded EHR data where diagnostic codes do not definitively identify a diagnosis or subtype. Notably, it should also be possible to further extend the utility of our approach by incorporating additional coded or uncoded data – concepts extracted by NLP, for example – where such data are available.
The gain in statistical power afforded by this approach is apparent. For a genotypic risk ratio of 1.5 with a minor allele frequency of 25% and a disease prevalence of 5%, nearly 80,000 cases are required to achieve 80% power after Bonferroni correction for 500 PheWAS phenotypes, versus nearly 800 cases with 50 topics, if each is analyzed as a dichotomous outcome. In reality, the increased case reliability that arises from integrating across related codes likely renders these estimates conservative, in some cases markedly so. Empirically, our results show that in no case would a single-code association have yielded stronger nominal association, independent of Bonferroni correction, than the topic-based association, and in most cases the association was markedly less.
Still, we note several important limitations. From a modern genomics perspective, the present cohorts are likely insufficient to robustly detect all but the largest associations. They are, however, large enough to demonstrate the feasibility and efficiency of using aggregated groups of diagnoses as an efficient complement to phenome-wide association. Additionally, the biobank cohorts studied here are insufficient to examine these associations in non–northern European populations; replication in other populations would be informative. We note that the topics in many cases include codes seemingly unrelated to the predominant diagnosis; while these may represent type I error or noise, they may also illustrate the power of topic modeling to detect co-occurring diagnoses where the physiologic relationship is not otherwise recognized. Our analysis of topic “heads” and “tails” suggests that, in most cases, topics are not well captured by a single code or small number of codes. As such, the names we apply represent a best guess at interpretation, and investigation of the mechanism of overlap (in vivo or in silico) represents an important next step. In particular, consideration of orthogonal biological data, such as investigating pathways or expression quantitative trait loci, could further clarify the way in which groups of associated diagnoses relate to one another mechanistically.
CONCLUSION
In sum, our results indicate the utility of an approach to large-scale biobank data that aggregates over groups of diagnostic codes by treating groups of codes as relating to underlying topics. This approach is superior to single-code association for diagnoses with shared liability or groups of diagnostic codes that more reliably identify an underlying phenotype. It identifies multiple apparently novel disease loci while replicating existing associations, and suggests multiple other regions as well as phenotypes that merit further investigation in biobank cohorts or registries.
Supplementary Material
ACKNOWLEDGMENTS
This study was funded by the National Human Genome Research Institute and the National Institute of Mental Health. The authors acknowledge the Partners HealthCare Biobank for providing genomic data and health information data.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
RHP has served on advisory boards for, or provided consulting to, Genomind, Healthrageous, Perfect Health, Pfizer, Psy Therapeutics, and RIDVentures.
Cite this article as: McCoy TH, et al. (2017) Efficient genome-wide association in biobanks using topic modeling identifies multiple novel disease loci. Mol. Med. 23:285–94.
REFERENCES
- 1.Antony A, et al. Translational upregulation of folate receptors is mediated by homocysteine via RNA-heterogeneous nuclear ribonucleoprotein E1 interactions. J Clin Invest. 2004;113:285–301. doi: 10.1172/JCI11548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perlis R, et al. Using electronic medical records to enable large-scale studies in psychiatry: treatment resistant depression as a model. Psychol Med. 2012;42:41–50. doi: 10.1017/S0033291711000997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castro VM, et al. Validation of electronic health record phenotyping of bipolar disorder cases and controls. Am J Psychiatr. 2015;172:363–72. doi: 10.1176/appi.ajp.2014.14030423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hallberg P, Sjöblom V. The use of selective serotonin reuptake inhibitors during pregnancy and breast-feeding: a review and clinical aspects. J Clin Psychopharmacol. 2005;25:59–73. doi: 10.1097/01.jcp.0000150228.61501.e4. [DOI] [PubMed] [Google Scholar]
- 5.American Psychiatric Association. Practice guidelines for the treatment of major depression. Washington, DC: American Psychiatric Press; 2010. [Google Scholar]
- 6.Cross-Disorder Group of the Psychiatric Genomics Consortium, et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013;45:984–94. doi: 10.1038/ng.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho JH, Feldman M. Heterogeneity of autoimmune diseases: pathophysiologic insights from genetics and implications for new therapies. Nat Med. 2015;21:730–38. doi: 10.1038/nm.3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elkin I, et al. Initial severity and differential treatment outcome in the National Institute of Mental Health Treatment of Depression Collaborative Research Program. J Consult Clin Psychol. 1995;63:841. doi: 10.1037//0022-006x.63.5.841. [DOI] [PubMed] [Google Scholar]
- 9.Blei DM, Ng AY, Jordan MI. Latent dirichlet allocation. J Mach Learn Res. 2003;3:993–1022. [Google Scholar]
- 10.Gainer VS, et al. The biobank portal for Partners Personalized Medicine: a query tool for working with consented biobank samples, genotypes, and phenotypes using i2b2. J Pers Med. 2016;6:11. doi: 10.3390/jpm6010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kent WJ. BLAT—the BLAST-like alignment tool. Genome Res. 2002;12:656–64. doi: 10.1101/gr.229202. Article published online before March 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henn BM, et al. Cryptic distant relatives are common in both isolated and cosmopolitan genetic samples. PLoS One. 2012;7(4):e34267. doi: 10.1371/journal.pone.0034267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuchsberger C, Abecasis GR, Hinds DA. minimac2: faster genotype imputation. Bioinformatics. 2015;31:782–84. doi: 10.1093/bioinformatics/btu704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minimac3. Available from http://genome.sph.umich.edu/wiki/Minimac3. Accessed May 1, 2017.
- 15.Michigan imputation server. Available from https://imputationserver.sph.umich.edu. Accessed May 1, 2017.
- 16.Delaneau O, Marchini J, Zagury JF. A linear complexity phasing method for thousands of genomes. Nat Methods. 2012;9:179–81. doi: 10.1038/nmeth.1785. [DOI] [PubMed] [Google Scholar]
- 17.Price AL, et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–09. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 18.Chang CC, et al. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience. 2015;4:1. doi: 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Purcell S, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denny JC, et al. PheWAS: demonstrating the feasibility of a phenome-wide scan to discover gene-disease associations. Bioinformatics. 2010;26:1205–10. doi: 10.1093/bioinformatics/btq126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rehurek R, Sojka P. Software framework for topic modelling with large corpora. Proceedings of the LREC 2010 Workshop on New Challenges for NLP Frameworks. 2010:45–50. [Google Scholar]
- 22.Hoffman M, Bach FR, Blei DM. Advances in Neural Information Processing systems. Cambridge, MA: MIT Press; 2010. Online learning for latent Dirichlet allocation; pp. 856–64. [Google Scholar]
- 23.Purcell S, Chang C. PLINK 1.9. 2015. Available from www.cog-genomics.org/plink2. Downloaded May 15, 2017.
- 24.Bulik-Sullivan BK, et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47:291–95. doi: 10.1038/ng.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prom-Wormley E, et al. Genetic and environmental contributions to the relationships between brain structure and average lifetime cigarette use. Behav Genet. 2015;45:157–70. doi: 10.1007/s10519-014-9704-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gene Page online. Cambridge, MA: Broad Institute; Available from www.gtexportal.org/home/gene/CLPX. Accessed March 8, 2017. [Google Scholar]
- 27.Plenge RM, et al. TRAF1-C5 as a risk locus for rheumatoid arthritis – a genomewide study. N Engl J Med. 2017;357:1199–1209. doi: 10.1056/NEJMoa073491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.International Multiple Sclerosis Genetics Consortium. Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med. 2007;357:851–62. doi: 10.1056/NEJMoa073493. [DOI] [PubMed] [Google Scholar]
- 29.Pavlova B, Perlis RH, Alda M, Uher R. Lifetime prevalence of anxiety disorders in people with bipolar disorder: a systematic review and meta-analysis. Lancet Psychiatr. 2015;2:710–17. doi: 10.1016/S2215-0366(15)00112-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.