Abstract
Understanding complex behavioral processes, both learned and innate, requires detailed characterization of the principles governing signal flow in corresponding neural circuits. Previous studies were hampered by the lack of appropriate tools needed to address the complexities of behavior-driving micro- and macrocircuits. The development and implementation of optogenetic methodologies revolutionized the field of behavioral neuroscience, allowing precise spatiotemporal control of specific, genetically defined neuronal populations and their functional connectivity both in vivo and ex vivo, thus providing unprecedented insights into the cellular and network-level mechanisms contributing to behavior. Here, we review recent pioneering advances in behavioral studies with optogenetic tools, focusing on mechanisms of fear-related behavioral processes with an emphasis on approaches which could be used to suppress fear when it is pathologically expressed. We also discuss limitations of these methodologies as well as review new technological developments which could be used in future mechanistic studies of fear behavior.
1. Introduction
Anxiety disorders are among the most commonly occurring mental illnesses in the United States and worldwide (Kessler et al., 2009). They include adult separation anxiety disorder, generalized anxiety, panic disorder, post-traumatic stress disorder (PTSD) and various types of phobias, including agoraphobia, social phobia and specific phobia (Kessler et al., 2009; DSM-5, 2013). Anxiety disorders are characterised by a greater degree of fear than required by the situation, often reflected in responses to a non-existent threat (LeDoux, 1998; LeDoux, 2000; Maren & Quirk, 2004). Accordingly, they can be considered as disorders involving a dysregulation of fear systems in the brain. Available treatments for anxiety disorders are relatively nonspecific and include cognitive behavioral therapy (CBT) and/or medication (e.g., benzodiazepines, beta blockers or antidepressants). In the case of exposure-based CBT, a patient is regularly presented with an intervention which triggers anxiety states to downregulate negative emotions toward these stimuli via desensitization. While much more complex compared to animal studies, the cognitive strategies for control of fear and anxiety in humans are conceptually similar to extinction of classical threat (fear) conditioning, that appears to be phylogenetically preserved across different species and shows significant overlap of underlying circuits (Phelps et al., 2004; Delgado et al., 2008; Milad & Quirk, 2012). For these reasons, fear extinction in rodents is generally accepted as a preclinical model for exposure-based behavioral therapy of anxiety disorders in human subjects.
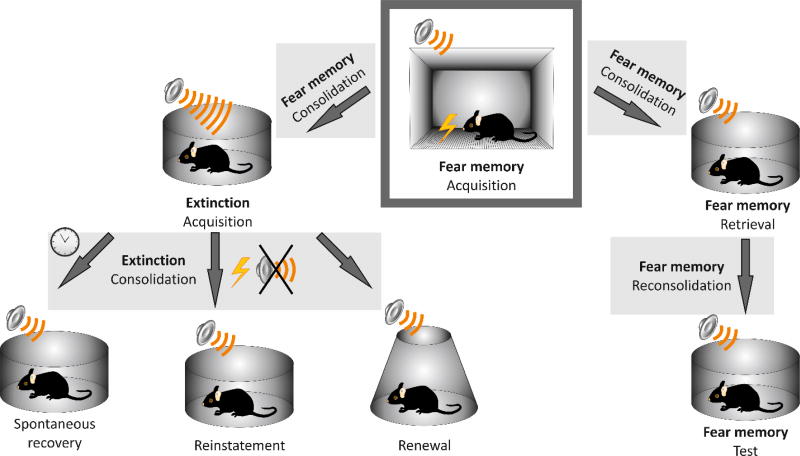
Classical conditioning and extinction paradigms were originally introduced in early twentieth century in experiments on appetitive responses in dogs (Pavlov, 1927). In rodent models, conditioned fear is induced experimentally by pairing an initially neutral stimulus (the conditioned stimulus or CS, such as tone, specific odor or light) with an aversive stimulus (the unconditioned stimulus or US - e.g., electric foot or tail shock, Fig. 1). If repeated several times, or even after a single CS and US pairing, this leads to the formation of a strong association between the US and CS — fear learning, allowing animals to predict the conditioned threat based on environmental cues. Thus, the presentation of the CS alone results in the fear response, which could be measured as a degree of freezing or an acoustic startle reflex. However, if the CS is presented repeatedly without an anticipated reinforcement (in the absence of US), the conditioned fear response gradually declines in a process of fear extinction (Myers & Davis, 2007). Fear extinction, which is a form of memory, is not permanent and often less stable than the initially acquired fear memory, which could reappear under different conditions in fear extinguished subjects. Thus, the extinguished fear can arise again in a process of spontaneous recovery (with a passage of time without any additional training), renewal (due to a change of context in which fear memory was extinguished) or reinstatement (with an exposure to the initial US) (Myers & Davis, 2007; Singewald et al., 2015). Re-emergence of fear memory is observed not only in rodent models, but also in humans (Dirikx et al., 2004; Hermans et al., 2005; Milad et al., 2005), bringing certain limitations for exposure-based CBT which need to be considered in clinical settings (e.g., Dunsmoor et al., 2014). The return of the original fear memory under different conditions supports an accepted view that extinction represents new learning that does not erase the initial CS-US association, underlying fear memory. Notably, it has been suggested that the efficiency of fear extinction in rats (new learning vs. erasure) may be determined by the time interval between fear conditioning and extinction training (Myers et al., 2006). However, this time dependence does not seem to be universal (Maren & Chang, 2006; Alvarez et al., 2007; Schiller et al., 2008; Woods & Bouton, 2008; Chang & Maren, 2009). Other studies suggest that “unlearning” might be a dominant mechanism of extinction in early development (Kim & Richardson, 2008).
Fig. 1. Mechanisms of threat (fear) conditioning and extinction.
Acquisition (initial learning during training sessions) of fear memory represents a training phase when an initially neutral stimulus (the conditioned stimulus or CS; in this example, an audible tone) is paired with an aversive stimulus (the unconditioned stimulus or US - electric foot shock). Following the training period, the conditioned fear memory goes through a consolidation phase. During this protein synthesis-dependent process, the newly acquired fear memory is converted into a long-term memory. Next, fear memory can be retrieved by presentation of the unreinforced CS or extinguished by repeated presentations of the CS. Retrieval of fear memory makes it transiently labile again and triggers another protein synthesis-dependent process known as memory reconsolidation (Nader et al., 2000). Fear memory reconsolidation is required to sustain the long-term memory; it may weaken or strengthen the original memory (Inda et al., 2011; Pedroso et al., 2013; Fukushima et al., 2014), therefore providing the window of opportunity for interventions aimed at suppressing the original fear memory (Li et al., 2013). Despite the fact that fear memory reconsolidation and extinction processes are initiated by the same stimulus (the unreinforced CS), they are mechanistically distinct processes which can be studied experimentally in isolation from each other (Eisenberg et al., 2003; Pedreira & Maldonado, 2003; Duvarci & Nader, 2004). The extinguished fear memory can return via three different mechanisms: spontaneous recovery (with the passage of time after fear conditioning), reinstatement (with an exposure to the initial US) or renewal (due to a change of extinction context) (Myers & Davis, 2007; Singewald et al., 2015).
Over the last decade, specific neural circuits and pathways involved in modulation of fear in the classical threat (fear) conditioning paradigm have been quite successfully dissected, though there still are many unanswered questions. There is significant evidence for the role of long-term synaptic plasticity at synapses in the neural pathways of fear learning in retention of conditioned threat (fear) memory (McKernan & Shinnick-Gallagher, 1997; Rogan et al., 1997; Tsvetkov et al., 2002; Rumpel et al., 2005; Dityatev & Bolshakov, 2005; Cho et al., 2012). Synaptic plasticity in afferent projections to the amygdala, providing conditioned stimulus information, is pathway-specific, thus helping to maintain functional independence of the convergent inputs (Tsvetkov et al., 2004; Shin et al., 2006; Shin et al., 2010). Previous studies also demonstrated that fear-related behavioral processes, both learned and innate, could be controlled by neural circuitry-specific gene expression (Shumyatsky et al., 2002; Shumyatsky et al., 2005; Riccio et al., 2009; Riccio et al., 2014).
The examination of neural circuits underlying innate fear, as opposed to learned fear (e.g., fear conditioning), and its regulation has been challenging and technically demanding due to the complexity and heterogeneity of brain structures and neural circuits involved. The bottom-up multilevel scientific approach, enabled by the development and implementation of optogenetic techniques in combination with advanced molecular biological and genetic tools, allowed the dissection of circuits governing expression and modulation of fear from the new perspectives (Johansen et al., 2012; Belzung et al., 2014; Lalumiere, 2014; Riga et al., 2014). By using genetically coded light-sensitive probes, optogenetic techniques allow precise temporal and spatial control of specific cells and their projections. Thus, the experimental possibilities are shifted from dissecting the roles of certain brain regions with somewhat limited pharmacological and lesion approaches, to the study of contributions of specific, either molecularly or anatomically defined, populations of neurons to behavioral processes in both freely moving animals and in ex vivo network-level studies.
In the current review, we provide a brief introduction to optogenetic techniques which have been used in studies of fear circuits. We then discuss recent advances and limitations of these techniques as well as other current technological developments which can potentially be applied to the study of the neural pathways controlling fear. Finally, we review recent discoveries utilising optogenetic tools, exploring neural circuits associated with both learned and innate fear and their modulation.
2. Optogenetics: summary and current developments
In the “pre-optogenetics” era, technological limitations made it almost impossible to study the individual elements (e.g., anatomically defined projections or genetically distinct subpopulations of neurons) of fear and extinction circuits. Optogenetic techniques, combined with advanced genetic tools, are extremely useful for probing the functions of specific cell types and distinct connections in neuronal networks guiding fear and anxiety. Detailed cell-specific characterization of neural circuits regulating fear and anxiety, as well as the possibility of precisely targeted manipulation of neuronal activity in defined cell populations, are critical for understanding the underlying neurobiological basis of both learned and innate fear systems. In addition, these advances may facilitate the development of controlled and specialized treatment paradigms for the disorders implicating dysregulation of fear mechanisms.
2.1 Optogenetic tools
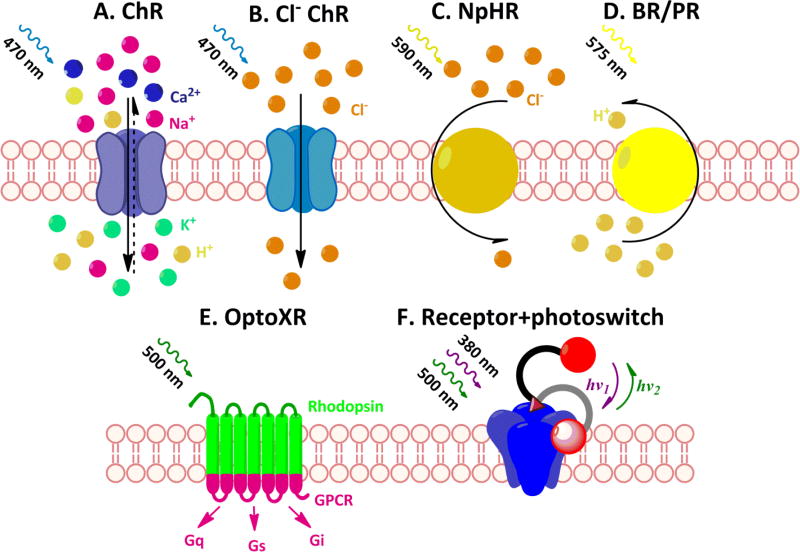
Optogenetics employs light-sensitive proteins (opsins) to enable millisecond-precision control of neuronal firing by photostimulation in genetically identifiable cell populations. Since channelrhodopsin-2 (ChR2), a rapidly gated light-sensitive cation channel derived from the green alga Chlamydomonas reinhardtii (Nagel et al., 2003), was shown to reliably induce action potential firing in hippocampal neurons in response to illumination with pulses of blue light (470 nm; Boyden et al., 2005), new optogenetic tools are continuously being developed. These tools enhance and broaden the ways to manipulate neuronal activity by photostimulation (Fig. 2; for review see Yizhar et al., 2011; Zhang et al., 2011; Zalocusky & Deisseroth, 2013; Guru et al., 2015). Neurons may be silenced by hyperpolarizing their membrane using light-activated ion pumps, which translocate chloride ions from the extracellular space into the cell (halorhodopsin from Natronomonas pharaonis or NpHR (Han & Boyden, 2007; Zhang et al., 2007) as well as its improved versions eNpHR2.0 and eNpHR3.0 (Gradinaru et al., 2008; Zhao et al., 2008; Gradinaru et al., 2010)) or by extruding protons from the cells (archaerhodopsin Halorubrum sodomense or Arch; Chow et al., 2010). Both Arch and NpHR silence neurons upon illumination with yellow light (Zhang et al., 2007; Chow et al., 2010); therefore, they do not overlap with the ChR2 excitation spectrum, allowing independent activation of ChR2 and Arch/NpHR for bidirectional modulation of neuronal activity. To achieve maximally deep penetration of light into the brain for in vivo studies and desirable future medical applications, efforts have been concentrated on the development of red-shifted inhibitory opsins. Recently, this work culminated in the engineering of a red-shifted cruxhalorhodopsin, Jaws, derived from Haloarcula (Halobacterium) salinarum (strain Shark), which noninvasively mediates transcranial optical inhibition of neurons located 1–3 mm below the brain’s surface in awake mice (Chuong et al., 2014). Despite such improvements, these inhibitory pumps are less efficient compared to excitatory channelrhodopsins (e.g., only a relatively small brain area could be silenced effectively in in vivo studies). Furthermore, they transport only one ion per photon and so require constant light illumination of high intensity coupled to high levels of photosensitive protein expression. In addition, NpHR can affect the GABAA receptor reversal potential, which may result in changes in synaptically evoked spiking following the photoactivation period (Raimondo et al., 2012). Finally, sustained Arch activation may increase spontaneous neurotransmitter release (Mahn et al., 2016); therefore, it affects neuronal firing rates and behavioral outcomes. Recently, helped by publication of the first high-resolution crystal structure of channelrhodopsin, two groups introduced the designed light-gated chloride channels for optogenetic inhibition (Berndt et al., 2014; Wietek et al., 2014), which should overcome many of the long-standing limitations mentioned above that hamper the efficiency of optogenetic inhibition.
Fig. 2. Tools for optical control of neuronal activity.
A. Cation-permeable channels such as channelrhodopsins (ChRs), activated by blue light, lead to membrane depolarization and cause spike firing (Nagel et al., 2003; Boyden et al., 2005). B. Recently engineered chloride-conducting channelrhodopsins, activated by blue light, efficiently hyperpolarize the membrane and therefore silence neurons (Berndt et al., 2014; Wietek et al., 2014). C. Halorhodopsin (NpHR), an inhibitory chloride pump, silence neurons upon illumination with yellow light (Han & Boyden, 2007; Zhang et al., 2007). D. Bacteriorhodopsins and proteorhodopsins (BR/PR), yellow light-activated proton pumps, with archaerhodopsin (Arch) as a most frequently used inhibitor of neuronal activity (Chow et al., 2010; Gradinaru et al., 2010; Han et al., 2011). E. OptoXR, a chimera of rhodopsin and G protein-coupled receptor, allowing modulation of intracellular signaling cascades by photostimulation with green light (Airan et al., 2009; Oh et al., 2010; Gunaydin et al., 2014). F. By attaching a photoswitchable tethered ligand (PTL) onto a genetically modified channel or receptor (a single cysteine substitution allows binding), such channel or receptor gains light-dependency. PTL flips between two conformations, bidirectionally controlled by different wavelengths, e.g., violet and green light, and activates or inhibits the channel or receptor. A number of light-controlled receptors and channels has been recently developed (see the main text).
Spatiotemporally precise control of intracellular signaling processes in neurons became possible with the development of opsin-receptor chimeras (Kim et al., 2005) between vertebrate rhodopsin and conventional ligand-gated G-protein-coupled receptors (GPCRs), such as the adrenergic, serotonin and dopamine receptors (the optoXR family) (Airan et al., 2009; Oh et al., 2010; Gunaydin et al., 2014; Singewald et al., 2015). These optoXRs activate native GPCR second messenger signaling in response to light instead of ligand-mediated activation (Zalocusky & Deisseroth, 2013) and many of them play modulatory roles in extinction processes (Mueller & Cahill, 2010; Homberg, 2012; Stiedl et al., 2015; Stockhorst & Antov, 2015). The drawback of all the above-listed strategies is that they involve expression of exogenous proteins (opsins), with natural endogenous neuronal firing patterns substituted by artificial spike firing patterns dictated by the experimenter.
Another approach, pioneered by Richard Kramer and termed optogenetic pharmacology, concentrates on modifying endogenous receptors and channels (Banghart et al., 2004). In this approach, a receptor or channel is genetically modified with a point mutation (a cysteine substitution) close to the neurotransmitter binding site or the pore-forming domain. This site is used to attach a cysteine-reactive photoswitchable tethered ligand (PTL), which changes its conformation in a light-dependent manner and either activates or inhibits the channel or receptor, respectively (Szobota & Isacoff, 2010; Kramer et al., 2013). Similar to optogenetics, this method provides cell-specific temporal and spatial control of neuronal activity, but adds bidirectionality and receptor-subunit specificity. To date, there are engineered light-regulated K+ channels (Banghart et al., 2004; Fortin et al., 2011; Sandoz et al., 2012); nicotinic acetylcholine receptor subtypes α3β4 and α4β2 (Tochitsky et al., 2012); P2X receptors (Lemoine et al., 2013; Browne et al., 2014); acid-sensing ion channels (Browne et al., 2014); group II metabotropic glutamate receptors (mGluRs), mGluR2 and mGluR3, and group III mGluR mGluR6 (Levitz et al., 2013); kainate subtype of ionotropic glutamate receptors GluK2 (Volgraf et al., 2006; Gorostiza et al., 2007) and the entire GABAA receptor family (Lin et al., 2014; Lin et al., 2015). In contrast to the mentioned single-component tools, optogenetic pharmacology is more challenging methodologically as it depends on two critical constituents: the chemical photoswitchable ligand and the gene encoding the receptor or channel of interest (Kramer et al., 2013). However, the development of knock-in mice where the wild-type channel is replaced by an engineered light-sensitive version should make the technical implementation of these tools easier and facilitate their usage (Lin et al., 2015).
2.2. Optogenetic targeting
The decoding of fear circuits using optogenetic techniques requires efficient targeted delivery and high levels of photosensitive proteins expression, which could be accomplished in several different ways. First, stereotactic virus-based introduction of opsin genes under direct control of specific promoters provides the brain region-specific targeting (Fig. 3). Spatially precise injections of viruses are used in situations where specific genetic targeting otherwise could not be achieved (e.g., targeting infralimbic versus prelimbic subregions in the mPFC). Depending on experimental requirements, different viruses could be used for gene delivery, such as the commonly used adeno-associated virus (AAV) and lentivirus, but also herpes simplex virus 1 (HSV-1) and rabies virus. The viral construct can include ubiquitous or neuronal promoters such as elongation factor 1-alpha (EF1), human thymocyte differentiation antigen 1 (Thy-1), neuron specific human synapsin 1 (hSyn) (Zhang et al., 2010; Rein & Deussing, 2012). The main constraint of viral transduction methods is the limited packaging capacity of viruses, which restricts the use of large promoters and lowers the number of cell types that can be targeted. Additionally, many cell-type specific promoters cannot achieve levels of opsin expression required for efficient photostimulation (Zhang et al., 2010). Despite these limitations, some cell-type specific promoters have been successfully employed for opsins delivery, including Ca2+/calmodulin-dependent protein kinase IIα (CaMKIIα) promoter to target excitatory glutamatergic neurons (e.g., Gradinaru et al., 2008), glial fibrillary acidic protein (GFAP) promoter for optogenetic astrocyte activation (Perea et al., 2014), Hcrt promoter for targeting hypocretin (orexin)-producing neurons in the lateral hypothalamus (Adamantidis et al., 2007) and D2SP promoter for transgene expression in dopamine receptor type 2 (D2R)-containing cells (Zalocusky et al., 2016). In rodents, functional levels of opsin expression at the viral injection sites are normally reached after 2–3 weeks (depending on the virus type). However, much longer times are required for expression in long-range terminal projections (>6 weeks; Zhang et al., 2010), limiting the use of this approach in developmental studies of fear-related behavioral processes.
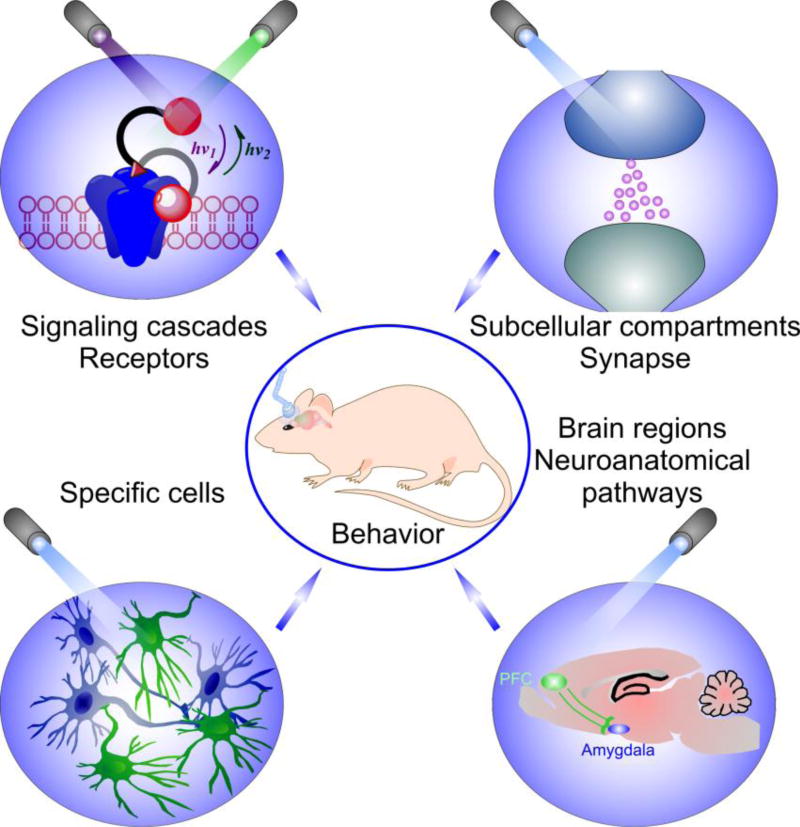
Fig. 3. Different levels of optogenetic control.
Optogenetics can be implemented at all functional levels; therefore, it provides a powerful experimental framework for dissection of different elements modulating fear (from distinct receptor subtypes on specific cell types to distinct neural circuits) and their links to behavior.
Different approaches have been implemented to achieve projection-specific opsin targeting. For instance, to perform mPFC projection-specific manipulations in amygdala, virus carrying opsin was injected into the medial prefrontal cortex (mPFC), while light was delivered to the amygdala via an implanted optical fiber in vivo or positioned LED source in slices ex vivo (Cho et al., 2013; Bukalo et al., 2015). Additionally, anterograde or retrograde labeling of specific projections, innervating either a targeted brain region or a targeted cell population, exploit such viral vectors as herpes simplex virus 1 (HSV-1), rabies virus, pseudorabies, vesicular stomatitis virus and designer AAV variant rAAV2-retro for retrograde gene delivery (Zhang et al., 2010; Zalocusky & Deisseroth, 2013; Tervo et al., 2016), whereas lymphocytic choriomeningitis virus glycoprotein (LCMV-G) and AAV serotypes AAV1 and AAV9 are used for trans-synaptic anterograde transduction (Zalocusky & Deisseroth, 2013; Zingg et al., 2016). An alternative approach, based on the use of a version of Cre-recombinase fused to wheat-germ agglutinin (WGA-Cre) or tetanus toxin fragment C (TTC-Cre), allows Cre recombinase to be trans-synaptically transported to anatomically connected neurons. Another subset of neurons or target structures, transduced with a Cre-dependent virus, will then express the opsin gene only upon Cre recombinase translocation (Gradinaru et al., 2010). Therefore, projection specific targeting combined with the cell-type specific (via genetic markers) and regional targeting provide versatile tools in the identification of truly distinct cell types by their anatomic, genetic and functional characteristics. It provides an additional degree of cellular and network-level specificity for dissection of fear circuits when other targeting methods are not available. For example, the differentiation of fear and extinction neurons in the basolateral nucleus of the amydgala (BLA) is based not only on their functional characteristics but also on their specific targeting of neurons in PL and IL subregions, respectively (Senn et al., 2014).
Viral transduction does not always result in the uniform expression of opsins. Furthermore, opsins are only expressed in cells that are receptive to viruses. For targeting cell types that require larger promoters, in order to obtain more consistent patterns of opsin expression, or for developmental studies using optogenetic tools, a good alternative to viral delivery of opsins is the use of genetically modified mice. There are several transgenic mouse lines that express opsins under control of a variety of different promoters, including Thy1 (Arenkiel et al., 2007; Zhao et al., 2008), vesicular glutamate transporter 2 (VGlut2) for glutamatergic neurons (Hägglund et al., 2010), vesicular GABA transporter (VGAT) promoter for opsin expression in GABAergic neurons, choline acetyl transferase (ChAT) promoter for cholinergic neurons, tryptophan hydroxylase 2 (TPH2) for serotonergic neurons, and Pvalb promoter for parvalbumin-positive interneurons (Zhao et al., 2011). Different mouse lines with robust Cre-dependent expression of the fluorescently tagged ChR2(H134R)-tdTomato, ChR2(H134R)-EYFP, Arch-EGFP-ER2, or eNpHR3.0-EYFP have been created recently. These mice allow optical control of neuronal activity for a broad range of cells when crossed with multiple available Cre-driver lines (Madisen et al., 2012). Additionally, the tetracycline transactivator (tTA)-tetracycline operator (tetO) promoter system can be used to generate tet-off bitransgenic mouse lines, allowing activity-dependent expression of the channelrhodopsin variant (ChEF) within a determined temporal window (e.g., corresponding to a specific behavior), with opsin expression restricted by administration of doxycycline outside of the required temporal window (Cowansage et al., 2014). Recently, methods of activity-dependent expression of opsins were extended to the CANE technology (Capturing Activated Neuronal Ensembles), utilizing both knock-in mice and engineered viruses. This method allows labelling of neurons activated during specific behavior or a set of behaviors with the subsequent manipulation of activity (both silencing and activation) of tagged neurons as well as mapping their efferent and afferent projections (Sakurai et al., 2016). These tools are capable of providing deep insights into molecular and cellular mechanisms of a specific behavior process, allowing photoactivation of neuronal ensembles specific for this behavior; therefore, such behavior can be “replayed” at the level of implicated neural circuits either ex vivo or in vivo.
To overcome the disadvantages of the somewhat limited cell-type specificity of applicable promoters, the following molecular tools have been used. Systems using flip-excision switch (FLEX) or double inverted open (DIO) reading frame AAVs combine the advantages of multiple available Cre-driver mouse lines (transgenic lines expressing Cre-recombinase under control of a cell type-specific promoter) and Cre-dependent AAV vectors in which an opsin gene is flanked by heterotypic, antiparallel loxP/lox2272 recombination sites. The open reading frame is only irreversibly inversed in Cre-expressing neurons; therefore cell-type specific opsin expression is enabled under the control of a strong promoter. Accordingly, if cells are infected by DIO/FLEX-AAV but lack Cre recombinase, loxP/lox2272, recombination sites will prevent transcriptional read-through and, consequently, will not express opsins carried by the viral construct (Atasoy et al., 2008; Sohal et al., 2009). In addition, a combination of Cre with other recombinases (such as Dre or Flp) have been recently implicated in the INTRSECT approach (INTronic Recombinase Sites Enabling Combinatorial Targeting), allowing simultaneous cell-type specific (several genetic markers) and/or projection-based targeting of several populations of neurons (Fenno et al., 2014).
Thus, due to the constantly developing and expanding experimental toolbox, optogenetic approaches provide unparalleled possibilities for selective manipulations of fear circuits in freely moving animals and network-level mechanistic studies of fear mechanisms in ex vivo experiments.
3. Neural circuits of extinction learning
Neural circuits of fear conditioning and extinction are quite distinct, although parts of the respective circuits overlap to a certain degree. There is an extensive body of work identifying brain regions and neuronal circuits engaged in fear extinction (reviewed in Sotres-Bayon et al., 2006; Myers & Davis, 2007; Quirk & Mueller, 2008; Herry et al., 2010; Milad & Quirk, 2012; Orsini & Maren, 2012; Bukalo et al., 2014; Furini et al., 2014; Milad et al., 2014; Baldi & Bucherelli, 2015; Tovote et al., 2015). Here, we will summarise the current views on networks underlying fear extinction and concentrate on new insights from studies adopting optogenetic approaches. The basic neural circuitry of fear extinction is comprised of three interconnected brain regions: amygdala, medial prefrontal cortex (mPFC) and hippocampus, although other brain regions such as the bed nucleus of the stria terminalis (BNST) and periaqueductal gray of the midbrain (PAG) may also play some roles. For instance, excitatory synaptic transmission in the BNST was implicated in the extinction of cocaine place preference (Conrad et al., 2012), with both extinction circuits for fear and addiction overlapping in prefrontal cortex (Peters et al., 2009). In addition, the BNST is important for the reinstatement of extinguished fear, but not for fear renewal per se (Goode et al., 2015). Aside from the established role of PAG in directing motor outputs toward the appropriate defensive behavior (i.e., freezing or fleeing) during fear learning, the ventrolateral PAG also plays a role in the acquisition of fear extinction mediated by opioid receptors (McNally et al., 2004; Parsons et al., 2010).
With respect to the amygdala and mPFC, both different and overlapping subregions of these brain areas are involved in fear and extinction learning. The lateral and basal nuclei of the amygdala and its central nucleus (CeA), including lateral (CeL) and medial (CeM) subdivisions, play roles in the acquisition and expression of fear memories. The BLA and intercalated cells (small GABAergic, densely packed cell clusters at the border between BLA and CeA) are important for the acquisition of extinction memory and its storage. CeA is the major output of the amygdala driving fear responses via its connections to the brain areas mediating physiological manifestations of fear. The infralimbic (IL) division of mPFC has been implicated in fear inhibition and fear extinction, whereas prelimbic (PL) mPFC is critical for fear acquisition and maintenance. In turn, the hippocampus, via its projections to both the mPFC and the BLA, contributes to contextual encoding and context-dependency of extinction retrieval, including the context-dependent renewal of extinguished fear memories (Herry et al., 2010; Pape & Pare, 2010; Orsini & Maren, 2012; Bukalo et al., 2014).
When the CS is presented in the extinction context, the hippocampus activates the IL. During recall of extinction memory, IL neurons exhibit increased neural firing in vivo (Milad & Quirk, 2002). Therefore, in the extinction context, enhanced activity of the IL subdivision of mPFC is proposed to control the signal flow in the BLA-CeA circuits, resulting in suppression of conditioned fear responses.
There are multiple pathways that could be involved in the modulation of extinction or extinction recall. First, IL may inhibit fear expression by driving activation of the medial ITC (ITCm) neurons (located between CeA and BLA), which, in turn, results in ITC-mediated feed-forward inhibition of the CeA neurons (Royer et al., 1999; Berretta et al., 2005; Likhtik et al., 2005; Likhtik et al., 2008). Notably, a recent study, using ex vivo optogenetic tools, did not identify a direct, monosynaptic innervation of ITC neurons by IL afferents, suggesting that IL control of mITC neurons during extinction may be mediated by inputs from the BLA (Strobel et al., 2015). However, it remains possible, that the failure to detect monosynaptic responses in the mITC upon photostimulation of the ChR2-expressing IL fibers in this study was due to the relatively low level of ChR2 expression. Indeed, the amplitude of excitatory postsynaptic currents in BLA neurons induced by photostimulation of IL fibers in the above-mentioned experiments was less than 100 pA (and even smaller in more dorsal parts of the BLA), whereas the maximal amplitude of synaptic responses in the mPFC-BLA projections was in the nanoampere range in the earlier study in which monosynaptic mPFC inputs to ITC were observed (Cho et al., 2013). Second, there is the possibility of differential targeting by afferent projections of distinct neuronal subpopulations in the BLA with specific temporal activity patterns. A recent study showed that a set of the basal nucleus (BA) neurons respond to the CS with the increased firing rate during fear memory retrieval (“fear neurons”), whereas a different, anatomically segregated population of BA neurons is activated during recall of extinction memory, i.e. they represent a distinct class of “extinction neurons”. Importantly, differences in the firing activity of these neuronal populations may predict the behavioral outcome. Thus, the increased firing of “extinction neurons” coupled with the decreased activity of “fear neurons” results in a reduction of freezing responses. Additionally, the hippocampus projects to “fear neurons” only, whereas “extinction neurons” are reciprocally connected with the mPFC (Herry et al., 2008). Therefore, IL may influence the neuronal activity in the CeA via “extinction neurons”, which can drive inhibitory CeL neurons (e.g., “fear-off”, PKCδ-positive cells) or mITC, or supress the activity of BA “fear neurons”. To clarify how extinction learning changes the signal flow in mPFC-amygdala projections, we combined ex vivo optogenetics with slice electrophysiology from behaviorally trained mice (Cho et al., 2013). Extinction learning was associated with decreased excitatory glutamatergic neurotransmission at mPFC-BLA synapses (both IL and PL have been activated), whereas feedforward GABA-mediated inhibition in these projections remained intact. The synaptic strength of mPFC projections to ITC cells as well as to BLA local circuit interneurons was unaffected by extinction training. As such, diminished excitatory drive in prefrontal projections to BLA neurons together with unchanged inhibition would result in a greater functional efficacy of inhibition in the BLA. The enhanced inhibition of BLA neurons output, combined with the retained ability of mPFC inputs to activate ITC neurons (which, in turn, project to CeA and inhibit it), would supress fear responses during CS presentation in fear-extinguished animals (Fig. 4). Moreover, activation of local GABAergic interneurons in the BLA caused frequency-dependent heterosynaptic inhibition in the pathway from the auditory cortex to the BLA (an auditory CS input), which, in turn, may diminish the CS-induced BLA activity and further supress fear expression during recall of extinction memory (Cho et al., 2013).
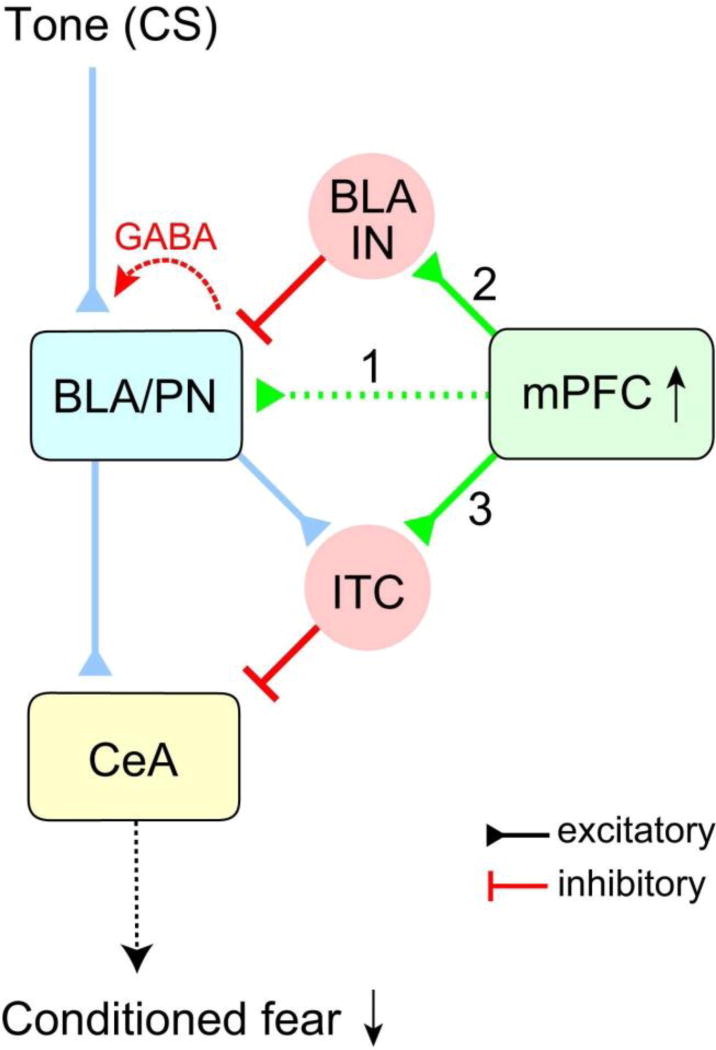
Fig. 4. The effect of fear extinction on the signal flow in mPFC-amygdala projections.
After fear extinction, the conditioned fear response is supressed by the following three mechanisms. Excitatory glutamatergic neurotransmission at mPFC synapses onto principal BLA neurons (BLA/PN) decreases (1), whereas feedforward GABA-mediated inhibition in these projections remains intact. This results in a reduced excitation/inhibition balance in the mPFC-BLA pathway, and a consequent increase in functional efficiency of inhibition in the BLA during the CS presentation. The synaptic efficacy at mPFC projections to BLA local circuit interneurons (2) as well as to ITC (3) is unaffected by extinction training. The activation of interneurons causes frequency-dependent heterosynaptic inhibition in the auditory cortex-BLA pathway (the input providing auditory CS information), also reducing fear response during the auditory CS presentation. Reproduced from Cho et al. (2013) with permission from Elsevier.
The notion that PL and IL mPFC subdivisions may contribute differentially to the control of fear-related behaviors is strongly supported by numerous previous studies using selective lesions, pharmacological inactivation, as well as in vivo electrophysiological recordings (reviewed in Maren & Quirk, 2004; Bukalo et al., 2014). It has been proposed that PL and IL may be acting antagonistically, with PL being implicated in control of fear mechanisms, whereas IL may contribute to control of fear extinction (Maren & Quirk, 2004; Herry et al., 2010; Pape & Pare, 2010; Orsini & Maren, 2012; Bukalo et al., 2014). The initial experimental support for this hypothesis was provided by the observation of increased CS-induced neuronal firing in IL during recall of fear extinction memory in rodents (Milad & Quirk, 2002) and humans (Phelps et al., 2004). However, the specific experimental targeting IL or PL, respectively, is technically challenging. Optogenetic techniques brought the desired capability for nuanced and precise spatiotemporal manipulations of IL or PL activities, and several studies employed these methods to test the respective contributions of either IL or PL in control of fear extinction. For instance, the manipulation of neuronal activity in IL during extinction training was shown to affect the formation of extinction memory. Optogenetic activation (with ChR2) or inhibition (with eNpHR) of IL neurons during extinction has opposite effects on freezing response assessed the following day. (Do-Monte et al., 2015). Specifically, photostimulation of ChR2-expressing IL mPFC inputs to the amygdala during extinction training facilitated the formation of extinction memory, whereas silencing by green light of ArchT-expressing IL inputs increased the freezing response, suggesting a deficit in extinction memory (Bukalo et al., 2015). Surprisingly, silencing of excitatory IL neurons with eNpHR (Do-Monte et al., 2015) or their projections to the amygdala with ArchT (Bukalo et al., 2015) during retrieval of extinction memory had no effect on freezing responses. One possible explanation for these unexpected observations might be the insufficient silencing of the IL (Do-Monte et al., 2015) or the importance of mPFC projections outside the amygdala in extinction retrieval (Bukalo et al., 2015). In support of the former, less than 50% of neurons have, in fact, exhibited decreased firing rates upon photostimulation of the eNpHR-expressing IL (Do-Monte et al., 2015). Another recent study suggested an alternative explanation as to why optogenetic silencing of IL might not affect extinction retrieval (Kim et al., 2016). In this work, photoinhibition of both excitatory neurons and inhibitory interneurons within the IL (by expressing eNpHR under control of the pan-neuronal hSyn promoter) during extinction retrieval resulted in impaired expression of fear extinction, supporting a previously proposed role of the IL in recall of extinction memory. However, optogenetic silencing of IL glutamatergic neurons alone (using eNpHR under the CaMKIIα promoter) did not produce similar effects on extinction. Notably, photostimulation-induced activation of IL excitatory neurons expressing ChR2 at the time of extinction retrieval was sufficient to enhance expression of fear extinction memory in both studies (Do-Monte et al., 2015; Kim et al., 2016). Based on these data, it has been proposed that both excitatory and GABAergic IL cells contribute to expression of fear extinction (Kim et al., 2016). It remains possible that IL interneurons may participate in extinction retrieval either via control of short-range projections to PL area or long-range projections to the BLA (Kim et al., 2016). Indeed, GABAergic projections from mPFC to subcortical structures, including the BLA, have been described recently (Lee et al., 2014). From a technical point of view, it remains unclear what proportion of excitatory and inhibitory IL neurons was silenced when the hSyn promoter was used. Whilst high titer AAV-hSyn infects both cortical excitatory and inhibitory neurons, lower titers, somewhat unexpectedly, preferentially transduce inhibitory neurons (Nathanson et al., 2009). Overall, additional experimental work might be needed to clarify the respective IL and PL mPFC contributions in encoding and recall of extinction memory.
Notably, not only changes in direct mPFC projections to the amygdala underlie mPFC-mediated mechanisms of extinction. Thus, Senn et al. (2014) evaluated whether BA neurons with distinct projection patterns to the mPFC are differentially recruited during fear conditioning and extinction. Fear conditioning activated c-fos expression (a commonly used marker of neuronal activation) primarily in PL-projecting BA neurons, whereas extinction triggered c-fos expression almost exclusively in IL-projecting BA neurons. This result was confirmed with in vivo recordings from optogenetically identified IL- and PL-projecting neurons during discriminative fear conditioning and extinction training (Senn et al., 2014). Specifically, the authors used the 2A peptide bridge as a tool for coexpression of ChR2 and NpHR (Tang et al., 2009), allowing bidirectional control of the activity in these subpopulations of neurons. By injecting a Cre-dependent virus to express opsins into BA and retrogradely transported viruses expressing Cre-recombinase into either IL or PL, they limited the expression of opsins specifically to IL- or PL-projecting BA neurons only (Senn et al., 2014). Using this approach, the authors showed that functionally characterized fear and extinction neurons (Herry et al., 2008) overlap with anatomically identified PL- and IL-projecting neurons, respectively. Whereas optogenetic inhibition of IL-projecting BA neurons impaired the retention of extinction memory, silencing PL-projecting cells produced an opposite outcome and promoted extinction. Interestingly, these optogenetic manipulations had no effect on the acquisition of extinction memory (within-session extinction) (Senn et al., 2014). In support of the modulatory roles of neural circuits within the mPFC in fear control, optical activation of IL neurons inhibited PL neurons with no effect on IL interneurons, suggesting that IL may also contribute to the impaired fear expression by downregulating PL activity (Ji & Neugebauer, 2012). Additionally, another study, using PV-IRES-Cre mice in combination with stereotaxic injections of AAV encoding Arch in the dorsomedial PFC (PL and Cg1 subregion of the anterior cingulate cortex), showed that, following fear extinction, the presentation of the tone CS together with optical inhibition of parvalbumin-positive (PV+) interneurons reinstated extinguished fear responses (Courtin et al., 2014).
Thus, whereas previous studies concentrated on understanding how mPFC projections to the BLA impact fear extinction, there is presently evidence that both BLA inputs to mPFC subdivisions and distinct connections within mPFC may play prominent roles in control of extinction-related behaviors. The previously mentioned studies indicate that while the same overlapping structures may be recruited in fear acquisition and fear extinction, there are different subsets of neurons and highly specific neuronal pathways mediating the fine balance of activities within the implicated neural networks, allowing reliable control of different aspects of fear-related behavior. These early optogenetic experiments emphasize how the appropriate techniques could help to elucidate how diverse structural components and pathways contribute to control of complex behavioral processes.
4. Molecular identity of neuronal populations modulating fear
An important mechanistic insight into the pathways of fear modulation is provided by recent optogenetic studies identifying different subpopulations of neurons in both BLA and CeA with distinct molecular biological characteristics which play opposite roles in fear learning and fear extinction. Thus, photostimulation of Thy1-expressing glutamatergic neurons within the BLA of Thy1-ChR2 mice during fear/extinction training led to impaired fear learning, but strengthened fear extinction retrieval, respectively, via inhibition of a subpopulation of neurons in the CeM (Jasnow et al., 2013). Optogenetic silencing of Thy1 neurons in Thy1-eNpHR mice during fear conditioning or fear extinction resulted in enhanced fear expression, but suppressed consolidation of fear extinction (McCullough et al., 2016). BLA Thy1-positive cells seem to represent a population of extinction neurons (Herry et al., 2008) that are selectively recruited during extinction training and extinction recall (Jasnow et al., 2013; McCullough et al., 2016). Furthermore, using RNA sequencing followed by pharmacological interventions, McCullough and colleagues identified neurotensin receptor 2 (NTSR2) as another potential molecular marker which could be used for the identification of Thy1-expressing BLA extinction neurons (McCullough et al., 2016).
Different subpopulations of neurons, which are characterized by specific molecular markers and allow modulation of conditioned fear, were recently identified in the CeA. Thus, in the CeL, which is a subdivision of the CeA, there are two functionally distinct populations of GABAergic neurons which exhibit either increased (CeLon cells) or decreased (CeLoff neurons) firing rates in response to the CS presentation (Ciocchi et al., 2010; Haubensak et al., 2010). CeLoff neurons, which inhibit output neurons in the CeM, regulate conditioned fear responses and overlap with a genetically defined neuronal population expressing protein kinase Cδ (PKCδ+ neurons) (Haubensak et al., 2010). In addition, spontaneous tonic activity of CeLoff neurons increased following fear conditioning, specifically with fear generalization in animals that exhibited poor discrimination between an auditory tone CS+ (paired with a foot shock) and a neutral CS−. In agreement with this finding, optogenetic stimulation in PKCδ-Cre animals infected with DIO-AAV-ChR2 during fear memory retrieval increased fear generalization (Botta et al., 2015). Conversely, spontaneous firing of CeLon cells showed almost no change following fear conditioning (Ciocchi et al, 2010). A recent study, in which the authors differentiated between neuronal populations of the CeA based on the gene expression pattern (with single-molecule fluorescence in situ hybridization (smFISH) of genes expressed in the CeA) in specific CeA subregions (Kim et al., 2017), suggests a more complex arrangement demonstrating the differences between PKCδ+ neurons in capsular nucleus of the CeA (CeC) and CeL. Specifically, c-fos expression was increased in CeC and CeL PKCδ+ neurons in response to footshocks/contextual fear recall and contextual fear extinction, respectively. The latter agrees with the proposed function of CeL PKCδ+ cells in inhibiting the defensive freezing response (Haubensak et al., 2010). In contrast, stimulation and inhibition of CeC PKCδ+ neurons (using ChR2 or Arch, respectively) increased freezing in a neutral conditioning chamber and reduced freezing (by ~15%) during contextual fear conditioning. Furthermore, on the following day during fear recall with no light inhibition, freezing remained reduced (by ~30%; Kim et al., 2017). Thus, PKCδ+ neurons in CeC show properties similar to those of previously identified fear-promoting fear-on cells (Ciocchi et al., 2010).
The role of PKCδ+ cells in innate anxiety-like behaviors is not completely elucidated. In keeping with the effect of optogenetic activation of PKCδ+ neurons on fear generalization, Botta et al (2015) demonstrated that optogenetic manipulation of spontaneous activity of PKCδ+ cells bidirectionally controls anxiety assessed in the elevated plus maze (EPM) and open field test (OPT): activation (ChR2) of PKCδ+ neurons had an anxiogenic effect, whereas yellow light (Arch) stimulation was anxiolytic (Botta et al., 2015). In contrast, a previous study showed an anxiolytic effect of optogenetic activation of CeL PKCδ+ cells in three different assays of anxiety, namely the EPM, OPT and light-dark box test (Cai et al., 2014).
In addition to PKCδ+ neurons, a population of somatostatin-positive (SOM+) neurons has been identified in the CeL. SOM+ and PKCδ+ cells are largely non-overlapping (only ~13% of SOM+ neurons express PKCδ; Li et al., 2013; ~2% when assessed with smFISH; Kim et al., 2017). Recent studies suggest SOM+ cells, at least to a certain degree, represent the previously described CeLon cells, exhibiting an increase in firing in response to the CS presentation. Thus, SOM-positive CeL neurons were preferentially activated during fear conditioning in response to the CS and their activation was both necessary and sufficient for acquisition of fear memories and fear expression (Li et al., 2013; Penzo et al., 2014; Penzo et al., 2015). Moreover, by using fiber-optic photometry combined with optogenetic and molecular techniques in behaving mice, Yu at al. (2016) demonstrated activation of SOM+ CeL neurons by threat-predicting sensory cues following fear conditioning (Yu et al., 2016).
A subpopulation of SOM+ CeL neurons sends long-range projections to extra-amygdala structures such as the PAG (among PAG-projecting neurons, the vast majority (~80–90%) are SOM+, <20% are PKCδ+ cells) and/or paraventricular nucleus of the thalamus. Fear conditioning potentiated excitatory synaptic transmission onto these long-range projecting neurons. This can directly disinhibit PAG output following fear conditioning (via CeA-mediated inhibition of GABAergic PAG cells leading to increased activity of glutamatergic PAG neurons; Tovote et al., 2016) and thereby drive fear expression by bypassing the classical CeL–CeM–PAG pathway (Penzo et al., 2014).
The tachykinin 2 (Tac2) gene, encoding neuropeptide neurokinin B (NkB), is specifically expressed in the CeA (with the highest expression level in the CeM), and marks another subpopulation of PKCδ− cells (only 6.4% of Tac2 neurons express PKCδ). Chemogenetic silencing of Tac2-expressing neurons (using Designer Receptors Exclusively Activated by a Designer Drug, DREADD) impaired fear consolidation, whereas optogenetic activation of Tac2+ neurons during fear acquisition enhanced consolidation of fear memory (Andero et al., 2014; Andero et al., 2016). Such studies could be expanded to evaluate the roles of these recently identified neuronal types located within the CeA in fear extinction as well.
Recent studies showed that distinct neuronal populations within CeA can be activated by CSs of different types and intensities. This may contribute to coding a threat’s proximity and guide transitions between appropriate active (flight) and passive (freezing) behavioral responses as well as the switch from discriminative to generalized fear (Fadok et al., 2017; Sanford et al., 2017). Corticotropin-releasing factor (CRF)-expressing neurons represent another distinct subpopulation showing little or no overlap with PKCδ+ and SOM+ CeL neurons as identified by immunostainings (Fadok et al., 2017; Sanford et al., 2017). However, a recent study using analysis of genes coexpression showed that ~70% of CRF neurons coexpress SOM gene when assayed with smFISH (Kim et al., 2017). Following fear conditioning to weak threats (low intensity US), CRF neurons undergo plastic changes and selectively respond to threat-predicting stimuli CS+ (fear-on cells). Release of the neuropeptide CRF facilitates such fear acquisition, but not expression, by enhancing excitatory synaptic transmission onto local CRF receptor 1-expressing neurons in LA-CeA pathway, including SOM+ neurons (Sanford et al., 2017). Fadok et al. (2017) showed that CRF cells mediate active behavioral responses to a threatening stimulus (conditioned flight), whereas activation of SOM+ neurons initiates passive freezing behavior by using fear conditioning protocols (high intensity US) to a serial compound auditory stimulus (a pure tone followed by white noise). This allows an analysis of both conditioned freezing (in response to a tone) and flight behavior (during the white noise) in the same animal. Notably, CRF+ and SOM+ cells reciprocally inhibit each other, allowing a rapid switch between active and passive fear responses. In agreement with this, optogenetic activation of ChR2-expressing CRF+ neurons during tone exposure decreased freezing and increased flight response, while activation of SOM+ neurons showed an opposite effect (Fadok et al., 2017). Based on these two studies, the CRF+ neurons appear to fine tune the CeA network by maintaining a delicate balance between direct GABAergic inhibition of SOM+ neurons (Fadok et al., 2017) and CRF-mediated modulation of excitatory synaptic transmission from LA to SOM+ neurons and other cell types in the CeA (Sanford et al., 2017), optimizing the signal flow within the CeA and its interactions with other components of behavior-controlling microcircuits.
5. Control of innate fear-related behavioral processes
The intrinsic ability of organisms to detect naturally threatening situations and respond to them accordingly is universal across species, including humans (e.g., Kendler et al., 2002). As opposed to learned fear, which may reflect individual fearful experiences throughout the subject’s lifetime, innate fear does not directly implicate learning mechanisms and can be triggered by threats, the behavioral sensitivity to which was acquired evolutionarily over many pre-existing generations. However, unlike neural substrates and circuit mechanisms of fear learning, the neural circuits and mechanisms of innate fear are still poorly understood.
5.1. Predator odor-induced fear
Predators and/or predator-like sensory cues induce fear and anxiety-like behaviors in rodents raised in the laboratories with no exposure to natural environmental threats for generations (Rosen, 2004; Rosen et al., 2008), emphasizing the evolutionary selected, instinctive nature of such behaviors. Recently, circuits underlying innate fear and threat detection have captured more attention, with particular emphasis on fear responses to predator odor as the dominant model used in the laboratory settings (Apfelbach et al., 2005; Takahashi et al., 2005; Rosen et al., 2015; Pereira & Moita, 2016). Putative neural pathways contributing to predator odor-induced innate fear have been previously described (Apfelbach et al., 2005; Takahashi et al., 2005; Rosen et al., 2008; Takahashi, 2014; Rosen et al., 2015; Pereira & Moita, 2016), with the medial nucleus of the amygdala (MeA) emerging as a key player in these behavioral processes. Notably, this region receives direct projections from the olfactory system, the bed nucleus of the stria terminalis (BNST) and ventral hippocampus (VHC). Additionally, some predator odors also activate the BLA, medial hypothalamic nuclei and mPFC. Furthermore, the dorsal periaqueductal gray (dPAG) and the hypothalamic paraventricular nucleus (PVN) are important for activation of the autonomic nervous system and the hypothalamic pituitary adrenal axis (HPA) by predator odors. However, most early studies relied on pharmacological inactivation and lesion approaches (Fendt et al., 2003; Li et al., 2004; Blanchard et al., 2005; Fendt et al., 2005; Pentkowski et al., 2006; Xu et al., 2012) or on the analysis of Fos expression (Dielenberg et al., 2001; Day et al., 2004; Perez-Gomez et al., 2015). Therefore, information about the functional inter-regional connectivity in diffused networks mediating innate fear is essentially lacking (Rosen et al., 2015). New studies using modern tracing and optogenetic methods shed light on these issues.
Recent experiments, combining in vivo optogenetics, behavioral assays and electrophysiology, identified new components in the predator odor-induced fear circuits. Thus, the laterodorsal tegmentum (LDT) was determined to be a key structure in the brain underlying innate fear induced by olfactory predator-like stimuli (Yang et al., 2016). Furthermore, there are distinct cell-type specific populations within the LDT promoting (PV+ interneurons) or suppressing (SOM+ cells) fear. The latter most likely occurs via disinhibition of fear-promoting PV+ neurons (Yang et al., 2016). Root and coauthors have recently developed an open field behavioral assay to test innate behavioral responses to different odors (avoidance versus attraction). In their experiments, 2,4,5-trimethyl-3-thiazoline (TMT), a widely used component of fox odor, not only evoked avoidance, but also caused a pronounced decrease in locomotor activity. This may represent a correlate of freezing and, therefore, a form of the innate fear response. Whereas the authors observed activity-dependent expression of ChR2 in the cortical amygdala, as well as neighboring areas of the piriform cortex and MeA following exposure to TMT, optogenetic activation of the cortical amygdala neurons alone (through an implanted optical fiber) recapitulated the avoidance and freezing behaviors similar to that induced by TMT (Root et al., 2014). To date, there is no direct experimental data addressing the downstream targets of the cortical amygdala that may underlie the observed behavior phenotype.
Unfortunately, the use of predator odor often gives inconsistent results that vary significantly depending on experimental conditions (e.g., the type of predator odor and behavioral chamber parameters) (Wallace & Rosen, 2000; McGregor et al., 2002; Blanchard et al., 2003; Fendt, 2006; Endres & Fendt, 2007; Rosen et al., 2008). Additionally, the parameters of the stimulus (odor), especially temporal and spatial characteristics of odor delivery, are difficult to control. This, for example, complicates the interpretation of habituation studies which also demonstrate mixed results, possibly due to differences in the odor intensity used in experiments (Takahashi et al., 2005). Furthermore, some of the previous studies did not observe freezing behavior in response to olfactory stimuli; therefore, fear responses were assessed by testing avoidance or physiological responses (e.g., measuring cardiovascular changes), which may show significant variability (Dielenberg et al., 2001; Fendt, 2006). Freezing can only be induced by odors in small confined test chambers (Wallace & Rosen, 2000; Rosen et al., 2008). Recently, by optimizing the chemical structure of TMT, Isosaka and coauthors designed an odorant which is capable of inducing freezing responses comparable to those observed during classical fear conditioning (Kobayakawa & Kobayakawa, 2011; Isosaka et al., 2015). The efficient and reproducible behavioral paradigms coupled with emergent methodologies are expected to facilitate understanding of the mechanisms of odor-induced innate fear in the near future.
5.2 Visual predator-induced fear
The advantages of cutting-edge techniques led to comprehensive studies of neural pathways mediating innate fear responses triggered by visual predator cues (Pereira & Moita, 2016). In laboratory settings, predator-like overhead visual cues induced innate defensive responses such as escape behavior or freezing in rodents (Yilmaz & Meister, 2013; Wei et al., 2015; De Franceschi et al., 2016). Unlike experiments with predator odor, the use of visual stimuli provides an opportunity to modulate behavioral outcome with precise temporal and spatial control, as well as with an adjustable range of stimulus parameters such as brightness, speed, contrast, and trajectory (Yilmaz & Meister, 2013). Interestingly, a recent study suggested that the visual stimuli with different parameters may induce different innate fear behaviors: a small moving disk, simulating a predator cruising overhead, generated a freezing response, whereas a shadow rapidly approaching from above (looming stimuli) produces flight responses (De Franceschi et al., 2016).
By using in vivo optogenetics and electrophysiology in combination with trans-synaptic viral tracing, Wei and coauthors identified a disynaptic circuit, consisting of projections from a subpopulation of glutamatergic neurons within the superior colliculus (SC, the medial region of the intermediate layers) to LA via the lateral posterior nucleus of the thalamus, underlying innate defensive behaviors elicited by visual predator cues in mice (Wei et al., 2015). Interestingly, another study identified PV+ excitatory neurons within the SC (mostly distributed in the superficial layer) as the primary neurons responsible for detection of looming stimuli that subsequently transmit innate fear-related visual cues to the CeA via the parabigeminal nucleus (Shang et al., 2015). Together, these two studies present an interesting example of the existence of parallel cortex-independent pathways for processing of innately aversive threats (Yuan & Su, 2015). However, it remains to be determined whether such pathways act in parallel or independently and how the modality of stimuli is coded (e.g., neutral vs. aversive stimuli).
Wei et al. (2015) did not detect any increases in c-fos expression in SC superficial layers after the presentation of visual stimuli approaching from above, which should be apparent based on the study of Shang et al. (2015). It is possible that the total number of PV+ neurons might be too low (representing only ~13% of SC neurons) to detect an increase in c-fos expression in these cells, or that transient activation of these neurons is not sufficient to induce c-fos expression, or, finally, that activation of different pathways could be attributed to specific characteristics of looming stimuli used in the experiments (Yilmaz & Meister, 2013). Indeed, Shang et al. used different visual stimuli with somewhat less naturalistic parameters, as an increase in firing rate of PV+ neurons was detected in response to collision threats (0.5–1.5 s before collision with a virtual soccer ball flying toward the eye) in anesthetized animals. Furthermore, in these experiments, initial optogenetic stimulation of SC induced immediate prolonged freezing, while activation of PV+ neurons manifested in impulsive escaping followed by long-lasting freezing, suggesting that other cells in the SC (possibly in intermediate layers) might promote freezing over escape during unspecific (in terms of the cell type) optogenetic activation of SC. Notably, both studies reported habituation of observed behaviors with repetition (Shang et al., 2015; Wei et al., 2015). Unlike neurons in SC, LA neurons exhibited adaptation in their firing rates with repetition which might underlie, at least in part, behavioral habituation (Wei et al., 2015).
However, the mentioned two studies are lacking inactivation experiments (Shang et al., 2015; Wei et al., 2015). Ideally, in order to provide evidence that a specific projection is implicated in control of a behavioral response, it is necessary to demonstrate the suppression of behavioral readouts (in this case, innate fear responses to looming visual stimuli) by blockade of neurotransmission between components of the studied pathways. Wei et al. (2015) showed that optogenetic silencing of ILSCm cell bodies suppresses the expression of freezing induced by threatening visual stimuli. However, when spike generation was suppressed in cell bodies, all SC efferent projections were affected, including those laying outside the studied pathway, which still could be implicated in control of innate defensive responses.
5.3. Innate fear responses triggered by stimuli of different sensory modalities and fear learning
In summary, converging evidence indicates that distinct neuronal circuits may be involved in control of innate fear induced by stimuli of different sensory modalities. Apparently, there are multiple, parallel neural mechanisms for detection of unimodal threat cues triggering innate fear responses (Perez-Gomez et al., 2015; Shang et al., 2015; Wei et al., 2015). The amygdala, with its numerous interconnected nuclei, could be a site where the information about stimuli of different sensory modalities converges and is subsequently integrated (Pereira & Moita, 2016). Whereas the role of the amygdala as a hub for the integration of different sensory signals in relation to control of fear behavior is well supported, a recent study described a pathway underlying innate sound-induced flight behavior which does not involve the amygdala (Xiong et al., 2015). Using both optogenetic activation and silencing of axon terminals, the authors showed that the auditory cortex may drive this innate fear-related behavior via the corticofugal projections to the cortex of the inferior colliculus, which, in turn, connects directly to the PAG, initiating the defensive flight response (Xiong et al., 2015).
The use of non-painful, predator-like stimuli as the US in studies of threat (fear)-conditioning and extinction could be more relevant for the understanding of neuronal mechanisms underlying emotional learning and memory (Takahashi et al., 2008). In several studies predator odor or exposure to a predator have been used in both cued and contextual fear conditioning, followed by extinction (Blanchard et al., 2001; Dielenberg et al., 2001; Do Monte et al., 2008; Takahashi et al., 2008; Clay et al., 2011), However, the interpretation of these experiments was also marred, as described above, by problems with spatiotemporal control of odor delivery.
Nevertheless, conditioning to innately aversive stimuli may provide critical insights into the principles of how distinct neural pathways, guiding learned and innate fear, may interact in order to achieve certain behavioral outcomes (Gross & Canteras, 2012; LeDoux, 2012). The first steps in this direction were taken recently, and the experimental findings revealed the existence of an unexpected hierarchy between innate and learned fear-related responses. This study tested the relationship between olfactory-induced learned and innate fear (Isosaka et al., 2015). By applying whole brain mapping and pharmacological screening, it demonstrated that a population of serotonin 2A receptor (Htr2a)-expressing cells are mainly located in the CeL, with the majority of these cells being SOM+, but PKCδ-negative. Chemogenetic (with DREADD) or optogenetic silencing of CeA Htr2a cells upregulated innate freezing responses and downregulated learned freezing responses. Conversely, chemogenetic or optogenetic activation of these cells diminished the innate freezing response with no effect on learned freezing. Moreover, activation of Htr2a cells reversed the suppressing effect of prior presentation of innate fear odorant on learned freezing behavior. By analyzing expression of immediately early genes (IEGs), this study demonstrated that, downstream of the amygdala, odor-induced innate and learned freezing behaviors are regulated in different subnuclei of the PAG (dPAG and vPAG, respectively). Thus, the presentation of an innate fear-inducing odorant led to inactivation of CeA Htr2a+ cells, which, in turn, increased the IEG expression in the dPAG and decreased the IEG expression in the vPAG. Through these mechanisms, the innate fear-associated freezing responses might be prioritized over any learned fear-associated freezing responses (Isosaka et al., 2015).
6. Emerging techniques and future directions
Optogenetics brought unique opportunities for precise spatiotemporal control of neuronal activity which was hitherto not possible. However, certain limitations persist, many of which have already been highlighted in recently published review articles (Zalocusky et al., 2013; Goshen, 2014; Häusser, 2014; Guru et al., 2015). Nevertheless, constant technical improvements resulted in steady methodological progress in the field by opening up new experimental possibilities and finding ways to overcome the previous limitations. We described some of the methodological pitfalls associated with optogenetic approaches and their suitable solutions (e.g., non-uniform expression of opsins with viral transduction can be circumvented by the use of transgenic mice with genetically encoded photosensitive proteins). Overall, depending on the experimenter’s needs, numerous optogenetic tools are available now, which can be adapted for specific goals of the study.
One of the current limitations of in vivo optogenetics is its inherent invasiveness. To control neuronal activity of a defined cell population, light has to physically reach these cells. Even when red-shifted opsins are used, the depth of light penetration is only several millimetres below the brain’s surface (Lin et al., 2013; Chuong et al., 2014). Therefore, for optogenetic manipulations of deep brain structures such as the amygdala in behaving animals, chronically implanted optical fibers have been used (Sparta et al., 2011). Usually, this limits the delivery of light to a single target within the brain, located immediately below the tip of the photostimulating fiber. Thus, optical fibers in combination with a bevelled guide cannula have been used to achieve preferential illumination of BLA terminals in the CeA, but not BLA cell bodies (Tye et al., 2011). In addition, the probes for delivery of light to multiple brain targets and distributed brain circuits have also been created (Zorzos et al., 2010; Zorzos et al., 2012). A significant step forward was the development and implementation of wireless injectable microscale inorganic light-emitting diodes (μ-ILEDs), comparable to the size of neurons (Kim et al., 2013; McCall et al., 2013). They are minimally invasive and may be more optimal for the spatially controlled manipulations of neuronal activity in vivo. Recently, an alternative solution, eliminating the need for the external light source, has been proposed. Specifically, intraperitoneal administration of luciferin (a compound emitting yellow light upon chemical reaction catalyzed by luciferase) inhibited neuronal firing as well as c-fos expression in the striatum and affected locomotor behavior in mice expressing virally transduced luciferase and eNpHR3.0 genes in the striatum (Land et al., 2014). These new approaches have significant potential, allowing more targeted manipulations of neuronal circuitries during complex behavioral paradigms within more natural environments. Furthermore, technological developments in the field of nanoscience are opening multiple avenues for controlling neuronal activity. For instance, gold nanoparticles, conjugated to high-avidity ligands, convert absorbed light energy into heat, which is followed by changes in membrane capacitance, leading to cellular depolarization and action potential firing (Carvalho-de-Souza et al., 2015). Minimally invasive nanoscale syringe-injectable mesh electronics developed in the Charles Lieber’s lab at Harvard (Hong et al., 2015; Liu et al., 2015) allow not only chronic recordings of activity from same neurons over long periods of time (up to 8 months), but also their chronic electric stimulation (Fu et al., 2016). The latter technique has a serious potential as a possible alternative to optogenetics, allowing easier access for manipulations and recordings from deep brain regions, such as the amygdala, hypothalamus and striatum, as well as permitting studies of different brain regions simultaneously (e.g., spatiotemporal patterns of their activity and their synchrony). However, at this current stage, nanomeshes cannot be used to manipulate the function of specific, genetically defined neuronal populations, which represents a major advantage of optogenetic techniques.
As light-induced neuronal firing patterns in optogenetic experiments are likely to be non-physiological, one desirable future direction might be closed-loop control of neural circuits. Optogenetic manipulation of neuronal activity could be based on feedback from simultaneous readouts of their activity in the course of behavioral tasks (Goshen, 2014; Häusser, 2014; Grosenick et al., 2015). To date, few studies successfully implemented a closed-loop optogenetic control, when photostimulation was automatically triggered based on the difference between desired and measured output, received from behavioral or electrophysiological readouts of activity (reviewed in Grosenick et al., 2015). In addition, all-optical, closed-loop control (combining expression of calcium or voltage sensors with opsins in the same neurons), although not yet implemented, could be possible in the near future (Grosenick et al., 2015; Emiliani et al., 2015).
At this stage, the studies of fear circuits and their modulation have adapted a limited range of optogenetic tools, mostly using brain region or projection specific targeting of activatory or inhibitory opsins. The ever-expanding optogenetic toolbox in combination with molecular biological techniques provides exciting new opportunities such as: the bidirectional control of neuronal activity (Tang et al., 2009; Kleinlogel et al., 2011), simultaneous projection-based targeting of different neuronal subpopulations (Fenno et al., 2014), targeting opsins not only to genetically identical cells, but to populations of cells activated during different behaviors (e.g., fear learning vs. fear extinction, innate vs. learned fear) (Sakurai et al., 2016), and manipulation of activity of specific receptor subtypes (Kramer et al., 2013). The studies of neural circuits underlying innate fear using advanced newly-developed techniques are still in their infancy, concentrating on the identification of specific cell populations and their interactions. Future experiments are needed in order to elucidate the mechanisms controlling innate fear, so that it can be modulated or suppressed when aberrantly expressed (e.g., in animal models of anxiety disorders). Thus, studies of fear circuits, engaging next generation optogenetic and other tools, have the capacity to uncover the fundamental mechanisms of complex behavioral processes, as exemplified by the mechanisms of fear-related behavior.
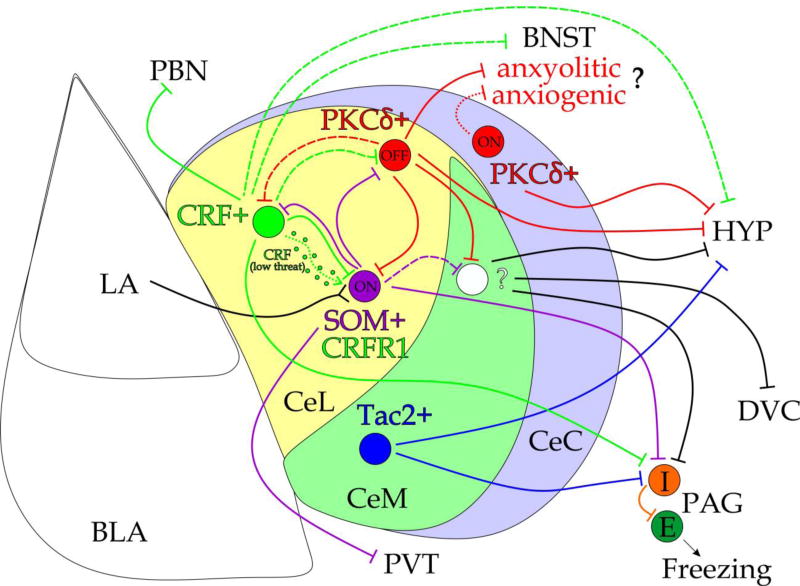
Fig. 5. Distinct neuronal populations in the CeA and corresponding circuitry implicated in fear and anxiety-related behaviors.
To promote or diminish fear and anxiety-related behaviors, distinct neuronal populations of the basolateral amygdala, BLA (including lateral amygdala, LA) activate diverse neuronal subpopulations within the lateral part of the central amygdala (CeL). There are two functionally different populations of neurons in the CeA, organized into inhibitory microcircuits: fear promoting fear-on (ON) and fear-inhibiting fear-off (OFF) cells, which respond to a CS following fear conditioning with increased and decreased firing, respectively (Ciocchi et al., 2010). Recent studies demonstrate that these functional ON and OFF units comprise distinct neuronal populations identified by specific molecular markers with differential projection patterns and roles in fear-related behaviors. CeL PKCδ+ cells represent fear-off cells and supress freezing responses by inhibiting PAG-projecting CeM cells (Ciocchi et al., 2010; Haubensak et al., 2010). PKCδ+ neurons project strongly to the BNST (Oh et al., 2014; Sanford et al., 2017). The role of PKCδ+ cells and their projections in the control of innate anxiety-related behaviors still needs further elucidation (Cai et al., 2014; Botta et al, 2015). In contrast, PKCδ+ cells in the CeC show characteristics of an opposing population of fear-on cells with their activation promoting freezing responses (Kim et al., 2017). SOM+ CeL neurons represent fear-on cells and are critical for fear acquisition and fear expression (Li et al., 2013; Penzo et al., 2014; Penzo et al., 2015; Yu et al., 2016). SOM+ cells do not directly inhibit CeM (15% of CeM-projecting cells are SOM+; Li et al., 2013) and promote freezing via their long-range projections to PAG (Penzo et al., 2014). CRF+ neurons are also activated in response to a CS (as fear-on cells; Sanford et al., 2017), but display a more complex modulatory function in the CeA, depending on the characteristics of the CS and US (Fadok et al., 2017; Sanford et al., 2017). While CRF+ neurons form reciprocal inhibitory connections with both PKCδ+ and SOM+ cells, they connect preferentially to the latter (Fadok et al, 2017). At high levels of threat (high intensity US) and/or for some CS parameters (e.g., in response to white noise), CRF+ network promotes conditioned flight, by directly inhibiting SOM+ cells, encoding freezing behavior (Fadok et al, 2017). At low levels of threat, CRF+ cells facilitate fear learning and promote freezing by releasing neuropeptide CRF, which acts on CRFR1-expressing cells in the CeA (in the medial and caudal CeA ~60% of CRFR1+ cells are SOM+ and ~20% are PKCδ+; Sanford et al., 2017). In addition to their strong projections within the CeA, CRF+ neurons also project to the hypothalamus, BNST, PAG and PBN (Oh et al., 2014; Fadok et al, 2017; Sanford et al., 2017). Tac2+ neurons depict a non-overlapping population of neurons in the CeM (in the CeL ~90% of Tac2+ neurons coexpress CRF gene; Kim et al., 2017), implicated in fear consolidation (Andero et al., 2014; Andero et al., 2016). According to the projection data from the Allen Brain Institute Mouse Connectivity Atlas (Oh et al., 2014), these neurons preferentially project to the HYP and PAG. LA, lateral amygdala; BLA, basolateral complex of the amygdala; CeL, lateral central nucleus of the amygdala; CeM, medial subdivision of central nucleus of the amygdala; CeC, capsular nucleus of the central amygdala; PBN, parabrachial nucleus; BNST, the bed nucleus of the stria terminalis; DVC, dorsal vagal complex; HYP, hypothalamus; PAG, periaqueductal gray of the midbrain; PVT, paraventricular nucleus of the thalamus; I, inhibitory GABAergic neuron; E, excitatory glutamatergic cell. Neuronal populations expressing following markers: PKCδ, protein kinase Cδ; SOM, somatostatin; CRF, corticotropin-releasing factor; CRFR1, corticotropin-releasing factor receptor 1; Tac2, tachykinin 2.
Highlights.
Overview of recent developments in optogenetic tools and emerging techniques
Review of the latest studies exploring fear circuits using these techniques
Emphasis on neural circuits of both learned and innate fear and their modulation
Acknowledgments
We thank Vernon Clarke and members of the laboratory for help and constructive discussions. This work was supported by a grant (R21MH108022) from NIMH to V.Y.B. and a grant (R01MH105851) from NIMH to V.Y.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450(7168):420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K. Temporally precise in vivo control of intracellular signalling. Nature. 2009;458(7241):1025–1029. doi: 10.1038/nature07926. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez RP, Johnson L, Grillon C. Contextual-specificity of short-delay extinction in humans: renewal of fear-potentiated startle in a virtual environment. Learn Memory. 2007;14:247–253. doi: 10.1101/lm.493707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andero R, Daniel S, Guo J-D, Bruner RC, Seth S, Marvar PJ, Rainnie D, Ressler KJ. Amygdala-Dependent Molecular Mechanisms of the Tac2 Pathway in Fear Learning. Neuropsychopharmacology. 2016;41:2714–2722. doi: 10.1038/npp.2016.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andero R, Dias BG, Ressler KJ. A role for Tac2, NkB, and Nk3 receptor in normal and dysregulated fear memory consolidation. Neuron. 2014;83(2):444–454. doi: 10.1016/j.neuron.2014.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apfelbach R, Blanchard CD, Blanchard RJ, Hayes RA, McGregor IS. The effects of predator odors in mammalian prey species: a review of field and laboratory studies. Neurosci Biobehav Rev. 2005;29(8):1123–1144. doi: 10.1016/j.neubiorev.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Arenkiel BR, Peca J, Davison IG, Feliciano C, Deisseroth K, Augustine GJ, Ehlers MD, Feng G. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron. 2007;54(2):205–218. doi: 10.1016/j.neuron.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atasoy D, Aponte Y, Su HH, Sternson SM. A FLEX switch targets Channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J Neurosci. 2008;28(28):7025–7030. doi: 10.1523/JNEUROSCI.1954-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baldi E, Bucherelli C. Brain sites involved in fear memory reconsolidation and extinction of rodents. Neurosci Biobehav Rev. 2015;53:160–190. doi: 10.1016/j.neubiorev.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Banghart M, Borges K, Isacoff E, Trauner D, Kramer RH. Light-activated ion channels for remote control of neuronal firing. Nat Neurosci. 2004;7(12):1381–1386. doi: 10.1038/nn1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belzung C, Turiault M, Griebel G. Optogenetics to study the circuits of fear- and depression-like behaviors: a critical analysis. Pharmacol Biochem Behav. 2014;122:144–157. doi: 10.1016/j.pbb.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Berndt A, Lee SY, Ramakrishnan C, Deisseroth K. Structure-guided transformation of channelrhodopsin into a light-activated chloride channel. Science. 2014;344(6182):420–424. doi: 10.1126/science.1252367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berretta S, Pantazopoulos H, Caldera M, Pantazopoulos P, Paré D. Infralimbic cortex activation increases c-Fos expression in intercalated neurons of the amygdala. Neuroscience. 2005;132(4):943–953. doi: 10.1016/j.neuroscience.2005.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanchard CD, Markham C, Yang M, Hubbard D, Madarang E, Blanchard RJ. Failure to produce conditioning with low-dose trimethylthiazoline or cat feces as unconditioned stimuli. Behav Neurosci. 2003;117:360–368. doi: 10.1037/0735-7044.117.2.360. [DOI] [PubMed] [Google Scholar]
- 15.Blanchard DC, Canteras NS, Markham CM, Pentkowski NS, Blanchard RJ. Lesions of structures showing FOS expression to cat presentation: effects on responsivity to a Cat, Cat odor, and nonpredator threat. Neurosci Biobehav Rev. 2005;29(8):1243–1253. doi: 10.1016/j.neubiorev.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 16.Blanchard RJ, Yang M, Li C-I, Gervacio A, Blanchard DC. Cue and context conditioning of defensive behaviors to cat odor stimuli. Neurosci Biobehav Rev. 2001;25:587–595. doi: 10.1016/s0149-7634(01)00043-4. [DOI] [PubMed] [Google Scholar]
- 17.Botta P, Demmou L, Kasugai Y, Markovic M, Xu C, Fadok JP, Lu T, Poe MM, Xu L, Cook JM, Rudolph U, Sah P, Ferraguti F, Lüthi A. Regulating anxiety with extrasynaptic inhibition. Nat Neurosci. 2015;18:1493–1500. doi: 10.1038/nn.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 19.Browne LE, Nunes JP, Sim JA, Chudasama V, Bragg L, Caddick S, North RA. Optical control of trimeric P2X receptors and acid-sensing ion channels. Proc Natl Acad Sci U S A. 2014;111(1):521–526. doi: 10.1073/pnas.1318582111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bukalo O, Pinard CR, Holmes A. Mechanisms to medicines: elucidating neural and molecular substrates of fear extinction to identify novel treatments for anxiety disorders. Br J Pharmacol. 2014;171:4690–4718. doi: 10.1111/bph.12779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bukalo O, Pinard CR, Silverstein S, Brehm C, Hartley ND, Whittle N, Colacicco G, Busch E, Patel S, Singewald N, Holmes A. Prefrontal inputs to the amygdala instruct fear extinction memory formation. Sci Adv. 2015;1(6) doi: 10.1126/sciadv.1500251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai H, Haubensak W, Anthony TE, Anderson DJ. Central amygdala PKC-δ(+) neurons mediate the influence of multiple anorexigenic signals. Nat Neurosci. 2014;17:1240–1248. doi: 10.1038/nn.3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carvalho-de-Souza JL, Treger JS, Dang B, Kent SB, Pepperberg DR, Bezanilla F. Photosensitivity of neurons enabled by cell-targeted gold nanoparticles. Neuron. 2015;86:207–217. doi: 10.1016/j.neuron.2015.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang C-h, Maren S. Early extinction after fear conditioning yields a context-independent and short-term suppression of conditional freezing in rats. Learn Memory. 2009;16(1):62–68. doi: 10.1101/lm.1085009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho J-H, Bayazitov IT, Meloni EG, Myers KM, Jr, W A, Zakharenko SS, Bolshakov VY. Coactivation of thalamic and cortical pathways induces input timing-dependent plasticity in amygdala. Nat Neurosci. 2012;15:113–122. doi: 10.1038/nn.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho J-H, Deisseroth K, Bolshakov VY. Synaptic encoding of fear extinction in mPFC-amygdala circuits. Neuron. 2013;80:1491–1507. doi: 10.1016/j.neuron.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chow BY, Han X, Dobry AS, Qian X, Chuong AS, Li M, Henninger MA, Belfort GM, Lin Y, Monahan PE, Boyden ES. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature. 2010;463(7277):98–102. doi: 10.1038/nature08652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chuong AS, Miri ML, Busskamp V, Matthews GA, Acker LC, Sørensen AT, Young A, Klapoetke NC, Henninger MA, Kodandaramaiah SB, Ogawa M, Ramanlal SB, Bandler RC, Allen BD, Forest CR, Chow BY, Han X, Lin Y, Tye KM, Roska B, Cardin JA, Boyden ES. Noninvasive optical inhibition with a red-shifted microbial rhodopsin. Nat Neurosci. 2014;17:1123–1129. doi: 10.1038/nn.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, Müller C, Lüthi A. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468:277–282. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- 30.Clay R, Hebert M, Gill G, Stapleton LA, Pridham A, Coady M, Bishop J, Adamec RE, Blundell JJ. Glucocorticoids are required for extinction of predator stress-induced hyperarousal. Neurobiol Learn Mem. 2011;96:367–377. doi: 10.1016/j.nlm.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 31.Conrad KL, Davis AR, Silberman Y, Sheffler DJ, Shields AD, Saleh SA, Sen N, Matthies HJG, Javitch JA, Lindsley CW, Winder DG. Yohimbine depresses excitatory transmission in BNST and impairs extinction of cocaine place preference through orexin-dependent, norepinephrine-independent processes. Neuropsychopharmacology. 2012;37:2253–2266. doi: 10.1038/npp.2012.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Courtin J, Chaudun F, Rozeske RR, Karalis N, Gonzalez-Campo C, Wurtz H, Abdi A, Baufreton J, Bienvenu TCM, Herry C. Prefrontal parvalbumin interneurons shape neuronal activity to drive fear expression. Nature. 2014;505:92–96. doi: 10.1038/nature12755. [DOI] [PubMed] [Google Scholar]
- 33.Cowansage KK, Shuman T, Dillingham BC, Chang A, Golshani P, Mayford M. Direct reactivation of a coherent neocortical memory of context. Neuron. 2014;84:432–441. doi: 10.1016/j.neuron.2014.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Day HE, Masini CV, Campeau S. The pattern of brain c-fos mRNA induced by a component of fox odor, 2,5-dihydro-2,4,5-Trimethylthiazoline (TMT), in rats, suggests both systemic and processive stress characteristics. Brain Res. 2004;1025:139–151. doi: 10.1016/j.brainres.2004.07.079. [DOI] [PubMed] [Google Scholar]
- 35.De Franceschi G, Vivattanasarn T, Saleem AB, Solomon SG. Vision Guides Selection of Freeze or Flight Defense Strategies in Mice. Curr Biol. 2016;26(16):2150–2154. doi: 10.1016/j.cub.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 36.Delgado MR, Nearing KI, Ledoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59:829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dielenberg RA, Carrive P, McGregor IS. The cardiovascular and behavioral response to cat odor in rats: unconditioned and conditioned effects. Brain Res. 2001;897(1–2):228–237. doi: 10.1016/s0006-8993(01)02227-2. [DOI] [PubMed] [Google Scholar]
- 38.Dielenberg RA, Hunt GE, McGregor IS. "When a rat smells a cat": the distribution of Fos immunoreactivity in rat brain following exposure to a predatory odor. Neuroscience. 2001;104(4):1085–1097. doi: 10.1016/s0306-4522(01)00150-6. [DOI] [PubMed] [Google Scholar]
- 39.Dirikx T, Hermans D, Vansteenwegen D, Baeyens F, Eelen P. Reinstatement of extinguished conditioned responses and negative stimulus valence as a pathway to return of fear in humans. Learn Memory. 2004;11:549–554. doi: 10.1101/lm.78004. [DOI] [PubMed] [Google Scholar]
- 40.Dityatev AE, Bolshakov VY. Amygdala, long-term potentiation, and fear conditioning. Neuroscientist. 2005;11:75–88. doi: 10.1177/1073858404270857. [DOI] [PubMed] [Google Scholar]
- 41.Do Monte FH, Canteras NS, Fernandes D, Assreuy J, Carobrez AP. New perspectives on beta-adrenergic mediation of innate and learned fear responses to predator odor. J Neurosci. 2008;28(49):13296–13302. doi: 10.1523/JNEUROSCI.2843-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Do-Monte FH, Manzano-Nieves G, Quiñones-Laracuente K, Ramos-Medina L, Quirk GJ. Revisiting the role of infralimbic cortex in fear extinction with optogenetics. J Neurosci. 2015;35:3607–3615. doi: 10.1523/JNEUROSCI.3137-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DSM-5. Diagnostic and Statistical Manual of Mental Disorders. 5. Anxiety Disorders; 2013. [Google Scholar]
- 44.Dunsmoor JE, Ahs F, Zielinski DJ, LaBar KS. Extinction in multiple virtual reality contexts diminishes fear reinstatement in humans. Neurobiol Learn Mem. 2014;113:157–164. doi: 10.1016/j.nlm.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duvarci S, Nader K. Characterization of Fear Memory Reconsolidation. J Neurosci. 2004;24:9269–9275. doi: 10.1523/JNEUROSCI.2971-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eisenberg M, Kobilo T, Berman DE, Dudai Y. Stability of retrieved memory: inverse correlation with trace dominance. Science. 2003;301(5636):1102–1104. doi: 10.1126/science.1086881. [DOI] [PubMed] [Google Scholar]
- 47.Emiliani V, Cohen AE, Deisseroth K, Häusser M. All-Optical Interrogation of Neural Circuits. J Neurosci. 2015;35:13917–13926. doi: 10.1523/JNEUROSCI.2916-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Endres T, Fendt M. Conditioned behavioral responses to a context paired with the predator odor trimethylthiazoline. Behav Neurosci. 2007;121:594–601. doi: 10.1037/0735-7044.121.3.594. [DOI] [PubMed] [Google Scholar]
- 49.Fadok JP, Krabbe S, Markovic M, Courtin J, Xu C, Massi L, Botta P, Bylund K, Müller C, Kovacevic A, Tovote P, Lüthi A. A competitive inhibitory circuit for selection of active and passive fear responses. Nature. 2017;542:96–100. doi: 10.1038/nature21047. doi:0.1038/nature21047. [DOI] [PubMed] [Google Scholar]
- 50.Fendt M. Exposure to urine of canids and felids, but not of herbivores, induces defensive behavior in laboratory rats. J Chem Ecol. 2006;32:2617–2627. doi: 10.1007/s10886-006-9186-9. [DOI] [PubMed] [Google Scholar]
- 51.Fendt M, Endres T, Apfelbach R. Temporary inactivation of the bed nucleus of the stria terminalis but not of the amygdala blocks freezing induced by trimethylthiazoline, a component of fox feces. J Neurosci. 2003;23(1):23–28. doi: 10.1523/JNEUROSCI.23-01-00023.2003. Retrieved from http://www.jneurosci.org/content/23/1/23.long. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fendt M, Siegl S, Steiniger-Brach B. Noradrenaline transmission within the ventral bed nucleus of the stria terminalis is critical for fear behavior induced by trimethylthiazoline, a component of fox odor. J Neurosci. 2005;25(25):5998–6004. doi: 10.1523/JNEUROSCI.1028-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fenno LE, Mattis J, Ramakrishnan C, Hyun M, Lee SY, He M, Tucciarone J, Selimbeyoglu A, Berndt A, Grosenick L, Zalocusky KA, Bernstein H, Swanson H, Perry C, Diester I, Boyce FM, Bass CE, Neve R, Huang ZJ, Deisseroth K. Targeting cells with single vectors using multiple-feature Boolean logic. Nature Methods. 2014;11(7):763–772. doi: 10.1038/nmeth.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fortin DL, Dunn TW, Fedorchak A, Allen D, Montpetit R, Banghart MR, Trauner D, Adelman JP, Kramer RH. Optogenetic photochemical control of designer K+ channels in mammalian neurons. J Neurophysiol. 2011;106:488–496. doi: 10.1152/jn.00251.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fu T-M, Hong G, Zhou T, Schuhmann TG, Viveros RD, Lieber CM. Stable long-term chronic brain mapping at the single-neuron level. Nature Methods. 2016;13(10):875–882. doi: 10.1038/nmeth.3969. [DOI] [PubMed] [Google Scholar]
- 56.Fukushima H, Zhang Y, Archbold G, Ishikawa R, Nader K, Kida S. Enhancement of fear memory by retrieval through reconsolidation. eLife. 2014;3:e02736. doi: 10.7554/eLife.02736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Furini C, Myskiw J, Izquierdo I. The learning of fear extinction. Neurosci Biobehav Rev. 2014;47:670–683. doi: 10.1016/j.neubiorev.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 58.Goode TD, Kim JJ, Maren S. Reversible Inactivation of the Bed Nucleus of the Stria Terminalis Prevents Reinstatement But Not Renewal of Extinguished Fear. eNeuro. 2015;2(3) doi: 10.1523/ENEURO.0037-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gorostiza P, Volgraf M, Numano R, Szobota S, Trauner D, Isacoff EY. Mechanisms of photoswitch conjugation and light activation of an ionotropic glutamate receptor. Proc Natl Acad Sci U S A. 2007;104:10865–10870. doi: 10.1073/pnas.0701274104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goshen I. The optogenetic revolution in memory research. Trends Neurosci. 2014;37:511–522. doi: 10.1016/j.tins.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 61.Gradinaru V, Thompson KR, Deisseroth K. eNpHR: a Natronomonas halorhodopsin enhanced for optogenetic applications. Brain Cell Biol. 2008;36(1–4):129–139. doi: 10.1007/s11068-008-9027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gradinaru V, Zhang F, Ramakrishnan C, Mattis J, Prakash R, Diester I, Goshen I, Thompson KR, Deisseroth K. Molecular and cellular approaches for diversifying and extending optogenetics. Cell. 2010;141:154–165. doi: 10.1016/j.cell.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grosenick L, Marshel JH, Deisseroth K. Closed-loop and activity-guided optogenetic control. Neuron. 2015;86(1):106–139. doi: 10.1016/j.neuron.2015.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gross CT, Canteras NS. The many paths to fear. Nat Rev Neurosci. 2012;13(9):651–658. doi: 10.1038/nrn3301. [DOI] [PubMed] [Google Scholar]
- 65.Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, Lammel S, Mirzabekov JJ, Airan RD, Zalocusky KA, Tye KM, Anikeeva P, Malenka RC, Deisseroth K. Natural neural projection dynamics underlying social behavior. Cell. 2014;157:1535–1551. doi: 10.1016/j.cell.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guru A, Post RJ, Ho Y-Y, Warden MR. Making Sense of Optogenetics. Int J Neuropsychopharmacol. 2015;18(11):pyv079. doi: 10.1093/ijnp/pyv079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hägglund M, Borgius L, Dougherty KJ, Kiehn O. Activation of groups of excitatory neurons in the mammalian spinal cord or hindbrain evokes locomotion. Nat Neurosci. 2010;13(2):246–252. doi: 10.1038/nn.2482. [DOI] [PubMed] [Google Scholar]
- 68.Han X, Boyden ES. Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution. PloS one. 2007;2(3):e299. doi: 10.1371/journal.pone.0000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Han X, Chow BY, Zhou H, Klapoetke NC, Chuong A, Rajimehr R, Yang A, Baratta MV, Winkle J, Desimone R, Boyden ES. A high-light sensitivity optical neural silencer: development and application to optogenetic control of non-human primate cortex. Front Syst Neurosci. 2011;5:18. doi: 10.3389/fnsys.2011.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong H-W, Deisseroth K, Callaway EM, Fanselow MS, Lüthi A, Anderson DJ. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468(7321):270–276. doi: 10.1038/nature09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Häusser M. Optogenetics: the age of light. Nature Methods. 2014;11:1012–1014. doi: 10.1038/nmeth.3111. [DOI] [PubMed] [Google Scholar]
- 72.Hermans D, Dirikx T, Vansteenwegen D, Vansteenwegenin D, Baeyens F, den Bergh OV, Eelen P. Reinstatement of fear responses in human aversive conditioning. Behav Res Ther. 2005;43:533–551. doi: 10.1016/j.brat.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 73.Herry C, Ciocchi S, Senn V, Demmou L, Müller C, Lüthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- 74.Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Lüthi A. Neuronal circuits of fear extinction. Eur J Neurosci. 2010;31:599–612. doi: 10.1111/j.1460-9568.2010.07101.x. [DOI] [PubMed] [Google Scholar]
- 75.Homberg JR. Serotonergic modulation of conditioned fear. Scientifica. 2012;2012:821549. doi: 10.6064/2012/821549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hong G, Fu T-M, Zhou T, Schuhmann TG, Huang J, Lieber CM. Syringe Injectable Electronics: Precise Targeted Delivery with Quantitative Input/Output Connectivity. Nano Lett. 2015;15:6979–6984. doi: 10.1021/acs.nanolett.5b02987. [DOI] [PubMed] [Google Scholar]
- 77.Inda MC, Muravieva EV, Alberini CM. Memory Retrieval and the Passage of Time: From Reconsolidation and Strengthening to Extinction. J Neurosci. 2011;31:1635–1643. doi: 10.1523/JNEUROSCI.4736-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Isosaka T, Matsuo T, Yamaguchi T, Funabiki K, Nakanishi S, Kobayakawa R, Kobayakawa K. Htr2a-Expressing Cells in the Central Amygdala Control the Hierarchy between Innate and Learned Fear. Cell. 2015;163(5):1153–1164. doi: 10.1016/j.cell.2015.10.047. [DOI] [PubMed] [Google Scholar]
- 79.Jasnow AM, Ehrlich DE, Choi DC, Dabrowska J, Bowers ME, McCullough KM, Rainnie DG, Ressler KJ. Thy1-expressing neurons in the basolateral amygdala may mediate fear inhibition. J Neurosci. 2013;33:10396–10404. doi: 10.1523/JNEUROSCI.5539-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ji G, Neugebauer V. Modulation of medial prefrontal cortical activity using in vivo recordings and optogenetics. Mol Brain. 2012;5:36. doi: 10.1186/1756-6606-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Johansen JP, Wolff SB, Lüthi A, LeDoux JE. Controlling the elements: an optogenetic approach to understanding the neural circuits of fear. Biol Psychiatry. 2012;71:1053–1060. doi: 10.1016/j.biopsych.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kendler KS, Myers J, Prescott CA. The etiology of phobias: an evaluation of the stress-diathesis model. Arch Gen Psychiatry. 2002;59(3):242–248. doi: 10.1001/archpsyc.59.3.242. [DOI] [PubMed] [Google Scholar]
- 83.Kessler RC, Aguilar-Gaxiola S, Alonso J, Chatterji S, Lee S, Ormel J, Ustün TB, Wang PS. The global burden of mental disorders: an update from the WHO World Mental Health (WMH) surveys. Epidemiol Psichiatr Soc. 2009;18(1):23–33. doi: 10.1017/s1121189x00001421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim H-S, Cho H-Y, Augustine GJ, Han J-H. Selective Control of Fear Expression by Optogenetic Manipulation of Infralimbic Cortex after Extinction. Neuropsychopharmacology. 2016;41:1261–1273. doi: 10.1038/npp.2015.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim J, Zhang X, Muralidhar S, LeBlanc SA, Tonegawa S. Basolateral to central amygdala neural circuits for appetitive behaviors. Neuron. 2017;93:1464–1479. doi: 10.1016/j.neuron.2017.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim JH, Richardson R. The effect of temporary amygdala inactivation on extinction and reextinction of fear in the developing rat: unlearning as a potential mechanism for extinction early in development. J Neurosci. 2008;28:1282–1290. doi: 10.1523/JNEUROSCI.4736-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim J-M, Hwa J, Garriga P, Reeves PJ, RajBhandary UL, Khorana HG. Light-driven activation of beta 2-adrenergic receptor signaling by a chimeric rhodopsin containing the beta 2-adrenergic receptor cytoplasmic loops. Biochemistry. 2005;44(7):2284–2292. doi: 10.1021/bi048328i. [DOI] [PubMed] [Google Scholar]
- 88.Kim T-i, McCall JG, Jung YH, Huang X, Siuda ER, Li Y, Song J, Song YM, Pao HA, Kim R-H, Lu C, Lee SD, Song I-S, Shin G, Al-Hasani R, Kim S, Tan MP, Huang Y, Omenetto FG, Rogers JA, Bruchas MR. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science. 2013;340:211–216. doi: 10.1126/science.1232437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kleinlogel S, Terpitz U, Legrum B, Gökbuget D, Boyden ES, Bamann C, Wood PG, Bamberg E. A gene-fusion strategy for stoichiometric and co-localized expression of light-gated membrane proteins. Nature Methods. 2011;8:1083–1088. doi: 10.1038/nmeth.1766. [DOI] [PubMed] [Google Scholar]
- 90.Kobayakawa K, Kobayakawa R. Animal Repellents. U.S. Patent 2011
- 91.Kramer RH, Mourot A, Adesnik H. Optogenetic pharmacology for control of native neuronal signaling proteins. Nat Neurosci. 2013;16:816–823. doi: 10.1038/nn.3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lalumiere R. Optogenetic dissection of amygdala functioning. Front Behav Neurosci. 2014;8 doi: 10.3389/fnbeh.2014.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Land B, Brayton C, Furman K, LaPalombara Z, DiLeone R. Optogenetic inhibition of neurons by internal light production. Front Behav Neurosci. 2014;8:108. doi: 10.3389/fnbeh.2014.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.LeDoux J. Fear and the brain: where have we been, and where are we going? Biol Psychiatry. 1998;44:1229–1238. doi: 10.1016/s0006-3223(98)00282-0. [DOI] [PubMed] [Google Scholar]
- 95.LeDoux J. Rethinking the emotional brain. Neuron. 2012;73(4):653–676. doi: 10.1016/j.neuron.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 97.Lee AT, Vogt D, Rubenstein JL, Sohal VS. A class of GABAergic neurons in the prefrontal cortex sends long-range projections to the nucleus accumbens and elicits acute avoidance behavior. J Neurosci. 2014;34(35):11519–11525. doi: 10.1523/JNEUROSCI.1157-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lemoine D, Habermacher C, Martz A, Méry P-F, Bouquier N, Diverchy F, Taly A, Rassendren F, Specht A, Grutter T. Optical control of an ion channel gate. Proc Natl Acad Sci U S A. 2013;110(51):20813–20818. doi: 10.1073/pnas.1318715110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Levitz J, Pantoja C, Gaub B, Janovjak H, Reiner A, Hoagland A, Schoppik D, Kane B, Stawski P, Schier AF, Trauner D, Isacoff EY. Optical control of metabotropic glutamate receptors. Nat Neurosci. 2013;16:507–516. doi: 10.1038/nn.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li C-I, Maglinao TL, Takahashi LK. Medial amygdala modulation of predator odor-induced unconditioned fear in the rat. Behav Neurosci. 2004;118:324–332. doi: 10.1037/0735-7044.118.2.324. [DOI] [PubMed] [Google Scholar]
- 101.Li H, Penzo MA, Taniguchi H, Kopec CD, Huang ZJ, Li B. Experience-dependent modification of a central amygdala fear circuit. Nat Neurosci. 2013;16(3):332–339. doi: 10.1038/nn.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li Y, Meloni EG, W A, Jr, Milad MR, Pitman RK, Nader K, Bolshakov VY. Learning and reconsolidation implicate different synaptic mechanisms. Proc Natl Acad Sci U S A. 2013;110:4798–4803. doi: 10.1073/pnas.1217878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Likhtik E, Pelletier JG, Paz R, Paré D. Prefrontal control of the amygdala. J Neurosci. 2005;25(32):7429–7437. doi: 10.1523/JNEUROSCI.2314-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Paré D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454(7204):642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lin JY, Knutsen PM, Muller A, Kleinfeld D, Tsien RY. ReaChR: a red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nat Neurosci. 2013;16:1499–1508. doi: 10.1038/nn.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lin W-C, Davenport CM, Mourot A, Vytla D, Smith CM, Medeiros KA, Chambers JJ, Kramer RH. Engineering a light-regulated GABAA receptor for optical control of neural inhibition. ACS Chem Biol. 2014;9:1414–1419. doi: 10.1021/cb500167u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lin W-C, Tsai M-C, Davenport CM, Smith CM, Veit J, Wilson NM, Adesnik H, Kramer RH. A Comprehensive Optogenetic Pharmacology Toolkit for In Vivo Control of GABAA Receptors and Synaptic Inhibition. Neuron. 2015;88(5):879–891. doi: 10.1016/j.neuron.2015.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu J, Fu T-M, Cheng Z, Hong G, Zhou T, Jin L, Duvvuri M, Jiang Z, Kruskal P, Xie C, Suo Z, Fang Y, Lieber CM. Syringe-injectable electronics. Nat Nanotechnol. 2015;10:629–636. doi: 10.1038/nnano.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Madisen L, Mao T, Koch H, Zhuo J-m, Berenyi A, Fujisawa S, Hsu Y-WA, A JG, 3rd, Gu X, Zanella S, Kidney J, Gu H, Mao Y, Hooks BM, Boyden ES, Buzsáki G, Ramirez JM, Jones AR, Svoboda K, Han X, Turner EE, Zeng H. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat Neurosci. 2012;15:793–802. doi: 10.1038/nn.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mahn M, Prigge M, Ron S, Levy R, Yizhar O. Biophysical constraints of optogenetic inhibition at presynaptic terminals. Nat Neurosci. 2016;19:554–556. doi: 10.1038/nn.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Maren S, Chang C-h. Recent fear is resistant to extinction. Proc Natl Acad Sci U S A. 2006;103(47):18020–18025. doi: 10.1073/pnas.0608398103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- 113.McCall JG, Kim T-i, Shin G, Huang X, Jung YH, Al-Hasani R, Omenetto FG, Bruchas MR, Rogers JA. Fabrication and application of flexible, multimodal light-emitting devices for wireless optogenetics. Nat Protoc. 2013;8:2413–2428. doi: 10.1038/nprot.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.McCullough KM, Choi D, Guo J, Zimmerman K, Walton J, Rainnie DG, Ressler KJ. Molecular characterization of Thy1 expressing fear-inhibiting neurons within the basolateral amygdala. Nat Commun. 2016;7:13149. doi: 10.1038/ncomms13149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McGregor IS, Schrama L, Ambermoon P, Dielenberg RA. Not all 'predator odours' are equal: cat odour but not 2,4,5 trimethylthiazoline (TMT; fox odour) elicits specific defensive behaviours in rats. Behav Brain Res. 2002;129(1–2):1–16. doi: 10.1016/s0166-4328(01)00324-2. Retrieved from http://www.sciencedirect.com/science/article/pii/S0166432801003242. [DOI] [PubMed] [Google Scholar]
- 116.McKernan MG, Shinnick-Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature. 1997;390(6660):607–611. doi: 10.1038/37605. [DOI] [PubMed] [Google Scholar]
- 117.McNally GP, Pigg M, Weidemann G. Opioid receptors in the midbrain periaqueductal gray regulate extinction of pavlovian fear conditioning. J Neurosci. 2004;24:6912–6919. doi: 10.1523/JNEUROSCI.1828-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 119.Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Milad MR, Orr SP, Pitman RK, Rauch SL. Context modulation of memory for fear extinction in humans. Psychophysiology. 2005;42:456–464. doi: 10.1111/j.1469-8986.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- 121.Milad MR, Rosenbaum BL, Simon NM. Neuroscience of fear extinction: implications for assessment and treatment of fear-based and anxiety related disorders. Behav Res Ther. 2014;62:17–23. doi: 10.1016/j.brat.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 122.Mueller D, Cahill SP. Noradrenergic modulation of extinction learning and exposure therapy. Behav Brain Res. 2010;208(1):1–11. doi: 10.1016/j.bbr.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 123.Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- 124.Myers KM, Ressler KJ, Davis M. Different mechanisms of fear extinction dependent on length of time since fear acquisition. Learn Memory. 2006;13:216–223. doi: 10.1101/lm.119806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nader K, Schafe GE, LeDoux JE. The labile nature of consolidation theory. Nature Rev Neurosci. 2000;1(3):216–219. doi: 10.1038/35044580. [DOI] [PubMed] [Google Scholar]
- 126.Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci U S A. 2003;100:13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nathanson JL, Yanagawa Y, Obata K, Callaway EM. Preferential labeling of inhibitory and excitatory cortical neurons by endogenous tropism of adeno-associated virus and lentivirus vectors. Neuroscience. 2009;161(2):441–450. doi: 10.1016/j.neuroscience.2009.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Oh E, Maejima T, Liu C, Deneris E, Herlitze S. Substitution of 5-HT1A receptor signaling by a light-activated G protein-coupled receptor. J Biol Chem. 2010;285(40):30825–30836. doi: 10.1074/jbc.M110.147298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Oh SW, Harris JA, Ng L, Winslow B, Cain N, Mihalas S, Wang Q, Lau C, Kuan L, Henry AM, Mortrud MT, Ouellette B, Nguyen TN, Sorensen SA, Slaughterbeck CR, Wakeman W, Li Y, Feng D, Ho A, Nicholas E, Hirokawa KE, Bohn P, Joines KM, Peng H, Hawrylycz MJ, Phillips JW, Hohmann JG, Wohnoutka P, Gerfen CR, Koch C, Bernard A, Dang C, Jones AR, Zeng H. A mesoscale connectome of the mouse brain. Nature. 2014;508:207–214. doi: 10.1038/nature13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Orsini CA, Maren S. Neural and cellular mechanisms of fear and extinction memory formation. Neurosci Biobehav Rev. 2012;36:1773–1802. doi: 10.1016/j.neubiorev.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pape H-C, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Parsons RG, Gafford GM, Helmstetter FJ. Regulation of extinction-related plasticity by opioid receptors in the ventrolateral periaqueductal gray matter. Front Behav Neurosci. 2010;4 doi: 10.3389/fnbeh.2010.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pavlov IP. Conditioned reflexes: An investigation of the physiological activity of the cerebral cortex. Oxford University Press; 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pedreira ME, Maldonado H. Protein synthesis subserves reconsolidation or extinction depending on reminder duration. Neuron. 2003;38(6):863–869. doi: 10.1016/s0896-6273(03)00352-0. [DOI] [PubMed] [Google Scholar]
- 135.Pedroso TR, Jobim PF, Carvalho LM, Christoff RR, Maurmann N, Reolon GK, Werenicz A, Roesler R. Inhibition of protein synthesis or mTOR in the basolateral amygdala blocks retrieval-induced memory strengthening. J Neural Transm. 2013;120:1525–1531. doi: 10.1007/s00702-013-1032-y. [DOI] [PubMed] [Google Scholar]
- 136.Pentkowski NS, Blanchard DC, Lever C, Litvin Y, Blanchard RJ. Effects of lesions to the dorsal and ventral hippocampus on defensive behaviors in rats. Eur J Neurosci. 2006;23(8):2185–2196. doi: 10.1111/j.1460-9568.2006.04754.x. [DOI] [PubMed] [Google Scholar]
- 137.Penzo MA, Robert V, Li B. Fear conditioning potentiates synaptic transmission onto long-range projection neurons in the lateral subdivision of central amygdala. J Neurosci. 2014;34:2432–2437. doi: 10.1523/JNEUROSCI.4166-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Penzo MA, Robert V, Tucciarone J, De Bundel D, Wang M, Van Aelst L, Darvas M, Parada LF, Palmiter RD, He M, Huang ZJ, Li B. The paraventricular thalamus controls a central amygdala fear circuit. Nature. 519:455–459. doi: 10.1038/nature13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Perea G, Yang A, Boyden ES, Sur M. Optogenetic astrocyte activation modulates response selectivity of visual cortex neurons in vivo. Nat Commun. 2014;5(3262) doi: 10.1038/ncomms4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pereira AG, Moita MA. Is there anybody out there? Neural circuits of threat detection in vertebrates. Curr Opin Neurobiol. 2016;41:179–187. doi: 10.1016/j.conb.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 141.Perez-Gomez A, Bleymehl K, Stein B, Pyrski M, Birnbaumer L, Munger SD, Leinders-Zufall T, Zufall F, Chamero P. Innate Predator Odor Aversion Driven by Parallel Olfactory Subsystems that Converge in the Ventromedial Hypothalamus. Curr Biol. 2015;25:1340–1346. doi: 10.1016/j.cub.2015.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Memory. 2009;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 144.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Raimondo JV, Kay L, Ellender TJ, Akerman CJ. Optogenetic silencing strategies differ in their effects on inhibitory synaptic transmission. Nat Neurosci. 2012;15:1102–1104. doi: 10.1038/nn.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rein ML, Deussing JM. The optogenetic (r)evolution. Mol Genet Genomics. 2012;287:95–109. doi: 10.1007/s00438-011-0663-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Riccio A, Li Y, Moon J, Kim K-S, Smith KS, Rudolph U, Gapon S, Yao GL, Tsvetkov E, Rodig SJ, Veer AV, Meloni EG, W AC, Jr, Bolshakov VY, Clapham DE. Essential role for TRPC5 in amygdala function and fear-related behavior. Cell. 2009;137:761–772. doi: 10.1016/j.cell.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Riccio A, Li Y, Tsvetkov E, Gapon S, Yao GL, Smith KS, Engin E, Rudolph U, Bolshakov VY, Clapham DE. Decreased anxiety-like behavior and Gαq/11-dependent responses in the amygdala of mice lacking TRPC4 channels. J Neurosci. 2014;34:3653–3667. doi: 10.1523/JNEUROSCI.2274-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Riga D, Matos MR, Glas A, Smit AB, Spijker S, Van, Michel Cd. Optogenetic dissection of medial prefrontal cortex circuitry. Front Syst Neurosci. 2014;8:230. doi: 10.3389/fnsys.2014.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rogan MT, Stäubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390(6660):604–607. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- 151.Root CM, Denny CA, Hen R, Axel R. The participation of cortical amygdala in innate, odour-driven behaviour. Nature. 2014;515:269–273. doi: 10.1038/nature13897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rosen JB. The neurobiology of conditioned and unconditioned fear: a neurobehavioral system analysis of the amygdala. Behav Cogn Neurosci Rev. 2004;3(1):23–41. doi: 10.1177/1534582304265945. [DOI] [PubMed] [Google Scholar]
- 153.Rosen JB, Asok A, Chakraborty T. The smell of fear: innate threat of 2,5-dihydro-2,4,5-trimethylthiazoline, a single molecule component of a predator odor. Front Neurosci. 2015;9:292. doi: 10.3389/fnins.2015.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rosen JB, Pagani JH, Rolla KL, Davis C. Analysis of behavioral constraints and the neuroanatomy of fear to the predator odor trimethylthiazoline: a model for animal phobias. Neurosci Biobehav Rev. 2008;32(7):1267–1276. doi: 10.1016/j.neubiorev.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 155.Royer S, Martina M, Paré D. An inhibitory interface gates impulse traffic between the input and output stations of the amygdala. J Neurosci. 1999;19(23):10575–10583. doi: 10.1523/JNEUROSCI.19-23-10575.1999. Retrieved from http://www.jneurosci.org/content/19/23/10575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308(5718):83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- 157.Sakurai K, Zhao S, Takatoh J, Rodriguez E, Lu J, Leavitt A, Fu M, Han B-X, Wang F. Capturing and Manipulating Activated Neuronal Ensembles with CANE Delineates a Hypothalamic Social-Fear Circuit. Neuron. 2016;92:739–753. doi: 10.1016/j.neuron.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sandoz G, Levitz J, Kramer RH, Isacoff EY. Optical control of endogenous proteins with a photoswitchable conditional subunit reveals a role for TREK1 in GABA(B) signaling. Neuron. 2012;74:1005–1014. doi: 10.1016/j.neuron.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sanford CA, Soden ME, Baird MA, Miller SM, Schulkin J, Palmiter RD, Clark M, Zweifel LS. A central amygdala CRF circuit facilitates learning about weak threats. Neuron. 2017;93:164–178. doi: 10.1016/j.neuron.2016.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Schiller D, Cain CK, Curley NG, Schwartz JS, Stern SA, Ledoux JE, Phelps EA. Evidence for recovery of fear following immediate extinction in rats and humans. Learn Memory. 2008;15:394–402. doi: 10.1101/lm.909208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Senn V, Wolff SB, Herry C, Grenier F, Ehrlich I, Gründemann J, Fadok JP, Müller C, Letzkus JJ, Lüthi A. Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron. 2014;81:428–437. doi: 10.1016/j.neuron.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 162.Shang C, Liu Z, Chen Z, Shi Y, Wang Q, Liu S, Li D, Cao P. BRAIN CIRCUITS. A parvalbumin-positive excitatory visual pathway to trigger fear responses in mice. Science. 2015;348(6242):1472–1477. doi: 10.1126/science.aaa8694. [DOI] [PubMed] [Google Scholar]
- 163.Shin R-M, Tsvetkov E, Bolshakov VY. Spatiotemporal asymmetry of associative synaptic plasticity in fear conditioning pathways. Neuron. 2006;52:883–896. doi: 10.1016/j.neuron.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Shin R-M, Tully K, Li Y, Cho J-H, Higuchi M, Suhara T, Bolshakov VY. Hierarchical order of coexisting pre- and postsynaptic forms of long-term potentiation at synapses in amygdala. Proc Natl Acad Sci U S A. 2010;107:19073–19078. doi: 10.1073/pnas.1009803107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shumyatsky GP, Malleret G, Shin R-M, Takizawa S, Tully K, Tsvetkov E, Zakharenko SS, Joseph J, Vronskaya S, Yin D, Schubart UK, Kandel ER, Bolshakov VY. stathmin, a gene enriched in the amygdala, controls both learned and innate fear. Cell. 2005;123:697–709. doi: 10.1016/j.cell.2005.08.038. [DOI] [PubMed] [Google Scholar]
- 166.Shumyatsky GP, Tsvetkov E, Malleret G, Vronskaya S, Hatton M, Hampton L, Battey JF, Dulac C, Kandel ER, Bolshakov VY. Identification of a signaling network in lateral nucleus of amygdala important for inhibiting memory specifically related to learned fear. Cell. 2002;111:905–918. doi: 10.1016/s0092-8674(02)01116-9. [DOI] [PubMed] [Google Scholar]
- 167.Singewald N, Schmuckermair C, Whittle N, Holmes A, Ressler KJ. Pharmacology of cognitive enhancers for exposure-based therapy of fear, anxiety and trauma-related disorders. Pharmacol Ther. 2015;149:150–190. doi: 10.1016/j.pharmthera.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sotres-Bayon F, Cain CK, LeDoux JE. Brain mechanisms of fear extinction: historical perspectives on the contribution of prefrontal cortex. Biol Psychiatry. 2006;60:329–336. doi: 10.1016/j.biopsych.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 170.Sparta DR, Stamatakis AM, Phillips JL, Hovelsø N, van Zessen R, Stuber GD. Construction of implantable optical fibers for long-term optogenetic manipulation of neural circuits. Nat Protoc. 2011;7(1):12–23. doi: 10.1038/nprot.2011.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Stiedl O, Pappa E, Konradsson-Geuken Å, Ögren SO. The role of the serotonin receptor subtypes 5-HT1A and 5-HT7 and its interaction in emotional learning and memory. Front Pharmacol. 2015;6:162. doi: 10.3389/fphar.2015.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Stockhorst U, Antov MI. Modulation of Fear Extinction by Stress, Stress Hormones and Estradiol: A Review. Front Behav Neurosci. 2015;9:359. doi: 10.3389/fnbeh.2015.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Strobel C, Marek R, Gooch HM, Sullivan RK, Sah P. Prefrontal and Auditory Input to Intercalated Neurons of the Amygdala. Cell Rep. 2015 doi: 10.1016/j.celrep.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 174.Szobota S, Isacoff EY. Optical control of neuronal activity. Annu Rev Biophys. 2010;39:329–348. doi: 10.1146/annurev.biophys.093008.131400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Takahashi LK. Olfactory systems and neural circuits that modulate predator odor fear. Front Behav Neurosci. 2014;8:72. doi: 10.3389/fnbeh.2014.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Takahashi LK, Chan MM, Pilar ML. Predator odor fear conditioning: current perspectives and new directions. Neurosci Biobehav Rev. 2008;32(7):1218–1227. doi: 10.1016/j.neubiorev.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Takahashi LK, Nakashima BR, Hong H, Watanabe K. The smell of danger: a behavioral and neural analysis of predator odor-induced fear. Neurosci Biobehav Rev. 2005;29:1157–1167. doi: 10.1016/j.neubiorev.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 178.Tang W, Ehrlich I, Wolff SB, Michalski A-M, Wölfl S, Hasan MT, Lüthi A, Sprengel R. Faithful expression of multiple proteins via 2A-peptide self-processing: a versatile and reliable method for manipulating brain circuits. J Neurosci. 2009;29(27):8621–8629. doi: 10.1523/JNEUROSCI.0359-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tervo DG, Hwang B-Y, Viswanathan S, Gaj T, Lavzin M, Ritola KD, Lindo S, Michael S, Kuleshova E, Ojala D, Huang C-C, Gerfen CR, Schiller J, Dudman JT, Hantman AW, Looger LL, Schaffer DV, Karpova AY. A Designer AAV Variant Permits Efficient Retrograde Access to Projection Neurons. Neuron. 2016;92(2):372–382. doi: 10.1016/j.neuron.2016.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tochitsky I, Banghart MR, Mourot A, Yao JZ, Gaub B, Kramer RH, Trauner D. Optochemical control of genetically engineered neuronal nicotinic acetylcholine receptors. Nat Chem. 2012;4:105–111. doi: 10.1038/nchem.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tovote P, Esposito MS, Botta P, Chaudun F, Fadok JP, Markovic M, Wolff SBE, Ramakrishnan C, Fenno L, Deisseroth K, Herry C, Arber S, Lüthi A. Midbrain circuits for defensive behaviour. Nature. 2016;534:206–212. doi: 10.1038/nature17996. [DOI] [PubMed] [Google Scholar]
- 182.Tovote P, Fadok JP, Lüthi A. Neuronal circuits for fear and anxiety. Nature Rev Neurosci. 2015;16(6):317–331. doi: 10.1038/nrn3945. [DOI] [PubMed] [Google Scholar]
- 183.Tsvetkov E, Carlezon WA, Benes FM, Kandel ER, Bolshakov VY. Fear conditioning occludes LTP-induced presynaptic enhancement of synaptic transmission in the cortical pathway to the lateral amygdala. Neuron. 2002;34:289–300. doi: 10.1016/s0896-6273(02)00645-1. [DOI] [PubMed] [Google Scholar]
- 184.Tsvetkov E, Shin RM, Bolshakov VY. Glutamate uptake determines pathway specificity of long-term potentiation in the neural circuitry of fear conditioning. Neuron. 2004;41:139–151. doi: 10.1016/s0896-6273(03)00800-6. [DOI] [PubMed] [Google Scholar]
- 185.Tye KM, Prakash R, Kim S-Y, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Volgraf M, Gorostiza P, Numano R, Kramer RH, Isacoff EY, Trauner D. Allosteric control of an ionotropic glutamate receptor with an optical switch. Nat Chem Biol. 2006;2:47–52. doi: 10.1038/nchembio756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wallace KJ, Rosen JB. Predator odor as an unconditioned fear stimulus in rats: elicitation of freezing by trimethylthiazoline, a component of fox feces. Behav Neurosci. 2000;114:912–922. doi: 10.1037//0735-7044.114.5.912. [DOI] [PubMed] [Google Scholar]
- 188.Wei P, Liu N, Zhang Z, Liu X, Tang Y, He X, Wu B, Zhou Z, Liu Y, Li J, Zhang Y, Zhou X, Xu L, Chen L, Bi G, Hu X, Xu F, Wang L. Processing of visually evoked innate fear by a non-canonical thalamic pathway. Nat Commun. 2015;6(6756) doi: 10.1038/ncomms7756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wietek J, Wiegert JS, Adeishvili N, Schneider F, Watanabe H, Tsunoda SP, Vogt A, Elstner M, Oertner TG, Hegemann P. Conversion of channelrhodopsin into a light-gated chloride channel. Science. 2014;344:409–412. doi: 10.1126/science.1249375. [DOI] [PubMed] [Google Scholar]
- 190.Woods AM, Bouton ME. Immediate extinction causes a less durable loss of performance than delayed extinction following either fear or appetitive conditioning. Learn Memory. 2008;15(12):909–920. doi: 10.1101/lm.1078508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Xiong XR, Liang F, Zingg B, Ji X-y, Ibrahim LA, Tao HW, Zhang LI. Auditory cortex controls sound-driven innate defense behaviour through corticofugal projections to inferior colliculus. Nat Commun. 2015;6:7224. doi: 10.1038/ncomms8224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Xu H-Y, Liu Y-J, Xu M-Y, Zhang Y-H, Zhang J-X, Wu Y-J. Inactivation of the bed nucleus of the stria terminalis suppresses the innate fear responses of rats induced by the odor of cat urine. Neuroscience. 2012;221:21–27. doi: 10.1016/j.neuroscience.2012.06.056. [DOI] [PubMed] [Google Scholar]
- 193.Yang H, Yang J, Xi W, Hao S, Luo B, He X, Zhu L, Lou H, Yu Y-q, Xu F, Duan S, Wang H. Laterodorsal tegmentum interneuron subtypes oppositely regulate olfactory cue-induced innate fear. Nat Neurosci. 2016;19:283–289. doi: 10.1038/nn.4208. [DOI] [PubMed] [Google Scholar]
- 194.Yilmaz M, Meister M. Rapid innate defensive responses of mice to looming visual stimuli. Curr Biol. 2013;23(20):2011–2015. doi: 10.1016/j.cub.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron. 2011;71(1):9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 196.Yu K, Garcia da Silva P, Albeanu DF, Li B. Central amygdala somatostatin neurons gate passive and active defensive behaviors. J Neurosci. 2016;36:6488–6496. doi: 10.1523/JNEUROSCI.4419-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yuan T-F, Su H. Fear learning through the two visual systems, a commentary on: "A parvalbumin-positive excitatory visual pathway to trigger fear responses in mice". Front Neural Circuits. 2015;9:56. doi: 10.3389/fncir.2015.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zalocusky KA, Fenno LE, Deisseroth K. In: Current challenges in Optogenetics. Hegemann P, Sigrist S, editors. Berlin/Boston: Walter de Gruyter GmbH; 2013. [Google Scholar]
- 199.Zalocusky KA, Ramakrishnan C, Lerner TN, Davidson TJ, Knutson B, Deisseroth K. Nucleus accumbens D2R cells signal prior outcomes and control risky decision-making. Nature. 2016;531:642–646. doi: 10.1038/nature17400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zalocusky K, Deisseroth K. Optogenetics in the behaving rat: integration of diverse new technologies in a vital animal model. Optogenetics. 2013;1:1–17. [Google Scholar]
- 201.Zhang F, Gradinaru V, Adamantidis AR, Durand R, Airan RD, de Lecea L, Deisseroth K. Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nat Protoc. 2010;5:439–456. doi: 10.1038/nprot.2009.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zhang F, Vierock J, Yizhar O, Fenno LE, Tsunoda S, Kianianmomeni A, Prigge M, Berndt A, Cushman J, Polle J, Magnuson J, Hegemann P, Deisseroth K. The microbial opsin family of optogenetic tools. Cell. 2011;147(7):1446–1457. doi: 10.1016/j.cell.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhang F, Wang L-P, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, Deisseroth K. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- 204.Zhao S, Cunha C, Zhang F, Liu Q, Gloss B, Deisseroth K, Augustine GJ, Feng G. Improved expression of halorhodopsin for light-induced silencing of neuronal activity. Brain cell biology. 2008;36(1–4):141–154. doi: 10.1007/s11068-008-9034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zhao S, Ting JT, Atallah HE, Qiu L, Tan J, Gloss B, Augustine GJ, Deisseroth K, Luo M, Graybiel AM, Feng G. Cell type–specific channelrhodopsin-2 transgenic mice for optogenetic dissection of neural circuitry function. Nature Methods. 2011;8:745–752. doi: 10.1038/nmeth.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zingg B, Chou X-L, Zhang Z-G, Mesik L, Liang F, Tao HW, Zhang LI. AAV-Mediated Anterograde Transsynaptic Tagging: Mapping Corticocollicular Input-Defined Neural Pathways for Defense Behaviors. Neuron. 2016 doi: 10.1016/j.neuron.2016.11.045. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zorzos AN, Boyden ES, Fonstad CG. Multiwaveguide implantable probe for light delivery to sets of distributed brain targets. Optics letters. 2010;35(24):4133–4135. doi: 10.1364/OL.35.004133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zorzos AN, Scholvin J, Boyden ES, Fonstad CG. Three-dimensional multiwaveguide probe array for light delivery to distributed brain circuits. Optics letters. 2012;37(23):4841–4843. doi: 10.1364/OL.37.004841. [DOI] [PMC free article] [PubMed] [Google Scholar]