Abstract
Alcohol consumption is a major risk factor for the acquisition of HIV/AIDS and is associated with greater disease burden and mortality among those who become HIV-infected. Of the extant pharmacological treatments for alcohol use disorders, naltrexone is recognized as one of the most efficacious, producing robust reductions in alcohol craving and use. Given that treatment with oral naltrexone has been limited by problems with adherence, which are particularly prevalent among individuals with multiple chronic, co-occurring conditions, long-acting formulations may be a promising approach for HIV-infected substance users. However, little is known about the barriers to initiation of extended-release naltrexone (XR-NTX) treatment among alcohol users living with HIV. In this report we present and discuss the content analysis of open-ended survey questions, as well as lessons learned, with regards to barriers to initiation and maintenance of XR-NTX treatment collected as part of an RCT evaluating a cognitive behavioral text messaging intervention for HIV-infected adults with alcohol use disorders. Barriers to initiation and maintenance of XR-NTX pharmacotherapy among HIV+ individuals with alcohol use disorders seem to fall in one of two categories: [1] barriers that are amenable to change, which include distance and transportation issues, fear of injections, belief that alcohol use does not warrant pharmacotherapy, and [2[ barriers that are not amenable to change, such as the potential interaction of XR-NTX with another medication regimen.
Keywords: Vivitrol, extended-release naltrexone, pharmacotherapy, barriers to implementation, HIV, SMS
1. Introduction
Alcohol use disorders and their associated consequences are significant public health problems. Individuals with alcohol use disorders, both alone and occurring with other drug use disorders, compromised nearly 36% and 48%, respectively, of all treatment admissions for substance abuse in 2014 (SAMHSA, 2016). Alcohol use remains the nation’s largest substance abuse problem with respect to treatment need, with over 17 million individuals in need of specialty intervention (CBHSQ, 2016).
Hazardous alcohol consumption is a major risk factor for HIV transmission. Evidence suggests that the disinhibiting effects of alcohol ingestion coupled with lowered cognitive functioning associated with intoxication puts heavy drinkers at an increased risk for HIV infection (Rehm et al., 2012). As a result, alcohol use has been associated with greater disease burden and mortality among those who become HIV-infected (Baliunas et al., 2010; Shuper et al., 2010). Alcohol use also increases the likelihood of sexual and injecting drug risk behaviors (Kalichman et al., 2007; Stein et al., 2002; Rehm et al., 2012), both of which are major risk factors for HIV transmission.
A growing body of literature suggests that pharmacotherapy treatments for drug and alcohol use disorders reduce substance use and HIV-risk behaviors (Metzger & Zhang, 2010). Moreover, participation in treatment for substance use disorders predicts improved access and adherence to antiretroviral (ART) treatment (Uhlmann et al., 2010), and retention in pharmacotherapy treatments in particular is also associated with long-term virologic suppression (Roux et al., 2009). Naltrexone (NTX) is considered one of the most efficacious pharmacological interventions targeting alcohol dependence, producing large reductions in alcohol craving and use (Monti et al., 1999; Volpicelli et al., 1992; O’Malley et al., 2002; Helstrom et al., 2016; Ray et al., 2010), although findings around its efficacy are mixed (Jonas et al., 2014). Jonas and colleagues, however, report that when NTX was either used alone, or combined with a psychosocial intervention, reductions in alcohol drinking outcomes along with improvements in health-related quality of life were observed. NTX also has beneficial effects on HIV treatment, as it inhibits alcohol-mediated enhancement of HIV infection of T cells. In fact, NTX may lessen the immunopathogenic effects of alcohol use on the progression of HIV, although these results have only been observed through in vitro research (Wang et al., 2006).
Despite these evident benefits, treatment persistence on oral NTX is low. The majority of patients discontinue treatment in one month (Harris & Thomas, 2004; McCarty et al., 2009), and more than 75% discontinue by six months (Kranzler et al., 2008). Barriers include difficulties with ordering and using the medication, cost, limited access to care, and lack of knowledge concerning NTX, by both providers and patients (Alanis-Hirsch et al., 2016). From the patients’ perspective, barriers to NTX use included concerns about stereotypes concerning alcoholism and addiction that may arise when taking medication for an alcohol-related problem (Alanis-Hirsch et al., 2016). Extended-release naltrexone (XR-NTX), which is administered monthly via injections, was developed partially in an attempt to address the adherence issues encountered with oral NTX (Bryson et al., 2011). However, due to the high cost of XR-NTX, the complexity of its administration, and lack of knowledge about the drug, prescribing of XR-NTX has been limited (Bryson et al., 2011). Initiation of and persistence on extended-release naltrexone is also problematic. In a feasibility and acceptability project by Friedmann and colleagues (2013), primary reasons found for low adherence rates to XR-NTX, as reported by participants, were unwillingness to receive injections, fear of needles, and unwillingness to alter drinking habits. Providers also report various barriers to XR-NTX implementation, including perceived insufficient training on how to use pharmacotherapy in individuals with alcohol dependence, lack of insurance, and insufficient research on medication efficacy as very major barriers (Chander et al., 2016).
Little is known about the barriers to initiation and maintenance of XR-NTX among HIV-infected individuals with alcohol use disorders. Across clinical settings, the majority of patients with HIV are never screened for alcohol use disorders, and of those who are screened, very few are offered referrals to substance use disorder treatment (NIAAA, 2005). In primary care settings, for example, one study found that only 10% of patients with alcohol use disorders received the recommended level of care of appropriate screening, brief intervention, and referral to treatment (McGlynn et al., 2003). With regard to XR-NTX in particular, in a study examining acceptability and retention on XR-NTX in a criminal justice population, 90% of HIV infected inmates with alcohol or opioid use disorders who were transitioning to the community received their first injection, indicating that the treatment was highly acceptable (Springer et al., 2015). However, 60% evidenced retention on XR-NTX, returning for a subsequent injection post-release. To extend these findings to a non-criminal justice population, in the present study, we report and discuss the content analysis of open ended survey questions concerning feasibility and acceptability of XR-NTX initiation among alcohol users with HIV collected as part of an ongoing RCT in which a cognitive behavioral text messaging intervention (TXT-CBT) targeting ART adherence and alcohol use is being evaluated (Glasner-Edwards, 2015). In that study, treatment with XR-NTX was offered to potential study participants as a pharmacotherapy platform. Here, we present findings concerning participants’ engagement in XR-NTX treatment, as well as barriers to initiation.
2. Methods
Data for this brief report was collected as part of a pilot study testing a 12 week, individually tailored, cognitive behavioral text messaging intervention (TXT-CBT) for HIV-positive adults with alcohol use disorders. The intervention program, designed to facilitate initiation to XR-NTX, optimize adherence to ART, and prevent relapse, included: 1) a single face-to-face CBT session designed to tailor the text messages to the participant’s needs; 2) CBT based daily messages focused on the top three barriers to adherence, as identified by the participant; 3) CBT based messages targeting relapse prevention, and 4) daily medication reminders throughout intervention period. A more extensive description of the TXT-CBT intervention has been published elsewhere (Glasner-Edwards & al., 2016).
Eligibility criteria were (i) age 18 years or older, (ii) alcohol use disorder as assessed by the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR), (iii) HIV-infected and currently taking ART prescribed within the past 30 days, (iv) own a cell phone that can send and receive text messages, (v) interested in receiving treatment to address problematic alcohol use and ART adherence, and (vi) ART adherence score of less than 90%, as assessed by unannounced pill counts (see Kalichman et al., 2007 for description of pill count procedures). An experienced research assistant attended a two-day training seminar in unannounced pill counts which involved didactic instruction, observation of phone based pill counts conducted in the context of an ongoing trial, and subsequent supervised practice for 10 cases. Exclusion criteria were (i) lack of proficiency in written and spoken English, (ii) dependence on an illicit substance for which medical detoxification is urgently needed, and (iii) presence of clinically significant psychiatric symptoms as assessed by the M.I.N.I. International Neuropsychiatric Interview such as psychosis or acute mania, which would make study compliance difficult. Potential participants were recruited through advertising, word of mouth, fliers posted in treatment programs and community locations, referrals from local substance abuse treatment and outreach programs, outpatient and inpatient alcohol and drug abuse clinics, primary care providers, local mental health centers, crisis clinics, public service announcements, hospital emergency rooms, and self-referral. Potential participants were either screened in person or over the phone by a trained research assistant. Both at the time of screening and at the time of informed consent, a well trained and experienced research assistant educated potential participants about alcohol use disorder pharmacotherapy and XR-NTX in particular using a standardized script developed based upon information from the National Institute on Alcohol Abuse and Alocholism (NIAAA, 2017) and made sure that all questions were answered prior to proceeding with screening or informed consent. In total, 46% of participants were recruited or referred to us from local substance use disorder treatment and outreach programs, 42 % from flyers or ads in local newspapers, and 12% were referred to the study by word of mouth. All participants were currently receiving HIV care from third party providers and on an ART regimen.
Information regarding barriers to XR-NTX initiation and maintenance were collected from two data sources: the initial screening process when eligibility of potential participants was determined and weekly qualitative phone calls conducted during the length of the study intervention. During the initial screening process participants were asked, in addition to other questions to determine their eligibility for the study, whether they had ever heard of extended release Naltrexone or Naltrexone. If participants reported familiarity with XR-NTX, they were asked if they were currently on XR-NTX or if they would be interested in learning more about XR-NTX to consider initiating a XR-NTX regimen. If participants endorsed interest in XR-NTX or in learning more about XR-NTX and met the other eligibility criteria, an appointment was scheduled to provide informed consent for the study procedures and initiate baseline assessments. Being on XR-NTX was not a necessary condition to be part of the TXT-CBT study.
Participants of the TXT-CBT study randomized to the intervention, texting, condition received weekly phone calls asking about their experience with the text messaging programs. They were also asked questions about their attitudes towards XR-NTX, regardless of whether or not they were currently on a XR-NTX regimen. The following questions were asked weekly to participants in the intervention group and at baseline, week 4, week 8, week 12 and week 24 for those in the control condition: Have you heard of extended release Naltrexone? Are you currently taking XR-NTX shots? If yes: When was your first shot?, How many shots have you received to date?, When is your next shot? If no: Are you interested in getting on XR-NTX? If no, why not?
Answers to open ended survey questions were recorded in an excel spreadsheet and categorized by topic area.
3. Results
Information about barriers to XR-NTX initiation and maintenance was recorded for 15 participants. Demographic information was however only available for participants who passed the screened and consented to be a part of the TXT-CBT intervention (n=9). Participant ages ranged from 28 to 66 (44 ± 11), 66% were male, 33% were Hispanic/Latino, 53% were never married, and the average number of years of education was 13.
3.1 Barriers to XR-NTX initiation
Of the 15 participants for whom information about barriers to XR-NTX initiation and maintenance was collected, only one initiated and then discontinued XR-NTX, leading us to the need to understand what barriers to XR-NTX initiation and maintenance are in a population of HIV-positive adults with alcohol dependence.
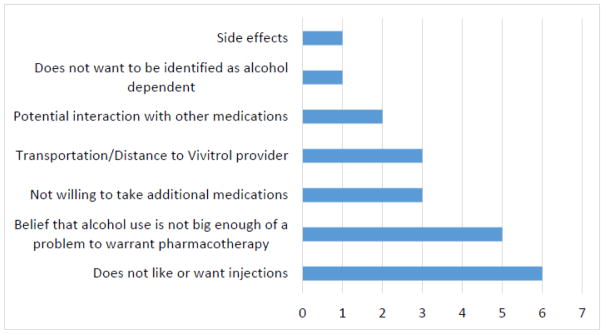
Reported barriers to XR-NTX initiation fell into one of six categories (Figure 1): dislike or fear of injections, belief that alcohol use is not big enough of a problem to warrant pharmacotherapy, transportation/distance to XR-NTX provider, not willing to take additional medications, potential interaction with other medications, and not wanting to be identified as alcohol dependent. Most participants reported multiple barriers to XR-NTX initiation.
Figure 1.
Frequencies of Reported Barriers to Vivitrol Initiation and Maintenance
Six out of fifteen participants (40%) voiced a dislike or fear of injections as a barrier to XR-NTX initiation. Participants expressed: “not sure about getting a shot”, “don’t want to take any shots”, and “worried about the shot.”
Five out of fifteen participants (30%) did not believe that their alcohol use was enough of a problem to warrant pharmacotherapy: “alcohol intake not bad, don’t want to take any shots”, “I don’t like taking things that I don’t need (…) I feel like if I needed it, I wouldn’t be opposed to it”, “alcohol is not a big enough problem to get on medication”, “I drink and stuff but I don’t have no problem.”
Transportation/distance to XR-NTX provider and not being willing to take additional medications were equally reported as a barrier to XR-NTX initiation by three participants (20%), followed by potential interaction with other medications for 13% of participants. With regards to transportation/distance to XR-NTX provider, one participant expressed not being able to get to either of the two XR-NTX providers used for the study using public transportation because of the distance and limited mobility. The two other participants who endorsed transportation/distance to provider as a barrier “did not want to go out there” to get XR-NTX. With regards to taking additional medication or potential interaction with other medications, participants stated “concerned about interaction with HIV meds”, “I don’t want to do no more meds”, “because I am already on a lot of other medications.”
3.2 Barriers to XR-NTX maintenance
One participant who did initiate the XR-NTX injections reported stopping after one injection because of both side effects “had side effects from it (…) it affected my libido, feeling nauseated, dubbed my sensations”, and not wanting to be identified as alcohol dependent or receiving treatment for alcohol dependence by his insurance. That participant, however, did report that XR-NTX helped curb alcohol use; “But it has helped me stay sober, that’s for sure.”
4. Discussion
Barriers to initiation and maintenance of XR-NTX pharmacotherapy among HIV+ individuals with alcohol use disorders seem to fall in one of two categories: [1] barriers that are amenable to change, which include distance and transportation issues, fear of injections, belief that alcohol use does not warrant pharmacotherapy, and [2[ barriers that are not amenable to change, such as the potential interaction of XR-NTX with another medication regimen. Barriers amenable to change could be targeted using motivational interventions to improve patient attitudes towards XR-NTX, clarify misconceptions, and motivate the desire to change alcohol use and use pharmacotherapy. Although the psychosocial intervention study from which these data were drawn had as an objective to initiate participants on XR-NTX, this component of the intervention was dropped due to the difficulties encountered recruiting both participants willing to get on XR-NTX and providers to initiate interested participants on XR-NTX. Once the XR-NTX condition was removed and only offered as an optional, supplementary pharmacotherapy platform, recruitment for the study greatly improved. This highlights the significant need for psychosocial and/or psychoeducational interventions that target barriers to XR-NTX initiation.
Another challenge encountered while trying to initiate participants on XR-NTX was to identify and enlist providers to offer XR-NTX injections to interested participants. Anecdotally, insurance status was part of the challenge faced, along with limited personnel resources to track participants. This is consistent with research indicating that routine use of XR-NTX in clinical practice for alcohol use disorders remains uncommon; according to a survey of addiction treatment providers, 16% reported having used XR-NTX for some patients (Abraham & Roman, 2010). Moreover, the 2013 National Survey of Substance Abuse Treatment Services indicated that less than 1% of the 1.2 million patients receiving care in March 2013 received XR-NTX (SAMHSA, 2014).
Although we have drawn valuable lessons from the qualitative data collected, several limitations warrant comment. First, this is a very small sample (n=15), and as such, there may be additional barriers not encountered or reported. Second, given the small sample size, there are limitations to the generalizability of these findings. Furthermore, these results may not be generalizable to other populations with HIV, as this data was collected as part of an intervention that specifically targeted for HIV-positive adults with alcohol dependence. Third, we did not collect information regarding participants’ interest in treatment for polysubstance use for substances other than alcohol. Having this information may have informed additional questions and allowed us to gather additional data with regards to barriers to initiation and maintenance to XR-NTX. Fourth, alcohol dependence data was collected using DSM-IV-TR and not DSM-V. As a result, we do not have data regarding severity of alcohol use disorder. This is relevant as XR-NTX is generally offered to patients with moderate or severe use disorder. Finally, coding of responses in content analysis of open ended survey questions can pose threats to the reliability and validity of the results (Krippendorff, 1980; Seidel & Kelle, 1995). However, our small sample size did enable us to carefully consider and verify our coding choices for each participant’s answers.
Important next steps should include acquiring providers’ input on barriers to pharmacotherapy initiation, collecting additional data about barriers to XR-NRT initiation and maintenance on a larger scale, and developing and testing psychosocial interventions that target initiation and maintenance of XR-NRT.
Highlights.
Barriers for HIV+ adults with alcohol use disorders fall in two categories
Barriers amenable to change such as fear of injections
Barriers not amenable to change such as the interaction with a medication regimen
Acknowledgments
The research presented in this paper was supported by NIAAA Grant 1R34AA022055.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham AJ, Roman PM. Early adoption of injectable naltrexone for alcohol use disorders: Findings in the private treatment sector. Journal of Studies on Alcohol and Drugs. 2010;71:460–466. doi: 10.15288/jsad.2010.71.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alanis-Hirsch K, Croff R, Ford JH, Johnson K, Chalk M, Schmidt L, McCarty D. Extended-Release Naltrexone: A Qualitative Analysis of Barriers to Routine Use. Journal of substance abuse treatment. 2016;62:68–73. doi: 10.1016/j.jsat.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliunas D, Rehm J, Irving H, Shuper P. Alcohol consumption and risk of incident human immunodeficiency virus infection: A meta-analysis. International Journal of Public Health. 2010;55(3):159–166. doi: 10.1007/s00038-009-0095-x. [DOI] [PubMed] [Google Scholar]
- Bryson WC, McConnell KJ, Korthuis PT, McCarty D. Extended-release naltrexone for alcohol dependence: persistence and healthcare costs and utilization. The American journal of managed care. 2011;17(Suppl 8):S222. [PMC free article] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality. 2015 National Survey on Drug Use and Health: Detailed Tables. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2016. [Google Scholar]
- Chander G, Monroe AK, Crane HM, Hutton HE, Saag MS, Cropsey K, Eron JJ, Quinlivan EB, Geng E, Mathews WC, Boswell S, Rodriquez B, Ellison M, Kitahata MM, Moore RD, McCaul ME. HIV primary care providers—Screening, knowledge, attitudes and behaviors related to alcohol interventions. Drug and alcohol dependence. 2016;161:59–66. doi: 10.1016/j.drugalcdep.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann PD, Mello D, Lonergan S, Bourgault C, O’Toole TP. Aversion to injection limits acceptability of extended-release naltrexone among homeless, alcohol-dependent patients. Substance abuse. 2013;34(2):94–96. doi: 10.1080/08897077.2012.763083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasner-Edwards S, Patrick K, Ybarra ML, Reback CJ, Rawson RA, Garneau HC, Chavez K, Venegas A. A Cognitive Behavioral Therapy–Based Text Messaging Intervention Versus Medical Management for HIV-Infected Substance Users: Study Protocol for a Pilot Randomized Trial. JMIR research protocols. 2016;5(2) doi: 10.2196/resprot.5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasner-Edwards S. Development of a text messaging intervention for HIV+ alcohol users. Paper presented at the 123rd annual meeting of the American Psychological Association; Toronto, Canada. 2015. Aug, [Google Scholar]
- Harris KM, Thomas C. Naltrexone and pharmacy benefit management. Journal of Addictive Diseases. 2004;23(4):11–29. doi: 10.1300/J069v23n04_02. [DOI] [PubMed] [Google Scholar]
- Helstrom AW, Blow FC, Slaymaker V, Kranzler HR, Leong S, Oslin D. Reductions in alcohol craving following naltrexone treatment for heavy drinking. Alcohol and alcoholism. 2016;51(5):562–6. doi: 10.1093/alcalc/agw038. [DOI] [PubMed] [Google Scholar]
- Jonas DE, Amick HR, Feltner C, Bobashev G, Thomas K, Wines R, Kim M, Shanahan E, Gass E, Rowe C, Garbutt JC. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. Jama. 2014;311(18):1889–1900. doi: 10.1001/jama.2014.3628. [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Simbayi LC, Kaufman M, Cain D, Jooste S. Alcohol use and sexual risks for HIV/AIDS in sub-Saharan Africa: Systematic review of empirical findings. Prevention Science. 2007;8(2):141–151. doi: 10.1007/s11121-006-0061-2. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Stephenson JJ, Montejano L, Wang S, Gastfriend DR. Persistence with oral naltrexone for alcohol treatment: Implications for health-care utilization. Addiction. 2008;103:1801–1808. doi: 10.1111/j.1360-0443.2008.02345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krippendorff K. Content analysis: An introduction to its methodology. Vol. 5. Newbury Park, CA: Sage; 1980. [Google Scholar]
- McCarty D, McConnell J, Leader D, Un H. Continued use of addiction medications and impacts on use of health care. Presented at Addiction Health Services Research Conference; San Francisco, CA. 2009. [Google Scholar]
- McGlynn EA, Asch SM, Adams J, Keesey J, Hicks J, DeCristofaro A, Kerr EA. The quality of health care delivered to adults in the United States. New England journal of medicine. 2003;348(26):2635–2645. doi: 10.1056/NEJMsa022615. [DOI] [PubMed] [Google Scholar]
- Metzger DS, Zhang Y. Drug treatment as HIV prevention: Expanding treatment options. Current HIV/AIDS Reports. 2010;7(4):220–225. doi: 10.1007/s11904-010-0059-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Hutchison KE, Swift RM, Mueller TI, Colby SM, Brown RA, Gulliver SB, Gordon A, Abrams DB. Naltrexone's effect on cue-elicited craving among alcoholics in treatment. Alcoholism: Clinical and Experimental Research. 1999;23(8):1386–1394. [PubMed] [Google Scholar]
- NIAAA. Helping Patients Who Drink Too Much: A Clinician’s Guide (Updated 2005 edition) U.S. Department of Health and Human Services, National Institutes of Health; 2015. [Google Scholar]
- NIAAA. Naltrexone. U.S. Department of Health and Human Services, National Institutes of Health; 2017. Answers to Frequently Asked Medication Questions. [Google Scholar]
- O'Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek M. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo–pituitary–adrenocortical axis. Psychopharmacology. 2002;160(1):19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Ray LA, Chin PF, Miotto K. Naltrexone for the treatment of alcoholism: clinical findings, mechanisms of action, and pharmacogenetics. CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders) 2010;9(1):13–22. doi: 10.2174/187152710790966704. [DOI] [PubMed] [Google Scholar]
- Rehm J, Shield KD, Joharchi N, Shuper PA. Alcohol consumption and the intention to engage in unprotected sex: Systematic review and meta-analysis of experimental studies. Addiction. 2012;107(1):51–59. doi: 10.1111/j.1360-0443.2011.03621.x. [DOI] [PubMed] [Google Scholar]
- Roux P, Carrieri MP, Cohen J, Ravaux I, Poizot-Martin I, Dallamonica P, Spire B. Retention in opioid substitution treatment: A major predictor of long-term virological success for HIV-infected injection drug users receiving antiretroviral treatment. Clinical Infectious Diseases. 2009;49(9):1433–1440. doi: 10.1086/630209. [DOI] [PubMed] [Google Scholar]
- SAMHSA. Data on substance abuse treatment facilities. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014. National Survey of Substance Abuse Treatment Services: 2013. HHS Publication No. SMA 14–4890. [Google Scholar]
- Seidel J, Kelle U. Different functions of coding in the analysis of textual data. In: Kelle U, editor. Computer-aided qualitative data analysis: Theory, methods, and practice. Thousand Oaks, CA: Sage; 1995. pp. 52–61. [Google Scholar]
- Shuper PA, Neuman M, Kanteres F, Baliunas D, Joharchi N, Rehm J. Causal considerations on alcohol and HIV/AIDS--a systematic review. Alcohol and Alcoholism. 2010;45(2):159–166. doi: 10.1093/alcalc/agp091. [DOI] [PubMed] [Google Scholar]
- Springer SA, Brown SE, Di Paola A, Altice FL. Correlates of retention on extended-release naltrexone among persons living with HIV infection transitioning to the community from the criminal justice system. Drug Alcohol Depend. 2015;1:157, 158–65. doi: 10.1016/j.drugalcdep.2015.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MD, Charuvastra A, Anderson B, Sobota M, Friedmann PD. Alcohol and HIV risk taking among intravenous drug users. Addictive behaviors. 2002;27(5):727–736. doi: 10.1016/s0306-4603(01)00205-2. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. National Admissions to Substance Abuse Treatment Services. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2016. Treatment Episode Data Set (TEDS): 2004–2014. BHSIS Series S-84, HHS Publication No. (SMA) 16–4986. [Google Scholar]
- Uhlmann S, Milloy MJ, Kerr T, Zhang R, Guillemi S, Marsh D, Hogg RS, Montaner JS, Wood E. Methadone maintenance therapy promotes initiation of antiretroviral therapy among injection drug users. Addiction. 2010;105(5):907–913. doi: 10.1111/j.1360-0443.2010.02905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli JR, Alterman AI, Hayashida M, O'Brien CP. Naltrexone in the treatment of alcohol dependence. Archives of General Psychiatry. 1992;49(11):876–880. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- Wang X, Douglas SD, Peng JS, Metzger DS, O’Brien CP, Zhang T, Ho WZ. Naltrexone inhibits alcohol-mediated enhancement of HIV infection of T lymphocytes. Journal of Leukocyte Biology. 2006;79(6):1166–1172. doi: 10.1189/jlb.1105642. [DOI] [PubMed] [Google Scholar]