Dear Editor,
Acute myeloid leukemia (AML) is a clinically and biologically heterogeneous disease. Cytarabine (Ara-C), a deoxycytidine analog, in combination with daunorubicin is the standard remission induction regimen used in the treatment of AML [1]. Drug resistance and drug-related toxicities are the major reasons of treatment failure, which could potentially be attributed to the defective or altered metabolism of the drugs [2]. Deoxycytidine kinase (DCK) is the rate limiting kinase involved in the activation of the pro-drug Ara-C to Ara-CTP. Decreased expression and activity of DCK in AML has been reported as a mechanism of clinical resistance to Ara-C [3]. Cytidine deaminase (CDA) is the major enzyme involved in the systemic degradation of Ara-C by deaminating the drug to its uracil derivative uracil arabinoside (Ara-U) [4]. Increased level of CDA has been shown to play an important role in the development of Ara-C resistance [5, 6]. Gemcitabine, another nucleoside analog, has been reported to cause severe drug toxicity when administered to patients with decreased CDA activity [7, 8]. CDA gene polymorphisms A79C and G208A are shown associated with decreased CDA activity there by resulting in severe drug-related toxicities [9]. However, there are no reports so far on Ara-C associated toxicity attributed to any of the Ara-C metabolizing enzymes expression or activity. We report a case of AML in which the patient developed severe cerebellar dysfunction following high-dose Ara-C based post induction consolidation.
A 43-year-old male was diagnosed as AML (FAB M1) with trisomy 21 as the sole cytogenetic abnormality (index case). He was started on standard remission induction chemotherapy for AML (Ara-C 200 mg/m2 for 7 days and daunorubicin 60 mg/m2 for 3 days). The induction phase was complicated by drop in blood counts leading to grade IV hematologic toxicity and neutropenic pyrexia which responded to sequential escalation of antibiotics and blood product support. Hematological recovery occurred by day 26 and he entered first complete remission (CR1). He was planned for post remission consolidation with 3 cycles of high-dose Ara-C chemotherapy (HiDAC–3 g/m2 twice daily on alternate days for 3 days, total dose of 18 g/m2 per course). On day five of the first high course of HiDAC, he developed features of cerebellar toxicity which included slurring of speech, unsteadiness of gait with dysmetria. Neuroimaging and cerebrospinal fluid analysis was normal. Cytarabine-induced cerebellitis was suspected. He improved on supportive care and occupational therapy. Further consolidation was done with a single course of 5 days each of mitoxantrone (10 mg/m2) and etoposide (65 mg/m2). The consolidation chemotherapy was complicated by myelosuppression and neutropenic pyrexia requiring sequential escalation of antibiotic support and growth factors. Hematological recovery was documented at the end of second week during both the consolidation cycles. Five weeks following the second consolidation chemotherapy, he was found to have suffered a medullary relapse of the leukemia. He opted for palliative therapy following diagnosis of relapse.
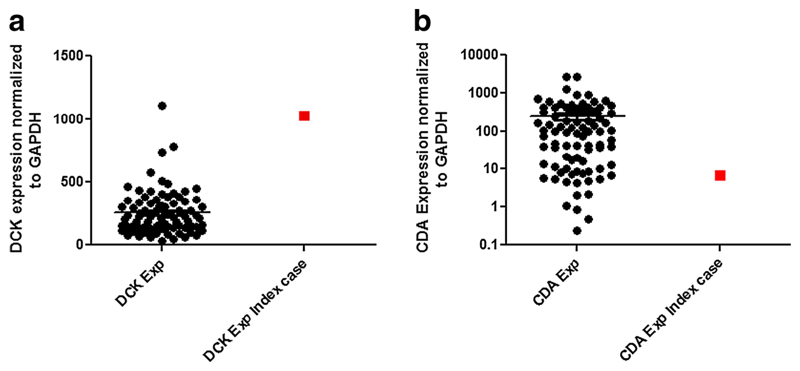
Expression of Ara-C metabolizing genes CDA and DCK were routinely evaluated in all AML patients as part of an ongoing study. Informed and written consent was obtained from all patients for this study; the index case reported here was also part of this study. RQ-PCR analysis of the index case revealed that he had very low messenger RNA (mRNA) levels for the drug inactivating enzyme CDA while RNA expression of DCK, the pivotal enzyme involved in the Ara-C biotransformation, was found to be higher when compared to other AML subjects analyzed (Fig. 1a and b). Ara-C cytotoxicity was determined using MTT assay, and the patient’s bone marrow mononuclear cells were found to be highly sensitive to Ara-C ex vivo. This ex vivo response as well as clinical toxicity profile can be correlated to the low and higher mRNA levels of CDA and DCK, respectively, displayed by the patient at presentation.
Fig. 1.
a, b RNA expression of Ara-C metabolizing genes DCK and CDA were determined using ΔΔCT method, where ΔCT is the difference in the threshold cycle (CT) values of target gene and the housekeeping gene GAPDH. Each sample was normalized to ΔCT value of the sample AML001. Index case is the patient documented with cerebellar toxicity. Median DCK expression was 214.4 (31.87–1113), whereas the index case DCK expression was determined to be 1030. Median CDA expression was 95.92 (0.238–2760), and the index case CDA expression was determined to be 6.56. Taqman-based gene expression assays used were Hs01040725_m1 (DCK), Hs00156401_m1 (CDA), and 4352934E for GAPDH
DCK/CDA expression ratio was calculated, for identifying those patients with strikingly high DCK and low CDA levels. A total of six patients with AML were identified with very high DCK/CDA expression ratio (Table-1) apart from the index patient. It was noted that four out of these six patients developed treatment-related mortality (death within 28 days of initiating therapy) secondary to infections due to grade IV myelosuppression. Four patients developed grade 2–3 hepatic dysfunction during the chemotherapy. One patient while on remission induction developed acute respiratory distress syndrome and died of pulmonary hemorrhage on day 14; the highest DCK/CDA expression ratio was noted in this patient. Pulmonary toxicity secondary to Ara-C infusion is a well described cytarabine-induced complication [10]. Complete remission (CR1) was documented in two patients; out of whom, one patient died of invasive pulmonary aspergillosis. One patient was lost to follow up post achieving CR post induction chemotherapy. The mean duration of neutropenia in these patients was 26 days.
Table 1.
Characteristics of patients with high DCK/CDA expression
| UPN | FAB | Cytogenetic risk group | DCK | CDA | DCK/CDAb | CDA SNP A79C | Day 28 status | Non-hematological toxicities encountered during chemotherapyc | Day of death/relapse | Cause of Death |
|---|---|---|---|---|---|---|---|---|---|---|
| 1a | M1 | Intermediate | 1030.3 | 6.560 | 157.041 | AA | CR1 | Grade 4 cerebellar toxicity | Relapsed 5 weeks after consolidation | Lost to follow-up |
| 2 | M2 | High | 219.36 | 0.238 | 918.528 | AC | TRM | Grade 4 pulmonary toxicity | Expired on day 14 | Pulmonary hemorrhage |
| 3 | M1 | Intermediate | 339.63 | 0.842 | 403.282 | AC | TRM | Grade 3 hyperbilirubinemia | Expired on day 13 | Gram negative sepsis |
| 4 | M1 | High | 330.61 | 1.072 | 308.356 | AA | TRM | Grade 2 hyperbilirubinemia | Expired on day 27 | Gram negative sepsis |
| 5 | M2 | Intermediate | 180.91 | 0.466 | 388.223 | AA | TRM | Nil | Expired on day 28 | Fungal encephalitis |
| 6 | M1 | High | 464.49 | 2.158 | 215.150 | AA | CR1 | Grade 3 hyperbilirubinemia Grade 2 transaminitis |
Expired on day 44 | Fungal pneumonia |
| 7 | M1 | Intermediate | 305.96 | 2.040 | 149.936 | AA | CR1 | Grade 2 hyperbilirubinemia | Lost to follow-up | Lost to follow-up |
FAB French-American-British AML classification, TRM treatment-related mortality, DCK deoxycytidine kinase, CDA cytidine deaminase, SNP single nucleotide polymorphism, AA wild type, AC heterozygous
Index case
Ratio of the expressions for DCK and CDA indicates those patients having low CDA expression and high DCK mRNA levels. Taqman-based gene expression assays used were Hs01040725_m1 (DCK), Hs00156401_m1 (CDA), and 4352934E for GAPDH
Toxicity grading as per NCI CTC toxicity scale version 2.0
As non-synonymous gene variants, A79C and G208A in CDA gene are known to be associated with Ara-C and gemcitabine-related toxicity [8, 9], CDA gene re-sequencing was done for all these patients, G208A was not detected, and A79C genotypes are as shown in the table-1. The index patient had trisomy 21 as the sole cytogenetic abnormality, hence, GATA1 exon two mutations, specifically associated with good prognosis in AML-M7 children with Down’s syndrome was evaluated [11]. GATA1 exon two mutations were absent in his blasts probably owing to the somatic origin of trisomy 21 rather than germ line nature. Ara-C-induced cerebellitis in this patient during the HiDAC consolidation can be attributed to low CDA expression in combination with high DCK, as systemic elimination of the drug is severely decreased due to low CDA levels. This report thus highlights the importance of evaluating Ara-C metabolizing gene expression especially for CDA and DCK in patients with AML who go for high dose cytosine consolidation. CDA and DCK levels are critical not only in determining the drug response but also be pivotal in predicting fatal drug-induced toxicities.
Acknowledgments
The authors would like to thank the Department of Biotechnology, India for the financial assistance given to the principal investigator PB (Grant no: BT/01/COE/08/03). AA and SK are recipients of fellowship from University Grants Commission (UGC), India. VM is supported by the Wellcome-DBT senior fellowship program.
Footnotes
Conflict of interest The authors have no conflicts of interest to disclose.
Author contributions AA performed the research, analyzed the data, and wrote the paper. AJD performed the clinical data accrual, analyzed the data, and wrote the paper. SV performed the research. SK performed the research. PB was the principal investigator, designed the study, performed the research, analyzed the data, and wrote the paper. VM performed the clinical data accrual, analyzed the data, and wrote the paper.
References
- 1.Rowe JM. Optimal induction and post-remission therapy for AML in first remission. Hematol Educ Program Am Soc Hematol Am Soc Hematol Educ Program. 2009:396–405. doi: 10.1182/asheducation-2009.1.396. [DOI] [PubMed] [Google Scholar]
- 2.Kaspers GJ, Zwaan CM. Pediatric acute myeloid leukemia: towards high-quality cure of all patients. Haematologica. 2007;92(11):1519–1532. doi: 10.3324/haematol.11203. [DOI] [PubMed] [Google Scholar]
- 3.Galmarini CM, Thomas X, Graham K, El Jafaari A, Cros E, Jordheim L, Mackey JR, Dumontet C. Deoxycytidine kinase and cN-II nucleotidase expression in blast cells predict survival in acute myeloid leukaemia patients treated with cytarabine. Br J Haematol. 2003;122(1):53–60. doi: 10.1046/j.1365-2141.2003.04386.x. [DOI] [PubMed] [Google Scholar]
- 4.Cohen SS. The lethality of aranucleotides. Med Biol. 1976;54(5):299–326. [PubMed] [Google Scholar]
- 5.Abraham A, Varatharajan S, Abbas S, Zhang W, Shaji RV, Ahmed R, George B, Srivastava A, Chandy M, Mathews V, Balasubramanian P. Cytidine deaminase genetic variants influence RNA expression and cytarabine cytotoxicity in acute myeloid leukemia. Pharmacogenomics. 2012;13(3):269–282. doi: 10.2217/pgs.11.149. [DOI] [PubMed] [Google Scholar]
- 6.Braess J, Pfortner J, Kern W, Hiddemann W, Schleyer E. Cytidine deaminase—the methodological relevance of AraC deamination for ex vivo experiments using cultured cell lines, fresh leukemic blasts, and normal bone marrow cells. Ann Hematol. 1999;78(11):514–520. doi: 10.1007/s002770050548. [DOI] [PubMed] [Google Scholar]
- 7.Ciccolini J, Dahan L, Andre N, Evrard A, Duluc M, Blesius A, Yang C, Giacometti S, Brunet C, Raynal C, Ortiz A, et al. Cytidine deaminase residual activity in serum is a predictive marker of early severe toxicities in adults after gemcitabine-based chemotherapies. J Clin Oncol. 2010;28(1):160–165. doi: 10.1200/JCO.2009.24.4491. [DOI] [PubMed] [Google Scholar]
- 8.Mercier C, Raynal C, Dahan L, Ortiz A, Evrard A, Dupuis C, Blesius A, Duluc M, Franceschini F, Giacometti S, Salas S, et al. Toxic death case in a patient undergoing gemcitabine-based chemotherapy in relation with cytidine deaminase downregulation. Pharmacogenet Genomics. 2007;17(10):841–844. doi: 10.1097/FPC.0b013e32825ea6e3. [DOI] [PubMed] [Google Scholar]
- 9.Bhatla D, Gerbing RB, Alonzo TA, Conner H, Ross JA, Meshinchi S, Zhai X, Zamzow T, Mehta PA, Geiger H, Perentesis J, et al. Cytidine deaminase genotype and toxicity of cytosine arabinoside therapy in children with acute myeloid leukemia. Br J Haematol. 2009;144(3):388–394. doi: 10.1111/j.1365-2141.2008.07461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forghieri F, Luppi M, Morselli M, Potenza L. Cytarabine-related lung infiltrates on high resolution computerized tomography: a possible complication with benign outcome in leukemic patients. Haematologica. 2007;92(9):e85–90. doi: 10.3324/haematol.11697. [DOI] [PubMed] [Google Scholar]
- 11.Ge Y, Jensen TL, Stout ML, Flatley RM, Grohar PJ, Ravindranath Y, Matherly LH, Taub JW. The role of cytidine deaminase and GATA1 mutations in the increased cytosine arabinoside sensitivity of Down syndrome myeloblasts and leukemia cell lines. Cancer Res. 2004;64(2):728–735. doi: 10.1158/0008-5472.can-03-2456. [DOI] [PubMed] [Google Scholar]