Abstract
Background
Identifying how relationships between variability of upper and lower body segments during walking are altered in persons with multiple sclerosis may uncover specific strategies for maintaining overall stability. The purpose of this study was to examine relationships between trunk and foot acceleration variability during walking in healthy controls and in persons with multiple sclerosis.
Methods
Linear and nonlinear variability measures were calculated for 40 healthy controls and 40 persons with multiple sclerosis from the acceleration time series recorded by inertial sensors attached to the trunk and foot while subjects walked on a treadmill at self-selected preferred pace.
Findings
No main effect of group was found for any variability measures. Main effect of location was found for all variability measures, with larger magnitudes of variability at the foot compared to the trunk, and more predictable variability patterns at the foot compared to the trunk. Differences in strength of correlations between trunk and foot accelerations were found between persons with multiple sclerosis and healthy controls in the frontal and sagittal plane. Sample entropy of accelerations at the feet and at the trunk correlated significantly higher in healthy controls than in persons with multiple sclerosis.
Interpretation
Relationships between variability of trunk and foot accelerations, which may provide a valuable comprehensive description of whole body stability during gait, showed minor changes in persons with MS compared to healthy controls.
Keywords: Gait, Accelerometers, Inertial sensors, Segmental control
1. Introduction
Multiple sclerosis (MS) is a progressive autoimmune disorder that affects an estimated 400,000 persons in North America (Evans et al., 2013). Gait deficits are reported in 80% of persons with MS (PwMS) (Souza et al., 2010), and fall risk is significantly increased with at least 50% of PwMS reporting at least one fall in a 2–6 month period (Nilsagard et al., 2009). Previous gait analysis studies have identified kinetic and kinematic differences in the gait of PwMS compared to healthy control subjects including reduced walking speed and stride length (Benedetti et al., 1999), decreased ankle and knee angular excursions (Gehlsen et al., 1986), altered trunk sway variability (Huisinga et al., 2013), altered step length and step width variability (Kaipust et al., 2012), and lower frequencies in vertical ground reaction forces (Wurdeman et al., 2011). These altered gait parameters in PwMS are influenced by decreased somatosensation (Cattaneo and Jonsdottir, 2009), altered sensorimotor responses (Huisinga et al., 2014), muscle asymmetry (Chung et al., 2008), and spasticity (Pau et al., 2015), and lead to altered gait stability and increased fall risk (Cameron et al., 2008; Huisinga et al., 2014; Nilsagard et al., 2009; Sosnoff et al., 2011).
Gait stability can be defined as the ability to maintain functional upright gait without falling (Bruijn et al., 2013). Maintaining upright stance requires a controlled interaction between the base of support (BoS) and center of mass (CoM) (Winter, 1995). The relationship between the BoS and CoM must be consistently maintained during walking through sensorimotor feedback loops controlling trunk sway and foot placement from step to step (Hurt et al., 2010; Lugade et al., 2011; Winter, 1995). However, as previous studies have shown, these sensorimotor responses are altered in PwMS (Cameron et al., 2008; Huisinga et al., 2014), which may provoke an altered relationship between the BoS and CoM.
Previous gait studies have used trunk motion to approximate CoM motion (Moe-Nilssen and Helbostad, 2002) and step length and width as indicators of dynamic balance (Maki, 1997). Wireless accelerometers can measure motion at the trunk segment and lower body segments simultaneously while also being highly portable and able to be used outside of a laboratory setting (Kavanagh and Menz, 2008). Wireless accelerometers have been used in previous studies on PwMS to measure accelerations at the trunk during walking (Huisinga et al., 2013; Solomon et al., 2015). However, these accelerometers do not allow for direct measurement of step placement or other position data. Alternatively, measuring the variability of accelerations patterns during gait can provide information about how the walking pattern is controlled in PwMS (Huisinga et al., 2013; Stergiou and Decker, 2011; Stergiou et al., 2006).
Measuring variability during gait can provide information about the sensorimotor control systems responsible for governing movement and balance during gait (Harbourne and Stergiou, 2009). Healthy persons exhibit an optimal gait pattern, and display optimal levels of variability (Stergiou et al., 2006). Compared to healthy controls, PwMS display greater variability in step width and single support time based on coefficient variation (Sosnoff et al., 2012). Linear measures of variability such as coefficient of variation or standard deviation provide information about the magnitude of variability within a time series (O’Connor et al., 2012; Terrier and Reynard, 2015). Nonlinear variability measures provide information on the temporal structure of variability within a time series (Harbourne and Stergiou, 2009; Stergiou and Decker, 2011). Nonlinear measures of variability are particularly helpful in quantifying the control systems governing time series related to movement (Stergiou and Decker, 2011; Stergiou et al., 2006). Nonlinear variability analyses showed that PwMS displayed more predictable and repeatable step length and step width during walking compared to healthy controls (Kaipust et al., 2012) while trunk acceleration displayed greater divergence in PwMS compared to healthy controls (Huisinga et al., 2013). These findings demonstrate that PwMS may be less adaptable to perturbations during gait since their foot placement is more predictable and point to a loss of complexity in their movement pattern (Kaipust et al., 2012; Stergiou and Decker, 2011; Stergiou et al., 2006).
Most studies examining variability during gait have focused on examining movement patterns of only one segment, either the trunk or the feet (Bruijn et al., 2010; Huisinga et al., 2013; Kaipust et al., 2012; Kang and Dingwell, 2009). However, stability requires coordination between upper and lower segments, so examining these segments simultaneously may provide a more comprehensive understanding of stability in a specific population. Since the coordination between segments is likely controlled in part by sensorimotor feedback loops (Hurt et al., 2010), it is possible that these segmental relationships will be altered in PwMS since we know their neural feedback loops are altered compared to healthy controls (Amadio et al., 2006; Cameron et al., 2008; Gagliardo et al., 2007; Huisinga et al., 2014).
The aim of this study was to examine the relationships between foot and trunk acceleration variability during walking in PwMS compared to the same relationships in healthy adults. Because PwMS have altered sensorimotor responses (Cameron et al., 2008; Huisinga et al., 2014) and many differences in spatial and temporal gait parameters compared to healthy controls (Benedetti et al., 1999; Gehlsen et al., 1986; Huisinga et al., 2013; Kaipust et al., 2012; Wurdeman et al., 2011), we hypothesized that (i) PwMS would exhibit different amounts and different structure of variability for foot and trunk accelerations during walking compared to healthy controls, and (ii) the strength of relationships between variability of accelerations at the foot and at the trunk during walking would be different in PwMS compared to healthy controls.
2. Methods
2.1 Participants
Forty PwMS between the ages of 20 and 60, and 40 age-matched healthy controls were recruited for this study (Table 1). Exclusion criteria for both groups were: additional neurological or orthopedic co-morbidities possessing the potential to alter balance or gait mechanics, female subjects who were pregnant, breastfeeding, or within 3 months post-partum at the time of data collection, persons with vestibular issues, diabetes, or a pre-existing condition which could make exercising difficult (i.e. myocardial infarction, chest pain, unusual shortness of breath, congestive heart failure, etc.), unable to walk a distance of 25 feet without the assistance of a mobility aide, or currently prescribed symptom specific medication therapies (i.e. Fampridine) which can directly affect gait. Additionally, PwMS were excluded if they had a Kurtzke Expanded Disability Status Scale (EDSS) (Kurtzke, 1983) score greater than 5.5. All PwMS enrolled in the study had relapsing-remitting MS.
Table 1.
Summary statistics of subject demographics; mean (standard deviation).
| Healthy Controls (n = 40) | PwMS (n = 40) | p-value | |
|---|---|---|---|
| Age (years) | 44 (10) | 44 (9) | 0.962 |
| Walking Speed (m/s) | 1.01 (0.34) | 0.88 (0.23) | 0.065 |
| EDSS | -- | 1.63 (0.7) | -- |
2.2 Protocol
Acceleration data was recorded by two APDM wireless sensors (Opal, APDM, Portland, OR, USA) secured by elastic strap to the right ankle and trunk. The trunk accelerometer was placed over the center of the sternum. The right ankle accelerometer was placed over anterior surface of the lower shank, on the distal most point of the shank, superior to the ankle joint. While the sensor was not placed directly on the foot, its position on the ankle is sufficient to approximate foot motion throughout the gait cycle, as each subsequent step is placed to maintain the subject’s BoS (Bruijn et al., 2012; Savin et al., 2014). Previous studies have shown that accelerations are attenuated inferiorly to superiorly within the trunk segment (Kavanagh et al., 2004); therefore the trunk accelerometer was placed over the sternum rather than the lumbar spine in order to measure accelerations that are maximally dampened by being in the superior portion of the trunk segment. Accelerations were recorded (128Hz) while subjects walked on a motorized treadmill (Woodway Bari-Mill, Eugene, OR, USA) at self-selected comfortable pace for 3 minutes.
The raw acceleration time series were exported to Matlab (MATLAB version R2013b, The MathWorks, Inc., Natick, Massachusetts, USA) and were initially translated from local 3-dimensional Cartesian coordinates to resultant frontal and sagittal plane time series. These resultant frontal and sagittal plane time series were not aligned to the global anatomical planes, but only local to the individual sensors. All subsequent processing took place on the resultant frontal and sagittal acceleration time series. For accurate analysis of the variability and complexity within the time series, data was left unfiltered (Mees and Judd, 1993). Since there were no statistical differences in stride velocity between the groups (Table 1) we did not need to normalize the outcome variables to walking speed. A custom Matlab program was used to calculate all variability measures.
Linear measures root mean square (RMS) and range were calculated from both the frontal and sagittal plane acceleration time series. Root mean square was calculated as the square root of the mean of squares of the numbers in the time series, and was used to quantify the dispersion of the acceleration traces. Range was calculated as the difference between the maximum and minimum acceleration values within the time series.
Nonlinear measures sample entropy (SaEn) and Lyapunov exponents (LyE) were calculated from both the frontal and sagittal plane acceleration time series. A thorough explanation of the SaEn calculation can be found in previous literature (Pincus, 1991; Richman and Moorman, 2000; Yentes et al., 2013). SaEn was calculated using customized Matlab software based on the methods of Pincus and Richman (Pincus, 1991; Richman and Moorman, 2000), using m=3 and r=0.2*(standard deviation of the time series). These parameters were selected after testing the algorithms on a selection of data, varying r from 0.1–0.3 in increments of 0.02. Relative consistency of results was apparent around r=0.2. The vector length m=3 was chosen after finding very inconsistent results using m=2 and m=4, regardless of r value. The LyE was calculated using customized Matlab software based on Wolf’s algorithm (Wolf et al., 1985) which calculates the largest LyE and provides information about the predictability of a system. Larger LyE values relative to optimal variability indicate more divergence in the system and less predictability of the system (Stergiou, 2016). The embedding dimension (edim) was calculated separately for each group and dataset using a Global False Nearest Neighbors analysis (Stergiou, 2016). For controls, edim was chosen as 11 for sagittal trunk, 11 for frontal trunk, 8 for sagittal foot, and 8 for frontal foot based on examination of a range of constants from each group. For PwMS, edim was chosen as 9 for sagittal trunk, 10 for frontal trunk, 10 for sagittal foot, and 8 for frontal foot. The time lag (τ) was calculated for every time series based on the Average Mutual Information Algorithm (Baker and Gollub, 1996; Gomez Garcia et al., 2012; Stergiou, 2016). Time lags ranged from 5 to 30 samples, with an average time lag of 12 (SD 5) samples. SaEn and LyE calculations used each time series’ specific time lag in order to appropriately capture the characteristics of each individual data set. In total, our processing resulted in four values per variability measure for each subject – trunk sagittal, trunk frontal, foot sagittal, and foot frontal.
2.3 Statistical Analysis
The assumption of data normality was accepted based on the results of Shapiro-Wilks tests. A two-way ANOVA was performed to investigate effects of Group (HC, PwMS) and Location (foot, trunk) on the results. Paired t-tests were used to analyze any significant interactions. Pearson’s correlations were used to assess the relationship between sensor locations within the same plane. The strength of relationship was directly compared between groups, with correlation coefficient of 0.3–0.5 indicating a low correlation, 0.5–0.7 indicating a moderate correlation, and greater than 0.7 indicating a strong correlation (Hinkle et al., 2003). To determine if correlations were significantly different between groups, a two-tailed test using Fischer’s r to z transformation was used. A significance level of 0.05 was used for all analyses, and all analyses were completed in SPSS 2013 (version 22, IBM Corp., Armonk, NY).
3. Results
3.1 ANOVA and Paired Tests
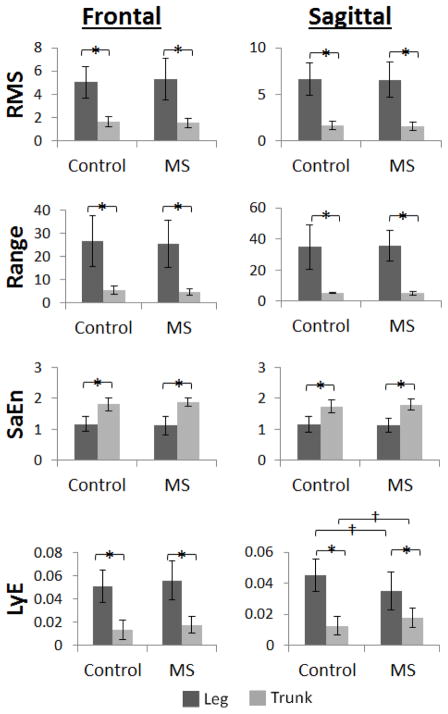
Both groups displayed larger magnitudes of variability (RMS, Range) at the feet compared to the trunk in the sagittal and frontal planes. Both groups displayed more regular accelerations (SaEn) at the trunk compared to the foot in both the sagittal and frontal planes. Both groups displayed more divergent accelerations (LyE) at the foot compared to the trunk in both the sagittal and frontal planes, and PwMS displayed more divergent accelerations at the foot and less divergent accelerations at the trunk compared to HC (Fig. 1).
Figure 1.
Acceleration variability results for healthy controls and persons with multiple sclerosis at the trunk and at the foot for the frontal and sagittal planes.
*Significant main effect for Location (p<0.05)
†Significant Location x Group interaction, post-hoc t-test (p<0.05)
3.2 Correlations
All segment correlation results are listed in Table 2. Healthy controls showed significant positive correlations between trunk and foot acceleration variability for all variables except sagittal plane Range and LyE. Of these significant correlations in HC, correlations were strong for range in the frontal plane and RMS in the sagittal plane, moderate for RMS and SaEn in the frontal plane, moderate for SaEn in the sagittal plane, and were low for LyE in the frontal plane. PwMS showed significant positive correlations between trunk and foot acceleration variability for all variables except frontal plane SaEn and sagittal plane SaEn. Of these significant correlations in PwMS, correlations were strong for RMS in the frontal plane and RMS in the sagittal plane, low for Range and LyE in the frontal plane, and low for range and LyE in the sagittal plane. Comparisons between groups using Fischer’s r to z transformation showed that the SaEn correlations in the frontal and sagittal plane were significantly different between groups.
Table 2.
Correlations to measure relationship between trunk and foot acceleration variability in frontal and sagittal planes in healthy control subjects (HC) and persons with MS (PwMS), and Fischer’s r to z transformation two-tailed comparison between groups; *Significant correlation, * p < 0.05.
| HC R-value (P-value) |
PwMS R-value (P-value) |
Fischer’s Transformation Z-statistic (P-value) | |||
|---|---|---|---|---|---|
| Frontal | Linear | RMS | 0.565 (< 0.01) * | 0.732 (< 0.01) * | −1.26 (0.207) |
| Range | 0.711 (< 0.01) * | 0.485 (< 0.01) * | 1.55 (0.121) | ||
|
| |||||
| Nonlinear | SaEn | 0.541 (< 0.01) * | 0.054 (0.740) | 2.37 (0.018) * | |
| LyE | 0.334 (0.046) * | 0.396 (0.020) * | −0.31 (0.757) | ||
|
| |||||
| Sagittal | Linear | RMS | 0.810 (< 0.01) * | 0.791 (< 0.01) * | 0.23 (0.818) |
| Range | 0.274 (0.111) | 0.372 (0.024) * | −0.47 (0.638) | ||
|
| |||||
| Nonlinear | SaEn | 0.510 (< 0.01) * | −0.098 (0.548) | 2.84 (< 0.01) * | |
| LyE | 0.259 (0.145) | 0.458 (< 0.01) * | −0.99 (0.322) | ||
4. Discussion
The goal of this study was to determine if differences exist in the relationships between foot and trunk acceleration variability during walking in PwMS and healthy adults. Acceleration data was collected from wireless sensors and analyzed using linear and nonlinear variability measures to quantify the amount and the structure of variability within the acceleration time series. We hypothesized i) that PwMS would exhibit different amounts and different structure of variability for foot and trunk accelerations during walking compared to healthy controls and ii) the strength of relationships between variability of accelerations at the foot and at the trunk during walking would be different in PwMS compared to healthy controls. Our results partially agree with the first hypothesis and fully agree with the second hypothesis.
The magnitude of acceleration variability was higher at the foot compared to the trunk in both HC and PwMS and accelerations at the feet were more regular but less dynamically stable compared to accelerations at the trunk in both HC and PwMS. In the frontal and sagittal plane, PwMS and HC showed greater magnitudes of variability at the foot compared to the trunk which agrees with previous findings that accelerations are attenuated from inferior to superior segments of the body during walking (Kavanagh et al., 2004). Measures of entropy also showed no differences between PwMS and HC in the sagittal and frontal planes and both groups showed significantly lower values of entropy at the foot than at the trunk in the sagittal and frontal plane. These results indicate that accelerations at the feet are more regular than accelerations at the trunk in HC and PwMS in agreement with previous work (Craig et al., 2016). Other studies have found that PwMS display altered variability of kinematics at the trunk and at the lower extremities during walking and quiet standing compared to HC (Cattaneo et al., 2015; Huisinga et al., 2013; Kaipust et al., 2012). It is possible that similar differences were not identified in the current study since the PwMS were mildly affected (EDSS: 1.63 (SD 0.7)) and had a similar preferred gait speed as HC. Progression of the disease may eventually alter the ability to attenuate accelerations from inferior to superior segments in PwMS as previous studies have shown that aging populations are less capable of attenuating accelerations from lower to upper segments during walking (Mazza et al., 2008). Future studies to examine segmental coordination would benefit from including PwMS with moderate to severe disability.
Both groups showed larger values of LyE at the foot compared to the trunk in the frontal and sagittal plane indicating less divergence or more predictable accelerations at the trunk compared to the feet in both groups. In the sagittal plane, LyE of accelerations at the feet was higher in HC compared to PwMS and LyE of accelerations at the trunk was lower in HC compared to PwMS which indicates that PwMS display greater predictability of foot accelerations in the sagittal plane compared to HC. It is possible that PwMS develop a more tightly controlled pattern of foot placement to reduce variability since their delayed sensorimotor responses may cause a slower response to any perturbations encountered during walking (Huisinga et al., 2014). In the sagittal plane, healthy controls exhibited significantly lower LyE at the trunk compared to PwMS, indicating that accelerations at the trunk are less dynamically stable for PwMS compared to HC. This finding is in agreement with previous studies which have shown that aging populations (Kang and Dingwell, 2009), populations with a known neuromuscular disorder (Hoogkamer et al., 2015; Huisinga et al., 2013), populations of fall-prone individuals (Lockhart and Liu, 2008), and PwMS all display higher LyE at the trunk compared to healthy control subjects. The present study showed that foot acceleration was less divergent in PwMS which may be a compensation for the more divergent acceleration pattern of the trunk. The combination of altered trunk and foot acceleration patterns may be indicative of fall risk in PwMS since both their CoM and BoS are altered during gait.
We hypothesized that the strength of relationships between variability of accelerations at the foot and at the trunk during walking would be different in PwMS compared to healthy controls and this was shown in our study. Correlations of acceleration variability between the feet and the trunk were similar in strength for both groups for all variables except SaEn. SaEn of accelerations at the feet and at the trunk correlated significantly higher in HC than in PwMS. This difference between the two groups indicates that more regular patterns of acceleration at the feet are related to more regular patterns of acceleration at the trunk in HC, but not in PwMS. The altered relationship between the trunk and feet in PwMS is likely reflective of disease-specific symptoms that contributed to gait deficits. PwMS experience muscle weakness (Hoang et al., 2014), strength asymmetries (Rudroff et al., 2014), and altered activation of trunk musculature (Ketelhut et al., 2015). This altered muscle functioning in PwMS, for example, may lead to an inability of the trunk to absorb accelerations via passive mechanisms, which typically attenuate higher frequency accelerations (Kavanagh et al., 2005). This change in trunk motion would affect trunk acceleration and may be represented as altered correlation between segment variability measures. These findings demonstrate the importance in studying upper and lower body motion simultaneously in order to better understand how relationships between segments contribute to walking.
During walking, motion of the frontal plane is governed by active feedback mechanisms in order to maintain proper step width to arrest and redirect the body’s center of mass from step to step (Bauby and Kuo, 2000; Hurt et al., 2010; O’Connor and Kuo, 2009). Previous studies have shown that PwMS have slowed sensorimotor responses (Cameron et al., 2008; Huisinga et al., 2014), which would directly impact these active control mechanisms in the frontal plane. In contrast to motion in the frontal plane during walking, motion in the sagittal plane is controlled by relatively passive mechanisms in healthy adults, similar to pendula motion (O’Connor and Kuo, 2009). However, this passive control may be altered in PwMS, as step length is actively constrained to increase their margin of stability from step to step (Peebles et al., 2016). As foot motion is altered, trunk motion must also be altered to compensate, leading to the observed differences in relationships between trunk and foot motion variability. In addition to previous work demonstrating relationships between trunk and foot motion, the results of the current study show that the relationship between movement variability of these segments is altered in PwMS compared to healthy adults.
A limitation of this study is that the data was collected on a motorized treadmill, which held the subjects’ gait at a constant speed and confined them to walk within the boundaries of the treadmill belt. While the treadmill could have eliminated some variability in the subjects’ gait, the use of the method was necessary to record a time series of sufficient length and the use of treadmill gait to assess variability of movement patterns is well established (Kaipust et al., 2012; Owings and Grabiner, 2004; Rosenblatt and Grabiner, 2010). Previous studies have also supported the validity of gait analysis using a treadmill, showing that treadmill gait is similar to over ground walking (Riley et al., 2007). Future studies should aim to expand this research to populations with other neuromuscular pathologies, which could further identify how coordination between foot and trunk motion during walking is affected by altered dynamic systems and to examine the effect of distorted visual or sensory feedback on the relationship between foot and trunk accelerations.
The current study revealed differences between upper and lower segment control during walking in HC and PwMS. Magnitude and structure of acceleration variability demonstrated differences between trunk and foot motion in both HC and PwMS, but there were few differences observed between groups. However, the relationships between trunk and foot accelerations revealed differences between HC and PwMS. These findings demonstrate the importance in studying how the relationships between upper and lower body motion contribute to walking. These differences highlight segmental control features that may contribute to increased fall occurrences in PwMS, which will be of interest in future development of fall risk assessments for clinical and home use.
Highlights.
Variability magnitude at foot is greater than at trunk in both groups.
Variability structure at foot is more predictable than at the trunk in both groups.
Correlations of foot and trunk variability are altered in multiple sclerosis group.
Acknowledgments
Funding:
This work was supported by the National Multiple Sclerosis Society RG 4914A1/2, the NIH National Center for Advancing Translational Science 1KL2TR00011, and the NIH Ruth L. Kirschstein National Research Service Award T32 HD057850 from the National Institute of Child Health and Human Development.
Footnotes
Authorship – The conception and design of the study (JC, JH), acquisition of data (JC, AB), analysis and interpretation of data (JC, AB, SL, JH), drafting the article (JC, JH), final approval of the version to be submitted (JC, AB, SL, JH).
Conflicts of Interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amadio S, Pluchino S, Brini E, Morana P, Guerriero R, Martinelli Boneschi F, Comi G, Zaratin P, Muzio V, del Carro U. Motor evoked potentials in a mouse model of chronic multiple sclerosis. Muscle & nerve. 2006;33:265–273. doi: 10.1002/mus.20463. [DOI] [PubMed] [Google Scholar]
- Baker GL, Gollub JP. Chaotic Dynamics: An Introduction. Cambridge University Press; 1996. [Google Scholar]
- Bauby CE, Kuo AD. Active control of lateral balance in human walking. Journal of biomechanics. 2000;33:1433–1440. doi: 10.1016/s0021-9290(00)00101-9. [DOI] [PubMed] [Google Scholar]
- Benedetti MG, Piperno R, Simoncini L, Bonato P, Tonini A, Giannini S. Gait abnormalities in minimally impaired multiple sclerosis patients. Multiple sclerosis. 1999;5:363–368. doi: 10.1177/135245859900500510. [DOI] [PubMed] [Google Scholar]
- Bruijn SM, Meijer OG, Beek PJ, van Dieen JH. Assessing the stability of human locomotion: a review of current measures. Journal of the Royal Society, Interface/the Royal Society. 2013;10:20120999. doi: 10.1098/rsif.2012.0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijn SM, Ten Kate WR, Faber GS, Meijer OG, Beek PJ, van Dieen JH. Estimating dynamic gait stability using data from non-aligned inertial sensors. Annals of biomedical engineering. 2010;38:2588–2593. doi: 10.1007/s10439-010-0018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijn SM, Van Impe A, Duysens J, Swinnen SP. Split-belt walking: adaptation differences between young and older adults. Journal of neurophysiology. 2012;108:1149–1157. doi: 10.1152/jn.00018.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron MH, Horak FB, Herndon RR, Bourdette D. Imbalance in multiple sclerosis: a result of slowed spinal somatosensory conduction. Somatosens Mot Res. 2008;25:113–122. doi: 10.1080/08990220802131127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo D, Carpinella I, Aprile I, Prosperini L, Montesano A, Jonsdottir J. Comparison of upright balance in stroke, Parkinson and multiple sclerosis. Acta Neurol Scand. 2015 doi: 10.1111/ane.12466. [DOI] [PubMed] [Google Scholar]
- Cattaneo D, Jonsdottir J. Sensory impairments in quiet standing in subjects with multiple sclerosis. Multiple sclerosis. 2009;15:59–67. doi: 10.1177/1352458508096874. [DOI] [PubMed] [Google Scholar]
- Chung LH, Remelius JG, Van Emmerik RE, Kent-Braun JA. Leg power asymmetry and postural control in women with multiple sclerosis. Medicine and science in sports and exercise. 2008;40:1717–1724. doi: 10.1249/MSS.0b013e31817e32a3. [DOI] [PubMed] [Google Scholar]
- Craig JJ, Bruetsch A, Huisinga JM. Relationship between trunk and foot accelerations during walking in healthy adults. Gait & posture. 2016;49:25–29. doi: 10.1016/j.gaitpost.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans C, Beland SG, Kulaga S, Wolfson C, Kingwell E, Marriott J, Koch M, Makhani N, Morrow S, Fisk J, Dykeman J, Jetté N, Pringsheim T, Marrie RA. Incidence and Prevalence of Multiple Sclerosis in the Americas: A Systematic Review. Neuroepidemiology. 2013;40:195–210. doi: 10.1159/000342779. [DOI] [PubMed] [Google Scholar]
- Gagliardo A, Galli F, Grippo A, Amantini A, Martinelli C, Amato MP, Borsini W. Motor evoked potentials in multiple sclerosis patients without walking limitation: amplitude vs. conduction time abnormalities. Journal of neurology. 2007;254:220–227. doi: 10.1007/s00415-006-0334-5. [DOI] [PubMed] [Google Scholar]
- Gehlsen G, Beekman K, Assmann N, Winant D, Seidle M, Carter A. Gait characteristics in multiple sclerosis: progressive changes and effects of exercise on parameters. Archives of physical medicine and rehabilitation. 1986;67:536–539. [PubMed] [Google Scholar]
- Gomez Garcia JA, Godino Llorente JI, Castellanos Dominguez G. Influence of delay time on regularity estimation for voice pathology detection. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:4217–4220. doi: 10.1109/EMBC.2012.6346897. [DOI] [PubMed] [Google Scholar]
- Harbourne RT, Stergiou N. Movement Variability and the Use of Nonlinear Tools: Principles to Guide Physical Therapist Practice. Physical Therapy. 2009;89:267–282. doi: 10.2522/ptj.20080130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkle DE, Wiersma W, Jurs SG. Applied Statistics for the Behavioral Sciences. Houghton Mifflin; 2003. [Google Scholar]
- Hoang PD, Gandevia SC, Herbert RD. Prevalence of joint contractures and muscle weakness in people with multiple sclerosis. Disability and rehabilitation. 2014;36:1588–1593. doi: 10.3109/09638288.2013.854841. [DOI] [PubMed] [Google Scholar]
- Hoogkamer W, Bruijn SM, Sunaert S, Swinnen SP, Van Calenbergh F, Duysens J. Toward new sensitive measures to evaluate gait stability in focal cerebellar lesion patients. Gait & posture. 2015 doi: 10.1016/j.gaitpost.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Huisinga JM, Mancini M, St George RJ, Horak FB. Accelerometry reveals differences in gait variability between patients with multiple sclerosis and healthy controls. Annals of biomedical engineering. 2013;41:1670–1679. doi: 10.1007/s10439-012-0697-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisinga JM, St George RJ, Spain R, Overs S, Horak FB. Postural response latencies are related to balance control during standing and walking in patients with multiple sclerosis. Archives of physical medicine and rehabilitation. 2014;95:1390–1397. doi: 10.1016/j.apmr.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt CP, Rosenblatt N, Crenshaw JR, Grabiner MD. Variation in trunk kinematics influences variation in step width during treadmill walking by older and younger adults. Gait & posture. 2010;31:461–464. doi: 10.1016/j.gaitpost.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Kaipust JP, Huisinga JM, Filipi M, Stergiou N. Gait variability measures reveal differences between multiple sclerosis patients and healthy controls. Motor control. 2012;16:229–244. doi: 10.1123/mcj.16.2.229. [DOI] [PubMed] [Google Scholar]
- Kang HG, Dingwell JB. Dynamic stability of superior vs. inferior segments during walking in young and older adults. Gait & posture. 2009;30:260–263. doi: 10.1016/j.gaitpost.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh JJ, Barrett RS, Morrison S. Upper body accelerations during walking in healthy young and elderly men. Gait & posture. 2004;20:291–298. doi: 10.1016/j.gaitpost.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Kavanagh JJ, Menz HB. Accelerometry: a technique for quantifying movement patterns during walking. Gait & posture. 2008;28:1–15. doi: 10.1016/j.gaitpost.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Kavanagh JJ, Morrison S, Barrett RS. Coordination of head and trunk accelerations during walking. European journal of applied physiology. 2005;94:468–475. doi: 10.1007/s00421-005-1328-1. [DOI] [PubMed] [Google Scholar]
- Ketelhut NB, Kindred JH, Manago MM, Hebert JR, Rudroff T. Core muscle characteristics during walking of patients with multiple sclerosis. J Rehabil Res Dev. 2015;52:713–724. doi: 10.1682/JRRD.2015.01.0006. [DOI] [PubMed] [Google Scholar]
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- Lockhart TE, Liu J. Differentiating fall-prone and healthy adults using local dynamic stability. Ergonomics. 2008;51:1860–1872. doi: 10.1080/00140130802567079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugade V, Lin V, Chou LS. Center of mass and base of support interaction during gait. Gait & posture. 2011;33:406–411. doi: 10.1016/j.gaitpost.2010.12.013. [DOI] [PubMed] [Google Scholar]
- Maki BE. Gait changes in older adults: predictors of falls or indicators of fear. 1997 doi: 10.1111/j.1532-5415.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- Mazza C, Iosa M, Pecoraro F, Cappozzo A. Control of the upper body accelerations in young and elderly women during level walking. Journal of neuroengineering and rehabilitation. 2008;5:30. doi: 10.1186/1743-0003-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mees AI, Judd K. Dangers of geometric filtering. Physica D: Nonlinear Phenomena. 1993;68:427–436. [Google Scholar]
- Moe-Nilssen R, Helbostad JL. Trunk accelerometry as a measure of balance control during quiet standing. 2002 doi: 10.1016/s0966-6362(01)00200-4. [DOI] [PubMed] [Google Scholar]
- Nilsagard Y, Lundholm C, Denison E, Gunnarsson LG. Predicting accidental falls in people with multiple sclerosis -- a longitudinal study. Clin Rehabil. 2009;23:259–269. doi: 10.1177/0269215508095087. [DOI] [PubMed] [Google Scholar]
- O’Connor SM, Kuo AD. Direction-dependent control of balance during walking and standing. Journal of neurophysiology. 2009;102:1411–1419. doi: 10.1152/jn.00131.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor SM, Xu HZ, Kuo AD. Energetic cost of walking with increased step variability. Gait & posture. 2012;36:102–107. doi: 10.1016/j.gaitpost.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owings TM, Grabiner MD. Step width variability, but not step length variability or step time variability, discriminates gait of healthy young and older adults during treadmill locomotion. Journal of biomechanics. 2004;37:935–938. doi: 10.1016/j.jbiomech.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Pau M, Coghe G, Corona F, Marrosu MG, Cocco E. Effect of spasticity on kinematics of gait and muscular activation in people with Multiple Sclerosis. J Neurol Sci. 2015;358:339–344. doi: 10.1016/j.jns.2015.09.352. [DOI] [PubMed] [Google Scholar]
- Peebles AT, Reinholdt A, Bruetsch AP, Lynch SG, Huisinga JM. Dynamic margin of stability during gait is altered in persons with multiple sclerosis. Journal of biomechanics. 2016 doi: 10.1016/j.jbiomech.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus SM. Approximate entropy as a measure of system complexity. Proc Natl Acad Sci U S A. 1991;88:2297–2301. doi: 10.1073/pnas.88.6.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman JS, Moorman JR. Physiological time-series analysis using approximate entropy and sample entropy. American journal of physiology Heart and circulatory physiology. 2000;278:H2039–2049. doi: 10.1152/ajpheart.2000.278.6.H2039. [DOI] [PubMed] [Google Scholar]
- Riley PO, Paolini G, Della Croce U, Paylo KW, Kerrigan DC. A kinematic and kinetic comparison of overground and treadmill walking in healthy subjects. Gait & posture. 2007;26:17–24. doi: 10.1016/j.gaitpost.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Rosenblatt NJ, Grabiner MD. Measures of frontal plane stability during treadmill and overground walking. Gait & posture. 2010;31:380–384. doi: 10.1016/j.gaitpost.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Rudroff T, Kindred JH, Koo PJ, Karki R, Hebert JR. Asymmetric glucose uptake in leg muscles of patients with Multiple Sclerosis during walking detected by [18F]-FDG PET/CT. NeuroRehabilitation. 2014;35:813–823. doi: 10.3233/NRE-141179. [DOI] [PubMed] [Google Scholar]
- Savin DN, Morton SM, Whitall J. Generalization of improved step length symmetry from treadmill to overground walking in persons with stroke and hemiparesis() Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2014;125:1012–1020. doi: 10.1016/j.clinph.2013.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon AJ, Jacobs JV, Lomond KV, Henry SM. Detection of postural sway abnormalities by wireless inertial sensors in minimally disabled patients with multiple sclerosis: a case-control study. Journal of neuroengineering and rehabilitation. 2015;12:74. doi: 10.1186/s12984-015-0066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosnoff JJ, Sandroff BM, Motl RW. Quantifying gait abnormalities in persons with multiple sclerosis with minimal disability. Gait & posture. 2012;36:154–156. doi: 10.1016/j.gaitpost.2011.11.027. [DOI] [PubMed] [Google Scholar]
- Sosnoff JJ, Socie MJ, Boes MK, Sandroff BM, Pula JH, Suh Y, Weikert M, Balantrapu S, Morrison S, Motl RW. Mobility, balance and falls in persons with multiple sclerosis. PloS one. 2011;6:e28021. doi: 10.1371/journal.pone.0028021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza A, Kelleher A, Cooper R, Cooper RA, Iezzoni LI, Collins DM. Multiple sclerosis and mobility-related assistive technology: systematic review of literature. J Rehabil Res Dev. 2010;47:213–223. doi: 10.1682/jrrd.2009.07.0096. [DOI] [PubMed] [Google Scholar]
- Stergiou N. Nonlinear Analysis for Human Movement Variability. CRC Press; 2016. [Google Scholar]
- Stergiou N, Decker LM. Human movement variability, nonlinear dynamics, and pathology: is there a connection? Human movement science. 2011;30:869–888. doi: 10.1016/j.humov.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stergiou N, Harbourne R, Cavanaugh J. Optimal movement variability: a new theoretical perspective for neurologic physical therapy. Journal of neurologic physical therapy: JNPT. 2006;30:120–129. doi: 10.1097/01.npt.0000281949.48193.d9. [DOI] [PubMed] [Google Scholar]
- Terrier P, Reynard F. Effect of age on the variability and stability of gait: a cross-sectional treadmill study in healthy individuals between 20 and 69 years of age. Gait & posture. 2015;41:170–174. doi: 10.1016/j.gaitpost.2014.09.024. [DOI] [PubMed] [Google Scholar]
- Winter DA. Human balance and posture control during standing and walking. Gait & posture. 1995;3:193–214. [Google Scholar]
- Wolf A, Swift JB, Swinney HL, Vastano JA. Determining Lyapunov exponents from a time series. Physica D: Nonlinear Phenomena. 1985;16:285–317. [Google Scholar]
- Wurdeman SR, Huisinga JM, Filipi M, Stergiou N. Multiple sclerosis affects the frequency content in the vertical ground reaction forces during walking. Clinical biomechanics. 2011;26:207–212. doi: 10.1016/j.clinbiomech.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yentes J, Hunt N, Schmid K, Kaipust J, McGrath D, Stergiou N. The Appropriate Use of Approximate Entropy and Sample Entropy with Short Data Sets. Annals of biomedical engineering. 2013;41:349–365. doi: 10.1007/s10439-012-0668-3. [DOI] [PMC free article] [PubMed] [Google Scholar]