Abstract
Glutamatergic signaling plays an important role in learning and memory. Using Pavlovian conditioned approach procedures, the mechanisms that drive stimulus-reward learning and memory have been investigated. However, there are instances where reward-predictive stimuli can function beyond being solely predictive and can be attributed with “motivational value” or incentive salience. Using a Pavlovian conditioned approach procedure consisting of two different but equally predictive stimuli (lever vs. tone) we investigated the role NMDA receptor function has in the attribution of incentive salience. The results revealed that the administration of MK-801, an NMDA receptor antagonist, during acquisition of Pavlovian conditioned approach promoted goal-tracking to a lever stimulus, while control animals learned to sign-track. Moreover, within the same animals, the use of a tone stimulus elicited goal-tracking responses that were unaffected by MK-801 pretreatments. Furthermore, a lever CS that elicited sign-tracking served as a more robust conditioned reinforcer than a tone CS that elicited goal-tracking or a lever CS that elicited goal-tracking via MK-801 pretreatments. Collectively, these results demonstrate that NMDA receptor antagonism can alter the stimulus-reward relationship learned and prevent the attribution of incentive salience, rather than impede general learning.
Keywords: incentive salience, glutamate, MK-801, sign-tracking, goal-tracking
1. Introduction
Glutamatergic signaling plays an important role in learning and memory. Specifically, NMDA receptor function is hypothesized to govern synaptic plasticity and underlie learning and the formation of associated memories (1–4). For example, the blockade of NMDA receptors results in the disruption of learning in a variety of behavioral procedures (5,6).
Studies using Pavlovian conditioning, via fear conditioning (7–9), have provided a vast amount of data regarding glutamatergic function in relation to learning and memory. While the associative processes involved in Pavlovian conditioning also encompass appetitive events, most of the reward-related learning literature has focused on dopaminergic signaling due to the associated dopamine-mediated neurobiological changes seen with stimulus-reward learning (see 10; 11,12). Beside dopamine, there is evidence that NMDA receptor function is also necessary for stimulus-reward learning; NMDA blockade disrupts the acquisition of stimulus-reward relationships (13–17).
In addition to their predictive function, there is a growing body of literature demonstrating that some conditioned stimuli (CS) can be more readily attributed with motivational value or “incentive salience” (18–20), and the predictive versus motivational aspects of a CS are typically identified via response topographies. For example, goal-tracking (approach to the location of reward delivery during CS presentation; 21) appears to be mediated by the predictive CS-US relationship, while sign-tracking (approach and interaction with the CS; 22,23) appears to be indicative of incentive salience attribution to the CS (19). More recent studies have revealed that dopaminergic function appears to be necessary for the attribution of incentive value to a CS, while it may not be necessary for the acquisition of the predictive CS-US relationship (18,24–26).
While there are dissociable dopaminergic mechanisms between the predictive and incentive function of a CS, little is known regarding the role of NMDA receptor function in these two processes. Thus, we investigated the role of NMDA receptor function in the acquisition of the predictive versus incentive function of a food-paired CS. To elucidate the role of NMDA receptor function, we utilized a 2-CS Pavlovian conditioned approach (PCA) procedure (see 27; 26) that incorporates two different but equally predictive stimuli that preferentially elicit sign- and goal-tracking responses within an individual. We reasoned that if NMDA receptor function is necessary for learning to occur more generally (as suggested by the literature), NMDA antagonism during CS-US acquisition should disrupt the development of stimulus-reward relationships altogether. However, if sign- and goal-tracking are indicative of distinct neurobehavioral processes, NMDA receptor blockade should preferentially affect a CS-US relationship that elicits sign-tracking (i.e., incentive salience) due to the role NMDA receptors have in relation to dopaminergic signaling (15).
2. Methods
2.1 Animals
Twenty-four adult male Sprague-Dawley rats (Harlan Inc., Indianapolis, IN), weighing ~250–275g upon arrival, were used. All rats were acclimated to the colony environment and handled daily for one week prior to experimentation. Rats were individually housed in a temperature-controlled environment with a 12:12 hr light:dark cycle and had ad libitum access to food and water in their home cage throughout experimentation. All experimentation was conducted during the light phase. All experimental protocols were conducted in accordance to the 2011, National Research Council: Guide for the Care and Use of Laboratory Animals (8th edition) and were approved by the Institutional Animal Care and Use Committee at the University of Kentucky.
2.2 Apparatus
Experiments were conducted in operant conditioning chambers (ENV-008; MED Associates, St. Albans, VT) and operated using MED-PC. Each operant chamber consisted of a recessed food-receptacle (ENV-200R2MA), outfitted with a head-entry detector (ENV-254-CB), located on the front response panel of the chamber which allowed for food pellets (45-mg Noyes Precision Pellets; Research Diets, Inc., New Brunswick, NJ) to be delivered via a dispenser (ENV-203M-45). Two retractable response levers were mounted on either side of the recessed food-receptacle (ENV-122CM), two white cue lights (ENV-221M) were mounted above each response lever, and a Sonalert© tone (ENV-223 AM) was located above the top left cue light. The back-response panel was outfitted with a single retractable response lever (ENV-122CM; located directly opposite of the food receptacle), two nosepoke response receptacles (ENV-114BM; directly opposite to front response levers) were mounted on either side of the retractable response lever, and a house-light (ENV-227M) was mounted at the top of the back panel above the response lever.
2.3 Drugs
(+)-MK-801 hydrogen maleate was purchased from Sigma-Aldrich (St. Louis, MO, USA) and mixed in sterile saline (0.9% NaCl).
2.4 Behavioral Procedures
2.4.1 Magazine Shaping
Animals were trained to retrieve food pellets from the food receptacle for two consecutive days, where rats were given 40 minutes to retrieve and consume 16 food pellets, delivered on a 60-s fixed time schedule.
2.4.2 2-CS PCA Training
Each trial began with the presentation of a single 8-s lever (located on the front-panel; balanced for side across animals) or a tone. Immediately following lever retraction or tone offset, a single food pellet was non-contingently delivered into the food receptacle. Stimulus presentations were separated by a 90-s variable-time inter-trial-interval (28). Each session contained 32 trials, consisting of 16 lever-only insertions and 16 tone-only presentations in pseudorandom order, where no more than four presentations of the same stimulus occurred consecutively. Rats were trained for 14 consecutive sessions. Sign-tracking responses were recorded as lever presses, while goal-tracking responses were recorded as breaks of a photo beam within the food receptacle during stimulus presentation. Additionally, goal-tracking responses made within 8s prior to either CS presentation (8s pre-CS) were also recorded. Fifteen minutes prior to each 2-CS PCA training session, rats (n=12/group) were pretreated (s.c. injection) with saline or MK-801 (0.1 mg/kg; dose was determined via pilot studies revealing suppression of pellet consumption at higher doses).
2.4.4 CS-only Test
To determine what was learned about the lever CS and tone CS during acquisition, a subset of animals (n=6/group), pretreated with saline or MK-801 during 2-CS PCA training, underwent a CS-test. The CS-test took place the next day following 2-CS PCA training and functioned similarly to 2-CS PCA training but only 8 stimulus presentations (4 lever and 4 tone; sans food delivery) occurred. Importantly, testing was conducted under drug-free conditions.
2.4.5 Conditioned Reinforcement
To determine the relative value attributed to the lever CS or tone CS, another subset of animals (n=6/group), pretreated with saline or MK-801 during 2-CS PCA training, were tested for conditioned reinforcement. Conditioned reinforcement took place over two subsequent days immediately following 2-CS PCA training. Conditioned reinforcement consisted of two 30-min sessions where rats were presented with an illuminated nosepoke (balanced for side), where a single response into the nosepoke resulted in the simultaneous offset of the nosepoke light and an 8s presentation of either the lever or tone (balanced across animals) that was used during 2-CS PCA training. After the offset of the 8-s stimulus (lever or tone), the nosepoke light turned on again. On the subsequent day, the opposite nosepoke light was illuminated, and a response produced either the lever or tone for 8s, opposite to what stimulus was presented the day before. Nosepoke-responses to the non-illuminated (i.e., inactive) receptacle were recorded during each test but had no consequence. Importantly, conditioned reinforcement sessions took place under drug-free conditions.
2.5 Analysis
Data were analyzed using linear mixed-effects modeling (29). Due to the absence of sign-tracking to a tone, sign-tracking response rates for 2-CS PCA training were analyzed alone with session (continuous) as a within-subject factor, treatment group (nominal) as a between-subject factor, and subject as a random factor (26,27). Goal-tracking to the CS and goal-tracking during the 8s pre-CS for 2-CS PCA training were analyzed with session (continuous) and stimulus (nominal) as within-subject factors, treatment group (nominal) as a between-subject factor, and subject as a random factor. For the CS-test, the responses per each stimulus were calculated as an average rate, with stimulus (nominal) as within-subject factor, treatment (nominal) as a between subject factor, and subject as a random factor. Conditioned reinforcement tests, with stimulus (nominal) and response type (i.e., active vs. inactive; nominal) as within-subject factors, treatment (nominal) as between-subject factor, and subject as a random factor. In addition, CS-dependent goal-tracking, for both the lever CS and tone CS, was directly compared to goal-tracking during the 8s pre-CS within each treatment group with session (continuous) and GT type (in presence vs. absence of CS; nominal) as within-subject factors, and subject as a random factor. Moreover, goal-tracking rates during CS presentations and the pre-CS periods were used to calculate the percent CS responding (goal-tracking during the 8s CS divided by goal-tracking during the 8s CS added to goal-tracking during the 8s pre-CS period) to further determine if a CS-US relationship had been learned. It should be noted that sign-tracking during the pre-CS period could not be measured since the lever was retracted; thus, all comparisons are related to goal-tracking behavior only. One-sample t-tests were conducted on the percent CS responding for the last day of 2-CS PCA acquisition and the CS-only test day to determine if goal-tracking responding was CS-dependent. One-sample t-tests were compared against 50%, which signifies no differences in the number of responses recorded during the presentation of the CS and the pre-CS period. Post hoc tests were conducted with Tukey HSD. For all tests, α was set at 0.05.
3. Results
3.1 2-CS PCA Training
Figure 1 illustrates the acquisition of sign- and goal-tracking response rates to the lever and tone CSs, goal-tracking responses made during an 8s pre-CS period, and the relative proportion of CS-dependent responses made during the 14-day acquisition period following pretreatments of saline or MK-801. Figure 1A illustrates sign-tracking rates to the lever CS. Linear mixed-effects analysis revealed a main effect of treatment [F(1,22) = 19.89, p < 0.05], indicating that MK-801 reduced sign-tracking behavior, and a main effect of session [F(1,22) = 10.61, p < 0.05], indicating that sign-tracking rates changed over sessions. Collectively, these results indicate that MK-801 significantly reduced sign-tracking to the lever CS over the course of the 14-day acquisition period.
Figure 1.
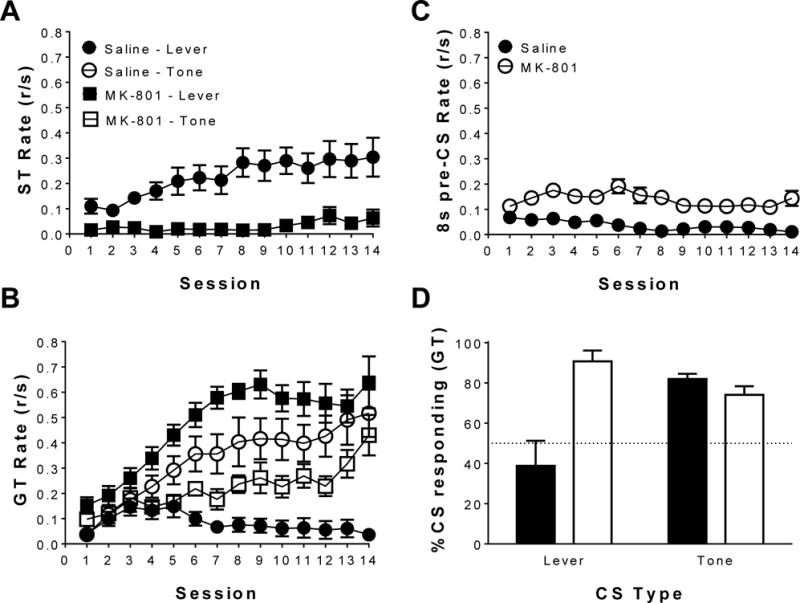
The effects of saline vs. MK-801, a NMDA antagonist, on 2-CS PCA acquisition. Mean (±SEM) response rate (responses/second; r/s) for saline pretreatments and MK-801 (0.1 mg/kg) pretreatments on (A) sign-tracking, (B) goal-tracking and (C) goal-tracking during an 8s pre-CS period. (D) Mean (±SEM) percent CS responding for CS-dependent goal-tracking relative to responding during an 8s pre-CS period for the last day of 2-CS PCA acquisition. Note: the line at 50% represents the point where the proportion of CS-dependent goal-tracking responses are equivalent to the number of goal-tracking responses emitted during an 8s pre-CS period.
Figure 1B illustrates goal-tracking rates to the lever CS and tone CS. Linear mixed-effects analysis revealed a main effect of treatment [F(1,22) = 10.06, p < 0.05], indicating goal-tracking rates varied depending on pretreatment, and a main effect of session [F(1,22) = 46.17, p < 0.05], indicating goal-tracking rates increased over the 14-day acquisition period. Analysis also revealed a significant treatment × stimulus interaction [F(1,22) = 61.71, p < 0.05], indicating that goal-tracking rates to the lever CS or tone CS were dependent on the pretreatments, and a significant treatment × session interaction [F(1,22) = 4.65, p < 0.05], indicating that changes in goal-tracking rates over sessions were pretreatment-dependent. Finally, there was a significant treatment × session × stimulus interaction [F(1,22) = 24.33, p < 0.05], indicating that changes in goal-tracking rates over the training sessions were dependent on both the CS type and the pretreatment. Collectively, these results indicate that, relative to saline, MK-801 attenuated goal-tracking to the tone CS while promoting goal-tracking to the lever CS.
Figure 1C illustrates goal-tracking response rates during the 8s pre-CS period. Linear mixed-effects analysis revealed there was a main effect of session [F(1,22) = 7.81, p < 0.05], indicating goal-tracking during the 8s pre-CS period decreased across sessions, and a main effect of treatment [F(1,22) = 40.95, p < 0.05], indicating goal-tracking rates during the 8s pre-CS period were greater in MK-801 pretreated animals. When CS-dependent goal-tracking and goal-tracking during the 8s pre-CS was directly compared within each treatment group, linear mixed-effects analysis revealed no differences between goal-tracking to the lever CS and 8s pre-CS for saline pretreated animals. Additionally, analysis revealed a main effect of session [F(1,11) = 15.96, p < 0.05], a main effect of GT type [F(1,11) = 24.48, p < 0.05], and a session × GT type interaction [F(1,11) = 22.73, p < 0.05], indicating goal-tracking rates to the tone CS was significantly greater than goal-tracking rates during the 8s pre-CS period for saline pretreated animals. Using linear mixed-effects analysis, a main effect of session [F(1,11) = 21.11, p < 0.05], a main effect of GT type [F(1,11) = 60.47, p < 0.05], and a session × GT type interaction [F(1,11) = 23.31, p < 0.05] for goal-tracking to the lever CS and goal-tracking during the 8s pre-CS in MK-801 pretreated animals was revealed, indicating that MK-801 pretreated animals had higher goal-tracking rates in the presence of the CS than in the absence of the CS. Finally, linear mixed-effects analysis revealed a main effect of session [F(1,11) = 13.72, p < 0.05], a main effect of GT type [F(1,11) = 7.49, p < 0.05], and a session × GT type interaction [F(1,11) = 16.67, p < 0.05], indicating goal-tracking rates to the tone CS was significantly greater than goal-tracking rates during the 8s pre-CS for MK-801 pretreated animals. Collectively, these results indicate that goal-tracking responses were learned and dependent upon CS presentation.
Figure 1D illustrates percent CS-dependent goal-tracking behavior as a proportion of all recorded goal-tracking responses to the lever CS and tone CS. One-sample t-tests on the last day of 2-CS PCA acquisition revealed no differences from 50% for goal-tracking to a lever CS in saline pretreated animals (46.30±13.69), indicating these animals did not learn to goal-track to a lever CS (sign-tracking to the lever CS instead). However, one-sample t-tests revealed a significant increase from 50% for goal-tracking to a tone CS in saline pretreated animals (90.73±5.41) [t(11) = 7.52, p <0.05]and goal-tracking to both the lever CS (82.00±2.55) [t(11)=12.55, p <0.05] and tone CS (74.08±4.29) [t(11) = 5.61, p <0.05] in MK-801 pretreated animals. Collectively, these results indicate that most of the goal-tracking responses recorded were made in the presence of the CS, further supporting that these responses were a product of a learned CS-US relationship.
3.2 CS-only Test
A drug-free, CS-only test session was utilized to probe what was learned regarding each stimulus during acquisition (30). Figure 2A and 2B illustrates the averaged sign- and goal-tracking rates to the lever and tone CS during the drug-free test session. Linear mixed-effects analysis revealed a significant treatment × stimulus interaction [F(2,20) = 13.12, p < 0.05], indicating that responding during the CS-test was dependent on pretreatment during 2-CS PCA acquisition and the type of CS presented. Post hoc analysis revealed that sign-tracking to the lever CS was greater in saline pretreated animals than MK-801. Additionally, post hoc analysis revealed that goal-tracking rates to the lever CS during the CS-test was greater in MK-801 pretreated animals than saline animals, while there were no differences in goal-tracking rates to the tone CS. Furthermore, post hoc analysis also revealed that there were no differences between goal-tracking rates to the lever or tone CS in MK-801 pretreated animals. Finally, Figure 2C illustrates goal-tracking response rates during the 8s pre-CS window on the CS-only test day. Linear mixed-effects analysis revealed no differences in goal-tracking rates during the 8s pre-CS between saline and MK-801 pretreated animals. Collectively, the results indicate that pretreatments of MK-801 during 2-CS PCA acquisition specifically prevented the learning of a CS-US relationship that results in sign-tracking to the lever, instead promoting the learning of a CS-US relationship that results in goal-tracking to the lever stimulus.
Figure 2.
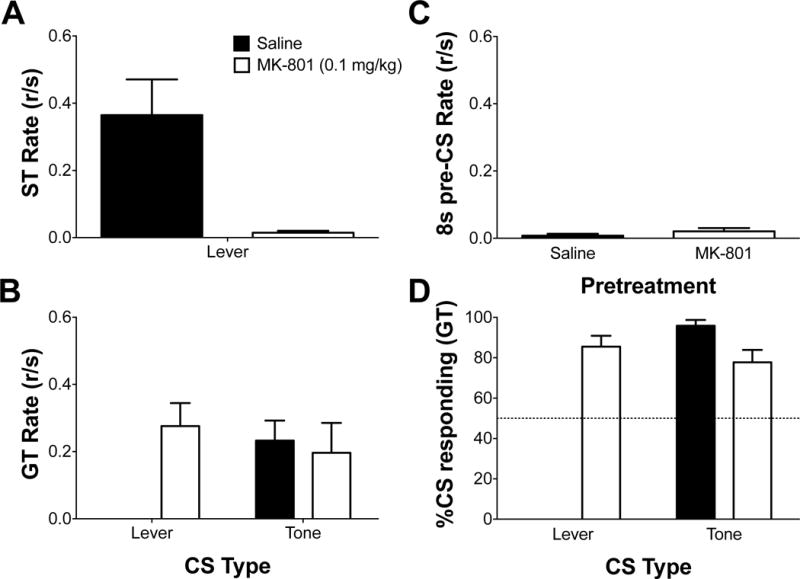
Sign- and goal-tracking response rates to a lever CS and tone CS during a drug-free CS-test to identify what was learned during 2-CS PCA acquisition. Mean (±SEM) response rate (responses/second; r/s) during the CS-test on (A) sign-tracking, (B) goal-tracking, and (C) goal-tracking during an 8s-preCS period for individuals pretreated with saline or MK-801 (0.1 mg/kg) during 2-CS PCA acquisition. (D) Mean (±SEM) percent CS responding during the CS-test for animals that were previously pretreated with saline and MK-801 during 2-CS PCA acquisition. Note: data not present (i.e., no bar in graph) directly reflects no responses made.
Figure 2D illustrates the percent responding to the CS during the CS-only test. One-sample t-tests revealed the percent CS responding to the tone for animals pretreated with saline (95.94±2.82) was significantly greater than 50% [t(5) = 16.29, p < 0.05]. One-sample t-tests also revealed that percent CS responding to the lever CS (85.46±6.96) [t(4) = 5.10, p < 0.05] and tone CS (78.33±7.88) [t(4) = 3.60, p < 0.05] for animals pretreated with MK-801 was also significantly greater than 50%. Collectively, these results indicate that during the drug-free CS-only test, animals were specifically responding in the presence of the CS. Thus, while MK-801 pretreatment promoted learning a goal-tracking response to the lever and tone, instead of sign-tracking to the lever and goal-tracking to the tone, both saline and MK-801 pretreated animals learned their respective conditioned responses equally well to both CSs.
3.3 Conditioned Reinforcement
Figure 3 illustrates the active and inactive nosepoke-responses during the post-acquisition, drug-free conditioned reinforcement test for the lever CS and tone CS. Figure 3A illustrates the number of active nosepokes for access to the lever CS and tone CSs alone, while Figure 3B illustrates the number of inactive nosepokes (i.e., responses produced no consequence). Linear mixed-effects analysis revealed that there was a main effect of treatment [F(1,10) = 18.26, p < 0.05], indicating that the pretreatments during acquisition affected the value attributed to the CSs, a main effect of stimulus [F(1,10) = 8.10, p < 0.05], indicating that the number of responses made during conditioned reinforcement was dependent on which previously trained CS was presented, and a main effect of response type [F(1,10) = 192.69, p < 0.05], indicating that there were more active nosepoke-responses than inactive nosepoke-responses. Analysis also revealed a treatment × stimulus interaction [F(1,10) = 8.73, p < 0.05], indicating previous pretreatments during 2-CS PCA acquisition and the stimulus presented following a nosepoke affected the number of nosepoke responses; a treatment × response type interaction [F(1,10) = 12.98, p < 0.05], indicating that responses into the nosepoke ports depended on the previous pretreatments from 2-CS PCA acquisition and whether or not the nosepoke port was active or inactive; and a stimulus × response type interaction [F(1,10) = 12.49, p < 0.05], indicating that the number of responses recorded depended on the stimulus presented following a nosepoke and whether or not the nosepoke port was active or inactive. Finally, linear mixed-effects analysis revealed a treatment × stimulus × response interaction [F(1,10) = 7.27, p < 0.05], indicating that the number of recorded nosepokes during conditioned reinforcement was dependent on the previous pretreatments received during 2-CS PCA training, which stimulus was presented following a nosepoke, and whether or not a nosepoke resulted in an event. Post hoc tests revealed that the number of active nosepokes was greater than all inactive nosepokes-responses in general. Furthermore, post hoc tests revealed that rats pretreated with saline during 2-CS PCA acquisition had more active responses for the lever CS than MK-801 pretreated animals; animals pretreated with saline during acquisition emitted more nosepokes for the lever CS than the tone CS. Finally, there were no differences in nosepokes for the tone CS between animals previously pretreated with saline or MK-801 during acquisition. Collectively, these results suggest that the lever CS served as a better conditioned reinforcer than the tone CS in animals previously pretreated with saline and learned to sign-track to the lever CS during acquisition, while MK-801 pretreatments that promoted the learning of a goal-tracking response to the lever CS during acquisition also prevented the attribution of incentive value for the lever CS.
Figure 3.
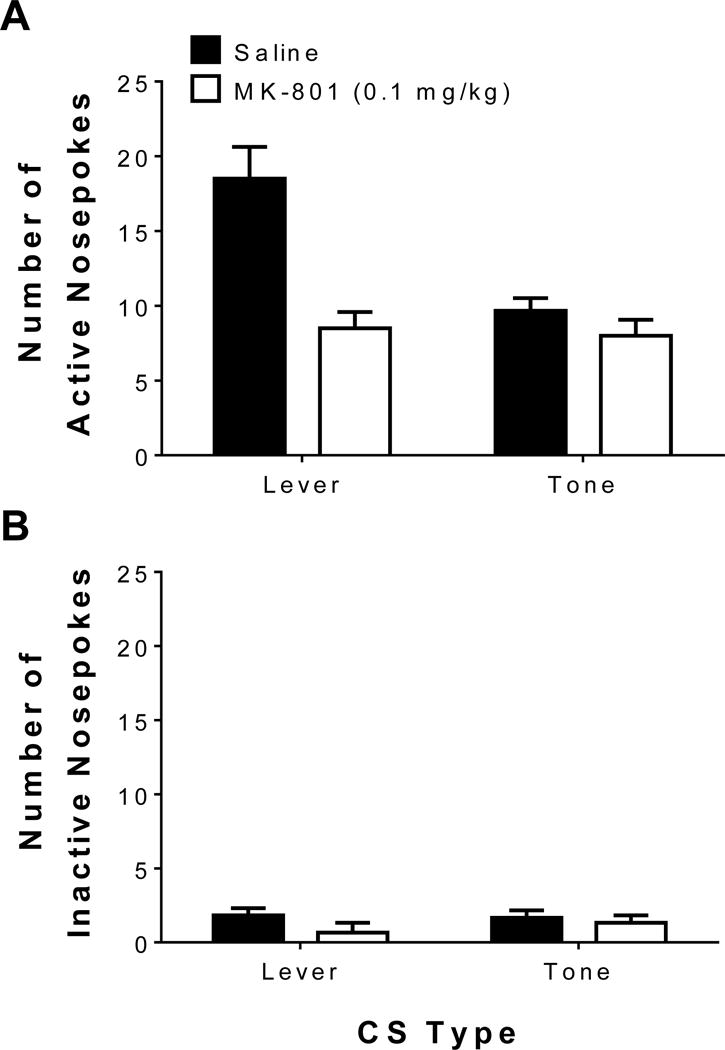
The relative conditioned reinforcing value attributed to the lever CS vs. the tone CS during drug-free tests following 2-CS PCA acquisition. Mean (±SEM) number of (A) active nosepokes, which produced the previously learned stimulus (i.e., lever or tone, depending on test-day), and (B) inactive nosepokes, which resulted in no consequences, for saline pretreated groups and MK-801 (0.1 mg/kg) pretreated groups during 2-CS PCA acquisition.
4. Discussion
While previous studies have examined the role of NMDA receptors in appetitive Pavlovian conditioning, most studies were conducted in relation to dopaminergic function (13–17). For example, in a study by Di Ciano et al. (13), it was demonstrated that localized infusions of AP-5, a NMDA antagonist, into the nucleus accumbens disrupted the acquisition of sign-tracking. Likewise, when NMDA receptors were disrupted in D1-expressing medium-spiny neurons goal-tracking to a lever CS was impaired (16). Unfortunately, neither of these studies reported effects on the alternate conditioned response (i.e., sign- or goal-tracking), making it difficult to determine if any effects of the manipulations used may have selectively affected the other response type, rather than attenuating stimulus-reward learning more generally.
Using a 2-CS procedure to isolate sign- and goal-tracking, the findings herein demonstrated that pretreatments of MK-801 during acquisition selectively promoted goal-tracking responses to the lever CS, instead of sign-tracking demonstrated by saline control animals. More importantly, during the post-acquisition drug-free CS-only test, animals pretreated with MK-801 during acquisition continued to goal-track to the lever CS, indicating that NMDA receptor antagonism altered the learned CS-US relationship and did not suppress sign-tracking performance during acquisition. Furthermore, post-acquisition drug-free conditioned reinforcement testing demonstrated that animals pretreated with MK-801 during acquisition exhibited similar levels of conditioned reinforcement for both the lever and tone CS, while the lever CS that elicited sign-tracking in saline-treated animals resulted in more robust conditioned reinforcement, an effect consistent with previous results demonstrating that sign- and goal-tracking behavior are representative of differential levels of conditioned reinforcement; a CS that elicits sign-tracking behavior functions as more robust conditioned reinforcer (20,26,27,31). To our knowledge, this is the first demonstration that NMDA antagonism during the acquisition of a Pavlovian conditioned approach procedure can preferentially shift what conditioned response is learned, primarily affecting the value attributed to the CS, rather than causing a general blockade of learning.
Of note, using a 2-CS PCA procedure, control animals herein learned to sign-track exclusively to the lever CS, replicating previous findings from our laboratory (26,27) and others (21, 32–36). However, there are reports (e.g., 20,38) that have found individual differences in sign-tracking or goal-tracking exclusively to a lever CS. One notable procedural difference is the 2-CS PCA procedure lasts for 14 days, while studies (e.g., 20,38) that find individual differences in conditioned responding to a lever CS typically last for 5 days. Interestingly, during the first 5 days of training, under the 2-CS PCA procedure used herein, there were no differences in sign- and goal-tracking rates to the lever CS in control animals. However, by the end of the 14 days of training, control animals sign-tracked exclusively to the lever CS, making it possible that more training sessions may result in a switch from goal- to sign-tracking (but see 39). Moreover, the use of multiple-stimulus designs that foster discrimination learning (cf. 36), could engender sign-tracking to the lever CS. Another notable procedural difference is the type of lever used. The lever used within our studies (e.g., 26,27) is a standard retractable lever, whereas studies reporting individual differences (e.g., 20,38) use an “illuminated lever”, or a lever that is outfitted with a backlight, making it a compound lever/light CS. Using a compound stimulus leaves open the possibility that individual differences in the conditioned response elicited to the “illuminated lever” could be a direct reflection of selective associations between the individual elements of the compound “illuminated lever” (cf. 40), where animals that sign-track attend to the lever, while animals that goal-track attend to the light, producing sign- and goal-trackers respectively. Future studies should systematically investigate procedural determinants of individual differences in sign- vs. goal-tracking to a lever CS.
Much of the work investigating the neurobiological systems governing the attribution of incentive salience (e.g., 12,25,26) has focused on the dopaminergic system. For example, previous findings from our laboratory, investigating the role of dopamine in the acquisition of incentive salience, demonstrated that pretreatments of SCH-23390, a D1 dopamine receptor antagonist, during 2-CS PCA acquisition results in the suppression both sign- and goal-tracking performance. Importantly, while the above D1 antagonist pretreatment effects could be interpreted as a blockade of learning in general, a post-acquisition CS-only test under drug-free conditions revealed that the drug was affecting the performance of conditioned responding while onboard, not inhibiting learning; more importantly, the drug-free, CS-only test revealed that the D1 antagonist prevented the attribution of value to a lever CS, promoting goal-tracking to the lever stimulus (26).
Using the same 2-CS PCA experimental design as above (e.g., 26), the results herein demonstrate NMDA receptor antagonism also prevented the attribution of incentive salience to a lever CS. Because a similar end-result can be seen between dopaminergic and glutamatergic receptor antagonism during 2-CS PCA acquisition, where pretreatments of a D1 dopamine receptor antagonist or a NMDA receptor antagonist during acquisition can bias the learned CS-US relationship toward one that engenders goal-tracking, it leaves open the possibility that systemic pretreatments of MK-801 have their effects via modulation of the dopaminergic system (15,16). However, it should also be noted that unlike dopaminergic antagonism, which disrupted both conditioned response topographies during acquisition (26), NMDA receptor antagonism selectively abolished sign-tracking while simultaneously promoting goal-tracking. Furthermore, the selective promotion of goal-tracking, via MK-801, to the lever CS, while leaving goal-tracking to the tone CS relatively unaffected, further supports the notion that incentive salience attribution and general reward-prediction learning may be two distinct neurobehavioral processes (41). Although the findings examining NMDA receptor function reveal some interesting insights regarding the glutamatergic system, future studies should further investigate how glutamatergic signaling might play a preferential role in promoting the attribution of incentive salience from reward-prediction learning.
In conclusion, the results found here demonstrate that NMDA receptor function can differentially affect the learning of stimulus-reward relationships; notably NMDA receptor function is necessary for the attribution of incentive salience. Furthermore, the current data provides evidence that blocking NMDA receptor function does not necessarily block learning altogether, but can alter the stimulus-reward relationship learned and the associated value attributed to reward-predictive stimuli. By understanding the neurobiological systems involved that underlie stimulus-reward learning, novel pharmacotherapies may be developed to preferentially treat or prevent the learning of stimuli specific stimulus-reward relations that are associated with substance-use disorder.
Highlights.
NMDA receptor inhibition prevents the attribution of incentive salience.
NMDA receptor inhibition promotes the acquisition of goal-tracking.
Showed within-subject dissociation in sign- and goal-tracking acquisition under MK801.
Acknowledgments
We would like to thank William T. McCuddy for their technical support. Funding was provided by the National Institute on Drug Abuse (NIDA), DA033373
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors report no conflicts of interest.
References
- 1.McGaugh JL. Memory–a century of consolidation. Science. 2000;287(5451):248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 2.Lynch MA. Long-term potentiation and memory. Physiological reviews. 2004;84(1):87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- 3.Lamprecht R, LeDoux J. Structural plasticity and memory. Nature reviews. Neuroscience. 2004;5(1):45. doi: 10.1038/nrn1301. [DOI] [PubMed] [Google Scholar]
- 4.Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nature reviews. Neuroscience. 2007;8(4):262. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- 5.Riedel G, Platt B, Micheau J. Glutamate receptor function in learning and memory. Behavioural brain research. 2003;140(1):1–47. doi: 10.1016/s0166-4328(02)00272-3. [DOI] [PubMed] [Google Scholar]
- 6.Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44(1):161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 7.Maren S. Neurobiology of Pavlovian fear conditioning. Annual review of neuroscience. 2001;24(1):897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- 8.Blair HT, Schafe GE, Bauer EP, Rodrigues SM, LeDoux JE. Synaptic plasticity in the lateral amygdala: a cellular hypothesis of fear conditioning. Learning & memory. 2001;8(5):229–242. doi: 10.1101/lm.30901. [DOI] [PubMed] [Google Scholar]
- 9.Kim JJ, Jung MW. Neural circuits and mechanisms involved in Pavlovian fear conditioning: a critical review. Neuroscience & Biobehavioral Reviews. 2006;30(2):188–202. doi: 10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- 11.Schultz W. Behavioral dopamine signals. Trends in neurosciences. 2007;30(5):203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Singer BF, Guptaroy B, Austin CJ, Wohl I, Lovic V, Seiler JL, Aragona BJ. Individual variation in incentive salience attribution and accumbens dopamine transporter expression and function. European Journal of Neuroscience. 2016;43(5):662–670. doi: 10.1111/ejn.13134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Ciano P, Cardinal RN, Cowell RA, Little SJ, Everitt BJ. Differential involvement of NMDA, AMPA/kainate, and dopamine receptors in the nucleus accumbens core in the acquisition and performance of pavlovian approach behavior. Journal of Neuroscience. 2001;21(23):9471–9477. doi: 10.1523/JNEUROSCI.21-23-09471.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalley JW, Lääne K, Theobald DE, Armstrong HC, Corlett PR, Chudasama Y, Robbins TW. Time-limited modulation of appetitive Pavlovian memory by D1 and NMDA receptors in the nucleus accumbens. Proceedings of the National Academy of Sciences. 2005;102(17):6189–6194. doi: 10.1073/pnas.0502080102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parker JG, Zweifel LS, Clark JJ, Evans SB, Phillips PE, Palmiter RD. Absence of NMDA receptors in dopamine neurons attenuates dopamine release but not conditioned approach during Pavlovian conditioning. Proceedings of the National Academy of Sciences. 2010;107(30):13491–13496. doi: 10.1073/pnas.1007827107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parker JG, Beutler LR, Palmiter RD. The contribution of NMDA receptor signaling in the corticobasal ganglia reward network to appetitive Pavlovian learning. Journal of Neuroscience. 2011;31(31):11362–11369. doi: 10.1523/JNEUROSCI.2411-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.James AS, Pennington ZT, Tran P, Jentsch JD. Compromised NMDA/Glutamate Receptor Expression in Dopaminergic Neurons Impairs Instrumental Learning, But Not Pavlovian Goal Tracking or Sign Tracking. eNeuro. 2015;2(3) doi: 10.1523/ENEURO.0040-14.2015. ENEURO-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson TE, Berridge KC. The incentive sensitization theory of addiction: some current issues. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2008;363(1507):3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson TE, Yager LM, Cogan ES, Saunders BT. On the motivational properties of reward cues: individual differences. Neuropharmacology. 2014;76:450–459. doi: 10.1016/j.neuropharm.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyer PJ, Cogan ES, Robinson TE. The form of a conditioned stimulus can influence the degree to which it acquires incentive motivational properties. PloS one. 2014;9(6):e98163. doi: 10.1371/journal.pone.0098163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boakes RA. Performance on learning to associate a stimulus with positive reinforcement. Operant-Pavlovian interactions. 1977:67–97. [Google Scholar]
- 22.Brown PL, Jenkins HM. Auto-shaping of the pigeon’s key-peck. Journal of the experimental analysis of behavior. 1968;11(1):1–8. doi: 10.1901/jeab.1968.11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hearst E, Jenkins HM. Sign-tracking: The stimulus-reinforcer relation and directed action. Psychonomic Society 1974 [Google Scholar]
- 24.Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akil H. A selective role for dopamine in reward learning. Nature. 2011;469(7328):53. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saunders BT, Robinson TE. The role of dopamine in the accumbens core in the expression of Pavlovian-conditioned responses. European Journal of Neuroscience. 2012;36(4):2521–2532. doi: 10.1111/j.1460-9568.2012.08217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chow JJ, Nickell JR, Darna M, Beckmann JS. Toward isolating the role of dopamine in the acquisition of incentive salience attribution. Neuropharmacology. 2016;109:320–331. doi: 10.1016/j.neuropharm.2016.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beckmann JS, Chow JJ. Isolating the incentive salience of reward-associated stimuli: value, choice, and persistence. Learning & Memory. 2015;22(2):116–127. doi: 10.1101/lm.037382.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fleshler M, Hoffman HS. A progression for generating variable-interval schedules. Journal of the experimental analysis of behavior. 1962;5(4):529. doi: 10.1901/jeab.1962.5-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gelman A, Hill J. Data analysis using regression and multilevelhierarchical models. Vol. 1. New York, NY, USA: Cambridge University Press; 2007. [Google Scholar]
- 30.Rescorla RA. Pavlovian conditioning and its proper control procedures. Psychological review. 1967;74(1):71. doi: 10.1037/h0024109. [DOI] [PubMed] [Google Scholar]
- 31.Robinson TE, Flagel SB. Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biological psychiatry. 2009;65(10):869–873. doi: 10.1016/j.biopsych.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davey GC, Cleland GG, Oakley DA. Applying Konorski’s model of classical conditioning to signal-centered behavior in the rat: some functional similarities between hunger CRs and sign-tracking. Learning & Behavior. 1982;10(2):257–262. [Google Scholar]
- 33.Chang SE, Wheeler DS, Holland PC. Roles of nucleus accumbens and basolateral amygdala in autoshaped lever pressing. Neurobiology of learning and memory. 2012;97(4):441–451. doi: 10.1016/j.nlm.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang SE, Holland PC. Effects of nucleus accumbens core and shell lesions on autoshaped lever-pressing. Behavioural brain research. 2013;256:36–42. doi: 10.1016/j.bbr.2013.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang SE. Effects of orbitofrontal cortex lesions on autoshaped lever pressing and reversal learning. Behavioural brain research. 2014;273:52–56. doi: 10.1016/j.bbr.2014.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holland PC, Asem JS, Galvin CP, Keeney CH, Hsu M, Miller A, Zhou V. Blocking in autoshaped lever-pressing procedures with rats. Learning & behavior. 2014;42(1):1–21. doi: 10.3758/s13420-013-0120-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyer PJ, Lovic V, Saunders BT, Yager LM, Flagel SB, Morrow JD, Robinson TE. Quantifying individual variation in the propensity to attribute incentive salience to reward cues. PloS one. 2012;7(6):e38987. doi: 10.1371/journal.pone.0038987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction. Neuropharmacology. 2009;56:139–148. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pearce JM, Kaye H, Hall G. Predictive accuracy and stimulus associability: Development of a model for Pavlovian learning. Quantitative analyses of behavior. 1982;3:241–255. [Google Scholar]
- 41.Clark JJ, Hollon NG, Phillips PE. Pavlovian valuation systems in learning and decision making. Current opinion in neurobiology. 2012;22(6):1054–1061. doi: 10.1016/j.conb.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]


